Abstract
Background:
There are no verified lytic Staphylococcus pseudintermedius phages in the literature and few temperate phage genomes in databases. S. pseudintermedius is an opportunistic zoonotic pathogen of great importance in veterinary and human medicine.
Materials and Methods:
We discovered phages against canine-derived S. pseudintermedius isolates by screening dog feces, hair, and skin swabs. Fourteen new phages were isolated and characterized by genomic analysis, transmission electron microscopy, and host range determination.
Results:
Three phages—DH2, DH5, and DS10, a phage K variant—were predicted lytic by sequencing, a designation supported by mitomycin C induction. All three are S. pseudintermedius and Staphylococcus aureus polyvalent phages, with DH2 and DS10 being strong killers of both species.
Conclusions:
We report discovery of the first verified lytic S. pseudintermedius phages and suggest dog hair as a novel reservoir. DH2, DH5, and DS10 are promising candidates toward developing an anti-Staphylococcal phage cocktail.
Keywords: virulent, lytic, pseudintermedius, canine, pyoderma
Introduction
Staphylococcus pseudintermedius presents a growing antibiotic resistant threat to human and companion pet populations. S. pseudintermedius is an opportunistic pathogen that colonizes the skin and mucosal membranes in dogs. Although its clinical significance was unknown when first classified separately from Staphylococcus intermedius,1 we now recognize its unique pathogenesis and virulence mechanisms in canine pyoderma, otitis externa, urinary tract infections, respiratory tract infections, and reproductive tract infections.2–5
Traditional penicillinase-stable-β-lactam antibiotics quickly became ineffective against S. pseudintermedius as multidrug resistance (MDR) spread.4,6–8 As such, the European Food Safety Authority now classifies S. pseudintermedius as the third most important MDR pathogen in the European Union, behind only to Escherichia coli and Pseudomonas aeruginosa.9
The rise of MDR pathogens coupled with the decline of antibiotic development highlights the need for alternative antimicrobials such as bacteriophage therapy. Consensus phage therapy guidelines dictate the use of strictly lytic phages without undesirable elements such as antibiotic resistance, integrases, toxins, or other virulence factors.10,11 However, as reported in the literature and congruent with our experience, discovery of lytic Staphylococcus aureus or S. pseudintermedius phages is difficult.5 Nonetheless, lytic phages from other members of the Staphylococcus genus have been isolated and characterized including for S. aureus and Staphylococcus epidermidis.12
The PhageAI database contains 26 S. pseudintermedius phage genomes whereas Zeman et al. reported 19 such genomes in the NCBI database, none of which are lytic.13,14 The reported paucity of S. pseudintermedius phages—whether virulent or temperate—demonstrates the need for discovery for potential clinical and veterinary applications.
We hypothesized that lytic phages may be found on companion pets such as dogs, due to the prevalence of Staphylococcal species on these animals. For instance, in superficial bacterial folliculitis—a type of canine pyoderma—the upper portion of the hair follicle is commonly infected with S. pseudintermedius.15 We hunted for S. pseudintermedius phages using canine feces, hair, and skin swabs. In this report, we isolate and characterize 14 phages from hair and skin swabs, of which 3 (DH2, DH5, and DS10) were predicted lytic.
Mitomycin C (MMC) induction supported this designation, whereas DH2 and DS10—a phage K variant—were polyvalent against dozens of S. pseudintermedius and S. aureus strains. This study describes the discovery and characterization of the first verified lytic S. pseudintermedius phages and offers insights to aid discovery of more lytic Staphylococcal phages.
Materials and Methods
Sample collection and processing
Fecal samples were collected using sterile gloves and placed in individual 1.5 mL centrifuge tubes, hair clippings from various dogs were collected in individual Ziploc bags, and skins swabs were collected from canine underbellies (where there is little hair) using Amies swabs soaked in 1.5 mL saline.
Each fecal and hair sample was mixed thoroughly with either 1 or 5 mL, respectively, of phage buffer (6.7 mM Tris-HCl, 3.2 mM Tris-base, 100 mM NaCl, 10 mM MgSO4·7H2O, pH 8.0, filter sterilized). No phage buffer was added to the skin swabs in saline. For the fecal and skin swab samples, 1 mL was transferred to a new sterile 1.5 mL tube and centrifuged at 17,000 g for 10 min at room temperature with supernatants filtered using a 0.22 μM syringe filter (SLGVR33RS; Millipore). The hair samples were pooled, and the mixture was filtered through a 0.22 μM bottle top filter (SCGPS02RE; Millipore). The pellets and the filtered supernatants were saved for bacterial and phage isolation, respectively, as described hereunder.
Bacterial isolation
Mannitol salt agar (MSA) (R01581; Thermo Scientific™) plates were used to select for possible S. aureus/S. pseudintermedius isolates. Pellets for each sample type were resuspended with 500 μL of lysogeny broth (LB) and titered (serially diluted in LB media). Next, 20 μL slants of each dilution were plated onto each plate and incubated overnight at 37°C. A single colony from each plate was picked and streaked onto a new plate to ensure purity and incubated overnight at 37°C. Lastly, 10% glycerol stocks were made from log phase LB cultures and stored at −80°C.
Isolates that grew on the MSA plates were further tested for catalase activity for possible identification as S. pseudintermedius or S. aureus. Bacterial cultures were submitted to the Center of Metagenomics and Microbiome Research at Baylor College of Medicine for 16S rRNA sequencing and species identification. Bacterial strains isolated through this method were labeled AZM19–32. Additional S. aureus/S. pseudintermedius isolates (AZM1–2, 33–38) were received from veterinary clinics through Anizome LLC. Other S. aureus isolates from human origin (LMB2, MW2, and TCH1516) were obtained from TAILΦR Labs' bacterial isolate library.
Phage isolation
Supernatants from each sample type were pooled and transferred to a 100 kDa centrifugal filter (ACS510024; Millipore) and centrifuged at 3000 g until the hold-up volume was concentrated by a factor of 100. The concentrated filtrate was transferred to a new sterile 1.5 mL tube and stored at 4°C until phage isolation. Phages were isolated on S. pseudintermedius lawns by spotting the concentrated hair, skin, or fecal filtrates through the double agar overlay method.16
Host range and efficiency of plating
Host range and efficiency of plating (EOP) were determined as previously described17 using 15 S. pseudintermedius and 10 S. aureus isolates. Phage plate lysates were titered (serially diluted in phage buffer) with 5 μL of each dilution spotted onto lawns of S. aureus or S. pseudintermedius in a double agar overlay assay and incubated overnight at 37°C. Resulting individual plaques were counted to determine the titer (PFU/mL).
Transmission electron microscopy
Bacteriophage samples were imaged at the Baylor College of Medicine Cryo-Electron Microscopy Advanced Technology Core Facility (Texas Medical Center, Houston, TX). Quantifoil 2/2 200Cu +2 nm ThinC (Quantifoil Micro Tools GmbH, Jena, Germany) grids were used for the negative stain preparation and imaging. A Pelco EasiGlow (Ted Pella, Inc.) was used to glow discharge the grids and create a hydrophilic surface for the sample and stain adsorption. The glow discharge process was done for 10 s with a current value of 15 μA and a vacuum of ∼200 psig.
Bacteriophage aliquots (5 μL) were applied to the grids and incubated for 1–3 min depending on the phage concentration. Whatman 541 (Whatman, Inc.) filter paper was used to absorb any excess buffer, water, and 2% uranyl acetate (Sigma Aldrich) during staining. The final stain required 1 min incubation to assure adequate contrast and stain quality in the images. Stained grids were dried overnight in a desiccator and imaged the following day.
Negative stained grids were imaged with either a JEOL1230 operating at 80 kV or a 200 kV JEOL 2100FS Electron Microscope (JEOL Ltd, Japan). The JEOL 1230 is outfitted with a 4k × 4k Gatan Ultrascan CCD Camera (Gatan, AMETEK) and the JEOL 2100 is outfitted with a DE-12 3k × 4k direct detecting camera (Direct Electron, San Diego, CA). To minimize astigmatism or other beam aberrations before imaging, each microscope was aligned on a neutral carbon grid. Gain and dark reference images were automatically applied by the cameras before saving the final images. Images were collected at various magnifications ranging from 5000 × to 60,000 × on each scope to assess the bacteriophage particle concentration, distribution, size, and structure. Further image visualization and correlative studies were performed using FIJI ImageJ software.
Genomic sequencing and bioinformatic analysis
Genomic DNA purified from plate lysates through E.Z.N.A.® Universal Pathogen Kit (Omega Bio-Tek) was submitted to Novogene (Sacramento, CA) for microbial whole genome sequencing using Illumina NovaSeq 6000-PE150 platform and 350 bp insert DNA library preparation (Q30 ≥ 85%) with a resolution of 1 Gb of raw data per sample.
Data were analyzed with software using default settings unless otherwise specified. Raw short reads were trimmed to a quality score of 30 and reads shorter than 50 bp in length were filtered out using BBDuk (version 38.84).18 After trimming, 3% of reads were randomly subsampled (resulting in ∼100,000–150,000 reads per sample) and assembled using Geneious assembler in Geneious 2022.0.1. To look for potential rare species in the samples, trimmed reads were also normalized to a target coverage level of 200 × and minimum depth of 10 × using BBNorm (version 38.84)18 and then assembled using Geneious assembler.
Assemblies from both normalized and subsampled reads were then compared using progressive Mauve (version 2015-02-26)19 to verify that they matched. Assemblies were validated by mapping all trimmed reads from each corresponding sample. Final assemblies were then compared using progressive Mauve (version 2015-02-26) and unique contigs kept for further analysis. Assemblies were annotated using RASTtk.20–22
Annotated assemblies were screened for antibiotic resistance and virulence genes by using BLAST23 (version 2.8.1) to align assemblies to the Comprehensive Antibiotic Resistance Database (database version 3.0.7),24 the Virulence Factor Database (database version 2021-09-24),25 the Victor VF database (downloaded 2020-11-18),26 and the PATRIC VF database (downloaded 2020-11-18).27 Genus was predicted using BLASTn (version 2.8.1) and the nr/nt database to determine the most closely related sequenced genomes and predicted the potential genus based on >70% nucleotide identity over >60% of the query.
Phage lifestyles were predicted with PhageAI14 by parsing annotated features for “integrase.” Assemblies were also analyzed using PHASTER28 to search for integrases and attachment sites using BLAST annotation software. PHASTER was used to predict prophage content in S. pseudintermedius ATCC 49051.
Induction assays
An overnight culture of the host strain was subcultured 1:100 in 20 mL of fresh LB medium and incubated at 37°C with shaking until OD 600 nm = 0.4. MMC (47582010MG; MilliporeSigma) was added to 10 μg/mL, covered with foil, and incubated at 37°C with continuous shaking for 2 h followed by addition of 2% chloroform for 10 min. The culture was centrifuged for 10 min at 3,000 g and the supernatant transferred to a new 15 mL conical tube. Fifty microliters of this induced lysate supernatant was mixed with 50 μL of the fresh host culture in 3 mL of 0.75% top agar, spread over an LB agar plate, and incubated at 37°C overnight. Plates were checked for plaque formation and served as the background lysogen plaque formation for that host strain.
Each phage was mixed with its host at a multiplicity of infection of 10, plated, and incubated overnight at 37°C. At least five resulting resistant colonies were restreaked twice onto a fresh LB agar plate and incubated at 37°C overnight to remove lytic phage. After the second streak, cultures from each colony were induced as already described. Resulting plates were checked for plaque formation and compared with the host baseline.
Results
S. pseudintermedius strains and phages isolated from canine hair and skin
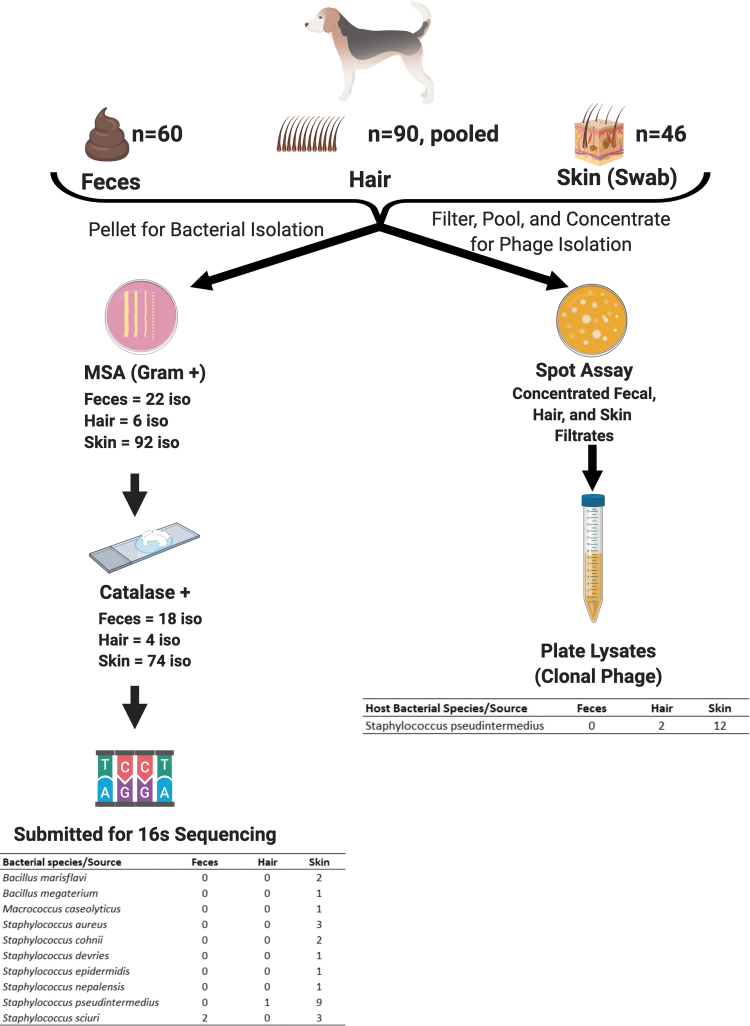
We collected 60 fecal samples, 90 hair samples, and 46 skin swabs from dogs at the 2019 Houston World Series of Dogs for isolation of S. aureus and S. pseudintermedius strains and phages. The 16S RNA sequencing of MSA-positive catalase-positive isolates identified 12 as S. pseudintermedius with 3 identified as S. aureus. All were isolated from skin swabs except for one S. pseudintermedius isolate recovered from hair. Other recovered Staphylococcal and Gram-positive species and their origins are listed in Figure 1.
FIG. 1.
Bacterial and phage isolation from random canine samples. Pellets were streaked onto mannitol salt agar plates, with isolates sequenced based on catalase positivity. Identified Staphylococcal and Gram-positive isolates are shown. A phage hunt against Staphylococcus aureus and Staphylococcus pseudintermedius isolates was performed using a double agar overlay spot assay with the concentrated filtrates. This figure was created with BioRender.com
A total of 14 phages against S. pseudintermedius were initially discovered. Twelve originated from the concentrated skin swab filtrates, but none were obtained from the concentrated fecal filtrates. Surprisingly, we recovered two phages from the concentrated hair filtrates. No S. aureus phages were initially recovered from any of the sources (Fig. 1).
Sequencing analysis predicted three lytic phages and coamplification of two prophages
The genomic analyses for all 12 phage plate lysates are summarized in Table 1. Genome sizes ranged from 35, 683 to 139,829 bp. Encoded open reading frames ranged from 17 to 217, and tRNAs ranged from 0 to 5. Genera represented included Twortvirus, Sextaecvirus, Andhravirus, Fibralongavirus, Coventryvirus, Kayvirus, Biseptimavirus, and two unknown. None of the genomes contained antibiotic resistance coding sequences (CDSs), whereas four phages (DS3, DS4, DS5, DS7) had at least one bacterial virulence CDS. Surprisingly, three phages were predicted to be lytic—a rarity for S. pseudintermedius phage.
Table 1.
Genomic Analysis of Isolated Staphylococcus pseudintermedius Phages
| Bacteriophage | DH2 | DH5 | DS1a | DS3b | DS4a,b | DS5a | DS6a | DS7a | DS8a | DS10 | DS11 | DS12 | DSP1 | DSP2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accession number | OM373548 | OM373549 | OM373550 | OM373551 | OM373552 | OM373553 | OM373554 | OM373555 | OM373556 | OM373557 | OM373558 | OM373559 | OM373560 | OM373561 |
| Host strain | AZM22 | AZM37 | AZM22 | AZM25 | AZM26 | AZM30 | AZM32 | AZM22 | AZM22 | AZM22 | AZM64 | AZM64 | AZM22 | AZM22 |
| Source | Hair | Hair | Skin | Skin | Skin | Skin | Skin | Skin | Skin | Skin | Skin | Skin | Skin | Skin |
| Morphology | Myovirus | Siphovirus | Myovirus | |||||||||||
| Genome size (bp) | 138,526 | 92,077 | 17,344 | 38,198 | 38,285 | 26,100 | 42,714 | 84,555 | 35,683 | 139,829 | 38,381 | 43,755 | 16,901 | 41,107 |
| G + C (%) | 30.8 | 30.3 | 33.3 | 35 | 30.2 | 37.8 | 35.4 | 37.3 | 35.8 | 30.4 | 36.2 | 36.4 | 28.1 | 35.2 |
| Open reading frames | 205 | 134 | 23 | 56 | 57 | 35 | 68 | 95 | 58 | 217 | 58 | 61 | 17 | 60 |
| Hypothetical proteins | 159 | 121 | 11 | 27 | 28 | 15 | 43 | 32 | 35 | 179 | 35 | 36 | 10 | 41 |
| Proteins with functional assignment | 46 | 13 | 12 | 29 | 29 | 20 | 35 | 63 | 23 | 38 | 23 | 25 | 7 | 19 |
| tRNAs | 1 | 5 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 4 | 0 | 0 | 0 | 0 |
| Genus | Twortvirus | Sextaecvirus | Fibralongavirus | Coventryvirus | Coventryvirus | Coventryvirus | Fibralongavirus | Unknown | Fibralongavirus | Kayvirus | Biseptimavirus | Unknown | Unknown | Fibralongavirus |
| Antibiotic resistance CDS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bacterial virulence CDS | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Closest sequenced relative | AY954970.1 | MW589261 | MK075001 | NC_055022 | NC_055022 | NC_055022 | MK075001 | CP065925 | MK075001 | KF766114 | KX827371 | NC_048192 | NC_047813 | MK075001 |
| Attachment sites | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 0 | 2 |
| Integrases | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| Lifestyle prediction | Virulent | Virulent | Temperate | Temperate | Temperate | Temperate | Temperate | Temperate | Temperate | Virulent | Temperate | Temperate | Temperate | Temperate |
Phage genomes were analyzed as described in the Materials and Methods section.
Contains DSP1.
Contains DSP2.
CDS, coding sequence.
They are deposited and named through the Adriaenssens and Brister method as Staphylococcal phages vB_SpsM-DH2, vB_SpsS-DH5, and vB_SpsM-DS10.29 Further investigation revealed DS10 to be a phage K variant, with an single nucleotide polymorphism causing a T523I mutation in a putative carbohydrate binding domain (locus tag: CPT_phageK_gp146; protein ID: YP_009041342.1). As expected, the other phages were predicted to be temperate, containing putative integrases and/or attachment sites. Sequencing coverage depth revealed that multiple preparations from presumably clonal plaques contained more than one genome.
For example, the DS1 preparation contained two contigs assembled into two different genomes, denoted DS1 and DSP1. In addition, two phages—DS9 and DS10—were identical, but DS9 had an additional contig (DSP2); thus, only DS10 is reported here. Interestingly, these two predicted prophages appeared to have coamplified with multiple phage isolates: DSP1 appeared in plate lysates of DS1 and DS4 through 8, whereas DSP2 appeared in lysates of DS3 and DS4. In total, 14 unique phage genomes were identified in DNA preparations from 12 plate lysates (Table 1).
Phages DH2, DH5, and DS10 are polyvalent S. pseudintermedius and S. aureus phages
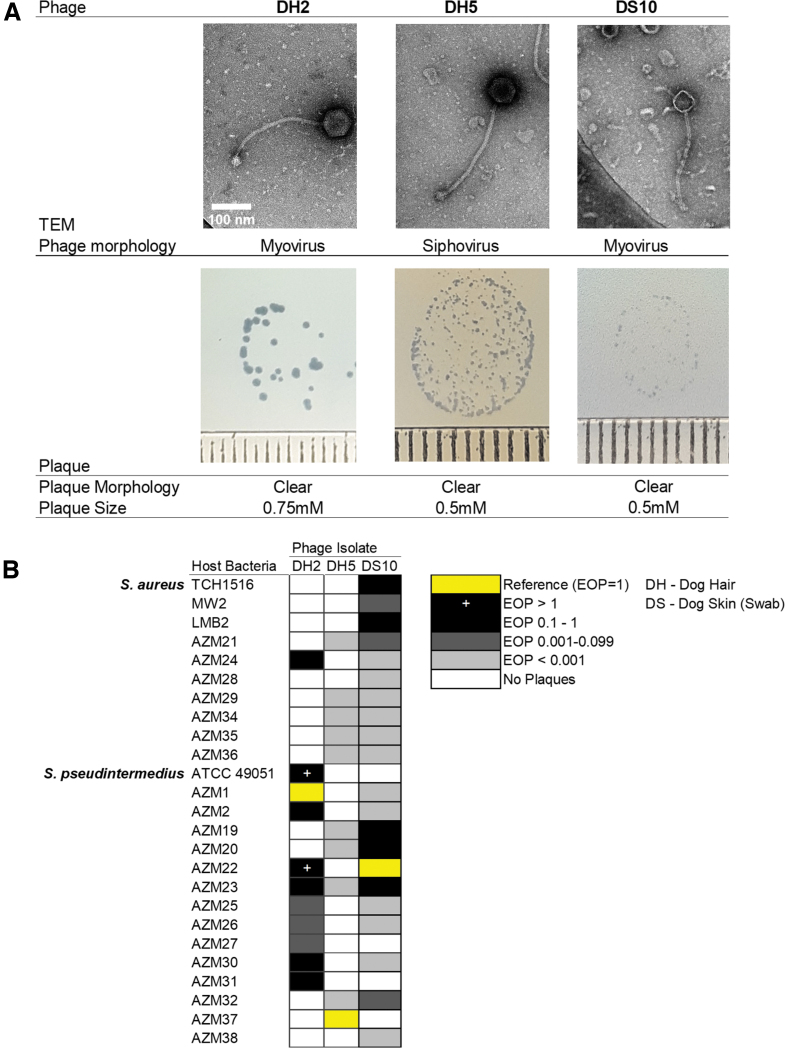
The absence of any confirmed lytic S. pseudintermedius phages in the literature compelled us to further investigate the predicted lytic phages (DH2, DH5, and DS10). Figure 2 displays the transmission electron microscopy (TEM) morphology, plaque morphology, host range, and EOP of S. pseudintermedius phages. As predicted from genomic analysis, DH2 and DS10 exhibited typical myovirus morphology with long contractile tails, whereas DH5 possessed a noncontractile tail with a large base plate (Fig. 2A). Their plaques varied in size (0.5–0.75 mm) but were all clear (Fig. 2A). A panel of 15 S. pseudintermedius and 10 S. aureus isolates was challenged with DH2, DH5, and DS10 to determine host range and EOP (Fig. 2B).
FIG. 2.
Morphology and host range of predicted lytic Staphylococcus pseudintermedius phages. (A) Top: Phages imaged by transmission electron microscopy. Bottom: Plaque size and morphology on strains AZM1, AZM37, and TCH1516, respectively. Each tick represents 1 mm. (B) Host range determined through efficiency of plating on 10 Staphylococcus. aureus and 15 S. pseudintermedius strains. This figure was created with BioRender.com
DH2 exhibited species specificity, plaquing on 10/15 (66.7%) S. pseudintermedius isolates and only 1/10 (10%) S. aureus isolates. DH5 plaqued on 5/15 (33.3%) of S. pseudintermedius isolates, and surprisingly on 5/10 (50%) of the S. aureus isolates. However, the EOP was <0.001 on all strains, indicative of lysis from without. Lastly, DS10 plaqued on 11/15 (73.3%) S. pseudintermedius, and all 10 S. aureus isolates—including the 3 clinical isolates TCH1516, MW2, and LMB2. TCH1516 and MW2 are USA 300 and 400 strains, respectively, whereas LMB2 is an uncharacterized patient isolate of TAILΦR Labs.30–32
MMC induction supports DH2, DH5, and DS10 as the first confirmed lytic S. pseudintermedius phages
DH2 is a Twortvirus with no antibiotic resistance or bacterial virulence CDSs, no attachment sites or integrases, but does harbor a putative antirepressor. DH5 is a Sextaecvirus without any antibiotic resistance or bacterial virulence CDS but contains putative attachment sites and an integrase. DS10 is a Kayvirus and like DH2 lacks antibiotic resistance and bacterial virulence CDS, attachment sites, and integrases. Given the ambiguity of two of the three genomes, we decided to test the predicted lytic lifestyle using induction assays. DH5 was propagated on AZM37, the only strain that produced a productive infection.
DH2 and DS10 were propagated on ATCC49051 and TCH1516, respectively. The strains were chosen because they are sequenced, their prophage content is known30 or predicted, and the phages are virulent on these strains as assessed by EOP assay. We also included phage K—a well-studied lytic phage33,34—with TCH1516 and predicted temperate phage DS3 with AZM22 as controls. Results of the MMC induction assay are given in Table 2. Surprisingly, DH5 did not exhibit plaque formation from 15 induced isolates tested. Likewise, DH2, DS10, and phage K did not exhibit plaque formation from five induced isolates tested for each. As predicted, DS3 formed plaques with 8 of 10 induced isolates.
Table 2.
Outcome of Mitomycin C Induction Assays
| Species | Strain—phage challenge | MMC only |
MMC+phage |
||
|---|---|---|---|---|---|
| Plaquing observed | Isolates tested | Plaquing observed | Isolates tested | ||
| Staphylococcus aureus | TCH1516—DS10, phage K | 0 | 5 | 0 | 5 each |
| Staphylococcus pseudintermedius | ATCC49051—DH2 | 0 | 5 | 0 | 5 |
| AZM37—DH5 | 1 | 8 | 0 | 15 | |
| AZM22—DS3 | 0 | 8 | 8 | 10 | |
MMC induction assays were performed with either host strain alone, or with phage as described in Materials and Methods section. Number of plates with plaques and number of biological isolates tested are reported. Predicted temperate phage DS3 and phage K were included as positive and negative controls for lysogeny, respectively.
MMC, mitomycin C.
Discussion
Here we report the discovery of the first lytic S. pseudintermedius phages. We opted for collecting feces, hair, and skin swabs from dogs, as methicillin-resistant S. aureus and methicillin-resistant S. pseudintermedius have been isolated from such samples.35 The Houston World Series of Dog Shows offered a unique opportunity to collect different samples from a variety of dog breeds, harboring a broad range of commensals and pathobionts. Although we did isolate S. aureus and S. pseudintermedius strains, we only found phages on S. pseudintermedius lawns. The preponderance of swab-derived phages is consistent with skin virome analysis and Staphylococci phage discovery,36,37 suggesting that the source rather than the method determines phage recovery.
Two phages, DSP1 and DSP2, are likely prophages from the bacterial hosts, and would explain their presence across several unique phage lysates. Three predicted lytic phages originated from concentrated hair and skin filtrates. These phages—DH2, DH5, and DS10—also targeted S. aureus strains as assessed by EOP assays. Predictably, the phage K variant DS10 plaqued on all S. aureus isolates tested. Interestingly, the DS10 gp146 mutation T523I is in a putative carbohydrate binding domain and not the annotated receptor binding protein of phage K, gp144. It is possible that gp146 is also important for receptor recognition, for which small mutations confer host range diversity. This rationale is supported by studies of Kayvirus mutants.38,39
As a Twortvirus, DH2 represents a unique and promising candidate for phage therapy. Although Kayviruses can expand their host range to S. pseudintermedius with limited lytic activity and infectivity, such ability is previously undocumented for Twortviruses.38,40,41 Although DH2 does possess a putative antirepressor, the virulent lifestyle prediction agrees with the Twortvirus designation and results of our induction assay.
Unlike myoviruses DH2 and DS10, siphovirus DH5 possessed elements of temperate phages, including an integrase and attachment sites. DH5 plaqued on S. pseudintermedius and S. aureus isolates but its EOP was very low (<0.001), indicating lysis from without rather than productive infection. MMC-induced isolates produced no plaques, supporting the virulent lifestyle prediction. A possible explanation for this is an effete integrase. Lysogeny modules are common among Staphylococcal phages with lytic variants arising due to mutations.33,42 DH5 may be one of these lytic variants, in support of this literature and our discovery of mostly temperate phages.
This project was undertaken to isolate and characterize lytic phages against S. pseudintermedius given their virtual absence in the literature.5 Moodley et al reported discovery of four phages from canine feces that appeared lytic against S. pseudintermedius and Staphylococcus schleiferi, however, genomic analysis revealed a lysogeny model in all four phages.43 To our knowledge, this is the first report of verified S. pseudintermedius lytic phages. Using canine hair and skin as novel sources, we discovered three lytic phages: DH5, DH2, and DS10, with the latter two capable of infecting both S. pseudintermedius and S. aureus strains.
Although skin is a known repository for phages, hair follicles harbor a rich microbiome including S. pseudintermedius during diseased states.15,44 This microenvironment might favor the evolution of lytic phages due to the proximity and density of potential hosts and explain why two novel lytic phages were found from dog hair clippings. Based on EOP analysis, a phage cocktail of DH2, DH5, and DS10 may plaque on all S. aureus and S. pseudintermedius strains tested. Future efforts will investigate the efficacy of these S. pseudintermedius phages in murine models of pyoderma.
Acknowledgments
Special thanks to Isaac Forrester and the staff at Baylor College of Medicine CryoEM Core for production of TEM images. We also thank Novogene for performing whole genome sequencing of these phages.
Authors' Contributions
A.T. and H.H.S. conceived and planned the experiments. H.H.S. performed the experiments. A.T. and H.H.S. contributed to the interpretation of the results. J.C. assembled the phage genomes and performed the bioinformatic analysis. H.H.S. wrote the article with support from A.T. and A.M. All authors have read and approved the final article.
Disclaimer
This article was submitted solely to this journal and is not published, in press, or submitted elsewhere.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported in part by Baylor College of Medicine seed funds, funds from the Mike Hogg Foundation, the National Institute of Health (U19 AI157981), and Anizome Biosciences.
References
- 1. Devriese LA, Vancanneyt M, Baele M, et al. Staphylococcus pseudintermedius Sp. Nov., a coagulase-positive species from animals. Int J Syst Evol Microbiol 2005;55(4):1569–1573; doi: 10.1099/ijs.0.63413-0 [DOI] [PubMed] [Google Scholar]
- 2. Ross Fitzgerald J. The Staphylococcus intermedius group of bacterial pathogens: Species re-classification, pathogenesis and the emergence of meticillin resistance: Recent studies of the staphylococcus intermedius group. Vet Dermatol 2009;20(5–6):490–495; doi: 10.1111/j.1365-3164.2009.00828.x [DOI] [PubMed] [Google Scholar]
- 3. Weese JS, van Duijkeren E. Methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Vet Microbiol 2010;140(3–4):418–429; doi: 10.1016/j.vetmic.2009.01.039 [DOI] [PubMed] [Google Scholar]
- 4. van Duijkeren E, Catry B, Greko C, et al. Review on methicillin-resistant Staphylococcus pseudintermedius. J Antimicrob Chemother 2011;66(12):2705–2714; doi: 10.1093/jac/dkr367 [DOI] [PubMed] [Google Scholar]
- 5. Lynch SA, Helbig KJ. The complex diseases of Staphylococcus pseudintermedius in canines: Where to next? Vet Sci 2021;8(1):11; doi: 10.3390/vetsci8010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moodley A, Damborg P, Nielsen SS. Antimicrobial resistance in methicillin susceptible and methicillin resistant Staphylococcus pseudintermedius of canine origin: Literature review from 1980 to 2013. Vet Microbiol 2014;171(3–4):337–341; doi: 10.1016/j.vetmic.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 7. Hartantyo SHP, Chau ML, Fillon L, et al. Sick pets as potential reservoirs of antibiotic-resistant bacteria in Singapore. Antimicrob Resist Infect Control 2018;7(1):106; doi: 10.1186/s13756-018-0399-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jantorn P, Heemmamad H, Soimala T, et al. Antibiotic resistance profile and biofilm production of Staphylococcus pseudintermedius isolated from dogs in Thailand. Pharmaceuticals 2021;14(6):592; doi: 10.3390/ph14060592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. EFSA Panel on Animal Health and Welfare (AHAW), Nielsen SS, Bicout DJ, et al. Assessment of animal diseases caused by bacteria resistant to antimicrobials: Dogs and cats. EFSA J 2021;19(6); doi: 10.2903/j.efsa.2021.6680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Principi N, Silvestri E, Esposito S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front Pharmacol 2019;10:513; doi: 10.3389/fphar.2019.00513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valente L, Prazak J, Que Y-A, et al. Progress and pitfalls of bacteriophage therapy in critical care: A concise definitive review. Crit Care Explor 2021;3(3):e0351; doi: 10.1097/CCE.0000000000000351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Melo LDR, Sillankorva S, Ackermann H-W, et al. Isolation and characterization of a new Staphylococcus epidermidis broad-spectrum bacteriophage. J Gen Virol 2014;95(2):506–515; doi: 10.1099/vir.0.060590-0 [DOI] [PubMed] [Google Scholar]
- 13. Zeman M, Bárdy P, Vrbovská V, et al. New genus fibralongavirus in siphoviridae phages of Staphylococcus pseudintermedius. Viruses 2019;11(12):1143; doi: 10.3390/v11121143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tynecki P, Guziński A, Kazimierczak J, et al. PhageAI—bacteriophage life cycle recognition with machine learning and natural language processing. bioRxiv 2020; doi: 10..1101/2020.07.11.198606 [Google Scholar]
- 15. Patel A. Superficial bacterial folliculitis. Companion Anim 2018;23(6):308–313; doi: 10.12968/coan.2018.23.6.308 [DOI] [Google Scholar]
- 16. Terwilliger AL, Gu Liu C, Green SI, et al. Tailored Antibacterials and Innovative Laboratories for Phage (Φ) Research: Personalized infectious disease medicine for the most vulnerable at-risk patients. PHAGE 2020;1(2):66–74; doi: 10.1089/phage.2020.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gibson SB, Green SI, Liu CG, et al. Constructing and characterizing bacteriophage libraries for phage therapy of human infections. Front Microbiol 2019;10:2537; doi: 10.3389/fmicb.2019.02537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bushnell B. BBMap. 38.84. https://sourceforge.net/projects/bbmap/ Accessed July 14, 2021.
- 19. Darling ACE. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res 2004;14(7):1394–1403; doi: 10.1101/gr.2289704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brettin T, Davis JJ, Disz T, et al. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci Rep 2015;5(1):8365; doi: 10.1038/srep08365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aziz RK, Bartels D, Best AA, et al. The RAST server: Rapid annotations using subsystems technology. BMC Genomics 2008;9(1):75; doi: 10.1186/1471-2164-9-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Overbeek R, Olson R, Pusch GD, et al. The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Res 2014;42(D1):D206–D214; doi: 10.1093/nar/gkt1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson M, Zaretskaya I, Raytselis Y, et al. NCBI BLAST: A better web interface. Nucleic Acids Res 2008;36(Web Server):W5–W9; doi: 10.1093/nar/gkn201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McArthur AG, Waglechner N, Nizam F, et al. The comprehensive antibiotic resistance database. Antimicrob Agents Chemother 2013;57(7):3348–3357; doi: 10.1128/AAC.00419-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen L, Zheng D, Liu B, et al. VFDB 2016: Hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res 2016;44(D1):D694–D697; doi: 10.1093/nar/gkv1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sayers S, Li L, Ong E, et al. Victors: A web-based knowledge base of virulence factors in human and animal pathogens. Nucleic Acids Res 2019;47(D1):D693–D700; doi: 10.1093/nar/gky999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mao C, Abraham D, Wattam AR, et al. Curation, integration and visualization of bacterial virulence factors in PATRIC. Bioinformatics 2015;31(2):252–258; doi: 10.1093/bioinformatics/btu631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arndt D, Grant JR, Marcu A, et al. PHASTER: A better, faster version of the phast phage search tool. Nucleic Acids Res 2016;44(W1):W16–W21; doi: 10.1093/nar/gkw387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adriaenssens E, Brister JR. How to name and classify your phage: An informal guide. Viruses 2017;9(4):70; doi: 10.3390/v9040070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Highlander SK, Hultén KG, Qin X, et al. Subtle genetic changes enhance virulence of methicillin resistant and sensitive Staphylococcus aureus BMC Microbiol 2007;7(1):99; doi: 10.1186/1471-2180-7-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Centers for Disease Control and Prevention (CDC). Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997–1999. MMWR Morb Mortal Wkly Rep. 1999 Aug 20;48(32):707-10. PMID: . [PubMed] [Google Scholar]
- 32. Baba T, Takeuchi F, Kuroda M, et al. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 2002;359(9320):1819–1827; doi: 10.1016/S0140-6736(02)08713-5 [DOI] [PubMed] [Google Scholar]
- 33. O'Flaherty S, Coffey A, Edwards R, et al. Genome of Staphylococcal phage K: A new lineage of myoviridae infecting gram-positive bacteria with a low G + C content. J Bacteriol 2004;186(9):2862–2871; doi: 10.1128/JB.186.9.2862-2871.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O'Flaherty S, Ross RP, Meaney W, et al. Potential of the polyvalent anti-Staphylococcus bacteriophage K for control of antibiotic-resistant staphylococci from hospitals. Appl Environ Microbiol 2005;71(4):1836–1842; doi: 10.1128/AEM.71.4.1836-1842.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beck KM, Waisglass SE, Dick HLN, et al. Prevalence of meticillin-resistant Staphylococcus pseudintermedius (MRSP) from skin and carriage sites of dogs after treatment of their meticillin-resistant or meticillin-sensitive staphylococcal pyoderma: MRSP in canine pyoderma. Vet Dermatol 2012;23(4):369–375, e66–67; doi: 10.1111/j.1365-3164.2012.01035.x [DOI] [PubMed] [Google Scholar]
- 36. Hannigan GD, Meisel JS, Tyldsley AS, et al. The human skin double-stranded DNA virome: Topographical and temporal diversity, genetic enrichment, and dynamic associations with the host microbiome. mBio 2015;6(5):e01578-15; doi: 10.1128/mBio.01578-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valente LG, Pitton M, Fürholz M, et al. Isolation and characterization of bacteriophages from the human skin microbiome that infect Staphylococcus epidermidis. FEMS Microbes 2021;2:xtab003; doi: 10.1093/femsmc/xtab003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Azam AH, Kadoi K, Miyanaga K, et al. Analysis host-recognition mechanism of Staphylococcal Kayvirus ΦSA039 reveals a novel strategy that protects Staphylococcus aureus against infection by Staphylococcus pseudintermedius Siphoviridae phages. Appl Microbiol Biotechnol 2019;103(16):6809–6823; doi: 10.1007/s00253-019-09940-7 [DOI] [PubMed] [Google Scholar]
- 39. Botka T, Pantůček R, Mašlaňová I, et al. Lytic and genomic properties of spontaneous host-range kayvirus mutants prove their suitability for upgrading phage therapeutics against Staphylococci. Sci Rep 2019;9(1):5475; doi: 10.1038/s41598-019-41868-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Junjappa RP, Desai SN, Roy P, et al. Efficacy of anti-staphylococcal protein P128 for the treatment of canine pyoderma: Potential applications. Vet Res Commun 2013;37(3):217–228; doi: 10.1007/s11259-013-9565-y [DOI] [PubMed] [Google Scholar]
- 41. Łobocka M, Hejnowicz MS, Dąbrowski K, et al. Genomics of Staphylococcal Twort-like phages—potential therapeutics of the post-antibiotic era. Adv Virus Res 2012;143–216; doi: 10.1016/B978-0-12-394438-2.00005-0 [DOI] [PubMed] [Google Scholar]
- 42. Deghorain M, Van Melderen L. The Staphylococci phages family: An overview. Viruses 2012;4(12):3316–3335; doi: 10.3390/v4123316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moodley A, Kot W, Nälgård S, et al. Isolation and characterization of bacteriophages active against methicillin-resistant Staphylococcus pseudintermedius. Res Vet Sci 2019;122:81–85; doi: 10.1016/j.rvsc.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 44. Polak-Witka K, Rudnicka L, Blume-Peytavi U, et al. The role of the microbiome in scalp hair follicle biology and disease. Exp Dermatol 2020;29(3):286–294; doi: 10.1111/exd.13935 [DOI] [PubMed] [Google Scholar]