Short abstract
Content available: Audio Recording
Listen to an audio presentation of this article.
BACKGROUND
Chronic hepatitis B virus (HBV) affects approximately 1.5 million people in the United States and 291 million people worldwide. 1 , 2 Appropriate diagnosis and antiviral treatment reduces the risk of progressive liver fibrosis, cirrhosis, and liver cancer. 3 A 2016 study of HBV care in the Veterans Health Administration (VHA) revealed gaps in HBV treatment among patients with cirrhosis. 4 While greater than 90% of patients receiving treatment had annual alanine transaminase (ALT) measurement, only 44% had annual HBV DNA testing. This report summarizes actions the VHA undertook to improve HBV care and describes subsequent HBV DNA testing and antiviral treatment rates with a population management dashboard.
METHODS
In August 2016, the Veterans Administration's (VA's) HIV, Hepatitis, and Related Conditions Program Office (HHRC)—the body charged with overseeing care for viral hepatitis within VA—convened an HBV strategic planning work group to address changes needed to improve HBV care. Subject matter experts (SMEs) included gastroenterologists, hepatologists, infectious disease specialists, primary care providers, clinical pharmacists, population health managers and national leaders within HHRC and the National Program for Prevention Policy. 5 The workgroup was tasked with developing evidence‐based HBV quality of care metrics for the VA healthcare system.
The group reviewed VHA's HBV continuum of care, evidence‐based literature and clinical guidelines applicable to Veterans with HBV. Quality of care metrics were selected and prioritized by consensus, quality of evidence, impact on patient care, and the ability to measure and collect data using VHA data infrastructure.
The workgroup identified seven HBV‐related quality of care outcomes: (1) HBV testing among high‐risk patients, (2) HBV treatment among patients with cirrhosis and chronic HBV, (3) liver cancer surveillance, (4) annual HBV DNA and ALT among patients receiving HBV antiviral therapy, (5) co‐testing for hepatitis A and C viruses in HIV in patients with HBV, (6) vaccination for HBV in selected high‐risk populations, and (7) prevention of HBV reactivation among patients receiving high‐risk immunosuppression (Table 1).
TABLE 1.
Table of deliverables for prioritization of HBV metrics
| Quality of care metric & guidelines | Definition |
|---|---|
| Metric 1 | Immunosuppressive treatment: |
| HBV Testing (HBsAg, HBcAb, and HBsAb) among high‐risk patients (e.g. HIV, HCV, B‐cell depleting agents) | Anthracycline derivatives: e.g., doxorubicin, epirubicin |
| Guidelines: (1–4) | B cell depleting agents: e.g., rituximab, ofatumumab, Obinutuzumab |
| Cytokine / Integrin inhibitor: e.g., abatacept, ustekinumab, natalizumab, Vedolizumab | |
| TNF‐alpha inhibitors: e.g., etanercept, adalimumab, certolizumab, infliximab | |
| Tyrosine kinase inhibitors: e.g., Imatinib, nilotinib | |
| Prednisone ≥10 mg daily, ≥4 weeks | |
| Metric 2 | Chronic HBV: HBsAg detectable and or HBV DNA detectable on two or more occasions at least 6 months apart. |
| HBV treatment among patients with chronic HBV and cirrhosis | Treatments: Acceptable HBV treatments include daily (or equivalent in CKD): entecavir, tenofovir alafenamide (TAF), or tenofovir disoproxil fumarate (TDF). If HIV+: combinations drugs that include these constituent medications are acceptable. |
| Guidelines: (1) | Cirrhosis: at least one inpatient or two outpatient visits with any of the following ICD codes for esophageal varices (primary or secondary) with or without bleeding, alcoholic cirrhosis of the liver, spontaneous bacterial peritonitis, hepatic coma or encephalopathy, cirrhosis of the liver (with or without ascites), chronic hepatic failure (with or without coma), other cirrhosis of the liver, portal hypertension. |
| Metric 3 | Surveillance: should be performed with ultrasound examination of the liver. Radiologic examination of liver with CT or MRI is not recommended for surveillance but will meet this criterion. AFP blood test will not meet this requirement. |
| HCC surveillance of patients with HBV‐related cirrhosis every 12 months | Chronic Hepatitis B: See definition for Metric 2 |
| Guidelines: (1) | Cirrhosis: see definition for Metric 2 |
| Metric 4 | HBV treatment: receipt of the following for any 30 or more days in the past 12 months (does not need to be consecutive): entecavir, TAF, or TDF on a daily (or equivalent in CKD) basis. |
| Among patients receiving HBV anti‐viral treatment for ≥30 days out of at least 12 months: | ALT: serum ALT performed at VA facility |
|
HBV DNA: Serum HBV DNA performed at VA facility |
| Guidelines: (1) | |
| Metric 5 | Chronic HBV: see definition for Metric 2 |
| Among patients with chronic HBV test for: | HCV: HCV antibody or HCV viral load (While HCV antibody is the preferred screening method, a negative HCV viral load will also meet this requirement). Individuals who have evidence of HCV infection are excluded from the requirement. |
|
HIV: HIV antibody of HIV viral load. (While HIV antibody is the preferred screening method, a negative HIV viral load will also meet this requirement.) Individuals who have prior evidence of HIV infection are excluded from this requirement. |
| Guidelines: (1) | |
| Metric 6* | Vaccination: |
| HBV vaccination for the following groups who are previously unvaccinated and are at risk for HBV (e.g., HBsAg‐, HBcAb‐, HBsAb‐, or HBV immune status unknown): | HBV: Receipt of one or more HBV vaccinations |
|
HAV: HAV antibody (IgG or total) or receipt of 1 HAV vaccine (hep A or twinrix at any time) |
| Guidelines: (5) | |
| Metric 7 | High risk immunosuppression: |
| HBV anti‐viral prophylaxis (with entecavir, TAF or TDF) to prevent HBV reactivation during high‐risk immunosuppression, and for 6–12 months post‐discontinuation (unless meeting criteria for chronic HBV treatment). | 1. If HBcAb positive (regardless of HBsAg or HBsAb): |
| High risk immunosuppression is defined as: |
|
|
|
| Guideline: (6) | 2. If HBsAg positive: |
| |
| Guidelines reviewed by workgroup in 2017: | |
| |
| References used by Workgroup in 2017: | |
| |
| ICD codes for cirrhosis: | |
|
Cirrhosis: At least one inpatient or two outpatient visits with any of the following ICD codes: |
I85.11 Secondary esophageal varices with bleeding |
| 456.0 Esophageal Varices With Bleeding | K65.2 Spontaneous Bacterial Peritonitis |
| 456.1 Esophageal Varices Without Mention Of Bleeding | K70.2 Alcoholic fibrosis of liver |
| 456.20 Esophageal Varices in Diseases Classified Elsewhere, with bleeding | K70.30 Alcoholic cirrhosis of liver without ascites |
| 567.2 Other Peritonitis (used through FY2006) | K70.31 Alcoholic cirrhosis of liver with ascites |
| 567.23 Spontaneous Bacterial Peritonitis (used after FY2006) | K70.40 Alcoholic hepatic failure without coma |
| 571.2 Alcoholic Cirrhosis Of Liver | K70.41 Alcoholic hepatic failure with coma |
| 571.5 Cirrhosis Of Liver Without Mention Of Alcohol | K72.10 Chronic hepatic failure without coma |
| 572.2 Hepatic Coma or hepatic encephalopathy | K72.11 Chronic hepatic failure with coma |
| B17.11 Unspecified viral hepatitis B with hepatic coma | K72.90 Hepatic failure, unspecified, without coma |
| B19.21 Unspecified viral hepatitis C with hepatic coma | K72.91 Hepatic failure, unspecified with coma |
| I85.00 Esophageal Varices Without Bleeding | K74.60 Unspecified cirrhosis of liver |
| I85.01 Esophageal Varices With Bleeding | K74.69 Other cirrhosis of liver |
| I85.10 Secondary esophageal varices without bleeding | K76.6 Portal hypertension |
Abbrevbiations: HBsAg, hepatitis B surface antigen; HBsAb, hepatitis B surface antibody; HBcAb, hepatitis B core antibody; PrEP, pre‐exposure prophylaxis; TNF, tumor necrosis factor; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; HCV, hepatitis C; HIV, human immunodeficiency virus; HAV, hepatitis A.
The workgroup identified several pathways for implementation of best practices (Figure 1). The pathways involved data infrastructure to display quarterly VHA population metrics, development of a population management tool to use for direct patient care, and improved educational programming such as face‐to‐face and virtual conferences. Educational programs were geared towards multi‐disciplinary teams in primary care and specialty care, (also known as the HIT, or Hepatic Innovation Teams, which have been well described previously. 6 , 7 The HITs had prior success with process improvement efforts related to hepatitis C elimination in VA.
FIGURE 1.
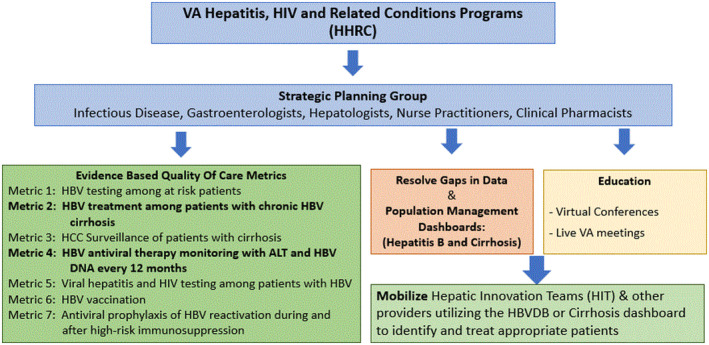
Pathway development. ALT, alanine aminotransferase; DNA, deoxyribonucleic acid; HBV, hepatitis B virus; HBVDB, hepatitis B dashboard; HCC, hepatocellular carcinoma; HIV, human immunodeficiency virus; VHA, Veterans Health Administration.
A crucial initial step was the deployment of a user‐friendly HBV dashboard (HBVDB) in December 2018 to specifically address gaps in care related to treatment of HBV in patients with cirrhosis and monitoring of HBV treatment with an annual ALT and HBV DNA. The HBVDB contained data derived from the VA Corporate Data Warehouse, a repository of ICD9/10 codes for diagnosis from clinical encounters, imaging, and lab data as well as pharmacy prescription data. The HBVDB displayed real time, clinically relevant laboratory data (e.g., HBV serology, ALT, platelets, HBV DNA, alpha fetoprotein), hepatocellular cancer surveillance, and HBV antiviral therapy prescription refill date (Figure 2). Chronic hepatitis B was defined as either two positive HBsAg and/or HBV DNA virus at least 6 months apart. Patients with a single known detectable HBV viral load or positive HBsAg were flagged for provider review and quick care prioritization. Abnormal results displayed as color coded flags to enable quick care prioritization. A list management functionality in the report enabled clinicians to sort patients by data of interest and track issues with quick notes and future review dates. A complementary summary report in the HBVDB enabled providers to track and self‐manage progress.
FIGURE 2.
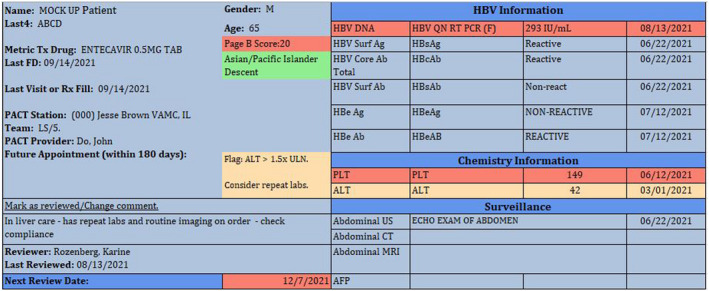
HBV dashboard view/Hepatitis B dashboard displays colored flags for abnormal labs (detectable HBV viral load (HBV DNA 293 IU/mL), low platelet count (PLT 149) and elevated alanine aminotransferase (ALT 42). ULN, upper limit of normal, high‐risk descent (Asian/Pacific islander) and overdue review date (12/7/21). Patient information: Last 4, patient identifier; Tx, treatment; FD, fill date; Rx, prescription; PACT, patient aligned care team (primary care). A mark as reviewed link allows user to add quick comments to the report (in liver care—has repeat labs), and to select a future review date if needed. HBV information contains: AB, antibody; AFP, alfa fetoprotein; CT, computerized tomography; HBcAb, hepatitis B core antibody; HBeAb/HBeAb, hepatitis B e antibody; HBeAg, hepatitis B e antigen; HBV DNA, hepatitis B virus deoxyribonucleic acid (DNA); HBV QN RT PCR (F), HBV quantitative polymerase chain reaction; IU/mL, International units/milliliters; HBV SurfAg/HBsAg, hepatitis B surface antigen; MRI, magnetic resonance imaging; surf ab/HBsAb, hepatitis B surface antibody; US, ultrasound of the abdomen.
Dashboard training was provided by the HBVDB development lead clinician and to the HITs who then facilitated training rapidly across VA facilities. Dashboard training was minimal as the HBVDB design and functionalities mirrored other liver dashboards already in use at the VA (namely hepatitis C and cirrhosis dashboards).
The other measures not included in the HBVDB were addressed through various other VA programs, which were highlighted during educational conferences and reported on the national VA SharePoint quarterly. For example, the VA Center for Medication Safety (VA MedSAFE) disseminates a monthly patient level report for patients on immunosuppressive therapy for prevention of HBV reactivation of at risk individuals and HCC surveillance is tracked in a cirrhosis dashboard for all cirrhosis etiologies.
RESULTS
Prior to release of the HBVDB, there were 7961 confirmed patients with chronic HBV, of whom 3011 (37.8%) were receiving antiviral treatment. Approximately 44% of patients prescribed antiviral therapy received annual ALT and HBV monitoring. Among 1362 patients with cirrhosis (defined by at least 1 inpatient or 2 outpatient ICD encounters for cirrhosis or complication of cirrhosis), 62.4% were receiving antiviral therapy. Three years later, there were 9670 patients with chronic HBV, of whom 3117 (32.2%) were receiving antiviral treatment, and 2269 (72.8%) had completed annual ALT and HBV DNA measurements. Receipt of antiviral medications among the 1247 patients with cirrhosis improved for 900 (72.2%) patients (Table 2).
TABLE 2.
Results: September 2021
| HBV metric | N (%) |
|---|---|
| Patients with chronic HBV (in care) | 9670 |
| HBV with cirrhosis | 1247 |
| With cirrhosis and prescribed antiviral therapy | 900 (72.2%) |
| Receiving HBV antiviral therapy | 3117 |
| Completed annual ALT & HBV laboratory monitoring | 2269 (72.8%) |
In fiscal year 2021, 79 of 140 VA facilities used the HBVDB on a regular basis, 4 facilities stopped using the report since 2020, and 62 facilities never used this tool. There were 237 HBVDB users identified of which 162 (68.3%) were categorized as providers, MD/DO, NP/PA, or clinical pharmacist. Infectious disease (ID) and gastroenterology (GI)/liver providers accounted for 120 (74%) of provider type users, while 18 (11%) were stratified as “other” and 19 (12%) as unknown clinic locations. Other users comprised 20 administrators, 31 nurses, and 24 users with unknown credentialing who either left the VA or had a marital status related name change (Tables 3). Clinical pharmacists were the largest user types followed by physician assistants, physicians, and nurse practitioners (Table 4).
TABLE 3.
Dashboard users by credentials and clinic location
| Clinic | Dashboard users (N = 237) | ||||
|---|---|---|---|---|---|
| Credentialed provider: MD/DO/NP/PA/PharmD | Admin | RN/nursing | N/A | Total | |
| ID | 20 | 1 | 21 | ||
| GI/Liver | 100 | 6 | 22 | 128 | |
| PACT | 5 | 2 | 7 | ||
| Other | 18 | 13 | 1 | 32 | |
| Unknown | 19 | 1 | 5 | 24 a | 49 |
| Total | 162 b | 20 | 31 | 24 | 237 |
Abbreviations: Admin, administrative (may include credentialed providers in an administrative role); GI, gastroenterology; ID, infectious disease; N/A, not available; PACT, patient aligned care team (primary care); RN, registered nurse.
Unknown credentials (position records unavailable due to leaving the VA or marital status name change).
162/237 (68.3%) of dashboard users were credentialed providers.
TABLE 4.
Dashboard providers by provider type and clinic location
| Clinic location | ||||||
|---|---|---|---|---|---|---|
| ID | Liver/GI | Other | PACT | Unknown | Total | |
| Credentials | 8 | 25 | 1 | 2 | 7 | 43 |
| MD/DO | 2 | 28 | 1 | 2 | 33 | |
| NP | 7 | 7 | ||||
| PA | 10 | 40 | 16 | 3 | 10 | 79 |
| PharmD | 20 (12%) | 100 (62%) | 18 (11%) | 5 (3%) | 19 (12%) | 162 |
Abbreviations: DO, doctor of osteopathic medicine; GI: gastroenterology clinic; ID, infectious disease clinic; MD: medical doctor; NP, nurse practitioner; PA, physician assistant; PACT: patient aligned care team (primary care); PharmD, doctor of pharmacy (clinical pharmacist).
Discussion
In 2018, the VA created a multidisciplinary HBV workgroup to develop evidence based metrics to measure HBV care for the VA health‐care system. The workgroup identified seven metrics and deployed a HBVDB to treat patients with cirrhosis and improve laboratory monitoring of antiviral treatments.
Improving HBV care requires changes to processes of patient care and a real‐time dashboard of patients with chronic HBV providing up‐to‐date information about the patient's liver tests, HBV tests, vaccinations, liver imaging, medications, and a diagnosis of cirrhosis. Thus, providers can identify patients who are overdue for ALT or HBV testing, or patients who have cirrhosis but are not receiving antiviral treatment. All providers in the VA had access to the dashboard all the time from their work/clinic computer connected to the VHA system. Three years after release of the HBVDB, 237 providers at 79 VA facilities (~55% of VA facilities) had used the HBVDB. During these 3 years, the number of patients who received annual ALT and HBV DNA testing increased from 44% to 72.8% while the number of patients with HBV cirrhosis receiving antiviral treatment increased from 62.4% to 72.2%.
Current VHA data systems do not capture HBV care at non‐VA facilities and therefore our data may underestimate the current state of HBV treatment and medication monitoring. Well‐designed population health dashboards, accessible national reporting tools, and implementation teams may enable large healthcare systems to proactively improve the quality of care for many patients. Additional analysis of dashboard usability and implementation teams is needed to determine if these efforts can be truly correlated to VHA's efforts in improving HBV care among Veterans.
CONFLICT OF INTEREST
Nothing to report.
ACKNOWLEDGMENT
HBV Dashboard Development Team: James Duvel, Samantha McClelland, Andrew David Jacob, and Christopher Sedgewick.
Rozenberg‐Ben‐Dror K, Maier MM, Beste L, Lowy E, Chartier M, Ross D, et al. Improving quality of hepatitis B care in the Veteran's Health Administration. Clinical Liver Disease. 2022;19:213–218. 10.1002/cld.1237
REFERENCES
- 1. Polaris Observatory Collaborators . Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383–403. [DOI] [PubMed] [Google Scholar]
- 2. Lim JK, Nguyen MH, Kim WR, Gish R, Perumalswami P, Jacobson IM. Prevalence of chronic Hepatitis B virus infection in the United States. Am J Gastroenterol. 2020. Sep;115(9):1429–38. 10.14309/ajg.0000000000000651 [DOI] [PubMed] [Google Scholar]
- 3. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. American Association for the Study of Liver Diseases. Update on prevention, diagnosis, and treatment of chronic Hepatitis B: AASLD 2018 Hepatitis B guidance. Hepatology. 2018;67:1560–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among US veterans with hepatitis B: A national cohort study. Hepatology. 2016. June;63:6, 28340. 10.1002/hep.28340 [DOI] [PubMed] [Google Scholar]
- 5. Chartier M, Maier MM, Morgan TM, Lowy E, Hoffman‐Hog, L , Ross D. et.al. Achieving excellence in Hepatitis B virus Care for Veterans in the veterans health administration. Fed Pract. 2018. Mar;35(Suppl 2):S49–S53. [PMC free article] [PubMed] [Google Scholar]
- 6. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing hepatitis C virus infection: best practices from the U.S. Department of Veterans Affairs. Ann Intern Med. 2017. Oct;167:499–504. 10.7326/M17-1073 [DOI] [PubMed] [Google Scholar]
- 7. Park A, Gonzalez R, Chartier M, Rogal S, Yakovchenko V, Ross D, et al. Screening and treating Hepatitis C in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018. July;35(7):24–9. [PMC free article] [PubMed] [Google Scholar]


