ABSTRACT
Introduction
Health care workers (HCWs) are disproportionately exposed to infectious diseases and play a role in nosocomial transmission, making them a key demographic for vaccination. HCW vaccination rates are not optimal in many countries; hence, compulsory vaccination policies have been implemented in some countries. Although these policies are effective and necessary under certain conditions, resolving HCWs’ hesitancies and misconceptions about vaccines is crucial. HCWs have the advantage of direct contact with patients; hence, they can respond to safety concerns, explain the benefits of vaccination, and counter antivaccine campaigns that escalate during pandemics, as has been observed with COVID-19.
Method
A short survey was carried out in May–June 2020 on the vaccination status of HCWs working with pediatric patients with COVID-19. The survey inquired about their vaccination status (mumps/measles/rubella [MMR], varicella, influenza, and diphtheria/tetanus [dT]) and willingness to receive hypothetical future COVID-19 vaccines. The respondents were grouped according to gender, age, occupation, and region.
Results
In total, 4927 HCWs responded to the survey. Most were young, healthy adults. The overall vaccination rates were 57.8% for dT in the past 10 years, 44.5% for MMR, 33.2% for varicella, and 13.5% for influenza. Vaccination rates were the highest among physicians. The majority of HCWs (81%) stated that they would be willing to receive COVID-19 vaccines.
Conclusion
Although vaccination rates for well-established vaccines were low, a majority of HCWs were willing to receive COVID-19 vaccines when available. Education and administrative trust should be enhanced to increase vaccination rates among HCWs.
KEYWORDS: Health care workers; vaccination; measles; influenza, SARS-CoV-2
Introduction
It has been two years since the world is affected by a highly contagious emerging pathogen that led to a pandemic in a very short time. The new virus compelled governments to lock down their countries and strained health care systems worldwide. Vaccination practices were made to prevent and control infectious disease outbreaks.1 Great advances have been achieved in a very short time; within the first year of the pandemic, vaccines started to become available. Nevertheless, even during a devastating pandemic, antivaccine lobbies and their unscientific, conspiracy-based arguments are widespread. Health care workers (HCWs) are responsible for evading and persuading against antivaccine arguments, which is more easily said than done. To ensure the success of vaccine campaigns, HCWs must set an example regarding vaccination, especially when skepticism toward vaccination is increasing, and safety rather than efficacy is receiving more public attention.2
HCWs are disproportionately exposed to infectious diseases and play a role in nosocomial transmission, which makes them a key demographic for vaccination. While many professional societies favor the vaccination of HCWs, recommendations by country; hence, vaccination rates of HCWs also vary. In most countries, HCW vaccination is less than optimal for vaccines against contagious diseases, such as measles,3–5 leading to nosocomial transmission among HCWs and patients.6–8
The vaccinations recommended and supplied for all HCW by the Turkish Ministry of Health are measles/mumps/rubella (MMR), seasonal influenza, diphtheria/Tetanus (dT) every 10-years, and Hepatitis B. Hepatitis A is recommended for those working in pediatrics or geriatric departments, and diphtheria/tetanus/acellular pertussis (dTap) is recommended for those working in neonatal units. It is also recommended for paramedics and microbiology laboratory workers to be vaccinated with conjugate meningococcus vaccines.9
Just before the rollout of COVID-19 vaccines, we aimed to determine the vaccination status of HCWs working with pediatric patients for routinely recommended vaccines that have been used for decades and the willingness of HCWs to receive COVID-19 vaccines when available.
Methods
The study was conducted among HCWs working in COVID-19 pediatric units at 32 hospitals in seven different regions of Turkey. The participants were enrolled in the study between May 25 and 10 June 2020. Physicians, including professors to residents, nurses, radiology technicians, and other medical staff, were enrolled.
Participants were informed about the study through staff meetings. HCWs who volunteered were asked to complete a brief printed survey. The survey included questions about age; sex; occupation; years in the profession; vaccination status against MMR, varicella, and dT; and COVID-19 vaccine willingness. Survey data were collected anonymously; each center was assigned a number, and survey results were collected in a box. The survey answers were entered and edited in a Microsoft Excel file. Data from all centers were collected and analyzed, and IBM SPSS software package version 26 was used for all statistical analyses. Categorical variables are expressed as frequencies and percentages, and continuous variables are expressed as medians with interquartile range. The Mann–Whitney U test was performed to compare two groups on the basis of gender, and Kruskal–Wallis analysis was performed to compare more than two groups on the basis of occupation, age group, and region. To determine the groups contributing to a statistically significant difference, Spearman pairwise comparison with Bonferroni’s correction was performed. A p-value <.05 was considered statistically significant.
Consensus agreements were obtained from all 32 centers, and the study was approved by the Institutional Review Board and the Ethics Committee of Hacettepe University (approval number 2020/11–57).
Results: population
A total of 7652 HCWs in the pediatric COVID-19 units of 32 health care centers were invited to participate; 4927 (64.4%) responded to the survey. The participants were mostly young adults, with 70.7% (n = 3482) under 40 years old. Their median age was 32 years (19–67), and 72.7% (n = 3583) were female. Most of the participants were physicians (43.1%, n = 2123), followed by nurses (34.6%, n = 1707) and other HCWs (22.3%, n = 1097).
Centers and regions
Thirty-two centers (18 state hospitals and 14 university hospitals) in seven geographic regions participated in the study. These included four university hospitals in central Anatolia, three university and three state hospitals in the Aegean region, one university and one state hospital in the Mediterranean region, one university and two state hospitals in the Black Sea region, four university and seven state hospitals in the Marmara region, one university and two state hospitals in southeast Anatolia, and two state hospitals in eastern Anatolia.
Vaccination rates of vaccines
The overall vaccination rates were 57.8% (n = 2662) for dT in the past 10 years, 44.5% (n = 2184) for MMR, 33.2% (n = 1631) for varicella, and 13.5% (n = 661) for influenza (Figure 1). Overall, the vaccination rate was the highest among physicians for all vaccines included in the survey. The vaccination status of participants varied depending on age, gender, occupation, and the region in which they were employed. The demographics and vaccination status of HCWs according to their occupation are presented in Table 1; vaccination status by occupation and age group is presented in Table 2; and vaccination status according to region is presented in Table 3.
Figure 1.
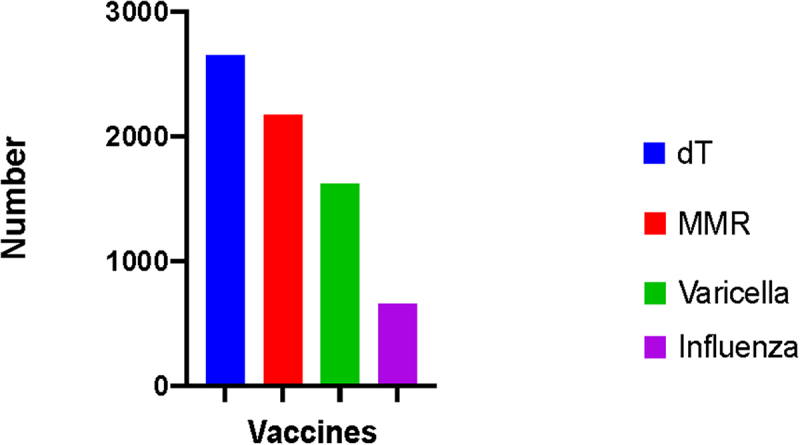
The number of vaccinated HCWs.
dT: diphtheria-Tetanus vaccine
MMR: mumps-measles- rubella vaccine.
Table 1.
Demographics and vaccination rates of health care workers by occupation.
| Occupation | n (%) | Age, median (range) |
Sex, F/M |
Vaccines (n) (%) |
|||
|---|---|---|---|---|---|---|---|
| Influenza | MMR | Varicella | dT | ||||
| Doctor | 2123 (43.1) |
32 | 1440/683 | ||||
|
Prof. Dr. Assoc.Prof. Dr Asst. Prof. Dr. Consult.Dr. Resident |
149 (3.0) 188 (3·8) 149 (3.0) 654 (13.3) 983 (20.0) |
52·7 (42–67) 44·17 (36–64) 36·01 (25–59) 38·64 (25–67) 28·25 (20–34) |
82/67 138/50 108/41 424/230 688/295 |
34 (22.8) 44 (23.7) 26 (17.7) 114 (17.5) 178 (18.1) |
52 (45) 89 (33.9) 71 (25.3) 320 (28.3) 591 (29.9) |
35 (23.5) 52 (28) 36 (24.7) 208 (31.8) 347 (35.3) |
54 (44.6) 105 (61.4) 84 (56.4) 377 (62.9) 577 (63) |
| Nurse | 1707 (34.6) |
32·38 (19–62) |
1490/217 | 118 (6.9) |
692 (30.7) |
590 (34.8) |
913 (56.2) |
| Others | 1097 (22.3) |
37·77 (19–64) |
651/446 | 143 (13.3) |
359 (25.8) |
351 (32.6) |
541 (52.9) |
| Total | 4927 | 34.2 | 3581/1346 | 657 (13,4) |
2174 (44,5) |
1619 (33,1) |
2651 (57,8) |
n: Number.
F : Female.
M: Male.
Table 2.
Vaccination status of HCW based on occupation.
| Occupation(n) | Age groups | Influenza, n (%) |
MMR, n (%) |
Varicella, n (%) |
dT, n (%) |
Total, n n* |
|---|---|---|---|---|---|---|
| Doctors |
19-29 n* |
157 (17.4) 885 |
539 (60.9) 885 |
42 (4.7) 885 |
534 (64.5) 828 |
1272 3483 |
|
30-39 n* |
109 (15.6) 697 |
379 (54.4) 697 |
201 (28.8) 697 |
415 (64.3) 645 |
1104 2736 |
|
|
40-49 n* |
77 (21.6) 357 |
152 (42.6) 357 |
101 (28.3) 357 |
190 (57.4) 331 |
520 1402 |
|
|
50-59 n* |
40 (28.8) 139 |
47 (33.8) 139 |
27 (19.4) 139 |
50 (41.3) 121 |
164 538 |
|
|
>60 n* |
13(32.5) 40 |
6 (15) 40 |
7 (17.5) 40 |
8 (25.8) 31 |
34 151 |
|
| Total n* | 2118 | 2118 | 2118 | 1956 | ||
| Nurses |
19-29 n* |
50 (6) 835 |
379 (45.4) 834 |
325 (39) 834 |
464 (57.4) 808 |
1218 2591 |
|
30-39 n* |
25 (5.9) 423 |
161 (38.2) 421 |
133 (31.7) 420 |
256 (66.8) 383 |
575 1647 |
|
|
40-49 n* |
36 (9.2) 391 |
141 (36.1) 391 |
121 (30.1) 391 |
178 (47.6) 374 |
476 1547 |
|
|
50-59 n* |
6 (11.3) 53 |
14 (26.4) 53 |
15 (28.3) 53 |
15 (31.9) 47 |
50 206 |
|
|
>60 n* |
1 (33.3) 3 |
0 3 |
0 3 |
2 (66.7) 3 |
3 12 |
|
| Total n* | 1705 | 1702 | 1701 | 1615 | ||
| Other |
19-29 n* |
26 (11.6) 225 |
112 (49.8) 225 |
100 (44.2) 226 |
127 (58.5) 217 |
264 893 |
|
30-39 n* |
55 (1.2) 408 |
113 (27.7) 408 |
131 (32.0) 409 |
216 (56.8) 380 |
515 1603 |
|
|
40-49 n* |
46 (13.6) 339 |
99 (29.5) 336 |
99 (29.4) 337 |
165 (50.6) 326 |
409 1338 |
|
|
50-59 n* |
19 (17.30 110 |
23 (20.7) 111 |
27 (24.3) 111 |
36 (34.3) 105 |
105 437 |
|
|
>60 n* |
1(12.5) 8 |
2 (25) 8 |
2 (25) 8 |
5(62.5) 8 |
10 32 |
|
| Total n* | 1090 | 1088 | 1091 | 1036 |
n: Number of vaccinated HCW.
n*: Number of HCW responding to the survey.
Table 3.
Vaccinations rates of HCW based on regions.
| |
VACCINES |
|||
|---|---|---|---|---|
| REGIONS | dT | MMR | Varicella | Influenza |
| Central Anatolia | 60.4 (422/699) | 44.3 (310/699) | 30.9 (216/699) | 13.3 (93/699) |
| Marmara | 60.9 (1283/2108) | 47.4 (1000/2111) | 37.4(789/2110) | 14.1 (297/2110) |
| Aegean | 48.4 (295/610) | 41.9 (372/887) | 20.4 (181/887) | 11.6 (103/887) |
| Mediterranean | 61.5 (126/205) | 50.2 (103/205) | 25.9(107/205) | 21 (43/205) |
| Black Sea | 54.2 (110/203) | 39.9 (81/203() | 28.1 (57/203) | 11.3 (23/203) |
| South-East Anatolia | 57 (304/533) | 42.8 (237/555) | 24.4 (136/557) | 14.1 (79/560) |
| East Anatolia | 49 (122/249) | 32.7 (81/248) | 16.1 (40/249) | 9.2 (23/249 |
| p >.05 | p <.05 | p <.001 | p <.05 | |
dT
dT vaccination data were available for 4607 participants. The highest vaccination rate was observed for dT: 56.4% of physicians were vaccinated against dT; 53.7% and 50% of nurses and other HCWs were vaccinated, respectively. The differences in d0.T vaccination status among HCWs did not differ significantly according to occupation (p = 1), age group (p = 1), gender (p = 1), or region (p = 1).
MMR
MMR vaccination data were available for 4908 participants. The vaccination rates of physicians, nurses, and other HCWs were 51.4%, 31.8%, and 16.8%, respectively. The vaccination rate of female HCWs was higher (74.8%) than that of males (25.2%) (p = .009). The vaccination rate of physicians was significantly higher than that of both nurses and other HCWs (p < .001 and p < .001), and a higher vaccination rate was observed for nurses than for other HCWs (p < .036). MMR vaccination also differed by age group. The highest vaccination rate was observed in those aged 19–29 years, followed by those aged 30–39 years. Significant differences were observed between the 19–29 year age group and all the remaining age groups (p < .001); the 30–39 year age group differed significantly from the 40–49 and 50–59 year age groups (p < .01 and p = .02, respectively) but not the >60 year age group (p = .08). No significant difference in MMR vaccination status was observed between those aged 40–49 years and the older age groups (p = .16 and p = .80) or between the 50–59 and >60 year age groups (p = 1). HCW vaccination rates differed by location. The highest vaccination rates were observed in the Marmara, Aegean, and Mediterranean regions, followed by southeast Anatolia. In paired comparisons, no significant differences were observed between the Marmara, Aegean, Mediterranean, and southeast Anatolia regions (p = .2, p = 1, p = 1, p = .01). However, vaccination rates were significantly higher in the Marmara, Aegean, and Mediterranean regions in the pairwise comparisons with central Anatolia (p < .001), east Anatolia (p < .001), and the Black Sea region (p = .02).
Varicella
Varicella vaccination data were available for 4910 respondents. The natural immunity against varicella was 40.3% (n = 1980), and the vaccination rate was 33.2% (n = 1631). The remaining 26.2% (n = 1299) of HCWs were neither vaccinated nor naturally immune. The vaccination rates were 57.9% for females and 50.5% for males (p < .001). The highest vaccination rate was among physicians (41.6%), followed by nurses (36.4%) and other HCWs (22%). Vaccination rates differed significantly between occupation groups; the vaccination rate of physicians was higher than that of nurses (p = .02) and other HCWs (p < .001), and the vaccination rate of nurses was higher than that of other HCWs (p < .001). The highest vaccination rate was observed in the 19–29 year age group and differed significantly from all other age groups (p < .001); the 30–39 year age group differed from the 40–49 (p = .03) and 50–59 (p < .01) year age groups but not the >60 year age group (p = .07). No significant difference in varicella vaccination status was observed between the 40–49 year age group and the older groups (p = .18, p = .80) or between the 50–59 and >60 year age groups (p = .64). No significant differences in varicella vaccination status were observed between the Marmara, Aegean, Mediterranean, central Anatolia, and southeast Anatolia regions (p > .005), whereas vaccination rates in the Black Sea and east Anatolia regions differed significantly (p = .02, p = .01).
Influenza
Influenza vaccination data were available for 4913 participants. The vaccination rate of male HCWs was higher than that of females (p = .01). The vaccination rate was the highest among physicians (18.7%), followed by other HCWs (13.5%) and nurses (6.9%). A statistically significant difference was observed among occupation groups. The vaccination rate of physicians was higher than that of nurses (p < .01) and other HCWs (p < .001), whereas the vaccination rate of other HCWs was higher than that of nurses (p < .001). A tendency for increased vaccination rates was observed among those over 50 years old. Although the vaccination rate among those aged 19–29 years differed significantly from those aged 50–59 and >60 years (p = .001, p = .003), no significant differences in influenza vaccination were observed for those aged 30–39 or 40–49 years.
COVID-19 vaccination
Just before the survey, it was announced that HCWs would be the first group to receive vaccines when available. Hence, HCWs were also questioned about their willingness to receive COVID-19 vaccines. A total of 4910 HCWs responded to the questions on COVID-19 vaccination willingness. Most respondents were physicians (43.1%), followed by nurses (34.6%) and other HCWs (22.2%). A total of 3977 (81%) respondents were willing to receive COVID-19 vaccines. Although most COVID-19 vaccine refusal was found among 19–29-year-olds (36%), no significant difference in vaccine willingness was observed according to gender, occupation, age group, or region (p = 1). The reluctance was mostly due to a lack of trust in the vaccine (since it was recently manufactured) and a lack of information on long-term side effects.
Discussion
HCWs are considered the most trustworthy sources of vaccine-related information for the public.10 They are in the best position to understand hesitant patients, respond to safety concerns, and explain the substantial benefits of vaccination. To accomplish this, HCWs should be well-educated so that any hesitancy or misconceptions about vaccines are resolved. One major finding of our study was the low overall immunity among HCWs from vaccines with well-established efficacy and safety. We observed the highest vaccination rates for dT, irrespective of occupation, age, gender, and location. This finding was attributed to the tetanus component of the vaccine, which is routinely administered after injuries as postexposure prophylaxis, rather than routine vaccination against both diphtheria and tetanus every 10 years. The measles vaccination rate among HCWs was low compared with the estimated critical coverage threshold value of >95% needed for herd immunity.11–13 Measles vaccination among HCW is crucial, especially in Turkey, which is close to war-bound countries from which immigration is ongoing. Small outbreaks of measles are encountered yearly, especially in regions with high immigration. In our study, although overall measles immunity among HCWs, acquired or through vaccination, was 73.0%, it was still too low for optimal prevention of disease transmission among susceptible HCWs and nosocomial outbreaks. The highest measles vaccination rate was observed among physicians aged 19–29 years (61%), followed by physicians aged 30–39 years (45.4%), most likely because measles or MMR vaccines were in their childhood vaccination schedules. The higher rate of immunization among female participants may be because the rubella component of the MMR vaccine is routinely checked before planned conceptions. The decline in the MMR vaccination rate with age could be attributed to the lower number of participants in older age groups and the high natural infection rates for measles, considering that the authorization dates of the measles and MMR vaccines for the national vaccination schedule were after their primary childhood vaccination dates (1972 and 1984, respectively). Vaccination rates differed according to geographical regions; western and southern coastal regions had the highest vaccination rates, suggesting that misconceptions due to cultural factors may outweigh education, except for southeast Anatolia. As one of the closest regions to Syria, and as the host to a large population of immigrants, southeast Anatolia experiences frequent measles outbreaks. Actual encounters with diseases and their consequences seem to be the strongest determinant of vaccination rates. Measles vaccination was strongly recommended by international and national health authorities because of possible transmission to susceptible patients and the increased risk of HCWs contracting the disease. It has been calculated that the risk for HCWs contracting measles is 2 to 19 times higher than that of the general population.6, 14, 15 Nosocomial outbreaks have been observed in several European countries; 17 of 30 countries strongly recommend measles vaccination for all HCWs, including Finland, where measles vaccination is mandatory.16,17
The varicella vaccine was added to the national vaccination schedule in 2013 and is currently administered as a single dose at 1 year of age; hence, no HCWs were vaccinated as part of their childhood vaccination schedule. Therefore, natural varicella immunity among HCWs was high (40.3%). The higher vaccination rate among young female HCWs can be attributed to the routine testing before planned conceptions. Single-dose administration of the varicella vaccine leads to annual wild-type infections, and nonimmune HCWs are at risk of being exposed to the disease and spreading it.
The overall vaccination rate against influenza among HCWs was 13.4%, slightly above that of the population (9%). Influenza vaccination rates among physicians and nurses tended to increase with age (p < .03). Influenza pandemics are the only times when influenza vaccination rates increase.18 Although the high fatality rate and the massive economic burden of seasonal influenza are well-established, prejudice against influenza vaccination exists throughout the world. In countries where influenza vaccination rates are high, influenza vaccination is compulsory for HCWs.19,20
Vaccination is the most effective way to eliminate infectious diseases, and eradication is the ultimate goal. Although vaccines are highly recommended for HCWs and supplied to them for free during or after working hours, misconceptions and false beliefs, mainly acquired through social media and the press, continue to spread. Our study revealed that influenza vaccination remained low, even during a pandemic. In the US, when all other methods failed, influenza vaccination was mandated among all employees in many centers, and only then did vaccination rates reach 80%.21
Because they are on the front line, many HCWs have lost their lives during the pandemic.22–24 Apart from the fear of death, HCWs experience fear of infection and spreading the virus to their patients, colleagues, friends, and families; however, they continue to demonstrate professional dedication. Many countries prioritized vaccinating HCWs against COVID-19. Our results on acceptance of COVID-19 vaccines aligned with a French study, in which 81.5% of HCWs expressed their intention to be vaccinated when vaccines became available.25 Several surveys carried out among non-HCWs revealed lower rates and male and elderly predominance of COVID-19 vaccine acceptance. The high rate of acceptance of a new vaccine compared with vaccination rates for well-established vaccines, such as MMR and influenza, among HCWs could be due to their daily reality of living, working, and surviving during a pandemic.26,27
Although the eradication or near-eradication of formerly deadly diseases, such as smallpox, diphtheria, and measles, in developed countries is due to effective vaccination strategies, most vaccine-preventable diseases carry a considerable risk of resurgence. Hence, continuity of vaccination programs and maintaining high levels of immunity is essential. Because of new, emerging pathogens and increasing rates of antibiotic resistance, antivaccine campaigns, and vaccine hesitancy and refusal, HCWs must understand that it is essential to resolve their hesitancies and misconceptions about vaccines in order to credibly recommend vaccines. Vaccines directly protect HCWs from occupational acquisition of vaccine-preventable diseases and indirectly protect their patients.
This study has several limitations. The largest limitation is that this survey was conducted in 2020, when COVID-19 vaccines were not yet available, whereas today, COVID-19 vaccines are available, and this could affect the behavior of HCWs. Furthermore, this study only included HCWs working with pediatric patients, who are more familiar with vaccines. Therefore, the fact that all HCWs were not represented should be considered. Moreover, the survey was self-administered, and it might be affected by recall bias. Another limitation is that none of the nonimmune HCWs were evaluated serologically. The percentages might be altered by the fact that in cases of measles and varicella, the disease may have occurred a long time ago and might not be recalled or may have been misdiagnosed or asymptomatic.
Conclusion
The low immunization rates for well-established vaccines among pediatric HCWs with is an alarming issue. While high acceptance rates for a new vaccine are promising, the hesitancy for effective and safe vaccines that are in circulation for decades must be addressed, and every step and action should be employed to achieve high levels of immunity against all vaccine-preventable diseases. The availability of vaccines for HCWs is not sufficient, and the vaccination status of all HCWs should be determined and recorded. Those who are unvaccinated must be followed up. Every effort should be employed, from education to one-on-one interviews, to address HCWs’ vaccine hesitancy and misconceptions.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Fedele F, Aria M, Esposito M, Micillo M, Cecere G, Spano M, De Marco G.. COVID-19 vaccine hesitancy: a survey in a population highly compliant to common vaccinations. Hum Vaccines Immunother. 2021;17(10):103348–6. doi: 10.1080/21645515.2021.1928460. PMID: 34096836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenwood B. The contribution of vaccination to global health: past, present, and future. Philos Trans R Soc B Biol Sci. 2014;369(1645):20130433. doi: 10.1098/rstb.2013.0433. PMID: 24821919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prato R, Tafuri S, Fortunato F, Martinelli D. Vaccination in healthcare workers: an Italian perspective. Expert Rev Vaccines. 2010;9(3):277–83. doi: 10.1586/erv.10.11. PMID: 20218856. [DOI] [PubMed] [Google Scholar]
- 4.Paya N, Pozzetto B, Berthelot P, Vallée J. Statut vaccinal des médecins généralistes dans le département de la Loire, France. Médecine Et Maladies Infectieuses. 2013;43(6):239–43. doi: 10.1016/j.medmal.2013.05.006. PMID: 23806507. [DOI] [PubMed] [Google Scholar]
- 5.Maltezou HC, Lourida A, Katragkou A, Grivea IN, Katerelos P, Wicker S, Syrogiannopoulos GA, Roilides E, Theodoridou M. Attitudes regarding occupational vaccines and vaccination coverage against vaccine-preventable diseases among healthcare workers working in pediatric departments in Greece. Pediatr Infect Dis J. 2012;31(6):623–25. doi: 10.1097/INF.0b013e31824ddc1e. PMID: 22333705. [DOI] [PubMed] [Google Scholar]
- 6.Genovese C, La Fauci V, Costa GB, Buda A, Nucera S, Antonuccio GM, Alessi V, Carnuccio S, Cristiano P, Laudani N, et al. A potential outbreak of measles and chickenpox among healthcare workers in a university hospital. EuroMediterranean Biomed J. 2019;14:145–48. [Google Scholar]
- 7.Maltezou HC, Wicker S. Measles in health-care settings. Am J Infect Control. 2013;41(7):661–63. doi: 10.1016/j.ajic.2012.09.017. PMID:23352075. [DOI] [PubMed] [Google Scholar]
- 8.Tajima K, Nishimura H, Hongo S, Hazawa M, Saotome-Nakamura A, Tomiyama K, Obara C, Kato T. Estimation of secondary measles transmission from a healthcare worker in a hospital setting. Int J Infect Dis. 2014;24:11–13. doi: 10.1016/j.ijid.2014.03.1377. PMID: 24780918. [DOI] [PubMed] [Google Scholar]
- 9.Turkish Ministry of Health. General Directorate of Public Health . Vaccination for occupational risks. Ankara: THSK; 2018. [Accessed 2021 Dec 6]. https://asi.saglik.gov.tr/asi-kimlere-yapilir/liste/32 [Google Scholar]
- 10.European Center for Disease Prevention and Control . Vaccine hesitancy among healthcare workers and their patients in Europe-a qualitative study. Stockholm: ECDC; 2015. Accessed 2021 Dec 6. [Google Scholar]
- 11.Coughlin MM, Beck AS, Bankamp B, Rota PA. Perspective on global measles epidemiology and control and the role of novel vaccination strategies. Viruses. 2017;9(11):1–17. doi: 10.3390/v9010011. PMID: 28106841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plans P, Torner N, Godoy P, Jané M. Lack of herd immunity against measles in individuals aged <35 years could explain re-emergence of measles in Catalonia (Spain). Int J Infect Dis. 2013;18:81–83. doi: 10.1016/j.ijid.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Thompson K, Odahowski CS. Systematic review of measles and rubella serology studies. Risk Anal. 2016;36(7):1459–86. doi: 10.1111/risa.12430. PMID: 26077609. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization . Measles vaccines: WHO position paper, April 2017 – recommendations. Vaccine. 2019;7(2):219–22. doi: 10.1016/j.vaccine.2017.07.066. [DOI] [PubMed] [Google Scholar]
- 15.Fiebelkorn AP, Seward JF, Orenstein WA. A global perspective of vaccination of healthcare personnel against measles: systematic review. Vaccine. 2014;27(38):4823–39. doi: 10.1016/j.vaccine.2013.11.005. PMID: 24280280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maltezou HC, Wicker S, Borg M, Heininger U, Puro V, Theodoridou M, Poland GA. Vaccination policies for health-care workers in acute health-care facilities in Europe. Vaccine. 2011;29:9557–62. doi: 10.1016/j.vaccine.2011.09.076. PMID: 24280280. [DOI] [PubMed] [Google Scholar]
- 17.Maltezou HC, Botelho-Nevers E, Brantsæter AB, Carlsson R, Heininger U, Hübschen JM, Josefsdottir KS, Kassianos G, Kyncl J, Ledda C, et al. Vaccination of healthcare personnel in Europe: Update to current policies. Vaccine. 2019;37(52):7576–84. doi: 10.1016/j.vaccine.2019.09.061. PMID: 31623916. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Yue N, Zheng M, Wang D, Duan C, Yu X, Zhang X, Bao C, Jin H. Influenza vaccination coverage of population and the factors influencing influenza vaccination in mainland China: A meta-analysis. Vaccine. 2018;36(48):7262–69. doi: 10.1016/j.vaccine.2018.10.045. PMID: 30340886. [DOI] [PubMed] [Google Scholar]
- 19.Macias AE, McElhaney JE, Chaves SS, Nealon, J., Nunes, M C., Samson, S I., Seet, B T., Weinke, T., Yu, H.. The disease burden of influenza beyond respiratory illness. Vaccine. 2021 Mar 15. 39Suppl 1:A6–A14. doi: 10.1016/j.vaccine.2020.09.048. PMID: 33041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lytras T, Kopsachilis F, Mouratidou E, Papamichail D, Bonovas S. Interventions to increase seasonal influenza vaccine coverage in healthcare workers: A systematic review and meta-regression analysis. Hum Vaccin Immunother. 2016;12(3):671–81. doi: 10.1080/21645515.2015.1106656. PMID: 26619125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babcock HM, Gemeinhart N, Jones M, Dunagan WC, Woeltje K. Mandatory Influenza Vaccination. Clin Infect Dis. 2010;50(4):459–65. doi: 10.1086/650752. PMID: 20064039. [DOI] [PubMed] [Google Scholar]
- 22.Zhan M, Qin Y, Xue X, Zhu S. Death from Covid-19 of 23 Health Care workers in China. N Engl J Med. 2020;382(23):2267–68. doi: 10.1056/NEJMc2005696. PMID: 32294342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng L, Wang X, Zhou C, Liu Q, Li S, Sun Q, Wang M, Zhou Q, Wang W. COVID-19 infection rate of HCWs in Wuhan. Clin Infect Dis. 2020;7:1109–13. doi: 10.1093/cid/ciaa588. PMID: 32409825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartmann S, Rubin Z, Sato H, Yong KO, Terashita D, Balter S. Coronavirus disease 2019 (COVID-19) infections among healthcare workers, Los Angeles County, February–May 2020. Clin Infec Dis. 2021;73(7):1850–54. doi: 10.1093/cid/ciaa1200. PMID: 32803237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Detoc M, Bruel S, Frappe P, Tardy B, Botelho-Nevers E, Gagneux-Brunon A. InteDntion to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine. 2020;38(45):7002–06. doi: 10.1016/j.vaccine.2020.09.041. PMID: 32988688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher KA, Bloomstone SJ, Walder J, Crawford S, Fouayzi H, Mazor KM. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S. Adults. Ann Intern Med. 2020;173(12):964–73. doi: 10.7326/M20-3569. PMID: 32886525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salali GD, Uysal MS. COVID-19 vaccine hesitancy is associated with beliefs on the origin of the novel coronavirus in the UK and Turkey. Psychol Med. 2020 Oct 19[Accessed 2021 Dec 5]. 1–3. doi: 10.1017/S0033291720004067. Epub ahead of print. PMID: 33070804. [DOI] [PMC free article] [PubMed] [Google Scholar]


