Abstract
A 46‐year‐old man 1 year after left‐sided pneumonectomy for squamous cell lung cancer presented with severely limited exercise tolerance and dyspnea corresponding to World Health Organization functional class IV (WHO Class IV). After right heart catheterization (RHC), mean pulmonary artery pressure (mPAP) was 43 mmHg and pulmonary vascular resistance (PVR) was 10.2 Wood units (WU). Arteriography revealed organized clots located at the proximal level of the right pulmonary artery, leading to a diagnosis of chronic thromboembolic pulmonary hypertension (CTEPH). The CTEPH team disqualified the patient from surgical treatment due to high perioperative risk and referred him for balloon pulmonary angioplasty (BPA) together with pulmonary hypertension‐specific pharmacotherapy (sildenafil). The patient underwent a cycle of nine BPA sessions and completed treatment without complications. Follow‐up showed sustained hemodynamic improvement in RHC (mPAP 23 mmHg, PVR 2.6 WU), improved physical capacity (WHO Class II), and relief of dyspnea symptoms.
Keywords: balloon pulmonary angioplasty, chronic thromboembolic pulmonary hypertension, lung cancer, single lung
INTRODUCTION
In chronic thromboembolic pulmonary hypertension (CTEPH) with proximal location of lesions, pulmonary endarterectomy (PEA) is the treatment of choice. 1 In cases of distal disease, high risk of cardiac surgery, or patient refusal to undergo complex surgery, treatment with pulmonary arterial hypertension‐like (PAH‐like) drugs with or without balloon pulmonary angioplasty (BPA) is recommended. 2 The decision on the optimal treatment strategy is made by the multidisciplinary CTEPH team. 3
CASE DESCRIPTION
A 46‐year‐old man diagnosed with squamous cell carcinoma of the left lung underwent left‐sided pneumonectomy in May 2018 (Figure 1a).
Figure 1.
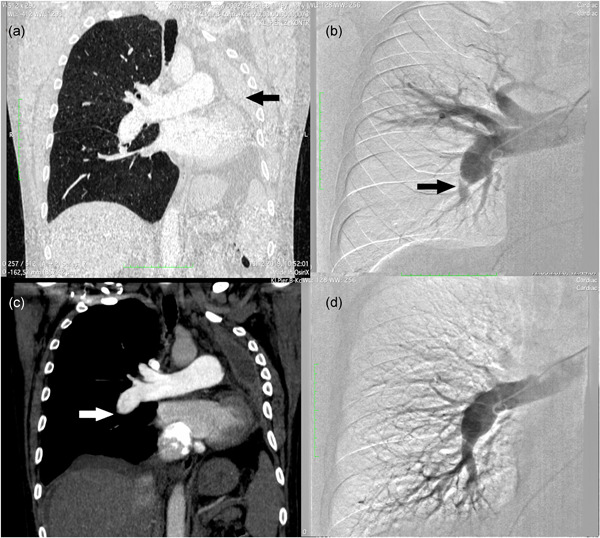
(a) Computed tomography showing reduced chest capacity due to left‐sided pneumonectomy (arrow). (b) Pulmonary angiography was performed before the first BPA session, showing an amputated right lower‐lobe artery (arrow). (c) Computed tomography was performed before the first BPA session, showing proximal occlusion of the right pulmonary artery (arrow). (d) Angiography was performed after the last BPA session with the improvement of distal perfusion. BPA, balloon pulmonary angioplasty.
In January 2019, the patient developed severely limited exercise tolerance and dyspnea. He was admitted with symptoms of World Health Organization (WHO) functional class IV. On physical examination, massive edema of the lower extremities was noted. Laboratory tests revealed elevated serum N‐terminal B‐type natriuretic peptide (NT‐proBNP) 2112 pg/ml and creatinine 1.28 mg/dl. Compressive ultrasound of the deep veins of the lower extremities did not show any thrombus.
After diuretic treatment, a weight loss of 13 kg was observed. The patient remained on continuous passive oxygen therapy with a flow of 3 l/min. The patient's general condition improved. He achieved 126 m in the 6‐min walk test (6MWT), but the test had to be terminated prematurely due to severe dyspnea. During the test, the patient desaturated from 100% to 89% and the respiratory rate increased from 16/min to 28/min. Right heart catheterization (RHC) was performed and resulted in increased mean pulmonary artery pressure (mPAP) 43 mmHg, pulmonary vascular resistance (PVR) 10.2 Wood units (WU), right atrial pressure (RAP) 12 mmHg, and a significantly decreased cardiac index (CI) 1.61 L/min/m2. The pulmonary angiography and angio‐computed tomography showed typical CTEPH lesions located at the proximal level of the right pulmonary artery (Figure 1b,c).
In accord with the RHC results and arteriography images, the patient was diagnosed with CTEPH. He was disqualified from surgery due to comorbidities and qualified for BPA procedures followed by specific pharmacotherapy. Before invasive treatment started, the patient received sildenafil 25 mg three times a day without any side effects.
The patient had a history of heparin‐induced thrombocytopenia (HIT), and for this reason, bivalirudin was used for perioperative bridging anticoagulant therapy. The patient underwent nine sessions of BPA between January 2019 and March 2020. No complications were observed during or after the sessions. A total of 26 vascular lesions were treated (avg. 2.9 per session) in all the segments of the right lung.
A total of 2110 ml of Ultravist 370 contrast medium (Bayer) was administered during the treatment period (avg. 235 ml per session). Renal function was monitored by creatinine measurement during the entire treatment period and remained normal. Follow‐up angiography showed almost complete revascularization of the lower lobe (Figure 1d).
According to the follow‐up protocol for CTEPH patients undergoing a series of BPAs, RHC was performed 4 months after the last session, in July 2020. Outcomes recorded were mPAP 23 mmHg, PVR 2.6 WU, mean right atrial pressure (mRAP) 2 mmHg, and CI 2.20 L/min/m2. At the 6MWT, the patient achieved 426 m (Borg class 0 pts); desaturation from 99% to 95% was noticed, with an increase in respiratory rate from 14/min to 24/min. Laboratory results were NT‐proBNP 203 pg/ml and creatinine 1.01 mg/dl.
The next follow‐up was 16 months after the last procedure, in July 2021. RHC was performed and showed mPAP 24 mmHg, PVR 2.6 WU, mRAP 5 mmHg, and CI 2.14 L/m2. In the 6MWT, the patient achieved 450 m with desaturation from 99% to 93% (Borg scale 3). Control laboratory samples showed mildly increased NT‐proBNP 151 pg/ml and creatinine 1.06 mg/dl (Table 1).
Table 1.
Baseline and follow‐up parameters in a single‐lung patient with proximal CTEPH
| Parameters | Baseline | 3 months after last BPA | 16 months after last BPA |
|---|---|---|---|
| HR | 72 | 72 | 74 |
| mPAP (mmHg) | 43 | 23 | 24 |
| CI (L/min/m2) | 1.6 | 2.2 | 2.1 |
| PVR WU (ARU) | 10.2 (813) | 2.6 (205) | 2.6 (209) |
| mRAP (mmHg) | 12 | 2 | 5 |
| PAWP (mmHg) | 7 | 10 | 11 |
| NT‐pro‐BNP (pg/ml) | 2533 | 145 | 150 |
| FC WHO | IV | II | II |
| TAPSE (mm) | 10 | 19 | 29 |
| AcT (ms) | 56 | 111 | 102 |
| RAA (mm2) | 40 | 25 | 26 |
| IVC (mm) | 32 | 13 | 18 |
Abbreviations: AcT, acceleration time; CI, cardiac index; FC WHO, functional class defined by World Health Organization; HR, heart rate; IVC, inferior vena cava; mPAP, mean pulmonary artery pressure; mRAP, mean right atrial pressure; NT‐proBNP, N‐terminal B‐type natriuretic peptide; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RAA, right atrial area; TAPSE, tricuspid annular plane systolic excursion.
Oncologic follow‐up in January 2021 showed no evidence of recurrence of the neoplastic disease.
DISCUSSION
To our knowledge, we present the first case of balloon pulmonary angioplasty in a single‐lung patient with significant hemodynamic and clinical sequelae in whom treatment of CTEPH with PEA was not possible due to too high risk associated with the surgical procedure and comorbidities. The efficacy of BPA in reducing PVR in the most experienced centers is above 50%, 4 while the efficacy of PAH‐like pharmacotherapy is around 30%. 5 (In our case, it declined from 10.2 to 2.6 WU, a 75% reduction.) Specific medical treatment may improve hemodynamics and functional parameters when used in patients with nonoperable CTEPH. Although riociguat and treprostinil currently are the only approved drugs to treat inoperable CTEPH, results from meta‐analysis suggested that other specific vasodilators may improve distance in 6MWD, reduce PVR, mPAP, and increase CI. 6 In the European multicenter prospective registry for BPA in CTEPH, 69.4% of patients received specific pharmacotherapy before the onset of BPA procedures. 7
In CTEPH with proximal localization of lesions, BPA is not a standard therapeutic option. BPA treatments, unlike PEA, do not evacuate thrombotic materials, but destroy intravascular flow‐obstructing structures. The possibility of successfully treating proximal thrombotic lesions with BPA was recently demonstrated. 8 Previous studies have shown that the efficacy and safety of BPA treatments are similar in operative and nonoperative CTEPH, with more sessions needed in patients with nonoperative CTEPH lesions. 9 In exceptional situations, the use of a vascular stent may be necessary. 10
BPA has a high level of safety even in patients with multiple burdens. Given his history of left lung resection, the occurrence of a reperfusion lung injury complication would have been a life‐threatening condition for the patient. Another key issue was anticoagulant treatment during the surgery period. Due to HIT, bivalirudin was used in a regimen. Despite the use of a large volume of contrast medium, the patient's renal function remains normal. 11 The staged BPA technique provides high procedural safety, 12 reducing the number of complications during and after the procedure.
AUTHOR CONTRIBUTIONS
Conceptualization: Szymon Darocha, Paweł Kurzyna, and Marcin Kurzyna. Patient care: Szymon Darocha, Marta Banaszkiewicz‐Cyganik, Piotr Kędzierski, Michał Florczyk, Arkadiusz Pietrasik, Adam Torbicki, and Marcin Kurzyna. Data curation: Szymon Darocha, Paweł Kurzyna, Marta Banaszkiewicz‐Cyganik, Piotr Kędzierski, and Michał Florczyk. Drafting the article: Szymon Darocha, Paweł Kurzyna, and Marcin Kurzyna. Treatment decisions: Dariusz Zieliński, Andrzej Biederman, Adam Torbicki, and Marcin Kurzyna. Supervision: Marcin Kurzyna
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
Informed consent was obtained from the patient.
ACKNOWLEDGMENT
This study was supported by funds from the statutory activity of the Centre of Postgraduate Medical Education in Warsaw, Warsaw, Poland (grant number 501‐1‐054‐25‐21).
Darocha S, Kurzyna P, Banaszkiewicz‐Cyganik M, Kędzierski P, Florczyk M, Pietrasik A, Zieliński D, Biederman A, Torbicki A, Kurzyna M. An unusual case of CTEPH treated by BPA in a patient with a single lung after cancer surgery. Pulmonary Circulation. 2022;12:e12064. 10.1002/pul2.12064
Szymon Darocha and Paweł Kurzyna contributed equally to this study.
REFERENCES
- 1. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document Group . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 2. Kim NH, Delcroix M, Jais X, Madani MM, Matsubara H, Mayer E, Ogo T, Tapson VF, Ghofrani HA, Jenkins DP. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53:1801915. 10.1183/13993003.01915-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siennicka A, Darocha S, Banaszkiewicz M, Kędzierski P, Dobosiewicz A, Błaszczak P, Peregud‐Pogorzelska M, Kasprzak JD, Tomaszewski M, Mroczek E, Zięba B, Karasek D, Ptaszyńska‐Kopczyńska K, Mizia‐Stec K, Mularek‐Kubzdela T, Doboszyńska A, Lewicka E, Ruchała M, Lewandowski M, Łukasik S, Chrzanowski Ł, Zieliński D, Torbicki A, Kurzyna M. Treatment of chronic thromboembolic pulmonary hypertension in a multidisciplinary team. Ther Adv Respir Dis. 2019;13:1753466619891529. 10.1177/1753466619891529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ogawa A, Satoh T, Fukuda T, Sugimura K, Fukumoto Y, Emoto N, Yamada N, Yao A, Ando M, Ogino H, Tanabe N, Tsujino I, Hanaoka M, Minatoya K, Ito H, Matsubara H. Balloon Pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: results of a multicenter registry. Circ Cardiovasc Qual Outcomes. 2017;10:e004029. 10.1161/CIRCOUTCOMES.117.004029 [DOI] [PubMed] [Google Scholar]
- 5. Ghofrani HA, D'Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, Neuser D, Weimann G, Wang C, CHEST‐1 Study Group. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–29. 10.1056/NEJMoa1209657 [DOI] [PubMed] [Google Scholar]
- 6. Kalra R, Duval S, Thenappan T, Raveendran G, Pritzker M, Prisco SZ, Prins KW. Comparison of balloon pulmonary angioplasty and pulmonary vasodilators for inoperable chronic thromboembolic pulmonary hypertension: a systematic review and meta‐analysis. Sci Rep. 2020;10:8870. 10.1038/s41598-020-65697-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Darocha S, Roik M, Kopeć G, Araszkiewicz A, Furdal M, Lewandowski M, Jacheć W, Grabka M, Banaszkiewicz M, Pietrasik A, Pietura R, Stępniewski J, Waligóra M, Magoń W, Jonas K, Łabyk A, Potępa M, Fudryna A, Jankiewicz S, Sławek‐Szmyt S, Mularek‐Kubzdela T, Lesiak M, Mroczek E, Orłowska J, Peregud‐Pogorzelska M, Tomasik A, Mizia‐Stec K, Przybylski R, Podolec P, Zieliński D, Biederman A, Torbicki A, Pruszczyk P, Kurzyna M. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension: a multicentre registry. EuroIntervention. 2022;17(13):1104–11. 10.4244/EIJ-D-21-00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Darocha S, Araszkiewicz A, Kurzyna M, Banaszkiewicz M, Jankiewicz S, Dobosiewicz A, Sławek‐Szmyt S, Janus M, Grymuza M, Pietrasik A, Mularek‐Kubzdela T, Kędzierski P, Pietura R, Zieliński D, Biederman A, Lesiak M, Torbicki A. Balloon pulmonary angioplasty in technically operable and technically inoperable chronic thromboembolic pulmonary hypertension. J Clin Med. 2021;10:1038. 10.3390/jcm10051038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Minatsuki S, Kiyosue A, Kodera S, Hara T, Saito A, Maki H, Hatano M, Takimoto E, Ando M, Komuro I. Effectiveness of balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension despite having lesion types suitable for surgical treatment. J Cardiol. 2020;75:182–8. [DOI] [PubMed] [Google Scholar]
- 10. Darocha S, Pietura R, Banaszkiewicz M, Pietrasik A, Kownacki Ł, Torbicki A, Kurzyna M. Balloon pulmonary angioplasty with stent implantation as a treatment of proximal chronic thromboembolic pulmonary hypertension. Diagnostics. 2020;10:363. 10.3390/diagnostics10060363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Darocha S, Banaszkiewicz M, Pietrasik A, Siennicka A, Piorunek M, Grochowska E, Piłka M, Dobosiewicz A, Florczyk M, Pietura R, Torbicki A, Kurzyna M. Changes in estimated glomerular filtration after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Cardiorenal Med. 2020;10:22–31. 10.1159/000502254 [DOI] [PubMed] [Google Scholar]
- 12. Kurzyna M, Darocha S, Pietura R, Pietrasik A, Norwa J, Mańczak R, Wieteska M, Biederman A, Matsubara H, Torbicki A. Changing the strategy of balloon pulmonary angioplasty resulted in a reduced complication rate in patients with chronic thromboembolic pulmonary hypertension. A single‐centre European experience. Kardiol Pol. 2017;75:645–54. 10.5603/KP.a2017.0091 [DOI] [PubMed] [Google Scholar]


