Introduction
Pulmonary neuroendocrine tumors (NETs) have a wide spectrum of clinical behaviors, ranging from indolent well-differentiated (WD) NETs (typical carcinoids) to aggressive, poorly differentiated neuroendocrine carcinomas (NECs) including large cell NEC and small-cell lung cancer (SCLC).1 Atypical carcinoids are an uncommon type of WD NET with an intermediate grade and prognosis. Compared with typical carcinoids, these tumors are more commonly nonfunctional and somatostatin receptor–negative and have worse prognosis.2
Therapy for advanced pulmonary carcinoids remains ill-defined, extrapolated from WD gastroenteropancreatic NETs and poorly differentiated lung NECs. Few prospective studies have included these neoplasms, and even the role of somatostatin analogs is uncertain. Everolimus was approved on the basis of a phase III trial not powered for the lung subgroup; practically, its use is restricted to patients with relatively indolent disease.3,4 The angiogenesis inhibitor surufatinib has activity in nonpancreatic NETs; however, lung NETs accounted for only 11.6% of patients in the pivotal trial.5 Although approved in SCLC, the role of immunotherapy in unselected WD lung NETs remains ill-defined.6-11 Data from small retrospective series suggest that platinum-etoposide, temozolomide (TMZ) monotherapy, and TMZ/capecitabine regimens have clinical activity in advanced pulmonary carcinoids.12-14
TMZ is an oral alkylating prodrug that methylates DNA at O6 guanine residues (O6-meG), causing mismatch pairing during DNA replication, leading to genomic instability, apoptosis, and cell death. TMZ cytotoxicity depends on an intact DNA mismatch repair (MMR) pathway and low levels of O6-methylguanine DNA methyltransferase (MGMT).15-17 MGMT-mediated repair is stoichiometrically limited, and in malignant gliomas and melanoma, MGMT deficiency is associated with TMZ response.18-23 This relationship is less clear in NETs.24-26 The absence of MGMT-mediated repair coupled with defective MMR (dMMR) leads to enrichment of C:G>A:T transitions throughout the genome, a marked increase in tumor mutational burden (TMB), and loss of TMZ-induced cytotoxicity, a resistance mechanism termed TMZ-associated hypermutation.27-33
TMZ-associated hypermutation is well-demonstrated in malignant glioma and is frequently associated with inactivating alterations in DNA MMR genes.34 The use of immunotherapy for TMZ-associated hypermutation falls under tumor-agnostic approvals of pembrolizumab for high TMB, microsatellite instability (MSI)-high, or dMMR solid tumors. There are few reports of TMZ-associated hypermutation in NETs, and none to our knowledge in atypical lung carcinoids.35 Here, we describe a patient with treatment-refractory atypical carcinoid tumor of the lung who developed TMZ-associated hypermutation that responded to checkpoint inhibitor immunotherapy.
Patient Consent Statement
Approval for the release of health information was obtained from the patient referenced in this report as requested by JCO Precision Oncology editorial.
Case Presentation
A 66-year-old man presented with right-sided chest pain. Computed tomography (CT) scan demonstrated a 3.8-cm right lung mass, mediastinal and hilar lymphadenopathy, and multiple hepatic masses up to 4.5 cm. Liver biopsy showed WD NET with immunohistochemistry (IHC) positive for synaptophysin, chromogranin, and thyroid transcription factor‐1 (3 mitoses per 2 mm2, Ki-67 14.4%; Fig 1, biopsy 1), consistent with metastatic atypical carcinoid tumor. Next-generation sequencing (NGS) of the liver metastasis (UCSF 500 Cancer Gene Panel [UCSF500], done retrospectively for research analysis) showed no pathogenic variants, a single variant of uncertain significance in EED, TMB 5.5 mutations/megabase (Mb), and no unstable microsatellites by MSIsensor (Table 1, t = 0).42 68Ga-DOTATATE scan revealed uptake in the lung mass but not the hepatic lesions, which demonstrated relatively high fluorodeoxyglucose avidity on 18F-labeled fluorodeoxyglucose positron emission tomography (standardized uptake value 13.2).
FIG 1.
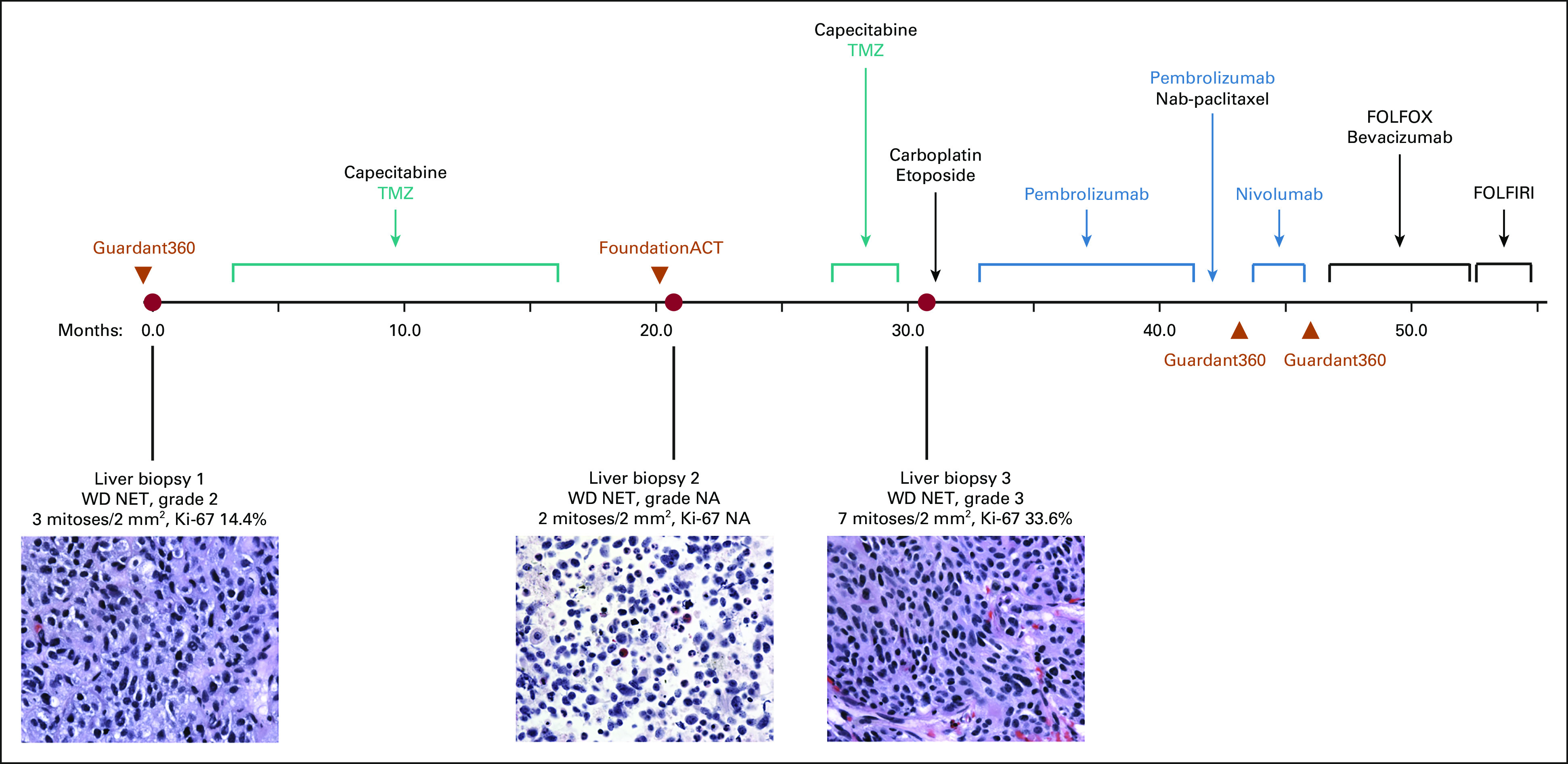
Chronological timeline of diagnostic liver biopsies (red circles), plasma ctDNA assessments (orange triangles), and treatment course of an atypical carcinoid tumor of the lung with TMZ-associated hypermutation treated with checkpoint inhibitor immunotherapy. Included is hematoxylin and eosin staining of histopathologic liver biopsy specimens at 60× magnification. ctDNA, circulating tumor DNA; FOLFIRI, fluorouracil, leucovorin, and irinotecan; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; NA, not available; NET, neuroendocrine tumor; TMZ, temozolomide; WD, well-differentiated.
TABLE 1.
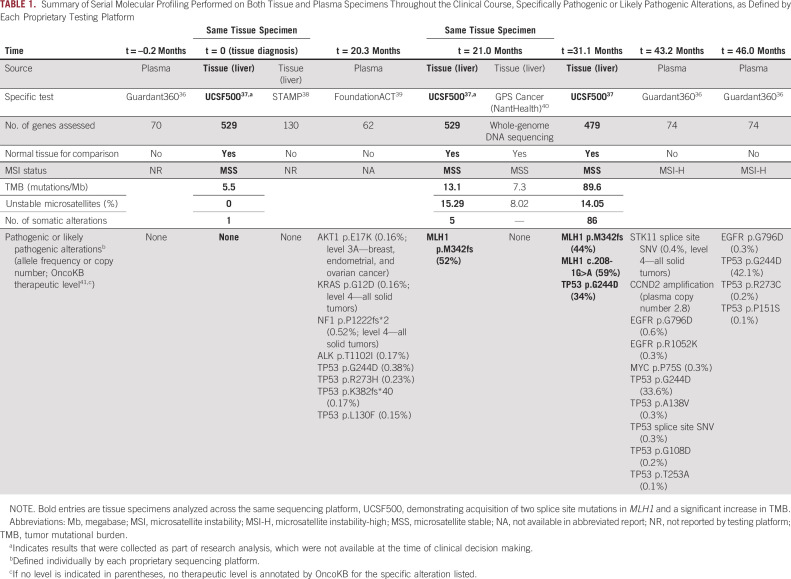
Summary of Serial Molecular Profiling Performed on Both Tissue and Plasma Specimens Throughout the Clinical Course, Specifically Pathogenic or Likely Pathogenic Alterations, as Defined by Each Proprietary Testing Platform
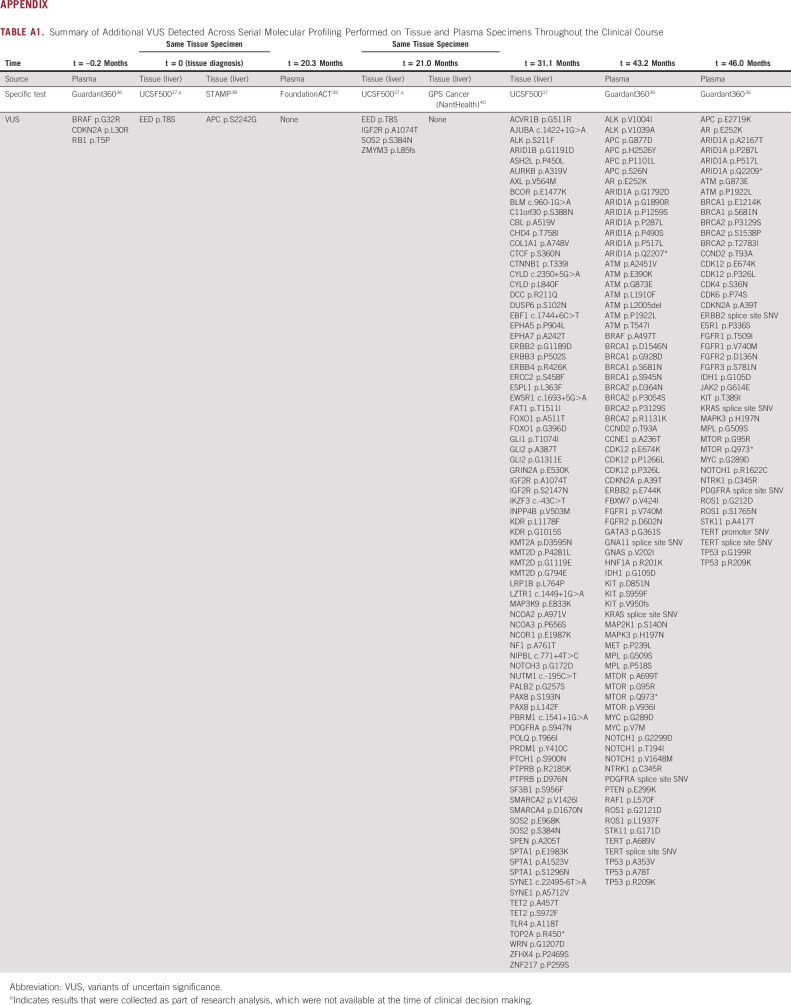
The patient received 14 cycles of TMZ/capecitabine with partial response in the lung and stable disease in the liver and retroperitoneal lymph nodes. The lung mass and thoracic lymphadenopathy were stable throughout the remaining clinical course. When a later CT scan showed a new 0.8 cm hepatic hypodensity, he underwent a second liver biopsy that revealed a WD NET (2 mitoses per 2 mm2, Ki-67 not available), with NGS (UCSF500, done retrospectively for research analysis) demonstrating the same variant of uncertain significance, < 10 somatic mutations, and a frameshift mutation in MLH1. TMB was 13.1 mutations/Mb, with 15% unstable microsatellites (Fig 1, biopsy 2; Table 1, t = 21.0 months). He subsequently developed rapidly progressive liver metastases and retroperitoneal lymphadenopathy, for which he received three additional cycles of TMZ/capecitabine without benefit. A third liver biopsy again demonstrated thyroid transcription factor‐1(+) WD NET (7 mitoses per 2 mm2), and the patient received three cycles of carboplatin/etoposide without benefit (Fig 2B). NGS (UCSF500) revealed > 80 somatic mutations, including two splice site mutations in MLH1 not present in the germline sample, consistent with a hypermutator phenotype from acquired dMMR (Table 1, t = 31.1 months; Appendix Table A1). Notably, nearly all the somatic mutations were C:G>T:A mutations. TMB had increased to 89.6 mutations/Mb with 14% unstable sites. Pathology review confirmed WD NET (Ki-67 33.6%), with the absence of MLH1 and PMS2 protein expression by IHC (Fig 1, biopsy 3).
FIG 2.
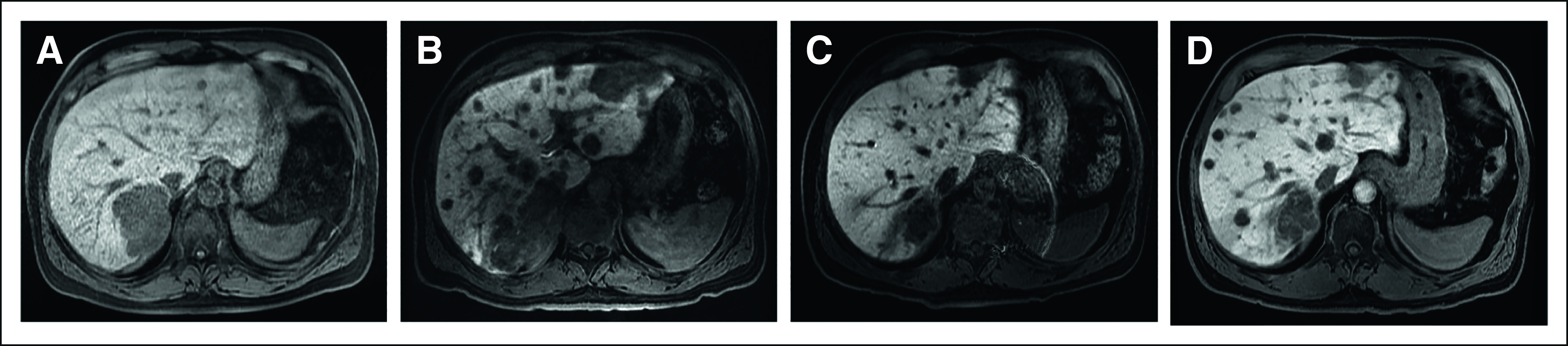
MRI of the abdomen/pelvis (T2-weighted, postgadolinium, LAVA) demonstrating progression of hepatic metastases on TMZ followed by partial response to pembrolizumab immunotherapy. Representative images (A) during treatment holiday before the last three cycles of TMZ/capecitabine (maximum lesion diameter: 7.3 cm); (B) after three cycles of carboplatin/etoposide, before initiation of pembrolizumab (maximum lesion diameter: 9.0 cm); (C) after eight cycles of pembrolizumab (maximum lesion diameter: 4.4 cm); and (D) after 12 cycles (9 months) of pembrolizumab (maximum lesion diameter: 7.0 cm). LAVA, liver acquisition volume acceleration; MRI, magnetic resonance imaging; TMZ, temozolomide.
The patient was treated with pembrolizumab (200 mg intravenously once every 3 weeks) for 9 months, with a marked interval decrease in the hepatic metastases and retroperitoneal lymphadenopathy after cycles 4 and 7 (Fig 2C). Magnetic resonance imaging after cycle 12 showed worsening hepatic and new osseous metastases (Fig 2D), for which nab-paclitaxel was added (three cycles). Faced with ongoing multifocal progression, the patient was switched to ipilimumab/nivolumab for 2 months without success.11 He was then treated with modified infusional fluorouracil, leucovorin, and oxaliplatin-6 plus bevacizumab for 6 months. After progressing on an irinotecan-based regimen, the patient succumbed to his disease almost five years after initial diagnosis.
Discussion
Emerging data support the use of TMZ-based therapy in NETs, with TMZ/capecitabine demonstrating superiority to single-agent TMZ in pancreatic tumors.43-45 There are few reports of TMZ-associated hypermutation beyond malignant glioma: two in pancreatic NETs, one in high-grade cervical NET, and one in pituitary carcinoma.35,46,47 For this patient, NGS identified two MLH1 splice site mutations absent in the treatment-naïve liver metastasis. IHC confirmed MLH1 loss in the hypermutated liver metastasis and loss of PMS2, the latter likely because of degradation of the undimerized partner of MLH1.48 There were no findings on germline analysis to explain the pattern of somatic hypermutation. Although MSI is a clinical biomarker for dMMR, it is defined on the basis of data from colorectal and endometrial carcinomas, and it remains unclear whether these traditional cutoffs apply to other tumor types. Technically, this dMMR lung NET was microsatellite stable. However, it has been shown that dMMR gliomas deemed microsatellite stable might actually have a MSI phenotype better characterized by single-cell whole-genome sequencing than standard NGS panels.49,50 This case demonstrated an increase from 0% unstable microsatellites in the first biopsy to 15% and 14% unstable microsatellites in the second and third biopsies, respectively. Taken together, these data suggest that TMZ-associated hypermutation occurs in NETs as a mechanism of resistance, and some, but not all, of the increased TMB is a result of increased MSI.
Few immunotherapy trials have enrolled pulmonary NETs other than SCLC, and none has explored immunotherapy for hypermutated tumors.11,51-54 Although results have generally been disappointing, a 17% overall response rate was observed for spartalizumab in bronchial NETs.6-8 Limited data suggest that combination therapy (eg, ipilimumab/nivolumab) may be more active, but additional information about the relationship between response and molecular markers (eg, TMB and MSI status) is needed. In gliomas, response to immunotherapy has been observed in a subset of dMMR gliomas, but overall response rate to programmed death-1 blockade was low in a series of 11 patients.50,55 At least two potential mechanisms underlie this observation. In contrast to colorectal cancer, dMMR gliomas lack significant T-cell infiltrates, despite a similar nonsynonymous mutational burden.50 Furthermore, hypermutation in gliomas with acquired dMMR tends to be subclonal and does not generate optimal antitumor T-cell responses. The use of immunotherapy for NETs with TMZ-associated hypermutation has only been reported in one other case (high-grade cervical NET).46 There, a subclonal MSH6 nonsense mutation was identified, but MMR deficiency was not confirmed with MSH6 loss by IHC or MSI testing.
In this case, serial tissue biopsy with concomitant NGS identified increasingly aggressive features (mitotic rate and proliferation index) and genomic evolution after treatment, most evident when analyzed retrospectively with a single platform (UCSF500) and akin to previous studies of pancreatic NETs.35 This patient had additional tissue and plasma NGS ordered in real time at various time points by different providers (Table 1), but the clinical utility of such testing was limited given significant heterogeneity between testing platforms (eg, limits of detection, number of genes assessed, and use of normal control) and lack of disease-specific guidance in this area.
In this case, the identification of a hypermutated phenotype with dMMR and high TMB prompted subsequent treatment with immunotherapy, which led to partial response and disease control for 9 months. Although the role of repeat tissue biopsy and molecular profiling in atypical bronchial carcinoid remains ill-defined, this case suggests that it is worth considering in patients treated with TMZ given the potential for identifying mutational signatures that could guide therapy. Additional studies are required to delineate the optimal strategy for molecular profiling, in both tissue and plasma. Furthermore, the association between the hypermutated phenotype and response to immunotherapy is not definitive in this case. Such a link could be further explored in a prospective study or at least a larger retrospective cohort (recognizing the response rate in biomarker-unselected bronchial NETs is low).6,7,9-11 Although the incidence of TMZ-associated hypermutation in NETs is unknown, its presence should alert clinicians to the potential value of immunotherapy, recognizing that the precise relationship between TMZ-associated hypermutation, dMMR, immune microenvironment, and response to immunotherapy requires further study.
APPENDIX
TABLE A1.
Summary of Additional VUS Detected Across Serial Molecular Profiling Performed on Tissue and Plasma Specimens Throughout the Clinical Course
Jessica Van Ziffle
Employment: Adaptive Biotechnologies (I)
Stock and Other Ownership Interests: Adaptive Biotechnologies (I)
Patents, Royalties, Other Intellectual Property: Adaptive Biotechnologies (I)
Claire K. Mulvey
Honoraria: OncLive/MJH Life Sciences
Research Funding: Genentech
Emily Bergsland
Stock and Other Ownership Interests: More Health (I), Exai Bio (I)
Consulting or Advisory Role: More Health (I)
Research Funding: Merck
Patents, Royalties, Other Intellectual Property: UpToDate
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health under award No. P30CA082103.
AUTHOR CONTRIBUTIONS
Conception and design: Fangdi Sun, Emily Bergsland
Collection and assembly of data: Fangdi Sun, Lisa Tan, Jessica Van Ziffle
Data analysis and interpretation: Fangdi Sun, James P. Grenert, Nancy M. Joseph, Claire K. Mulvey, Emily Bergsland
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jessica Van Ziffle
Employment: Adaptive Biotechnologies (I)
Stock and Other Ownership Interests: Adaptive Biotechnologies (I)
Patents, Royalties, Other Intellectual Property: Adaptive Biotechnologies (I)
Claire K. Mulvey
Honoraria: OncLive/MJH Life Sciences
Research Funding: Genentech
Emily Bergsland
Stock and Other Ownership Interests: More Health (I), Exai Bio (I)
Consulting or Advisory Role: More Health (I)
Research Funding: Merck
Patents, Royalties, Other Intellectual Property: UpToDate
No other potential conflicts of interest were reported.
REFERENCES
- 1. Travis WD, Rush W, Flieder DB, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 1998;22:934–944. doi: 10.1097/00000478-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 2. García-Yuste M, Matilla JM, Cueto A, et al. Typical and atypical carcinoid tumours: Analysis of the experience of the Spanish Multi-centric Study of Neuroendocrine Tumours of the Lung. Eur J Cardiothoracic Surg. 2007;31:192–197. doi: 10.1016/j.ejcts.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 3. Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968–977. doi: 10.1016/S0140-6736(15)00817-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fazio N, Buzzoni R, Delle Fave G, et al. Everolimus in advanced, progressive, well-differentiated, non-functional neuroendocrine tumors: RADIANT-4 lung subgroup analysis. Cancer Sci. 2018;109:174–181. doi: 10.1111/cas.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu J, Shen L, Zhou Z, et al. Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:1500–1512. doi: 10.1016/S1470-2045(20)30496-4. [DOI] [PubMed] [Google Scholar]
- 6. Strosberg J, Mizuno N, Doi T, et al. Efficacy and safety of pembrolizumab in previously treated advanced neuroendocrine tumors: Results from the phase II KEYNOTE-158 study. Clin Cancer Res. 2020;26:2124–2130. doi: 10.1158/1078-0432.CCR-19-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yao J, Strosberg J, Fazio N, et al. Spartalizumab in metastatic, well/poorly-differentiated neuroendocrine neoplasms. Endocr Relat Cancer. 2021;28:161–172. doi: 10.1530/ERC-20-0382. [DOI] [PubMed] [Google Scholar]
- 8. Mehnert JM, Bergsland E, O'Neil BH, et al. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer. 2020;126:3021–3030. doi: 10.1002/cncr.32883. [DOI] [PubMed] [Google Scholar]
- 9. Klein O, Kee D, Markman B, et al. Immunotherapy of ipilimumab and nivolumab in patients with advanced neuroendocrine tumors: A subgroup analysis of the CA209-538 clinical trial for rare cancers. Clin Cancer Res. 2020;26:4454–4459. doi: 10.1158/1078-0432.CCR-20-0621. [DOI] [PubMed] [Google Scholar]
- 10. Capdevila J, Teule A, López C, et al. A multi-cohort phase II study of durvalumab plus tremelimumab for the treatment of patients (pts) with advanced neuroendocrine neoplasms (NENs) of gastroenteropancreatic or lung origin: The DUNE trial (GETNE 1601) Ann Oncol. 2020;31:S770–S771. [Google Scholar]
- 11. Patel SP, Othus M, Chae YK, et al. A phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART SWOG 1609) in patients with nonpancreatic neuroendocrine tumors. Clin Cancer Res. 2020;26:2290–2296. doi: 10.1158/1078-0432.CCR-19-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shah MH, Goldner WS, Halfdanarson TR, et al. Neuroendocrine and adrenal tumors, version 2.2018 featured updates to the NCCN guidelines. J Natl Compr Cancer Netw. 2018;16:693–702. doi: 10.6004/jnccn.2018.0056. [DOI] [PubMed] [Google Scholar]
- 13. Chong CR, Wirth LJ, Nishino M, et al. Chemotherapy for locally advanced and metastatic pulmonary carcinoid tumors. Lung Cancer. 2014;86:241–246. doi: 10.1016/j.lungcan.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wirth LJ, Carter MR, Jänne PA, et al. Outcome of patients with pulmonary carcinoid tumors receiving chemotherapy or chemoradiotherapy. Lung Cancer. 2004;44:213–220. doi: 10.1016/j.lungcan.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 15. Daniel P, Sabri S, Chaddad A, et al. Temozolomide induced hypermutation in glioma: Evolutionary mechanisms and therapeutic opportunities. Front Oncol. 2019;9:41. doi: 10.3389/fonc.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karran P, Marinus MG. Mismatch correction at O6-methylguanine residues in E. coli DNA. Nature. 1982;296:868–869. doi: 10.1038/296868a0. [DOI] [PubMed] [Google Scholar]
- 17. Margison GP, Santibáñez Koref MF, Povey AC. Mechanisms of carcinogenicity/chemotherapy by O6-methylguanine. Mutagenesis. 2002;17:483–487. doi: 10.1093/mutage/17.6.483. [DOI] [PubMed] [Google Scholar]
- 18. Kulke MH, Hornick JL, Frauenhoffer C, et al. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res. 2009;15:338–345. doi: 10.1158/1078-0432.CCR-08-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 20. Brandes AA, Tosoni A, Cavallo G, et al. Correlations between O6-methylguanine DNA methyltransferase promoter methylation status, 1p and 19q deletions, and response to temozolomide in anaplastic and recurrent oligodendroglioma: A prospective GICNO study. J Clin Oncol. 2006;24:4746–4753. doi: 10.1200/JCO.2006.06.3891. [DOI] [PubMed] [Google Scholar]
- 21. Chinot OL, Barrié M, Fuentes S, et al. Correlation between O6-methylguanine-DNA methyltransferase and survival in inoperable newly diagnosed glioblastoma patients treated with neoadjuvant temozolomide. J Clin Oncol. 2007;25:1470–1475. doi: 10.1200/JCO.2006.07.4807. [DOI] [PubMed] [Google Scholar]
- 22. Middleton MR, Lunn JM, Morris C, et al. O6-methylguanine-DNA methyltransferase in pretreatment tumour biopsies as a predictor of response to temozolomide in melanoma. Br J Cancer. 1998;78:1199–1202. doi: 10.1038/bjc.1998.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 24. Cros J, Hentic O, Rebours V, et al. MGMT expression predicts response to temozolomide in pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2016;23:625–633. doi: 10.1530/ERC-16-0117. [DOI] [PubMed] [Google Scholar]
- 25. Krug S, Boch M, Rexin P, et al. Impact of therapy sequence with alkylating agents and MGMT status in patients with advanced neuroendocrine tumors. Anticancer Res. 2017;37:2491–2500. doi: 10.21873/anticanres.11590. [DOI] [PubMed] [Google Scholar]
- 26. Cives M, Ghayouri M, Morse B, et al. Analysis of potential response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2016;23:759–767. doi: 10.1530/ERC-16-0147. [DOI] [PubMed] [Google Scholar]
- 27. Yip S, Miao J, Cahill DP, et al. MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin Cancer Res. 2009;15:4622–4629. doi: 10.1158/1078-0432.CCR-08-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marra G, D'Atri S, Corti C, et al. Tolerance of human MSH2+/– lymphoblastoid cells to the methylating agent temozolomide. Proc Natl Acad Sci USA. 2001;98:7164–7169. doi: 10.1073/pnas.121136498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fink D, Aebi S, Howell SB. The role of DNA mismatch repair in drug resistance. Clin Cancer Res. 1998;4:1–6. [PubMed] [Google Scholar]
- 30. Felsberg J, Thon N, Eigenbrod S, et al. Promoter methylation and expression of MGMT and the DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2 in paired primary and recurrent glioblastomas. Int J Cancer. 2011;129:659–670. doi: 10.1002/ijc.26083. [DOI] [PubMed] [Google Scholar]
- 31. Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343:189–193. doi: 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cahill DP, Levine KK, Betensky RA, et al. Loss of the mismatch repair protein MSH6 in human glioblastomas is associated with tumor progression during temozolomide treatment. Clin Cancer Res. 2007;13:2038–2045. doi: 10.1158/1078-0432.CCR-06-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choi S, Yu Y, Grimmer MR, et al. Temozolomide-associated hypermutation in gliomas. Neuro Oncol. 2018;20:1300–1309. doi: 10.1093/neuonc/noy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang J, Cazzato E, Ladewig E, et al. Clonal evolution of glioblastoma under therapy. Nat Genet. 2016;48:768–776. doi: 10.1038/ng.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Raj N, Shah R, Stadler Z, et al. Real-time genomic characterization of metastatic pancreatic neuroendocrine tumors has prognostic implications and identifies potential germline actionability. JCO Precis Oncol. 2018;2018:1–18. doi: 10.1200/PO.17.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guardant Health . Guardant360® CDx. https://guardant360cdx.com/ [Google Scholar]
- 37.UCSF Health Center for Clinical Genetics and Genomics . UCSF 500 Cancer Gene Panel Test (UCSF500/UC500) https://genomics.ucsf.edu/content/ucsf-500-cancer-gene-panel-test-ucsf500-uc500 [Google Scholar]
- 38.Stanford Health Care . Molecular Genetic Pathology—Stanford Tumor Actionable Mutation Pattern for Solid Tumors (STAMP) https://stanfordlab.com/content/stanfordlab/en/molecular-pathology/molecular-genetic-pathology.html/ [Google Scholar]
- 39.Foundation Medicine . Foundation Medicine Introduces FoundationOne®Liquid, the Latest Advance in the Company’s Liquid Biopsy Test for Solid Tumors in Patients With Advanced Cancer (FoundationACTTM, Assay for Circulating Tumor DNA, Has Since Been Superceded by FoundationOne® Liquid CDx) 2018. https://www.foundationmedicine.com/press-releases/d67a6b76-e9a9-4f5f-9a0a-c47831980163 [Google Scholar]
- 40.NantOmics . GPS CancerTM. https://nantomics.com/gpscancer/ [Google Scholar]
- 41.Chakravarty D, Gao J, Phillips SM, et al. OncoKB: A precision oncology knowledge base JCO Precis Oncol 1PO.17.000112017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Niu B, Ye K, Zhang Q, et al. MSIsensor: Microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics. 2014;30:1015–1016. doi: 10.1093/bioinformatics/btt755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Al‐Toubah T, Morse B, Strosberg J. Capecitabine and temozolomide in advanced lung neuroendocrine neoplasms. Oncologist. 2020;25:e48–e52. doi: 10.1634/theoncologist.2019-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koumarianou A, Kaltsas G, Kulke MH, et al. Temozolomide in advanced neuroendocrine neoplasms: Pharmacological and clinical aspects. Neuroendocrinology. 2015;101:274–288. doi: 10.1159/000430816. [DOI] [PubMed] [Google Scholar]
- 45. Kunz PL, Catalano PJ, Nimeiri H, et al. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: A trial of the ECOG-ACRIN Cancer Research Group (E2211) J Clin Oncol. 2018;36 doi: 10.1200/JCO.22.01013. suppl 15; abstr 4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Klempner SJ, Hendifar A, Waters KM, et al. Exploiting temozolomide-induced hypermutation with pembrolizumab in a refractory high-grade neuroendocrine neoplasm: A proof-of-concept case. JCO Precis Oncol. 2020;4:614–619. doi: 10.1200/PO.20.00034. [DOI] [PubMed] [Google Scholar]
- 47. Lin AL, Jonsson P, Tabar V, et al. Marked response of a hypermutated ACTH-secreting pituitary carcinoma to ipilimumab and nivolumab. J Clin Endocrinol Metab. 2018;103:3925–3930. doi: 10.1210/jc.2018-01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen W, Frankel WL. A practical guide to biomarkers for the evaluation of colorectal cancer. Mod Pathol. 2019;32:1–15. doi: 10.1038/s41379-018-0136-1. suppl 1. [DOI] [PubMed] [Google Scholar]
- 49. Indraccolo S, Lombardi G, Fassan M, et al. Genetic, epigenetic, and immunologic profiling of MMR-deficient relapsed glioblastoma. Clin Cancer Res. 2019;25:1828–1837. doi: 10.1158/1078-0432.CCR-18-1892. [DOI] [PubMed] [Google Scholar]
- 50. Touat M, Li YY, Boynton AN, et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020;580:517–523. doi: 10.1038/s41586-020-2209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lu M, Zhang P, Zhang Y, et al. Efficacy, safety, and biomarkers of toripalimab in patients with recurrent or metastatic neuroendocrine neoplasms: A multiple-center phase Ib trial. Clin Cancer Res. 2020;26:2337–2345. doi: 10.1158/1078-0432.CCR-19-4000. [DOI] [PubMed] [Google Scholar]
- 52. Maggio I, Manuzzi L, Lamberti G, et al. Landscape and future perspectives of immunotherapy in neuroendocrine neoplasia. Cancers (Basel) 2020;12:832. doi: 10.3390/cancers12040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Halperin DM, Liu S, Dasari A, et al. A phase II trial of atezolizumab and bevacizumab in patients with advanced, progressive neuroendocrine tumors (NETs) J Clin Oncol. 2020;38 suppl 4; abstr 619. [Google Scholar]
- 54. Yao JC, Strosberg J, Fazio N, et al. Activity & safety of spartalizumab (PDR001) in patients (pts) with advanced neuroendocrine tumors (NET) of pancreatic (Pan), gastrointestinal (GI), or thoracic (T) origin, & gastroenteropancreatic neuroendocrine carcinoma (GEP NEC) who have progressed on prior treatment (Tx) Ann Oncol. 2018;29:viii467–viii468. [Google Scholar]
- 55. Hypermutated gliomas respond poorly to immunotherapy. Cancer Discov. 2020;10:OF5. doi: 10.1158/2159-8290.CD-NB2020-045. [DOI] [PubMed] [Google Scholar]