Abstract
OBJECTIVE:
Conyza bonariensis is an ornamental medicinal weed. This experiment was planned to explore the outcome of petroleum ether extract of C. bonariensis (PECB) leaves on scopolamine-induced amnesia in rats.
MATERIALS AND METHODS:
For impairing memory, 0.4 mg/kg (i. p.) of scopolamine was given. Fifty to 200 mg/kg of PECB was fed orally to rats and 3 mg/kg (i. p.) of tacrine was given as a standard drug. Anti-amnesic property was evaluated in Barnes maze using ANY-maze software. Following a behavioral study, acetylcholinesterase (AChE), β-amyloid1-41, antioxidant enzymes, and cytokine levels were measured. Furthermore, reverse transcription–polymerase chain reaction was done for expression of the marker genes such as AChE, Nrf2, NF-κB, PP2A, and HO-1, whereas BDNF, TrkB, caspase-3, and Bax were measured by Western blotting.
RESULTS:
PECB and tacrine significantly improved memory dysfunction by decreasing escape latency in Barnes maze. At the highest dose, treatment with PECB altered the scopolamine-induced hyperactivation of AChE and β-amyloid1-41 activity. PECB elevated the levels of superoxide dismutase, glutathione, and catalase and decreased lipid peroxidation and nitric oxide dose dependently. PECB attenuated scopolamine-induced increase of tumor necrosis factor-α and interleukin (IL)-1β concentrations in the hippocampus with reversed diminished IL-10 level toward normal in the brain. Nrf2, HO-1, PP2A, BDNF, and TrkB were significantly upregulated with downregulation of AChE, NF-κB, Tau, Bax, and caspase-3. Different components such as beta-amyrin and alpha-amyrin were isolated from leaves of the plant.
CONCLUSION:
The results indicated that PECB might be a potential curative drug for the treatment of cognitive impairment.
Keywords: Acetylcholinesterase, Conyza bonariensis, scopolamine, tacrine
Introduction
For long, medicinal plants were used in the treatment of various diseases due to their remedial value. The treatment of Alzheimer's disease (AD) with allopathic medicine is not yet to be established, hence the world is thinking to investigate the benefits of medicinal herbs in the therapy of AD. The major reasons of AD arise to be decreased cholinergic activity, beta-amyloid peptides deposition in the brain, formation of neurofibrillary tangles inside nerve cells, and oxidative stress.[1] Till date, no drug can permanently cure AD, and the available drugs can only improve the symptoms or temporarily slow down its progression. The key approach for AD treatment is inhibition of acetylcholinesterase (AChE) and other cognitive impairments.[2,3]
Conyza bonariensis (Family: Asteraceae) is a perennial erect herb of cosmopolitan distribution. The plant appears in Northeast India as a weed of upland situations in the late winter and summer seasons. The leaves of the plant have a laxative effect,[4] while flowers as aphrodisiac and emollient.[5] The phytochemical constituents, i.e., flavonoids, flavonoid glycosides, stigmasterol, ergosterol,[6] cyclopropenoids,[7] and anthocyanin pigments,[6] are found in C. bonariensis. The anticonvulsant,[4] antitumor,[8] hypoglycemic,[9] and estrogenic activities[10] of the plant have been evaluated. Antidiarrheal, antioxidant, anti-inflammatory, antibacterial, antifungal, and cytotoxic activities of C. bonariensis have also been reported.[11,12,13,14,15] Anti-inflammatory activity is mainly due to the presence of phytochemicals, mostly concentrated in the hexane fraction of the extract.[16] In mice, C. bonariensis attenuated lipopolysaccharide-induced depressive-like behavior.[17]
Scopolamine is a muscarinic receptor antagonist and can cause a transient disruption of memory in experimental animals by increasing the AChE activity, which decreases the endogenous acetylcholine content at cholinergic synapses resulting memory loss.[18,19]
In view of properties such as antidepressant, antioxidant, and anti-inflammatory and the presence of phytoconstituent like flavonoids, C. bonariensis could possess memory-enhancing activity and this forms the basis of this study.
Preliminary behavioral studies were performed for different extracts (ethanol, hydroethanolic, aqueous, petroleum ether, n-hexane, ethyl acetate, and chloroform) using Morris water maze to investigate scopolamine-induced amnesia. Among them, petroleum ether extract of C. bonariensis (PECB) was found to be more active than the other extracts, hence the present study investigates the memory-enhancing activity of PECB using Barnes maze. The biochemical parameters and molecular mechanisms of PECB in scopolamine-induced impaired memory were also evaluated.
Materials and Methods
Drugs and reagents
Drugs/reagents were purchased from different sources. Scopolamine hydrobromide, tacrine hydrochloride (Sigma-Aldrich, USA), enzyme-linked immunosorbent assay (ELISA) kit (Ray Bio®, USA), TRIzol® reagent (Invitrogen, USA), and cDNA synthesis kit (Thermo Scientific, EU, Lithuania).
Preparation of extract
C. bonariensis leaves were taken from Jorhat, Assam, India. Dr. Iswar Chandra Barua, Principal Scientist, AAU, has authenticated it (voucher no: 5189 dated May 20, 2016). The leaves were dried at 35–40°C for a week and pulverized. Petroleum ether extract was prepared as per standard methods in Soxhlet extractor and rotary evaporator.
Acute toxicity study
To evaluate the acute toxicity, PECB (2000 mg/kg) p. o. was given to Swiss albino mice as per the protocol of OECD 423. They were observed for 24 h for any sign of gross abnormality or mortality and then till 14 days. There was no mortality at 2000 mg/kg dose in the oral acute toxicity study. Therefore, we have selected the following doses of the extract, i. e., 50, 100, and 200 mg/kg, p. o.
Experimental design
Male Wistar rats were selected for the study. The feeding and drinking water were provided ad libitum. Experimental works were done as per guidelines recommended by CPCSEA and approved by the Institutional Animal Ethical Committee (770/ac/CPCSEA/FVSc, AAU/IAEC/16-17/452).
Treatment schedule
A total of 36 rats were equally distributed into six groups with six numbers in each.
Group 1: Normal control (Tween 80 and saline p. o.)
Group 2: Negative control (scopolamine 0.4 mg/kg i. p.)
Group 3: Standard control (tacrine 3 mg/kg i. p. and scopolamine 0.4 mg/kg i. p.)
Group 4: PECB 50 mg/kg p. o. and scopolamine 0.4 mg/kg i. p.
Group 5: PECB 100 mg/kg p. o. and scopolamine 0.4 mg/kg i. p.
Group 6: PECB 200 mg/kg p. o. and scopolamine 0.4 mg/kg i. p.
Scopolamine was injected into rats after 45 min of respective drug treatment. Following an hour of scopolamine administration, the behavioral study was performed and the rats were sacrificed under i. m. injection of ketamine (80 mg/kg) and xylazine (10 mg/kg).[20,21] The brains were rapidly dissected out, and the hippocampus was isolated and stored at −80°C until detection.
Gas chromatography–mass spectrometry method
PECB extract was studied by utilizing a Shimadzu 17A-GC chromatograph. For separation, a fused silica column SPB coated with poly (dimethylsiloxane) was used. The temperature of injector port and detector was 225°C and 250°C, respectively. The temperature of the oven was fixed at 60°C and elevated to 225°C. Recognition of compounds by comparing the retention indices and their mass spectral fragmentation patterns with database in spectrometer.
Barnes maze
To assess spatial learning Barnes maze was used,[22] which is a circular platform having 21 holes located 6 cm from the edge and distributed equally. The maze uses rat's natural aversion to open illuminated places, so the subjects were motivated by bright light to locate an escape hole which leads to a dark box. On days 1 and 2, a pretrial was arranged before the start of the trial. Total latency describes the time taken by the rat to enter the escape hole on the maze were monitored and recorded via a digital video tracking system ANY-maze™ (Sloelting and Co.,USA).
Estimation of acetylcholinesterase activity
As per the procedure depicted by Ellman et al.,[23] AChE assay was done.
Estimation of β-amyloid1-42
The level of β-amyloid1-42 in the brain tissue was estimated by sandwich ELISA using rat β-amyloid1-42 (Bioassay Technology Laboratory, Shanghai, China) kit.
Assessment of antioxidant parameters
Lipid peroxidation (LPO),[24] nitric oxide (NO),[25] reduced glutathione (GSH),[26] superoxide dismutase (SOD),[27] and catalase (CAT)[28] were measured spectrophotometrically (Multiskan Go, Thermo Fisher) as per standard protocol.
Evaluation of inflammatory cytokines
Amounts of interleukin (IL)-1β, tumor necrosis factor-α (TNF-α), and IL-10 in the brain tissues were estimated by using cytokine ELISA kits.
Reverse transcription–polymerase chain reaction analysis
The mRNA expression levels of genes encoding AChE, Nrf2, Tau, PP2A, HO-1, and NF-κB in the hippocampus were measured by reverse transcription–polymerase chain reaction (RT-PCR). Amplification was performed in a thermal cycler using a standard protocol (Applied Biosystems–Veriti Thermal Cycler).
SDS-polyacrylamide gel electrophoresis immunoblot analysis
Western blotting was performed to analyze the expression of TrkB, BDNF, Bax, and caspase-3 proteins in rats’ hippocampus. The 50 μg of total extracted proteins of the samples were added with an equivalent amount of Laemmli buffer and then boiled and cooled. Preparation was loaded and separated in 10% polyacrylamide gels having sodium dodecyl sulfate. For immunoblot analysis, the separated proteins were transferred to PVDF membranes. Blocking was done at room temperature for 1 h with the help of 3% bovine serum albumin in 1X TBST and incubated the membranes with primary antibodies (TrkB, BDNF, Bax, and caspase-3) at a dilution of 1:500 at 4°C for overnight. On the subsequent day, membrane was washed with 1X TBST and incubated with respective secondary antibodies conjugated to HRP at a dilution of 1:5000 for 1 h. The bands were visualized using TMB blotting solution and quantified by using ImageJ software.
Statistical analysis
Data were revealed as mean ± standard error of the mean. The statistical study was carried out by one-way analysis of variance succeeded by post hoc Tukey's multiple range tests. Results were found significant when P < 0.05.
Results
Chemistry
The major constituents in the petroleum ether extract were found to be 64.25% of the identified compounds (1–18); of these, beta-amyrin (12.7%), alpha-amyrin (8.9%), l-(+)-ascorbic acid 2,6-dihexadecanoate (4.6%), caryophyllene oxide (5.6%) and spathulenol (7.7%) were the major constituents [Figure 1].
Figure 1.

The structure of different identified compounds in the petroleum ether extract of Conyza bonariensis (PECB) by gas chromatography–mass spectrometry
Effect of PECB on escape latency
A significant increase in EL was observed in scopolamine-treated (P < 0.001) rats than the control group. Conversely, pretreatment with standard drug tacrine (P < 0.001) and PECB at all the three doses significantly reduced EL than the scopolamine-administered group [Figure 2a].
Figure 2.
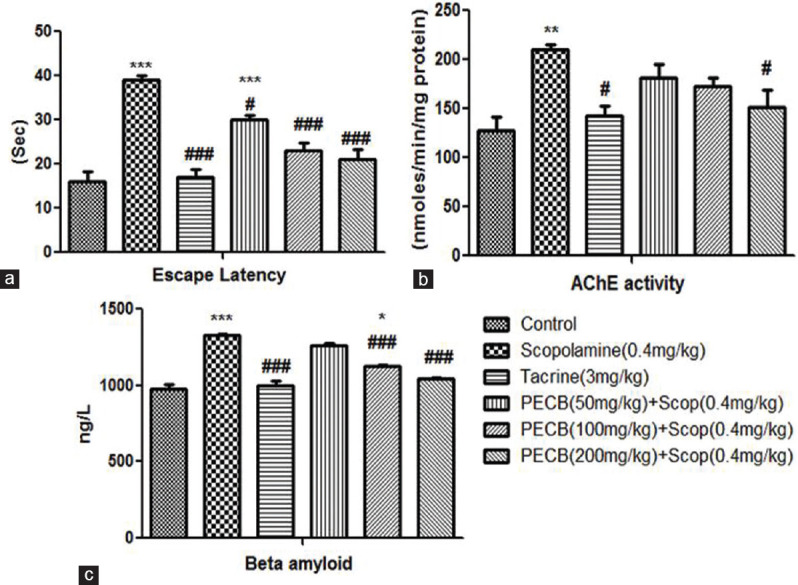
Effect of PECB pretreatment on scopolamine-induced amnesia by Barnes maze model in rats. (a) Escape latency, (b) acetylcholinesterase activity, (c) β amyloid1-42 activity. Values are expressed as mean ± standard error of the mean (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 compared with normal control group; #P < 0.05, ###P < 0.001 compared with scopolamine-treated group
Effect of PECB on brain acetylcholinesterase activity
When compared with the control group, scopolamine administration revealed a marked elevation of AChE activity in rats’ brain tissues (P < 0.01). However, pretreatment with tacrine and PECB at the highest dose revealed a significant (P < 0.05) decrease in AChE activity than the scopolamine-administered group [Figure 2b].
Effect of PECB on β-amyloid1-42 activity
In the brain of the scopolamine-treated group, the β-amyloid1-42 activity was significantly (P < 0.001) higher than the control group. In tacrine pretreated groups, β-amyloid1-42 activity in hippocampus tissue was markedly lower than scopolamine-administered batch. Pretreatment with PECB considerably (P < 0.001) reduced β-amyloid1-42 activity dose dependently in contrast to the scopolamine-administered group [Figure 2c].
Effect of PECB on antioxidant parameters
LPO and NO levels increased significantly in the scopolamine-treated group (P < 0.001) than the control group. Standard drug tacrine (P < 0.001) and test drug PECB at the dose of 100 and 200 mg/kg treated groups revealed a significant decline in LPO [Figure 3a] and nitrite level than scopolamine-administered batch [Figure 3b]. SOD and CAT levels were markedly reduced (P < 0.001) in the brain of scopolamine-treated rats than the control group. Conversely, pretreatment with standard drug tacrine and the extract at the highest dose increased the levels of SOD (P < 0.001) [Figure 3c] and CAT (P < 0.05) [Figure 3d] than the scopolamine-treated group. GSH level was decreased significantly in the hippocampus of scopolamine (P < 0.001) treated rats than the control group. Depletion in GSH level was improved by pretreatment with tacrine and all the three doses of extract dose dependently than the scopolamine-administered group [Figure 3e].
Figure 3.

Effect of PECB pretreatment on scopolamine-induced changes on in vivo antioxidant parameters. (a) Lipid peroxidation, (b) nitric oxide, (c) superoxide dismutase, (d) catalase, (e) glutathione. Values are expressed as mean ± standard error of the mean (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 compared with normal control group; # P < 0.05, ##P < 0.01, ###P < 0.001 compared with scopolamine-treated group
Effect of PECB on inflammatory cytokines
TNF-α and IL-1β levels were markedly elevated (P < 0.001) in scopolamine-administered rats than control batch. Standard drug tacrine (P < 0.001 and P < 0.001), and the extract at the dose of 100 and 200 mg/kg, could markedly restore TNF-α [Figure 4a] and IL-1β levels [Figure 4b] as compared with scopolamine-administered group. IL-10 level significantly declined in the scopolamine-administered group than the control group. This significant fall in the IL-10 level was improved by treatment with standard drug tacrine and test drug PECB at the highest dose [Figure 4c].
Figure 4.
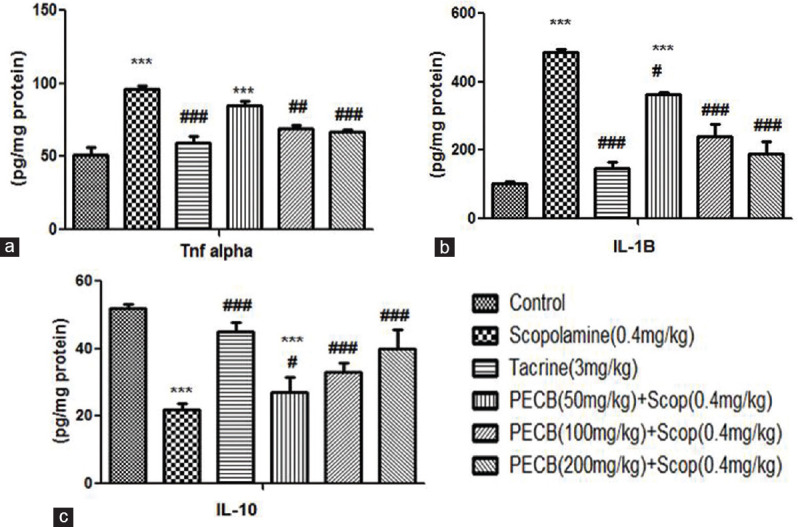
Effect of PECB pretreatment on pro- and anti-inflammatory cytokine activity on scopolamine-induced amnesia in rats. (a) Tumor necrosis factor-α, (b) interleukin-1β, (c) interleukin-10. Values are expressed as mean ± standard error of the mean (n = 6). ***P < 0.001 compared with normal control group; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with scopolamine-treated group
Effects of PECB on acetylcholinesterase, Nrf2, HO-1, NF-κB, PP2A, and Tau gene expression
The RT-PCR assay of AChE, Nrf2, HO-1, NF-κB, PP2A, and Tau genes is presented in Figure 5a. The mRNA expression of AChE, NF-κB, and Tau was significantly (P < 0.001) upregulated in scopolamine-administered rats than the control group. Administration of PECB significantly downregulated the mRNA expression of AChE [Figure 5b], NF-κB [Figure 5c], and Tau [Figure 5d] genes in the hippocampus of rats similar to standard drug tacrine in comparison to scopolamine-administered rats. Conversely, mRNA expression of Nrf2, HO-1, and PP2A were markedly downregulated (P < 0.001) in the scopolamine-administered batch than the control group. PECB markedly upregulated the expression of Nrf2 [Figure 5e], HO-1 [Figure 5f], and PP2A [Figure 5g] genes in the hippocampus of rats, similar to tacrine, than scopolamine-administered rats.
Figure 5.

Effect of PECB pretreatment on scopolamine-induced amnesia on acetylcholinesterase, NF-κB, Tau Nrf2, HO-1, and PP2A mRNA expression in the hippocampus of rats. (a) Lane 1: DNA marker, lane 2: normal control, lane 3: scopolamine, lane 4: tacrine + scopolamine, lane 5: PECB (50 mg/kg) + scopolamine, lane 6: PECB (100 mg/kg) + scopolamine, lane 7: PECB (200 mg/kg) + scopolamine. Quantitative data expression of (b) acetylcholinesterase, (c) NF-κB, (d) Tau, (e) Nrf2, (f) HO-1, and (g) PP2A. **P < 0.01, ***P < 0.001 compared with normal control group; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with scopolamine-treated group
Effect of PECB on BDNF, TrkB, Bax, and caspase-3 expression by immunoblotting
The immunoblotting assay of BDNF, TrkB, Bax, and caspase-3 is presented in [Figure 6a]. Scopolamine significantly (P < 0.001) downregulated BDNF and TrkB protein expression in rats’ hippocampus than control batch. Pretreatment with PECB at higher doses markedly upregulated BDNF (P < 0.05) [Figure 6b] and TrkB (P < 0.01) protein expression [Figure 6c]. Scopolamine administration (P < 0.001) markedly upregulated the protein expression of caspase-3 and Bax in comparison to control rats. Pretreatment with PECB in scopolamine-pretreated rats significantly downregulated Bax [Figure 6d] and caspase-3 [Figure 6e] protein in rats’ hippocampal tissues similar to standard drug tacrine (P < 0.001) than scopolamine-treated rats.
Figure 6.
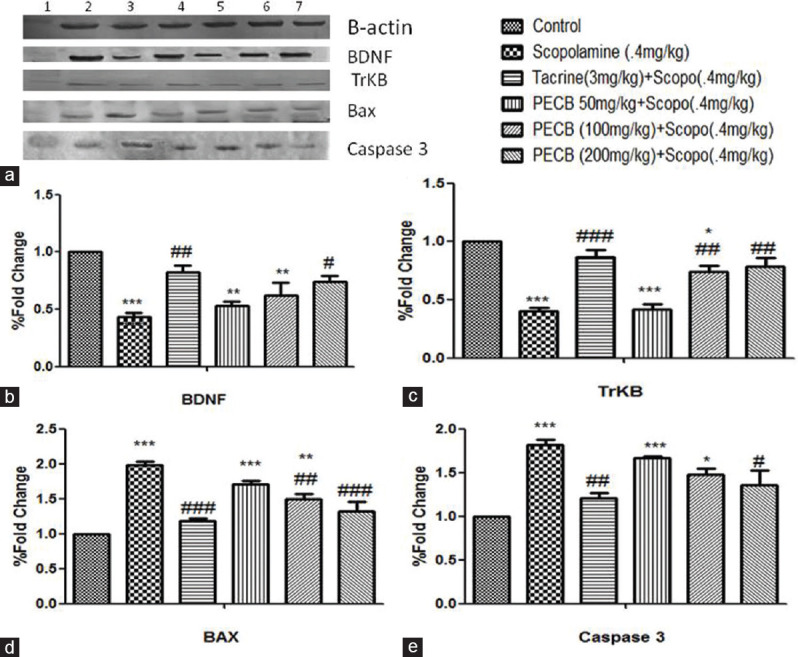
Effect of PECB pretreatment on scopolamine-induced amnesia on BDNF, TrkB, Bax, and caspase-3 protein expression in rats. (a) lane 1: DNA marker, lane 2: control, lane 3: scopolamine, lane 4: tacrine + scopolamine, lane 5: PECB (50 mg/kg) + scopolamine, lane 6: PECB (100 mg/kg) + scopolamine, lane 7: PECB (200 mg/kg) + scopolamine. Quantitative data expression of (b) BDNF, (c) TrkB, (d) Bax, and (e) caspase-3 protein levels was assessed using ImageJ software. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with scopolamine-treated group
Discussion
The typical symptoms of AD were progressive impairment of cognition and affective disorder. In AD, the cognitive deficiency is mainly related to cholinergic neurotransmission disorder in the cerebral hippocampus.
To evaluate the effects of C. bonariensis on spatial learning and memory, Barnes maze task was performed in rats. Pretreatment with PECB 200 mg/kg reduces the escape latency which suggested that C. bonariensis has an anti-amnesic effect in the scopolamine-induced model. The effect was also dose dependent, the maximum effect was observed at 200 mg/kg oral dose, which significantly reversed the elevated AChE level. Thus, the result suggests that cognition-enhancing property of PECB could be due to the inhibition of AChE enzyme.
Aggregation of β-amyloid protein in the brain tissue leads to cognition impairment which is characterized by plaque deposition in extracellular spaces, and β-amyloid1-42 is predominant in the brain. Pretreatment with PECB at the given dose resulted in a significant decrease in the amount of β-amyloid1-42 in the brain.
In various neurodegenerative diseases, oxidative stress plays an important role.[29] The hippocampus is highly vulnerable to oxidative stress and plays a vital role in short- and long-term memory.[30] Our results show that scopolamine induction increased the levels of LPO and NO; following administration of PECB or tacrine, a significant attenuation of their levels was visible. SOD and CAT protect the cells from oxidative stress by detoxifying the superoxide anion and hydrogen peroxide, respectively.[31] In our study, scopolamine significantly decreased the antioxidant capacity of SOD, CAT, and GSH in the hippocampus, whereas pretreatment with PECB significantly ameliorated these levels to restore neuronal plasticity and memory function dose dependently.
PECB caused a decrease in Nrf2 level and prevented the scopolamine-induced increase in TNF-α and IL-1β levels dose dependently. The extract also prevented the loss of anti-inflammatory cytokine IL-10, showing anti-inflammatory potential.
PECB restored PP2A activity/mRNA expression and decreased Tau hyperphosphorylation which prevents neurofibrillary tangles formation and deposition. BDNF act as an indicative biomarker in mild cognitive impairment and early AD patients.[32] Treatment with PECB reinstates the amount of BDNF proteins with its receptor, TrkB in the hippocampus, noticeably downregulated by scopolamine.
One of the major causes of neurodegeneration is apoptosis. Caspase-3 is the terminal executing enzyme in apoptosis.[33] Bcl-2 plays an important role in anti-apoptosis, whereas Bax protein is responsible for promoting cell apoptosis.[34] Our study showed that PECB remarkably downregulated Bax and caspase-3 expressions in the hippocampus.
Conclusion
Our results signified that PECB has an anti-amnesic effect by mechanism such as antioxidant action, cholinergic property, hippocampal neurogenesis, and its BDNF pathway. This suggests that the C. bonariensis may be a promising alternative treatment for patients with neurodegenerative disorders. The weed is mostly recognized as ornamental herb, but our study is in a new direction out of serendipity. Since the active compounds have been identified, further study with the pure compound may yield new drug molecule in future.
Financial support and sponsorship
This study was financially supported by the Defence Research and Development Organisation, Government of India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank the Director of Research (Vety), AAU, Khanapara.
References
- 1.Mohandas E, Rajmohan V, Raghunath B. Neurobiology of Alzheimer's disease. Indian J Psychiatry. 2009;51:55–61. doi: 10.4103/0019-5545.44908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lane RM, Potkin SG, Enz A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int J Neuropsychopharmacol. 2006;9:101–24. doi: 10.1017/S1461145705005833. [DOI] [PubMed] [Google Scholar]
- 3.Giacobini E. Cholinesterase inhibitors: New roles and therapeutic alternatives. Pharmacol Res. 2004;50:433–40. doi: 10.1016/j.phrs.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Kasture VS, Chopde CT, Deshmukh VK. Anticonvulsive activity of Albizzia lebbeck, Hibiscus rosa sinesis and Butea monosperma in experimental animals. J Ethnopharmacol. 2000;71:65–75. doi: 10.1016/s0378-8741(99)00192-0. [DOI] [PubMed] [Google Scholar]
- 5.Pullaiah T. New Delhi: Regency Publication; 2002. Medicinal Plants in Andhra Pradesh; pp. 143–4. [Google Scholar]
- 6.Prajapati ND, Purohit SS, Sharma AK, Kumar T. India: Agrobios; 2003. A Hand Book of Medicinal Plants. A Complete Source Book. [Google Scholar]
- 7.Nakatani M, Matsuoka K, Uchio Y, Hase T. Two aliphatic enone ethers from Conyza bonariensis. Phytochem. 1994;35:1245–7. [Google Scholar]
- 8.Jain SC, Purohit M. Anticancerous reagents from some selected Indian medicinal plants. I: Screening studies against sarcoma 180 ascites. J Res Ayurveda Siddha. 1987;8:70–3. [Google Scholar]
- 9.Sachdewa A, Khemani LD. A preliminary investigation of the possible hypoglycemic activity of Hibiscus rosa-sinensis. Biomed Environ Sci. 1999;12:222–6. [PubMed] [Google Scholar]
- 10.Murthy DR, Reddy CM, Patil SB. Effect of benzene extract of Hibiscus rosa sinensis on the estrous cycle and ovarian activity in albino mice. Biol Pharm Bull. 1997;20:756–8. doi: 10.1248/bpb.20.756. [DOI] [PubMed] [Google Scholar]
- 11.Bukhari IA, Shah AJ, Khan RA, Meo SA, Khan A, Gilani AH. Gut modulator effects of Conyza bonariensis explain its traditional use in constipation and diarrhea. Eur Rev Med Pharmacol Sci. 2013;17:552–8. [PubMed] [Google Scholar]
- 12.Maia JG, da Silva MH, Zoghbi MD, Andrade EH. Composition of the essential oils of Conyza bonariensis (L.) Cronquist. J Essent Oil Res. 2002;14:325–6. [Google Scholar]
- 13.Hayet E, Maha M, Samia A, Ali MM, Souhir B, Abderaouf K, et al. Antibacterial, antioxidant and cytotoxic activities of extracts of Conyza canadensis (L.) Cronquist growing in Tunisia. Med Chem Res. 2009;18:447–54. [Google Scholar]
- 14.Shah NZ, Muhammad N, Khan AZ, Muhammad S, Khan H, Azeem S, et al. Phytochemical analysis and antioxidant studies of Conyza bonariensis. Afr J Plant Sci. 2013;6:109–12. [Google Scholar]
- 15.Shah NZ, Muhammad N, Khan AZ, Muhammad S, Khan H, Azeem S, et al. Antimicrobial and phytotoxic properties of Conyza bonariensis. Pharm Pharmacol Res. 2013;1:8–11. [Google Scholar]
- 16.Bukhari IA, Sheikh SA, Shaikh NA, Assiri AM, Gilani AH. Peripheral analgesic and anti-inflammatory Activities of the methanolic extracts of Conyza bonariensis and its fractions in rodents models. Int J Pharmacol. 2018;14:144–50. [Google Scholar]
- 17.Barua CC, Sulakhiya K, Haloi P, Buragohain L, Saikia B, Barua IC, et al. Erigeron linifolius attenuates lipopolysachharide-induced depressive-like behavior in mice by impeding neuroinflammation, oxido-nitrosative stress, and upregulation of tropomyosin receptor kinase B-derived neurotrophic factor and monoaminergic pathway in the hippocampus. Pharmacogn Mag. 2019;15:92–103. [Google Scholar]
- 18.Golechha M, Bhatia J, Arya DS. Studies on effects of Emblica officinalis (Amla) on oxidative stress and cholinergic function in scopolamine induced amnesia in mice. J Environ Biol. 2012;33:95–100. [PubMed] [Google Scholar]
- 19.Reddy P. Organofluorine Compounds in Biology and Medicine. 1st ed. Elsevier; 2015. Fluorinated Compounds in Enzyme catalysed reactions; pp. 29–57. [Google Scholar]
- 20.Villard V, Espallergues J, Keller E, Alkam T, Nitta A, Yamada K, et al. Antiamnesic and neuroprotective effects of the aminotetrahydrofuran derivative ANAVEX1-41 against amyloid beta (25-35)-induced toxicity in mice. Neuropsychopharmacology. 2009;34:1552–66. doi: 10.1038/npp.2008.212. [DOI] [PubMed] [Google Scholar]
- 21.Bhuvanendran S, Kumari Y, Othman I, Shaikh MF. Amelioration of cognitive deficit by embelin in a scopolamine-induced Alzheimer's disease-like condition in a rat model. Front Pharmacol. 2018;9:665. doi: 10.3389/fphar.2018.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes CA. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 23.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 24.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidation in animal tissues by the thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 25.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 26.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 27.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 28.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 29.Polidori MC, Mecocci P. Plasma susceptibility to free radical-induced antioxidant consumption and lipid peroxidation is increased in very old subjects with Alzheimer disease. J Alzheimers Dis. 2002;4:517–22. doi: 10.3233/jad-2002-4608. [DOI] [PubMed] [Google Scholar]
- 30.Mattson MP, Pedersen WA, Duan W, Culmsee C, Camandola S. Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer's and Parkinson's diseases. Ann N Y Acad Sci. 1999;893:154–75. doi: 10.1111/j.1749-6632.1999.tb07824.x. [DOI] [PubMed] [Google Scholar]
- 31.Liu RH, Runyon RS, Wang YC, Oliver SG, Fan TP, Zhang WD. Deciphering ancient combinatorial formulas: The Shexiang Baoxin Pill. Science. 2015;347:540–2. [Google Scholar]
- 32.O’Bryant SE, Hobson V, Hall JR, Waring SC, Chan W, Massman P, et al. Brain-derived neurotrophic factor levels in Alzheimer's disease. J Alzheimers Dis. 2009;17:337–41. doi: 10.3233/JAD-2009-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nuñez G, Benedict MA, Hu Y, Inohara N. Caspases: The proteases of the apoptotic pathway. Oncogene. 1998;17:3237–45. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- 34.Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang HG, Reed JC. Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol. 1994;145:1323–36. [PMC free article] [PubMed] [Google Scholar]


