Abstract
Protein kinase inhibitors (PKIs) have been implicated in pulmonary vascular toxicities including risk factors for at least three of the five World Health Organization groups of pulmonary hypertension (PH). These toxicities include direct drug‐induced pulmonary arterial hypertension, an increase in cardiomyopathies, and an increase in interstitial lung disease. On‐ and off‐target toxicities are common within multitargeted PKIs leading to cardiopulmonary toxicities. This review highlights the incidence, possible mechanisms, and management strategies for each group of possible PKI‐induced PH. Future identification and clarification of protein kinase pathways for both mechanisms of toxicity and pathophysiology for PH could lead to improvements in patient care in oncology and pulmonary vascular diseases.
Keywords: cancer, dasatinib, protein kinase, pulmonary arterial hypertension, tyrosine kinase
Abbreviations
- ACE
angiotensin converting enzyme
- ALK
anaplastic lymphoma kinase
- ALL
acute lymphoblastic leukemia
- ARB
angiotensin receptor blocker
- ARTEMIS‐IPF
Ambrisentan in Subjects with Pulmonary Hypertension Associated with Idiopathic Pulmonary Fibrosis
- ATP
adenosine triphosphate
- BCR‐ABL1
breakpoint cluster region‐Abelson leukemia gene
- BMP
bone morphogenic protein
- BNP
brain‐natriuretic peptide
- BTK
Bruton's tyrosine kinase
- CCL2
CC ligand chemokine 2
- CDK
cyclin‐dependent kinase
- CML
chronic myeloid leukemia
- CT
computed tomography
- CTEPH
chronic thromboembolic pulmonary hypertension
- EGFR
epidermal growth factor receptor
- ET‐1
endothelin‐1
- FDA
Food and Drug Administration
- FGFR
fibroblast growth factor receptor
- FLT3
FMS‐like tyrosine kinase‐3
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- IGF1
insulin‐like growth factor 1
- ILD
interstitial lung disease
- IMPRES
Imatinib in Pulmonary Arterial Hypertension, a Randomized, Efficacy Study
- INCREASE
Inhaled Treprostinil in Pulmonary Hypertension Due to Interstitial Lung Disease
- IPF
idiopathic pulmonary fibrosis
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- MAPK
mitogen activated protein kinases
- mPAP
mean pulmonary artery pressure
- NO
nitric oxide
- PAH
pulmonary arterial hypertension
- PDGFR
platelet‐derived growth factor
- PGI2
prostacyclin
- PH
pulmonary hypertension
- PI3K
phosphoinositide‐3 kinase
- PKI
protein kinase inhibitor
- PVR
pulmonary vascular resistance
- RCC
renal cell carcinoma
- RHC
right heart catheterization
- RISE‐IIP
Riociguat for idiopathic interstitial pneumonia‐associated pulmonary hypertension
- ROS
reactive oxygen species
- sE‐selectin =
soluble E‐selectin
- sICAM‐1
soluble intercellular adhesion molecule
- sVCAM‐1
soluble vascular cell adhesion molecule
- TGFB1
tumor growth factor‐beta 1
- VEGF
vascular endothelial growth factor
- VTE
venous thromboembolism
- WHO
World Health Organization
INTRODUCTION
Pulmonary hypertension (PH) is defined by the sixth World Symposium as a mean pulmonary artery pressure of greater than 20 mmHg. 1 PH is classified into five different groups based on etiology and pathophysiology as defined by the World Health Organization (WHO). Over the last 30 years, advances in the understanding of pathophysiology, prognosis, and treatment paradigms have led to improved management of patients with PH. 2 Similarly, innovations within cancer therapeutics have led to an improvement in progression‐free survival for cancer survivors. Due to improvements in mortality among cancer survivors, complications from cancer treatment are more prevalent with cardiotoxicities as one of the leading causes of death. 3
Protein kinases inhibitors (PKIs) have become ubiquitous in the field of oncology, being used for treating leukemias, lung cancer, and melanoma, among other forms of malignancy (Table 1). Protein kinases work by transferring phosphoryl groups from adenosine triphosphate (ATP) to proteins. Phosphorylation of these kinases is crucial in cell signaling, proliferation, and survival and disruption can lead to cell death. PKIs inhibit this pathway. 95 , 96 , 97 Two classes of PKIs currently exist based on the binding properties and selectivity of the drug. Type I inhibitors inhibit phosphorylation via competitive binding within the ATP pocket of the substrate. Due to the ubiquity of ATP binding sites, Type I inhibitors exhibit low selectivity. Type II inhibitors demonstrate higher selectivity by binding to both the ATP pocket and an adjacent binding site. 98 , 99
Table 1.
FDA‐approved PKIs with on‐ and off‐target receptors, uses, and adverse drug effects according to WHO Group (as of February 2022)
| Drug | Receptors 4 , 5 | Year approved | Uses | Group 1 risk factors | Group 2 risk factors | Group 3 risk factors |
|---|---|---|---|---|---|---|
| ALK inhibitors | ||||||
| Alectiniba, 6 | ALK | 2015 | NSCLC | ILD | ||
| Brigatiniba, 7 , 8 | ALK, ErbB1 | 2017 | NSCLC | PAH | HTN | ILD |
| Ceritiniba, 9 , 10 | ALK | 2014 | NSCLC | PAH | ILD | |
| Crizotiniba, 11 , 12 | ALK, MET | 2011 | NSCLC | PAH | ILD | |
| Lorlatinib 9 , 13 | ALK, ROS1 | 2018 | NSCLC | PAH | ILD | |
| BTK inhibitors | ||||||
| Acalabrutiniba, 14 | BTK | 2017 | CLL, SLL, MCL | HTN, AF | ||
| Ibrutiniba, 15 | BTK | 2013 | CLL, GVHD, MCL, MZL, SLL, WMG | HTN, AF, HFrEF | ||
| Zanubutinib 16 | BTK | 2019 | MCL | HTN, AF | ||
| BCR‐ABL1 inhibitors | ||||||
| Asciminib 17 | BCR‐ABL1, STAMP | 2021 | CML | HTN, HFrEF | ||
| Bosutiniba, 18 , 19 , 20 , 21 | BCR‐ABL1, Src, FGFR1‐3, VEGFR1‐2, FLT3, PDGFRα/β | 2012 | CML | PAH | HTN, HFrEF | |
| Dasatiniba, 22 , 23 , 24 , 25 , 26 | BCR‐ABL1, FGFR, KIT, PDGFRα/β, Src | 2006 | ALL, CML, GIST | PAH | HTN, HFrEF | |
| Imatinib 27 | BCR‐ABL1, FLT3, KIT, PDGFRα/β | 2001 | ALL, ASM, CEL, CML, DFSP, HES, GIST, MDS/MPD | HTN, HFrEF | ||
| Nilotinib 28 , 29 , 30 | BCR‐ABL1, FLT3, KIT, PDGFRα/β | 2007 | ALL, CML, GIST | PAH | AF | |
| Ponatinib 29 , 31 , 32 , 33 | BCR‐ABL1, FGFR, VEGFR1‐3, FLT3, KIT, PDGFRα/β, Src, TIE2 | 2012 | ALL, CML | PAH | HTN, HFrEF | |
| BRAF/MEK inhibitors | ||||||
| Binimetiniba, 34 | MEK1/2 | 2018 | Melanoma, CRC | HFrEF, HTN | ILD | |
| Cobimetinib 35 | MEK1 | 2015 | Melanoma | HFrEF, HTN | ||
| Dabrafeniba, 36 | BRAF | 2013 | Melanoma, NSCLC, TC | HFrEF | ||
| Encorafeniba, 37 | BRAF | 2018 | CRC, Melanoma | |||
| Selumetinib 38 | MEK1/2 | 2020 | NF1 | HFrEF, HTN | ||
| Trametinib 39 | MEK1/2 | 2013 | Melanoma, NSCLC, TC | HTN, HFrEF | ILD | |
| Vemurafeniba, 40 | BRAF | 2011 | Melanoma, ECD, NSCLC | AF, HTN | ||
| CDK‐4/6 inhibitors | ||||||
| Abemacicliba, 41 | CDK‐4/6 | 2017 | BC | ILD | ||
| Palbocicliba, 42 | CDK‐4/6 | 2015 | BC | ILD | ||
| Ribocicliba, 43 | CDK‐4/6 | 2017 | BC | ILD | ||
| Trilaciclib 44 | CDK‐4/6 | 2021 | Chemo‐induced myelosuppression | ILD | ||
| ErbB inhibitors | ||||||
| Afatiniba, 45 | ErbB1, ErbB2, ErbB4 | 2013 | NSCLC | HFrEF | ILD | |
| Dacomitinib 46 | ErbB1, ErbB2, ErbB4 | 2018 | NSCLC | ILD | ||
| Erlotiniba, 47 | ErbB1 | 2004 | NSCLC, PC | ILD | ||
| Gefitiniba, 48 , 49 | ErbB1 | 2015 | NSCLC | ILD | ||
| Lapatiniba, 50 | ErbB1, ErbB2, ErbB4 | 2007 | BC | HFrEF | ILD | |
| Mobocertinib 51 | ErbB1, ErbB2, ErbB4 | 2021 | NSCLC | AF, HTN, HFrEF | ILD | |
| Neratiniba, 52 | ErbB1, ErbB2 | 2017 | BC | |||
| Osimertiniba, 53 | ErbB1 | 2015 | NSCLC | HFrEF | ILD | |
| Tucatinib 54 | ErbB2 | 2020 | BC | |||
| FGFR inhibitors | ||||||
| Erdafitinib 55 | FGFR | 2019 | UC | HFrEF | ||
| Infigratinib 56 | FGFR | 2021 | Cholangio‐carcinoma | |||
| Nintedaniba, 57 | FGFR, VEGFR1‐3, Src, PDGFR, CSF1 | 2014 | ILD/IPF | HTN | ||
| Pemigatinib 58 | FGFR | 2020 | Cholangio‐carcinoma | |||
| FLT3 inhibitors | ||||||
| Gilteritinib 59 | FLT3, AXL, ALK | 2018 | AML | HFrEF | ILD | |
| Midostaurina, 60 | FLT3, VEGFR2, KIT, PDGFR | 2017 | AML, MCL, ASM | HTN, HFrEF | ILD | |
| JAK inhibitors | ||||||
| Abrocitinib 61 | JAK 1 | 2022 | Atopic dermatitis | HTN | ||
| Baricitiniba, 62 | JAK1/2 | 2018 | RA | |||
| Fedratinib 63 | JAK2, FLT3 | 2019 | Myelofibrosis | HTN, HFrEF | ||
| Ruxolitiniba, 29 , 64 , 65 | JAK1/2 | 2011 | Atopic dermatitis, GVHD, Myelofibrosis, PV | PAH | HTN | |
| Tofacitiniba, 66 | JAK1‐3 | 2012 | RA, PsA, Ulcerative colitis | HTN | ILD | |
| MET inhibitors | ||||||
| Capmatinib 67 | MET | 2020 | NSCLC | ILD | ||
| Tepotinib 68 | MET | 2021 | NSCLC, thyroid cancer | |||
| mTOR inhibitors | ||||||
| Everolimus 69 | mTOR | 2009 | BC, NT, RCC, TS, transplants, WMG | HTN | ILD | |
| Sirolimus 70 | mTOR | 1999 | GVHD, LAM, transplants | HTN | ILD | |
| Temsirolimus 71 | mTOR | 2007 | Endometrial cancer, RCC | HTN | ILD | |
| PDGFR inhibitors | ||||||
| Avapritinib 72 | PDGFRα, KIT | 2020 | GIST | HTN | ||
| Ripretinib 73 | PDGFRα, KIT | 2020 | GIST | HTN, HFrEF | ||
| PI3K‐δ inhibitors | ||||||
| Copanlisiba, 74 | PI3K‐δ | 2017 | FL | HTN | ILD | |
| Idelalisiba, 75 | PI3K‐δ | 2014 | CLL, FL, SLL | ILD | ||
| Umbralisib 76 | PI3K‐δ | 2021 | FL, MZL | ILD | ||
| RET inhibitors | ||||||
| Pralsetinib 77 | RET, DDR1, JAK1/2, TRKA/C, PDGFRβ, FGFR | 2020 | NSCLC, TC | HTN | ILD | |
| Selpercatinib 78 | RET, VEGFR1/3, FGFR | 2020 | NSCLC, TC | HTN | ||
| Vandetaniba, 79 | RET, ErbB1, VEGFR2, TIE2, Src | 2011 | TC | HTN, HFrEF | ILD | |
| TRK inhibitors | ||||||
| Entrectinib 80 | TRKA/B/C, ROS1, ALK | 2019 | NSCLC, NTRK + solid tumors | HTN, HFrEF | ||
| Larotrectinib 81 | TRKA/B/C | 2018 | NTRK + solid tumors | HTN | ||
| VEGF inhibitors | ||||||
| Axitiniba, 82 | VEGF1‐3, FGFR | 2012 | RCC, TC | HTN, HFrEF | ||
| Cabozantinib 83 | VEGFR1‐3, MET, RET, KIT, FLT3, TIE2, TRKB, AXL | 2012 | HCC, RCC, TC | HTN | ||
| Lenvatiniba, 84 | VEGFR1‐3, FGFR, PDGFRα, KIT, RET | 2015 | Endometrial cancer, HCC, RCC, TC | HTN, HFrEF | ||
| Pazopaniba, 85 | VEGFR1‐3, KIT, PDGFRβ | 2009 | RCC, Soft tissue sarcoma, TC | HTN, HFrEF | ILD | |
| Regorafenib 86 | VEGFR2/3, RET, KIT, PDFGR, BRAF | 2012 | CRC, GIST, HCC, osteosarcoma | HTN | ||
| Sorafenib 87 | VEGFR1‐3, FLT3, PDGFRα/β, BCR‐ABL1, FGFR | 2005 | Angiosarcoma, GIST, HCC, RCC, TC | HTN, HFrEF | ILD | |
| Sunitiniba, 88 | VEGFR1‐3, FLT3, PDGFRα/β, BCR‐ABL1, FGFR, Src | 2006 | GIST, PC, RCC | HTN, HFrEF | ||
| Tivozanib 89 | VEGF | 2021 | RCC | HTN, HFrEF | ||
| Other | ||||||
| Belumosudil 90 | ROCK1, ROCK2 | 2021 | GVHD | HTN | ||
| Fostamatiniba, 91 | Syk | 2018 | ITP | HTN | ||
| Netarsudil 92 | Rho | 2017 | Glaucoma | |||
| Pexidartinib 93 | CSF1, KIT, FLT3 | 2019 | Tenosynovial giant cell tumor | HTN |
Abbreviations: ALK, anaplastic lymphoma kinase; ALL, acute lymphoblastic leukemia; ASM, aggressive systemic mastocytosis; AXL, AXL oncogene; BC, breast cancer; BCR‐ABL1, breakpoint cluster region‐Abelson leukemia gene; BTK, Bruton's tyrosine kinase; BRAF, b‐Raf oncogene; CEL, chronic eosinophilic leukemia; CDK, cyclin‐dependent kinase; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; CRC, colorectal cancer; CSF1, colony‐stimulating factor 1; DFSP, dermatofibrosarcoma protuberans; ECD, Erdheim‐Chester disease; ErbB1/EGFR, epidermal growth factor receptor; ErbB2/HER2, human epidermal growth factor receptor 2; ErbB4/HER4, human epidermal growth factor receptor 4; FGFR, fibroblast growth factor receptor; FL, follicular lymphoma; FLT3, Fms‐like tyrosine kinase 3; GIST, gastrointestinal stromal tumor; GVHD, graft versus host disease; HTN, hypertension, HFrEF, heart failure with reduced rejection fraction, HES, hypereosinophilic syndrome; HA, hemolytic anemia; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; ITP, immune thrombocytopenia; JAK, Janus kinase; KIT, c‐KIT oncogene; LAM, lymphangioleiomyomatosis; MCL, mantle cell lymphoma; MEK1/MAPK, mitogen‐activated protein kinase kinase 1; MET/HGHR, hepatocyte growth factor receptor; MDS/MPD, myelodysplastic/myeloproliferative disorder; mTOR, mechanistic target of rapamycin; MZL, marginal zone lymphoma; NF1, neurofibromatosis type 1; NSCLC, non‐small cell lung cancer; NT, neuroendocarine tumor; NTRK, neurotrophic receptor kinase; PAH, pulmonary arterial hypertension; PC, pancreatic cancer; PDGFR, platelet‐derived growth factor receptor; PI3K‐δ, phosphoinositide‐3 kinase delta; PsA, Psoriatic arthritis; PV, polycythemia vera; RA, rheumatoid arthritis; RET, rearranged during transfection oncogene; Rho, Rhodopsin oncogene; ROCK, rho‐associated, coiled‐coil containing protein kinase; ROS1, C‐ros oncogene 1; SLL, small lymphocytic lymphoma; Src, Src oncogene; STAMP, specifically targeting the ABL myristoyl pocket; Syk, Spleen‐associated tyrosine kinase; TC, thyroid cancer; TE, thromboembolic event; TIE2, tyrosine kinase with Ig and EGF homology domains 2; TRK, tropomyosin receptor kinase; TS, tuberous sclerosis; UC, urothelial carcinoma; VEGFR, vascular endothelial growth factor receptor; WMG, Waldenström macroglobulinemia.
Denotes known Type I inhibitor. 94
PKIs have been implicated in the development of PH. Different on‐target and off‐target toxicities of PKIs can lead to the development of PH through a variety of factors that contribute to one or multiple of the PH WHO groups. 22 , 23 Therefore, when a patient develops PH having been treated with a PKI, it can be challenging to determine the group of PH they fall into and the underlying etiology. specifically. 1 , 100 , 101 , 102 , 103 In this review, we discuss the various PKIs, explore their role in the development of Group 1 pulmonary arterial hypertension (PAH), Group 2 PH due to left‐sided heart disease, and Group 3 PH due to lung disease and/or hypoxia. In addition, we will offer guidance as to how to clinically approach patients who develop PH in the setting of PKI treatment, based on the WHO PH classification system, and discuss management strategies.
PULMONARY ARTERIAL HYPERTENSION
WHO Group 1 PAH accounts for <3%–14%% of all cases of PH. 104 , 105 The pathophysiology of PAH is an imbalance in endothelial proliferation, inflammation, and remodeling within the pulmonary vasculature via three main pathways: nitric oxide (NO), endothelin‐1 (ET‐1), and prostacyclin (PGI2) pathways. 106 Prolonged disproportionality of these pathways leads to dysregulation of inflammation, apoptosis, and proliferation of the smooth muscle and endothelial cells of the pulmonary artery causing an increase in mean pulmonary artery pressure (mPAP). 107 While the complexity of PAH pathophysiology extends beyond the NO, ET‐1, and PGI2 pathways, PKIs can contribute to disparities in these pathways potentially leading to direct drug‐induced PAH (Table 1).
PKIs that have been implicated in Group 1 PAH are dasatinib, bosutinib, ponatinib, and nilotinib. These agents, along with imatinib, are breakpoint cluster region‐Abelson leukemia gene (BCR‐ABL1) inhibitors. They have been groundbreaking for the treatment of chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL) and have varying cardiovascular profiles. Imatinib, the first Food and Drug Administration (FDA) approved BCR‐ABL1 inhibitor, has been associated with improvements in hemodynamics of pulmonary pressures and was studied for the treatment of PAH in the IMPRES trial (Imatinib in Pulmonary Arterial Hypertension, a Randomized, Efficacy Study) demonstrating an improvement in functional capacity and hemodynamics. 108 , 109 , 110 Although serious adverse events and study drug discontinuations were seen with imatinib in PAH, limiting its clinical use, the proposed mechanisms through which imatinib effects the pulmonary vasculature include, inhibition of platelet‐derived growth factor‐α/β (PDGFR‐α/β), decrease proliferation of pulmonary artery smooth muscle cells, and a decrease in calcium influx resulting in pulmonary artery vasodilation. 4 , 111 , 112
In contrast to imatinib, dasatinib, a second‐generation BCR‐ABL1 inhibitor has been associated with rare, but fatal PAH. 22 , 24 Inflammation of the pulmonary artery smooth muscle cells and elevations of T lymphocytes, leukocytes, monocytes, and macrophages is thought to be the primary mechanism of dasatinib toxicity. Rat studies have demonstrated that dasatinib predisposes those with chronic hypoxia or monocrotaline with an exaggerated worsening of pulmonary pressures. 23 Additionally, increased endothelial dysfunction as indicated by elevated levels of reactive oxygen species (ROS), soluble intercellular adhesion molecule (sICAM)‐1, soluble vascular cell adhesion molecule (sVCAM)‐1, and soluble E‐selectin (sE‐selectin) may be contributing. 112 , 113 Furthermore, potential off‐target inhibition of Src, a non‐receptor tyrosine kinase family, can lead to pulmonary vascular remodeling. 114 The Src kinase family is instrumental in phosphorylating and activating TWIK‐related acid‐sensitive potassium channel‐1 (TASK‐1) on the pulmonary artery smooth muscle cells leading to vasodilation. 114 Inhibition of the TASK‐1 channels causes a depolarization of the smooth muscle cell leading to an increase in intracellular calcium via L‐type voltage‐gated calcium channels. This Src kinase inhibition could explain why imatinib might have a therapeutic effect in PAH, whereas dasatinib has been shown to cause PAH. 112 (Figure 1) The PKI pathway continues to be explored therapeutically in PAH. One such example is with a novel inhaled PDGFR kinase inhibitor, seralutinib (Gb002), which in animal studies improved hemodynamics, NT‐proBNP, and pulmonary vascular remodeling. 115 Imatinib is also being explored in aerolized forms. In theory, both of these agents will be expected to have less adverse events due to the localized delivery system and are currently being studied in clinical trials. 116 , 117 In addition, oral imatinib remains under consideration as a potential PAH therapy.
Figure 1.
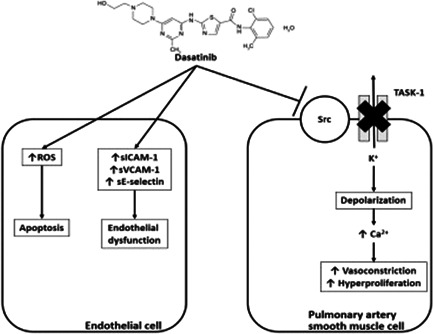
Mechanism of dasatinib‐induced PAH. PAH, pulmonary arterial hypertension; ROS, reactive oxygen species; sE‐selectin, soluble E‐selectin; sICAM‐1, soluble intercellular adhesion molecule; sVCAM‐1, soluble vascular cell adhesion molecule
Bosutinib, ponatinib, and nilotinib have less evidence with only rare case reports or limited of worsening pre‐existing PAH, some of which only found echocardiographic evidence of PH. 18 , 19 , 20 , 28 , 31 , 118 , 119 A recent pharmacovigilance study supports the Src family kinase postulation indicating that the c‐Src, c‐yes, Lck, and Lyn genes (members of the Src kinase family) are implicated in a disproportionately high incidence of PAH within the BCR‐ABL1 inhibitors that are dose‐related. 29
Anaplastic lymphoma kinase (ALK)‐inhibitors, brigatinib, ceritinib, crizotinib, lorlatinib are used in the treatment of non‐small cell lung cancer and have also been implicated in the development of PAH, with lorlatinib being the most implicated. 7 , 9 , 11 The causative mechanism of action of these agents in the development of pulmonary vascular disease is unknown although typical histologic findings of PAH are seen, namely intimal hyperplasia, medial hypertrophy, and angioproliferative plexiform lesions, plus sporadic peripheral arterial thrombosis in situ. 7
The difficulty of predicting the long‐term effects of PKIs on pulmonary vascular toxicity may result in a failure to prevent adverse effects and may delay the use of life‐saving PAH therapies. For example, several guidelines recommend regular monitoring for the development of cardiotoxicity with some chemotherapy drugs, but specific recommendations are not provided for pulmonary vascular toxicity. 120 , 121 Within our program, the practice is to perform echocardiograms every 3 months on patients receiving PKIs. If patients have evidence of PH on echocardiogram, accompanied by rapid symptom onset or progression, they would be referred for urgent right heart catheterization (RHC) to evaluate for the presence of PAH. If PH is uncovered on echocardiogram and the patients are minimally symptomatic and display marginally progressive echocardiogram features, then these patients can be followed with serial echocardiograms to observe the development of early right ventricular failure or signs of early clinical decompensation. Patients are referred for RHC only if there is significant progression of disease or if there are questions regarding optimum oncological therapy. If patients have significant risk factors for Group 2 PH, then they are followed serially rather than referred for invasive hemodynamics, unless there is concern for the concomitant development of Group 1 PAH. 122
Early discontinuation of the culprit agent can lead to a reversal of the pulmonary vascular disease. 18 , 19 , 20 , 22 , 25 , 28 , 118 Rapid clinical and hemodynamic improvements were noted within 4 months of discontinuation of dasatinib, although a more recent study found that PAH persisted in approximately one‐third of patients. 22 , 25
The management of PKI‐induced PAH varies depending on the long‐term complications of the therapy. Pharmacotherapy for persistent PAH revolves around standard PAH‐therapy protocols. 101 In the absence of high‐risk features, or the development of right heart failure, our practice is to start upfront dual combination therapy. 102 , 123 This involves an endothelin receptor antagonist (ERA) combined with a phosphodiesterase 5 inhibitor (PDE5i). Within our program, we combine ambrisentan or macitentan with either sildenafil or tadalafil, once PAH is confirmed on RHC. If there is a concern regarding acute right heart failure, this requires initiation of parenteral prostacyclins. However, with the exception of dasatinib, most cases of PKI‐induced PAH are low‐ to intermediate‐risk, and cessation of the PKI combined with long‐term use of dual combination therapy is sufficient to prevent or postpone clinical demise.
PH DUE TO LEFT HEART DISEASE
Group 2 PH is the most prevalent form of PH accounting for upwards of 68.5% of PH patients, encompassing heart failure with reduced ejection fraction (HFrEF), heart failure with preserved (HFpEF), and valvular heart disease. 105 , 124 The association between PKI therapy and heart failure appears to be indirect and mediated by increase in left ventricular end‐diastolic pressure from elevated blood pressure. 125 Alternatively, a direct effect may be the antiangiogenesis in capillarization of the myocardium itself, which impairs the preservation of functional status. 126 Of note, while bilateral pleural effusions may be associated with heart failure, certain PKIs, for example, dasatinib, have been shown to increase the permeability of endothelial cells leading to effusions independent of a heart failure diagnosis. 127 Hypertension remains the commonest modifiable risk factor for the development of heart failure and almost every group of PKIs is associated with the development of hypertension and heart failure (Table 1). 128 The pathophysiology contrasts with Group 1 and these cases can be distinguished by an increased left ventricular end‐diastolic pressure. In this setting, if there is concern for PH secondary to PKI therapies, an RHC is warranted to determine the optimum treatment strategy and a need to distinguish between Group 1 and Group 2 PH, 124 although, combined pre‐ and post‐capillary PH secondary to dasatinib has been reported. 25
Hypertension and atrial fibrillation are co‐morbidities linked to the development of HFpEF. 129 Select PKIs, specifically VEGF inhibitors, such as sorafenib and sunitinib, and Bruton's tyrosine kinase inhibitors (BTKs), such as ibrutinib and acalabrutinib, are known to increase hypertension and atrial fibrillation. VEGF inhibitor‐induced hypertension is a multifactorial mechanism. First, vasodilation occurs from VEGFR2 activation of phosphoinositide‐3 kinase (PI3K) increasing downstream endothelial NO synthase phosphorylation and thus NO release. 130 VEGFR2 activation also leads to increases PGI2 via activation of mitogen‐activated protein kinases (MAPKs). 131 Antagonism of these pathways in addition to glomerular damage from increased ET‐1 production lead to the on‐target toxicity from VEGF inhibitors. 130 Decreases in microvascular and myocardial capillary density could lead to increases in vascular resistance and endothelial dysfunction. 131 Additionally, some PKIs, such as ponatinib, have off‐target VEGF inhibition not related to their therapeutic target. The most effective blood pressuring lowering agent is unknown, but both calcium channel blockers and angiotensin‐converting enzyme (ACE) inhibitors appear effective. 132
The BTK inhibitors, acalabrutinib, ibrutinib, and zanubutinib. may increase the risk of HFpEF by elevating blood pressure, inducing atrial fibrillation, and other off‐target effects involving C‐terminal Src kinase inhibition causing left atrial inflammation, fibrosis, and enlargement. 133 Ibrutinib can cause hypertension within a few months of treatment and is associated with upwards of 75% of patients developing or worsening hypertension. 134 Atrial fibrillation has a 16% occurrence rate with ibrutinib. 135 Of note, more selective BTK inhibitors, acalabrutinib and zanubrutinib, do not carry the same risk of hypertension or atrial fibrillation to the extent of ibrutinib.
More recently, PKIs have been related to the development of valvular dysfunction. One culprit is the BCR‐ABL1 inhibitor, nilotinib which has been associated with rapid progression of aortic valve stenosis. The mechanism behind this is theorized to be related to an increase in BMP2 related valvular interstitial cell calcification. 136
VEGF inhibiting PKIs have also been associated with the development of HFrEF. While the incidence is difficult to estimate, two meta‐analyses described a higher risk of developing cardiomyopathy among people treated with VEGF inhibitors (odds ratio: 1.35 (95% confidence interval [CI]: 1.06–1.70) and 2.53 (95% CI: 1.79–3.57)). 137 , 138 Sunitinib treats renal cell carcinoma and targets the VEGF receptors to produce antiproliferative and antiangiogenesis effects (RR: 2.96; 95% CI: 1.93–4.53) and has the highest risk of causing cardiomyopathy (prevalence of ~10%). 137 , 138 Due to its wide selectivity, sunitinib also inhibits PDGFR‐α/β, FMS‐like tyrosine kinase‐3 (FLT3), fibroblast growth factor receptors (FGFR), and multiple other receptors. While the exact mechanism of left ventricle (LV) dysfunction from sunitinib is unknown and is likely multifactorial; it could be a sequela of hypertension associated with VEGF inhibition (on‐target) or due to inhibition of FGFR, which are important to LV functionality (off‐target). 139 , 140 , 141 Fortunately, in patients who develop LV dysfunction from sunitinib, withdrawal of the medication appears to lead to improvement in LV dysfunction and heart failure symptoms. 141
Management of PKI‐induced Group 2 PH includes screening left ventricular ejection fraction (LVEF) and blood pressure along with management of any baseline cardiovascular risk factors. 142 For hypertension, ACE inhibitors and angiotensin receptor blockers (ARBs) are the preferred agents, as calcium channel blockers (specifically verapamil and diltiazem) can cause CYP3A4 interactions. A recent publication compiled the recommendations from European and American guidelines for the management of cardiotoxicities in cancer patients. 142 Laboratory screening of biomarkers such as brain‐natriuretic peptide (BNP)/NT‐proBNP and troponin can also be considered. After initial evaluation, follow‐up screening can be considered every 3 months during treatment or sooner if symptoms develop. In patients who experience a decrease in LVEF, a referral for a cardio‐oncological evaluation and the initiation of ACE inhibitors and β‐blockers are recommended (Figure 2), in addition to other guideline‐directed medical therapy for cardiomyopathy as needed. If the patient is symptomatic and/or the LVEF is <40%, discontinuation of the therapy is recommended. If the patient is asymptomatic with an LVEF ≥ 40%, continuation of therapy can be considered with close monitoring. Consultation from an expert cardio‐oncology center is recommended to navigate the complex treatment environment associated with Group 2 PH and cancer. 143
Figure 2.
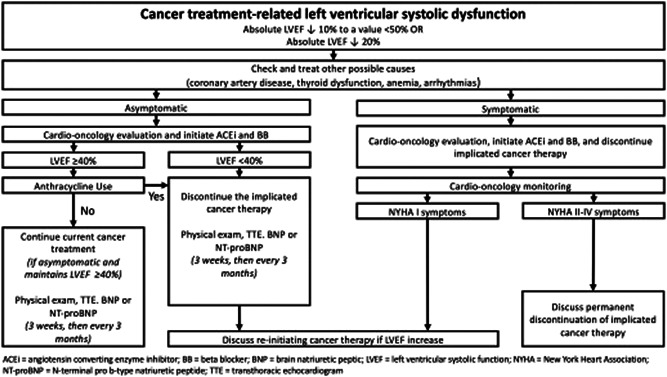
Definitions and management of overt cancer therapy‐related left ventricular systolic dysfunction (adapted from ref. [142]). ACEi, angiotensin‐converting enzyme inhibitor; BB, beta‐blocker; BNP, brain natriuretic peptic; LVEF, left ventricular systolic function; NYHA, New York Heart Association; NT‐proBNP, N‐terminal pro b‐type natriuretic peptide; TTE, transthoracic echocardiogram
PH DUE TO CHRONIC LUNG DISEASE AND HYPOXIA
PH due to chronic lung disease and hypoxia is the second most common form of PH. 105 , 144 PKIs are implicated in interstitial lung disease (ILD) and idiopathic pulmonary fibrosis (IPF), purely in the parenchymal space. 145 ILD was first noted as a complication of gefitinib, an epidermal growth factor receptor (EGFR) inhibitor in the early 2000s. 146 ILD developed typically within days of initiation, but could occur up to 3 months after starting therapy. The prevalence of ILD with gefitinib was <1%, but with a high mortality of up to 35%. 48 , 145 In IPF, a PH has been reported in 8%–15% of patients upon initial diagnosis with increasing prevalence up to >60% in advanced and end‐stage disease. 147 , 148 , 149 Additionally, a high prevalence of PH in ILD was noted in an echocardiographic study. 150 In this manner, it is reasonable to assume that PKI‐induced ILD could be associated with PH, no least through hypoxic pulmonary vasoconstriction alone.
Cyclin‐dependent kinase (CDK)‐4/6 inhibitors, ErbB inhibitors, and FLT3 inhibitors are the commonest causes of PKI‐induced ILD (Table 1), but can also occur with the use of ALK inhibitors, such as brigatinib. 151 Contrary to most forms of ILD, PKI‐induced ILD has nonspecific changes with the parenchymal tissue on high‐resolution computed tomography (CT) that is difficult to diagnose. 152 Nonspecific areas with ground‐glass opacities without loss of lung volume are the most common pattern accounting for 50% of PKI‐induced ILD. The toxicity does not appear to be dose related, and the mechanism remains largely unknown. 153 Recent bioinformatics studies indicate that the four genes with the highest association with ILD development include EGFR, tumor growth factor β‐1 (TFGB1), insulin‐like growth factor 1 (IGF1), and CC ligand chemokine 2 (CCL2). 154 Further investigation into the exact mechanism of these pathways may elucidate the on‐ and off‐target toxicities of PKIs that lead to ILD.
For patients on PKIs that develop pulmonary symptoms or suspected ILD, the PKI should be held. 145 Furthermore, switching to another PKI appears to be safe with no recurrence of ILD, indicating a lack of cross‐reactivity between agents. 145 Also, as symptoms improve, one can consider rechallenging the person with the PKI after discussing the risk versus benefit of treatment. 155 The use of high‐dose corticosteroids has been used in other forms of drug‐induced ILD (i.e., taxanes and gemcitabine) and may be useful in PKI‐induced ILD. 146 Otherwise, there is conflicting evidence in using PAH‐specific therapies Group 3 PH in this population. 144 Riociguat and ambrisentan have been shown to be harmful in patients with idiopathic interstitial pneumonia as noted in the RISE‐IIP and ARTEMIS‐IPF studies. 156 , 157 There is evidence that inhaled treprostinil (INCREASE trial) improves symptoms in ILD‐associated PH. 158 Although not specifically studied in PKI‐induced ILD, inhaled treprostinil could be considered in this group, especially if withdrawal of the offending agent does not result in clinical improvement.
CONCLUSION
Targeted PKI therapies for malignancies have revolutionized treatment for many patients with cancer. However, increasing cardiotoxicities are being identified as both on‐ and off‐target effects, including effects on the pulmonary vasculature. Cardiac screening and cardio‐oncology programs have typically focused on the effects of cancer therapeutics on the left ventricle and systemic vasculature. 143 , 159 Growing awareness of PH and the risks associated with PKIs and other novel targeted therapies is important in this population, as is understanding which agents are implicated in the different forms of PH (Figure 3). This review provides a roadmap for the management of PH in the setting of PKI therapy and highlights the ongoing challenges that these patients face.
Figure 3.
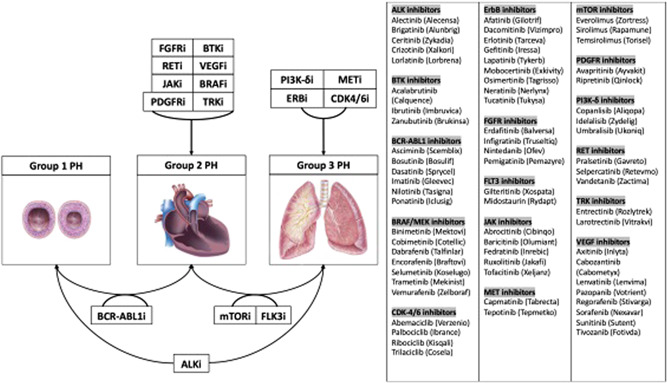
Connecting PKI groups with the likely WHO PH class. ALK, anaplastic lymphoma kinase; BCR‐ABL1, breakpoint cluster region‐Abelson leukemia gene; BRAF, b‐Raf oncogene; BTK, Bruton's tyrosine kinase; CDK, cyclin‐dependent kinase; ErbB1/EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; FLT3, Fms‐like tyrosine kinase 3; JAK, Janus kinase; MEK, mitogen‐activated protein kinase; MET/HGHR, hepatocyte growth factor receptor; mTOR, mechanistic target of rapamycin; PDGFR, platelet‐derived growth factor receptor; PI3K‐δ, phosphoinositide‐3 kinase delta; RET, rearranged during transfection; TRK, tropomyosin receptor kinase; VEGFR, vascular endothelial growth factor receptor
FUTURE DIRECTIONS
The ongoing identification of PKI toxicities plays a crucial role in determining treatment options for patients who develop PH while undergoing cancer treatment. Advancements in bioinformatics and genomics research, in conjunction with large electronic databases, improve detection of PKI toxicities, 29 , 160 as it pertains to the pulmonary vasculature. Such advances may also help identify PKIs with therapeutic potential. 112
AUTHOR CONTRIBUTIONS
Joshua A. Jacobs contributed by designing the concept, writing, correcting the manuscript, and creating tables. John J. Ryan contributed by designing the concept, writing, correcting the manuscript, and creating figures. Eiman Jahangir contributed by writing and correcting the manuscript. All authors have reviewed and acknowledged the accuracy of this review paper.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
The ethics statement is not available.
ACKNOWLEDGMENTS
Dr. John J. Ryan and his research team would like to thank The Reagan Corporation, The Gordon Family, and The Cushman Family for their support and funding from R01HL093081.
Jacobs JA, Jahangir E, Ryan JJ. Differentiating pulmonary hypertension associated with protein kinase inhibitors. Pulmonary Circulation. 2022;12:e12075. 10.1002/pul2.12075
REFERENCES
- 1. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Galiè N, McLaughlin VV, Rubin LJ, Simonneau G. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53(1):1802148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Henson KE, Reulen RC, Winter DL, Bright CJ, Fidler MM, Frobisher C, Guha J, Wong KF, Kelly J, Edgar AB, McCabe MG, Whelan J, Cutter DJ, Darby SC, Hawkins MM. Cardiac mortality among 200000 five‐year survivors of cancer diagnosed at 15 to 39 years of age: the teenage and young adult cancer survivor study. Circulation. 2016;134(20):1519–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moslehi JJ, Deininger M. Tyrosine kinase inhibitor‐associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol. 2015;33(35):4210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Center for Biotechnology Information . 2021. Available from: https://pubchem.ncbi.nlm.nih.gov/compound
- 6. Alecensa (alectinib) [prescribing information]. South San Francisco, CA: Genentech USA Inc; 2021. [Google Scholar]
- 7. Tabbò F, D'Aveni A, Tota D, Pignataro D, Bironzo P, Carnio S, Cappia S, Cortese G, Righi L, Novello S. Pulmonary arterial hypertension in ALK receptor tyrosine kinase‐positive lung cancer patient: adverse event or disease spread? J Thorac Oncol. 2019;14(2):e38–40. [DOI] [PubMed] [Google Scholar]
- 8. Alunbrig (brigatinib) [prescribing information]. Cambridge, MA: Ariad Pharmaceuticals Inc; 2021. [Google Scholar]
- 9. Khouri C, Hlavaty A, Roustit M, Cracowski JL, Chaumais MC, Humbert M, Montani D. Investigating the association between ALK receptor tyrosine kinase inhibitors and pulmonary arterial hypertension: a disproportionality analysis from the WHO pharmacovigilance database. Eur Respir J. 2021;58. [DOI] [PubMed] [Google Scholar]
- 10. Xalkori (crizotinib) [prescribing information]. New York, NY: Pfizer Labs; 2021. [Google Scholar]
- 11. Awada C, Grobs Y, Wu WH, Habbout K, Romanet C, Breuils‐Bonnet S, Tremblay E, Martineau S, Paulin R, Bonnet S, Provencher S, Potus F, Boucherat O. R‐Crizotinib predisposes to and exacerbates pulmonary arterial hypertension in animal models. Eur Respir J. 2021;57(5):2003271. [DOI] [PubMed] [Google Scholar]
- 12. Xalkori (crizotinib) [package insert]. New York City, NY: Pfizer Inc; 2021. [Google Scholar]
- 13. Lorbrena (lorlatinib) [prescribing information]. New York, NY: Pfizer Labs; 2021. [Google Scholar]
- 14. Calquence (acalabrutinib) [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2019. [Google Scholar]
- 15. Imbruvica (ibrutinib) [prescribing information]. South San Francisco, CA: Pharmacyclics LLC; 2021. [Google Scholar]
- 16. Brukinsa (zanubrutinib) [prescribing information]. San Mateo, CA: BeiGene USA Inc; 2021. [Google Scholar]
- 17. Scemblix (asciminib) tablets [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2021. [Google Scholar]
- 18. Yo S, Thenganatt J, Lipton J, Granton J. Incident pulmonary arterial hypertension associated with Bosutinib. Pulm Circ. 2020;10(3):2045894020936913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hickey PM, Thompson AA, Charalampopoulos A, Elliot CA, Hamilton N, Kiely DG, Lawrie A, Sabroe I, Condliffe R. Bosutinib therapy resulting in severe deterioration of pre‐existing pulmonary arterial hypertension. Eur Respir J. 2016;48(5):1514–6. [DOI] [PubMed] [Google Scholar]
- 20. Riou M, Seferian A, Savale L, Chaumais MC, Guignabert C, Canuet M, Magro P, Rea D, Sitbon O, Jaïs X, Humbert M, Montani D. Deterioration of pulmonary hypertension and pleural effusion with bosutinib following dasatinib lung toxicity. Eur Respir J. 2016;48(5):1517–9. [DOI] [PubMed] [Google Scholar]
- 21. Bosulif (bosutinib) [prescribing information]. New York, NY: Pfizer; 2021. [Google Scholar]
- 22. Montani D, Bergot E, Günther S, Savale L, Bergeron A, Bourdin A, Bouvaist H, Canuet M, Pison C, Macro M, Poubeau P, Girerd B, Natali D, Guignabert C, Perros F, O'Callaghan DS, Jaïs X, Tubert‐Bitter P, Zalcman G, Sitbon O, Simonneau G, Humbert M. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation. 2012;125(17):2128–37. [DOI] [PubMed] [Google Scholar]
- 23. Guignabert C, Phan C, Seferian A, Huertas A, Tu L, Thuillet R, Sattler C, Le Hiress M, Tamura Y, Jutant EM, Chaumais MC, Bouchet S, Manéglier B, Molimard M, Rousselot P, Sitbon O, Simonneau G, Montani D, Humbert M. Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J Clin Invest. 2016;126(9):3207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morishita S, Hagihara M, Itabashi M, Ishii Y, Yamamoto W, Numata A, Motohashi K, Matsumoto K, Fujisawa S, Nakajima H. Development of pulmonary arterial hypertension during oral dasatinib therapy for chronic myelogenous leukemia. Rinsho Ketsueki. 2016;57(8):999–1003. [DOI] [PubMed] [Google Scholar]
- 25. Weatherald J, Chaumais MC, Savale L, Jaïs X, Seferian A, Canuet M, Bouvaist H, Magro P, Bergeron A, Guignabert C, Sitbon O, Simonneau G, Humbert M, Montani D. Long‐term outcomes of dasatinib‐induced pulmonary arterial hypertension: a population‐based study. Eur Respir J. 2017;50(1):1700217. [DOI] [PubMed] [Google Scholar]
- 26. Sprycel (dasatinib) [prescribing information]. Princeton, NJ: Bristol‐Myers Squibb Company; 2021. [Google Scholar]
- 27. Gleevec (imatinib) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals; 2020. [Google Scholar]
- 28. Zakrzewski D, Seferynska I, Warzocha K, Hryniewiecki T. Elevation of pulmonary artery pressure as a complication of nilotinib therapy for chronic myeloid leukemia. Int J Hematol. 2012;96(1):132–5. [DOI] [PubMed] [Google Scholar]
- 29. Cornet L, Khouri C, Roustit M, Guignabert C, Chaumais MC, Humbert M, Revol B, Despas F, Montani D, Cracowski JL. Pulmonary arterial hypertension associated with protein kinase inhibitors: a pharmacovigilance‐pharmacodynamic study. Eur Respir J. 2019;53(5):1802472. [DOI] [PubMed] [Google Scholar]
- 30. Tasigna (nilotinib) [prescribing information]. East Hanover, NJ: Novartis; 2021. [Google Scholar]
- 31. Quilot FM, Georges M, Favrolt N, Beltramo G, Foignot C, Grandvuillemin A, Montani D, Bonniaud P, Camus P. Pulmonary hypertension associated with ponatinib therapy. Eur Respir J. 2016;47(2):676–9. [DOI] [PubMed] [Google Scholar]
- 32. Iclusig (ponatinib) [prescribing information]. Lexington, MA: Takeda Pharmaceuticals America Inc; 2021. [Google Scholar]
- 33. Spina E, Renna R, Lanterna LA, Colleoni ML, Andreone V. Progressive thrombosis of cervical and intracranial arteries related to Ponatinib treatment for Chronic Myeloid Leukemia. J Stroke Cerebrovasc Dis. 2020;29(9):105085. [DOI] [PubMed] [Google Scholar]
- 34. Mektovi (binimetinib) [prescribing information]. Boulder, CO: Array BioPharma Inc; 2020. [Google Scholar]
- 35. Cotellic (cobimetinib) [prescribing information]. South San Francisco, CA: Genentech USA, Inc; 2018. [Google Scholar]
- 36. Tafinlar (dabrafenib) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2021. [Google Scholar]
- 37. Braftovi (encorafenib) [prescribing information]. Boulder, CO: Array BioPharma Inc; 2020. [Google Scholar]
- 38. Koselugo (selumetinib) [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2021. [Google Scholar]
- 39. Mekinist (trametinib) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2021. [Google Scholar]
- 40. Zelboraf (vemurafenib) [prescribing information]. South San Francisco, CA: Genentech USA Inc; 2020. [Google Scholar]
- 41. erzenio (abemaciclib) [prescribing information]. Indianapolis, IN: Lilly USA, LLC; 2021. [Google Scholar]
- 42. Brance (palbociclib) [prescribing information]. New York, NY: Pfizer Labs; 2019. [Google Scholar]
- 43. Isqali (ribociclib) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2021. [Google Scholar]
- 44. Cosela (trilaciclib) [prescribing information]. Durham, NC: G1 Therapeutics Inc; 2021. [Google Scholar]
- 45. Gilotrif (afatinib) [prescribing information]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; 2019. [Google Scholar]
- 46. Vizimpro (dacomitinib) [prescribing information]. New York, NY: Pfizer Labs; 2020. [Google Scholar]
- 47. Tarceva (erlotinib) [prescribing information]. South San Francisco, CA: Genetech USA Inc; 2016. [Google Scholar]
- 48. Cohen MH, Williams GA, Sridhara R, Chen G, McGuinn WD Jr, Morse D, Abraham S, Rahman A, Liang C, Lostritto R, Baird A, Pazdur R. United States Food and Drug Administration Drug Approval summary: Gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 2004;10(4):1212–8. [DOI] [PubMed] [Google Scholar]
- 49. Iressa (gefitinib) [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2021. [Google Scholar]
- 50. Tykerb (lapatinib) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2021. [Google Scholar]
- 51. Exkivity (mobocertinib) [prescribing information]. Lexington, MA: Takeda Pharmaceuticals America Inc; 2021. [Google Scholar]
- 52. Nerlynx (neratinib) [prescribing information]. Los Angeles, CA: Puma Biotechnology Inc; 2021. [Google Scholar]
- 53. Tagrisso (osimertinib) [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2020. [Google Scholar]
- 54. Tukysa (tucatinib) [prescribing information]. Bothell, WA: Seattle Genetics Inc; 2020. [Google Scholar]
- 55. Balversa (erdafitinib) [prescribing information]. Horsham, PA: Janssen Products, LP; 2020. [Google Scholar]
- 56. Truseltiq (infigratinib) [prescribing information]. Brisbane, CA: QED Therapeutics Inc; 2021. [Google Scholar]
- 57. Ofev (nintedanib) [prescribing information]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; 2020. [Google Scholar]
- 58. Pemazyre (pemigatinib) [prescribing information]. Wilmington, DE: Incyte Corporation; 2021. [Google Scholar]
- 59. Xospata (gilteritinib) [prescribing information]. Northbrook, IL: Astellas Pharma US, Inc; 2019. [Google Scholar]
- 60. Rydapt (midostaurin) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2021. [Google Scholar]
- 61. Cibinqo (abrocitinib) [prescribing information]. New York, NY: Pfizer Labs; 2022. [Google Scholar]
- 62. Olumiant (baricitinib) [prescribing information]. Indianapolis, IN: Lilly USA LLC; 2021. [Google Scholar]
- 63. Inrebic (fedratinib) [prescribing information]. Summit, NJ: Celgene Corporation; 2021. [Google Scholar]
- 64. Jakafi (ruxolitinib) [prescribing information]. Wilmington, DE: Incyte Corporation; 2021. [Google Scholar]
- 65. Opzelura (ruxolitinib) [prescribing information]. Wilmington, DE: Incyte Corporation; 2021. [Google Scholar]
- 66. Xeljanz/Xeljanz XR (tofacitinib) [prescribing information]. New York, NY: Pfizer Inc; 2021. [Google Scholar]
- 67. Tabrecta (capmatinib) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2020. [Google Scholar]
- 68. Tepmetko (tepotinib) [prescribing information]. Rockland, MA: EMD Serono Inc; 2021. [Google Scholar]
- 69. Zortress (everolimus) [prescribing information]. East Hanover, NJ: Novartis Pharmaceutical Corporation; 2021. [Google Scholar]
- 70. Rapamune (sirolimus) [prescribing information]. Philadelphia, PA: Wyeth Pharmaceuticals LLC; 2021. [Google Scholar]
- 71. Torisel (temsirolimus) [prescribing information]. Philadelphia, PA: Pfizer Inc; 2018. [Google Scholar]
- 72. Ayvakit (avapritinib) [prescribing information]. Cambridge, MA: Blueprint Medicines Corporation; 2021. [Google Scholar]
- 73. Qinlock (ripretinib) [prescribing information]. Waltham, MA: Deciphera Pharmaceuticals LLC; 2021. [Google Scholar]
- 74. Aliqopa (copanlisib) [prescribing information]. Whippany, NJ: Bayer Healthcare Pharmaceuticals Inc; 2021. [Google Scholar]
- 75. Zydelig (idelalisib) [prescribing information]. Foster City, CA: Gilead Sciences Inc; 2020. [Google Scholar]
- 76. Ukoniq (umbralisib) [prescribing information]. Edison, NJ: TG Therapeutics Inc; 2021. [Google Scholar]
- 77. Gavreto (pralsetinib) [prescribing information]. South San Francisco, CA: Genentech Inc; 2021. [Google Scholar]
- 78. Retevmo (selpercatinib) [prescribing information]. Indianapolis, IN: Lilly USA LLC; 2021. [Google Scholar]
- 79. Caprelsa (vandetanib) [prescribing information]. Cambridge, MA: Genzyme Corporation; 2021. [Google Scholar]
- 80. Rozlytrek (entrectinib) [prescribing information]. South San Francisco, CA: Genentech USA Inc; 2021. [Google Scholar]
- 81. Vitrakvi (larotrectinib) [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2021. [Google Scholar]
- 82. Inlyta (axitinib) [prescribing information]. New York, NY: Pfizer Inc; 2020. [Google Scholar]
- 83. Cabometyx (cabozantinib) tablet [prescribing information]. Alameda, CA: Exelixis Inc; 2021. [Google Scholar]
- 84. Lenvima (lenvatinib) [prescribing information]. Woodcliff Lake, NJ: Eisai Inc; 2021. [Google Scholar]
- 85. Votrient (pazopanib) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2021. [Google Scholar]
- 86. Stivarga (regorafenib) [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2020. [Google Scholar]
- 87. Nexavar (sorafenib) [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals; 2020. [Google Scholar]
- 88. Sutent (sunitinib) [prescribing information]. New York, NY: Pfizer Labs; 2021. [Google Scholar]
- 89. Fotivda (tivozanib) [prescribing information]. Boston, MA: AVEO Pharmaceuticals Inc; 2021. [Google Scholar]
- 90. Rezurock (belumosudil) [prescribing information]. Warrendale, PA: Kadmon Pharmaceuticals LLC; 2021. [Google Scholar]
- 91. Tavalisse (fostamatinib) [prescribing information]. South San Francisco, CA: Rigel Pharmaceuticals, Inc; 2020. [Google Scholar]
- 92. Rhopressa (netarsudil) [prescribing information]. Irvine, CA: Aerie Pharmaceuticals; 2019. [Google Scholar]
- 93. Turalio (pexidartinib) [prescribing information]. Basking Ridge, NJ: Daiichi Sankyo Inc; 2021. [Google Scholar]
- 94. Zhao Z, Bourne PE. Overview of current type i/ii kinase inhibitors. In: Shapiro P, editor. Next generation kinase inhibitors: moving beyond the ATP binding/catalytic sites. Cham: Springer International Publishing; 2020. p. 13–28. [Google Scholar]
- 95. Wang Z, Cole PA. Catalytic mechanisms and regulation of protein kinases. Methods Enzymol. 2014;548:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Roskoski R Jr. Properties of FDA‐approved small molecule protein kinase inhibitors: A 2020 update. Pharmacol Res. 2020;152:104609. [DOI] [PubMed] [Google Scholar]
- 97. Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol. 2011;29(11):1046–51. [DOI] [PubMed] [Google Scholar]
- 98. Ferguson FM, Gray NS. Kinase inhibitors: the road ahead. Nat Rev Drug Discov. 2018;17(5):353–77. [DOI] [PubMed] [Google Scholar]
- 99. Chen MH, Kerkelä R, Force T. Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circulation. 2008;118(1):84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Taichman DB, Ornelas J, Chung L, Klinger JR, Lewis S, Mandel J, Palevsky HI, Rich S, Sood N, Rosenzweig EB, Trow TK, Yung R, Elliott CG, Badesch DB. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146(2):449–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document Group . ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2015;37(1):67–119. [DOI] [PubMed] [Google Scholar]
- 102. Klinger JR, Elliott CG, Levine DJ, Bossone E, Duvall L, Fagan K, Frantsve‐Hawley J, Kawut SM, Ryan JJ, Rosenzweig EB, Sederstrom N, Steen VD, Badesch DB. Therapy for pulmonary arterial hypertension in adults: update of the CHEST Guideline and Expert Panel Report. Chest. 2019;155(3):565–86. [DOI] [PubMed] [Google Scholar]
- 103. Rosenzweig EB, Abman SH, Adatia I, Beghetti M, Bonnet D, Haworth S, Ivy DD, Berger R. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53(1):1801916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Strange G, Playford D, Stewart S, Deague JA, Nelson H, Kent A, Gabbay E. Pulmonary hypertension: prevalence and mortality in the Armadale echocardiography cohort. Heart. 2012;98(24):1805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wijeratne DT, Lajkosz K, Brogly SB, Lougheed MD, Jiang L, Housin A, Barber D, Johnson A, Doliszny KM, Archer SL. Increasing incidence and prevalence of World Health Organization groups 1 to 4 pulmonary hypertension: a population‐based cohort study in Ontario, Canada. Circ Cardiovasc Qual Outcomes. 2018;11(2):e003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. McLaughlin VV, Shah SJ, Souza R, Humbert M. Management of pulmonary arterial hypertension. J Am Coll Cardiol. 2015;65(18):1976–97. [DOI] [PubMed] [Google Scholar]
- 107. Mayeux JD, Pan IZ, Dechand J, Jacobs JA, Jones TL, McKellar SH, Beck E, Hatton ND, Ryan JJ. Management of pulmonary arterial hypertension. Curr Cardiovasc Risk Rep. 2021;15(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115(10):2811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med. 2005;353(13):1412–3. [DOI] [PubMed] [Google Scholar]
- 110. Hoeper MM, Barst RJ, Bourge RC, Feldman J, Frost AE, Galié N, Gómez‐Sánchez MA, Grimminger F, Grünig E, Hassoun PM, Morrell NW, Peacock AJ, Satoh T, Simonneau G, Tapson VF, Torres F, Lawrence D, Quinn DA, Ghofrani HA. Imatinib mesylate as add‐on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circulation. 2013;127(10):1128–38. [DOI] [PubMed] [Google Scholar]
- 111. Rieg AD, Bünting NA, Cranen C, Suleiman S, Spillner JW, Schnöring H, Schröder T, von Stillfried S, Braunschweig T, Manley PW, Schälte G, Rossaint R, Uhlig S, Martin C. Tyrosine kinase inhibitors relax pulmonary arteries in human and murine precision‐cut lung slices. Respir Res. 2019;20(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ryan JJ. Tyrosine kinase inhibitors in pulmonary vascular disease. JACC Basic Transl Sci. 2016;1(7):684–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. El‐Dabh A, Acharya D. EXPRESS: pulmonary hypertension with dasatinib and other tyrosine kinase inhibitors. Pulm Circ. 2019;9(3):2045894019865704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Nagaraj C, Tang B, Bálint Z, Wygrecka M, Hrzenjak A, Kwapiszewska G, Stacher E, Lindenmann J, Weir EK, Olschewski H, Olschewski A. Src tyrosine kinase is crucial for potassium channel function in human pulmonary arteries. Eur Respir J. 2013;41(1):85–95. [DOI] [PubMed] [Google Scholar]
- 115. Galkin A, Clemons B, Garcia E, Brooks J, Slee D, Salter‐Cid L, Zisman L. Abstract 11102: Gb002, a novel inhaled PDGFR kinase inhibitor, demonstrates efficacy in the Su5416 hypoxia rat model of pulmonary arterial hypertension (PAH). Circulation. 2019;140(Suppl_1):A11102. [Google Scholar]
- 116. clinicaltrials.gov: National Institute of Health U.S. National Library of Medicine. 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT04456998
- 117. https://clinicaltrials.gov/ct2/show/NCT05036135 clinicaltrials.gov: National Institute of Health U.S. National Library of Medicine. 2022. https://clinicaltrials.gov/ct2/show/NCT05036135
- 118. Seegobin K, Babbar A, Ferreira J, Lyons B, Cury J, Seeram V. A case of worsening pulmonary arterial hypertension and pleural effusions by bosutinib after prior treatment with dasatinib. Pulm Circ. 2017;7(4):808–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. https://clinicaltrials.gov/ct2/show/NCT01179737 clinicaltrials.gov: National Institute of Health U.S. National Library of Medicine. 2014. https://clinicaltrials.gov/ct2/show/NCT01179737
- 120. Lenihan DJ, Kowey PR. Overview and management of cardiac adverse events associated with tyrosine kinase inhibitors. Oncologist. 2013;18(8):900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip G, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM, ESC Scientific Document G . ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768–801. [DOI] [PubMed] [Google Scholar]
- 122. Jansen SMA, Huis In 't Veld AE, Jacobs W, Jacobs W, Grotjohan HP, Waskowsky M, van der Maten J, van der Weerdt A, Hoekstra R, Overbeek MJ, Mollema SA, Tolen P, Hassan El Bouazzaoui LH, Vriend J, Roorda J, de Nooijer R, van der Lee I, Voogel B, Peels K, Macken T, Aerts JM, Vonk Noordegraaf A, Handoko ML, de Man FS, Bogaard HJ. Noninvasive prediction of elevated wedge pressure in pulmonary hypertension patients without clear signs of left‐sided heart disease: external validation of the OPTICS risk score. J Am Heart Assoc. 2020;9(15):e015992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Galiè N, Barberà JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, Peacock AJ, Simonneau G, Vachiery JL, Grünig E, Oudiz RJ, Vonk‐Noordegraaf A, White RJ, Blair C, Gillies H, Miller KL, Harris JH, Langley J, Rubin LJ, AMBITION Investigators . Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373(9):834–44. [DOI] [PubMed] [Google Scholar]
- 124. Vachiéry JL, Tedford RJ, Rosenkranz S, Palazzini M, Lang I, Guazzi M, Coghlan G, Chazova I, De Marco T. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Manouchehri A, Kanu E, Mauro MJ, Aday AW, Lindner JR, Moslehi J. Tyrosine kinase inhibitors in leukemia and cardiovascular events: from mechanism to patient care. Arterioscler Thromb Vasc Biol. 2020;40(2):301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kaza E, Ablasser K, Poutias D, Griffiths ER, Saad FA, Hofstaetter JG, del Nido PJ, Friehs I. Up‐regulation of soluble vascular endothelial growth factor receptor‐1 prevents angiogenesis in hypertrophied myocardium. Cardiovasc Res. 2011;89(2):410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Phan C, Jutant EM, Tu L, Thuillet R, Seferian A, Montani D, Huertas A, Bezu JV, Breijer F, Vonk Noordegraaf A, Humbert M, Aman J, Guignabert C. Dasatinib increases endothelial permeability leading to pleural effusion. Eur Respir J. 2018;51(1):1701096. [DOI] [PubMed] [Google Scholar]
- 128. Tackling G, Borhade MB. Hypertensive heart disease. Treasure Island, FL: StatPearls Publishing LLC; 2021. [PubMed] [Google Scholar]
- 129. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype‐specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134(1):73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Pandey AK, Singhi EK, Arroyo JP, Ikizler TA, Gould ER, Brown J, Beckman JA, Harrison DG, Moslehi J. Mechanisms of VEGF (vascular endothelial growth factor) inhibitor‐associated hypertension and vascular disease. Hypertension. 2018;71(2):e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Robinson ES, Khankin EV, Karumanchi SA, Humphreys BD. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: mechanisms and potential use as a biomarker. Semin Nephrol. 2010;30(6):591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Waliany S, Sainani KL, Park LS, Zhang CA, Srinivas S, Witteles RM. Increase in blood pressure associated with tyrosine kinase inhibitors targeting vascular endothelial growth factor. JACC CardioOncol. 2019;1(1):24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Xiao L, Salem JE, Clauss S, Hanley A, Bapat A, Hulsmans M, Iwamoto Y, Wojtkiewicz G, Cetinbas M, Schloss MJ, Tedeschi J, Lebrun‐Vignes B, Lundby A, Sadreyev RI, Moslehi J, Nahrendorf M, Ellinor PT, Milan DJ. Ibrutinib‐mediated atrial fibrillation attributable to inhibition of C‐terminal Src Kinase. Circulation. 2020;142(25):2443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Dickerson T, Wiczer T, Waller A, Philippon J, Porter K, Haddad D, Guha A, Rogers KA, Bhat S, Byrd JC, Woyach JA, Awan F, Addison D. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood. 2019;134(22):1919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ganatra S, Sharma A, Shah S, Chaudhry GM, Martin DT, Neilan TG, Mahmood SS, Barac A, Groarke JD, Hayek SS, Dani S, Venesy D, Patten R, Nohria A. Ibrutinib‐associated atrial fibrillation. JACC Clin Electrophysiol. 2018;4(12):1491–500. [DOI] [PubMed] [Google Scholar]
- 136. Carracedo M, Stenke L, Franco‐Cereceda A, Bäck M. Aortic stenosis and the tyrosine kinase inhibitor nilotinib in chronic myeloid leukemia. JACC CardioOncol. 2020;2(1):123–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Abdel‐Qadir H, Ethier JL, Lee DS, Thavendiranathan P, Amir E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: a systematic review and meta‐analysis. Cancer Treat Rev. 2017;53:120–7. [DOI] [PubMed] [Google Scholar]
- 138. Totzeck M, Mincu RI, Mrotzek S, Schadendorf D, Rassaf T. Cardiovascular diseases in patients receiving small molecules with anti‐vascular endothelial growth factor activity: a meta‐analysis of approximately 29,000 cancer patients. Eur J Prev Cardiol. 2018;25(5):482–94. [DOI] [PubMed] [Google Scholar]
- 139. Narayan V, Keefe S, Haas N, Wang L, Puzanov I, Putt M, Catino A, Fang J, Agarwal N, Hyman D, Smith AM, Finkelman BS, Narayan HK, Ewer S, ElAmm C, Lenihan D, Ky B. Prospective evaluation of sunitinib‐induced cardiotoxicity in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2017;23(14):3601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Catino AB, Hubbard RA, Chirinos JA, Townsend R, Keefe S, Haas NB, Puzanov I, Fang JC, Agarwal N, Hyman D, Smith AM, Gordon M, Plappert T, Englefield V, Narayan V, Ewer S, ElAmm C, Lenihan D, Ky B. Longitudinal assessment of vascular function with sunitinib in patients with metastatic renal cell carcinoma. Circ Heart Fail. 2018;11(3):e004408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J, George S, Morgan JA, Harris DM, Ismail NS, Chen JH, Schoen FJ, Van den Abbeele AD, Demetri GD, Force T, Chen MH. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370(9604):2011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Alexandre J, Cautela J, Ederhy S, Damaj GL, Salem JE, Barlesi F, Farnault L, Charbonnier A, Mirabel M, Champiat S, Cohen‐Solal A, Cohen A, Dolladille C, Thuny F. Cardiovascular toxicity related to cancer treatment: a pragmatic approach to the American and European Cardio‐Oncology Guidelines. J Am Heart Assoc. 2020;9(18):e018403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Tuzovic M, Brown SA, Yang EH, West BH, Bassi NS, Park S, Guha A, Ghosh AK, Ganatra S, Hayek SS, Moslehi J, Jahangir E. Implementation of cardio‐oncology training for cardiology fellows. JACC CardioOncol. 2020;2(5):795–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Nathan SD, Barbera JA, Gaine SP, Harari S, Martinez FJ, Olschewski H, Olsson KM, Peacock AJ, Pepke‐Zaba J, Provencher S, Weissmann N, Seeger W. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. 2019;53:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Shah RR. Tyrosine kinase inhibitor‐induced interstitial lung disease: clinical features, diagnostic challenges, and therapeutic dilemmas. Drug Saf. 2016;39(11):1073–91. [DOI] [PubMed] [Google Scholar]
- 146. Müller NL, White DA, Jiang H, Gemma A. Diagnosis and management of drug‐associated interstitial lung disease. Br J Cancer. 2004;91(Suppl 2):S24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kimura M, Taniguchi H, Kondoh Y, Kimura T, Kataoka K, Nishiyama O, Aso H, Sakamoto K, Hasegawa Y. Pulmonary hypertension as a prognostic indicator at the initial evaluation in idiopathic pulmonary fibrosis. Respiration. 2013;85(6):456–63. [DOI] [PubMed] [Google Scholar]
- 148. Raghu G, Nathan SD, Behr J, Brown KK, Egan JJ, Kawut SM, Flaherty KR, Martinez FJ, Wells AU, Shao L, Zhou H, Henig N, Szwarcberg J, Gillies H, Montgomery AB, O'Riordan TG. Pulmonary hypertension in idiopathic pulmonary fibrosis with mild‐to‐moderate restriction. Eur Respir J. 2015;46(5):1370–7. [DOI] [PubMed] [Google Scholar]
- 149. Shorr AF, Wainright JL, Cors CS, Lettieri CJ, Nathan SD. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur Respir J. 2007;30(4):715–21. [DOI] [PubMed] [Google Scholar]
- 150. Nadrous HF, Pellikka PA, Krowka MJ, Swanson KL, Chaowalit N, Decker PA, Ryu JH. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest. 2005;128(4):2393–9. [DOI] [PubMed] [Google Scholar]
- 151. Ng TL, Narasimhan N, Gupta N, Venkatakrishnan K, Kerstein D, Camidge DR. Early‐onset pulmonary events associated with brigatinib use in advanced NSCLC. J Thorac Oncol. 2020;15(7):1190–9. [DOI] [PubMed] [Google Scholar]
- 152. Gemma A, Kudoh S, Ando M, Ohe Y, Nakagawa K, Johkoh T, Yamazaki N, Arakawa H, Inoue Y, Ebina M, Kusumoto M, Kuwano K, Sakai F, Taniguchi H, Fukuda Y, Seki A, Ishii T, Fukuoka M. Final safety and efficacy of erlotinib in the phase 4 POLARSTAR surveillance study of 10 708 Japanese patients with non‐small‐cell lung cancer. Cancer Sci. 2014;105(12):1584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ohnishi K, Sakai F, Kudoh S, Ohno R. Twenty‐seven cases of drug‐induced interstitial lung disease associated with imatinib mesylate. Leukemia. 2006;20(6):1162–4. [DOI] [PubMed] [Google Scholar]
- 154. Lu Y, Li A, Lai X, Jiang J, Zhang L, Zhong Z, Zhao W, Tang P, Zhao H, Ren X. Identification of differentially expressed genes and signaling pathways using bioinformatics in interstitial lung disease due to tyrosine kinase inhibitors targeting the epidermal growth factor receptor. Invest New Drugs. 2019;37(2):384–400. [DOI] [PubMed] [Google Scholar]
- 155. Weatherald J, Bondeelle L, Chaumais MC, Guignabert C, Savale L, Jaïs X, Sitbon O, Rousselot P, Humbert M, Bergeron A, Montani D. Pulmonary complications of Bcr‐Abl tyrosine kinase inhibitors. Eur Respir J. 2020;56(4). [DOI] [PubMed] [Google Scholar]
- 156. Nathan SD, Behr J, Collard HR, Cottin V, Hoeper MM, Martinez FJ, Corte TJ, Keogh AM, Leuchte H, Mogulkoc N, Ulrich S, Wuyts WA, Yao Z, Boateng F, Wells AU. Riociguat for idiopathic interstitial pneumonia‐associated pulmonary hypertension (RISE‐IIP): a randomised, placebo‐controlled phase 2b study. Lancet Respir Med. 2019;7(9):780–90. [DOI] [PubMed] [Google Scholar]
- 157. Raghu G, Behr J, Brown KK, Egan JJ, Kawut SM, Flaherty KR, Martinez FJ, Nathan SD, Wells AU, Collard HR, Costabel U, Richeldi L, de Andrade J, Khalil N, Morrison LD, Lederer DJ, Shao L, Li X, Pedersen PS, Montgomery AB, Chien JW, O'Riordan TG, ARTEMIS‐IPF I. Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med. 2013;158(9):641–9. [DOI] [PubMed] [Google Scholar]
- 158. Waxman A, Restrepo‐Jaramillo R, Thenappan T, Ravichandran A, Engel P, Bajwa A, Allen R, Feldman J, Argula R, Smith P, Rollins K, Deng C, Peterson L, Bell H, Tapson V, Nathan SD. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med. 2021;384(4):325–34. [DOI] [PubMed] [Google Scholar]
- 159. Gujral DM, Lloyd G, Bhattacharyya S. Provision and clinical utility of cardio‐oncology services for detection of cardiac toxicity in cancer patients. J Am Coll Cardiol. 2016;67(12):1499–500. [DOI] [PubMed] [Google Scholar]
- 160. Beck EM, Hatton ND, Ryan JJ. Novel techniques for advancing our understanding of pulmonary arterial hypertension. Eur Respir J. 2019;53(5). [DOI] [PubMed] [Google Scholar]


