Abstract
This paper presents a classification and definitions for types of nystagmus and other oscillatory eye movements relevant to evaluation of patients with vestibular and neurological disorders, formulated by the Classification Committee of the Bárány Society, to facilitate identification and communication for research and clinical care. Terminology surrounding the numerous attributes and influencing factors necessary to characterize nystagmus are outlined and defined. The classification first organizes the complex nomenclature of nystagmus around phenomenology, while also considering knowledge of anatomy, pathophysiology, and etiology. Nystagmus is distinguished from various other nystagmus-like movements including saccadic intrusions and oscillations.
View accompanying videos at http://www.jvr-web.org/ICVD.html
1. Introduction
The following classification and definitions for nystagmus and nystagmus-like movements are part of the International Classification of Vestibular Disorders (ICVD), an initiative by the Bárány Society to develop a comprehensive classification scheme and definitions of individual vestibular disorders that is acceptable worldwide [23]. The structure of the ICVD includes 4 layers: 1) Symptoms and signs, 2) Syndromes, 3) Disorders and diseases, and 4) Mechanisms. As a first step, the Classification Committee of the Bárány Society published a consensus document defining vestibular symptoms [21]. Details of the international, cross-disciplinary consensus-building process may be found in that document. Briefly, small workgroups composed of representatives from neurology and otolaryngology from at least three continents are charged with developing consensus definitions for relevant terms linked to a vestibular theme. These definitions are then subjected to scrutiny and open comment by vestibular experts prior to being published as consensus definitions or criteria.
The Subcommittee for Vestibular Signs and Examination Techniques is charged with developing consensus definitions for vestibular signs and examination techniques used frequently in the assessment of patients with vestibular symptoms. The Subcommittee felt that the number and complexity of definitions required more than one consensus document, beginning by defining the nomenclature around nystagmus, organizing the types of nystagmus based on their many qualities, and distinguishing them from saccadic intrusions and other nystagmus-like movements. A future document will address other vestibular and ocular motor examination signs and techniques.
2. Methods
In 2006 the Bárány Society convened the first meeting of a Classification Committee to begin structuring the approach to developing the ICVD [23]. The group created a conceptual framework, a list of initial topics, and a process for consensus building. Key vestibular symptoms were formally defined and published in 2009 [21]. In 2012, the Subcommittee for Vestibular Signs and Examination Techniques was convened and began drafting a classification and definitions for terms related to nystagmus and its evaluation. In keeping with established procedures for multinational and multidisciplinary consensus building [6], the subcommittee included neurologists (S.E., A.B., M.vB., D.Z., J.S.K., M.W., D.N-T.) and otolaryngologists (N.P., C.D.S.) from 4 continents (Europe, Asia, Australia, and North America). The chair (S.E.) drafted an initial document, which led to subsequent deliberations and numerous iterative changes. Working drafts were presented for discussion by the general membership during the Bárány Society’s biennial congresses in Uppsala, Sweden in June 2012 and Buenos Aires, Argentina in May 2014 as well as at Classification Committee meetings in Mondorf-les-Bains, Luxembourg in November 2013 and Berlin, Germany in March 2017. These deliberations led to a consensus document endorsed by the subcommittee members (authors) that was made available online for comment by all members of the Bárány Society.
3. Basic definitions and etymology
3.1. Definition
Nystagmus is an involuntary, rapid, rhythmic, oscillatory eye movement with at least one slow phase. Jerk nystagmus is nystagmus with a slow phase and a fast phase. Pendular nystagmus is nystagmus with only slow phases.
3.2. General comments
-
•
Pathologic forms of nystagmus have many causes but generally result from diseases affecting the peripheral vestibular apparatus, brainstem, or the cerebellum and less commonly the anterior visual pathways or cerebral hemispheres.
-
•
Nystagmus differs from saccadic intrusions and oscillations, such as square wave jerks, ocular flutter, and opsoclonus, in which inappropriate saccades (fast eye movements) take the eye away from a target during intended fixation.
-
•
Nystagmus may be continuous or episodic. Episodes of nystagmus may occur spontaneously, may occur in only certain gaze positions or viewing conditions, or may be triggered by particular maneuvers.
-
•
Nystagmus is characterized by numerous phenomenological attributes, many of which are linked to specific underlying pathologic mechanisms. Specific forms of nystagmus are sometimes given special names based on combinations of these attributes.
3.3. Etymology
The term nystagmus is derived from the head nodding motion seen in people who are dozing off (New Latin, from Greek nustagmos (nodding, drowsiness), from nystazein (nod, be sleepy, to doze)) [4, 5]. Following from this literal definition, jerk nystagmus consists of repetitive cycles of a slow drift of the eyes (the slow phase) followed by an oppositely directed quick movement (the fast phase). When pathologic, jerk nystagmus prevents steady visual fixation. It classically begins with a slow drift of the eyes taking the line of sight away from the object of regard before it is brought back toward the object of regard with the fast phase. Jerk nystagmus also may be physiologic, such as during natural rotation of the head, and in this circumstance the slow phase ensures rather than disrupts steady fixation. Although deviating from the original derivation of the word, the term nystagmus has also come to include pathologic oscillations of the eyes in which cycles consist of slow drifts of the eyes that reverse direction periodically, usually at a frequency between 1 and 10 Hz. The appearance of this oscillation resembles the sinusoidal motion of a pendulum and hence is called pendular nystagmus. While some refer to these as “pendular oscillations,” the term pendular nystagmus is too well established in the medical literature to replace it.
3.4. Differentiation from other oscillatory eye movements
Nystagmus is distinguished from other types of oscillatory eye movements, such as those in which the primary disturbance is in the saccadic system. Saccades are rapid eye movements that shift the line of sight between successive points of fixation [109]. Saccades include voluntary and reflexive shifts of fixation as well as fast phases of nystagmus and rapid eye movements during REM sleep. Normally, clinically-visible saccades are suppressed during steady fixation. Saccadic intrusions and oscillations are rapid movements that take the eye away from the target during attempted fixation in the absence of a novel distracting visual stimulus [130]. With saccadic intrusions, the initial eye movement away from the target is a saccade, as opposed to nystagmus in which the initial eye movement is generally a slow phase. More importantly, unlike nystagmus, saccadic intrusions (except for saccadic pulses (3.1.3)) have no slow phase drift. Examples of saccadic intrusions include square wave jerks, in which the oscillations are separated by an intersaccadic interval (pause), and back-to-back saccadic oscillations such as ocular flutter and opsoclonus in which there is no intersaccadic interval. Ocular flutter and opsoclonus differ from nystagmus not only physiologically but also etiologically—both have a strong association with paraneoplastic or parainfectious encephalitis [59, 97] that makes the distinction in terminology between nystagmus and saccadic oscillations of particular clinical relevance.
Some other types of oscillatory eye movements are less well understood and not so easily classified. We will describe specific characteristics of these disorders in this classification so they may be recognized clinically. By our definitions here, the ocular oscillations of spasmus nutans, convergence retraction nystagmus, ocular bobbing, superior oblique myokymia, and some other related phenomena are referred to as “nystagmus-like” given their uncertain nature.
3.5. Prior definitions for nystagmus
-
•
“A rapid, involuntary, oscillatory motion of the eyeball” [3]
-
•
“An involuntary, rapid, rhythmic movement of the eyeball, which may be horizontal, vertical, rotatory, or mixed” [1]
-
•
“Nystagmus is a rhythmic regular oscillation of the eyes” [16]
-
•
“Repetitive to-and-fro movements of the eyes that are initiated by slow phases” [109]
The committee reviewed prior definitions for nystagmus listed above. The first three definitions were considered inadequate because they fail to discriminate nystagmus from saccadic oscillations such as ocular flutter and opsoclonus. The fourth definition does not clarify the involuntary and rapid nature of the oscillation; it also demands that nystagmus be initiated by slow phases, but this distinction may not be clear at the bedside. For example, epileptic nystagmus may be initiated either by slow phases or fast phases [109]. Similarly, despite their clinical and etiologic similarity, half of the variants of ocular bobbing and dipping are thought to be initiated by slow phases while the other half are thought to be initiated by fast phases [109].
4. Nystagmus trajectory: Reference frames, axes, planes, and directions
When the eye is pointing straight ahead with the line of sight perpendicular to the frontal plane, it is said to be in the “straight-ahead position” or “center gaze position” (Box 1). When describing motion of the eye in nystagmus, the path taken from starting eye position to ending eye position is known as the trajectory [153]. Although the term “vector” is sometimes used synonymously with the term “trajectory”, we advise use of the term trajectory so as not to be confused with the related (but slightly different) concept of the rotation vector [71], which has been defined as a straight line from starting eye position to ending eye position during one half cycle of movement [164]. Describing this trajectory in three dimensions requires choosing and specifying a reference frame [144]. Eye-referenced coordinates describe eye rotation around three axes that intersect at the center of the globe. Thus, an eye rotation can be described by the axis around which the eye is rotating (e.g., craniocaudal axis rotation) or the plane perpendicular to the axis of rotation (e.g., yaw plane rotation) (Table 1). However, depending on the clinical or scientific circumstances, rather than an eye-referenced coordinate system (e.g., vertical-torsional nystagmus), it may be more appropriate to use a head-referenced coordinate system (e.g., nystagmus in the plane of the posterior semicircular canal) or even an earth-referenced coordinate system (e.g., “geotropic” nystagmus for that which beats toward the ground) to describe the motion of the eyes.
Table 1.
Eye movement axes and planes of rotation in three dimensions
| Axis | Anatomy & Radiology Plane | Aeronautics & Vestibular Plane | Ocular Motor Direction with eyes in straight-ahead position |
| Craniocaudal, superoinferior, or Z* | Horizontal or Axial | Yaw | Horizontal: Right vs. Left |
| Interaural or Y | Sagittal | Pitch | Vertical: Up vs. Down |
| Naso-occipital, anteroposterior or X | Coronal or Frontal | Roll | Torsional: Top pole toward right ear vs. left ear |
| RALP | 45° from midsagittal, perpendicular to the mean plane of RA and LP SCCs | RALP: right anterior – left posterior SCC plane | Mixed vertical-torsional: Up with torsion moving the top of the globe toward left ear – down with torsion moving the top of the globe toward right ear |
| LARP | 45° from midsagittal, perpendicular to the mean plane of LA and RP SCCs | LARP: left anterior – right posterior SCC plane | Mixed vertical-torsional: Up with torsion moving the top of the globe toward right ear – down with torsion moving the top of the globe toward left ear |
LA, left anterior; LP, left posterior; RA, right anterior; RP, right posterior; LARP, left anterior-right posterior; RALP, right anterior-left posterior; SCC, semicircular canal. *Two slightly different XYZ axis coordinates may be used, each maintaining the interaural Y axis. Head-referenced coordinates (Reid’s coordinates) define the craniocaudal +Z axis as upward along the vector perpendicular to Reid’s horizontal plane, which in turn is defined by containing the midpoint of the entrance of the bony ear canal and the cephalic edge of the inferior orbital rim bilaterally; the +Y axis is the leftward end of the interaural axis; and the +X axis is anterior along a naso-occipital axis perpendicular to +Y and +Z. The canal frame of reference also uses the leftward interaural axis as +Y, but the +Z axis is perpendicular to the mean horizontal SCC plane (which is about 20 deg pitch-nose-up from Reid’s horizontal plane) and the +X axis is parallel to the horizontal SCC plane and perpendicular to +Y and +Z [43].
For spontaneous nystagmus in the straight-ahead position, the farther the eye is moved into an eccentric position, the greater the eye-referenced and head-referenced frames’ descriptions of the nystagmus will differ [127]. Consider a patient with horizontal nystagmus in the straight-ahead gaze position. If the nystagmus remains “horizontal” in upgaze and downgaze in eye-referenced coordinates (horizontal with respect to the patient’s changing visual axis), the nystagmus would now appear to have a torsional component if described in head-referenced coordinates, which could imply a central etiology like infantile nystagmus (2.1.3.1.) rather than a peripheral vestibular nystagmus (2.1.1.) whose axis should remain fixed in head or labyrinthine coordinates. Similarly, if downbeat nystagmus (with respect to the head) were due to an imbalance in the rotational vestibulo-ocular reflex, then an examiner face to face with the patient should observe a torsional component (with respect to the patient’s eye) when the patient looks laterally to either side, because the slow phase is still rotating around the head-fixed interaural axis (pitch plane). If instead the downbeat nystagmus is in an eye-referenced frame, the nystagmus would appear vertical (with respect to the eye) in all horizontal eye positions. Thus, for spontaneous horizontal or vertical nystagmus in the straight-ahead position, one can detect whether the dysfunctional signals engendering the nystagmus are eye- or head-fixed by examining the nystagmus when the patient looks in a direction orthogonal to the spontaneous nystagmus. Although all frames of reference are equally valid, the frame of reference that most efficiently describes a given pathologic nystagmus is typically the one most closely linked to the mechanism or site causing the nystagmus; therefore, when describing the nystagmus, the examiner should specify the frame of reference being used, both to avoid ambiguity and to facilitate diagnosis.
Box 1.
Listing’s law
Listing’s law states that any eye position can be described by rotation of the eye from the primary position about a single axis lying in the equatorial plane. Strictly speaking, “primary position” is defined with reference to Listing’s law as the unique position at which the line of sight is perpendicular to Listing’s plane and the position from which purely horizontal or purely vertical rotations of the eye to a new position are unassociated with any torsion [80, 109]. This is usually close to, but not always exactly, straight ahead with visual fixation directed at a distant target (e.g., as when driving an automobile). While indistinguishable clinically, careful eye movement recordings have confirmed that Listing’s law is approximately obeyed for saccades, pursuit, and eye movement responses to head translations, but less so for vestibular eye movements from head rotation. Thus, whether eye movements of a given form of nystagmus obey Listing’s law may have implications for whether the pathogenesis lies in the pursuit system, translational vestibulo-ocular reflex (VOR), or rotational VOR. Therefore, we have chosen to use the terms “straight-ahead position” or “center gaze position” for clinical observation in this document. We recognize, however, that “primary position” is a standard term used in ophthalmology as it relates to strabismus [99], and its meaning there is often intended as synonymous with these other terms that refer to an approximate eye position (rather than a very specific anatomic locus with reference to Listing’s plane).
The direction of all eye movements, including nystagmus, should be described from the vantage point of the subject, not the examiner (e.g., right-beating nystagmus beats toward the subject’s right ear, not the examiner’s right ear, when the examiner is face-to-face in front of the patient). Conventionally, the direction of jerk nystagmus is described with reference to the fast phase, despite the fact that the fast phase is usually not the primary cause of the nystagmus but a movement that corrects for the abnormal slow-phase drift away from fixation. The committee agreed this convention is too deeply entrenched in clinical practice to change. Thus, left-beating nystagmus comprises a rightward slow phase and leftward fast phase.
Eye movements in an eye- or head-referenced frame are described as such regardless of head position with respect to gravity. Thus, nystagmus beating toward the forehead when the head is in the Dix-Hallpike head hanging position (i.e., full neck extension while lying supine) is still upbeating, despite the fact that the movement is, in some sense, “downward” with respect to gravity. In patients with positional forms of nystagmus, particularly horizontal positional nystagmus (2.3.1.1.2.), an earth-referenced frame is often adopted to describe the direction of the nystagmus. In the supine position with the right ear “down” (i.e., supine subject with neck rotated toward the right shoulder), nystagmus that beats toward the earth is often referred to as “geotropic” rather than “right-beating,” especially if its direction with respect to the head reverses after the head is reoriented to the left ear “down” supine position (i.e., now “left-beating” but still “geotropic”). In this nomenclature and using an earth-referenced frame, nystagmus beating toward the earth is called geotropic while that which beats away from the earth is called apogeotropic (sometimes mistakenly called “ageotropic”). Such terminology is restricted to use with positional testing, since referring to downbeat nystagmus in center gaze while the patient is seated or standing upright as “geotropic,” while technically correct, would be misleading.
Since the movements of nystagmus are not typically confined to only rotations about one of the three cardinal anatomic axes (e.g., purely roll, pitch, or yaw rotations, respectively, about axes parallel to the anteroposterior, interaural, or superoinferior axes of the eye or head), each of the 3 directional components should be described, along with the coordinate system (e.g., “with respect to the head”, “with respect to the eye” or “geotropic”), except in special cases for which the components not described are very small relative to the largest component. In many cases, one can choose a coordinate frame of reference for which one directional component dominates the other two. That coordinate frame will typically provide not only the most parsimonious description but also the most intuitive model of the underlying pathology.
For example, benign paroxysmal positional vertigo from the left posterior semicircular canal elicits eye movements about an axis parallel to the affected canal’s axis (which is approximately 45° from the anterior and left ends of the naso-occipital and interaural axes, respectively), regardless of the direction of gaze [38, 43]. When the subject’s gaze is directed 45° to the left of center, the nystagmus with respect to the eye appears purely torsional about the line of sight, but when the subject’s gaze is directed 45° to the right of center, the nystagmus with respect to the eye appears purely vertical. Using an eye frame of reference would therefore result in a confusing description complicated by dependence on instantaneous eye orientation. Choosing a head frame of reference is only modestly better, because the nystagmus will include two nearly equal components (roll and pitch), neither of which is intuitively linked to the source of dysfunction. In contrast, choosing a canal frame of reference (i.e., three mutually perpendicular axes comprising the mean axes of the left-anterior/right-posterior [LARP], right-anterior/left-posterior [RALP] and left-horizontal/right-horizontal [LHRH] canal pairs) greatly simplifies the description, because the nystagmus is almost entirely about the RALP axis.
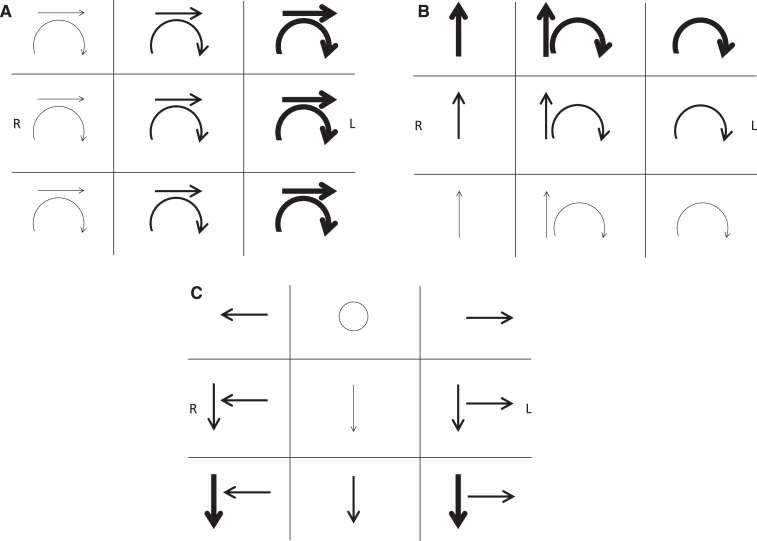
Describing torsional eye movements requires special care. Use of the terms “rotary” or “rotatory” is discouraged, since nearly all eye movements are technically “rotations” (i.e., angular movements of the eye rather than linear ones). Torsional direction is most unambiguously described by the ear toward which the top or upper pole of the eye (the 12 o’clock position) rotates (e.g., top pole of the eye beating toward the right ear). We caution against use of the terms “clockwise” and “counterclockwise” since, despite appearing intuitive, they are a common source of miscommunication (e.g., “clockwise” from the examiner’s perspective is properly called “counterclockwise” from the subject’s viewpoint). If used, the fast versus slow phase direction must be specified, and it must be emphasized that the direction is from the perspective of the subject rather than the examiner (e.g., “torsional nystagmus beating counterclockwise from the patient’s perspective”). Schematic notation of jerk nystagmus is illustrated in (Fig. 1).
Fig.1.
Jerk nystagmus schematic notation. Representing the important attributes of three-dimensional eye movements on a two-dimensional page presents several challenges requiring care to avoid ambiguity. As with describing nystagmus, its schematic notation should document the direction and intensity in the 9 cardinal gaze positions. If the nystagmus is not conjugate, each eye’s characteristics can be documented separately. By convention, eye movements are drawn from the vantage point of the examiner in front of the patient. Text should indicate whether arrows represent the slow or fast phase direction. In A-C, the arrows denote the direction of the fast phases, with the boldness of the arrows reflecting the intensity of the nystagmus. The frame of reference must be specified in every schematic in order to clarify whether the arrows for a given gaze position refer to eye movements being described in head-fixed coordinates (as viewed face to face with the patient) or eye-fixed coordinates (as viewed along the patient’s visual axis). The frame of reference that most efficiently describes a given pathologic nystagmus is typically the one most closely linked to the mechanism or site causing the nystagmus. Examples are shown: (A) Spontaneous third-degree horizontal-torsional left-beating peripheral vestibular nystagmus. This mixed horizontal-torsional nystagmus in straight-ahead gaze that obeys Alexander’s law (increases when looking in the fast phase direction and decreases when looking in the slow phase direction) is typical for an uncompensated right unilateral vestibular lesion. In this case the nystagmus directional arrows are in head-referenced coordinates, reflecting the combined effect of injury to all three semicircular canals and producing nystagmus that remains in a fixed direction with respect to the head and labyrinths regardless of gaze position (see Fig. 3). (B) Left posterior semicircular canal benign paroxysmal positional nystagmus. Nystagmus elicited in the left Dix-Hallpike position consists of mixed upbeat and torsional nystagmus with the upper pole of the eyes beating toward the left ear in straight-ahead gaze. Since the nystagmus direction is fixed in the plane of the left posterior canal, it appears predominantly vertical in rightward gaze and predominantly torsional in leftward gaze when observed along the patient’s visual axis (as documented in each box representing eye-referenced coordinates in this schematic), as well as obeying Alexander’s law. Note that the torsional fast phases could be confusingly described as clockwise from the examiner’s perspective (incorrect, but commonly used) but counterclockwise from the patient’s perspective (correct, but inconsistently used). (C) Spontaneous downbeat and bilateral gaze-holding nystagmus. Low-intensity pure downbeat nystagmus in straight-ahead gaze increases in lateral and downgaze and is associated with pathologic bilateral gaze-holding nystagmus. This schematic uses head-referenced coordinates, indicating that the downbeat component appears to remain fixed to labyrinthine coordinates regardless of gaze position. This may imply that the downbeat nystagmus is coming from a vestibular tone imbalance of the vertical rotational vestibulo-ocular reflex.
For monocular or disconjugate movements, intorsion refers to rotation of the top pole of the eye toward the nose; extorsion refers to rotation of the top pole of the eye toward the ear. With conjugate torsional eye movements (as is typical with nystagmus that has a torsional component to the movement), one eye intorts while the other extorts. In head-referenced coordinates, conjugate torsion should be described as rotating the top pole toward the right ear or left ear. Note that conjugate torsional displacement of the eyes may be dynamic (as seen in torsional nystagmus) or static (as seen in the pathologic ocular tilt reaction due to otolith pathway imbalance). In contrast, involuntary static horizontal or vertical displacements are generally referred to as “forced gaze deviations,” “conjugate gaze deviations,” or simply “gaze deviations,” rather than “static” horizontal or vertical displacements and are generally not due to otolith imbalance.
5. Additional nystagmus attributes
In addition to plane/axis and direction, several attributes of nystagmus (and of other eye movements) are typically described either qualitatively at the bedside or quantitatively using oculographic equipment (Table 2):
Table 2.
Checklist of attributes for nystagmus characterization
| Nystagmus Trajectory: axis or plane of rotation and direction in straight-ahead (center) gaze position including horizontal, vertical, and torsional components |
| Binocularity: monocular or binocular |
| Conjugacy: conjugate or disconjugate (dissociated or disjunctive) |
| Velocity: quantitative measurement of slow-phase velocity |
| Waveform: pendular or jerk |
| Frequency: most useful for low-frequency (<3 Hz) forms of pendular nystagmus |
| Intensity: qualitative assessment as product of amplitude and frequency |
| Eccentric gaze influence on presence or attributes of nystagmus including direction (from a head-referenced or eye-referenced coordinate system) |
| Effect of convergence |
| Influence of permitting versus blocking visual fixation |
| Effect of provocative maneuvers |
| Age of first appearance |
| Temporal profile: intermittent, continuous, or changing over time |
-
1.
Binocularity: Nystagmus may be monocular (in one eye) or binocular (in both eyes). Most nystagmus is binocular and should be presumed so unless specifically noted to be monocular. The terms “unilateral” or “bilateral” are reserved to describe pathologic horizontal gaze-evoked nystagmus present when looking to only one side or to both sides.
-
2.
Conjugacy: Conjugate eye movements rotate both eyes together in the same direction by the same amount. Movements are disconjugate if the eyes do not rotate in the same plane and direction by the same amount. Disconjugate movements are considered dissociated if the velocity or amplitude of the movements are different in the two eyes, whereas they are referred to as disjunctive if the two eyes simultaneously rotate in different directions, such as with convergence or divergence. Generally speaking, conjugacy is determined clinically, and minor amounts of disconjugacy that might be measured with quantitative ocular motor recordings are disregarded. Clinically evident disconjugacy, however, should be noted when describing nystagmus attributes. Most nystagmus is largely conjugate and should be presumed so unless specifically noted to be otherwise.
-
3.
Velocity: Slow-phase velocity (measured in degrees per second) is the most useful measurement variable for quantifying the intensity of nystagmus. It is the standard descriptor when quantifying spontaneous or evoked nystagmus using oculographic recordings [56, 73, 85, 143]. Though velocity can be described qualitatively at the bedside, it is generally assessed indirectly by combining frequency and amplitude (see below), since it is difficult to estimate slow-phase velocity by inspection alone.
-
4.Waveform: The nystagmus “waveform” is the oscillatory appearance of nystagmus on an oculographic trace (Fig. 2). It reflects changes in nystagmus velocity (and direction) over time (unlike trajectory, which reflects changes in eye position over time). Aspects of the waveform, such as pendular vs. jerk nystagmus, are readily evident to visual inspection at the bedside, while other aspects (e.g., increasing or decreasing velocity during the slow phase of jerk nystagmus) may be difficult to appreciate.
-
a.Pendular nystagmus consists of back and forth slow-phase oscillations that are often approximately sinusoidal in an oculographic trace.
-
b.Jerk nystagmus consists of a slow phase followed by a fast phase. The slow phase can have a constant velocity (linear “sawtooth” appearance), sometimes called “ramp” or “ramp-like”, decreasing velocity, or increasing velocity waveform in oculographic recordings.
-
a.
-
5.
Frequency: Though measuring beats per second is generally of little value in characterizing vestibular nystagmus, describing the nystagmus frequency (in cycles per second or Hertz) is useful in certain forms of nystagmus such as oculopalatal tremor, oculomasticatory myorhythmia, and the monocular vertical oscillations associated with loss of vision in one eye (Heimann-Bielschowsky phenomenon), which have characteristically low frequencies of 2 Hz or less. Qualitative descriptions of higher-frequency (e.g., >3–5 Hz) eye movements are generally unhelpful, since it is virtually impossible to accurately differentiate these by visual inspection alone. Generally, the oscillations of nystagmus are rhythmic even if their frequency varies somewhat beat-to-beat over time or in different gaze positions. This differs from saccadic oscillations (e.g., opsoclonus), which are often non-rhythmic and irregular in their frequency.
-
6.
Amplitude: Jerk nystagmus amplitude refers to the magnitude (in degrees) of nystagmus from the base of a nystagmus beat to its peak (i.e. the distance the eye rotates during an individual slow phase). While this attribute may be described qualitatively at the bedside, amplitude in isolation provides little useful information since both small- and large-amplitude nystagmus may have the same velocity if the frequencies are different. For example, a small-amplitude (sometimes called “fine”) nystagmus with high frequency may have the same velocity as a large-amplitude (sometimes called “coarse”) nystagmus with low frequency.
-
7.
Intensity: The mathematical product of amplitude and frequency is velocity. Qualitatively, when velocity cannot be measured directly, velocity is reflected by what is sometimes called the intensity of the nystagmus, which may be gauged clinically by considering amplitude and frequency together. Intensity is often reported clinically by the boldness of arrows drawn in nystagmus diagrams (see Fig. 1).
-
8.
Temporal profile: If the nystagmus is intermittent rather than continuous or if it changes characteristics over time, the temporal profile should be described. In addition to the duration and frequency of episodes, temporal features may include changes in direction over time (e.g., as in periodic alternating nystagmus or recovery nystagmus), damping (i.e., decaying after a period of time), crescendo-decrescendo profile (e.g. in nystagmus of benign paroxysmal positional vertigo), or fatiguing (i.e., habituating or becoming less intense with repeated provocative maneuvers).
-
9.
Age of first appearance: Generally, nystagmus is referred to as congenital or infantile if present since birth or early life and acquired if it develops at some later age. This distinction may be challenging at times, particularly if the nystagmus is subtle or only appears under certain conditions of visual fixation (e.g., latent nystagmus), in which case it might simply not have been recognized in early life. Nevertheless, knowing when nystagmus first appeared often helps identify the underlying cause.
Fig.2.
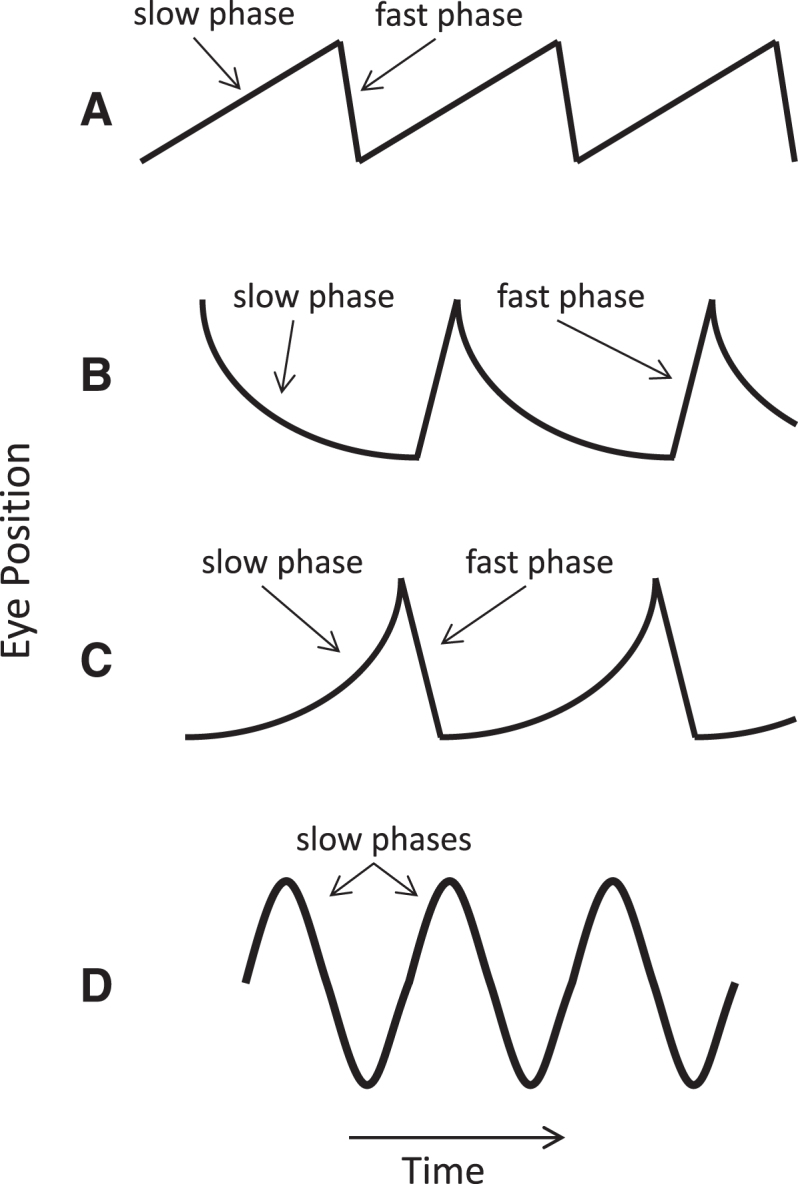
Common nystagmus slow-phase waveforms. (A) Constant velocity (linear) waveform, with added fast phases producing a sawtooth appearance characteristic of vestibular or cerebral hemispheric disease. (B) Decreasing velocity waveform with a negative exponential time course typical of pathologic gaze-evoked nystagmus from an impaired neural integrator. (C) Increasing velocity waveform suggesting an unstable neural integrator. (D) Pendular nystagmus, consisting of only slow phases. Adapted from Leigh and Zee [109].
6. Influencing factors
Nystagmus trajectory and other attributes may be influenced by several factors:
6.1. Gaze positions
Nystagmus characteristics can be affected by eye position within the orbit. The presence, intensity, and direction of nystagmus should be evaluated in the nine cardinal gaze positions (center, right, left, up, down, and the four oblique eccentricities) as well as in convergence. The vantage point of the examiner relative to the direction in which the subject is looking (the subject’s line of sight) can influence the apparent directional components of the nystagmus. Consider spontaneous jerk nystagmus from a peripheral vestibular cause: Such vestibular nystagmus has a fixed plane and axis of rotation relative to one or more semicircular canals (a head-referenced frame [144]). Thus, the apparent eye rotation trajectory (the clinical appearance of the horizontal, vertical, and torsional components) referenced from the subject’s line of sight may shift depending on the position of the eye in the orbit. This effect is most profound with gaze positions that cause the globe to rotate around an axis perpendicular to the head-referenced plane of motion. For example, horizontal vestibular nystagmus in straight-ahead gaze appears to develop a torsional component in far up or down gaze positions if described from the vantage point of the subject’s line of sight, an eye-referenced frame, despite the fact that the eyes are still rotating around the same yaw (vertical) axis in a head-referenced frame. Conversely, infantile nystagmus that appears horizontal in straight-ahead gaze typically still appears horizontal in upward gaze to the examiner when viewed in an eye-referenced (visual axis) coordinate system. This means that the axis around which the eye is rotating relative to the head has changed from the original yaw axis, and if now described from a head-referenced straight-ahead viewpoint the nystagmus would have acquired a torsional component even though it remains horizontal relative to the visual axis. Clinicians must be careful not to misinterpret these small trajectory shifts with respect to orbital eye position as meaningful alterations in the nystagmus characteristics, though their presence or absence may be helpful in determining the origin of the nystagmus (e.g., from the semicircular canals or their projections when the nystagmus is fixed to a head-referenced plane or from other central structures when the nystagmus is fixed to an eye-referenced plane).
6.2. Visual fixation
Visual fixation can influence the characteristics of nystagmus including its presence or absence, intensity, and direction. Conditions of visual fixation include:
-
1)
Binocular fixation—both eyes open viewing a target.
-
2)
Monocular occlusion (monocular fixation)—fixation is blocked in one eye while the other is fixating.
-
3)
Binocular occlusion (visual fixation blocked)—fixation is blocked in both eyes.
It is important to remember that the ability to visually fixate is influenced by baseline visual acuity (a blind eye cannot fixate even if the eye is open and not occluded) and developmental conditions such as alternating esotropia where fixation switches between eyes. Thus, under these circumstances, “both eyes open and not occluded” does not necessarily imply a state of “binocular fixation”. When problems with visual acuity or fixation are present, the examiner should carefully note the state of fixation during the eye movement exam.
Methods of blocking or preventing fixation partially or completely include the following:
-
1)
Closing or covering one eye.
-
2)
Occlusive ophthalmoscopy—observing one optic disc with an ophthalmoscope for nystagmus or saccadic intrusions while the other eye alternately fixates or is covered [166].
-
3)
Penlight-cover test—shining a penlight directly into one eye to “blind it” from fixating and observing that eye while covering and then uncovering the other eye (or occluding one eye while intermittently shining a penlight into the fellow eye by swinging the light into and out of the visual axis of that eye) [121].
-
4)
Ganzfeld technique—staring at a large featureless field of uniform color (e.g., plain white paper).
-
5)
Frenzel goggles—internally-illuminated high-diopter lenses preventing fixation while providing the examiner a magnified view of the eyes.
-
6)
Darkness (examining with oculographic equipment such as infrared video goggles or electro-oculography).
-
7)
Eyelid closure (observing movement of the corneal bulge under lids or with oculographic equipment).
Note that attempted visual fixation may influence eye movements even in total darkness if patients are asked to imagine a visual target [141].
6.3. Effect of vergence
Some forms of nystagmus may have a convergence-divergence component, especially acquired pendular nystagmus (2.1.3.2.) associated with multiple sclerosis, oculopalatal tremor (2.1.3.2.1.), and oculomasticatory myorhythmia (2.1.3.2.2.). In patients with conjugate forms of nystagmus, note should be made of the effect of convergence on the nystagmus. Convergence often supresses infantile nystagmus (2.1.3.1.). Convergence may accentuate, suppress, or reverse the direction of vertical nystagmus.
6.4. Provocative maneuvers
Some forms of nystagmus may be triggered by natural activities or by provocative maneuvers at the bedside. Discussed in detail in the classification, common triggers include changes in position, sound, Valsalva, headshaking, vibration, and hyperventilation.
Appendix
Classification of nystagmus and nystagmus-like movements
1. PHYSIOLOGIC NYSTAGMUS
1.1 Physiologic end-point nystagmus
1.2 Per-rotational nystagmus
1.3 Post-rotational nystagmus
1.4 Optokinetic nystagmus
1.5 Optokinetic after-nystagmus
1.6 Caloric nystagmus
1.7 Magnetic vestibular stimulation
(MVS)-induced nystagmus
2. PATHOLOGIC NYSTAGMUS
2.1. Spontaneous nystagmus
2.1.1. Spontaneous peripheral vestibular nystagmus
2.1.1.1. Spontaneous peripheral vestibular nystagmus, inhibitory type
2.1.1.2. Spontaneous peripheral vestibular nystagmus, excitatory type
2.1.1.3. Recovery nystagmus
2.1.2. Spontaneous central vestibular nystagmus
2.1.2.1. Predominantly horizontal central vestibular nystagmus
2.1.2.1.1. Direction-fixed horizontal central vestibular nystagmus
2.1.2.1.2. Periodic alternating nystagmus
2.1.2.1.3. Latent nystagmus
2.1.2.2. Predominantly vertical or torsional central vestibular nystagmus
2.1.2.2.1. Downbeat nystagmus
2.1.2.2.2. Upbeat nystagmus
2.1.2.2.3. Torsional nystagmus
2.1.3. Other spontaneous central nystagmus forms
2.1.3.1. Infantile nystagmus
2.1.3.2. Acquired pendular nystagmus
2.1.3.2.1. Oculopalatal tremor
2.1.3.2.2. Oculomasticatory myorhythmia
2.1.3.3. Seesaw nystagmus
2.1.3.4. Epileptic nystagmus
2.1.3.5. Pursuit-paretic nystagmus
2.2. Gaze-evoked nystagmus
2.2.1. Gaze-holding nystagmus (unilateral, bilateral, vertical)
2.2.2. First degree vestibular nystagmus
2.2.3. Vestibular plus gaze-holding nystagmus
2.2.4. Rebound nystagmus
2.2.5. Centripetal nystagmus
2.3. Triggered nystagmus
2.3.1. Positional nystagmus
2.3.1.1. Benign paroxysmal positional nystagmus (BPPN)
2.3.1.1.1. Posterior semicircular canal BPPN
2.3.1.1.2. Horizontal semicircular canal BPPN
2.3.1.1.2.1. Pseudo-spontaneous nystagmus
2.3.1.1.3. Anterior semicircular canal BPPN
2.3.1.2. Other forms of peripheral positional nystagmus
2.3.1.3. Central positional nystagmus
2.3.2. Headshaking-induced nystagmus
2.3.3. Cross-coupled nystagmus
2.3.4. Sound-induced nystagmus
2.3.5. Valsalva-induced nystagmus
2.3.6. Pressure-induced nystagmus
2.3.7. Vibration-induced nystagmus
2.3.8. Hyperventilation-induced nystagmus
2.3.9. Pursuit-induced nystagmus
3. NYSTAGMUS-LIKE MOVEMENTS
3.1. Saccadic intrusions and oscillations
3.1.1. Square-wave jerks
3.1.2. Macrosaccadic oscillations
3.1.3. Saccadic pulses
3.1.4. Ocular flutter
3.1.5. Opsoclonus
3.1.6. Voluntary saccadic oscillations
3.2. Other nystagmus-like movements
3.2.1. Convergence-retraction nystagmus
3.2.2. Ocular oscillations in spasmus nutans
3.2.3. Ocular bobbing and its variants
3.2.4. Superior oblique myokymia
3.2.5. Ping-pong gaze
3.2.6. Pendular pseudonystagmus
Nystagmus can be classified in many different ways. Any one type of nystagmus might be classified differently depending on the context or scheme being used (anatomically-based, direction-based, physiologic vs. pathologic, jerk vs. pendular, peripheral vs. central, congenital vs. acquired). No classification scheme perfectly accommodates every form of nystagmus. Since the primary goal of this document is to establish uniform definitions for the examination of and types of nystagmus in order to facilitate communication for research and clinical care, we attempted to categorize the different pathologic forms of nystagmus phenomenologically as much as possible, based on whether they are present spontaneously in the upright straight-ahead gaze position, evoked by change in gaze position, or induced only by specific triggers. Within that construct, it became unavoidable to mix terms that are defined by different characteristics such as direction (downbeat nystagmus), presumed localization (vestibular nystagmus), or etiology (epileptic nystagmus) in order to include all of the relevant forms. Video simulation examples from patient eye movement recording data have been created to illustrate selected nystagmus types and will be expanded as data become available. Videos may be found at http://www.jvr-web.org/ICVD.html.
1. Physiologic nystagmus: Nystagmus occurring in normal individuals in the absence of pathology as part of natural behavior or in response to a physiologic stimulus.
1.1. Physiologic end-point nystagmus: Gaze-evoked nystagmus in the absence of pathology, attributed to normal variation in gaze-holding ability.
Comment: Physiologic end-point nystagmus is typically seen with extreme lateral gaze or occasionally upgaze and is generally low amplitude, low frequency, binocular, symmetric in right and left gaze, poorly sustained (damps within a few seconds), and unassociated with other ocular motor or neurological abnormalities [8, 49, 138]. It may occasionally be sustained, asymmetric, or slightly dissociated [138]. Absent baseline oculographic or video recordings before illness onset, it may not always be possible to differentiate physiologic end-point nystagmus from subtle, newly acquired pathologic gaze-evoked nystagmus.
Terms not recommended: End-gaze nystagmus; extreme-gaze nystagmus
1.2. Per-rotational nystagmus: Nystagmus occurring during sustained head and body rotation, with fast phases in the direction of rotation.
Comment: In darkness with rapid acceleration to a constant-velocity rotation, this vestibular nystagmus is greatest at the start of rotation, with the slow-phase velocity exponentially declining as the cupula returns to its resting position. In the light with a view of the environmental surround, per-rotational nystagmus is a combination of vestibular and optokinetic nystagmus, with the vestibular component exponentially decaying during constant-velocity rotation.
Term not recommended: per-rotatory nystagmus
1.3. Post-rotational nystagmus: Vestibular nystagmus triggered by suddenly stopping after sustained rotation.
Comment: Fast phases are directed opposite the original direction of rotation. Such a stimulus is the equivalent of an impulse of acceleration in the opposite direction of the sustained rotation.
Term not recommended: post-rotatory nystagmus
1.4. Optokinetic nystagmus: Nystagmus induced by a moving full-field visual stimulus either during sustained self-rotation in the light or by the visual stimulus rotating around the subject. Slow phases are in the direction of visual motion and can be horizontal, vertical, or torsional depending on the stimulus.
Comment: Circularvection refers to the subject’s compelling sensation of self-rotation induced by a sustained rotating visual stimulus. Translational vection refers to the subject’s sensation of linear self-motion induced by a translational optic flow stimulus (e.g., watching a passing train or handheld optokinetic tape or drum). With circularvection, the smooth pursuit system contributes to the initial ocular following response at the onset of optokinetic nystagmus, but the optokinetic system takes over with a sustained rotating stimulus. By contrast, with translational vection, the eye movement kinematics are primarily consistent with activation of the pursuit system alone [118, 119, 149].
1.5. Optokinetic after-nystagmus: Nystagmus that persists in the same direction as optokinetic nystagmus in darkness after the optokinetic stimulus has ceased.
Comment: Such nystagmus persists for seconds with declining slow-phase velocity and is attributed to the vestibular velocity storage mechanism.
1.6. Caloric nystagmus: Vestibular nystagmus induced by irrigation of water or insufflation of air against the tympanic membrane that is different from body temperature.
Comment: Conventionally, irrigation temperatures of 7°C away from body temperature (30°C and 44°C) or ice water are used in the laboratory. When supine, a cold caloric produces a predominantly horizontal nystagmus with fast phases directed opposite the side of the stimulus. A warm caloric produces a predominantly horizontal nystagmus with fast phases directed toward the side of the stimulus.
1.7. Magnetic vestibular stimulation (MVS)-induced nystagmus: Vestibular nystagmus induced by the effect of a strong magnetic field on the labyrinth.
Comment: A strong static magnetic field, such as within an MRI scanner, induces nystagmus in subjects with normal vestibular function that is proportional to the magnetic field strength and whose direction is dependent on magnetic field polarity and head orientation. This nystagmus has been attributed to Lorentz forces from interaction between the magnetic field and naturally occurring ionic currents in the endolymph fluid acting to push the semicircular canal cupula to a new position [81, 133, 160].
2. Pathologic nystagmus: Nystagmus due to disease affecting one or more ocular motor systems.
Comment: Pathologic nystagmus generally results from disruption of one or more mechanisms that normally hold gaze steady—disturbance of the vestibulo-ocular reflexes, failure or instability of the mechanism for gaze-holding (the neural integrator), or disorders of the visual pathways that impair the ability to suppress eye drifts during attempted fixation. Such disturbances may be acquired or may be due to congenital or early developmental abnormalities.
2.1. Spontaneous nystagmus: Nystagmus present while looking in the straight-ahead (center) gaze position with the head stationary in the upright and neutral position (not turned or tilted), not triggered by positional or other provocative maneuvers.
Comment: Spontaneous nystagmus is typically also present, but may be modified (e.g., increased or decreased in intensity), in one or more other gaze positions. Note that some forms of vestibular pathology (particularly horizontal canal cupulolithiasis) may produce nystagmus in the straight-ahead gaze position referred to as “pseudo-spontaneous” (2.3.1.1.2.1.). Such nystagmus should disappear by pitching the head forward 30° to place the horizontal canal in the horizontal plane with respect to gravity and should reverse directions by pitching forward even further.
2.1.1. Spontaneous peripheral vestibular nystagmus: Spontaneous jerk nystagmus due to an imbalance in vestibular tone between the labyrinths or vestibular nerves.
Comment: Spontaneous peripheral vestibular nystagmus should have the following characteristics: 1) binocular and conjugate in head-referenced coordinates; 2) beats in a single plane and direction in head-referenced coordinates, regardless of gaze position; 3) obeys Alexander’s law [134], 4) suppressed by visual fixation (enhanced by blocking fixation), and 5) constant-velocity slow phases if recorded by oculographic equipment.
Spontaneous peripheral vestibular nystagmus should be direction-fixed, i.e. it should beat in only one direction (in head-referenced coordinates) rather than change directions in different gaze positions. It usually results from acute structural or physiologic lesions of the peripheral vestibular system affecting the labyrinth, vestibular ganglion, or vestibular nerve. The nystagmus trajectory should align with the approximate plane of the affected semicircular canal(s) or its/their afferent connections. When a horizontal canal or its afferents are involved (as is often the case), in straight-ahead gaze the nystagmus usually appears predominantly horizontal with additional small torsional and/or vertical components (depending on what additional structures are affected, if any).
Spontaneous horizontal-torsional nystagmus (in straight-ahead gaze) is expected in a unilateral process damaging the entire vestibular labyrinth, all three semicircular canals, or their afferents. This is because the vertical contributions to the slow phases of nystagmus from the anterior and posterior canals of the intact side cancel one another, leaving unopposed horizontal and torsional vestibular slow phases toward the lesioned side (see Fig 3). Though most commonly resulting from a peripheral vestibular lesion, the same horizontal-torsional vestibular nystagmus may result from a lesion affecting the intrapontine vestibular nerve fascicles [129], vestibular nucleus [92], or cerebellar structures modulating resting vestibular tone [103]. Sometimes the torsional component may be difficult to appreciate, making the nystagmus appear predominantly horizontal as from a process affecting only the horizontal canal or its afferents. Such horizontal nystagmus must be distinguished from pseudo-spontaneous nystagmus (2.3.1.1.2.1.), infantile nystagmus (2.1.3.1.), or rare central causes such as Chiari malformation [17] and observed long enough to exclude periodic alternating nystagmus (2.1.2.1.2.).
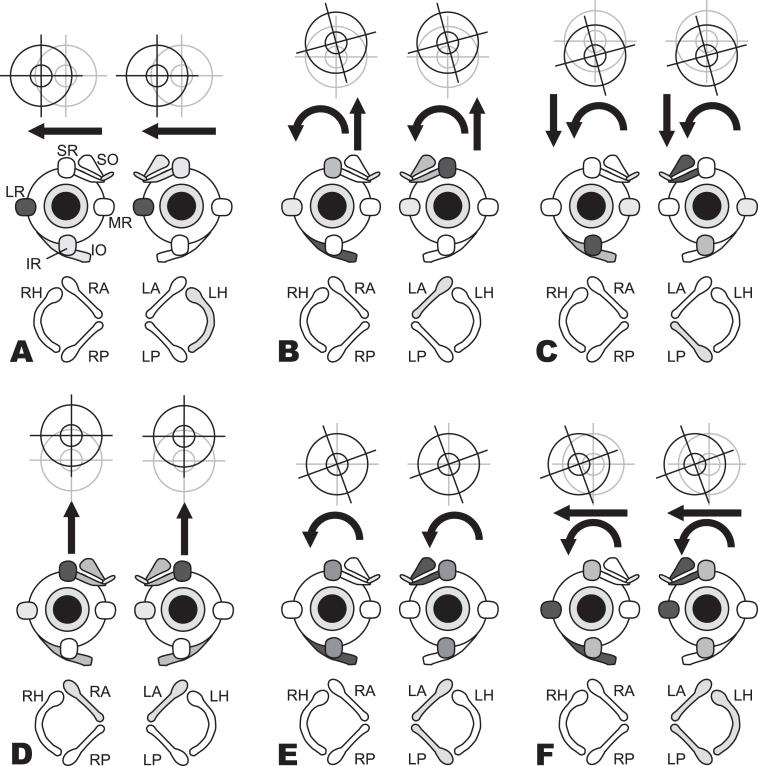
Fig.3.
Nystagmus slow phases observed for excitation of individual semicircular canals. In the bottom row of each panel (A through F), shading indicates the excited canals. In the second row, a diagram of the extraocular muscles depicts which muscles are activated (darker shading indicates stronger activation). In the top row, the resultant yaw, pitch, and/or roll eye movements are indicated. (A) Excitation of the left horizontal (LH) canal causes rightward slow phases mainly as a result of strong activation of right lateral rectus (LR) and left medial rectus (MR). (B) Excitation of the left anterior (LA) canal causes upward/clockwise (from patient’s perspective) slow phase because of the combined action of the right inferior oblique (IO) and superior rectus (SR) and the left superior oblique (SO) and SR. (C) Excitation of the left posterior (LP) canal causes downward/clockwise (from patient’s perspective) slow phases as a result of the combined action of the right IO and inferior rectus (IR) and the left SO and IR. (D) Combined equal excitation of both the left anterior (LA) and right anterior (RA) canals activates bilateral SR and oblique muscles and causes purely upward slow phases since the torsional components from each canal cancel each other. (E) Combined equal excitation of left anterior (LA) and left posterior (LP) canals excites muscle activity that is the sum of each canal’s individual effect; upward and downward pulls cancel, which results in a purely torsional nystagmus. (F) Combined equal excitation of all three left canals causes a right-clockwise (from patient’s perspective) slow phase, the expected result of summing activity for each individual canal. (Modified from Cohen et al [37]; adapted from Cummings Otolaryngology: Head and Neck Surgery. Flint, Haughey (eds.). Sixth edition [61]. Chapter 163: Principles of applied vestibular physiology. Carey JP, Della Santina CC. ISBN: 978-1-4557-4696-5. Data adjusted to human head frame of reference.)
Spontaneous vertical-torsional nystagmus occurs in conditions affecting one vertical (anterior [superior] or posterior [inferior]) semicircular canal or its afferents. For example, isolated inferior vestibular neuritis produces torsional-downbeat nystagmus due to dysfunction of the afferents from the posterior canal [95]. Spontaneous pulse-synchronous torsional or vertical-torsional nystagmus has been described in superior canal dehiscence syndrome [44, 66, 151, 165].
Spontaneous horizontal-vertical-torsional nystagmus is seen most often in superior division vestibular neuritis, where dysfunction in the horizontal canal afferents contributes to the horizontal component and dysfunction in the anterior canal afferents contributes to the torsional and small upbeat components [52, 163]. Note, however, that a similar nystagmus trajectory can occur in central disorders such as the lateral medullary (Wallenberg) syndrome [14].
The nystagmus intensity is dictated by the degree of asymmetry in vestibular tone. Alexander’s law describes how the intensity of peripheral vestibular nystagmus varies predictably in different gaze positions relative to the direction the nystagmus is beating—greatest when looking in the direction of the fast phases and least when looking in the direction of the slow phases. Three “degrees” of nystagmus can thus be defined: first degree vestibular nystagmus is present only when looking toward the fast phase direction; second degree vestibular nystagmus is also present in the straight-ahead gaze position; and third degree vestibular nystagmus is also present when looking toward the slow phase direction. Note that first degree vestibular nystagmus (2.2.2.) in this nomenclature is not truly a form of ‘spontaneous’ vestibular nystagmus since it is not present in straight-ahead gaze and may not always be distinguishable from unilateral gaze-holding nystagmus (2.2.1.). Although the three ‘degrees’ are sometimes used to describe vertical nystagmus [57], they are more typically used to describe predominantly horizontal nystagmus [45].
Central lesions (e.g., stroke) in the brainstem or cerebellum may sometimes produce spontaneous, predominantly horizontal vestibular nystagmus that is indistinguishable from the nystagmus of acute peripheral vestibulopathies. Other times, however, central lesions produce nystagmus that differs in one or more of the features described above. Acute spontaneous jerk nystagmus that does not follow these characteristics precisely (e.g., changes direction in different gaze positions or is purely vertical or torsional) has been associated with central rather than peripheral localization [89, 146]. The term direction-changing horizontal nystagmus [89, 120, 146] has been applied in this context to refer to spontaneous jerk nystagmus in straight-ahead gaze that changes directions in different gaze positions (though the term bilateral gaze-holding nystagmus (2.2.2). should be applied when no nystagmus exists in straight-ahead gaze and a pathologic horizontal nystagmus is present in both right and left gaze). Spontaneous peripheral vestibular nystagmus may co-exist with bilateral gaze-holding nystagmus in conditions simultaneously causing an imbalance of vestibular tone as well as impaired gaze holding (see 2.2.3.) or following central adaptation to acute unilateral loss of peripheral vestibular function [134].
2.1.1.1. Spontaneous peripheral vestibular nystagmus, inhibitory type: Spontaneous peripheral vestibular nystagmus whose vestibular tone asymmetry is due to a unilateral reduction or loss of vestibular function.
Comment: The direction of the linear slow phases in vestibular nystagmus is determined by the imbalance in tonic neural activity at the level of the vestibular nuclei. If a process reduces unilateral vestibular tone, activity from the vestibular nuclei on the unaffected side will drive the eyes in a slow phase drift toward the affected side, and the nystagmus will beat away from the affected side of reduced function. The same is true for triggered forms of vestibular nystagmus. Though spontaneous peripheral vestibular nystagmus is most often inhibitory (with examples of exceptions being during attacks of Menière’s disease or vestibular paroxysmia), one generally cannot determine whether the nystagmus is inhibitory or excitatory from its characteristics alone; this must generally be inferred based on other clinical (e.g., side of hearing loss) or radiographic (e.g., lesion side by imaging) features, or using knowledge of the underlying pathophysiology.
Previous term: Paretic nystagmus
2.1.1.2. Spontaneous peripheral vestibular nystagmus, excitatory type: Spontaneous peripheral vestibular nystagmus whose vestibular tone asymmetry is due to a unilateral increase vestibular activity.
Comment: The direction of the linear slow phases in vestibular nystagmus is determined by the imbalance in tonic neural activity at the level of the vestibular nuclei. If a process increases unilateral vestibular tone, activity from the vestibular nuclei on that side will drive the eyes in a slow phase drift away from the affected side, and the nystagmus will beat toward the affected side of increased activity (Fig. 3). The same is true for triggered forms of vestibular nystagmus. Spontaneous peripheral vestibular nystagmus of the excitatory subtype is most often seen in Menière’s disease or vestibular paroxysmia.
Previous term: Irritative nystagmus
2.1.1.3. Recovery nystagmus: Spontaneous peripheral vestibular nystagmus that has reversed direction after a period of time (usually hours or days, depending on the cause) and is attributed to recovery from an underlying vestibular disorder causing an initial inhibitory nystagmus.
Comment: Recovery nystagmus results from the persistence of a degree of central compensation for an initial imbalance in vestibular tone after the need for this amount of compensation is lessened or absent. Recovery nystagmus primarily occurs when the initial disease phase is sustained, often for hours or days, and the recovery is rapid (minutes to hours, perhaps days). It does not generally occur when nystagmus is very brief or paroxysmal. Examples of recovery nystagmus include attacks of M nière’s disease in which nystagmus beating away from the affected labyrinth may be followed by a “recovery” nystagmus beating toward the affected labyrinth [69, 114] and transient reversal of nystagmus following MVS-induced nystagmus (1.7) [133]. A triggered form of recovery nystagmus can occur in vestibular schwannoma when hyperventilation (2.3.8.) induces a nystagmus beating ipsilesionally, attributed to transient improvement of axonal conduction in a partially demyelinated vestibular nerve [34]. In a similar way, if a unilateral labyrinthine lesion is followed later by destruction of the second labyrinth, nystagmus occurs as if the originally damaged labyrinth were intact. This Bechterew’s phenomenon occurs because after a rebalancing of central vestibular tone following the first lesion, the second lesion creates a new imbalance reflecting the prior adaptive mechanisms [168].
2.1.2. Spontaneous central vestibular nystagmus: Spontaneous jerk nystagmus due to dysfunction of the central nervous system circuits that contribute to the vestibulo-ocular reflexes or adaptive control of these reflexes.
Comment: Spontaneous central vestibular nystagmus may have any trajectory. In some cases, the central nature of the nystagmus is apparent because the trajectory cannot be readily explained by common forms of peripheral vestibular dysfunction affecting one or more semicircular canals or their afferents (e.g., purely downbeat, upbeat, or torsional nystagmus). In other cases, the central nature of the disorder is only obvious because of additional nystagmus attributes, accompanying central ocular motor or neurologic signs, or the overall clinical context.
2.1.2.1. Predominantly horizontal central vestibular nystagmus: Spontaneous central vestibular nystagmus that is predominantly horizontal in the straight-ahead gaze position.
2.1.2.1.1. Direction-fixed horizontal central vestibular nystagmus: Spontaneous central vestibular nystagmus that is predominantly horizontal and remains direction-fixed in the straight-ahead gaze position.
Comment: In acute vertigo syndromes, central vestibular nystagmus often has a predominantly horizontal trajectory (pure or mixed horizontal-torsional), so it can be easily mistaken for spontaneous peripheral vestibular nystagmus (2.1.1.) normally seen with vestibular neuritis or other conditions [89, 104]. Most commonly this is due to cerebellar or brainstem stroke [89, 105]. In this setting, other clues to a central disorder are a normal vestibulo-ocular reflex, gaze-evoked nystagmus (2.2.1), skew deviation (usually a central sign) [146], impaired vertical smooth pursuit, or impaired suppression of the vestibulo-ocular reflex. Allowing or eliminating visual fixation usually does not influence the slow phase velocity to the same degree that it does with spontaneous peripheral vestibular nystagmus [104, 120]. Head shaking or positional changes may cause the nystagmus to reverse direction, but it does not reverse spontaneously (as with periodic alternating nystagmus 2.1.2.1.2) or under different visual fixation states (as with latent nystagmus 2.1.2.1.3) [76].
2.1.2.1.2. Periodic alternating nystagmus: Conjugate binocular horizontal jerk nystagmus that spontaneously reverses direction, usually every 90 to 120 seconds.
Comment: Downbeat nystagmus and square-wave jerks may become more apparent during the brief null phase when the horizontal nystagmus reverses direction. Acquired periodic alternating nystagmus (PAN) is most commonly associated with dysfunction of the cerebellar nodulus or uvula [63, 82]. Congenital PAN reverses direction with less regular timing than the acquired form and often has accelerating slow-phase waveforms more typical of infantile nystagmus (2.1.3.1.) [64]. If the condition causing PAN also impairs the brainstem mechanism for generating fast phases, patients may develop periodic alternating gaze deviation, consisting of horizontal conjugate deviation of the eyes alternating right and left approximately every 2 minutes. This latter condition differs from ping-pong gaze (3.2.5., which also occurs without saccades), in which there is a continuous, conjugate side-to-side movement of the eyes with a frequency of approximately 0.25 Hz (too low to be considered pendular nystagmus) [79]. Windmill nystagmus may be considered a rare variant of periodic alternating nystagmus in which a periodic alternating horizontal nystagmus has a superimposed periodic alternating vertical nystagmus with the periods of the two oscillations being 90 degrees out of phase, producing a clock-like rotation of the beating direction. Windmill nystagmus and its variants are described in patients with prolonged visual loss, suggesting that impairment in gaze-stabilizing networks as well as velocity-storage mechanisms important for short-term adaptation could explain why the periodicity is less regular in windmill nystagmus than in typical PAN [35].
2.1.2.1.3. Latent nystagmus: Conjugate horizontal jerk nystagmus absent during binocular viewing that appears with monocular occlusion. Fast phases of both eyes beat towards the side opposite the covered eye.
Comment: Latent nystagmus is a visuo-vestibular disorder associated with strabismus (usually esotropia), amblyopia, and dissociated vertical deviation (also called alternating sursumduction, in which the covered eye elevates) as a part of the fusional maldevelopment nystagmus syndrome [2, 26]. Manifest latent nystagmus is more common than true latent nystagmus and is present during binocular viewing, though it may increase in intensity or reverse direction with monocular occlusion or even with attempting to look out of one eye or the other. Like latent nystagmus, manifest latent nystagmus typically occurs with congenital strabismus and failed development of binocular vision (amblyopia). Thus, even without monocular occlusion, vision in one eye may be impaired sufficiently to produce a functional occlusion and “manifest” latent nystagmus. Occasionally patients can release and suppress their latent nystagmus at will [98]. Latent nystagmus slow phases have a constant or decreasing velocity waveform and usually follow Alexander’s law.
Term not recommended: Occlusion nystagmus
2.1.2.2. Predominantly vertical or torsional central vestibular nystagmus: Spontaneous central vestibular nystagmus that is predominantly vertical or torsional in the straight-ahead gaze position.
2.1.2.2.1. Downbeat nystagmus: Spontaneous central vestibular nystagmus that is predominantly downbeating in the straight-ahead gaze position.
Comment: Downbeat nystagmus is typically due to vestibulocerebellar dysfunction. It commonly increases in lateral and downward gaze or might only become evident in lateral gaze and is often accompanied by bilateral gaze-holding nystagmus (2.2.1.). Slow phases are commonly linear and obey Alexander’s law (least intense in upgaze, most in downgaze), though sometimes downbeat nystagmus is greatest in upgaze and associated with increasing-velocity slow phases. It is usually poorly suppressed by visual fixation. It may increase, suppress, or convert to upbeat nystagmus with convergence or with adopting a supine or prone head position (central positional nystagmus 2.3.1.3.). It may also increase after vigorous horizontal or vertical headshaking (2.3.2.) or hyperventilation (2.3.8.). There may be an associated divergence-beating component.
2.1.2.2.2. Upbeat nystagmus: Spontaneous central vestibular nystagmus that is predominantly upbeating in the straight-ahead gaze position.
Comment: Upbeat nystagmus in straight-ahead gaze is less common and less well localizing than downbeat nystagmus but most often occurs with lesions of the paramedian medulla. It does not typically increase in lateral gaze but may be influenced by convergence. It often obeys Alexander’s law (increasing in upgaze) but may instead increase in downgaze and have linear-, increasing-, or decreasing-velocity waveforms. Like downbeat nystagmus, it may be influenced by head position but generally not by visual fixation. Upbeat nystagmus sometimes has a small horizontal component that causes an oblique upward fast phase alternating to the right and left, creating the trajectory of bowtie nystagmus [33].
2.1.2.2.3. Torsional nystagmus: Spontaneous central vestibular nystagmus that is predominantly torsional in the straight-ahead gaze position.
Comment: Purely torsional nystagmus in straight-ahead gaze is usually due to disease affecting central vestibular pathways, most often from medullary or midbrain lesions [113]. It may accompany the ocular tilt reaction or internuclear ophthalmoplegia. It may also be triggered by vertical pursuit in lesions of the middle cerebellar peduncle (2.3.9.) [60]. A predominantly torsional-appearing nystagmus can also occur because fixation mechanisms are more effective at suppressing the horizontal and vertical components of peripheral vestibular nystagmus.
2.1.3. Other spontaneous central nystagmus forms: Spontaneous nystagmus due to central nervous system dysfunction that can have either pendular or jerk waveforms but is not necessarily due to impairment of central vestibular circuits.
2.1.3.1. Infantile nystagmus: Conjugate horizontal nystagmus present at birth or developing during infancy.
Comment: The infantile nystagmus syndrome consists of conjugate, mainly horizontal nystagmus (remaining horizontal in eye-referenced coordinates in upward or downward gaze) with coexisting increasing-velocity jerk and pendular waveforms, each punctuated by brief foveation periods during which the eyes can transiently fixate on an object of interest. Infantile nystagmus may be accentuated by visual attention or arousal and suppressed by convergence, inattention, eye closure, or sleep. The amplitude, frequency, and waveform can vary with eye position, typically increasing on lateral gaze (right-beating in right gaze, left-beating in left gaze) but diminishing in intensity in a null zone, leading individuals to adopt a head turn that minimizes the nystagmus during fixation on a target. Inversion of optokinetic responses is commonly observed, with quick phases in the same direction as a moving hand-held optokinetic tape or drum [70]. Infantile nystagmus may occur in the setting of other visual sensory disorders or with a normal visual system [2].
Previous term: Congenital nystagmus. Though the term congenital nystagmus is entrenched in the literature, infantile nystagmus better reflects the fact that it develops more often between 8 and 12 weeks or later rather than being present at birth. Some prefer the more descriptive name “infantile-onset” nystagmus to indicate that the syndrome generally persists into adulthood, but the committee felt infantile nystagmus was too well established as a term.
2.1.3.2. Acquired pendular nystagmus: Pendular nystagmus developing after infancy that may have horizontal, vertical, and torsional components.
Comment: The amplitude and phase relationship determines the nystagmus trajectory, which may be oblique, elliptical, or circular. Horizontal and vertical components can be in phase, resulting in a diagonal trajectory, or 90 degrees out of phase, resulting in a circular trajectory (if the amplitudes of horizontal and vertical movements are the same) or an elliptical trajectory (if the amplitude of the horizontal and vertical components differ). A phase difference of 180 degrees between the two eyes produces a disjunctive nystagmus in which the two eyes move in opposite directions: if horizontal, a convergent-divergent nystagmus; if torsional, a cyclovergent nystagmus (both eyes intort simultaneously); and if mixed vertical-torsional, a seesaw nystagmus. Nystagmus may be monocular or have differing amplitudes (dissociated acquired pendular nystagmus) or trajectories (e.g., disjunctive acquired pendular nystagmus with a vergence component) between the two eyes. Acquired pendular nystagmus associated with multiple sclerosis typically has a higher frequency (>4 Hz) and lower amplitude (<4°) than that associated with oculopalatal tremor [150].
2.1.3.2.1. Oculopalatal tremor: A form of acquired pendular nystagmus characterized most commonly by large amplitude, low frequency (1–3 Hz), and often disconjugate vertical, torsional and horizontal oscillations [96, 150] that may be enhanced by eye closure [112]. The syndrome of oculopalatal tremor includes synchronous movements of the soft palate and sometimes other muscles derived from the same branchial arch.
Comment: The presence of oculopalatal tremor implies dysfunction in the brainstem or cerebellum within the Guillain-Mollaret triangle (the dentato-rubro-olivary tract) and is associated with hypertrophic degeneration of the inferior olivary nucleus. The nystagmus waveform is variable, being less smooth and sinusoidal than the acquired pendular nystagmus typically seen in demyelinating diseases.
Term not recommended: Oculopalatal myoclonus
2.1.3.2.2. Oculomasticatory myorhythmia: A form of disjunctive acquired pendular nystagmus characterized by pendular convergence-divergence oscillations at about 1 Hz often associated with synchronous oscillatory movements of the jaw, face, or limbs.
Comment: Oculomasticatory myorhythmia is generally accompanied by vertical saccadic palsy and has thus far only been described in central nervous system Whipple’s disease [132, 135].
2.1.3.3. Seesaw nystagmus: A disconjugate nystagmus in which one half-cycle consists of a slow phase elevation and intorsion of one eye and synchronous depression and extorsion of the other eye. The next half-cycle consists of slow or fast phases in the opposite direction.
Comment: Seesaw nystagmus occurs in either jerk or pendular form. In jerk seesaw nystagmus, one half-cycle consists of a slow phase elevation and intorsion of one eye and synchronous depression and extorsion of the other eye, while the next half-cycle consists of fast phases in the opposite directions. In pendular seesaw nystagmus, both half-cycles are slow phase movements. Jerk seesaw nystagmus may be due to interruption of otolithic vestibular inputs to the interstitial nucleus of Cajal, which is responsible for vertical and torsional gaze holding, or due to impaired connections from the anterior and posterior semicircular canals on one side to the their oculomotor and trochlear nucleus targets in the midbrain [68, 126]. Pendular seesaw nystagmus has most often been associated with large parasellar tumors compressing the optic chiasm or with other disorders affecting the retina or optic chiasm producing visual loss [40, 42].
Term not recommended for jerk seesaw nystagmus: Hemi-seesaw nystagmus
2.1.3.4. Epileptic nystagmus: Nystagmus attributed to epileptic seizure activity.
Comment: Epileptic seizures can cause conjugate gaze deviations and skew deviation but can also cause nystagmus. Epileptic nystagmus is considered separately here since the localization and appearance are distinct from other forms of nystagmus discussed. It is usually horizontal and conjugate, though monocular or vertical epileptic nystagmus has been described [161]. Temporo-occipito-parietal seizure foci most commonly cause an initial contraversive gaze deviation followed shortly by centripetal drift and contraversive nystagmus fast phases due to epileptic activation of the cortical saccade region [86, 87, 148]. The slow phases are due to centripetal drift from missing activation of the gaze-holding mechanism. However, sometimes the eyes will initially deviate toward the side of the seizure, followed by nystagmus with ipsiversive slow phases, suggesting that activation of pursuit mechanisms at the occipito-temporo-parietal junction is responsible for both the gaze deviation and slow phases, with the nystagmus fast phases generated reflexively [87, 152]. One patient with a left temporo-parietal seizure focus developed vertigo and right-beating nystagmus with linear slow phases but without any preceding gaze deviation, suggesting cortical involvement of the vestibular and pursuit structures [62].
2.1.3.5. Pursuit paretic nystagmus: Low amplitude horizontal jerk nystagmus in response to slow ocular drift due to the marked asymmetry of horizontal smooth pursuit resulting from large cerebral hemispheric lesions.
Comment: Large unilateral hemispheric lesions can cause abnormally high contralesional smooth pursuit gain and low ipsilesional pursuit gain. This can cause slow ocular drift away from the damaged side and pursuit paretic nystagmus fast phases toward the damaged side [139, 141]. This term should not be used to describe the saccadic breakdown of impaired smooth pursuit in which saccades occur in the same direction as the intended pursuit of a moving target.
2.2. Gaze-evoked nystagmus: Jerk nystagmus induced by moving the eye into an eccentric position in the orbit, with fast phases most often beating in the direction of gaze.
Comment: Gaze-evoked nystagmus may be physiologic, indicate peripheral or central vestibular dysfunction, or result from central disturbances of gaze-holding mechanisms, depending on the characteristics. In general, if it is seen only with extreme lateral gaze, is low amplitude, low frequency, binocular, symmetric in right and left gaze, poorly sustained (damps within a few seconds), and unassociated with other ocular motor or neurological abnormalities, it typically represents physiologic end-point nystagmus (1.1.). Occasionally physiologic and pathologic gaze-evoked nystagmus can be difficult to distinguish. If nystagmus is present in the straight-ahead position, then one of the forms of spontaneous nystagmus (2.1.) should be applied, though spontaneous nystagmus may co-exist with gaze-evoked nystagmus (see 2.2.3).
Gaze-evoked nystagmus is usually binocular but may be dissociated or monocular (usually in circumstances where the movement of one eye is pathologically reduced). Dissociated or monocular gaze-evoked nystagmus may be more prominent in the laterally-placed eye (abducting nystagmus) or medially-placed eye (adducting nystagmus). Abducting nystagmus is typically seen when gazing in the direction contralateral to an eye with impaired adduction, as in internuclear ophthalmoplegia, partial third nerve palsy, myasthenia gravis, or restrictive orbitopathy. Adducting nystagmus is typically seen when gazing in the direction toward an eye with impaired abduction, as in sixth nerve palsy, myasthenia gravis, or restrictive orbitopathy. The term gaze-paretic nystagmus should only be applied in cases of gaze-evoked nystagmus associated with paresis of gaze (from brainstem or hemispheric lesions or extraocular muscle weakness such as in myasthenia gravis).
2.2.1. Gaze-holding nystagmus: Pathologic gaze-evoked nystagmus attributed to an impaired neural integrator.
Comment: Pathologic gaze-evoked nystagmus is most often linked to impaired gaze-holding function [11] from lesions in the brainstem and cerebellar networks that control eye position commands (the neural integrator), in which case it may be referred to as gaze-holding nystagmus. This manifests as drift of the eyes back from an eccentric to central position with decreasing-velocity slow-phase waveforms and corrective fast phases of nystagmus. Gaze-holding nystagmus is often simply referred to phenomenologically as gaze-evoked nystagmus, even when the mechanism is known, despite the fact that other types of gaze-evoked nystagmus exist.
Gaze-holding nystagmus may be horizontal, vertical, or both. The nystagmus fast phase generally beats in the direction of gaze, but occasionally may not (e.g., torsional nystagmus elicited by horizontal or vertical eccentric gaze). When it is purely horizontal, the nystagmus may be bilateral (present in both right gaze and left gaze) or unilateral (present in either rightward gaze or leftward gaze but not both). When it is purely vertical, the nystagmus may be present in upward gaze, downward gaze, or both. Rarely downbeat nystagmus is elicited by upward gaze and has increasing velocity slow phases indicating instability of vertical gaze holding [9, 167]. Sometimes downbeat nystagmus is evoked only in lateral gaze, and spontaneous downbeat nystagmus (2.1.2.2.1.) commonly increases looking down and lateral in association with bilateral horizontal gaze-holding nystagmus to produce an oblique appearance.
2.2.2. First degree vestibular nystagmus: Unilateral gaze-evoked nystagmus due to a peripheral or central imbalance of vestibular tone in which nystagmus is present only while looking away from the side of reduced vestibular function but is not present in the straight-ahead gaze position.
Comment: First degree vestibular nystagmus is a consequence of Alexander’s law (see 2.1.1. for discussion). Distinguishing unilateral gaze-holding nystagmus from first degree vestibular nystagmus can be challenging. Gaze-holding nystagmus has a decreasing-velocity slow phase waveform, while vestibular nystagmus has a constant velocity waveform, but this is difficult to appreciate at the bedside. The co-existence of either rebound nystagmus (2.2.4.) or vertical gaze-evoked nystagmus would generally confirm an impaired gaze-holding mechanism, while additional horizontal headshaking-induced nystagmus in the same direction (as well as an abnormal head impulse test to the opposite side) is common in first degree vestibular nystagmus.
2.2.3. Vestibular plus gaze-holding nystagmus: Bilateral gaze-evoked nystagmus occurring from the combination of an imbalance in vestibular tone and impaired gaze-holding mechanisms.
Comment: The vestibular tone imbalance may be due to either peripheral or central vestibular dysfunction. The rare entity Bruns nystagmus classically occurs with a cerebellopontine angle tumor affecting the vestibular nerve and adjacent cerebellum. This mixed nystagmus consists of gaze-evoked nystagmus looking ipsilesionally due to defective gaze holding and first degree peripheral vestibular nystagmus looking contralesionally due to an imbalance in vestibular tone [111, 155]. The vestibular tone imbalance may be sufficient to also produce second degree spontaneous peripheral vestibular nystagmus of the inhibitory type (2.1.1.1.) in the straight-ahead position beating away from the side of the lesion.
A similar nystagmus may occur when central vestibular and gaze-holding structures are affected directly in the brainstem or cerebellum (as with stroke or demyelinating disease). Because the term Bruns nystagmus is historically linked to posterior fossa tumors (and mixed peripheral rather than central vestibular nystagmus), it is preferable to use the term “central vestibular plus gaze-holding nystagmus” or “mixed central vestibular and gaze-holding nystagmus” when this nystagmus pattern occurs from purely central lesions.
2.2.4. Rebound nystagmus: Nystagmus appearing transiently upon return to the straight-ahead gaze position after sustained eccentric gaze, with the fast phases beating away from the original direction of eccentric gaze.
Comment: Rebound nystagmus is typically associated with gaze-holding nystagmus though has been demonstrated in normal subjects with physiologic end-point nystagmus [138].
2.2.5. Centripetal nystagmus: A form of gaze-evoked nystagmus developing during eccentric gaze in which the eyes drift centrifugally (eccentrically), and fast phases are directed toward the straight-ahead position.
Comment: Centripetal nystagmus is a rare form of nystagmus that typically begins once gaze-holding nystagmus quiets down during sustained eccentric gaze [107]. It is usually associated with strong rebound nystagmus (2.2.4.).
2.3. Triggered nystagmus: Nystagmus induced by an identifiable trigger other than gaze position, such as a change in head position or other provocative maneuver.
Comment: The trigger should be specified. Note should be made whether triggered nystagmus is present during visual fixation versus only present or increased with fixation blocked. Though usually not essential for evaluating positional nystagmus, blocking visual fixation is often necessary to elicit nystagmus with headshaking, sound, Valsalva, pressure, vibration, or hyperventilation.
2.3.1. Positional nystagmus: Nystagmus triggered by and occurring after a change in head position with respect to gravity.
Comment: Positional nystagmus may be transient, usually lasting <1 minute (as seen with the canalolithiasis form of benign paroxysmal positional vertigo) or persistent, usually >1 minute (as seen with cupulolithiasis, positional alcohol nystagmus (2.3.1.2.) [24, 53], and some forms of central positional nystagmus). In keeping with prior terminology for vestibular symptoms [21], we do not separate “positioning” and “positional” nystagmus in order to distinguish nystagmus triggered by the head movement itself from that triggered by the final head position with respect to gravity [156]. Note that the normal physiologic nystagmus that occurs momentarily while the patient’s head is being moved into the first step of a positional test (e.g., the Dix-Hallpike maneuver) represents per-rotational nystagmus (1.2.) rather than positional nystagmus. In addition to the forms discussed below, positional nystagmus of low intensity that is not accompanied by vestibular and autonomic symptoms may sometimes be observed in normal subjects.
Previous term: positioning nystagmus
2.3.1.1. Benign paroxysmal positional nystagmus (BPPN): Positional nystagmus attributed to benign paroxysmal positional vertigo (BPPV), either canalolithiasis or cupulolithiasis.
Comment: Such positional nystagmus should rotate about an axis perpendicular to the plane of the affected semicircular canal(s). While by definition, affected patients experience positional vertigo or dizziness [156], occasionally patients with BPPN report few or no positional vestibular symptoms, but their positional nystagmus may resolve with repositioning maneuvers.
2.3.1.1.1. Posterior semicircular canal BPPN: Positional nystagmus, attributed to BPPV, elicited after a latency of one or few seconds by the Dix-Hallpike maneuver or side-lying maneuver (Semont diagnostic maneuver). The nystagmus beats torsionally with the upper pole of the eye to the lower ear and vertically upward (to the forehead) [156].
Comment: If fixation is not blocked, the nystagmus may appear predominantly torsional since visual fixation suppresses vertical more than torsional nystagmus. The direction of the patient’s gaze may influence the appearance of positional nystagmus. If gaze is directed to the lower ear, nystagmus appears to be predominately torsional about the line of sight; if directed to the upper ear, it is predominantly vertical in eye-referenced coordinates [24]. Independent of orbital eye position, the eye movement in a head-referenced coordinate system occurs about an axis perpendicular to the plane of the posterior semicircular canal. In canalolithiasis, positional nystagmus usually increases rapidly in intensity and then declines more slowly (crescendo-decrescendo); the duration typically does not exceed 40 seconds [12]; and the direction may briefly reverse after the patient returns to the upright position. In the rare cupulolithiasis variant, positional nystagmus is optimally elicited after a brief or no latency by a “half Dix-Hallpike maneuver” and lasts > 1 minute. A “half Dix-Hallpike maneuver” is performed with the head resting slightly raised from supine. This position is best suited to bring the affected cupula to an earth-horizontal position to be maximally deflected by the gravitational force [50, 78, 123].
2.3.1.1.2. Horizontal semicircular canal BPPN: Positional nystagmus, attributed to BPPV, elicited after a brief or no latency by the supine roll test, changing directions to beat horizontally toward either the undermost ear (geotropic form) or uppermost ear (apogeotropic form) with the head turned to either side [156].
Comment: The geotropic form of horizontal canal BPPV is attributed to canalolithiasis. It beats horizontally toward the undermost ear (with a smaller torsional component beating with the upper pole of the eye toward the undermost ear) with the head turned to either side and generally lasts ∼1 minute (range ∼30–90 seconds) [10]. It is more intense when the head is turned toward the affected ear, though the intensity is greater and latency shorter with larger and faster head turns in the supine roll test [13, 145], so the net angle and acceleration of the head rotation should be similar for head turns to the right and left to allow for comparison of nystagmus intensity. In the upright position, flexing the head 90° forward (‘bow’) may elicit a transient nystagmus beating toward the affected ear, while looking up or lying backward from the sitting position (‘lean’) may provoke transient nystagmus beating toward the healthy ear [36].
The apogeotropic form of horizontal BPPV is generally attributed to cupulolithasis (though can occur in horizontal canalolithiasis, in which case it may rapidly convert to the geotropic form during the supine roll test [54, 78, 124]). It beats horizontally toward the uppermost ear (with a smaller torsional component beating with the upper pole of the eye to the uppermost ear) with the head turned to either side. Typically the intensity builds up slowly over approximately 30 seconds and then gradually decays over a longer period of several minutes [10, 15]. The intensity is usually stronger with the head turned away from the affected ear in the supine roll test [31], so the net angle and acceleration of the head rotation should be similar for head turns to the right and left to allow for comparison of nystagmus intensity. In the supine position a weak persistent nystagmus beating toward the affected ear may be observed (ipsilesional-beating “lying down nystagmus”) that subsides when the head is turned slightly to that side [22]. Pseudo-spontaneous nystagmus (2.3.1.1.2.1.) may also be observed in the upright head position.
2.3.1.1.2.1. Pseudo-spontaneous nystagmus: Positional nystagmus that appears spontaneous (horizontal jerk nystagmus in the upright head position) secondary to canalolithiasis or cupulolithiasis of the horizontal semicircular canal.
Comment: Pseudo-spontaneous nystagmus is attributed to the 30° inclination between the horizontal canal and the horizontal gravitational plane when in the upright position that places the ampulla in a higher position than the rest of the canal [22, 41, 122]. Gravity allows otoconia either floating in the canal or attached to the cupula to deflect the cupula and produce horizontal nystagmus, the direction of which depends on the side and location of the otoconia and thus the direction of deflection of the cupula. Pseudo-spontaneous nystagmus is therefore a form of positional nystagmus that happens to occur with the head in the neutral position and the eyes straight ahead, making it appear similar to horizontal spontaneous peripheral vestibular nystagmus (2.1.1.). It will generally beat toward the affected ear in the apogeotropic type but toward either ear in the geotropic type [106]. Pseudo-spontaneous nystagmus should disappear by pitching the head forward 30° to place the horizontal canal in the horizontal plane with respect to gravity and should reverse directions by pitching forward even further.
2.3.1.1.3. Anterior semicircular canal BPPN: Positional nystagmus, attributed to BPPV, elicited immediately or after a latency of one or few seconds by the Dix-Hallpike maneuver or in the supine straight head-hanging position. The nystagmus beats predominantly downward but with a small torsional component in which the upper pole of the eye beats toward the affected ear [10, 156].
Comment: Canalolithiasis affecting the anterior canal is a rare variant of BPPV that must be distinguished from central positional nystagmus (2.3.1.3.) and should have a duration of less than 1 minute during positional testing. It is sometimes the apogeotropic variant of posterior semicircular canal BPPV [154].
2.3.1.2. Other forms of peripheral vestibular positional nystagmus: Positional nystagmus due to a peripheral vestibular disorder other than BPPV.
Comment: Ingestion of alcohol (or other substances with a specific gravity different from endolymph) can produce horizontal positional nystagmus. According to the buoyancy hypothesis, compounds of different specific gravity diffuse into and out of the cupula and endolymph at different rates, causing a transient density gradient and gravity-dependent deflection of the cupula. During initial alcohol intoxication, the cupula becomes relatively lighter than the surrounding endolymph, producing geotropic horizontal positional alcohol nystagmus. While sobering up, the cupula becomes relatively heavy, resulting in apogeotropic nystagmus [24].
Other peripheral vestibular disorders have been reported to occasionally cause positional nystagmus that must be differentiated from BPPV based on additional symptoms and signs, including vestibular paroxysmia (neurovascular cross-compression of the vestibulocochlear nerve) [25, 74], vestibular schwannoma [147], Menière’s disease [101], and vestibular atelectasis [162].
2.3.1.3. Central positional nystagmus: Positional nystagmus attributed to disease affecting the central nervous system.
Comment: Positional nystagmus can be caused by neurological diseases affecting the central vestibulocerebellar pathways, such as cerebellar degeneration, multiple sclerosis, posterior fossa neoplasm, Chiari malformation, stroke, vestibular migraine, or a paraneoplastic syndrome. Additional neurological or ocular motor symptoms or signs are typically present to suggest a diagnosis other than BPPV [19, 31, 32]. In their absence, several clues suggest a central cause. The nystagmus may have any trajectory, but pure downbeat and pure horizontal forms are far more common than upbeat, torsional, or mixed forms [120]. Positional nystagmus whose trajectory does not align with a semicircular canal in head-referenced coordinates, such as purely vertical or purely torsional positional nystagmus (provided visual fixation is blocked), suggests central nervous system disease [18, 19, 28, 29, 48, 100, 157]. Failure to fatigue after repeated positional testing or resolve after appropriate canalith repositioning procedures suggests a central cause [20, 55]. Intense positional nystagmus with little to no vertiginous sensation may also suggest a central cause.
Positional downbeat nystagmus is particularly likely to have a central cause [18, 19], though a small torsional component with the upper pole of the eye beating toward the affected ear may suggest anterior canal BPPN (2.3.1.1.3.) [10].
Central horizontal positional nystagmus (particularly apogeotropic) is sometimes indistinguishable from the nystagmus of horizontal canal BPPV at the bedside [19, 31, 83, 91], though in central positional nystagmus the intensity is typically at its peak initially and decreases exponentially over time [32], rather than gradually building up over 10–20 seconds and then decreasing slowly over time as in horizontal cupulolithiasis [15]. With fixation removed, patients with apogeotropic central positional nystagmus usually also have low-intensity horizontal central vestibular nystagmus that is similar in both the sitting and supine positions. With respect to the nystagmus direction while supine, the apogeotropic nystagmus evoked in the ear-down position with the supine roll test may be called “ipsiversive” if it continues to beat in the same direction or “contraversive” if it beats in the opposite direction. Unlike in apogeotropic horizontal canal BPPN (2.3.1.1.2.), the nystagmus intensity of apogeotropic central positional nystagmus is usually similar in the right- and left-ear down positions [31].
2.3.2. Headshaking-induced nystagmus: Nystagmus triggered by and occurring after repetitive headshaking.
Comment: Headshaking-induced nystagmus is typically elicited by shaking the upright subject’s head vigorously at about 2 Hz horizontally (preferably with the head pitched forward 30° to align the horizontal semicircular canals with the plane of head rotation) for about 20 cycles and then stopping abruptly to look for jerk nystagmus [30]. Note that if the examiner moves the head in a circular or elliptical fashion instead of back and forth, this will induce post-rotational nystagmus (1.3.) rather than headshaking-induced nystagmus [72]. Headshaking-induced nystagmus may be monophasic and in a unilateral inhibitory vestibular lesion usually beats toward the better ear. Sometimes the first phase is followed by a weaker second phase beating toward the hypofunctioning side that decays more slowly [67, 88]. Vertical headshaking may produce nystagmus beating toward the paretic ear due to activation of the posterior canal, which produces a horizontal component. Headshaking-induced nystagmus may also occur in central vestibular disorders, in which it may beat toward the side of the lesion, quickly reverse direction, or show a cross-coupled nystagmus (2.3.3.) [75].
Terms not recommended: Post-headshaking nystagmus; headshaking nystagmus
2.3.3. Cross-coupled nystagmus: Nystagmus developing in a plane different from the plane stimulated by caloric irrigation, head rotation, or following headshaking.
Comment: Cross-coupled nystagmus most commonly occurs as vertical (usually downbeat) nystagmus following horizontal canal stimulation in the setting of central vestibular lesions [75, 93, 158].
Previous term: Perverted nystagmus
2.3.4. Sound-induced nystagmus: Nystagmus triggered by an auditory stimulus.
Comment: Sound-induced nystagmus occurs most commonly in superior canal dehiscence syndrome [116], in which case the nystagmus trajectory aligns with the affected semicircular canal plane [39]. The frequency and amplitude of the sound stimulus and the ear(s) to which the stimulus was applied should be specified. Sound-induced nystagmus may be elicited by external sound sources or by the patient’s own voice, e.g. during singing or humming. For the latter clinical sign, the term “fremitus nystagmus” has been proposed [65].
Previous term: Tullio phenomenon
2.3.5. Valsalva-induced nystagmus: Nystagmus triggered by any bodily maneuver that increases intracranial or middle ear pressure.
Comment: Valsalva-induced nystagmus occurs most commonly in superior canal dehiscence syndrome [116]. Actions that reduce venous return from the intracranial space by raising intrathoracic pressure against a closed glottis (glottic Valsalva) include coughing, sneezing, straining, or lifting heavy objects. By contrast, nose-pinched Valsalva forces air directly into the middle ear cavity without a significant change in intrathoracic pressure. Note should be made whether nystagmus is triggered by glottic Valsalva, nose-pinched Valsalva, or both, and the nystagmus direction with each. Nystagmus induced by pneumatic otoscopy/insufflation and other “extrinsic” pressure changes should be classified as pressure-induced nystagmus (2.3.6.).
2.3.6. Pressure-induced nystagmus: Nystagmus triggered by extrinsic pressure changes such as insufflation with pneumatic otoscopy or tragal compression.
Comment: Nystagmus occurs in conditions where external ear pressure changes can be transmitted across the tympanic membrane to the middle and inner ear (e.g. in superior canal dehiscence syndrome, in which the nystagmus occurs in the plane of the affected canal). Note should be made whether nystagmus is triggered by positive or negative pressure in the external ear canal. Nystagmus triggered by bodily maneuvers that increase intracranial or middle ear pressure should be classified as Valsalva-induced nystagmus (2.3.5.).
Previous term: Hennebert’s sign
Term not recommended: Fistula test
2.3.7. Vibration-induced nystagmus: Nystagmus triggered by vibration applied to the head or neck.
Comment: Typically, a hand-held vibrator is firmly applied to the head (generally the mastoid processes and the vertex) or neck (generally the sternocleidomastoid muscle) for 10 seconds, observing for nystagmus with visual fixation blocked. Vibration-induced nystagmus most commonly occurs with unilateral vestibular loss [30, 46]. The location of vibrator application and direction of nystagmus with each stimulus should be recorded.
2.3.8. Hyperventilation-induced nystagmus: Nystagmus triggered by hyperventilation.
Comment: Hyperventilation may induce nystagmus in some central or peripheral vestibular disorders [30, 34, 115, 159]. Hyperventilation-induced nystagmus that beats toward the side of reduced hearing or vestibular dysfunction may alert one to the presence of a cerebellopontine angle tumor [34]. Subjects are typically instructed to rapidly take many large breaths through the mouth for 30 to 90 seconds and observed with visual fixation blocked.
2.3.9. Pursuit-induced nystagmus: Nystagmus triggered by and occurring during smooth pursuit of a moving target in which the nystagmus occurs in a different plane than the pursuit.
Comment: Torsional nystagmus has been reported to develop during vertical pursuit in the setting of a cavernous angioma of the middle cerebellar peduncle [60]. Such nystagmus has been attributed to asymmetric damage to vertical pursuit pathways that are purportedly encoded in a labyrinthine (vertical semicircular canal) coordinate system. Pursuit-induced nystagmus differs from the saccadic breakdown of impaired smooth pursuit in which saccades occur in the same plane and direction as the moving target stimulus.
3. Nystagmus-like movements: Some oscillatory eye movements may resemble nystagmus but instead represent saccadic intrusions or remain ambiguous.
3.1. Saccadic intrusions and oscillations: Inappropriate saccades that disrupt foveal vision by taking the eye away from a target during intended fixation.
Comment: Saccades are normally suppressed during steady visual fixation. Saccadic intrusions should be distinguished from excessive distractibility, in which case novel but behaviorally irrelevant visual stimuli evoke inappropriate reflexive saccades. The spectrum of saccadic intrusions ranges from small square wave jerks in normal subjects to the large pathologic saccadic oscillations of ocular flutter and opsoclonus [110]. Ocular flutter and opsoclonus are of particular clinical relevance to distinguish from nystagmus and other oscillations because of their strong association with paraneoplastic or parainfectious encephalitis [59, 97].
3.1.1. Square-wave jerks: Pairs of small horizontal conjugate saccades (typically < 2 degrees) that take the eyes away from the fixation position and then return them after an interval of about 200 to 400 ms.
Comment: Square wave jerks often occur in series and are common in normal subjects, though may be more frequent or larger in elderly subjects. They may become almost continuous, though still <2 Hz, in certain cerebellar, brainstem, and cerebral hemispheric diseases, in which case they have been called square-wave oscillations. Macrosquare-wave jerks refer to large (typically >5 degrees) 2-3 Hz saccadic oscillations with an intersaccadic interval of about 70–150 ms that often occur in bursts of varying amplitude.
3.1.2. Macrosaccadic oscillations: Oscillations around a fixation point due to saccadic hypermetria, typically consisting of runs of (usually horizontal) saccades that build up and then decrease in amplitude, with intersaccadic intervals of about 200 ms.
Comment: Macrosaccadic oscillations reflect saccadic dysmetria in which primary and corrective saccades are so hypermetric that they continue overshooting the target and oscillate around the intended fixation point. They are most commonly associated with lesions affecting the cerebellar fastigial nucleus or its output pathways in the superior cerebellar peduncle.
3.1.3. Saccadic Pulses: Saccadic pulses are brief intrusions upon steady fixation caused by an unintended saccade away from the fixation position, sometimes followed by an immediate drift back.
Comment: Single saccadic pulses are followed by a drift back to the original position, suggesting absence of a step signal (pulse-step mismatch) [102]. Double saccadic pulses consist of an initial saccade away from fixation followed immediately by a return saccade back to fixation [7, 94]. Saccadic pulses may sometimes occur in a series or alternate directions.
3.1.4. Ocular flutter: Intermittent bursts of conjugate horizontal saccades without an intersaccadic interval, often beginning after a voluntary saccade.
Comment: The oscillation frequency is typically 10–25 Hz, higher with small-sized movements. If very small (microflutter), they might be only seen with an ophthalmoscope or recording device.
3.1.5. Opsoclonus: Combined conjugate multidirectional (horizontal, vertical, and torsional) saccadic oscillations without an intersaccadic interval that interfere with steady fixation.
Comment: The saccadic oscillations of opsoclonus are typically large and are present during pursuit, convergence, blinks, eyelid closure, and sleep.
3.1.6. Voluntary saccadic oscillations: Also referred to as voluntary flutter or voluntary nystagmus, some normal subjects can voluntarily induce conjugate high-frequency saccadic oscillations that are usually confined to the horizontal plane.
Comment: The same saccadic oscillations sometimes occur “involuntarily” as a functional disorder and may be superimposed upon smooth pursuit movements. Such oscillations can generally be distinguished from pathologic ocular flutter since they are usually not sustained and are often accompanied by convergence (and pupillary constriction), facial grimacing, or eyelid flutter.
Terms not recommended: Voluntary nystagmus (since the oscillations occur without a slow phase movement and so are technically not a form of nystagmus); psychogenic flutter
3.2. Other nystagmus-like movements: Some oscillatory eye movements do not solely represent saccadic movements but also do not fit the strict definition of jerk or pendular nystagmus.
3.2.1. Convergence-retraction nystagmus: A condition in which upward saccades or fast phases are replaced by rapid convergent or retractory (pulling the globes into the orbit) eye movements, or both.
Comment: Convergence-retraction nystagmus is almost invariably caused by lesions of the dorsal midbrain in the region of the posterior commissure and is associated with impaired upgaze. Convergence-retraction nystagmus is typically elicited by having the patient attempt to make a voluntary upward saccade or track a downwardly moving optokinetic tape or drum, thus inducing rapid upward convergent or retractory eye movements. The retraction can best be seen by watching the globe while observing the patient from the side. It remains unsettled scientifically whether the convergent movements are abnormal opposed adducting saccades or a disorder of the vergence system or both [125, 131]. It resembles but is not truly a jerk nystagmus. Pretectal pseudobobbing, which may be a form of convergence nystagmus seen in acute obstructive hydrocephalus, consists of non-rhythmic, rapid movements that carry the eyes downward and medially with a frequency of about 0.3 to 2 Hz, followed by a slow return to central position [90].
3.2.2. Ocular oscillations in spasmus nutans: Intermittent, small amplitude, high frequency, predominantly horizontal pendular oscillations that vary in their amplitude and phase relationship between the two eyes attributed to spasmus nutans.
Comment: These “shimmering oscillations” usually develop in the first year of life as part of an early-life syndromic triad including irregular head nodding and anomalous head positions, then remit after a few years. Spasmus nutans can be distinguished from infantile or latent nystagmus by its intermittency, vertical component, high frequency, and dissociated features.
3.2.3. Ocular bobbing and its variants: Several related types of spontaneous eye movements may be encountered in patients with decreased levels of consciousness (Fig. 4). Though their pathophysiology may differ from other forms of spontaneous jerk nystagmus, their waveforms have both slow and fast components and have similar frequencies and amplitudes to each other.
Fig.4.
Schematic summary of the waveforms of ocular bobbing and its variants. Adapted from Leigh and Zee [109].
Comments: Classic ocular bobbing consists of intermittent, usually conjugate, rapid downward eye movements followed by a slower return to the straight-ahead position. Reflex horizontal eye movements are usually absent. Classic ocular bobbing is usually a sign of intrinsic pontine lesions such as hemorrhage or extrinsic compression. Ocular dipping consists of intermittent, usually conjugate, slow downward eye movements followed by a rapid return to the straight-ahead position. Reverse ocular bobbing consists of intermittent, usually conjugate, rapid upward eye movements followed by a slower return to the straight-ahead position. Reverse ocular dipping consists of intermittent, usually conjugate, slow upward eye movements followed by a rapid return to the straight-ahead position. Ocular bobbing and reverse ocular bobbing may be forms of single saccadic pulses [140].
Previous term for ocular dipping: Inverse ocular bobbing
Previous term for reverse ocular dipping: Converse bobbing
3.2.4. Superior oblique myokymia: Bursts of small, irregular, high-frequency monocular oscillations attributed to abnormal discharges of the trochlear nerve.
Comment: Eye movement recordings have demonstrated initial intorsion and depression followed by irregular small oscillations [108]. These tiny oscillations may only be visible with ophthalmoscopy or close inspection of the conjunctival vessels during attacks.
3.2.5. Ping-pong gaze: Slow continuous horizontal conjugate deviation of the eyes, alternating every few seconds [58, 79].
Comment: Ping-pong gaze is typically seen in obtunded patients with severe bilateral cerebral hemispheric dysfunction but has also been described in collicular infarction [117]. It may be smooth in deeply obtunded patients or comprised of many small saccades in patients whose level of consciousness is higher [84, 142]. The frequency of approximately 0.25 Hz is too low to be considered pendular nystagmus. Ping-pong gaze should be distinguished from periodic alternating gaze deviation (2.1.2.1.2.), consisting of horizontal conjugate deviation of the eyes alternating right and left approximately every 2 minutes [136].
Term not recommended: Ocular clonus, a term that has been used in criteria for serotonin toxicity [47] but is likely referring to ping-pong gaze [51, 77, 128].
3.2.6. Pendular pseudonystagmus: Pendular ocular oscillations due to the combination of head tremor and vestibular hypofunction.
Comment: Pendular pseudonystagmus is due to an inadequate vestibulo-ocular reflex that is unable to stabilize gaze during the oscillating movements of head tremor. Patients may experience oscillopsia, and though no nystagmus is present on examination, the examiner may mistakenly diagnose pendular nystagmus due to oscillation of the optic disc during ophthalmoscopy. It is “pseudo” nystagmus because it disappears once the head is stabilized. It can occur with central vestibular disorders such as multiple sclerosis or in those with bilateral peripheral vestibulopathy [27, 137].
Acknowledgments
Thank you to Michael Brodsky for input on infantile nystagmus and latent nystagmus.
References
- [1]. Nystagmus, in: Dorland’s Illustrated Medical Dictionary, W.B. Saunders Comany, Philadelphia, 1994, pp. 1164. [Google Scholar]
- [2].Committee for the Classification of Eye Movement Abnormalities and Strabismus (CEMAS) Workshop, in: A Classification of Eye Movement Abnormalities and Strabismus (CEMAS), National Eye Institute, 2003. Accessed 23 January 2017. https://www.nei.nih.gov/sites/default/files/nei-pdfs/cemas.pdf
- [3]. Nystagmus, in: The American Heritage Dictionary of the English Language, Houghton Mifflin Harcourt, 2011. [Google Scholar]
- [4]. Nystagmus, in: Merriam-Webster Online Edition, Merriam-Webster, Inc., 2013. Accessed 31 August 2013. www.merriam-webster.com/dictionary/nystagmus [Google Scholar]
- [5]. Nystagmus, in: Oxford Dictionaries, Oxford University Press, 2013. [Google Scholar]
- [6].Bárány Society initiative for the establishment of the International Classification of Vestibular Disorders (ICVD), Journal of Vestibular Research, 2014. Accessed 26 January 2017. http://www.jvr-web.org/images/InstructionsforICVDsubcommittees_as_of_19Oct2014.pdf
- [7]. Abadi R.V., Gowen E., Characteristics of saccadic intrusions, Vision research 44 (2004), 2675–2690. [DOI] [PubMed] [Google Scholar]
- [8]. Abel L.A., Parker L., Daroff R.B., Dell’Osso L.F., End-point nystagmus, Investigative ophthalmology & visual science 17 (1978), 539–544. [PubMed] [Google Scholar]
- [9]. Abel L.A., Traccis S., Dell’Osso L.F., Ansevin C.F., Variable waveforms in downbeat nystagmus imply short-term gain changes, Annals of Neurology 13 (1983), 616–620. [DOI] [PubMed] [Google Scholar]
- [10]. Aw S.T., Todd M.J., Aw G.E., McGarvie L.A., Halmagyi G.M., Benign positional nystagmus: A study of its three-dimensional spatio-temporal characteristics, Neurology 64 (2005), 1897–1905. [DOI] [PubMed] [Google Scholar]
- [11]. Baier B., Dieterich M., Incidence and anatomy of gaze-evoked nystagmus in patients with cerebellar lesions, Neurology 76 (2011), 361–365. [DOI] [PubMed] [Google Scholar]
- [12]. Baloh R.W., Honrubia V., Jacobson K., Benign positional vertigo: Clinical and oculographic features in 240 cases, Neurology 37 (1987), 371–378. [DOI] [PubMed] [Google Scholar]
- [13]. Baloh R.W., Jacobson K., Honrubia V., Horizontal semicircular canal variant of benign positional vertigo, Neurology 43 (1993), 2542–2549. [DOI] [PubMed] [Google Scholar]
- [14]. Baloh R.W., Yee R.D., Honrubia V., Eye movements in patients with Wallenberg’s syndrome, Annals of the New York Academy of Sciences 374 (1981), 600–613. [DOI] [PubMed] [Google Scholar]
- [15]. Baloh R.W., Yue Q., Jacobson K.M., Honrubia V., Persistent direction-changing positional nystagmus: Another variant of benign positional nystagmus? Neurology 45 (1995), 1297–1301. [DOI] [PubMed] [Google Scholar]
- [16]. Barton J., Overview of Nystagmus, in: UpToDate, Brazis P. ed., Wolters Kluwer Health, 2013. Accessed 4 October 2013. [Google Scholar]
- [17]. Barton J.J., Sharpe J.A., Oscillopsia and horizontal nystagmus with accelerating slow phases following lumbar puncture in the Arnold-Chiari malformation, Annals of Neurology 33 (1993), 418–421. [DOI] [PubMed] [Google Scholar]
- [18]. Bertholon P., Bronstein A.M., Davies R.A., Rudge P., Thilo K.V., Positional down beating nystagmus in 50 patients: Cerebellar disorders and possible anterior semicircular canalithiasis, Journal of Neurology, Neurosurgery, and Psychiatry 72 (2002), 366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Bertholon P., Tringali S., Faye M.B., Antoine J.C., Martin C., Prospective study of positional nystagmus in 100 consecutive patients, Ann Otol Rhinol Laryngol 115 (2006), 587–594. [DOI] [PubMed] [Google Scholar]
- [20]. Bhattacharyya N., Baugh R.F., Orvidas L., Barrs D., Bronston L.J., Cass S., Chalian A.A., Desmond A.L., Earll J.M., Fife T.D., Fuller D.C., Judge J.O., Mann N.R., Rosenfeld R.M., Schuring L.T., Steiner R.W., Whitney S.L., Haidari J., Clinical practice guideline: Benign paroxysmal positional vertigo, Otolaryngology–head and neck surgery: Official journal of American Academy of Otolaryngology-Head and Neck Surgery 139 (2008), S47–81. [DOI] [PubMed] [Google Scholar]
- [21]. Bisdorff A., Von Brevern M., Lempert T. and Newman-Toker D.E., Classification of vestibular symptoms: Towards an international classification of vestibular disorders, Journal of Vestibular Research: Equilibrium & Orientation 19 (2009), 1–13. [DOI] [PubMed] [Google Scholar]
- [22]. Bisdorff A.R., Debatisse D., Localizing signs in positional vertigo due to lateral canal cupulolithiasis, Neurology 57 (2001), 1085–1088. [DOI] [PubMed] [Google Scholar]
- [23]. Bisdorff A.R., Staab J.P., Newman-Toker D.E., Overview of the International Classification of Vestibular Disorders, Neurologic Clinics 33 (2015), 541–550, vii. [DOI] [PubMed] [Google Scholar]
- [24]. Brandt T., Positional and positioning vertigo and nystagmus, J Neurol Sci 95 (1990), 3–28. [DOI] [PubMed] [Google Scholar]
- [25]. Brandt T., Strupp M., Dieterich M., Vestibular paroxysmia: A treatable neurovascular cross-compression syndrome, Journal of Neurology 263(Suppl 1) (2016), S90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Brodsky M.C., Visuo-vestibular eye movements: Infantile strabismus in 3 dimensions, Archives of Ophthalmology 123 (2005), 837–842. [DOI] [PubMed] [Google Scholar]
- [27]. Bronstein A.M., Gresty M.A., Mossman S.S., Pendular pseudonystagmus arising as a combination of head tremor and vestibular failure, Neurology 42 (1992), 1527–1531. [DOI] [PubMed] [Google Scholar]
- [28]. Buttner U., Helmchen C., Brandt T., Diagnostic criteria for central versus peripheral positioning nystagmus and vertigo: A review, Acta Oto-laryngologica 119 (1999), 1–5. [DOI] [PubMed] [Google Scholar]
- [29]. Chang M.B., Bath A.P., Rutka J.A., Are all atypical positional nystagmus patterns reflective of central pathology? J Otolaryngol 30 (2001), 280–282. [DOI] [PubMed] [Google Scholar]
- [30]. Cherchi M., Hain T., Provocative maneuvers for vestibular disorders, in: Vertigo and Imbalance: Clinical Neurophysiology of the Vestibular System, Eggers S. and Zee D. eds. Elsevier, Amsterdam, 2010, pp. 111–134. [Google Scholar]
- [31]. Choi J.Y., Glasauer S., Kim J.H., Zee D.S., Kim J.S., Characteristics and mechanism of apogeotropic central positional nystagmus, Brain: A Journal of Neurology 141(3) (2018), 762–775. doi: 10.1093/brain/awx381 [DOI] [PubMed] [Google Scholar]
- [32]. Choi J.Y., Kim J.H., Kim H.J., Glasauer S., Kim J.S., Central paroxysmal positional nystagmus: Characteristics and possible mechanisms, Neurology 84 (2015), 2238–2246. [DOI] [PubMed] [Google Scholar]
- [33]. Choi K.D., Jung D.S., Park K.P., Jo J.W., Kim J.S., Bowtie and upbeat nystagmus evolving into hemi-seesaw nystagmus in medial medullary infarction: Possible anatomic mechanisms, Neurology 62 (2004), 663–665. [DOI] [PubMed] [Google Scholar]
- [34]. Choi K.D., Kim J.S., Kim H.J., Koo J.W., Kim J.H., Kim C.Y., Oh C.W., Kee H.J., Hyperventilation-induced nystagmus in peripheral vestibulopathy and cerebellopontine angle tumor, Neurology 69 (2007), 1050–1059. [DOI] [PubMed] [Google Scholar]
- [35]. Choi K.D., Shin H.K., Kim J.S., Kim S.H., Choi J.H., Kim H.J., Zee D.S., Variants of windmill nystagmus, Journal of Neurology 263 (2016), 1375–1381. [DOI] [PubMed] [Google Scholar]
- [36]. Choung Y.H., Shin Y.R., Kahng H., Park K., Choi S.J., ‘Bow and lean test’ to determine the affected ear of horizontal canal benign paroxysmal positional vertigo, Laryngoscope 116 (2006), 1776–1781. [DOI] [PubMed] [Google Scholar]
- [37]. Cohen B., Suzuki J.I., Bender M.B., Eye Movements from Semicircular Canal Nerve Stimulation in the Cat, Ann Otol Rhinol Laryngol 73 (1964), 153–169. [DOI] [PubMed] [Google Scholar]
- [38]. Cremer P.D., Migliaccio A.A., Pohl D.V., Curthoys I.S., Davies L., Yavor R.A., Halmagyi G.M., Posterior semicircular canal nystagmus is conjugate and its axis is parallel to that of the canal, Neurology 54 (2000), 2016–2020. [DOI] [PubMed] [Google Scholar]
- [39]. Cremer P.D., Minor L.B., Carey J.P., Della Santina C.C., Eye movements in patients with superior canal dehiscence syndrome align with the abnormal canal, Neurology 55 (2000), 1833–1841. [DOI] [PubMed] [Google Scholar]
- [40]. Daroff R.B., See-Saw Nystagmus, Neurology 15 (1965), 874–877. [DOI] [PubMed] [Google Scholar]
- [41]. De Stefano A., Kulamarva G., Citraro L., Neri G. and Croce A., Spontaneous nystagmus in benign paroxysmal positional vertigo, American Journal of Otolaryngology 32 (2011), 185–189. [DOI] [PubMed] [Google Scholar]
- [42]. Dell’Osso L.F., Daroff R.B., Two additional scenarios for see-saw nystagmus: Achiasma and hemichiasma, Journal of Neuro-Ophthalmology: The Official Journal of the North American Neuro-Ophthalmology Society 18 (1998), 112–113. [PubMed] [Google Scholar]
- [43]. Della Santina C.C., Potyagaylo V., Migliaccio A.A., Minor L.B. and Carey J.P., Orientation of human semicircular canals measured by three-dimensional multiplanar CT reconstruction, J Assoc Res Otolaryngol 6 (2005), 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Deutschlander A., Jahn K., Strupp M., Brandt T., Pulse-synchronous rotational and vertical pendular eye movements in superior canal dehiscence syndrome, European Journal of Neurology: The Official Journal of the European Federation of Neurological Societies 14 (2007), e29. [DOI] [PubMed] [Google Scholar]
- [45]. Doslak M.J., Dell’Osso L.F., Daroff R.B., A model of Alexander’s law of vestibular nystagmus, Biol Cybern 34 (1979), 181–186. [DOI] [PubMed] [Google Scholar]
- [46]. Dumas G., Karkas A., Perrin P., Chahine K., Schmerber S., High-frequency skull vibration-induced nystagmus test in partial vestibular lesions, Otol Neurotol 32 (2011), 1291–1301. [DOI] [PubMed] [Google Scholar]
- [47]. Dunkley E.J., Isbister G.K., Sibbritt D., Dawson A.H., Whyte I.M., The Hunter Serotonin Toxicity Criteria: Simple and accurate diagnostic decision rules for serotonin toxicity, QJM 96 (2003), 635–642. [DOI] [PubMed] [Google Scholar]
- [48]. Eggers S.D., Pittock S.J., Shepard N.T., Habermann T.M., Neff B.A., Klebig R.R., Positional periodic alternating vertical nystagmus with PCA-Tr antibodies in Hodgkin lymphoma, Neurology 78 (2012), 1800–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Eizenman M., Cheng P., Sharpe J.A., Frecker R.C., End-point nystagmus and ocular drift: An experimental and theoretical study, Vision Research 30 (1990), 863–877. [DOI] [PubMed] [Google Scholar]
- [50]. Epley J.M., Human experience with canalith repositioning maneuvers, Annals of the New York Academy of Sciences 942 (2001), 179–191. [DOI] [PubMed] [Google Scholar]
- [51]. Erich J.L., Shih R.D., O’Connor R.E., “Ping-pong” gaze in severe monoamine oxidase inhibitor toxicity, J Emerg Med 13 (1995), 653–655. [DOI] [PubMed] [Google Scholar]
- [52]. Fetter M., Dichgans J., Vestibular neuritis spares the inferior division of the vestibular nerve, Brain: A Journal of Neurology 119 (Pt 3) (1996), 755–763. [DOI] [PubMed] [Google Scholar]
- [53]. Fetter M., Haslwanter T., Bork M., Dichgans J., New insights into positional alcohol nystagmus using three-dimensional eye-movement analysis, Annals of Neurology 45 (1999), 216–223. [DOI] [PubMed] [Google Scholar]
- [54]. Fife T.D., Recognition and management of horizontal canal benign positional vertigo, Am J Otol 19 (1998), 345–351. [PubMed] [Google Scholar]
- [55]. Fife T.D., Iverson D.J., Lempert T., Furman J.M., Baloh R.W., Tusa R.J., Hain T.C., Herdman S., Morrow M.J., Gronseth G.S., Practice parameter: Therapies for benign paroxysmal positional vertigo (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology, Neurology 70 (2008), 2067–2074. [DOI] [PubMed] [Google Scholar]
- [56]. Fife T.D., Tusa R.J., Furman J.M., Zee D.S., Frohman E., Baloh R.W., Hain T., Goebel J., Demer J., Eviatar L., Assessment: Vestibular testing techniques in adults and children: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology, Neurology 55 (2000), 1431–1441. [DOI] [PubMed] [Google Scholar]
- [57]. Fisher A., Gresty M., Chambers B., Rudge P., Primary position upbeating nystagmus. A variety of central positional nystagmus, Brain: A Journal of Neurology 106 (Pt 4) (1983), 949–964. [DOI] [PubMed] [Google Scholar]
- [58]. Fisher C.M., Some neuro-ophthalmological observations, Journal of Neurology, Neurosurgery, and Psychiatry 30 (1967), 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Fisher P.G., Wechsler D.S., Singer H.S., Anti-Hu antibody in a neuroblastoma-associated paraneoplastic syndrome, Pediatr Neurol 10 (1994), 309–312. [DOI] [PubMed] [Google Scholar]
- [60]. FitzGibbon E.J., Calvert P.C., Dieterich M., Brandt T., Zee D.S., Torsional nystagmus during vertical pursuit, Journal of neuro-ophthalmology: The official journal of the North American Neuro-Ophthalmology Society 16 (1996), 79–90. [PubMed] [Google Scholar]
- [61]. Flint P.W., Cummings otolaryngology–head & neck surgery, Elsevier/Saunders, Philadelphia, PA, 2015. [Google Scholar]
- [62]. Furman J.M., Crumrine P.K., Reinmuth O.M., Epileptic nystagmus, Annals of Neurology 27 (1990), 686–688. [DOI] [PubMed] [Google Scholar]
- [63]. Furman J.M., Wall C. 3rd and Pang D.L., Vestibular function in periodic alternating nystagmus, Brain: A Journal of Neurology 113 (Pt 5) (1990), 1425–1439. [DOI] [PubMed] [Google Scholar]
- [64]. Gradstein L., Reinecke R.D., Wizov S.S., Goldstein H.P., Congenital periodic alternating nystagmus. Diagnosis and Management, Ophthalmology 104 (1997), 918–928; discussion 928-919. [DOI] [PubMed] [Google Scholar]
- [65]. Gurkov R., Jerin C., Flatz W., Maxwell R., Superior canal dehiscence syndrome: Diagnosis with vestibular evoked myogenic potentials and fremitus nystagmus, HNO 66 (2018), 28–33. [DOI] [PubMed] [Google Scholar]
- [66]. Hain T.C., Cherchi M., Pulse-synchronous torsional pendular nystagmus in unilateral superior canal dehiscence, Neurology 70 (2008), 1217–1218. [DOI] [PubMed] [Google Scholar]
- [67]. Hain T.C., Fetter M., Zee D.S., Head-shaking nystagmus in patients with unilateral peripheral vestibular lesions, American Journal of Otolaryngology 8 (1987), 36–47. [DOI] [PubMed] [Google Scholar]
- [68]. Halmagyi G.M., Aw S.T., Dehaene I., Curthoys I.S., Todd M.J., Jerk-waveform see-saw nystagmus due to unilateral meso-diencephalic lesion, Brain: A Journal of Neurology 117 (Pt 4) (1994), 789–803. [DOI] [PubMed] [Google Scholar]
- [69]. Halmagyi G.M., Cremer P.D., Anderson J., Murofushi T., Curthoys I.S., Isolated directional preponderance of caloric nystagmus: I. Clinical significance, Am J Otol 21 (2000), 559–567. [PubMed] [Google Scholar]
- [70]. Halmagyi G.M., Gresty M.A., Leech J., Reversed optokinetic nystagmus (OKN): Mechanism and clinical significance, Annals of Neurology 7 (1980), 429–435. [DOI] [PubMed] [Google Scholar]
- [71]. Haslwanter T., Mathematics of three-dimensional eye rotations, Vision Research 35 (1995), 1727–1739. [DOI] [PubMed] [Google Scholar]
- [72]. Haslwanter T., Minor L.B., Nystagmus induced by circular head shaking in normal human subjects, Exp Brain Res 124 (1999), 25–32. [DOI] [PubMed] [Google Scholar]
- [73]. Henriksson N.G., The correlation between the speed of the eye in the slow phase of nystagmus and vestibular stimulus, Acta Oto-laryngologica 45 (1955), 120–136. [DOI] [PubMed] [Google Scholar]
- [74]. Hufner K., Barresi D., Glaser M., Linn J., Adrion C., Mansmann U., Brandt T., Strupp M., Vestibular paroxysmia: Diagnostic features and medical treatment, Neurology 71 (2008), 1006–1014. [DOI] [PubMed] [Google Scholar]
- [75]. Huh Y.E., Kim J.S., Patterns of spontaneous and head-shaking nystagmus in cerebellar infarction: Imaging correlations, Brain: A Journal of Neurology 134 (2011), 3662–3671. [DOI] [PubMed] [Google Scholar]
- [76]. Huh Y.E., Koo J.W., Lee H., Kim J.S., Head-shaking aids in the diagnosis of acute audiovestibular loss due to anterior inferior cerebellar artery infarction, Audiol Neurootol 18 (2013), 114–124. [DOI] [PubMed] [Google Scholar]
- [77]. Hunter B., Kleinert M.M., Osatnik J., Soria E., Serotonergic syndrome and abnormal ocular movements: Worsening of rigidity by remifentanil? Anesth Analg 102 (2006), 1589. [DOI] [PubMed] [Google Scholar]
- [78]. Imai T., Takeda N., Ito M., Sekine K., Sato G., Midoh Y., Nakamae K., Kubo T., 3D analysis of benign positional nystagmus due to cupulolithiasis in posterior semicircular canal, Acta Oto-Laryngologica 129 (2009), 1044–1049. [DOI] [PubMed] [Google Scholar]
- [79]. Ishikawa H., Ishikawa S., Mukuno K., Short-cycle periodic alternating (ping-pong) gaze, Neurology 43 (1993), 1067–1070. [DOI] [PubMed] [Google Scholar]
- [80]. Jampel R.S., Shi D.X., The primary position of the eyes, the resetting saccade, and the transverse visual head plane. Head movements around the cervical joints, Investigative Ophthalmology & Visual Science 33 (1992), 2501–2510. [PubMed] [Google Scholar]
- [81]. Jareonsettasin P., Otero-Millan J., Ward B.K., Roberts D.C., Schubert M.C., Zee D.S., Multiple Time Courses of Vestibular Set-Point Adaptation Revealed by Sustained Magnetic Field Stimulation of the Labyrinth, Current Biology: CB 26 (2016), 1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82]. Jeong H.S., Oh J.Y., Kim J.S., Kim J., Lee A.Y., Oh S.Y., Periodic alternating nystagmus in isolated nodular infarction, Neurology 68 (2007), 956–957. [DOI] [PubMed] [Google Scholar]
- [83]. Johkura K., Central paroxysmal positional vertigo: Isolated dizziness caused by small cerebellar hemorrhage, Stroke 38 (2007), e26–27; author reply e28. [DOI] [PubMed] [Google Scholar]
- [84]. Johkura K., Komiyama A., Tobita M., Hasegawa O., Saccadic ping-pong gaze, Journal of Neuro-ophthalmology: The Official Journal of the North American Neuro-Ophthalmology Society 18 (1998), 43–46. [PubMed] [Google Scholar]
- [85]. Jongkees L.B., [Caloric test; general considerations], Acta oto-rhino-laryngologica Belgica 4 (1950), 376–382. [PubMed] [Google Scholar]
- [86]. Kaplan P.W., Lesser R.P., Vertical and horizontal epileptic gaze deviation and nystagmus, Neurology 39 (1989), 1391–1393. [DOI] [PubMed] [Google Scholar]
- [87]. Kaplan P.W., Tusa R.J., Neurophysiologic and clinical correlations of epileptic nystagmus, Neurology 43 (1993), 2508–2514. [DOI] [PubMed] [Google Scholar]
- [88]. Katsarkas A., Smith H., Galiana H., Head-shaking nystagmus (HSN): The theoretical explanation and the experimental proof, Acta Oto-laryngologica 120 (2000), 177–181. [DOI] [PubMed] [Google Scholar]
- [89]. Kattah J.C., Talkad A.V., Wang D.Z., Hsieh Y.H., Newman-Toker D.E., HINTS to diagnose stroke in the acute vestibular syndrome: Three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging, Stroke 40 (2009), 3504–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90]. Keane J.R., Pretectal pseudobobbing. Five patients with ‘V’-pattern convergence nystagmus, Archives of Neurology 42 (1985), 592–594. [DOI] [PubMed] [Google Scholar]
- [91]. Kim H.A., Yi H.A., Lee H., Apogeotropic central positional nystagmus as a sole sign of nodular infarction, Neurol Sci 33 (2012), 1189–1191. [DOI] [PubMed] [Google Scholar]
- [92]. Kim H.J., Lee S.H., Park J.H., Choi J.Y., Kim J.S., Isolated vestibular nuclear infarction: Report of two cases and review of the literature, Journal of Neurology 261 (2014), 121–129. [DOI] [PubMed] [Google Scholar]
- [93]. Kim J.S., Ahn K.W., Moon S.Y., Choi K.D., Park S.H., Koo J.W., Isolated perverted head-shaking nystagmus in focal cerebellar infarction, Neurology 64 (2005), 575–576. [DOI] [PubMed] [Google Scholar]
- [94]. Kim J.S., Choi K.D., Oh S.Y., Jeong S.H., Oh Y.M., Kim H.J., Park S.H., Ahn J.Y., Double saccadic pulses and macrosaccadic oscillations from a focal brainstem lesion, J Neurol Sci 263 (2007), 118–123. [DOI] [PubMed] [Google Scholar]
- [95]. Kim J.S., Kim H.J., Inferior vestibular neuritis, Journal of Neurology 259 (2012), 1553–1560. [DOI] [PubMed] [Google Scholar]
- [96]. Kim J.S., Moon S.Y., Choi K.D., Kim J.H., Sharpe J.A., Patterns of ocular oscillation in oculopalatal tremor: Imaging correlations, Neurology 68 (2007), 1128–1135. [DOI] [PubMed] [Google Scholar]
- [97]. Klaas J.P., Ahlskog J.E., Pittock S.J., Matsumoto J.Y., Aksamit A.J., Bartleson J.D., Kumar R., McEvoy K.F., McKeon A., Adult-onset opsoclonus-myoclonus syndrome, Archives of neurology 69 (2012), 1598–1607. [DOI] [PubMed] [Google Scholar]
- [98]. Kommerell G., Zee D.S., Latent nystagmus. Release and suppression at will, Investigative Ophthalmology & visual Science 34 (1993), 1785–1792. [PubMed] [Google Scholar]
- [99]. Krimsky E., The seven primary positions of gaze, The British Journal of Ophthalmology 51 (1967), 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100]. Lawson J., Bamiou D.E., Cohen H.S., Newton J., Positional vertigo in a Falls Service, Age Ageing 37 (2008), 585–589. [DOI] [PubMed] [Google Scholar]
- [101]. Lechner C., Taylor R.L., Todd C., Macdougall H., Yavor R., Halmagyi G.M., Welgampola M.S., Causes and characteristics of horizontal positional nystagmus, Journal of Neurology 261 (2014), 1009–1017. [DOI] [PubMed] [Google Scholar]
- [102]. Lee E.S., Choi J.Y., Kim J.S., Teaching Video NeuroImages: Alternating horizontal single saccadic pulses in progressive supranuclear palsy, Neurology 88 (2017), e32– e33. [DOI] [PubMed] [Google Scholar]
- [103]. Lee H., Cho Y.W., A case of isolated nodulus infarction presenting as a vestibular neuritis, J Neurol Sci 221 (2004), 117–119. [DOI] [PubMed] [Google Scholar]
- [104]. Lee H., Kim H.A., Nystagmus in SCA territory cerebellar infarction: Pattern and a possible mechanism, Journal of Neurology, Neurosurgery, and Psychiatry 84 (2013), 446–451. [DOI] [PubMed] [Google Scholar]
- [105]. Lee H., Sohn S.I., Cho Y.W., Lee S.R., Ahn B.H., Park B.R., Baloh R.W., Cerebellar infarction presenting isolated vertigo: Frequency and vascular topographical patterns, Neurology 67 (2006), 1178–1183. [DOI] [PubMed] [Google Scholar]
- [106]. Lee S.U., Kim H.J., Kim J.S., Pseudo-spontaneous and head-shaking nystagmus in horizontal canal benign paroxysmal positional vertigo, Otol Neurotol 35 (2014), 495–500. [DOI] [PubMed] [Google Scholar]
- [107]. Leech J., Gresty M., Hess K., Rudge P., Gaze failure, drifting eye movements, and centripetal nystagmus in cerebellar disease, The British Journal of Ophthalmology 61 (1977), 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108]. Leigh R.J., Tomsak R.L., Seidman S.H., Dell’Osso L.F., Superior oblique myokymia. Quantitative characteristics of the eye movements in three patients, Archives of Ophthalmology 109 (1991), 1710–1713. [DOI] [PubMed] [Google Scholar]
- [109]. Leigh R.J., Zee D.S., The neurology of eye movements, Oxford University Press, New York, 2015. [Google Scholar]
- [110]. Lemos J., Eggenberger E., Saccadic intrusions: Review and update, Current Opinion in Neurology 26 (2013), 59–66. [DOI] [PubMed] [Google Scholar]
- [111]. Lloyd S.K., Baguley D.M., Butler K., Donnelly N., Moffat D.A., Bruns’ nystagmus in patients with vestibular schwannoma, Otol Neurotol 30 (2009), 625–628. [DOI] [PubMed] [Google Scholar]
- [112]. Lopez J.L., Zee D.S., Levi L., Eye closure and oculopalatal tremor, Neurology 77 (2011), 1929. [DOI] [PubMed] [Google Scholar]
- [113]. Lopez L., Bronstein A.M., Gresty M.A., Rudge P., du Boulay E.P., Torsional nystagmus. A neuro-otological and MRI study of thirty-five cases, Brain: A Journal of Neurology 115 (Pt 4) (1992), 1107–1124. [DOI] [PubMed] [Google Scholar]
- [114]. McClure J.A., Copp J.C., Lycett P., Recovery nystagmus in Meniere’s disease, Laryngoscope 91 (1981), 1727–1737. [DOI] [PubMed] [Google Scholar]
- [115]. Minor L.B., Haslwanter T., Straumann D., Zee D.S., Hyperventilation-induced nystagmus in patients with vestibular schwannoma, Neurology 53 (1999), 2158–2168. [DOI] [PubMed] [Google Scholar]
- [116]. Minor L.B., Solomon D., Zinreich J.S., Zee D.S., Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal, Arch Otolaryngol Head Neck Surg 124 (1998), 249–258. [DOI] [PubMed] [Google Scholar]
- [117]. Moccia M., Allocca R., Erro R., Barone P., Vitale C., Ping-pong gaze: Sherrington would not have done it better, JAMA Neurol 71 (2014), 1450. [DOI] [PubMed] [Google Scholar]
- [118]. Mossman S.S., Bronstein A.M., Hood J.D., Linear and angular optokinetic nystagmus in labyrinthine and central nervous system lesions, Acta Oto-laryngologica. Supplementum 481 (1991), 352–356. [DOI] [PubMed] [Google Scholar]
- [119]. Mossman S.S., Bronstein A.M., Hood J.D., Sacares P., The interaction of angular and linear optokinetic stimuli with an angular vestibular stimulus, Annals of the New York Academy of Sciences 656 (1992), 868–870. [DOI] [PubMed] [Google Scholar]
- [120]. Newman-Toker D.E., Symptoms and signs of neuro-otologic disorders, Continuum (Minneap Minn) 18 (2012), 1016–1040. [DOI] [PubMed] [Google Scholar]
- [121]. Newman-Toker D.E., Sharma P., Chowdhury M., Clemons T.M., Zee D.S., Della Santina C.C., Penlight-cover test: A new bedside method to unmask nystagmus, Journal of Neurology, Neurosurgery, and Psychiatry 80 (2009), 900–903. [DOI] [PubMed] [Google Scholar]
- [122]. Nuti D., Mandala M., Salerni L., Lateral canal paroxysmal positional vertigo revisited, Annals of the New York Academy of Sciences 1164 (2009), 316–323. [DOI] [PubMed] [Google Scholar]
- [123]. Nuti D., Masini M., Mandala M., Benign paroxysmal positional vertigo and its variants, Handb Clin Neurol 137 (2016), 241–256. [DOI] [PubMed] [Google Scholar]
- [124]. Nuti D., Vannucchi P., Pagnini P., Benign paroxysmal positional vertigo of the horizontal canal: A form of canalolithiasis with variable clinical features, Journal of vestibular research: Equilibrium & Oorientation 6 (1996), 173–184. [PubMed] [Google Scholar]
- [125]. Ochs A.L., Stark L., Hoyt W.F., D’Amico D., Opposed adducting saccades in convergence-retraction nystagmus: A patient with sylvian aqueduct syndrome, Brain: A Journal of Neurology 102 (1979), 497–508. [DOI] [PubMed] [Google Scholar]
- [126]. Oh K., Chang J.H., Park K.W., Lee D.H., Choi K.D., Kim J.S., Jerky seesaw nystagmus in isolated internuclear ophthalmoplegia from focal pontine lesion, Neurology 64 (2005), 1313–1314. [DOI] [PubMed] [Google Scholar]
- [127]. Patel V.R., Zee D.S., The cerebellum in eye movement control: Nystagmus, coordinate frames and disconjugacy, Eye (Lond) 29 (2015), 191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128]. Prueter C., Schiefer J., Norra C., Podoll K., Sass H., Ping-pong gaze in combined intoxication with tranylcypromine, thioridazine, and clomipramine, Neuropsychiatry Neuropsychol Behav Neurol 14 (2001), 246–247. [PubMed] [Google Scholar]
- [129]. Pula J.H., Newman-Toker D.E., Kattah J.C., Multiple sclerosis as a cause of the acute vestibular syndrome, Journal of Neurology 260 (2013), 1649–1654. [DOI] [PubMed] [Google Scholar]
- [130]. Ramat S., Leigh R.J., Zee D.S., Optican L.M., What clinical disorders tell us about the neural control of saccadic eye movements, Brain: A Journal of Neurology 130 (2007), 10–35. [DOI] [PubMed] [Google Scholar]
- [131]. Rambold H., Kompf D., Helmchen C., Convergence retraction nystagmus: A disorder of vergence? Annals of Neurology 50 (2001), 677–681. [DOI] [PubMed] [Google Scholar]
- [132]. Revilla F.J., de la Cruz R., Khardori N. and Espay A.J., Teaching NeuroImage: Oculomasticatory myorhythmia: Pathognomonic phenomenology of Whipple disease, Neurology 70 (2008), e25. [DOI] [PubMed] [Google Scholar]
- [133]. Roberts D.C., Marcelli V., Gillen J.S., Carey J.P., Della Santina C.C. and Zee D.S., MRI magnetic field stimulates rotational sensors of the brain, Current Biology: CB 21 (2011), 1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134]. Robinson D.A., Zee D.S., Hain T.C., Holmes A., Rosenberg L.F., Alexander’s law: Its behavior and origin in the human vestibulo-ocular reflex, Annals of Neurology 16 (1984), 714–722. [DOI] [PubMed] [Google Scholar]
- [135]. Schwartz M.A., Selhorst J.B., Ochs A.L., Beck R.W., Campbell W.W., Harris J.K., Waters B., Velasco M.E., Oculomasticatory myorhythmia: A unique movement disorder occurring in Whipple’s disease, Annals of Neurology 20 (1986), 677–683. [DOI] [PubMed] [Google Scholar]
- [136]. Senelick R.C., “Ping-pong” gaze. Periodic alternating gaze deviation, Neurology 26 (1976), 532–535. [DOI] [PubMed] [Google Scholar]
- [137]. Shaikh A.G., Reich S., Zee D.S., Pseudonystagmus–clinical features and quantitative characteristics, Nature Reviews. Neurology 6 (2010), 519–523. [DOI] [PubMed] [Google Scholar]
- [138]. Shallo-Hoffmann J., Schwarze H., Simonsz H.J., Muhlendyck H., A reexamination of end-point and rebound nystagmus in normals, Investigative Ophthalmology & Visual Science 31 (1990), 388–392. [PubMed] [Google Scholar]
- [139]. Sharpe J.A., Neurophysiology and neuroanatomy of smooth pursuit: Lesion studies, Brain Cogn 68 (2008), 241–254. [DOI] [PubMed] [Google Scholar]
- [140]. Sharpe J.A., Fletcher W.A., Saccadic intrusions and oscillations, Can J Neurol Sci 11 (1984), 426–433. [DOI] [PubMed] [Google Scholar]
- [141]. Sharpe J.A., Lo A.W., Voluntary and visual control of the vestibuloocular reflex after cerebral hemidecortication, Annals of Neurology 10 (1981), 164–172. [DOI] [PubMed] [Google Scholar]
- [142]. Sieben A., Crevits L., Santens P., Saccadic ping pong gaze in coma, Neurologist 13 (2007), 161–163. [DOI] [PubMed] [Google Scholar]
- [143]. Sills A.W., Baloh R.W., Honrubia V., Caloric testing 2. results in normal subjects, The Annals of Otology, Rhinology & Laryngology. Supplement 86 (1977), 7–23. [DOI] [PubMed] [Google Scholar]
- [144]. Soechting J.F., Flanders M., Moving in three-dimensional space: Frames of reference, vectors, and coordinate systems, Annual Review of Neuroscience 15 (1992), 167–191. [DOI] [PubMed] [Google Scholar]
- [145]. Steddin S., Ing D., Brandt T., Horizontal canal benign paroxysmal positioning vertigo (h-BPPV): Transition of canalolithiasis to cupulolithiasis, Annals of Neurology 40 (1996), 918–922. [DOI] [PubMed] [Google Scholar]
- [146]. Tarnutzer A.A., Berkowitz A.L., Robinson K.A., Hsieh Y.H., Newman-Toker D.E., Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome, CMAJ 183 (2011), E571–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147]. Taylor R.L., Chen L., Lechner C., Aw S.T., Welgampola M.S., Vestibular schwannoma mimicking horizontal cupulolithiasis, Journal of clinical neuroscience: Official journal of the Neurosurgical Society of Australasia 20 (2013), 1170–1173. [DOI] [PubMed] [Google Scholar]
- [148]. Thurston S.E., Leigh R.J., Osorio I., Epileptic gaze deviation and nystagmus, Neurology 35 (1985), 1518–1521. [DOI] [PubMed] [Google Scholar]
- [149]. Tian J., Zee D.S., Walker M.F., Rotational and translational optokinetic nystagmus have different kinematics, Vision Research 47 (2007), 1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150]. Tilikete C., Jasse L., Pelisson D., Vukusic S., Durand-Dubief F., Urquizar C., Vighetto A., Acquired pendular nystagmus in multiple sclerosis and oculopalatal tremor, Neurology 76 (2011), 1650–1657. [DOI] [PubMed] [Google Scholar]
- [151]. Tilikete C., Krolak-Salmon P., Truy E., Vighetto A., Pulse-synchronous eye oscillations revealing bone superior canal dehiscence, Annals of Neurology 56 (2004), 556–560. [DOI] [PubMed] [Google Scholar]
- [152]. Tusa R.J., Kaplan P.W., Hain T.C., Naidu S., Ipsiversive eye deviation and epileptic nystagmus, Neurology 40 (1990), 662–665. [DOI] [PubMed] [Google Scholar]
- [153]. Van der Stigchel S., Meeter M. and Theeuwes J., Eye movement trajectories and what they tell us, Neurosci Biobehav Rev 30 (2006), 666–679. [DOI] [PubMed] [Google Scholar]
- [154]. Vannucchi P., Pecci R., Giannoni B., Di Giustino F., Santimone R. and Mengucci A., Apogeotropic Posterior Semicircular Canal Benign Paroxysmal Positional Vertigo: Some Clinical and Therapeutic Considerations, Audiol Res 5 (2015), 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155]. Venkateswaran R., Gupta R., Swaminathan R.P., Bruns nystagmus in cerebellopontine angle tumor, JAMA Neurol 70 (2013), 646–647. [DOI] [PubMed] [Google Scholar]
- [156]. von Brevern M., Bertholon P., Brandt T., Fife T., Imai T., Nuti D. and Newman-Toker D., Benign paroxysmal positional vertigo: Diagnostic criteria, Journal of Vestibular Research: Equilibrium & Orientation 25 (2015), 105–117. [DOI] [PubMed] [Google Scholar]
- [157]. von Brevern M., Radtke A., Clarke A.H. and Lempert T., Migrainous vertigo presenting as episodic positional vertigo, Neurology 62 (2004), 469–472. [DOI] [PubMed] [Google Scholar]
- [158]. Walker M.F., Zee D.S., Directional abnormalities of vestibular and optokinetic responses in cerebellar disease, Annals of the New York Academy of Sciences 871 (1999), 205–220. [DOI] [PubMed] [Google Scholar]
- [159]. Walker M.F., Zee D.S., The effect of hyperventilation on downbeat nystagmus in cerebellar disorders, Neurology 53 (1999), 1576–1579. [DOI] [PubMed] [Google Scholar]
- [160]. Ward B.K., Otero-Millan J., Jareonsettasin P., Schubert M.C., Roberts D.C., Zee D.S., Magnetic Vestibular Stimulation (MVS) As a Technique for Understanding the Normal and Diseased Labyrinth, Frontiers in Neurology 8 (2017), 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161]. Weber Y.G., Roesche J., Lerche H., Epileptic nystagmus: Two case reports, clinical and pathophysiological review of the literature, Journal of Neurology 253 (2006), 767–771. [DOI] [PubMed] [Google Scholar]
- [162]. Wenzel A., Ward B.K., Schubert M.C., Kheradmand A., Zee D.S., Mantokoudis G., Carey J.P., Patients with vestibular loss, tullio phenomenon, and pressure-induced nystagmus: Vestibular atelectasis? Otol Neurotol 35 (2014), 866–872. [DOI] [PubMed] [Google Scholar]
- [163]. Yagi T., Koizumi Y., Sugizaki K., 3D analysis of spontaneous nystagmus in early stage of vestibular neuritis, Auris Nasus Larynx 37 (2010), 167–172. [DOI] [PubMed] [Google Scholar]
- [164]. Yagi T., Koizumi Y., Sugizaki K., Rotation vectors of slow and quick phase of caloric nystagmus, Auris Nasus Larynx 39 (2012), 475–478. [DOI] [PubMed] [Google Scholar]
- [165]. Younge B.R., Khabie N., Brey R.H., Driscoll C.L., Rotatory nystagmus synchronous with heartbeat: A treatable form of nystagmus, Trans Am Ophthalmol Soc 101 (2003), 113–117; discussion 117-118. [PMC free article] [PubMed] [Google Scholar]
- [166]. Zee D.S., Ophthalmoscopy in examination of patients with vestibular disorders, Annals of Neurology 3 (1978), 373–374. [DOI] [PubMed] [Google Scholar]
- [167]. Zee D.S., Leigh R.J., Mathieu-Millaire F., Cerebellar control of ocular gaze stability, Annals of Neurology 7 (1980), 37–40. [DOI] [PubMed] [Google Scholar]
- [168]. Zee D.S., Preziosi T.J., Proctor L.R., Bechterew’s phenomenon in a human patient, Annals of Neurology 12 (1982), 495–496. [DOI] [PubMed] [Google Scholar]