Abstract
Cardiovascular disease (CVD) is a broad term that incorporated a group of conditions that affect the blood vessels and the heart. CVD is a foremost cause of fatalities around the world. Multiple pathophysiological mechanisms are involved in CVD; however, oxidative stress plays a vital role in generating reactive oxygen species (ROS). Oxidative stress occurs when the concentration of oxidants exceeds the potency of antioxidants within the body while producing reactive nitrogen species (RNS). ROS generated by oxidative stress disrupts cell signaling, DNA damage, lipids, and proteins, thereby resulting in inflammation and apoptosis. Mitochondria is the primary source of ROS production within cells. Increased ROS production reduces nitric oxide (NO) bioavailability, which elevates vasoconstriction within the arteries and contributes to the development of hypertension. ROS production has also been linked to the development of atherosclerotic plaque. Antioxidants can decrease oxidative stress in the body; however, various therapeutic drugs have been designed to treat oxidative stress damage due to CVD. The present review provides a detailed narrative of the oxidative stress and ROS generation with a primary focus on the oxidative stress biomarker and its association with CVD. We have also discussed the complex relationship between inflammation and endothelial dysfunction in CVD as well as oxidative stress-induced obesity in CVD. Finally, we discussed the role of antioxidants in reducing oxidative stress in CVD.
1. Introduction
Cardiovascular diseases (CVDs) are a group of ailments that affect the heart and the blood vessels. Primary types of CVDs include coronary heart disease, rheumatic heart disease, peripheral arterial disease, cerebrovascular disease, and stroke. According to the World Health Organization (WHO), CVD is a complex disease and a leading cause of death worldwide accounting for 31% of all fatalities. CVD is mainly caused by the deposition of fatty acids in the inner wall of the blood vessels. Approximately, 17.6 million people have died due to CVD in 2016. According to a report from the Royal College of the cardiologist, the number of CVD-related facilities increased by 14.5 percent in the United Kingdom between 2006 and 2016 [1]. Several factors contribute to CVD such as high cholesterol, smoking, and elevated blood pressure but a major contributing factor still remains oxidative stress. Oxidative stress generates free radicals and reactive oxygen species (ROS); when these are abundant, the primary structures that are affected in cells are DNA, lipids, and proteins, resulting in cell death [2]. The processes such as neurological system deregulation, immune cell degeneration, and response control are known to be affected by ROS exhibiting both positive and negative effects. Oxidative damage to the cell in CVD causes myocyte dysfunction, which leads to cell death [3]. ROS affect contractile function directly by altering the proteins that are involved in excitation-contraction coupling. Therefore, reduced ROS formation should be employed to prevent and treat cardiovascular disease [4]. However, analyzing redox systems is difficult due to significant subcellular variations in redox potential and the short lifetime of ROS. The discovery of different biomarkers of oxidative stress warrants further investigation to evaluate CVD [5]. Despite significant efforts, elucidating the pathophysiologic pathways driving the onset and progression of CVD still remains a work in progress [6–8].
The purpose of this review is to demonstrate the relationship between oxidative stress and CVD with emphasis on the following points: oxidative enzymes cause endothelial dysfunction, oxidative stress biomarkers that are involved in CVD, the link between oxidative stress-induced inflammation and CVD, and the vital role of different antioxidants in lowering free radical levels in CVD.
2. Oxidative Stress and ROS
Oxidative stress is characterized as a natural imbalance between the production and accumulation of ROS, which plays a role in the ageing process [9, 10]. ROS are oxidants and an excessive ROS induces oxidative stress. ROS generation and the intracellular immune system are important regulators of intracellular oxidative equilibrium. Increased cellular ROS production plays a vital role in LDL oxidation, endothelial dysfunction, and inflammatory processes [11]. Excessive ROS production exhibits a negative impact on the biological system by serving as a second messenger in cellular signaling and affecting natural components like lipids, proteins, and DNA [12]. Various environmental elements, such as UV rays, radiation, smoking cigarettes, and heavy alcohol use, encourage ROS generation that leads to the emergence of several illnesses like cancer and CVD [13–15].
3. CVD Concerning Oxidative Stress
According to the WHO, CVDs are complex diseases that cause a significant number of deaths worldwide. An increase in ROS production has been linked to various cardiovascular disorders [16–19]. Oxidative stress has been strongly linked to myocardial infarction (MI), ischemia/reperfusion, and heart failure [20]. Heart attacks and strokes are unexpected occurrences that are caused due to the lack of blood supply to reach the heart or brain as a result og blockage. Atherosclerosis is the most prevalent cause of CVD. It is caused by artery hardness and constriction, which results in decreased oxygen and blood distribution throughout the body [21]. Plaque development in the inner walls of the coronary artery is characterized by the deposition of large amounts of LDL cholesterol, cellular debris, and other elements [22]. Lipids, especially polyunsaturated fatty acids (PUFAs) and cholesterol, are critical oxidative stress target substrates as lipids that make up cell membranes. Various reactive aldehydes are generated during lipid oxidation depending on the type of PUFA trans-4-hydroxy-2-nominal, isoprostanes, and malondialdehyde [23]. Within macrophages, oxidized LDLs are endocytosed, which is a gradual process that leads to a build-up in the intima and culminats in the atherosclerosis. Studies on endothelial cell damage produced by LDL modifiers have shown that glycated LDL (gLDL) and oxidized LDL (oxLDL) are associated with atherogenic processes. Modified natural LDL components such as apoβ, a surface protein of LDL, improve the ability of LDL to attach to its receptor [24]. In contractile failure, the contractility is altered by the oxidation of Sarco and endoplasmic Ca+2-ATPase as well as contractile proteins, including tropomyosin and actin [25, 26]. The impact of ROS on endothelium underpinning molecules that can induce death, inflammation, and therefore clotting plaque formation in atherosclerosis makes oxidative stress a critical characteristic of CVD, which is also characterized as an initial causal component [27, 28].
4. Sources of ROS in CVD
Both endogenous and exogenous factors produce ROS in CVD. Mitochondria are the primary source of endogenous ROS via the electron transport chain (ETC) and oxidative phosphorylation that produce by-products in CVD [29]. Mitochondria is the primary source of ATP synthesis involving complexes I to V. The mitochondria's respiratory chain is disturbed during pathological conditions. Oxygen drains into the electron to form superoxide [30]. Isoproterenol or Ang II leads to increased mitochondrial superoxide anion generation during cardiomyocyte hypertrophy [31, 32]. Increased oxidative stress and ROS generation are linked to mitochondrial respiratory chain abnormalities; as a result, many transcription factors and protein kinase signaling pathways that are involved in cardiac hypertrophic are activated [33]. Mitochondria is the primary generator of ROS, especially in cardiac cells [27]. The two most prevalent ROS generating sources in the ETC are complex I as well as complex III to facilitate pore opening (KATP and mPTP) in mitochondrial outer and inner membranes [34]. Cyclosporin is the inhibitor of mPTP [35]. In a recent study, complex II ROS possesses cardioprotective properties, whereas complex I ROS generation causes damage and is linked to CVD [36]. Complex III-produced ROS causes delayed channel opening and is associated with improved functional recovery following injury to the ischemic-reperfusion, which is identified as a major risk factor of oxidative stress [37]. Xanthine oxidase, lipoxygenase, myeloperoxidase (MPO), noncoupling nitric oxide synthase (NOS), and NADPH oxidase (NOX) are known to be associated with ROS generation [38] (Figure 1). Several investigations have revealed that the mitochondria and NOX are both significant ROS producers and hyperglycemia is linked to mitochondria-NOX ROS interaction [39]. Further, mitochondrial biogenesis and mitophagy pathways that regulate mitochondrial dynamics are critical in maintaining healthy cardiomyocytes [40]. Ketogenic diet can accelerate cardiac fibrosis by reducing mitochondria-associated membranes and inhibiting mitochondrial function [41]. Secreted frizzled-related protein 2 (SFRP2) that interfere with Wnt is known to modulate mitochondrial dynamics and mitochondrial biogenesis as well as exert cardioprotective effects within diabetic cardiomyopathy [42].
Figure 1.
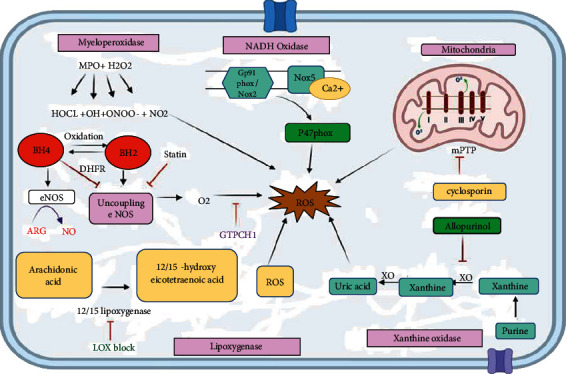
Schematic representation and molecular mechanism of the sources of ROS generation in CVD. Mitochondria is the leading cause of ROS generation; complexes I and III produce superoxides and open mPTP pores in the mitochondrial membrane through ROS release. NOX2/gp91phox generates O2•, and other members of the NOX1, NOX4, and NOX5 family are known to be involved in ROS generation. Xanthine oxidase accepts an electron from O2 and produces O2• while its action can be blocked by allopurinol. Lipoxygenase produces ROS by acting on arachidonic acid into HETE, and LOXBlock-1 blocks this step. When eNOS is uncoupled, it forms O2• instead of NO and helps in ROS production. MPO is the critical oxidative stress biomarker that reacts with H2O2 and produces various super radicals, which are the primary source of ROS.
4.1. NADPH Oxidase (NOX)
NADPH oxidase is the major enzyme involved in ROS production within the mitochondria. NADPH oxidase produced in mammalian phagocytes is the first known enzyme specialized to generate O2 [43]. NOX uses NADPH or NADH as an electron donor to catalyze the conversion of dioxygen into superoxide anion. NOX family members responsible for the generation of ROS in CVD are NOX1, NOX2, NOX4, and NOX5. The oxygen synthesis mainly characterizes NOX enzymes; however, NOX4 is an outlier due to its primary hydrogen peroxide production (H2O2) [21]. NOX2 and NOX4 were shown to generate ROS in cardiomyocytes and fibroblasts, whereas NOX1, NOX4, and NOX5 were identified in vascular smooth muscle cells [44]. The control of NOX2, also called gp91phox, is being extensively researched. NOX2 is activated by other NOX subunits such as p22phox, p67phox, p40phox, and p47phox. p47phox activation is caused by the phosphorylation at Ser303, Ser304, and Ser328. The stimulated p47phox then interacts with p22phox, allowing NOX2 to access p40phox and p67phox, culminating in NOX2 activation [45]. Upon NOX2 activation, NADPH can bind to intracellular C- terminus, leading to ROS on the extracellular membrane. Furthermore, secondary oxidase systems such as NOS uncoupling, mitochondrial malfunction, and XO activation are stimulated by NOX-derived ROS [12, 46]. NOX5 is calcium dependent and controlled by calcium stimuli; its involvement in CVD is considerably more complicated. NOX4 remains active within the cell in the presence of O2 [47].
NOX4 has been found to enhance ROS production upon TGF-β stimulation [48]. NOX4 regulates smooth muscle-actin upon being expressed by inactivating p38MAPK through MKP-1 oxidization. Moreover, NOX4 activates the RhoA protein kinase, which activates the serum response factor (SRF) and controls the level of myocardin-related transcription factor (MRTF). Subsequently, this leads to the activation of spinal muscular atrophy (SMA). As a result, when NOX4-ROS inhibits the activation of MKP-1 and p38MAPK, p38MAPK phosphorylates SRF, thereby causing VSMC differentiation that is linked to vascular damage [21]. Several lines of evidences indicate that the prediabetic patients with mildly elevated glucose levels are found to be linked with increased risk of heart failure [49, 50] and worse prognosis in patients with heart failure (HF) [51]. High glucose levels have been linked to increased NOX4 synthesis in cardiomyocytes, contributing to heart injury. Overexpression of NOX4 has also found to lower baseline blood pressure, which is connected to H2O2 generation [52].
NOX2 and NOX4 levels are elevated in pulmonary artery hypertension (PAH), and its blockage has shown to revere PAH in animal models [53]. Neutrophil-NOX synthesis is a potent antibacterial agent, which is identified to reduce molecular oxygen into O2, dismutation into H2O2, NOX 4, and chloride ion in hypochlorous acid [54]. Further, upregulation of NOX2, NOX4, and NOX5 has been linked to CVD and atherosclerosis during the early stages of the disease [21].
4.2. Xanthin Oxidase (XO)
The dehydrogenase form of XOs accepts electrons from both molecular oxygen and NAD+, whereas the oxidase form of XOs accepts electrons solely from the molecular oxygen [55]. XO is the first biological process recognized as a source of ROS. Endothelial dysfunction has been related to the generation of XO-ROS, which is caused by the interaction of O2 and NO, resulting in the formation of OONO− that causes cellular damage. In CVD patients, hyperuricemia or elevated uric acid levels are a common occurrence. Xanthine oxidoreductase is an enzyme that is responsible for the synthesis of uric acid and plays an important role in the pathophysiology of CVD [21]. Elevated uric acid is a biomarker for fatal heart failure; hence, XO inhibition will assist in positively treating patients with heart defects [56]. NOX activity is required for Ang II in order to activate XO, as Ang II-induced superoxide generation could be prevented by NOX inhibition [55]. Endothelial XO activity has been shown to be elevated in individuals with coronary artery disease. Allopurinol is an XO inhibitor that may benefit HF patients with endothelial dysfunction [57].
Furthermore, XO's product can be used as a biomarker to detect cardiovascular disorders [58]. Pulmonary hypertension has been linked to reduced XO activity through the early growth response-1 (Egr-1) signaling pathway [59]. Erg-1 expression is induced by XO-ROS, which ultimately leads to CVD [60].
4.3. Lipoxygenase
Lipoxygenase (LOX) oxidizes arachidonic acid (AA), a polyunsaturated fatty acid produced by the plasma membrane following phosphatidylcholine hydrolysis, in order to produce hydroperoxides. 12-LOX and 15-LOX convert arachidonic acid to 12- and 15-hydroxyeicosatetraenoic acid, respectively, and generate ROS (ROS). LOXBlock-1 is the inhibitor of 12/15 lipoxygenase [61]. Hyperglycemia activation of 12/15-LOX is linked to increased cardiovascular oxidative stress and diabetic cardiomyopathy [39]. In rat fibroblasts, cPLA2-arachidonic acid-related cascade might lead to a rise in ROS production via the lipoxygenase pathway. Metabolism of AA with the help of 5-lipoxygenase and cPLA2 activation was shown to be involved in the production of ROS induced by tumor necrosis factor-α (TNF-α) [62]. NSAIDs induce leukotrienes and 5-lipoxygenase levels in the body and increase ROS generation [62]. Through LOX, Ang II causes NOX to be produced in VSMCs and AA catalysis results in ROS being produced in vascular cells. 5-LOX is elevated by cytotoxicity and oxidative stress (NOX or mitochondrial ROS), while 5-LOX catalysis of AA includes leukotriene (LTA4) and 5-HETE lipid mediators [63]. Several proinflammatory molecules are produced by LTA4 metabolism including LTB4, LTC4, LTD4, and LTE4 that subsequently connect with and activate other cells like endothelial cells, neutrophils, mast cells, macrophages, foam cells, and T-cell. Upon activation, these cells release cytokines and metalloproteinase, which exhibit proatherosclerotic functions [64]. LDL molecule oxygenation is a defining feature of 12/15-lipoxygenase action. In diabetic heart patients, inhibition of 12/15-LO leads to a decrease in 4-hydroxy-2-nonenol (4-HEN), an oxidative stress marker, ROS generation, and NOX4 related to 12/15-LO activity [65].
5. Noncoupling Nitric Oxide Synthase
Nitric oxide synthase (NOS) occurs in three forms: inducible NOS (iNOS), endothelial NOS (eNOS), and neuronal NOS. NOS activity produces NO in the cardiovascular system (nNOS) [56]. NO has been included in several clinical studies including CVD, diabetes, and hypersensitive stress, in order to support vasodilation and enzyme failure linked to NO generation. Endothelial nitric oxide synthase may produce NO from l-arginine. BH4 deficiency due to vascular homeostasis regulation caused by eNOS leads to the production of free radicals [62]. When exposed to oxidative stress, BH4 is transformed to 7,8-dihydrobiopterin (BH2), which can bind to eNOS, induced uncoupling, and resulted in the production of O2• rather than NO, which causes oxidative stress to develop [66].
Upregulation of eNOS has been proven to protect against heart dysfunction. It has been shown that during coronary artery ligation, better left ventricle function and decreased left ventricle hypertrophy in transgenic mice are increased by 30-fold during cardiomyocyte eNOS activity [67]. In contrast, cardiomyocyte-specific NOS mutant mice were reported to have dramatically improved survival following heart failure caused by MI, suggesting that iNOS may be implicated in the etiology of heart failure [68].
•NO interacts with molecular oxygen and ROS (ROS) to produce various oxidation products, including RNS. The interaction of •NO with superoxide (O2•) can make peroxynitrite (ONOO−) at virtually diffusion-limited rates, one of the most significant RNS-generating reactions. It also acts as an oxidant when protonated, undergoing homolytic scission to produce nitrogen dioxide and hydroxyl radical (•OH) [69].
6. Myeloperoxidase (MPO)
Myeloperoxidase is a member of the heme peroxidase superfamily [70]. MPO is implicated in both inflammatory and oxidative stress pathways in CVD development. MPO is found in large amounts in neutrophils and at lower levels in monocytes. Neutrophils also produce superoxide, which can oxidize other molecules or transform into hydrogen peroxide by SOD. MPO employs hydrogen peroxide to generate several potent oxides, including hypochlorous acid (HOCL), hydroxyl radicals (OH), nitrogen dioxide (NO2), and peroxynitrite (ONOO) [71]. MPO produces ROS, which can alter lipids, lipoproteins, and proteins. SCN is produced in large quantities by smoking and its interaction with hydrogen peroxide (H2O2). MPO causes considerably an elevated hypothyocianous acid (HOSCN) level [72]. HOSCN may also alter LDL and HDL to OXLDL and OXHDL as well as oxidize NO, resulting in cyanate, which interacts with amides to produce a CVD prediction marker [21]. Recent research has found a connection between IR with increased MPO production in leucocytes and enhanced ROS formation in polycystic ovary syndrome (PCOS) patients, emphasizing MPO's role in oxidative stress events [73].
7. Endothelial Dysfunction in CVD
Endothelial dysfunction is a proinflammatory and prothrombotic condition characterized by the production of cell surface adhesion molecules that are required for the recruitment and attachment of inflammatory cells [74, 75]. Endothelial dysfunction is linked to oxidative stress and inflammatory processes. All these factors are all involved in the etiology of cardiovascular mortality and morbidity [76, 77]. Endothelial cells have a vascular disease-fighting enzyme called endothelial nitric oxide synthase (eNOS) [78]. This chemical diffuses vascular smooth muscle cells, while activating the cGMP pathway [79]. Nitric oxide (NO) is the most critical factor in maintaining vascular homeostasis in endothelial cells [80]. Endothelial dysfunction is marked by decreased NO bioavailability due to decreased NO synthesis or NO breakdown by superoxide anion. When the superoxide anion interacts with NO, peroxynitrite ONOO− is produce [81]. The inactivation of NO by various oxidative enzyme systems is a critical process that leads to endothelial dysfunction via an increase in superoxide anion levels [81]. NADPH oxidase and eNOS uncoupling are the primary sources of O2 generation that gives rise to vascular oxidative stress [82].
Several investigations have found that oxidative stress influences cytokine synthesis and release [83–85]. Increased levels of proinflammatory cytokines such as tumor necrosis factor (TNF), interferon-gamma, and interleukin 1 (IL-1) have been linked to aging-related endothelial dysfunction [86]. Inflammatory cytokines, ROS, lipid, and mechanical forces acting on the endothelium wall of blood vessels can activate the NF-κB pathway [5]. TNF-α-induced CD40 expression has been shown to change the certain adhesion molecules' level of expression and increase the inflammatory reaction [87]. Resveratrol therapy reduces CD4 expression; it also suppresses the generation of ROS triggered by TNF-α via stimulating the activity of sirtuin-1, a histone deacetylase associated with inflammation suppression and protecting cells from damage caused by inflammatory stimuli [88].
Apelin is a peptide encoded by the APLN gene in humans. Apelin is one of two endogenous ligands for the G-protein-coupled APJ receptor found on the surface cells. Many studies have found that apelin plays a vital role in the endothelial cells and is well known for its impact on cardiac myocytes [89–91]. Apelin increases NO bioavailability in endothelial cells [92]. Apelin is an anti-inflammatory drug as it inhibits the synthesis of inflammatory mediators, including interleukin-1 and TNF-α [93]. Apelin protects against oxidative stress and reduces I/R in cardiac cells by inhibiting mitochondrial oxidative stress damage and peroxidation of lipids [94]. Therefore, an increase in the oxidative stress in apelin-deficient heart tissues causes decreased NO bioavailability and activation of the inflammatory protein, which ultimately leads to endothelial dysfunction.
Endothelial dysfunction caused by mental stress is an initial cause of developing severe CVD [95]. Glucocorticoid and endothelin-1 (ET-1) play a role in causing stress [81]. Extreme stress reduces eNOS mRNA and protein expression and increases the ROS level by depleting BH4 and activating NADPH oxidase. OxLDL, a cardiovascular risk factor, increases endothelin-1 synthesis [96]. ET-1 suppresses the vasodilating effects of NO by activating ETA receptors and exerting a vasoconstrictive effect. Endothelial dysfunction indicators include lipid peroxide, nitrotyrosine, and NO [97] (Figure 2).
Figure 2.
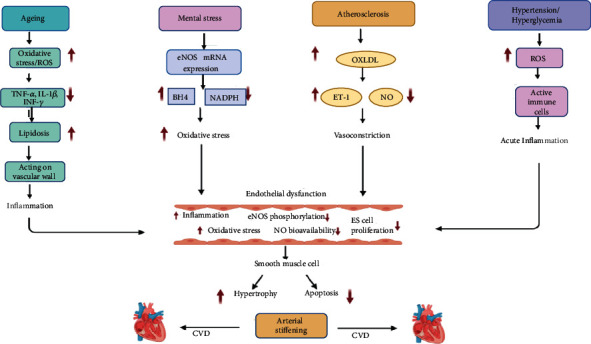
Schematic representation of the primary mechanisms involved in endothelial dysfunction in CVD. Endothelial cell rich in eNOS enzyme that produces NO is an essential compound in the endothelial function. eNOS acts on the cGMP pathway in VSMC and causes vasodilation. When eNOS is uncoupled, it reduces NO's production, which leads to decreased vasodilation properties and causes endothelial dysfunction. Oxidative stress is enhanced during aging, elevating the release of cytokines and ROS production; these act on the vascular wall and cause inflammation. Mental stress in CVD reduces eNOS mRNA expression and decreases BH4 activity, enhancing ROS generation and reducing NO bioavailability. OXLDL levels are high during atherosclerosis and increase the release of the ET-1 vasoconstrictor, which is also a significant factor of endothelial dysfunction. Other CVD risk factors like smoking, hypertension, and hypercholesterolemia generate ROS, causing inflammation and ultimately leads to endothelial dysfunction.
8. Biomarkers of Oxidative Stress in CVD
The National Institute of Health defines a biomarker as “an objectively measured and assessed feature that serves as an indication of normal biological process, pathogenic process or pharmacological reactions to a therapeutic intervention.” The evidence for directing therapy and improving patient outcomes defines a biomarker's clinical usefulness [98]. Biomarkers that closely correspond to the disease pathophysiological pathway are the most favorable ones. ROS has been quantified as a potential biomarker for disease progression because of its significant role in oxidative stress in CVD pathogenesis [99]. Molecules that are altered during its interaction with ROS and the antioxidant system molecules that vary in response to increasing redox stress are the two diverse biomarkers for oxidative stress [100]. Over 20 distinct markers have been discovered in meta-analyses of diseases like depression; however, the number is likely to be greater, making it impossible to draw significant conclusions [101].
To date, several vital biomarkers of CVD oxidative stress have been identified. Since the oxidative hypothesis of atherogenesis has been proposed, the importance of free radical oxidation of cellular components in CVD has been recognized [102]. Lipid peroxidation (isoprostane and malondialdehyde), oxidative protein modification (nitrotyrosine, S-glutathionylation), myeloperoxidase, oxidized low-density lipoprotein, and oxidized lipoprotein are the sources of oxidative stress biomarkers. Detailed biomarkers and pathways are depicted in Table 1.
Table 1.
Characteristics of the included studies investigating the association between oxidative stress biomarkers in CVD.
| Biomarkers | Study design | Pathway involvement | Laboratory methods | Model | Findings | Specificity | Reference |
|---|---|---|---|---|---|---|---|
| Isoprostane | Case-control | Peroxidation of polyunsaturated fatty acid catalyzed by free radical | GC/MS, ELISA, urine sample, radioimmune assay | In vivo, in vitro, ex vivo | F2-isoprostanes are predictive of peripheral and coronary artery disease | Nonspecific | [105, 126–128] |
| Malondialdehyde (MDA) | Cross-sectional | Peroxidation of polyunsaturated fatty acid, a side product of the thromboxane A2 pathway | Calorimetric assay, TBRS assay, ELISA, HPLC | In vivo | TRBAS blood serum levels in cardiovascular event | Specific | [103, 104, 109] |
| S-Glutathionylation | Case-control | Protein oxidation, glutathionylation pathway of protein | Western blotting, ELISA with monoclonal anti-glutathione antibody, MS | In vivo | It causes changes in intracellular Na+ and Ca2+ processing and other critical signaling pathways of CVD | Specific | [129–131] |
| Nitrotyrosine | Case-control | Tyrosine nitrate-mediated protein oxidation. ERK1/2 pathway | MS/MS, GC/MS, HPLC, immunocytochemical and immune histochemical assay based on monoclonal and polyclonal antibodies | In vivo | Nitrotyrosine enhanced fibrinogen activity and clot formation speed. Plasma protein-bound nitrotyrosine values are higher in coronary artery disease | Specific | [132–135] |
| OX-LDL | Nested case-control, cohort study | Autophagy-lysosome pathway, lipoxygenase-catalyzed oxidation of LDL | Monoclonal antibody technique, OX-LDL-EO6, LFL-DLH 3, OX-LDL-4E6 sandwich ELISA | In vivo | CVD endpoint predicted by OX-LDL; its level also indicates MI. In vivo OX-LDL link to atherosclerosis and its level in CVD individuals is more | Specificity of OXLDL is questionable | [113, 136–139] |
| Myeloperoxidase | Cohort study, case-control | Inflammatory neutrophil and basophil activate MPO, MAPK/NF-κB signaling | Peroxidation assay, spectrophotometrically, ELISA | In vivo and in vitro | MPO linked to acute MI, CAD | Specific | [71, 140–143] |
8.1. Lipid Peroxidation
Numerous plasma markers of lipid peroxidation are available, such as malondialdehyde (MDA), lipid hydroperoxides, oxysterols, and F2-α isoprostanes [71]. Isoprostanes and MDA have widely researched indicators for lipid peroxidation. Isoprostanes and MDA are formed by the peroxidation of polyunsaturated fatty acids that are found in the phospholipids of the cell membrane [103]. Further, phospholipids release isoprostane from the cell membrane that is measured in tissue, blood, and urine [104]. Its concentration is determined using gas chromatography-mass spectrometry (GC/MS), ELISA, liquid chromatography-mass spectrometry (LC/MS), urine sample, and radio immune assay in plasma [105]. MDA can be measured using a colorimetric assay, an ELISA, an HPLC, or a TBRS assay. Different antioxidants like lipoic acid and aminoguanidine can adjust plasma TBRS concentration [106].
8.2. Protein Oxidation
Protein oxidation can be triggered selectively by ROS (ROS) and nitrogen species (RNS) in multiple ways [107]. Nitrotyrosine and S-glutathionylation are the two most essential biomarkers of CVD. The reaction of protein tyrosine nitrate is mediated by reactive nitrogen species such as peroxynitrite (ONOO) and nitrogen dioxide (NO2) with its transition metalcore, MPO that can respond with ONOO to produce the oxo metal complex and NO2 to facilitate the nitrogen response [103]. The current gold standard method for quantifying free nitrotyrosine (3-NO2-TYR) is tandem mass spectrometry (MS/MS) combined with GC or HPLC. The immune cytochemical and histochemical assays are based on monoclonal or polyclonal anti-3-NO2-TYR antibodies [108]. The level of plasma protein-bound nitrotyrosine was very significant for CVD patients, even after adjusting conventional CVD and CR risk factors [109]. Trimethylamine N-oxide (TMAO) is a tiny amine oxide produced by gut microbial metabolism. The elevated plasma TMAO level in patients with HF is associated with poor prognoses [110].
S-Glutathionylation creates a disulfide bridge between the cellular tripeptide glutathione and reactive cysteine residue [111]. By this oxidative modification, the tertiary structure of protein gets changed and redox regulation of various activities including ryanodine receptor, eNOS; Na + k + pump etc. This impacts proteins and can alter the intracellular Na + −K + pump and the signaling pathway of CVD [112]. The level of S-glutathionylation of sensitive protein can be measured by Western blotting [113], ELISA with monoclonal anti-glutathione antibody, and MS technique [114].
8.3. OX-LDL and Oxidized Phospholipids
Low-density lipoprotein oxidation is possible nonenzymatically, or enzymes like 12/15 lipoxygenase can catalyze the LDL [115]. OXLDL can be quantified using monoclonal antibodies that detect unusual oxidation based on a specific epitope; currently, available plasma OXLDL ELISA techniques are OXLDL-EO6 ELISA assay (quantify oxidation of phospholipid on apo-B-100), LDL-DLH 3 (quantify apoB100 on oxidized phospholipids), and OXLDL-4E6-sandwich ELISA (for MAD-LDL and copper OXLDL detection). It has been observed that OXLDL levels are higher in CVD patients [103]. Multiple studies have indicated that the long-chain (LC) omega-3 polyunsaturated fatty acids (n-3 PUFAs) significantly decrease the risk of fatal coronary heart disease [116]. However, no association between α-linolenic acid (ALA) and the risk of HF was documented [117].
8.4. Myeloperoxidase (MPO)
MPO functions as a master heme enzyme that helps generate ROS by converting hydrogen peroxide (H2O2) into OH, ONOO, NO2, and HOCL. ROS generated by this process can alter lipid, protein, and lipoprotein [118]. The MPO level can be quantified by peroxidase assay, spectrophotometry, and ELISA. The presence of CAD was related to higher circulating MPO levels [119]. This leads to chest pain, increases the risk for MI, and can even be fatal. Because of this, MPO is the most effective biomarker of CVD [120].
9. Secreted Frizzled-Related Proteins (SFRPs)
Secreted frizzled-related proteins (SFRPs) are Wnt antagonists. By interfering with the Wnt signaling pathway, it downregulates axin-related protein 2 (AXIN2) and matrix metalloproteinase-7 (MMP7) proteins [121]. Sfrps family members influence cellular apoptosis, angiogenesis, differentiation, the inflammatory process, and cardiac remodelling [122]. SFRP2 can regulate cardiac development and CVD [123]. Further, SFRP2 remains an independent biomarker for myocardial fibrosis [124]. Measurement of secreted frizzled-related protein 5 (SFRP5) levels in HF patients with or without T2DM indicated that elevated SFRP5 levels decreased rehospitalization or all-cause mortality in HF patients with T2DM [125].
10. Clinical Significance of Biomarkers
Despite its physiopathological significance and promising preclinical findings, no redox biomarkers such as myeloperoxidase, protein and lipid oxidation, advanced glycation end products (AGEs), and their soluble receptor have made it to clinical practice. This could be attributed in part to a lack of data, the redox balance's complexity, and high level of interaction between these indicators and several established risk factors (age, renal failure, smoking, inflammatory disease, and drugs) [144].
11. Disadvantages of Biomarkers
The prevalence of a wide range of oxidative stress biomarkers does not imply superiority of any particular biomarker over the other; however, it is critical to compare the oxidative stress assessment's value with commonly used risk scores. This property must be considered when deciding the biomarker to be used in a specific situation [145]. The nitrotyrosine levels in the circulatory system differ from those in the tissues; besides, S-glutathionylation detection is susceptible to methodological artifact [103]. Subsequently, it has been revealed that MDA is not a specific biomarker as the TBARS assay can detect aldehydes except MDA [146].
11.1. Oxidative Stress, Obesity, and CVD
Obesity is a primary risk factor for metabolic and CVD. Oxidative stress is essential for obesity-related diseases, including dyslipidemia and hypertension in CVD [147]. Adipokines and other types of bioactive chemicals are released from the adipose tissue, and its adipokines include interleukin-6 (IL-6), TNF-α, monocyte chemo attachment protein-1 (MCP-1), and adiponectin [147, 148]. Obesity combined with dyslipidemia has been proven to enhance the onset of CVD associated with oxidative stress [149]. Low-density lipoprotein (VLDL) increases the production of ROS (ROS) in the endothelium, which can disrupt the lipid and protein signaling pathway [150]. ROS-mediated changes in lipids such as oxidized LDL (OX-LDL) and peroxidized lipoproteins either directly or indirectly stimulate adipocyte growth by increasing monocyte/macrophage infiltration [151]. Activating lipoprotein lipase causes fatty acid build-up in the adipocytes [152]. OX-LDL reduces the release of adiponectin from adipose tissue limiting ROS formation. Reduced adiponectin levels have been linked to coronary artery disease (CAD) [153].
An increase in ROS substantially impacts the biology of white adipose tissue, leading to unregulated production of inflammatory cytokines like tumor necrosis factor (TNF-α), thereby contributing to CVD [154]. Adiposite is associated with oxidative stress while mitochondria are the primary source of ROS. However, the mitochondrial action in the dysfunction of adipose tissue during obesity is a critical event in obesity. In this context, the elevated level of oxidative stress is a primary cause of CVD [155]. Adiponectin can reduce the production of adhesion molecules like intracellular adhesion molecule-1 by blocking TNF-mediated activation of nuclear factor kappa B (NF-κB) endothelial cells. This results in the inhibition of monocyte adhesion, which is an early stage of atherosclerotic CVD [156]. Adiponectin decreases atherosclerosis, reducing pressure overload-induced myocardial hypertrophy and angiotensin-II-induced cardiac fibrosis, and protects the heart from ischemia [157]. Further, oxidative stress activates SNS in the brain that plays a significant role in the pathogenesis of obesity-related hypertension [158].
11.2. Antioxidants in CVD
Antioxidants are chemicals that protect the damaged cellular components via oxidative stress chain reaction [159]. Antioxidants limit ROS production by eliminating free radicals and enhancing cellular structures like lipid, protein, and DNA damage by ROS [160]. Antioxidants can be classified into enzymatic and nonenzymatic antioxidants. Examples of enzymatic antioxidants are superoxide dismutase (SOD), glutathione reductase (GRX), glutathione peroxidase (GPX), and catalase (CAT). Nonenzymatic antioxidants include endogenous antioxidants like glutathione (GSH), uric acid, albumin, and exogenous antioxidant like ascorbic acid (Vit-C), carotenoids, flavonoid, and vitamin-E [161].
11.2.1. SOD
Superoxide dismutase is catalyzed by SOD and converted to H2O2 and O2. Catalase is an enzyme that adds in the conversion of H2O2 into H2O and O2 [162]. SOD has been proven to slow the progression of atherosclerosis [163].
11.2.2. GPX
Red cell GPX isoform-1 exhibits significance in patients with coronary artery disease in addition to conventional risk factors [164]. The paraoxonase enzyme is linked to high-density lipoprotein (HDL). They have peroxide-like activity and protect lipoprotein against oxidation [165].
11.2.3. TRX
TRX can scavenge ROS (ROS) such as H2O2 and ONOO− [164], while oxidative damage is elevated upon TRX inhibition. TRX has reduced disulfide component, which is increased under oxidative stress. A study suggests that fibrosis, oxidative stress, and apoptosis are reduced in cardiomyocytes and endothelial cells as compared to the diabetic group with MI, a group lacking TRX1 overexpression [166].
11.2.4. Vitamin E
The role of vitamin E supplementation in preventing CVD is controversial. When vitamin E was administered alone, MI was significantly reduced [167]. Vitamin E and vitamin C act as an inhibitor for LDL oxidation by reducing ROS formation and increasing NO bioavailability [168].
11.2.5. Vitamin C
Vitamin C is an electron donor (reducer) with an antioxidant effect due to its ability to reduce oxidized species or oxidant radicals. Through various mechanisms, vitamin C contributes to the overall pool of nitric oxide (NO) and increases the bioavailability of NO. Vitamin C neutralizes superoxide radicals, stabilizes BH4, increases eNOS activity, and maintains L-arginine and cGMP levels [169]. It partly mediates antiapoptotic and antioxidant functions and exerts protective effects on human umbilical vein endothelial cells through the regulation of miRNA/mRNA axis expression [170]. Growing evidence also shows that H2O2-mediated endothelial cell senescence may be associated with abnormally expressed Piwi-interacting RNAs (piRNAs) and PIWI proteins. Furthermore, vitamin C is known to attenuate H2O2-mediated abnormal expression of Piwi-interacting RNAs (piRNAs) [171].
11.2.6. Vitamin A
Vitamin A has been found to influence apolipoprotein on MI risk [29].
11.2.7. Nuts
Nuts are known to lower the risk of CVD as it can inhibit lipid peroxidation and LDL oxidation in LDL particles [5, 172]. The EURAMIC trial discovered a minor link between the adipose-carotene content and the MI risk; however, the effect was lost after accounting for several confounding variables. Findings from a recent trial including 1031 Finnish males revealed a vital link between serum-carotene and a lower risk of MI [173]. Statin, α-tocopherol, and ascorbic acid have been used to prevent CVDs which are also antioxidant drugs [161]. It has been claimed that using antioxidants indiscriminately might be harmful. The quantity of antioxidants and oxidative stress should be in a careful equilibrium in our cells, which is crucial for optimal cell function. Disrupting this equilibrium can have unfavorable consequences. Furthermore, natural antioxidant defenses may be considerably more essential than antioxidants obtained from food or supplements to humans [174]. The use of antioxidant supplements as a treatment for oxidative stress is still debatable. However, it should be emphasized that antioxidants cannot repair damage in CVD patients but can only control disease consequences.
12. Conclusion and Future Perspective
Elevated oxidative stress in the body may lead to various pathophysiological conditions such as CVD. Involvement of free radicals like ROS and NOS affects the cardiovascular system causing multiple cardiac disorders. Some superoxide causes endothelial dysfunction of vascular tissue by reducing the bioavailability of NO. Oxidative stress causes cytokine secretion and promotes inflammation in the vascular tissue. Adhesion molecules act on the endothelial blood vessel wall and are the major cause of atherosclerotic plaque formation. Several oxidative stress biomarkers can detect CVD; however, these biomarkers are not enough to identify CVD and additional research is warranted. Antioxidants can reduce the effect of oxidative stress on CVDs. Many antioxidant drugs have been discovered so far that assist in decreasing the CVD mortality rate. There is an urgent need to identify and discover new methodologies and effective biomarkers that can be implemented to better understand the ROS mechanism towards CVD pathophysiology.
Acknowledgments
The corresponding authors thank Universiti Malaysia Sabah for providing the funding support.
Contributor Information
Bhaskar V. K. S. Lakkakula, Email: lvksbhaskar@gmail.com.
Pasupuleti Visweswara Rao, Email: visweswararao@reva.edu.in.
Conflicts of Interest
The authors declare no potential conflict of interest.
Authors' Contributions
BL, PVR, and HKV designed the review. PP and SL performed the literature search as well as collected and assembled the data. HKV and PP analyzed the retrieved articles. PM, HKV, SL, PVR, and BL prepared the manuscript. PP, HKV, SL, PVR, and BL revised the manuscript critically. All authors have read and agreed to the published version of the manuscript. The complete article was created under the supervision of BL.
References
- 1.Benjamin E. J., Muntner P., Alonso A., et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation . 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.van der Pol A., van Gilst W., Voors A. A., van der Meer P. Treating oxidative stress in heart failure: past, present and future. European Journal of Heart Failure . 2019;21(4):425–435. doi: 10.1002/ejhf.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma W., Wei S., Zhang B., Li W. Molecular mechanisms of cardiomyocyte death in drug-induced cardiotoxicity. Frontiers in Cell and Developmental Biology . 2020;8(434) doi: 10.3389/fcell.2020.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippi G., Plebani M. Statins for primary prevention of cardiovascular disease. Trends in pharmacological sciences . 2017;38(2):111–112. doi: 10.1016/j.tips.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Senoner T., Dichtl W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients . 2019;11(9):p. 2090. doi: 10.3390/nu11092090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huynh D. T. N., Heo K.-S. Therapeutic targets for endothelial dysfunction in vascular diseases. Archives of Pharmacal Research . 2019;42(10):848–861. doi: 10.1007/s12272-019-01180-7. [DOI] [PubMed] [Google Scholar]
- 7.Volobueva A., Grechko A., Yet S. F., Sobenin I., Orekhov A. Changes in mitochondrial genome associated with predisposition to atherosclerosis and related disease. Biomolecules . 2019;9(8):p. 377. doi: 10.3390/biom9080377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anwar M. A., Al Disi S. S., Eid A. H. Anti-hypertensive herbs and their mechanisms of action: Part II. Frontiers in pharmacology . 2016;7(50) doi: 10.3389/fphar.2016.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biology . 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siti H. N., Kamisah Y., Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review) Vascular Pharmacology . 2015;71:40–56. doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Chen K., Keaney J. F., Jr. Evolving concepts of oxidative stress and reactive oxygen species in cardiovascular disease. Current Atherosclerosis Reports . 2012;14(5):476–483. doi: 10.1007/s11883-012-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W., Kang P. M. Oxidative Stress and Antioxidant Treatments in Cardiovascular Diseases. Antioxidants (Basel) . 2020;9(12):p. 1292. doi: 10.3390/antiox9121292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Hariri M., Zibara K., Farhat W., et al. Cigarette smoking-induced cardiac hypertrophy, vascular inflammation and injury are attenuated by antioxidant supplementation in an animal model. Frontiers in pharmacology . 2016;7(397) doi: 10.3389/fphar.2016.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dikalov S., Itani H., Richmond B., et al. Tobacco smoking induces cardiovascular mitochondrial oxidative stress, promotes endothelial dysfunction, and enhances hypertension. American Journal of Physiology. Heart and Circulatory Physiology . 2019;316(3):H639–h646. doi: 10.1152/ajpheart.00595.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S. C., Hevia D., Patchva S., Park B., Koh W., Aggarwal B. B. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxidants & Redox Signaling . 2012;16:1295–1322. doi: 10.1089/ars.2011.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsutsui H., Kinugawa S., Matsushima S. Oxidative stress and heart failure. American Journal of Physiology. Heart and Circulatory Physiology . 2011;301(6):H2181–H2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 17.Samman Tahhan A., Sandesara P. B., Hayek S. S., et al. Association between oxidative stress and atrial fibrillation. Heart Rhythm . 2017;14(12):1849–1855. doi: 10.1016/j.hrthm.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baradaran A., Nasri H., Rafieian-Kopaei M. Oxidative stress and hypertension: possibility of hypertension therapy with antioxidants. Journal of Research in Medical Sciences : The Official Journal of Isfahan University of Medical Sciences . 2014;19(4):358–367. [PMC free article] [PubMed] [Google Scholar]
- 19.Kattoor A. J., Pothineni N. V. K., Palagiri D., Mehta J. L. Oxidative stress in atherosclerosis. Current Atherosclerosis Reports . 2017;19(11):p. 42. doi: 10.1007/s11883-017-0678-6. [DOI] [PubMed] [Google Scholar]
- 20.Dubois-Deruy E., Peugnet V., Turkieh A., Pinet F. Oxidative stress in cardiovascular diseases. Antioxidants (Basel) . 2020;9(9):p. 864. doi: 10.3390/antiox9090864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cervantes Gracia K., Llanas-Cornejo D., Husi H. CVD and oxidative stress. Journal of Clinical Medicine . 2017;6(2):p. 22. doi: 10.3390/jcm6020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bentzon J. F., Otsuka F., Virmani R., Falk E. Mechanisms of plaque formation and rupture. Circulation Research . 2014;114(12):1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 23.Spickett C. M. The lipid peroxidation product 4-hydroxy-2-nonenal: advances in chemistry and analysis. Redox Biology . 2013;1(1):145–152. doi: 10.1016/j.redox.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee A.-S., Chen W. Y., Chan H. C., et al. Gender disparity in LDL-induced cardiovascular damage and the protective role of estrogens against electronegative LDL. Cardiovascular Diabetology . 2014;13(1):p. 64. doi: 10.1186/1475-2840-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toya T., Ito K., Kagami K., et al. Impact of oxidative posttranslational modifications of SERCA2 on heart failure exacerbation in young patients with non-ischemic cardiomyopathy: a pilot study. International Journal of Cardiology Heart & Vasculature . 2020;26, article 100437 doi: 10.1016/j.ijcha.2019.100437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinberg S. F. Oxidative stress and sarcomeric proteins. Circulation Research . 2013;112(2):393–405. doi: 10.1161/CIRCRESAHA.111.300496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assies J., Mocking R. J. T., Lok A., Ruhé H. G., Pouwer F., Schene A. H. Effects of oxidative stress on fatty acid- and one-carbon-metabolism in psychiatric and cardiovascular disease comorbidity. Acta Psychiatrica Scandinavica . 2014;130(3):163–180. doi: 10.1111/acps.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panth N., Paudel K. R., Parajuli K. Reactive oxygen species: a key hallmark of cardiovascular disease. Advances in medicine . 2016;2016:12. doi: 10.1155/2016/9152732.9152732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Circu M. L., Aw T. Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radical Biology & Medicine . 2010;48(6):749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabharwal S. S., Schumacker P. T. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel? Nature Reviews Cancer . 2014;14(11):709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubois-Deruy E., Cuvelliez M., Fiedler J., et al. MicroRNAs regulating superoxide dismutase 2 are new circulating biomarkers of heart failure. Scientific Reports . 2017;7(1, article 14747) doi: 10.1038/s41598-017-15011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dikalov S. I., Nazarewicz R. R. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxidants & Redox Signaling . 2013;19(10):1085–1094. doi: 10.1089/ars.2012.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bugger H., Pfeil K. Mitochondrial ROS in myocardial ischemia reperfusion and remodeling. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease . 2020;1866(7, article 165768) doi: 10.1016/j.bbadis.2020.165768. [DOI] [PubMed] [Google Scholar]
- 34.Mikhed Y., Daiber A., Steven S. Mitochondrial oxidative stress, mitochondrial DNA damage and their role in age-related vascular dysfunction. International Journal of Molecular Sciences . 2015;16(7):15918–15953. doi: 10.3390/ijms160715918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yingzhong C., Lin C., Chunbin W. Clinical effects of cyclosporine A on reperfusion injury in myocardial infarction: a meta-analysis of randomized controlled trials. Springer Plus . 2016;5(1):1117–1117. doi: 10.1186/s40064-016-2751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madungwe N. B., Zilberstein N. F., Feng Y., Bopassa J. C. Critical role of mitochondrial ROS is dependent on their site of production on the electron transport chain in ischemic heart. American journal of cardiovascular disease . 2016;6(3):93–108. [PMC free article] [PubMed] [Google Scholar]
- 37.Mozaffari M. S., Baban B., Liu J. Y., Abebe W., Sullivan J. C., el-Marakby A. Mitochondrial complex I and NAD(P) H oxidase are major sources of exacerbated oxidative stress in pressure-overloaded ischemic-reperfused hearts. Basic Research in Cardiology . 2011;106(2):287–297. doi: 10.1007/s00395-011-0150-7. [DOI] [PubMed] [Google Scholar]
- 38.Tejero J., Shiva S., Gladwin M. T. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiological Reviews . 2019;99(1):311–379. doi: 10.1152/physrev.00036.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kayama Y., Raaz U., Jagger A., et al. Diabetic cardiovascular disease induced by oxidative stress. International Journal of Molecular Sciences . 2015;16(10):25234–25263. doi: 10.3390/ijms161025234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng H., Zhu H., Liu X., Huang X., Huang A., Huang Y. Mitophagy in diabetic cardiomyopathy: roles and mechanisms. Frontiers in Cell and Development Biology . 2021;9, article 750382 doi: 10.3389/fcell.2021.750382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tao J., Chen H., Wang Y. J., et al. Ketogenic diet suppressed T-regulatory cells and promoted cardiac fibrosis via reducing mitochondria-associated membranes and inhibiting mitochondrial function. Oxidative Medicine and Cellular Longevity . 2021;2021:15. doi: 10.1155/2021/5512322.5512322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma T., Huang X., Zheng H., et al. SFRP2 improves mitochondrial dynamics and mitochondrial biogenesis, oxidative stress, and apoptosis in diabetic cardiomyopathy. Oxidative Medicine and Cellular Longevity . 2021;2021:18. doi: 10.1155/2021/9265016.9265016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuroda J., Sadoshima J. NADPH oxidase and cardiac failure. Journal of Cardiovascular Translational Research . 2010;3(4):314–320. doi: 10.1007/s12265-010-9184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braunersreuther V., Montecucco F., Ashri M., et al. Role of NADPH oxidase isoforms NOX1, NOX2 and NOX4 in myocardial ischemia/reperfusion injury. Journal of Molecular and Cellular Cardiology . 2013;64:99–107. doi: 10.1016/j.yjmcc.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Pick E. Role of the Rho GTPase Rac in the activation of the phagocyte NADPH oxidase: outsourcing a key task. Small GTPases . 2014;5(1, article e27952) doi: 10.4161/sgtp.27952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y., Murugesan P., Huang K., Cai H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: novel therapeutic targets. Nature Reviews Cardiology . 2020;17(3):170–194. doi: 10.1038/s41569-019-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thiriet M. Cardiovascular disease: an introduction. In: Thiriet M., editor. Vasculopathies: Behavioral, Chemical, Environmental, and Genetic Factors . Cham: Springer International Publishing; 2018. pp. 1–90. [Google Scholar]
- 48.Tobar N., Guerrero J., Smith P. C., Martínez J. NOX4-dependent ROS production by stromal mammary cells modulates epithelial MCF-7 cell migration. British Journal of Cancer . 2010;103(7):1040–1047. doi: 10.1038/sj.bjc.6605847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai X., Zhang Y., Li M., et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ . 2020;370, article m2297 doi: 10.1136/bmj.m2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai X., Liu X., Sun L., et al. Prediabetes and the risk of heart failure: a meta-analysis. Diabetes, Obesity & Metabolism . 2021;23(8):1746–1753. doi: 10.1111/dom.14388. [DOI] [PubMed] [Google Scholar]
- 51.Mai L., Wen W., Qiu M., et al. Association between prediabetes and adverse outcomes in heart failure. Diabetes, Obesity & Metabolism . 2021;23(11):2476–2483. doi: 10.1111/dom.14490. [DOI] [PubMed] [Google Scholar]
- 52.Maalouf R. M., Eid A. A., Gorin Y. C., et al. Nox4-derived reactive oxygen species mediate cardiomyocyte injury in early type 1 diabetes. American Journal of Physiology. Cell Physiology . 2012;302(3):C597–C604. doi: 10.1152/ajpcell.00331.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dick A. S., Ivanovska J., Kantores C., Belcastro R., Keith Tanswell A., Jankov R. P. Cyclic stretch stimulates nitric oxide synthase-1-dependent peroxynitrite formation by neonatal rat pulmonary artery smooth muscle. Free Radical Biology and Medicine . 2013;61:310–319. doi: 10.1016/j.freeradbiomed.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 54.Vatansever F., de Melo W. C. M. A., Avci P., et al. Antimicrobial strategies centered around reactive oxygen species--bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiology Reviews . 2013;37(6):955–989. doi: 10.1111/1574-6976.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu T., Ding W., Ji X., et al. Oxidative stress in cell death and cardiovascular diseases. Oxidative Medicine and Cellular Longevity . 2019;2019:11. doi: 10.1155/2019/9030563.9030563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faria A., Persaud S. J. Cardiac oxidative stress in diabetes: mechanisms and therapeutic potential. Pharmacology & Therapeutics . 2017;172:50–62. doi: 10.1016/j.pharmthera.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 57.White W. B. Gout, xanthine oxidase inhibition, and cardiovascular outcomes. Circulation . 2018;138(11):1127–1129. doi: 10.1161/CIRCULATIONAHA.118.036148. [DOI] [PubMed] [Google Scholar]
- 58.Grassi D., Ferri L., Desideri G., et al. Chronic hyperuricemia, uric acid deposit and cardiovascular risk. Current Pharmaceutical Design . 2013;19(13):2432–2438. doi: 10.2174/1381612811319130011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghasemzadeh N., Patel R. S., Eapen D. J., et al. Oxidative stress is associated with increased pulmonary artery systolic pressure in humans. Hypertension . 2014;63(6):1270–1275. doi: 10.1161/HYPERTENSIONAHA.113.02360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Givertz M. M., Mann D. L., Lee K. L., et al. Xanthine oxidase inhibition for hyperuricemic heart failure patients: design and rationale of the EXACT-HF study. Circulation. Heart Failure . 2013;6(4):862–868. doi: 10.1161/CIRCHEARTFAILURE.113.000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Leyen K., Holman T. R., Maloney D. J. The potential of 12/15-lipoxygenase inhibitors in stroke therapy. Future Medicinal Chemistry . 2014;6(17):1853–1855. doi: 10.4155/fmc.14.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghosh R., Alajbegovic A., Gomes A. V. NSAIDs and cardiovascular diseases: role of reactive oxygen species. Oxidative Medicine and Cellular Longevity . 2015;2015:25. doi: 10.1155/2015/536962.536962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chou V. P., Holman T. R., Manning-Bog A. B. Differential contribution of lipoxygenase isozymes to nigrostriatal vulnerability. Neuroscience . 2013;228:73–82. doi: 10.1016/j.neuroscience.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang B., Wu L., Chen J., et al. Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Signal Transduction and Targeted Therapy . 2021;6(1):p. 94. doi: 10.1038/s41392-020-00443-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suzuki H., Kayama Y., Sakamoto M., et al. Arachidonate 12/15-lipoxygenase-induced inflammation and oxidative stress are involved in the development of diabetic cardiomyopathy. Diabetes . 2015;64(2):618–630. doi: 10.2337/db13-1896. [DOI] [PubMed] [Google Scholar]
- 66.Crabtree M. J., Hale A. B., Channon K. M. Dihydrofolate reductase protects endothelial nitric oxide synthase from uncoupling in tetrahydrobiopterin deficiency. Free Radical Biology & Medicine . 2011;50(11):1639–1646. doi: 10.1016/j.freeradbiomed.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Janssens H. J., Fransen J., van de Lisdonk E., van Riel P., van Weel C., Janssen M. A diagnostic rule for acute gouty arthritis in primary care without joint fluid analysis. Archives of Internal Medicine . 2010;170(13):1120–1126. doi: 10.1001/archinternmed.2010.196. [DOI] [PubMed] [Google Scholar]
- 68.Yang G., Fang Z., Liu Y., et al. Protective effects of Chinese traditional medicine Buyang Huanwu decoction on myocardial injury. Evidence-based Complementary and Alternative Medicine . 2011;2011:7. doi: 10.1093/ecam/nep013.930324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rochette L., Lorin J., Zeller M., et al. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets? Pharmacology & Therapeutics . 2013;140(3):239–257. doi: 10.1016/j.pharmthera.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 70.Ndrepepa G. Myeloperoxidase - a bridge linking inflammation and oxidative stress with cardiovascular disease. Clinica Chimica Acta . 2019;493:36–51. doi: 10.1016/j.cca.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 71.Strobel N. A., Fassett R. G., Marsh S. A., Coombes J. S. Oxidative stress biomarkers as predictors of cardiovascular disease. International Journal of Cardiology . 2011;147(2):191–201. doi: 10.1016/j.ijcard.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 72.Rayner B. S., Love D. T., Hawkins C. L. Comparative reactivity of myeloperoxidase-derived oxidants with mammalian cells. Free Radical Biology & Medicine . 2014;71:240–255. doi: 10.1016/j.freeradbiomed.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 73.Victor V. M., Rovira-Llopis S., Bañuls C., et al. Insulin resistance in PCOS patients enhances oxidative stress and leukocyte adhesion: role of myeloperoxidase. PLoS One . 2016;11(3, article e0151960) doi: 10.1371/journal.pone.0151960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mittal M., Siddiqui M. R., Tran K., Reddy S. P., Malik A. B. Reactive oxygen species in inflammation and tissue injury. Antioxidants & Redox Signaling . 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liao J. K. Linking endothelial dysfunction with endothelial cell activation. The Journal of Clinical Investigation . 2013;123(2):540–541. doi: 10.1172/JCI66843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Münzel T., Daiber A. Environmental stressors and their impact on health and disease with focus on oxidative stress. Antioxidants & Redox Signaling . 2018;28(9):735–740. doi: 10.1089/ars.2017.7488. [DOI] [PubMed] [Google Scholar]
- 77.Steven S., Frenis K., Oelze M., et al. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxidative Medicine and Cellular Longevity . 2019;2019:26. doi: 10.1155/2019/7092151.7092151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yagita Y., Kitagawa K., Oyama N., et al. Functional deterioration of endothelial nitric oxide synthase after focal cerebral ischemia. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism . 2013;33(10):1532–1539. doi: 10.1038/jcbfm.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolin M. S., Gupte S. A., Neo B. H., Gao Q., Ahmad M. Oxidant-redox regulation of pulmonary vascular responses to hypoxia and nitric oxide-cGMP signaling. Cardiology in Review . 2010;18(2):89–93. doi: 10.1097/CRD.0b013e3181c9f088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tousoulis D., Kampoli A. M., Tentolouris Nikolaos Papageorgiou C., Stefanadis C. The role of nitric oxide on endothelial function. Current Vascular Pharmacology . 2012;10(1):4–18. doi: 10.2174/157016112798829760. [DOI] [PubMed] [Google Scholar]
- 81.Incalza M. A., D'Oria R., Natalicchio A., Perrini S., Laviola L., Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascular Pharmacology . 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 82.Münzel T., Camici G. G., Maack C., Bonetti N. R., Fuster V., Kovacic J. C. Impact of oxidative stress on the heart and vasculature: part 2 of a 3-part series. Journal of the American College of Cardiology . 2017;70(2):212–229. doi: 10.1016/j.jacc.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bulua A. C., Simon A., Maddipati R., et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) The Journal of Experimental Medicine . 2011;208(3):519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakahira K., Haspel J. A., Rathinam V. A. K., et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature Immunology . 2011;12(3):222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou R., Yazdi A. S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature . 2011;469(7329):221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 86.Herrera M. D., Mingorance C., Rodríguez-Rodríguez R., Alvarez de Sotomayor M. Endothelial dysfunction and aging: an update. Ageing Research Reviews . 2010;9(2):142–152. doi: 10.1016/j.arr.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 87.Parameswaran N., Patial S. Tumor necrosis factor-α signaling in macrophages. Critical Reviews in Eukaryotic Gene Expression . 2010;20(2):87–103. doi: 10.1615/CritRevEukarGeneExpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan W., Yu H., Huang S., Zhu P. Resveratrol protects against TNF-α-induced injury in human umbilical endothelial cells through promoting sirtuin-1-induced repression of NF-KB and p38 MAPK. PLoS One . 2016;11(1, article e0147034) doi: 10.1371/journal.pone.0147034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.del Toro R., Prahst C., Mathivet T., et al. Identification and functional analysis of endothelial tip cell-enriched genes. Blood . 2010;116(19):4025–4033. doi: 10.1182/blood-2010-02-270819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Charo D. N., Ho M., Fajardo G., et al. Endogenous regulation of cardiovascular function by apelin-APJ. American Journal of Physiology. Heart and Circulatory Physiology . 2009;297(5):H1904–H1913. doi: 10.1152/ajpheart.00686.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scimia M. C., Hurtado C., Ray S., et al. APJ acts as a dual receptor in cardiac hypertrophy. Nature . 2012;488(7411):394–398. doi: 10.1038/nature11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kwon M. H., Tuvshintur B., Kim W. J., et al. Expression of the Apelin-APJ Pathway and Effects on Erectile Function in a Mouse Model of Vasculogenic Erectile Dysfunction. The Journal of Sexual Medicine . 2013;10(12):2928–2941. doi: 10.1111/jsm.12158. [DOI] [PubMed] [Google Scholar]
- 93.Masoumi J., Abbasloui M., Parvan R., et al. Apelin, a promising target for Alzheimer disease prevention and treatment. Neuropeptides . 2018;70:76–86. doi: 10.1016/j.npep.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 94.Zhou Q., Cao J., Chen L. Apelin/APJ system: a novel therapeutic target for oxidative stress-related inflammatory diseases (review) International Journal of Molecular Medicine . 2016;37(5):1159–1169. doi: 10.3892/ijmm.2016.2544. [DOI] [PubMed] [Google Scholar]
- 95.Toda N., Nakanishi-Toda M. How mental stress affects endothelial function. Pflügers Archiv-European Journal of Physiology . 2011;462(6):779–794. doi: 10.1007/s00424-011-1022-6. [DOI] [PubMed] [Google Scholar]
- 96.Gradinaru D., Borsa C., Ionescu C., Prada G. I. Oxidized LDL and NO synthesis--Biomarkers of endothelial dysfunction and ageing. Mechanisms of Ageing and Development . 2015;151:101–113. doi: 10.1016/j.mad.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 97.Scioli M. G., Storti G., D’Amico F., et al. Oxidative stress and new pathogenetic mechanisms in endothelial dysfunction: potential diagnostic biomarkers and therapeutic targets. Journal of Clinical Medicine . 2020;9(6):p. 1995. doi: 10.3390/jcm9061995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ge Y., Wang T. J. Circulating, imaging, and genetic biomarkers in cardiovascular risk prediction. Trends in Cardiovascular Medicine . 2011;21(4):105–112. doi: 10.1016/j.tcm.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Griendling K., FitzGerald G. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation . 2003;108(16):1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 100.De Rosa S., Cirillo P., Paglia A., Sasso L., Palma V., Chiariello M. Reactive oxygen species and antioxidants in the pathophysiology of cardiovascular disease: does the actual knowledge justify a clinical approach? Current Vascular Pharmacology . 2010;8(2):259–275. doi: 10.2174/157016110790887009. [DOI] [PubMed] [Google Scholar]
- 101.Black C. N., Bot M., Scheffer P. G., Cuijpers P., Penninx B. W. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology . 2015;51:164–175. doi: 10.1016/j.psyneuen.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 102.Zhong S., Li L., Shen X., et al. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radical Biology and Medicine . 2019;144:266–278. doi: 10.1016/j.freeradbiomed.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 103.Ho E., Karimi Galougahi K., Liu C. C., Bhindi R., Figtree G. A. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biology . 2013;1(1):483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ayala A., Muñoz M. F., Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Medicine and Cellular Longevity . 2014;2014:31. doi: 10.1155/2014/360438.360438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thomas D., Suo J., Ulshöfer T., et al. Nano-LC-MS/MS for the quantitation of prostanoids in immune cells. Analytical and Bioanalytical Chemistry . 2014;406(28):7103–7116. doi: 10.1007/s00216-014-8134-8. [DOI] [PubMed] [Google Scholar]
- 106.Shah D., Mahajan N., Sah S., Nath S. K., Paudyal B. Oxidative stress and its biomarkers in systemic lupus erythematosus. Journal of Biomedical Science . 2014;21(1):p. 23. doi: 10.1186/1423-0127-21-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moldogazieva N. T., Lutsenko S. V., Terentiev A. A. Reactive oxygen and nitrogen species–induced protein modifications: implication in carcinogenesis and anticancer therapy. Cancer Research . 2018;78(21):6040–6047. doi: 10.1158/0008-5472.CAN-18-0980. [DOI] [PubMed] [Google Scholar]
- 108.Chen H.-J. C., Yang Y. F., Lai P. Y., Chen P. F. Analysis of chlorination, nitration, and nitrosylation of tyrosine and oxidation of methionine and cysteine in hemoglobin from type 2 diabetes mellitus patients by nanoflow liquid chromatography tandem mass spectrometry. Analytical Chemistry . 2016;88(18):9276–9284. doi: 10.1021/acs.analchem.6b02663. [DOI] [PubMed] [Google Scholar]
- 109.Daiber A., Hahad O., Andreadou I., Steven S., Daub S., Münzel T. Redox-related biomarkers in human cardiovascular disease - classical footprints and beyond. Redox Biology . 2021;42, article 101875 doi: 10.1016/j.redox.2021.101875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li W., Huang A., Zhu H., et al. Gut microbiota-derived trimethylamine N-oxide is associated with poor prognosis in patients with heart failure. The Medical Journal of Australia . 2020;213(8):374–379. doi: 10.5694/mja2.50781. [DOI] [PubMed] [Google Scholar]
- 111.Kalinina E., Novichkova M. Glutathione in protein redox modulation through S-glutathionylation and S-nitrosylation. Molecules . 2021;26(2):p. 435. doi: 10.3390/molecules26020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Figtree G. A., Keyvan Karimi G., Liu C. C., Rasmussen H. H. Oxidative regulation of the Na+-K+ pump in the cardiovascular system. Free Radical Biology & Medicine . 2012;53(12):2263–2268. doi: 10.1016/j.freeradbiomed.2012.10.539. [DOI] [PubMed] [Google Scholar]
- 113.Jerng H. H., Pfaffinger P. J. S-Glutathionylation of an auxiliary subunit confers redox sensitivity to Kv4 channel inactivation. PLoS One . 2014;9(3, article e93315) doi: 10.1371/journal.pone.0093315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Su D., Gaffrey M. J., Guo J., et al. Proteomic identification and quantification of S-glutathionylation in mouse macrophages using resin-assisted enrichment and isobaric labeling. Free Radical Biology and Medicine . 2014;67:460–470. doi: 10.1016/j.freeradbiomed.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sugamura K., Keaney J. F. Reactive oxygen species in cardiovascular disease. Free Radical Biology & Medicine . 2011;51(5):978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zheng S., Qiu M., Wu J. H., et al. Long-chain omega-3 polyunsaturated fatty acids and the risk of heart failure. Therapeutic advances in chronic disease . 2022;13 doi: 10.1177/20406223221081616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu J., Qiu M., Sun L., et al. α-Linolenic acid and risk of heart failure: a meta-analysis. Frontiers in cardiovascular medicine . 2021;8, article 788452 doi: 10.3389/fcvm.2021.788452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gaupp R., Ledala N., Somerville G. Staphylococcal response to oxidative stress. Frontiers in cardiovascular medicine . 2012;2:p. 33. doi: 10.3389/fcimb.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fong S. W., Few L. L., See Too W. C., et al. Systemic and coronary levels of CRP, MPO, sCD40L and PlGF in patients with coronary artery disease. BMC Research Notes . 2015;8(1):679–679. doi: 10.1186/s13104-015-1677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Razzouk L., Fusaro M., Esquitin R. Novel biomarkers for risk stratification and identification of life-threatening cardiovascular disease: troponin and beyond. Current Cardiology Reviews . 2012;8(2):109–115. doi: 10.2174/157340312801784943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jin L., Cao Y., Yu G., et al. SFRP2 enhances the osteogenic differentiation of apical papilla stem cells by antagonizing the canonical WNT pathway. Cellular & Molecular Biology Letters . 2017;22(1):p. 14. doi: 10.1186/s11658-017-0044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang A., Huang Y. Role of Sfrps in cardiovascular disease. Therapeutic Advances in Chronic Disease . 2020;11 doi: 10.1177/2040622320901990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu Y., Liu X., Zheng H., et al. Multiple roles of sFRP2 in cardiac development and cardiovascular disease. International Journal of Biological Sciences . 2020;16(5):730–738. doi: 10.7150/ijbs.40923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang S., Chen H., Tan K., et al. Secreted frizzled-related protein 2 and extracellular volume fraction in patients with heart failure. Oxidative Medicine and Cellular Longevity . 2020;2020:9. doi: 10.1155/2020/2563508.2563508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu J., Zheng H., Liu X., et al. Prognostic value of secreted frizzled-related protein 5 in heart failure patients with and without type 2 diabetes mellitus. Circulation: Heart Failure . 2020;13(9, article e007054) doi: 10.1161/CIRCHEARTFAILURE.120.007054. [DOI] [PubMed] [Google Scholar]
- 126.Cooke J. P., Wilson A. M. Biomarkers of peripheral arterial disease. Journal of the American College of Cardiology . 2010;55(19):2017–2023. doi: 10.1016/j.jacc.2009.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bauer J., Ripperger A., Frantz S., Ergün S., Schwedhelm E., Benndorf R. A. Pathophysiology of isoprostanes in the cardiovascular system: implications of isoprostane-mediated thromboxane A2 receptor activation. British Journal of Pharmacology . 2014;171(13):3115–3131. doi: 10.1111/bph.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang Z. J. Systematic review on the association between F2-isoprostanes and cardiovascular disease. Annals of Clinical Biochemistry . 2013;50(2):108–114. doi: 10.1258/acb.2012.011263. [DOI] [PubMed] [Google Scholar]
- 129.Piccirillo S., Castaldo P., Macrì M. L., Amoroso S., Magi S. Glutamate as a potential "survival factor" in an in vitro model of neuronal hypoxia/reoxygenation injury: leading role of the Na+/Ca2+ exchanger. Cell Death & Disease . 2018;9(7):p. 731. doi: 10.1038/s41419-018-0784-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rashdan N. A., Shrestha B., Pattillo C. B. S-Glutathionylation, friend or foe in cardiovascular health and disease. Redox Biology . 2020;37, article 101693 doi: 10.1016/j.redox.2020.101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bajic V. P., van Neste C., Obradovic M., et al. Glutathione “redox homeostasis” and its relation to cardiovascular disease. Oxidative Medicine and Cellular Longevity . 2019;2019:14. doi: 10.1155/2019/5028181.5028181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Daiber A., Münzel T. Increased circulating levels of 3-nitrotyrosine autoantibodies: marker for or maker of cardiovascular disease? Circulation . 2012;126(20):2371–2373. doi: 10.1161/CIRCULATIONAHA.112.143214. [DOI] [PubMed] [Google Scholar]
- 133.Dominguez-Rodriguez A., Abreu-Gonzalez P., Consuegra-Sanchez L., Avanzas P., Sanchez-Grande A., Conesa-Zamora P. Thrombus aspirated from patients with ST-elevation myocardial infarction: association between 3-nitrotyrosine and inflammatory markers-insights from ARTERIA study. International Journal of Medical Sciences . 2016;13(7):477–482. doi: 10.7150/ijms.15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mu H., Wang X., Lin P., Yao Q., Chen C. Nitrotyrosine promotes human aortic smooth muscle cell migration through oxidative stress and ERK1/2 activation. Biochimica et Biophysica Acta . 2008;1783(9):1576–1584. doi: 10.1016/j.bbamcr.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pourfarzam M., Movahedian A., Sarrafzadegan N., Basati G., Samsamshariat S. Z. Association between plasma myeloperoxidase and free 3-nitrotyrosine levels in patients with coronary artery disease. International Journal of Clinical Medicine . 2013;4(3):158–164. doi: 10.4236/ijcm.2013.43028. [DOI] [Google Scholar]
- 136.Itabe H., Obama T., Kato R. The dynamics of oxidized LDL during atherogenesis. Journal of Lipids . 2011;2011:9. doi: 10.1155/2011/418313.418313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hernáez Á., Soria-Florido M. T., Schröder H., et al. Role of HDL function and LDL atherogenicity on cardiovascular risk: a comprehensive examination. PLoS One . 2019;14(6):p. e0218533. doi: 10.1371/journal.pone.0218533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang A., Li S., Zhang N., et al. Oxidized low-density lipoprotein to high-density lipoprotein ratio predicts recurrent stroke in minor stroke or transient ischemic attack. Stroke . 2018;49(11):2637–2642. doi: 10.1161/STROKEAHA.118.022077. [DOI] [PubMed] [Google Scholar]
- 139.Zhang Y. L., Cao Y. J., Zhang X., et al. The autophagy-lysosome pathway: a novel mechanism involved in the processing of oxidized LDL in human vascular endothelial cells. Biochemical and Biophysical Research Communications . 2010;394(2):377–382. doi: 10.1016/j.bbrc.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 140.Li D. Y., Wilson W. H. Tang, chapter 21- cardiovascular biomarker assessment across glycemic status. In: Watson R. R., Dokken B. B., editors. Glucose Intake and Utilization in Pre-Diabetes and Diabetes . Boston: Academic Press; 2015. pp. 245–268. [DOI] [Google Scholar]
- 141.Wang J., Tan G. J., Han L. N., Bai Y. Y., He M., Liu H. B. Novel biomarkers for cardiovascular risk prediction. Journal of Geriatric Cardiology: JGC . 2017;14(2):135–150. doi: 10.11909/j.issn.1671-5411.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhong C., Quanzhong Y., Genshan M., et al. Myeloperoxidase gene-463G > a polymorphism and premature coronary artery disease. Genetics and Molecular Biology . 2009;32(2):260–263. doi: 10.1590/S1415-47572009005000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sun P., Zhou K., Wang S., et al. Involvement of MAPK/NF-κB signaling in the activation of the cholinergic anti-inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PLoS One . 2013;8(8, article e69424) doi: 10.1371/journal.pone.0069424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cournot M., Burillo E., Saulnier P. J., et al. Circulating concentrations of redox biomarkers do not improve the prediction of adverse cardiovascular events in patients with type 2 diabetes mellitus. Journal of the American Heart Association . 2018;7(5, article e007397) doi: 10.1161/JAHA.117.007397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gaggini M., Sabatino L., Vassalle C. Conventional and innovative methods to assess oxidative stress biomarkers in the clinical cardiovascular setting. BioTechniques . 2020;68(4):223–231. doi: 10.2144/btn-2019-0138. [DOI] [PubMed] [Google Scholar]
- 146.Khoubnasabjafari M., Ansarin K., Jouyban A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. BioImpacts: BI . 2015;5(3):123–127. doi: 10.15171/bi.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Matsuda M., Shimomura I. Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Reviews in Endocrine and Metabolic Disorders . 2014;15(1):1–10. doi: 10.1007/s11154-013-9271-7. [DOI] [PubMed] [Google Scholar]
- 148.Makowski L., Chaib M., Rathmell J. C. Immunometabolism: from basic mechanisms to translation. Immunological Reviews . 2020;295(1):5–14. doi: 10.1111/imr.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cercato C., Fonseca F. A. Cardiovascular risk and obesity. Diabetology & Metabolic Syndrome . 2019;11(1):p. 74. doi: 10.1186/s13098-019-0468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Marseglia L., Manti S., Angelo G., et al. Oxidative stress in obesity: a critical component in human diseases. International Journal of Molecular Sciences . 2015;16(1):378–400. doi: 10.3390/ijms16010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hulsmans M., Holvoet P. The vicious circle between oxidative stress and inflammation in atherosclerosis. Journal of Cellular and Molecular Medicine . 2010;14(1-2):70–78. doi: 10.1111/j.1582-4934.2009.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bartelt A., Weigelt C., Cherradi M. L., et al. Effects of adipocyte lipoprotein lipase on de novo lipogenesis and white adipose tissue browning. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids . 2013;1831(5):934–942. doi: 10.1016/j.bbalip.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 153.Villarreal-Molina M. T., Antuna-Puente B. Adiponectin: anti-inflammatory and cardioprotective effects. Biochimie . 2012;94(10):2143–2149. doi: 10.1016/j.biochi.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 154.De Marchi E., Baldassari F., Bononi A., Wieckowski M. R., Pinton P. Oxidative stress in cardiovascular diseases and obesity: role of p66Shc and protein kinase C. Oxidative Medicine and Cellular Longevity . 2013;2013:11. doi: 10.1155/2013/564961.564961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Manna P., Jain S. K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metabolic Syndrome and Related Disorders . 2015;13(10):423–444. doi: 10.1089/met.2015.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kapetanovic S., Griner R., Zeldow B., et al. Biomarkers and neurodevelopment in perinatally HIV-infected or exposed youth: a structural equation model analysis. AIDS (London, England) . 2014;28(3):355–364. doi: 10.1097/QAD.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Motobayashi Y., Izawa-Ishizawa Y., Ishizawa K., et al. Adiponectin inhibits insulin-like growth factor-1-induced cell migration by the suppression of extracellular signal-regulated kinase 1/2 activation, but not Akt in vascular smooth muscle cells. Hypertension Research . 2009;32(3):188–193. doi: 10.1038/hr.2008.19. [DOI] [PubMed] [Google Scholar]
- 158.Jiang S.-Z., Lu W., Zong X. F., Ruan H. Y., Liu Y. Obesity and hypertension. Experimental and Therapeutic Medicine . 2016;12(4):2395–2399. doi: 10.3892/etm.2016.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Whayne T. F., Saha S. P., Mukherjee D. Antioxidants in the practice of medicine; what should the clinician know? Cardiovascular & Hematological Disorders Drug Targets . 2016;16(1):13–20. doi: 10.2174/1871529X16666160614015533. [DOI] [PubMed] [Google Scholar]
- 160.Hyderali B. N., Mala K. Oxidative stress and cardiovascular complications in polycystic ovarian syndrome. European Journal of Obstetrics, Gynecology, and Reproductive Biology . 2015;191:15–22. doi: 10.1016/j.ejogrb.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 161.Birben E., Sahiner U. M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organization Journal . 2012;5(1):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tiwari M. K., Hägglund P. M., Møller I. M., Davies M. J., Bjerrum M. J. Copper ion / H2O2 oxidation of Cu/Zn-Superoxide dismutase: Implications for enzymatic activity and antioxidant action. Redox Biology . 2019;26, article 101262 doi: 10.1016/j.redox.2019.101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ighodaro O. M., Akinloye O. A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine . 2018;54(4):287–293. doi: 10.1016/j.ajme.2017.09.001. [DOI] [Google Scholar]
- 164.Lee R., Margaritis M., Channon K., Antoniades C. Evaluating oxidative stress in human cardiovascular disease: methodological aspects and considerations. Current Medicinal Chemistry . 2012;19(16):2504–2520. doi: 10.2174/092986712800493057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Boshtam M., Emami Razavi A., Pourfarzam M., et al. Serum paraoxonase 1 activity is associated with fatty acid composition of high density lipoprotein. Disease Markers . 2013;35(4):p. 280. doi: 10.1155/2013/612035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Whayne T. F., Parinandi N., Maulik N. Thioredoxins in cardiovascular disease. Canadian Journal of Physiology and Pharmacology . 2015;93(11):903–1011. doi: 10.1139/cjpp-2015-0105. [DOI] [PubMed] [Google Scholar]
- 167.Loffredo L., Perri L., di Castelnuovo A., Iacoviello L., de Gaetano G., Violi F. Supplementation with vitamin E alone is associated with reduced myocardial infarction: a meta-analysis. Nutrition, Metabolism, and Cardiovascular Diseases . 2015;25(4):354–363. doi: 10.1016/j.numecd.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 168.Maruyama K., Iso H. Chapter 21- overview of the role of antioxidant vitamins as protection against cardiovascular disease: implications for aging. In: Preedy V. R., editor. Aging . San Diego: Academic Press; 2014. pp. 213–224. [Google Scholar]
- 169.Ashor A. W., Brown R., Keenan P. D., Willis N. D., Siervo M., Mathers J. C. Limited evidence for a beneficial effect of vitamin C supplementation on biomarkers of cardiovascular diseases: an umbrella review of systematic reviews and meta-analyses. Nutrition Research . 2019;61:1–12. doi: 10.1016/j.nutres.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 170.Wu J., Liang J., Li M., et al. Modulation of miRNAs by vitamin C in H2O2-exposed human umbilical vein endothelial cells. International Journal of Molecular Medicine . 2020;46(6):2150–2160. doi: 10.3892/ijmm.2020.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zheng S., Zheng H., Huang A., et al. Piwi-interacting RNAs play a role in vitamin C-mediated effects on endothelial aging. International Journal of Medical Sciences . 2020;17(7):946–952. doi: 10.7150/ijms.42586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Aune D., Keum N. N., Giovannucci E., et al. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Medicine . 2016;14(1):p. 207. doi: 10.1186/s12916-016-0730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Goszcz K., Deakin S. J., Duthie G. G., Stewart D., Leslie S. J., Megson I. L. Antioxidants in cardiovascular therapy: panacea or false hope? Frontiers in Cardiovascular Medicine . 2015;2:29–29. doi: 10.3389/fcvm.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacognosy Reviews . 2010;4(8):118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]


