Abstract
Permeabilized cells of a highly enriched, toluene-mineralizing, methanogenic culture catalyzed the addition of toluene to fumarate to form benzylsuccinate under anaerobic conditions. The specific in vitro rate of benzylsuccinate formation was >85% of the specific in vivo rate of toluene consumption. This is the first report of benzylsuccinate synthase activity in a methanogenic culture; the activity has previously been reported to occur in denitrifying, sulfate-reducing, and anoxygenic phototrophic bacteria.
Benzylsuccinate synthase, a recently discovered enzyme that catalyzes the addition of the methyl carbon of toluene to the double bond of fumarate, has been reported in toluene-degrading, denitrifying (2, 8), sulfate-reducing (3, 18), and anoxygenic phototrophic (20) bacteria. Several lines of evidence suggest that the activation of toluene via benzylsuccinate synthase is the first step of anaerobic toluene mineralization, and subsequent steps in the mineralization pathway have been proposed based on biochemical and genetic studies (2, 4, 8, 15, 16). This article describes the detection of high benzylsuccinate synthase activity during in vitro assays with a highly enriched, toluene-degrading, methanogenic culture. The methanogenic culture, which has been maintained for 10 years with toluene as the sole carbon source and electron donor, is dominated by two archaeal species (members of the Methanosaeta and Methanospirillum genera), a eubacterial species belonging to the genus Desulfotomaculum, and a eubacterial organism whose 16S rRNA sequence does not correspond well to known species (13). The last bacterium has been hypothesized to be responsible for toluene activation for two reasons (13): (i) the methanogens are known to have a limited substrate range and (ii) the addition of sulfate retards toluene degradation in this culture, which would not be expected if the Desulfotomaculum strain could activate toluene.
In preparation for this study, the methanogenic culture was grown to a relatively high density (ca. 17 mg of protein per liter) with toluene as the sole carbon source in medium that has been described previously (11). The culture, which has been maintained in batch mode and amended with approximately 1 mM toluene every 2 weeks, has consistently produced 85 to 100% of the theoretical methane yield (4.3 mol of methane/mol of toluene). In vivo and in vitro kinetic experiments were performed at 30°C in an anaerobic glove box (Coy Laboratory Products, Inc., Grass Lake, Mich.) with a gas composition of 80% N2, 10% CO2, and 10% H2. The in vivo rate of toluene consumption was determined immediately prior to the harvesting of cells for in vitro assays of benzylsuccinate synthase activity. In vivo toluene consumption was measured by a static headspace method similar to that described previously (3, 6) except that gas chromatography (GC)-flame ionization detection was used rather than GC-photoionization detection. After the in vivo rate was determined, the culture was harvested and permeabilized cell assays were performed in a manner similar to that used previously for denitrifying and sulfate-reducing cultures (2–5). Briefly, 350-ml batches of cells were harvested anaerobically by centrifugation (7,900 × g, 4°C, 20 min) in sealed polycarbonate bottles (Nalge Co., Rochester, N.Y.), suspended in 2 ml of degassed morpholinepropanesulfonic acid (MOPS) buffer (20 mM MOPS, 10 mM MgSO4 [pH 7.2] [2]), and then permeabilized with Triton X-100 (2% [vol/vol] final concentration). The assay mixtures (total volume, 1.15 ml) contained toluene-d8 (0.33 μmol; 100 atom% D; Sigma Chemical Co., St. Louis, Mo.), sodium fumarate (1.3 μmol), dithiothreitol (as a reductant; 1.5 μmol), and 0.45 ml of permeabilized cells. The reaction was halted at selected time intervals by rapid cooling on ice and injection with air (the enzyme is inactivated by molecular oxygen). After incubation, assay mixtures were treated with DNase I, acidified with concentrated HCl, and extracted four times with high-purity diethyl ether. The ether extracts were dried with anhydrous sodium sulfate, derivatized with ethereal diazomethane to convert carboxylic acids into methyl esters, exchanged into high-purity CH2Cl2, and analyzed by capillary GC-mass spectrometry (MS) in electron ionization mode (2, 6). Since previous studies with very similar in vitro assay procedures have demonstrated that toluene-fumarate addition is enzyme dependent (2, 5), control experiments with heat-treated permeabilized cells were not conducted for this study.
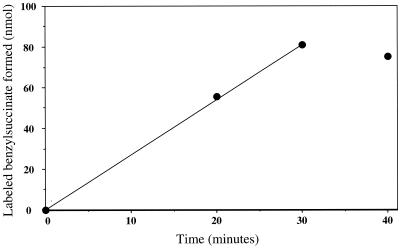
Permeabilized cells of the methanogenic culture catalyzed the formation of deuterium-labeled benzylsuccinate from labeled toluene and fumarate. The results of a kinetic study of this reaction are depicted in Fig. 1. A linear regression of these data (0 to 30 min; r2 >0.999) indicated a rate of 2.7 nmol · min−1, or an estimated specific rate of 15 to 16 nmol · min−1 · mg of protein−1. The activity appeared to cease after 30 min, as the benzylsuccinate yields at 30 and 40 min were essentially the same (Fig. 1). To estimate the specific rate, it was assumed that only one of the two dominant eubacteria in the culture was responsible for toluene activation by benzylsuccinate synthase; a recent molecular phylogenetic study of this culture (13) showed that each eubacterial species accounted for approximately 20% of the total cells (and, presumably, an equivalent percentage of the total protein). If in fact both eubacteria had benzylsuccinate synthase activity, then the specific activity would be half the value cited above. A specific, in vitro benzylsuccinate synthase rate of 15 to 16 nmol · min−1 · mg of protein−1 (or even half that rate) is among the highest values reported to date. The highest value reported previously, 8 nmol · min−1 · mg of protein−1, was obtained with crude cell extracts of denitrifying Azoarcus sp. strain T (5). Notably, the rate observed for the methanogenic culture may be an underestimate, as a deuterium kinetic isotope effect appears to markedly retard benzylsuccinate synthase activity on methyl-deuterated toluene relative to that on unlabeled toluene (14).
FIG. 1.
Kinetics of in vitro benzylsuccinate-d8 formation from toluene-d8 and fumarate by permeabilized cells of a methanogenic enrichment culture. The data points represent the averages of results of duplicate assays. The linear regression used to calculate the rate of benzylsuccinate formation is shown.
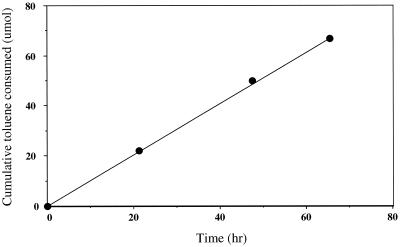
The results of a kinetic study of in vivo toluene consumption by the methanogenic culture are depicted in Fig. 2. Toluene consumption was zero order (r2 >0.999 for the regression line shown in Fig. 2) and resulted in a specific in vivo rate of approximately 17 to 18 nmol · min−1 · mg of protein−1 (again estimating that the toluene-activating organism accounted for 20% of the total protein). This rate is within the range of values observed for other anaerobic, toluene-degrading cultures (2, 3, 7, 18, 20). The specific in vitro rate of benzylsuccinate formation from toluene and fumarate accounted for >85% of the specific in vivo rate of toluene consumption by this culture. This in vitro/in vivo ratio is high relative to the ratios reported for other anaerobic, toluene-degrading cultures as well as ratios for anaerobic, m-xylene- or m-cresol-degrading cultures that perform analogous fumarate addition reactions (Table 1).
FIG. 2.
Cumulative toluene consumption by whole cells of a methanogenic enrichment culture. The linear regression used to calculate the rate of toluene consumption is shown.
TABLE 1.
Relative rates of specific in vitro benzylsuccinate synthase activitya for a range of bacterial cultures
| Bacterial culture and substrateb | In vitro/in vivo rate (%) | Reference |
|---|---|---|
| Methanogenic consortium | 85–90 | This study |
| Denitrifying bacteria | ||
| Azoarcus sp. strain T | 30–50 | 2, 5 |
| Azoarcus sp. strain T (m-xylene) | 15 | 14 |
| Thauera aromatica strain K172 | 1–2 | 7, 8 |
| Sulfate-reducing bacteria | ||
| Strain PRTOL1 | ∼7 | 3 |
| Desulfobacula toluolica | 0.3 | 18 |
| Desulfobacterium cetonicum (m-cresol) | ∼7 | 17 |
| Phototrophic bacterium | ||
| Blastochloris sulfoviridis strain ToP1 | ∼1 | 20 |
[(Specific in vitro fumarate addition activity)/(specific in vivo substrate consumption rate)], where toluene is the substrate (and thus, benzylsuccinate synthase is the activity), unless specified otherwise.
The substrate is toluene unless indicated otherwise.
A distinctive characteristic of the benzylsuccinate synthase reaction observed during in vitro assays performed with denitrifying Azoarcus sp. strain T (2, 14) and sulfate-reducing strain PRTOL1 (3) is that the H atom abstracted from the toluene methyl group during addition to fumarate is retained in the succinyl moiety of benzylsuccinate. This characteristic, which is consistent with the proposed reaction mechanism for benzylsuccinate synthase (4, 16), was also observed in the present study. Specifically, mass spectral data showed conclusively that benzylsuccinate-d8 (with a molecular ion at m/z 244 and a tropylium ion at m/z 98) was formed from toluene-d8 and fumarate by permeabilized cells of the methanogenic culture (data not shown). The mass spectrum of benzylsuccinate-d8 obtained in this study is very similar to that shown previously for another toluene-degrading culture (1).
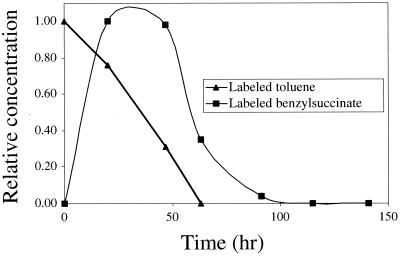
The syntrophic associations in this methanogenic consortium are not well understood, particularly with respect to interspecies metabolite transfer. Benzylsuccinate, which is apparently the first metabolite of toluene mineralization in this culture, is one of a number of candidates for interspecies metabolite transfer. In many toluene-degrading cultures, a small amount of benzylsuccinate (representing ∼0.5 to 7% of the toluene consumed) accumulated in the spent medium (1, 6, 9, 12, 19). Notably, in this study, benzylsuccinate appeared only transiently and at a very low concentration in the culture medium during toluene degradation (Fig. 3). For the experiment represented in Fig. 3, the methanogenic culture was amended with toluene-d8 in the absence of unlabeled toluene. Liquid chromatography-MS-MS analysis was used to measure benzylsuccinate-d8 in the culture medium during and after degradation of toluene-d8; unlabeled benzylsuccinate, which was present in the medium as a result of maintenance for months on unlabeled toluene, was also measured. At its maximum concentration, benzylsuccinate-d8 accounted for <0.01 mol% of the toluene-d8 consumed. Similarly, the concentration of unlabeled benzylsuccinate represented a very small fraction of the unlabeled toluene that had been consumed by the culture prior to the experiment represented in Fig. 3. Accordingly, radiolabeled benzylsuccinate was not detected (detection limit, 10 nM) in a 1992 study of this culture that involved degradation of radiolabeled toluene (10). Such low extracellular benzylsuccinate yields and the transient occurrence of benzylsuccinate during toluene degradation (Fig. 3) differ markedly from observations made for pure, toluene-degrading cultures and are consistent with interspecies benzylsuccinate transfer in this methanogenic consortium.
FIG. 3.
Normalized plot of toluene-d8 and benzylsuccinate-d8 concentration in a methanogenic enrichment culture. Toluene concentration is normalized to the total consumed (∼500 μM), and labeled benzylsuccinate is normalized to its maximum observed concentration (∼0.01 μM). Benzylsuccinate-d8 was quantified by liquid chromatography-MS-MS, and toluene-d8 was quantified by GC-flame ionization detection.
Acknowledgments
We thank Emmanuel Francois for maintaining the methanogenic culture.
Funding was provided by the U.S. Department of Energy through a contract to the Lawrence Livermore National Laboratory.
REFERENCES
- 1.Beller H R, Reinhard M, Grbic-Galic D. Metabolic by-products of anaerobic toluene degradation by sulfate-reducing enrichment cultures. Appl Environ Microbiol. 1992;58:3192–3195. doi: 10.1128/aem.58.9.3192-3195.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beller H R, Spormann A M. Anaerobic activation of toluene and o-xylene by addition to fumarate in denitrifying strain T. J Bacteriol. 1997;179:670–676. doi: 10.1128/jb.179.3.670-676.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beller H R, Spormann A M. Benzylsuccinate formation as a means of anaerobic toluene activation by sulfate-reducing strain PRTOL1. Appl Environ Microbiol. 1997;63:3729–3731. doi: 10.1128/aem.63.9.3729-3731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beller H R, Spormann A M. Analysis of the novel benzylsuccinate synthase reaction for anaerobic toluene activation based on structural studies of the product. J Bacteriol. 1998;180:5454–5457. doi: 10.1128/jb.180.20.5454-5457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beller H R, Spormann A M. Substrate range of benzylsuccinate synthase from Azoarcus sp. strain T. FEMS Microbiol Lett. 1999;178:147–153. doi: 10.1111/j.1574-6968.1999.tb13771.x. [DOI] [PubMed] [Google Scholar]
- 6.Beller H R, Spormann A M, Sharma P K, Cole J R, Reinhard M. Isolation and characterization of a novel toluene-degrading, sulfate-reducing bacterium. Appl Environ Microbiol. 1996;62:1188–1196. doi: 10.1128/aem.62.4.1188-1196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biegert T, Fuchs G. Anaerobic oxidation of toluene (analogues) to benzoate (analogues) by whole cells and by cell extracts of a denitrifying Thauera sp. Arch Microbiol. 1995;163:407–417. doi: 10.1007/BF00272130. [DOI] [PubMed] [Google Scholar]
- 8.Biegert T, Fuchs G, Heider J. Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur J Biochem. 1996;238:661–668. doi: 10.1111/j.1432-1033.1996.0661w.x. [DOI] [PubMed] [Google Scholar]
- 9.Chee-Sanford J C, Frost J W, Fries M R, Zhou J, Tiedje J M. Evidence for acetyl coenzyme A and cinnamoyl coenzyme A in the anaerobic toluene mineralization pathway in Azoarcus tolulyticus Tol-4. Appl Environ Microbiol. 1996;62:964–973. doi: 10.1128/aem.62.3.964-973.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards E A, Edwards A M, Grbic-Galic D. A method for the detection of metabolites at very low concentration: application to the detection of metabolites of anaerobic toluene degradation. Appl Environ Microbiol. 1994;60:323–327. doi: 10.1128/aem.60.1.323-327.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards E A, Grbic-Galic D. Anaerobic degradation of toluene and o-xylene by a methanogenic consortium. Appl Environ Microbiol. 1994;60:313–322. doi: 10.1128/aem.60.1.313-322.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans P J, Ling W, Goldschmidt B, Ritter E R, Young L Y. Metabolites formed during anaerobic transformation of toluene and o-xylene and their proposed relationship to the initial steps of toluene mineralization. Appl Environ Microbiol. 1992;58:496–501. doi: 10.1128/aem.58.2.496-501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ficker M, Krastel K, Orlicky S, Edwards E. Molecular characterization of a toluene-degrading methanogenic consortium. Appl Environ Microbiol. 1999;65:5576–5585. doi: 10.1128/aem.65.12.5576-5585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krieger C J, Beller H R, Reinhard M, Spormann A M. Initial reactions in anaerobic oxidation of m-xylene by the denitrifying bacterium Azoarcus sp. strain T. J Bacteriol. 1999;181:6403–6410. doi: 10.1128/jb.181.20.6403-6410.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leuthner B, Heider J. Anaerobic toluene catabolism of Thauera aromatica: the bbs operon codes for enzymes of β oxidation of the intermediate benzylsuccinate. J Bacteriol. 2000;182:272–277. doi: 10.1128/jb.182.2.272-277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leuthner B, Leutwein C, Schulz H, Hörth P, Haehnel W, Schiltz E, Schägger H, Heider J. Biochemical and genetic characterization of benzylsuccinate synthase from Thauera aromatica: a new glycyl radical enzyme catalysing the first step in anaerobic toluene metabolism. Mol Microbiol. 1998;28:615–628. doi: 10.1046/j.1365-2958.1998.00826.x. [DOI] [PubMed] [Google Scholar]
- 17.Müller J A, Galushko A S, Kappler A, Schink B. Anaerobic degradation of m-cresol by Desulfobacterium cetonicum is initiated by formation of 3-hydroxybenzylsuccinate. Arch Microbiol. 1999;172:287–294. doi: 10.1007/s002030050782. [DOI] [PubMed] [Google Scholar]
- 18.Rabus R, Heider J. Initial reactions of anaerobic metabolism of alkylbenzenes in denitrifying and sulfate-reducing bacteria. Arch Microbiol. 1998;170:377–384. [Google Scholar]
- 19.Seyfried B, Glod G, Schocher R, Tschech A, Zeyer J. Initial reactions in the anaerobic oxidation of toluene and m-xylene by denitrifying bacteria. Appl Environ Microbiol. 1994;60:4047–4052. doi: 10.1128/aem.60.11.4047-4052.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zengler K, Heider J, Rossello-Mora R, Widdell F. Phototrophic utilization of toluene under anoxic conditions by a new strain of Blastochloris sulfoviridis. Arch Microbiol. 1999;172:204–212. doi: 10.1007/s002030050761. [DOI] [PubMed] [Google Scholar]