Abstract
OBJECTIVE:
To inform the design of open-source ventilators, we performed a systematic review of clinical practice guidelines (CPGs) to consolidate the evidence on mechanical ventilation strategies that result in improved patient-important outcomes for acute hypoxic respiratory failure.
DATA SOURCES:
We developed a search strategy to identify relevant CPGs from Ovid Medline, Ovid Medline In-Process & Other Non-Indexed Citations, Embase, the Cochrane Library, Mendeley, and Google scholar from 2010 to February 17, 2022.
STUDY SELECTION:
Using a two-step screening process with two independent reviewers, we included CPGs that made recommendations on mechanical ventilation strategies of interest. Guidelines that reported at least one recommendation about mechanical ventilation in ICU patients with acute hypoxic respiratory failure were included.
DATA EXTRACTION:
From the 13 eligible guidelines, we collected data on country, aim, patient population, impact on morbidity and mortality (effect size and CIs), recommendations, strength of Recommendation (as per Grading of Recommendations, Assessment, Development and Evaluations), and details of supporting evidence base.
DATA SYNTHESIS:
We identified three ventilation strategies that confer a mortality and morbidity benefit for ventilated patients with acute hypoxic respiratory failure: low-tidal volume ventilation, plateau pressures of less than 30 cm H2O, and higher positive end-expiratory pressure (PEEP). These moderate-to-strong recommendations were based on moderate-to-high certainty in evidence. We identified several other recommendations with no or minimal certainty in evidence.
CONCLUSIONS:
Our systematic review of international CPGs identified no recommendations favoring specific mode of ventilation and three ventilation strategies that confer mortality and morbidity benefits, backed by moderate-to-strong evidence. Ventilator design teams must include the ability to consistently provide and measure low-tidal volume ventilation, plateau pressures of less than 30 cm H2O, and higher PEEP into their designs. Based on our findings, we provide the first public framework for open-source ventilator design.
Keywords: COVID-19, guidelines, hypoxic respiratory failure, open-source, systematic review, ventilator
The COVID-19 pandemic has resulted in an extraordinary number of patients requiring ICU admission for mechanical ventilation (1). Due to risk of ventilator demand outpacing available supplies, public and private design teams have embarked to develop open-source ventilators that can be rapidly deployed to address regional shortages during ongoing waves of COVID-19, or future medical disasters and pandemics (2).
To achieve this goal, ventilator design teams may balance “comprehensiveness,” which tries to incorporate the key features of modern ICU ventilators, and “minimalism,” which aims to provide the simplest (and therefore cheapest and easiest to operate) viable product to achieve desired results (3). Although minimalistic designs are desirable, the design teams are often unclear regarding what features of a modern ventilator are essential for safe and effective management of the most prevalent indication for mechanical ventilation-acute hypoxic respiratory failure (4). Safety features aside, they faced a question: what should an open-source ventilator be able to do to reduce morbidity and mortality in patients with acute hypoxic respiratory failure? To date, this question remains unanswered. Although some publications have listed safety features and engineering components for developing emergency-use ventilators (5, 6), there has been no clear guidance on the evidence-based ventilation strategies that should be incorporated in the designs of these open-source ventilators to improve patient outcomes.
To inform the design of open-source ventilators, we conducted a systematic review of international clinical practice guidelines (CPGs) on the management of acute hypoxic respiratory failure to identify and summarize mechanical ventilation strategies that confer survival and morbidity benefits.
The objective of the study is to summarize evidence-based mechanical ventilation strategies from international CPGs that reduce morbidity and increase survival in patients with acute hypoxic respiratory failure in order to inform the design of open-source ventilators.
MATERIALS AND METHODS
Protocol
Our systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis guideline (www.prisma-statement.org) (Supplemental Digital Content 6, http://links.lww.com/CCX/B22) (7). We have registered our protocol in Prospero (CRD42022311815).
Population, Inclusion, and Exclusion Criteria
The target population was adult patients (≥18 yr old) receiving mechanical ventilation for acute hypoxic respiratory failure (e.g., due to acute respiratory distress syndrome [ARDS], COVID-19, pneumonia, and congestive heart failure) as this is the most common indication for mechanical ventilation in the ICU worldwide (8). We included guidelines that provided recommendations involving strategies for invasive mechanical ventilation (e.g., mode of ventilation, adjustable parameters such as positive end-expiratory pressure [PEEP], and inspiratory pressure). To be included, the CPG must have reported at least one recommendation regarding invasive mechanical ventilation and had to include a method for assessing the evidence behind these recommendations (e.g., Grading of Recommendations, Assessment, Development and Evaluations [GRADE]). We excluded guidelines that focused on noninvasive mechanical ventilation (including high-flow nasal oxygen), weaning from mechanical ventilation, or recommendations involving adjunctive care (e.g., head of bed elevation, use muscle relaxants, and use of esophageal balloon to titrate PEEP). To capture the latest recommendations and summaries of most recent evidence, texts prior to 2010 were excluded.
Search Strategy and Study Selection
Guided by a health information specialist (Alla Iansavitchene), we performed searches in Ovid Medline, Ovid Medline In-Process & Other Non-Indexed Citations, Embase, Cochrane Library, Mendeley, and Google Scholar, from 2010 to February 17, 2022. The search strategy included a combination of the following keywords: clinical, guidelines, acute hypoxic respiratory failure, mechanical ventilation, and ventilator. Acknowledging the greater burden of ventilator shortages in resource-limited settings and that guideline recommendations may vary between jurisdictions, we purposefully broadened our search to include international guidelines outside of North America and Europe. Furthermore, inclusion of international guidelines should minimize the bias that may be present in the evidence-to-decision framework employed by key critical care societies in making recommendations.
Two reviewers (C.D., A.S.) independently screened articles in two stages via Covidence (Covidence.org, Australia). We screened titles/abstracts in the first stage, followed by a full-text review of relevant guidelines in the second stage. Disagreements were resolved by a third reviewer (M.S.). Our search strategy is summarized in Supplemental Digital Content 1 (http://links.lww.com/CCX/B22).
Data Extraction
Two reviewers (C.D., A.S.) independently extracted data using a standardized excel spreadsheet that included: funding, country/region, aim, patient population, society endorsement, assessed strength of recommendation and details regarding evidence base used to reach recommendation, professional recommendations and their description, impact on morbidity, impact on mortality, level of recommendation (as per GRADE), certainty in effect estimate, source of evidence, number of studies included, number of participants, mortality effect size, type of morbidity and morbidity effect size, and lower CI and upper confidence level. Disagreements were resolved through consensus.
Rating the Quality of Clinical Practice Guidelines
To address the variability in quality of CPGs, we used The Appraisal of Guidelines for Research & Evaluation Instrument (AGREE II) tool, which has been validated for use in quality assessment of CPGs (9). Each domain that scored greater than or equal to 70% was considered effectively addressed. CPGs were considered ‘‘high quality” if they scored greater than or equal to 70% in at least three of six AGREE II domains, including domain 3. If three domains or more were assessed a score of greater than or equal to 70%, except domain 3, they were considered to be of ‘‘moderate’’ overall quality. ‘‘Low-quality” CPGs scored less than 70% in two or more domains and scored less than 60% in domain 3.
RESULTS
Study Selection and Characteristics
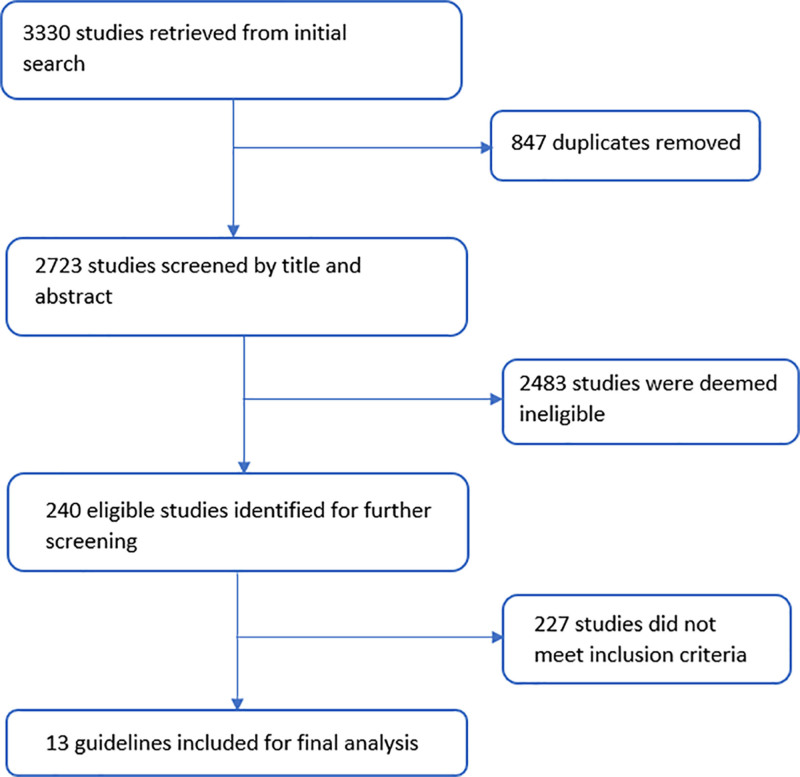
Our search strategy yielded 3,330 results, of which 847 duplicates were removed. Of the remaining 2,723 studies, 240 studies met the initial screening criteria. Subsequently, 31 guidelines that met inclusion criteria underwent full-text review. From these, 13 CPGs (10–22) met our inclusion and exclusion criteria (Fig. 1).
Figure 1.
Literature search and study inclusion.
All 13 included guidelines reported recommendations on ICU patients, and six guidelines were published and/or supported by a professional society. The focus of seven guidelines was on ARDS management (10–14), two on sepsis management (15, 16), and one each on management of COVID-19 (17), mechanical ventilation for acute respiratory failure and COVID-19 in low-middle income countries (LMICs) (18), multiple organ failure in critically ill patients (19), and ventilation in ICU (20). Two guidelines were from North America (10, 17), two from Germany (12, 14), and one each from the United Kingdom (11), France (20), Scandinavia (21), Pakistan (16), Korea (22), and Japan (13). Three guidelines were international as it included collaboration from multiple countries’ professional societies (15, 19) or an international task force (18).
Methodological Quality
The AGREE-II domain scores for each guideline can be found in Supplemental Digital Content 2 (http://links.lww.com/CCX/B22). Nine of 12 guidelines were deemed high-quality based on greater than or equal to 70% quality score in three or more domains, including domain 3 (10–15, 17, 21, 23). Eight of these high-quality guidelines had a quality score greater than or equal to 70% in all domains (10–15, 17, 22). One guideline was qualified as moderate quality based on quality score greater than or equal to 70% in three or more domains excluding domain 3 (16). Two guidelines were deemed low quality based on scores less than 70% in two or more domains (19, 20). Eleven of 13 guidelines scored greater than 70% for domains of scope/purpose and clarity/presentation (8–15, 17–19), and 10 guidelines scored greater than 70% for rigor of development and applicability (10–15, 17, 18, 21, 22).
Summary of Recommendations
All guidelines included data from randomized control trials (RCTs) in their assessment of strength of recommendations and seven of the guidelines conducted their own meta-analyses and presented relative risk of the recommendation/intervention on outcomes (mortality and morbidity) (10–13, 15, 17, 21). Summary of guideline characteristics and recommendations can be found in Supplemental Digital Content 3 (http://links.lww.com/CCX/B22).
Mode of Ventilation
None of the included guidelines made strong recommendations favoring specific mode of ventilation in patients with acute hypoxic respiratory failure. Eight guidelines provided strong recommendations against the use of high-frequency oscillation ventilation, given strong evidence of increased mortality and morbidity (10–15, 21, 22). One guideline provided a conditional recommendation against the use of high-frequency oscillation ventilation based on a meta-analysis of RCTs that showed no reduction in the risk of mortality (RR, 1.05; 95% CI, 0.82–1.36) (13). One guideline provided a conditional recommendation for the use of both volume-controlled or pressure-controlled modes of ventilation in patients with ARDS or a partial mode of ventilatory support (e.g., pressure support mode, where a spontaneous breath is initiated by the patient), based on low quality of evidence (21). This conditional recommendation was based on three RCTs with 136 participants that showed a trend toward decreased relative mortality risk of 0.78 (CI, 0.57–1.07) in patients ventilated using pressure-control over volume-control mode. The included RCTs and meta-analyses conducted by the authors for each guideline differ and so do their calculated effect on mortality and morbidity and subsequent strength of recommendation. Complete details are presented in Supplemental Digital Content 4 (http://links.lww.com/CCX/B22).
Low Tidal Volume Ventilation
Eleven guidelines made a strong recommendation for lower tidal volume (LTV; 4–8 mL/kg of predicted body weight) ventilation in patients with acute hypoxic respiratory failure based on moderate-to-high-quality evidence. These recommendations were based on a meta-analysis of trials, showing that LTV reduced the risk of mortality ranging from 0.73 (CI, 0.63–0.85) to 0.87 (CI, 0.7–1.08) (11, 13–18, 21, 22). The morbidity outcomes assessed were ventilator-free days (10, 11, 13, 21), nosocomial pneumonia (11), development of ARDS (in sepsis-induced respiratory failure without ARDS [15]), barotrauma (10, 13, 21), and new or worsening renal failure (19). Four guidelines did not assess morbidity outcomes (12, 14, 17, 22). Two guidelines did not make any recommendations regarding tidal volume targets (19, 20).
Positive End-Expiratory Pressure
Eleven guidelines recommended using higher PEEP (defined as greater than 5 cm H2O) in patients with hypoxemic respiratory failure due to moderate-to-severe ARDS (Pao2/Fio2 ratio <200) (10–17, 20–22). Five guidelines made a strong (12, 14, 16, 17, 20) recommendation, three guidelines made a moderate strength recommendation (13, 21, 22), and three guidelines made a conditional recommendation about employing high PEEP (10, 11, 15). Two guidelines provided guidance on PEEP in the setting of COVID-19 ARDS. One guideline strongly recommended employing higher PEEP (17), whereas the other guideline that is focused on the management of COVID-19 in LMICs made a moderate recommendation of using a “low PEEP/high Fio2 table,” noting the lack of the survival benefit from a high PEEP strategy in patients with ARDS based on low-quality evidence. For the guidelines that made strong recommendations, mortality outcomes were prioritized; however, no independent meta-analysis was conducted, and no effect size or relative risk were reported (12, 14, 16, 17, 20). The guidelines that assessed morbidity outcomes assessed barotrauma (three guidelines) (11, 13, 21), ventilator-free days (three guidelines) (10, 13, 21), ICU-free days (one guideline) (11), use of rescue therapies (two guidelines) (17, 21), and death after rescue therapy (one guideline) (21). Three guidelines did not include any information on morbidity outcomes (15, 20, 22).
One guideline made a conditional recommendation based on six RCTs with 2580 patients and an individual patient data meta-analysis that demonstrated mortality RR of 0.91 (CI, 0.81–1.03), and additionally looked at oxygenation, barotrauma, new organ failure, and ventilator-free days as morbidity outcomes and noted no difference (10). One guideline employed different terminologies for their recommendation: “adequate PEEP to avoid alveolar collapse” and did not present any mortality or morbidity analyses (16).
Inspiratory Pressure
Six of 13 guidelines made a strong recommendation to limit plateau pressures (Pplat) to less than 30 cm H2O (10, 14–17, 21) in all patients with acute hypoxic respiratory failure. Plateau pressure was defined as the pressure recorded after a 0.5 second inspiratory pause. All six guidelines concluded there was strong evidence supporting this recommendation with good quality data to show a mortality and morbidity benefit. In one meta-analysis (n = 1,629), Pplat < 30 cm H2O reduced the risk of death when compared with high Pplat (RR, 0.8; 95% CI, 0.66–0.98) (17). Four guidelines conducted independent meta-analysis for mortality outcomes, and the relative risk ranged from 0.8 (CI, 0.66–0.98) to 0.87 (CI, 0.7–1.08) (10, 13, 17, 21). Three guidelines assessed morbidity outcomes in their meta-analysis—specific outcomes assessed were barotrauma (10, 13, 21) and ventilator-free days (10, 13, 21). Four of the guidelines that made strong recommendations did not include any morbidity outcomes (14–17). One guideline provided a conditional recommendation based on a meta-analysis of four RCTs with 1,132 patients that found a mortality RR of 0.84 (CI, 0.62–1.15), relative risk of barotrauma 0.92 (CI, 0.65–1.31), and increase in mean ventilator-free days by 2.5 days (CI, 0.51–4.49 d) (13).
Eight guidelines provided recommendations regarding lung recruitment maneuvers (LRMs) (10, 12, 15, 16, 18, 20–22). LRM was described as a prolonged high continuous positive airway pressure (30–40 cm H2O), progressive incremental increases in PEEP at constant driving pressure, and high driving pressures for a limited duration (approximately 30 s). Of these, five guidelines provided a conditional recommendation for LRM with moderate-to-low certainty in effect size (12, 15, 16, 21, 22), whereas one guideline provided a strong recommendation for LRM (20), one guideline provided a conditional recommendation (10), and one provided a moderate recommendation against LRM (18).
DISCUSSION
The sudden onset of the COVID-19 pandemic triggered fears of ventilator shortages worldwide. In response, several public and private initiatives sought to develop and deploy open-source ventilators (24). To contain design and production costs, ventilator project teams had to balance comprehensiveness with minimalism and decide what features of modern ventilators to include and which ones to forgo. Three publications have provided guidance on the safety features and engineering aspects for developing ventilators for emergency use (4–6). However, there has been no clear guidance on the ventilation strategies that must be incorporated in the design of these open-source ventilators to improve patient outcomes. Given that the goal of these open-source ventilators is to improve patient outcomes, we collated the existing knowledge regarding ventilator strategies that confer survival and morbidity benefits in patients with acute hypoxic respiratory failure. Our systematic review of international practice guidelines is the first to address this question. Our results suggest that although there is no single recommended mode of ventilation, three ventilation strategies confer survival and morbidity benefit in patients with hypoxic respiratory failure and should be incorporated in the design of open-source ventilators. By collating existing recommendations regarding ventilation strategies that confer mortality and morbidity benefits across international CPGs, our work supports ventilator development teams by providing the first public framework for developing evidence-informed open-source ventilators (Supplemental Digital Content 5, http://links.lww.com/CCX/B22).
First, open-source ventilators should be able to consistently deliver low tidal volume (4–8 mL/kg of predicted body weight) ventilation, with 11 of 13 guidelines making strong recommendations based on moderate-to-high level of evidence. To achieve this, open-source ventilators should be able to: 1) deliver low tidal volumes, 2) consistently measure delivered tidal volumes, and 3) alarm the clinician when this is not possible (e.g., low- and high-volume alarms) or dangerous (high-pressure alarms). In short, open-source ventilators should ensure that breath-to-breath tidal volumes are reliably delivered and measured. Second, open-source ventilators should be able to measure plateau pressure, so that it can be limited to less than 30 cm H2O, with six of 13 guidelines making a strong recommendation based on high quality of evidence. To achieve this, ventilators should continuously measure and display airway pressures, and be able to institute an inspiratory pause to measure plateau pressure. To enhance the portability and minimalism of the ventilator, the design teams may choose to display the airway curves and pressures on a smartphone via the development of an accompanying software application. Third, open-source ventilators should enable setting of higher PEEP, with 11 of 13 guidelines making variable strength recommendation for use of higher PEEP in patients with moderate-to-severe ARDS (Pao2/Fio2 ratio < 200) based on moderate level of evidence. To achieve this, open-source ventilators should have the ability to regulate and monitor the level of PEEP with options for higher settings (PEEP of 20–24) and ensure that appropriate alarms are in place to limit lung overdistension (e.g., high-pressure alarms). In efforts to incorporate the above three parameters, the ventilator development teams may be faced with a notable hurdle: the significant variability in the actual delivery of pressure and volume to the lungs. Several bench studies have reported that even in sophisticated ventilators, commonly employed in the ICU setting, there are several factors that can influence tidal volume delivery and PEEP (including leaks, nature of gas delivery, humidification performance, and circuit compliance) (25, 26). The design teams should take into consideration these potential influences as well as the other necessary aspects in the provision of mechanical ventilation (3), such as infrastructure and trained personnel, while designing and testing their ventilator for specific clinical settings.
Although the above three recommendations appeared in majority of guidelines, we identified tangible differences in the strength of recommendations that were based on the similar set of primary studies, which may reflect differences in evidence-to-decision framework (where jurisdictions for guidelines may have different priorities, costs, and healthcare systems that affect the strength of a recommendation), bias from certain local practices, and a lack of robust data. This difference between jurisdictions was most evident in two guidelines regarding COVID-19 ARDS management. One guideline developed by the Society of Critical Care of Medicine and European Society of Intensive Care Medicine strongly recommended a high PEEP strategy in patients with moderate-to-severe ARDS (17), whereas another guideline published by an international and multidisciplinary collaboration with the focus on LMICs recommended “low-PEEP/high-Fio2” strategy (18). The differences in evidence-to-decision were also evident with respect to the recommendation of higher PEEP in patients with moderate-to-severe ARDS, where six guidelines made a weak recommendation, two studies made a strong recommendation, and one study made a conditional recommendation using very similar evidence base. Interestingly, the guidelines that made strong recommendation for higher PEEP were the ones that did not conduct an independent meta-analysis for this specific question (12, 14, 16, 17, 20).
Although there were no recommendations regarding what mode of ventilation is preferred, there were strong recommendations (eight of 12 guidelines) against using high-frequency oscillation ventilation based on moderate-to-high-quality evidence (RCTs and meta-analysis), suggesting higher patient mortality and morbidity. In the absence of strong evidence favoring volume over pressure control, open-source ventilators should consider both modes.
The heart of frugal innovation is to develop health technologies that provide comparable clinical outcomes at a fraction of the cost. One method of analyzing cost-effectiveness is to estimate incremental cost-effectiveness ratio (ICER), which is expressed as the additional cost associated with gaining an additional unit of effectiveness (e.g., quality-adjusted life year and mortality) to quantify improvement in outcomes associated with that cost (27). A healthcare intervention with low ICER is more favorable as it maximizes patient benefit at a lower cost. Furthermore, different countries employ different ICER thresholds to allow new health technologies to be implemented (28). Hence, inexpensive and evidence-based open-source ventilators may serve as the optimal solution for the meeting the varying ICER thresholds in different LMICs, thus improving the frequency and outcomes of mechanical ventilation in LMICs. Designing an open-source ventilator that incorporates the three ventilation strategies identified earlier provides an evidence-based approach to improve patients’ outcomes, while facilitating usability through minimalism and potentially maximizing cost-effectiveness in LMICs settings. We have provided an evidence-based checklist to standardize the development and design of open-source ventilators (Supplemental Digital Content 4, http://links.lww.com/CCX/B22).
Our study has limitations. Our research question was focused on identifying the ventilation strategies shown to provide patient benefit and does not include other strategies in the care of a ventilated patient that may confer additional benefits such as weaning strategies, head-of-bed elevation, daily spontaneous breathing trials, and others. Specific studies assessing ventilator strategies may have been missed if they were not included at the time of guideline publication. Our systematic review focused on adult patient population. Given that ventilation recommendations and associated volume and pressure targets may differ in children and neonates, a similar review should be conducted in pediatric populations to inform ventilator design teams. Although other aspects of mechanical ventilation, such as mechanical power, may affect patient outcomes, they were not reported in the extracted guidelines and, therefore, were not included in our results (29). Future studies should incorporate these additional determinants of clinical outcomes into ventilator design frameworks.
CONCLUSIONS
Our systematic review of international CPGs on mechanical ventilation in acute hypoxic respiratory failure identified no recommendations favoring specific mode of ventilation. We identified three ventilation strategies conferring survival and morbidity benefits that are supported by moderate-to-strong evidence. Ventilator design teams should incorporate the ability to provide low tidal volume ventilation, plateau pressures of less than 30 cm H2O, and higher PEEP into their ventilator designs. Based on our findings, we provide the first public framework for open-source ventilator design. Future research is needed to establish whether incorporation of these strategies into open-source ventilator designs translates into improved patient outcomes.
ACKNOWLEDGMENT
We thank Alla Iansavitchene for her assistance with the design and execution of the search strategy.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Drs. Dave, Basmaji, and Slessarev conceived and designed the study. Dr. Dave and Mr. Sivajohan completed the screening of studies and data extraction. Dr. Dave was responsible for the article draft. Mr. Sivajohan, Dr. Basmaji, and Dr. Slessarev contributed to critical revisions. All authors read and approved the final article.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Fried MW, Crawford JM, Mospan AR, et al. Patient characteristics and outcomes of 11 721 patients with coronavirus disease 2019 (COVID-19) hospitalized across the United States. Clin Infect Dis 2021; 72:e558–e565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.PubInv. PubInv/covid19-Vent-List. GitHub. Available at: %3Cgithub.com/PubInv/covid19-vent-list%3E. Accessed February 12, 2022. [Google Scholar]
- 3.Dave C, Cameron P, Basmaji J, et al. Frugal innovation: Enabling mechanical ventilation during coronavirus disease 2019 pandemic in resource-limited settings. Crit Care Explor 2021; 3:e0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearce JM. A review of open source ventilators for COVID-19 and future pandemics. F1000Research 2020; 9:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Administration FD. Enforcement Policy for Ventilators and Accessories and Other Respiratory Devices During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency. FDA. 2020. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-ventilators-and-accessories-and-other-respiratory-devices-during-coronavirus. Accessed February 12, 2022. [Google Scholar]
- 6.Medicines and Healthcare products Regulatory Agency. Specification for ventilators to be used in UK hospitals during the coronavirus (COVID-19) outbreak.. [Google Scholar]
- 7.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esteban A, Anzueto A, Alía I, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med 2000; 161:1450–1458 [DOI] [PubMed] [Google Scholar]
- 9.Brouwers MC, Kho ME, Browman GP, et al. AGREE Next Steps Consortium: AGREE II: Advancing guideline development, reporting, and evaluation in health care. Prev Med 2010; 51:421–424 [DOI] [PubMed] [Google Scholar]
- 10.Fan E, Del Sorbo L, Goligher EC, et al. American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine: An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2017; 195:1253–1263 [DOI] [PubMed] [Google Scholar]
- 11.Griffiths MJD, McAuley DF, Perkins GD, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir Res 2019; 6:e000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papazian L, Aubron C, Brochard L, et al. Formal guidelines: Management of acute respiratory distress syndrome. Ann Intensive Care 2019; 9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashimoto S, Sanui M, Egi M, et al. ARDS Clinical Practice Guideline Committee from the Japanese Society of Respiratory Care Medicine and the Japanese Society of Intensive Care Medicine: The clinical practice guideline for the management of ARDS in Japan. J Intensive Care 2017; 5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fichtner F, Moerer O, Laudi S, et al. Investigators and the Guideline Group on Mechanical Ventilation and Extracorporeal Membrane Oxygenation in Acute Respiratory Insufficiency: Mechanical ventilation and extracorporeal membrane oxygenation in acute respiratory insufficiency. Dtsch Arztebl Int 2018; 115:840–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock. 2016; 45:2017 [DOI] [PubMed] [Google Scholar]
- 16.Hashmi M, Khan FH, Bin Sarwar Zubairi A, et al. Developing local guidelines for management of sepsis in adults: Sepsis Guidelines for Pakistan (SGP): Endorsed by global sepsis alliance. Anaesth Pain Intensive Care 2015; 19:196–208 [Google Scholar]
- 17.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med 2020; 46:854–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serpa Neto A, Checkley W, Sivakorn C, et al. Pragmatic recommendations for the management of acute respiratory failure and mechanical ventilation in patients with COVID-19 in low- and middle-income countries. Am J Trop Med Hyg. 2021; 104(3_Suppl):60–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joannidis M, Forni LG, Klein SJ, et al. Lung-kidney interactions in critically ill patients: Consensus report of the Acute Disease Quality Initiative (ADQI) 21 Workgroup. Intensive Care Med 2020; 46:654–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quintard H, l’Her E, Pottecher J, et al. Experts’ guidelines of intubation and extubation of the ICU patient of French Society of Anaesthesia and Intensive Care Medicine (SFAR) and French-speaking Intensive Care Society (SRLF): In collaboration with the pediatric Association of French-speaking Anaesthetists and Intensivists (ADARPEF), French-speaking Group of Intensive Care and Paediatric emergencies (GFRUP) and Intensive Care physiotherapy society (SKR). Ann Intensive Care 2019; 9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Claesson J, Freundlich M, Gunnarsson I, et al. ; Scandinavian Society of Anaesthesiology and Intensive Care Medicine. Scandinavian clinical practice guideline on mechanical ventilation in adults with the acute respiratory distress syndrome. Acta Anaesthesiol Scand 2015; 59:286–297 [DOI] [PubMed] [Google Scholar]
- 22.Cho Y-J, Young Moon J, Shin E-S, et al. ARDS clinical practice guideline of acute respiratory distress syndrome guideline of mechanically ventilated patients and ARDS. Tuberc Respir Dis 2016; 79:214–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang YS, Ahn SY, Yoo HS, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: Phase 1 dose-escalation clinical trial. J Pediatr 2014; 164:966–972.e6 [DOI] [PubMed] [Google Scholar]
- 24.White DB, Lo B. Mitigating inequities and saving lives with ICU triage during the COVID-19 pandemic. Am J Respir Crit Care Med 2021; 203:287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garnier M, Quesnel C, Fulgencio JP, et al. Multifaceted bench comparative evaluation of latest intensive care unit ventilators. Br J Anaesth 2015; 115:89–98 [DOI] [PubMed] [Google Scholar]
- 26.Lyazidi A, Thille AW, Carteaux G, et al. Bench test evaluation of volume delivered by modern ICU ventilators during volume-controlled ventilation. Intensive Care Med 2010; 36:2074–2080 [DOI] [PubMed] [Google Scholar]
- 27.Institute for Clinical and Economic Review. A Guide to ICER’s Methods for Health Technology Assessment. 2020. Available at: https://icer.org/wp-content/uploads/2021/01/ICER_HTA_Guide_102720.pdf. Accessed February 17, 2022
- 28.Nanavaty M, Kaura S, Mwamburi M, et al. The use of incremental cost-effectiveness ratio thresholds in health technology assessment decisions. J Clin Pathways 2015; 8 [Google Scholar]
- 29.Costa ELV, Slutsky AS, Brochard LJ, et al. Ventilatory variables and mechanical power in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2021; 204:303–311 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.