Abstract
Background
and purpose: Radiation recall dermatitis is an adverse event predominantly due to systemic therapy administration after a previous radiation therapy course. Few case reports describe radiation recall dermatitis in breast cancer patients treated with postoperative radiation therapy following COVID-19 vaccination. In this study we investigated the incidence and severity of radiation recall dermatitis after COVID-19 vaccination in irradiated breast cancer patients.
Methods
Patients that received at least one COVID-19 vaccination dose during the year after the end of postoperative breast radiation therapy were included in this observational monocentric study. Local symptoms occurring inside the radiation field after vaccination were patient-reported and scored according to the PRO-CTCAE questionnaire. Descriptive data of radiation recall dermatitis incidence and severity, and potential risk factors were evaluated.
Results
A cohort of 361 patients with 756 administered COVID-19 vaccinations was analyzed. Breast symptoms were reported by 7.5% of patients, while radiation recall dermatitis was considered for 5.5%. The incidence of radiation recall dermatitis per single dose of vaccine was 2.6%, with a higher risk for the first dose compared to the second/third (4.4% vs 1%, p = 0.003), especially when administered within the first month after the end of irradiation (12.5% vs 2.2%, p = 0.0004). Local symptoms were generally self-limited and a few cases required anti-inflammatory drugs.
Conclusions
Radiation recall dermatitis is an uncommon but not rare phenomenon in breast cancer patients that received COVID-19 vaccination within one year after breast irradiation. However, symptoms severity were generally low/mild and reversible. These findings can be useful for patient counseling.
Keywords: Breast cancer, Radiation therapy, Radiation recall dermatitis, COVID-19 vaccination
Abbreviations: BC, breast cancer; RRD, radiation recall dermatitis; RRP, radiation recall phenomenon; EBRT, external beam radiation therapy
Highlights
-
•
First study that reported the incidence of radiation recall dermatitis after COVID-19 vaccination in breast cancer patients.
-
•
Radiation recall dermatitis after COVID-19 vaccination was not a rare event in breast cancer patients.
-
•
Symptom severity of radiation recall dermatitis after COVID-19 vaccination was low/moderate and self-limiting.
1. Introduction
Radiation therapy is an integral part of a multidisciplinary management of breast cancer (BC) treatment and depending on cancer severity, often involves the use of other systemic therapies, such as chemotherapy, targeted therapies and endocrine therapies. Every treatment is associated with valuable clinical benefit but not always overlapping, with profiles of tolerability. For instance, the radiation recall phenomenon (RRP) is a complication which can occur in a previously irradiated site, weeks to months or even years after radiation [1]. RRP is triggered by the administration of systemic drugs, most frequently chemotherapy and immunotherapy drugs, but also other drugs, like antibiotics, after radiation treatment [1]. Generally, it has the histopathological and the clinical features of an acute inflammation and can occur in a variety of tissues, the commonest being the skin (defined as radiation recall dermatitis (RRD)), which accounts for two third of the reported cases [2].
To date, the radiation recall triggering mechanism remains poorly understood and its estimated incidence accounts for approximately 8.8% with cytotoxic chemotherapy [3]. Previous evidence reported a mixed non-specific inflammatory infiltrate as a common histopathologic criteria [4]. This phenomenon is probably under-reported since most of the published data are presented as case reports limiting the opportunity to determine the actual risk of occurrence.
Also, COVID-19 vaccines are reported as a cause of RRD. Starting from December 2020 COVID-19 vaccination was progressively available in EU countries. According to the European Medical Agency (EMA) COVID-19 vaccines mRNA-based (Pfizer-BioNTech [5] and Moderna) [6] and, following, adenoviral vector-based (Astrazeneca [7] and Janssen COVID-19 vaccine) [8] were approved also for patients with cancer. Specifically, in BC patients, postoperative radiation therapy was not associated with leucopenia, therefore, no specific recommendations regarding the type of the available vaccination platforms or the vaccination timing or other precautions were advanced [9]. To date, the actual incidence of RRD related to the administration of COVID-19 vaccine is unknown and only a few case reports described this phenomenon related to COVID-19 vaccination in cancer patients [10].
In the present study we evaluated the incidence of RRD after COVID-19 vaccination in BC patients previously treated with postoperative radiation therapy. Also, the potential effect of factors, like dosing regimen or vaccination timing, on RRD was investigated. To the best of our knowledge this is the first study that reports the incidence and severity of RRD in BC patients treated with radiation therapy prior to vaccination.
2. Materials and methods
2.1. Radiation therapy treatment
All patients received postoperative external beam radiation therapy (EBRT) after lumpectomy or mastectomy. Radiation therapy indication and volume definition were consistent with the Italian Association of Radiotherapy and Clinical Oncology (AIRO) guideline [11]. EBRT was performed with intensity modulated radiation therapy in all patients. Conventional fractionation (50Gy in 25 fractions), moderate hypofractionation (40.05Gy in 15 fractions) and ultra-hypofractionation (26Gy in 5 fractions) were used. Skin was considered as organ at risk and was contoured by subtraction of 0.5 mm from body surface. During treatment planning no dose hotspot were allowed inside the skin volume. Boost on tumor bed, when indicated, was performed with intraoperative radiation therapy (IORT) or with EBRT with photons. Electron based EBRT was never used. None of the patients received chemotherapy during or after EBRT treatment. Anti-HER2 target therapies and/or endocrine therapies were allowed concomitantly and after irradiation. All patients were advised to use local moistures during and after EBRT to reduce skin dermatitis and itching.
2.2. COVID-19 vaccination
Starting from December 2020 COVID-19 vaccines were available in Europe. Following, EMA approved four different vaccines, two mRNA-based (Comirnaty (Pfizer/Biontech) and Spikevax (Moderna)) and two adenoviral vector-based (Vaxzevria (Astrazeneca) and Janssen COVID-19 vaccine (Johnson & Johnson)). All vaccines were allowed to be used in cancer patients. The third dose (booster) was administered only with mRNA-based (Pfizer/Biontech; Moderna) vaccines at least 5 months after second vaccination or after COVID-19 infection. There were no specific cautions or administration timing for patients undergoing breast postoperative radiation therapy.
2.3. Study population and data collection
The study included BC patients treated with postoperative breast EBRT from May 2020 to December 2021 and had received at least one COVID-19 vaccine dose after EBRT. None of the patients received chemotherapy after the EBRT treatment.
Exclusion criteria were: i) patients treated exclusively with partial breast irradiation with IORT or EBRT since the skin dose was very low or limited on a small volume and, ii) patients without COVID-19 vaccination or with all vaccination doses administered before/during EBRT. RRD can potentially occur also years after EBRT, however for study purposes we decided to limit the study population to patients that received at least a dose of COVID-19 vaccine within the year after the end of EBRT.
Patient selection and oncological data were collected through the hospital medical record at the Radiation Oncology Department of the Centro di Riferimento Oncologico of Aviano (CRO) IRCCS, Aviano (Italy). Clinical parameters such as age, EBRT volume and dose, chemotherapy administration before EBRT, presence of breast implants, type of vaccine, interval time between end of EBRT and vaccination, corticosteroid intake concomitant to vaccination, and previous COVID-19 infection were assessed.
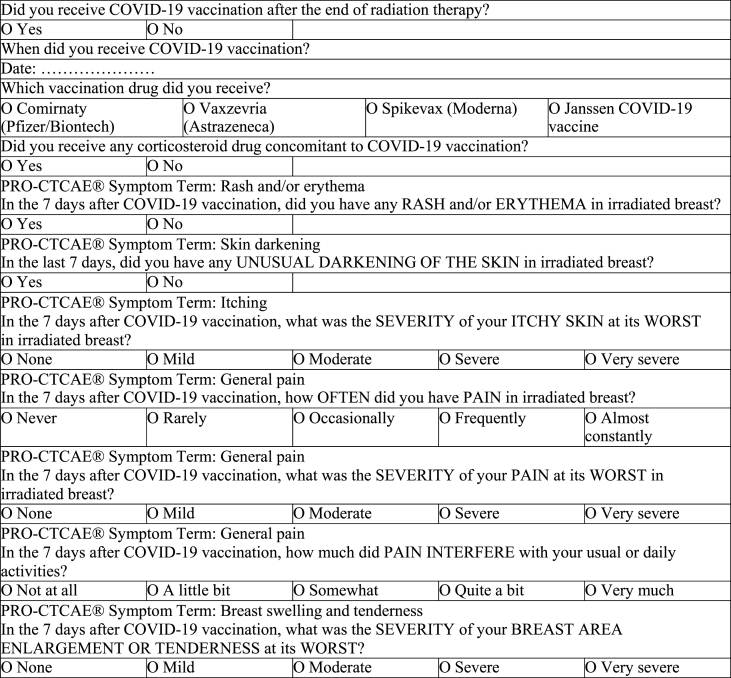
Breast cancer patients were telephone interviewed by a nurse or a medical doctor and responses to the survey questions were recorded. The validated Italian version of the Patient Reported Outcome – Common Terminology Criteria of Adverse Events (PRO-CTCAE) was administered [12]. For study purposes, only the symptoms of the irradiated area, like rash and/or erythema, itching, skin darkening, pain, and breast swelling and tenderness were evaluated (Table 1). All patients that reported one or more local symptoms were re-examined by a radiation oncologist. Patients with symptoms that did not improve in one week after vaccination were invited for a clinical check-up. This analysis was a preliminary evaluation of a trial approved by the Friuli Venezia Giulia regional ethics committee and registered at number CRO-2021-50. Privacy consent was obtained before EBRT treatment, whereas informed consent was obtained verbally during the telephone interview.
Table 1.
Questionnaire used for telephone interview with PRO-CTCAE scale (adapted).
PRO-CTCAE (adapted) questionnaire used for the interview. Items of acute local toxicity as rash, itching pain, skin darkening and breast swelling and tenderness were referred to the week after COVID-19 vaccination.
2.4. Study definitions and statistical analysis
RRD was defined as the acute skin inflammation in the previously irradiated field that occurred at the first two weeks after the COVID-19 vaccine administration. An RRD event was registered when local rash and/or erythema were present, alone or associated with other symptoms, like pain, itching or breast swelling. A minimum interval time of 15 days from the end of EBRT and COVID-19 vaccination was considered necessary to avoid misclassification of radiosensitization or delayed radiation-induced skin dermatitis for an RRD event. Additionally, RRD was registered only if recall symptoms developed after resolution of acute radiation toxicity.
The calculated RRD incidence was correlated with patient characteristics, EBRT parameters and timing of COVID-19 vaccine administration. Statistical analyses were performed using SPSS software, Version 20. Age subgroups, chemotherapy administration, EBRT schedule, EBRT boost administration, breast implant presence, COVID-19 vaccine type and timing were considered as possible risk factors for RRD. Pearson's chi-square test and Fisher's exact test were used to test for differences between subgroups and p-values <0.05 were considered statistically significant.
3. Results
3.1. Radiation therapy and COVID-19 vaccination characteristics
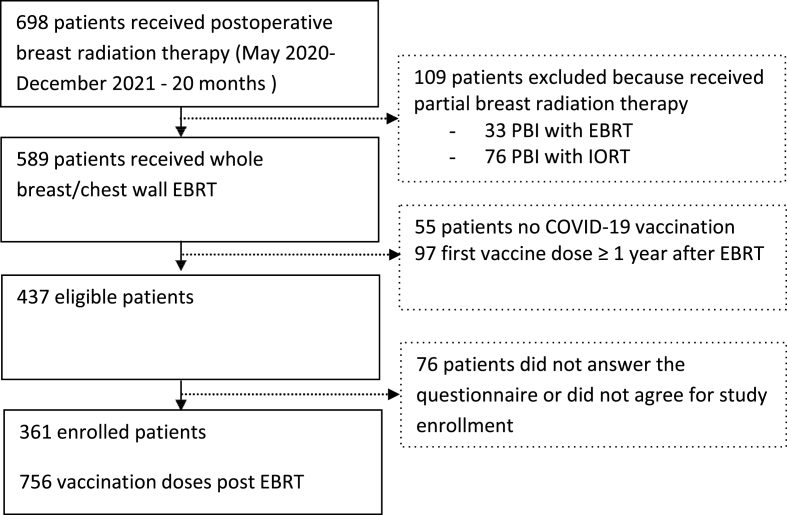
Of 698 patients treated between May 2020 and December 2021, 437 were eligible for this study. All patients were phone called; complete questionnaires and informed consents were available for 361 patients. Patients' selection process flowchart is depicted in Fig. 1. All patients were females, with a median age of 58 years (range, 30–85 years) at the beginning of EBRT. Treatment characteristics are summarized in Table 2.
Fig. 1.
Patients' selection process flowchart
EBRT = External Beam Radiation Therapy; IORT= Intraoperative Radiation Therapy.
Table 2.
External Beam Radiation Therapy (EBRT) characteristics.
| Treatment type | N° patients |
| EBRT side | |
| Left | 172 (47.6%) |
| Right | 183 (50.7%) |
| Bilateral | 6 (1.7%) |
| Dose schedule: | |
| Conventional fractionation (50Gy/25 fractions) | 3 (0.8%) |
| Moderate hypofractionation (40.05Gy/15 fractions) | 261 (72.3%) |
| Ultra-hypofractionation (26Gy/5 fractions) | 97 (26.9%) |
| Boost schedule: | |
| Without boost | 208 (57.6%) |
| With concomitant boost (48Gy) | 92 (25.5%) |
| With anticipated IORT boost (20Gy in single fraction) | 35 (9.7%) |
| With consecutive boost (16Gy in 8 fractions) | 26 (7.2%) |
| Systemic therapies | |
| Anthracycline-based chemotherapy | 109 (30.1%) |
| Taxane-based chemotherapy | 119 (33%) |
| Anti-HER2 drugs | 33 (0.8%) |
| Endocrine therapy | 260 (72%) |
| No systemic therapy | 45 (12.5%) |
| Presence of tissue expander before EBRT | |
| Yes | 17 (4.7%) |
| No | 344 (95.3%) |
Postoperative breast radiation therapy characteristics were described. Systemic therapies before or concomitant to irradiation and presence of breast implants were evaluated as possible risk factors for skin toxicity.
Of 361 eligible BC patients, 8 (2.2%) patients received only 1 dose of COVID-19 vaccination, 53 (14.7%) received 2 vaccination doses, while 300 (83.1%) patients received the complete vaccination (3 doses). A total number of 1012 vaccine doses were administered, 256 (25%) doses were administered before or during EBRT and 756 (75%) doses after EBRT. Median time between the end of EBRT and the vaccination was 186 days (range:15–365 days). A total of 963 (95%) of the administered vaccine doses were mRNA-based (745 doses Comirnaty and 218 doses Spikevax), while 51 (5%) were adenoviral vector-based (46 doses Vaxzevria and 5 doses Janssen COVID-19 vaccine). Fourteen (3.9%) patients assumed corticosteroid concomitantly to vaccination, while 347 (96.1%) did not assume any medication.
3.2. Radiation recall dermatitis incidence and characteristics
Overall, 27 BC patients reported at least one local symptom in the irradiated area during the two weeks after COVID-19 vaccination. Of these, after evaluation by a radiation oncologist, RRD was diagnosed for 20 (5.5%) patients, where 4 patients reported dermatitis alone and 16 patients reported dermatitis associated with other symptoms (itching, local pain or breast swelling) in the irradiated area. Seven (1.9%) patients that reported only itching (3 cases) or local pain (4 cases) and without signs of dermatitis were not considered as RRD.
The mean timing between dose administration and beginning of RRD was 4 days (range, 1–14 days). For 16 patients, the reported time interval was 2–4 days. Dermatitis and the other symptoms were self-limiting and did not require any medication in 15 (75%) cases, while 5 patients assumed analgesic or steroid medication. All symptoms completely recovered in few weeks except two patients, which developed chronic hypercromia in the irradiated field. Additional details regarding patients' characteristics are summarized in Table 3.
Table 3.
Patients' reported symptoms in the irradiated field.
| Rash and/or erythema | |
|---|---|
| No | 341 (94.5%) |
| Yes | 20 (5.5%) |
| Itching | |
| None | 349 (96.7%) |
| Mild | 6 (1.7%) |
| Moderate | 2 (0.5%) |
| Severe | 3 (0.8%) |
| Very severe | 1 (0.3%) |
| Skin darkening | |
| Yes | 2 (0.6%) |
| No | 359 (99.4%) |
| Pain | |
| Frequency | |
| Never | 346 (95.8%) |
| Rarely | 2 (0.6%) |
| Occasionally | 5 (1.4%) |
| Frequently | 5 (1.4%) |
| Almost constantly | 3 (0.8%) |
| Severity | |
| None | 346 (95.8%) |
| Mild | 5 (1.4%) |
| Moderate | 7 (1.9%) |
| Severe | 2 (0.6%) |
| Very severe | 1 (0.3%) |
| Interference with usual/daily activity | |
| Not at all | 351 (97.2%) |
| A little bit | 6 (1.7%) |
| Somewhat | 3 (0.8%) |
| Quite a bit | 1 (0.3%) |
| Very much | 0 |
| Breast swelling and tenderness | |
| None | 353 (97.8%) |
| Mild | 4 (1.1%) |
| Moderate | 3 (0.8%) |
| Severe | 1 (0.3%) |
| Very severe | 0 |
Patient reported effects in irradiated field were scored according the PRO-CTCAE scale.
3.3. Risk factors for radiation recall dermatitis
For this analysis 756 post EBRT COVID-19 vaccine doses were considered. The RRD incidence per single dose of vaccination was 2.6%. RRD occurred in 80% (16 out of 20) of patients after receiving the first dose of COVID-19 vaccine, while in 20% (4 out of 20) of patients after receiving the second dose of vaccine. The incidence of RRD was 4.4% after the administration of the first dose of COVID-19 vaccine and 1% after the administration of 2nd or 3rd doses (p = 0.003). All patients that reported RRD during the first or the second dose did not experience a second RRD during the subsequent doses. The timing between end of EBRT and RRD ranged between 15 and 453 days (median: 146 days). The incidence of RRD was significantly higher when the vaccination occurred in the first 30 days after the completion of EBRT (12.5%) compared to the incidence after the first 30 days (2.2%) (p = 0.0004). Of 4 patients that experienced RRD during the first month after EBRT, 3 patients received 40.05Gy/15 fractions and 1 patient received 26Gy/5fractions. All cases were classified as RRD and not as delayed skin radiotoxicity since all cases had a Grade 1 radiodermitis that completely resolved in 5–10 days after EBRT and a new local rash appeared 3–5 days after vaccination. Other risk factors as EBRT schedule, anthracycline-based chemotherapy, taxane-based chemotherapy, anti-HER2 drugs, endocrine therapy, breast reconstruction, EBRT boost administration, type of vaccination platform, COVID-19 infection and corticosteroid intake concomitant to vaccination were not associated with an increased RRD risk at univariate analysis (Table 4).
Table 4.
Risk factors and radiation recall dermatitis (RRD) univariate analysis.
| Risk factors | N° events/n° exposed | % | P value |
|---|---|---|---|
| Timing end EBRT-vaccination | |||
| 15–30 days | 4/32 (doses) | 12.5 | 0.0004 |
| >30 days | 16/724 | 2.2 | |
| Vaccination n° after EBRT | |||
| First vaccination after EBRT | 16/361 (doses) | 4.4 | 0.003 |
| Second/third vaccination after EBRT | 4/395 | 1 | |
| Vaccination drug | |||
| Comirnaty (Pfizer/Biontech) | 14/529 (doses) | 2.4 | NS |
| Spikevax (Moderna) | 6/194 | 3.1 | |
| Vaxzevria (Astrazeneca) | 0/28 | 0 | |
| Janssen COVID-19 vaccine | 0/5 | 0 | |
| EBRT schedule | |||
| 50Gy/25fr. or 40.05Gy/15fr. | 16/264 (patients) | 6.1 | NS |
| 26Gy/5fr | 4/97 | 4.1 | |
| Tumor bed boost | |||
| Yes | 10/208 (patients) | 4.8 | NS |
| No | 10/153 | 6.5 | |
| Anthracycline-based chemotherapy | |||
| Yes | 5/109 (patients) | 4.6 | NS |
| No | 15/252 | 6 | |
| Taxane-based chemotherapy | |||
| Yes | 3/119 (patients) | 2.5 | NS |
| No | 17/242 | 7 | |
| Anti-HER2 drugs | |||
| Yes | 1/33 (patients) | 3 | NS |
| No | 19/328 | 5.8 | |
| Endocrine therapy | |||
| Yes | 16/260 (patients) | 6.1 | NS |
| No | 4/101 | 4 | |
| Age at EBRT beginning | |||
| ≥70 years | 3/62 (patients) | 4.8 | NS |
| <70 years | 17/202 | 8.4 | |
| Delayed breast reconstruction | |||
| Yes | 1/17 (patients) | 5.9 | NS |
| No | 19/344 | 5.5 | |
| COVID-19 infection | |||
| Yes | 2/61 (patients) | 3.3 | NS |
| No | 18/300 | 6 | |
| Corticosteroid intake concomitant vaccination | |||
| Yes | 1/14 (patients) | 7.1 | NS |
| No | 19/347 | 5.5 | |
Univariate analysis for potential risk factors was calculated by chi square-test or fisher-exact test as appropriate. P-value < 0.05 was considered statistically significant.
NS = not significant.
4. Discussion
We described the incidence and severity of RRD, potentially induced by COVID-19 vaccination, in BC patients treated with postoperative EBRT. Patients' local symptoms in the irradiated area were self-reported. RRD was defined as the presence of dermatitis alone or associated with other symptoms like, rash, itching, pain or breast swelling. Local symptoms were considered as an RRD event when present after 15 days from the EBRT completion, in order to avoid misclassification due to EBRT-induced side effects in the irradiated area.
Overall, local symptoms were reported by 7.5% (27 cases) of the BC patients treated with postoperative EBRT. RRD was diagnosed for 5.5% (20 cases) of patients. The incidence of RRD per single dose of vaccine was 2.6%. This risk was significantly higher after the administration of the first dose of COVID-19 vaccine compared to the administration of second or third dose (4.4% vs 1%, respectively, p = 0.003), independent of the timing administration after the EBRT completion. Moreover, the risk of having RRD further increased when the first dose of COVID-19 vaccine was administered within the first month after the completion of the postoperative EBRT treatment compared to the following period, 12.5% vs 2.2%, respectively, and was found to be statistically significant (p = 0.0004).
Although anticancer agents like anthracyclines and taxanes have been identified as the major contributors to RRD onset, COVID-19 vaccine has recently been reported as a RRD triggering agent in cancer patients. The first two cases were described by Soyfer et al. [10], which reported the RRD event in two BC patients after receiving 2 doses of mRNA-based vaccination (Comirnaty, Pfizer/Biontech). With the increase of COVID-19 vaccination other case reports described similar findings, also for different vaccination platforms, i.e. viral vector-based vaccines [[13], [14], [15], [16]].
RRD incidence is difficult to report as the literature is primarily limited to single cases, with a few studies reporting an incidence of 7%–8.8% [1,3]. Furthermore, due to the recent introduction of COVID-19 vaccination the RRD risk is even less known. To date, RRD was considered a rare event but from our study it appears that in irradiated BC patients the occurrence of RRD is “not rare”, accordingly to the frequency scale for adverse drug reactions of Council for International Organization of Medical Science [17].
In our dataset we found the highest risk of having RRD when the first dose of COVID-19 vaccine was administered within the first 30 days after treatment with postoperative EBRT. Even though there is no data regarding the specific timing for RRD development, one paper estimated that most cases occurred within 40 days Camidge et al. [18], in agreement with our results. As reported in the literature [19], we also found that the risk of having RRD did not depend on the postoperative radiation dose (dose schedule or boost addiction), age, breast reconstruction or previous chemotherapy or by a previous COVID-19 infection. Similarly to what observed for drugs, RRD potentially triggered by COVID-19 vaccination appear also to be self-limiting. In fact, in our study only 4 out of 20 patients required anti-inflammatory drugs whereas for the other patients the acute symptoms recovered spontaneously. Although the majority of patients had low-mild symptoms, they must be informed and monitored as there are no accurate predictors of their occurrence.
Our study presents some limitations. Firstly, we based our RRD incidence analysis on patient reported symptoms obtained through telephone surveys. Secondly, not all patients received three doses of COVID-19 vaccination (83% of patients), thus the RRD incidence could be under-estimated. Also, we could not evaluate potential differences between the different COVID-19 vaccination platforms since the adenoviral vector-based vaccines were used in a small fraction of the study population. Thirdly, delayed skin radiotoxicity can occur between 1 and 6 weeks after EBRT [20] and this could potentially overestimate the RRD incidence in our study. However, the 4 cases with RRD that occurred within the 6 weeks after EBRT were re-evaluated and confirmed as RRD events since all cases had a recall of symptoms after resolution of a previous radiation dermatitis.
Lastly, we restricted our analysis to patients that received the first vaccination dose within one year after the treatment with postoperative EBRT, thus we could miss the onset of later RRD event.
To date, the radiation recall triggering mechanism remains still unknown. Different hypotheses have been proposed, as stem cell dysfunction in the irradiated area, keratinocyte necrosis, activation of inflammatory pathways, or even a hypersensitivity reaction to the inducing agent [18,19]. Although, investigating the triggering mechanism of RRD potentially induced by COVID-19 vaccination was not the aim of the study, we hypothesize that the release of the inflammatory cytokines could be a potential major player. For instance, it was shown an increased expression of IL-2 and IL-6 cytokines in BC patients treated with radiation therapy within one month after treatment, whereas TNF-alpha levels were stable up to 6 months after treatment [21]. These three pro-inflammatory cytokines could be further up-regulated also by COVID-19 vaccination [22] as a consequence of a local hypersensitivity reaction [18] induced by the vaccine. Additional studies in cancer patients undergoing EBRT and COVID-19 vaccination should be performed to clarify the pathophysiology of RRD.
5. Conclusions
To the best our best knowledge, this is the first and largest study that estimates the incidence of RRD induced by COVID-19 vaccination in patients treated with postoperative breast radiation therapy. We found that the frequency of this phenomenon is not rare, occurs most often after 1st vaccination dose, with low-mild severity and generally with self-limiting symptoms. Current COVID-19 vaccination management remains safe and this new data could be used for proper patients' information.
Funding
This work was supported by Ministero della Salute Ricerca Corrente.
Author contributions
Concept and design: Vinante, Baboci. Acquisition, analysis, or interpretation of data: Vinante, Marson, Giroldi, Caroli, Relevant, Bertini. Drafting of the manuscript: Vinante, Baboci. Critical revision of the manuscript for important intellectual content: All authors.
Declaration of competing interest
The authors Vinante L., Caroli A., Bertini F., Relevant A., Giroldi A., Marson M., Franchin G., Muraro E., Brisotto G., Steffan A., Baboci L. disclosed no potential conflicts of interest.
Acknowledgments
Not applicable.
References
- 1.Burris H.A., 3rd, Hurtig J. Radiation recall with anticancer agents. Oncol. 2010;15:1227–1237. doi: 10.1634/theoncologist.2009-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drost L., Li N., Vesprini D., Sangha A., Lee J., Leung E., et al. Prospective study of breast radiation dermatitis. Clin Breast Cancer. 2018;18:e789–e795. doi: 10.1016/j.clbc.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Kodym E., Kalinska R., Ehringfeld C., Sterbik-Lamina A., Kodym R., Hohenberg G. Frequency of radiation recall dermatitis in adult cancer patients. Onkologie. 2005;28:18–21. doi: 10.1159/000082175. [DOI] [PubMed] [Google Scholar]
- 4.Azria D., Magne N., Zouhair A., Castadot P., Culine S., Ychou M., et al. Radiation recall: a well recognized but neglected phenomenon. Cancer Treat Rev. 2005;31:555–570. doi: 10.1016/j.ctrv.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 5.https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty
- 6.https://www.ema.europa.eu/en/medicines/human/EPAR/spikevax
- 7.https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-covid-19-vaccine- astrazeneca
- 8.https://www.ema.europa.eu/en/medicines/human/EPAR/covid-19-vaccine-janssen
- 9.https://www.esmo.org/covid-19-and-cancer/covid-19-vaccination
- 10.Soyfer V., Gutfeld O., Shamai S., Schlocker A., Merimsky O. COVID-19 vaccine-induced radiation recall phenomenon. Int J Radiat Oncol Biol Phys. 2021;110:957–961. doi: 10.1016/j.ijrobp.2021.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruppo AIRO mammella . 2019. GdcA. Best Clinical Practice nella radioterapia dei tumori della mammella.https://www.radioterapiaitalia.it/wp-content/uploads/2019/09/Best-Clinical-Practice-nella-radioterapia-dei-tumori-della-mammella-2019.pdf [Google Scholar]
- 12.https://healthcaredelivery.cancer.gov/pro-ctcae/measurement.html
- 13.Afacan E., Ogut B., Ustun P., Senturk E., Yazici O., Adisen E. Radiation recall dermatitis triggered by inactivated COVID-19 vaccine. Clin Exp Dermatol. 2021;46:1582–1584. doi: 10.1111/ced.14786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart R., McDowell L. Radiation recall phenomenon following COVID-19 vaccination. Int J Radiat Oncol Biol Phys. 2021;111:835–836. doi: 10.1016/j.ijrobp.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishikawa Y., Umezawa R., Yamamoto T., Takahashi N., Takeda K., Suzuki Y., et al. Radiation recall phenomenon after administration of the mRNA-1273 SARS-CoV-2 vaccine. Int Cancer Conf J. 2022:1–5. doi: 10.1007/s13691-021-00528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross R.B., Rabinovitch R.A. Radiation recall after COVID-19 infection. Lancet Oncol. 2022;23(4):e197. doi: 10.1016/S1470-2045(22)00038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.https://cioms.ch
- 18.Camidge R., Price A. Characterizing the phenomenon of radiation recall dermatitis. Radiotherapy and oncology. J Eur Soc Therap Radiol Oncol. 2001;59:237–245. doi: 10.1016/s0167-8140(01)00328-0. [DOI] [PubMed] [Google Scholar]
- 19.McKay M.J., Foster R. Radiation recall reactions: an oncologic enigma. Crit Rev Oncol-Hematol. 2021;168 doi: 10.1016/j.critrevonc.2021.103527. [DOI] [PubMed] [Google Scholar]
- 20.Brunt A.M., Wheatley D., Yarnold J., Somaiah N., Kelly S., Harnett A., et al. FAST-Forward Trial Management Group. Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the UK FAST-Forward Trial. Radiother Oncol. 2016;120(1):114–118. doi: 10.1016/j.radonc.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marconi R., Serafini A., Giovanetti A., Bartoleschi C., Pardini M.C., Bossi G., et al. Cytokine modulation in breast cancer patients undergoing radiotherapy: a revision of the most recent studies. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20020382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergamaschi C., Terpos E., Rosati M., Angel M., Bear J., Stellas D., et al. Systemic IL-15, IFN- gamma, and IP-10/CXCL10 signature associated with effective immune response to SARS-CoV-2 in BNT162b2 mRNA vaccine recipients. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109504. [DOI] [PMC free article] [PubMed] [Google Scholar]