Abstract
Spices and herbs are widely used food ingredients that enhance most organoleptic features of prepared foods. They are also used for medicinal and preservative purposes. Spices and herbs are potential carriers of bacteria, yeasts, and molds due to the nature of cultivation, harvest methods, storage conditions, packaging procedures, distribution, sale, and general handling. Although some fungi have been identified to be associated with most spices and herbs elsewhere in the world, little has been done on the presence of fungi in spices and herbs in Ghana. This study sought to identify the toxicogenic fungal profiles, mycotoxins (aflatoxins) present in some herbs, bay leaf (Laurus nobilis) and garden egg leaves (“gboma”) (Solanum macrocarpon), and spices, ginger (Zingiber officinale) and “dawadawa”(Parkia biglobosa), as well as to investigate the antimicrobial properties of the selected herbs and spices. The decimal reduction technique was used to plate onto Dichloran Rose Bengal Chloramphenicol (DRBC) agar media plates for fungal growth. Aflatoxin detection was carried out with high-performance liquid chromatographer connected to a fluorescence detector (HPLC-FLD). Antimicrobial properties were carried out using the agar diffusion method on solidified, freshly prepared Mueller-Hinton agar. A total of 12 species belonging to 7 genera, Aspergillus (niger, flavus, fumigatus, and ochraceus), Fusarium (oxysporum, verticillioides), Mucor (racemosus), Penicillium (digitatum, expansum), Rhizopus (stolonifer), Rhodotorula sp., and Trichoderma harzianum, were identified as fungal contaminants. Fusarium oxysporum was the most predominant species identified. Fresh ginger recorded the greatest number of colony-forming units (3.71 log10 CFU/g) with bay leaves recording the least number of colony counts (2.36 log10 CFU/g). Mycotoxin concentration detected in gboma was2.06 ± 0.07 μg/kgand in dawadawa was2.13 ± 0.09 μg/kg; however, mycotoxins were not detected in bay leaf and ginger. Ginger exhibited antibacterial activity against all bacteria ranging from 7.0 ± 0.0 mm to 12.0 ± 5.66 mm zones of inhibition. Ginger, bay leaf, and gboma extracts displayed fair antimicrobial activity against the bacteria investigated. On the other hand, dawadawa generally produced the least resistance against the five bacterial species but exhibited the highest zone of inhibition. All samples were slightly acidic with pH readings ranging from 5.81 to 6.76.
1. Introduction
Spices and herbs are products of plant origin which are used as ingredients in foods to perform several organoleptic functions, taste, flavor, smell, color, etc., as well as dietetic functions [1]. Spices have been known and used for years among all regions and groups of people in the world [2]. They are cultivated, harvested, and sold out in very large to small quantities to the general population. Most spices and herbs have great nutritional properties; they have been identified as natural antioxidant sources and hence play a crucial role in the chemoprevention of illnesses caused by lipid peroxidation [3]. Most of these spices are rich in phytochemicals [3].
Spices and herbs can be contaminated with toxigenic fungi and may lead to the production of mycotoxins [4]. Sufficient evidence has proven that spices are commonly heavily contaminated with xerophilic storage molds and bacteria [5, 6]. Additionally, inappropriate handling and other unfavourable conditions after harvesting, storage, processing, packaging, and distribution could leave these food products with traces of fungi on them [5].
In the Ghanaian market, there are numerous mixtures of spices that are well patronized. Common spices such as onion, pepper, ginger, nutmeg, rosemary, and garlic are used in the preparation of household cookery and foods sold at restaurants and food joints or chop bars (local eateries) [7]. Nutmeg and aniseed cloves are ocimum, which are most frequently used in the food industry as food additives for flavoring and health effects [8], while dawadawa is widely used in the northern parts but scarcely used domestically in the southern parts of Ghana. Spices and their extracts, particularly their oils, are receiving attention and are being widely studied as potential antimicrobial agents, which may be important for food preservation and the control of human diseases of microbial origin. Previous work done by some researchers in Ghana reported these local spices to have several activities against microbial infections [7, 9–11]. This may probably be due to the bioactive phytochemicals such as terpenoids, flavonoids, phenolics, and alkaloids present in these herbs and spices, which makes it possible to reduce pathogen populations and curb mycotoxin proliferations in foods.
Some fungi produce secondary metabolites known as mycotoxins, which are capable of causing several diseases in humans and some animals as well. Mycotoxins are inevitable contaminants found in food grains, feeds, medicinal herbs, and spices, and they pose a health risk to both animals and people [12]. As spices and herbs are widely used for various needs by Ghanaians, it has become important to determine the possible species of fungi that are associated with different local spices. Spices and herbs are gaining importance in recent years as potential sources of natural food preservatives due to the growing interest in the development of safe and effective natural food preservation [13]. Efforts are being made by food scientists to gradually reduce the use of artificial synthetic preservatives in the food industry because they are known to cause some adverse health effects, especially cancer. A thorough look must be made into ensuring the maximum safety of food products that are preserved by these natural preservatives: spices, herbs, and their extracts.
This study is aimed at updating the fungal profile, mycotoxins, and antimicrobial properties of spices and herbs from three selected markets in the Ho municipality, Ghana.
2. Materials and Methods
2.1. Study Design
A quantitative experimental study design was employed in this study.
2.2. Study Site
Samples were obtained from three selected markets in the Ho municipality, namely, Ho main market (1), Ahoe satellite market (2), and Dome market (3). All other procedures were carried out in the University of Health and Allied Sciences (UHAS) food laboratory located on the Dave campus.
2.3. Sampling and Sample Preparation
Samples of spices and herbs are, namely, bay leaf (BL) (fresh), ginger (fresh ginger = GF, powdered ginger = GP), dawadawa (DAW), and gboma (GB) (fresh) (Table 1). A sample size of forty-five (45) individual units was used. Five different spice and herb samples were purchased at three points per each of the three different markets. The samples were randomly sampled over a two-week period in July and August 2020. Approximately 100 g of each sample was collected and stored in sterile specimen containers (Nasco, USA) and transported in an ice chest freezer (Thermos 7750, China) with cold packs at a temperature of 10°C under aseptic conditions to the UHAS laboratory for microbiological analysis within 2 hours of collection [14].
Table 1.
Spices and herb samples and their corresponding interpretations.
| Code | Interpretation |
|---|---|
| GP1 | Ginger powder/vendor 1 |
| GP2 | Ginger powder/vendor 2 |
| GP3 | Ginger powder/vendor 3 |
| GF1 | Ginger fresh/vendor 1 |
| GF2 | Ginger fresh/vendor 2 |
| GF3 | Ginger fresh/vendor 3 |
| BL1 | Bay leaf/vendor 1 |
| BL2 | Bay leaf/vendor 2 |
| BL3 | Bay leaf/vendor 3 |
| GB1 | Gboma/vendor 1 |
| GB2 | Gboma/vendor 2 |
| GB3 | Gboma/vendor 3 |
| DAW1 | Bambara bean/vendor 1 |
| DAW2 | Bambara beans/vendor 2 |
| DAW3 | Bambara beans/vendor 3 |
Differences in moisture contents of the samples were corrected by using the formula
| (1) |
2.3.1. Fungal Plating
One gram (1 g) of each sample was transferred into 9 ml of distilled water. The samples were then vortexed for 1 minute at a moderate speed. One milliliter of each sample was plated onto the media plates and incubated at 25°C for 7 days. All samples were weighed using an electronic balance (OHAUS®) with a readability of 0.01 g. Each stock solution was serially diluted in 9 ml of peptone (0.1%) water in tenfold increments up to 10−3. One milliliter (1 ml) of each serial dilution was plated onto Dichloran Rose Bengal Chloramphenicol (DRBC) (Oxoid CM727, United Kingdom). Agar media plates were prepared according to the manufacturer's instructions and incubated at 25°C for 5-7 days [15].
2.3.2. Fungal Enumeration and Identification
Enumeration was carried out by a colony counter. Fungal counts were recorded in standard form and later transformed into a logarithmic scale. Colony-forming unit (CFU) per gram was calculated using the formula [14, 16]
| (2) |
Percentage occurrence of fungal species was calculated using the formula
| (3) |
2.3.3. Lactophenol Cotton Blue Tease Procedure
A drop of Lactophenol Cotton Blue (LPCB) dye was placed on the slide, and a sterile iron needle was used to transfer a tiny piece of a colony of Lactophenol Cotton Blue Dye on the slide. The colony was then teased into minuscule pieces using an iron needle. The slide was covered with a coverslip.
Identification of fungi was done macroscopically (texture and color of the plate) and microscopically by observation of morphological features under the microscope (Figures 1–3).
Figure 1.
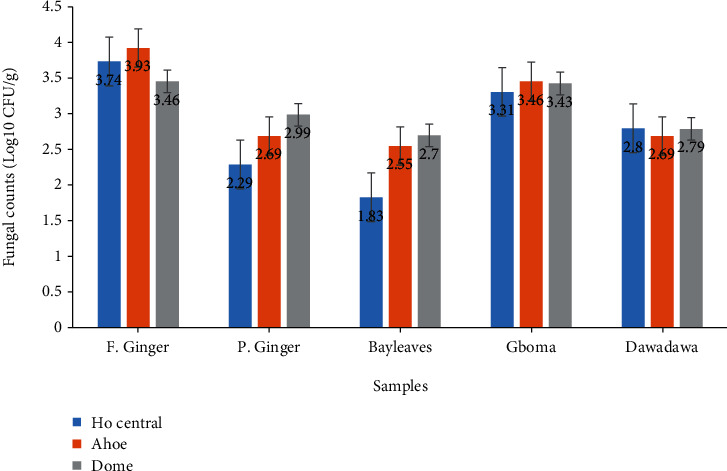
Mean fungal counts on samples from the three markets. Data presented are means ± standard error.
Figure 2.
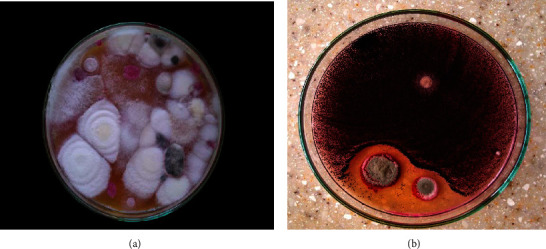
Macroscopic view of fungal species: (a) Fusarium oxysporum on gboma and (b) Aspergillus niger in dawadawa on DRBC agar.
Figure 3.
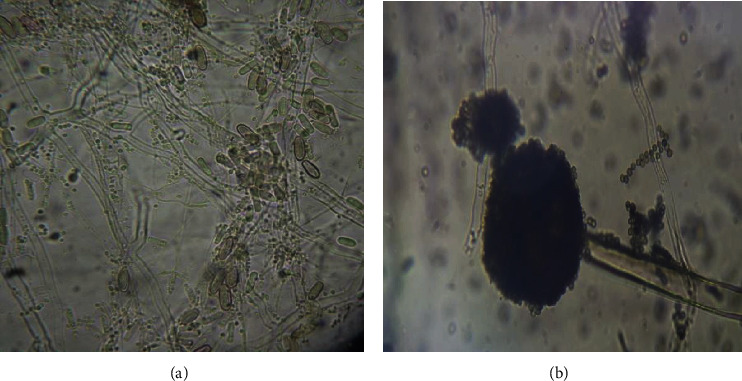
Microscopic views of (a) Fusarium oxysporum (×400) and (b) Aspergillus niger (×400).
2.4. Identification
Molds that appeared were identified by their culture and morphological characteristics (Figures 2 and 3) using standard identification manuals [17, 18].
2.5. Aflatoxin Analysis
2.5.1. Extraction of Samples
The European Committee for Standardization (CEN) official method EN14123 [19] was used to extract and quantify AFB1, AFB2, AFG1, and AFG2 from the samples. Methanol in water (200 ml) (8 + 2) and 5 g NaCl were used to extract 20 g of sample. Fat samples containing more than 50% fat were extracted with 100 ml of hexane in the normal methanol extraction solvent. One hundred milliliters (100 ml) of hexane was added to 200 ml of methanol. After homogenization, a separation funnel was used to separate the hexane, which became the upper layer. The mixture was homogenized for 3 min at 3000 rpm (2 min) and 3500 rpm (1 min). The extracts were filtered and 10 ml of the filtrate added to 60 ml of phosphate buffer saline (PBS) for solid-phase extraction using a preconditioned immune affinity column specific for AFB1, AFB2, AFG1, and AFG2. The 70 ml filtrate-PBS mixture was loaded onto the preconditioned column and allowed to elute by gravity at a flow rate of 1 ml min−1. This was followed by a cleanup with 15 ml distilled water at a flow rate of 5 ml min−1. Aflatoxins were eluted in two steps into a 5 ml volumetric flask with 0.5 ml followed by 0.75 ml of methanol (HPLC grade) and allowed to elute by gravity. Deionized water was used to make up the volume of eluate to 5 ml and eluate vortexed and 2 ml pipetted into HPLC vials for quantification.
2.5.2. HPLC Parameters
Injection volume: 10 μl, flow rate: 1 ml/min, column temperature: 35°C, excitation wavelength: 360 nm, emission wavelength: 440 nm, mobile phase composition: water/acetonitrile/MeOH (65 : 15 : 20 v/v/v), postcolumn derivatization: Kobra cells. HPLC column specification Spherisorb ODS1-Excel (4.6 mm × 25 cm), 5 μm particle size, 250A pore size.
2.5.3. Limit of Detection/Quantification (LOD/LOQ)
The limit of detection and quantification (LOD/LOQ) of the HPLC was estimated by making a calibration curve around the standard used for spiking 5 μg/kg (the lowest concentration range of the calibration curve). Blank did not produce any signal, so LOD and LOQ were calculated as
| (4) |
2.5.4. Measurement Accuracy
Spiking of pure aflatoxin standard solution was done to ensure the measurement accuracy of the analysis. Three levels of spiking were done at the lower, middle, and upper concentration range of the calibration curve concentrations (5 μg/kg, 15 μg/kg, and 30 μg/kg). Spike volumes of pure standards were calculated as
| (5) |
Spike volumes were distributed evenly on aflatoxin-free sample (blank), and the spiked sample was analyzed for percentage recovery which was calculated as
| (6) |
2.5.5. Measurement Precision
Repeatability and intermediate precision analyses of an Internal Reference Material (IRM) were used to ensure the measurement precision of the method. For repeatability analysis, 10 parallel extractions of the IRM were done by the same analyst at the same time using the same HPLC, and the relative standard deviation between the results was calculated. For intermediate precision, 10 extractions of the IRM were done on different days by different analysts, and the relative standard deviation between the results was calculated according to Kortei et al. [20]. The relative standard deviations were calculated as
| (7) |
2.6. Antimicrobial Sensitivity
2.6.1. Plant Extraction for Antimicrobial Sensitivity Test
All samples (ginger, bay leaf, gboma, and dawadawa) were pulverized after which an initial mass (100 g) was weighed into clean conical flasks. About 200 ml of methanol was added to each sample of powdered spice in conical flasks. The conical flasks were covered with aluminium foil to prevent evaporation and kept for 48 hours at 28°C away from light and subjected to occasional shaking/stirring to enhance the extraction. The resulting solution was filtered using filter paper, and the filtrates were evaporated to dryness in a Heratherm oven at a temperature of 40°C for 48 hours. The dried weights of the extracts were determined and stored in falcon tubes. Plant extracts in falcon tubes were kept in a refrigerator at 8°C for further analysis.
2.6.2. Determining the Antimicrobial Activity of Samples
Using the agar diffusion method, the extracts were concentrated and tested for antibacterial activity against Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus, Salmonella typhi, and Streptococcus mutans obtained from the Microbiology Unit, Department of Biomedical Sciences, University of Health and Allied Sciences. The agar diffusion method as previously used by Perez [21] and modified by Ahmad and Beg [22] was used. The test organisms were subcultured on Mueller-Hinton agar at 37°C between 18 and 24 hours. An inoculum (500 μl) was adjusted to 1.5 × 108 CFU/ml using 0.5 McFarland standard. On each Mueller-Hinton agar plate, each test organism was evenly spread using a sterile microbiological loop. The plate was incubated at room temperature (approx. 25°C) for 10 minutes. Subsequently, seven wells (diameter: 8 mm) were aseptically created into the agar medium. Four concentrations of each sample (500 mg/ml, 250 mg/ml, 125 mg/ml, and 62.5 mg/ml) of 70% methanol extraction were prepared. Into each of the wells, 100 μl (5 mg/ml) spice and herb extracts was aseptically dispensed into six individual wells while 30 μl of a reference drug (chloramphenicol (CPL) (concentration 5 mg/ml)) was dispensed aseptically into the seventh well. The Mueller-Hinton agar plates were incubated aerobically between 18 and 24 hours. The zones of inhibition of the plant extracts were measured and compared with those of the reference drug.
2.7. Ethical Considerations
Ethical clearance was sought from the University of Health and Allied Sciences Research and Ethics Committee (UHAS-REC) prior to the conduct of the research (protocol ID UHAS-REC A.9 [47] 20-21). All COVID-19 safety protocols were observed throughout the study.
2.8. Statistical Analysis
Linear regression analysis was used to calculate aflatoxin concentrations. Fungal counts were done in standard form but were transformed into logarithm. Antimicrobial activity results were presented as means and standard deviation. One-way ANOVA was performed, and means were separated using Duncan's Multiple Range Test (DMRT). A p value less than 0.05 was considered significant. Analyses were done using SPSS version 23 (USA, Virginia).
3. Results
3.1. Fungal Counts
There was an average fungal count ranging from 3.46 log10 CFU/g to 3.93 log10 CFU/g for fresh ginger samples. Fungal counts of fresh ginger samples from Ahoe and Ho central were comparable (p > 0.05). Similarly, counts from Ahoe and Dome were not comparable (p > 0.05). However, samples from Ho central and Dome were not significantly different (p < 0.05). There was observed comparatively higher counts for fresh ginger samples obtained from all three markets (Ho central, Ahoe, and Dome) than any other spice or herb (Figure 1).
The mean fungal counts obtained on DRBC agar media for powdered ginger ranged from a minimum value of 2.29 log10 CFU/g to a maximum of 2.99 log10 CFU/g (Figure 1). Ahoe and Dome showed no significant difference (p > 0.05). Ho central and Dome were comparable (p > 0.05).
For bay leaf samples, there was a mean fungal count range of 1.83 log10 CFU/g to 2.70 log10 CFU/g. Gboma samples from the three markets had a mean fungal count range of 3.31 log10 CFU/g to 3.46 log10 CFU/g. Counts from Ho central market were significantly (p < 0.05) lower than the other two markets.
There was no statistically significant difference (p > 0.05) in fungal counts among the three markets (Ho Central, Ahoe, and Dome) for all spices of gboma and dawadawa. For dawadawa samples, there was a mean fungal count ranging from 2.69 log10 CFU/g to 2.81 log10 CFU/g.
3.2. Percentage Occurrence of Fungal Species
A pooled data of fungi in herbs and spices from all three markets (Ho central, Ahoe, and Dome) comprising a total of twelve (12) fungal species were isolated and identified. These belonged to seven (7) genera: Aspergillus, Fusarium, Mucor, Penicillium, Rhizopus, Rhodotorula, and Trichoderma. The species were Aspergillus niger, A. flavus, A. fumigatus, A. ochraceus, Fusarium oxysporum, F. verticillioides, Mucor racemosus, Penicillium digitatum, P. expansum, Rhizopus stolonifer, Rhodotorula species, and Trichoderma harzianum (Tables 2 and 3). All species identified were molds with the exception of Rhodotorula species which was the only yeast among the fungi.
Table 2.
Percentage occurrence (%) per sample of different fungal species identified. TPOfs = total percentage occurrence of each fungal species among all samples.
| Fungal species | Percentage occurrence (%) | TPOfs 100% |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GP1 | GP2 | GP3 | GF1 | GF2 | GF3 | BL1 | BL2 | BL3 | GB1 | GB2 | GB3 | DAW1 | DAW2 | DAW3 | ||
| Aspergillus niger | — | 55.0 | — | 15.0 | — | 7.0 | 25.0 | — | 42.0 | 56.0 | 33.0 | — | 5.3 | — | 5.3 | 16.24 |
| Aspergillus flavus | — | — | — | 24.0 | 3.3 | — | — | — | 20.0 | — | 13.8 | — | — | — | — | 4.07 |
| Aspergillus fumigatus | — | — | 70.0 | 17.0 | — | — | 15.0 | — | — | 29.0 | — | — | 5.4 | — | 5.5 | 9.46 |
| Aspergillus ochraceus | — | 15.5 | — | — | — | — | — | — | 8.0 | — | 13.9 | — | 8.9 | — | — | 3.09 |
| Fusarium oxysporum | 100.0 | 29.5 | 30.0 | — | 25.3 | — | 45.0 | — | 16.0 | 15.0 | 35.0 | — | 25.0 | 16.7 | 16.1 | 23.54 |
| Fusarium verticillioides | — | — | — | 12.0 | — | 71.0 | — | — | — | — | — | 27.0 | — | — | — | 7.33 |
| Mucor racemosus | — | — | — | — | — | — | — | 40.0 | 11.0 | — | — | — | — | — | 3.4 | 3.63 |
| Penicillium digitatum | — | — | — | — | 6.0 | 22.0 | 15.0 | — | — | — | — | — | 55.3 | — | — | 6.55 |
| Penicillium expansum | — | — | — | — | — | — | — | — | — | — | — | 73.0 | — | — | — | 4.90 |
| Rhizopus stolonifer | — | — | — | — | 65.4 | — | — | 60.0 | — | — | — | — | — | 56.7 | 46.5 | 15.18 |
| Rhodotorula sp. | — | — | — | 21.0 | — | — | — | — | — | — | 4.3 | — | — | — | 3.8 | 1.94 |
| Trichoderma harzianum | — | — | — | 11.0 | — | — | — | — | 3.0 | — | — | — | — | 26.6 | 19.4 | 4.00 |
| Total no. of species | 1 | 3 | 2 | 6 | 4 | 3 | 4 | 2 | 6 | 3 | 5 | 2 | 5 | 3 | 7 | |
Table 3.
Pooled data of total fungi isolated from ginger, dawadawa, bay leaf, and gboma.
| Aspergillus niger Van TieghemGP2,GF1,GF3,BL1,BL3,GB1,GB2,DAW1,DAW3 |
| A. flavus LinkGF1,GF2,BL3,GB2 |
| A. fumigatus FresenGP3,GF1,BL1,GB1,DAW1 |
| A. ochraceus WilhelmGP2,BL3,GB2,DAW1 |
| Fusarium oxysporum SchlechtGP1,GP2,GP3,GF2,BL1,BL3,GB1,GB2,DAW1,DAW2,DAW3 |
| Fusarium verticillioides GF1,GF3,GB3 |
| Mucor racemosus Fres.BL2,BL3,DAW3 |
| Penicillium digitatum Sacc.GF3,BL1 |
| Penicillium expansum DierckxGB3 |
| Rhizopus stolonifer (Ehrenb.) Lind.GF2,BL2,DAW2,DAW3 |
| Rhodotorula mucilaginosa (A. Jorg) F.C. HarrisonGF1,GB2,DAW3 |
| Trichoderma harzianum RifaiGF1,BL3,DAW2,DAW3 |
For the powdered ginger sample, four fungal species were identified, namely, A. niger (18.3%), A. fumigatus (23.3%), A. ochraceus (5.2%), and Fusarium oxysporum (53.2%). A total of nine fungal species were identified on fresh ginger samples: A. niger (7.3%), A. flavus (9.1%), A. fumigatus (5.7%), F. oxysporum (8.4%), F. verticillioides (27.7%), Penicillium digitatum (9.3%), Rhizopus stolonifer (21.8%), Rhodotorula spp. (7.0%), and Trichoderma harzianum (3.7%). A total of nine fungal species, namely, A. niger (22.3%), Fusarium oxysporum (20.3%), A. fumigatus (5.0%), Penicillium digitatum (5.0%), A. flavus (6.7%), Trichoderma harzianum (1.0%), Mucor racemosus (17.0%), A. ochraceus (2.7%), and Rhizopus stolonifer (20.0%), were isolated for bay leaf samples. Gboma samples had a total number of eight fungal species identified: A. niger (30.0%), A. flavus (4.3%), A. fumigatus (9.7%), F. oxysporum (16.6%), F. verticillioides (9.0%), Penicillium expansum (24.4%), Rhodotorula spp. (1.4%), and Trichoderma harzianum (3.7%), and in dawadawa samples, nine species of fungi were identified. These were A. niger (3.5%), A. fumigatus (3.6%), A. ochraceus (3.0%), Fusarium oxysporum (19.3%), Mucor racemosus (1.1%), Penicillium digitatum (18.4%), R. stolonifer (34.4%), Rhodotorula spp. (1.3%), and Trichoderma harzianum (15.3%) (Table 2).
Fusarium oxysporum had the highest percentage occurrence in all samples (23.54%) (Figures 2 and 3) compared to the other species identified, occurring at least once in each of the samples tested. On the other hand, Rhodotorula sp. occurred the least in all samples compared to the other species identified.
3.3. Antibacterial Activities of Samples
The antibacterial activity of extracts from the various samples is presented in Table 4. Methanolic extracts of the four spices and herbs as tested against five bacterial species produced the following results: ginger extract showed inhibitory zones ranging from 4.5 ± 6.36 mm to 9.5 ± 0.70 mm against Pseudomonas aeruginosa and 10.5 ± 3.54 mm to 12.0 ± 5.66 mm. There was no significant (p = 0.16) difference observed. Bay leaf extract exhibited growth inhibition against Pseudomonas aeruginosa (6 ± 0.00 mm for 62.5 mg/ml to 9 ± 0.00 mm at 500 mg/ml) (Figure 4) and showed no significant (p = 0.10) difference. Ginger methanolic extract was potent against all bacteria investigated (Table 4). There was no zone of inhibition for all concentrations of bay leaf extract as tested against Klebsiella pneumoniae and Salmonella typhi (Table 4).
Table 4.
Pooled data of antimicrobial activities of spices and herbs against microorganisms.
| Samples | Concentration (mg/ml) | Zone of inhibition (mean in mm ± std) | ||||
|---|---|---|---|---|---|---|
| PA | KP | SA | SM | ST | ||
| Ginger | 500 | 4.50 ± 6.36 | NZI | 11.50 ± 2.12 | 10.50 ± 0.71 | NZI |
| 250 | 8.00 ± 0.00 | 9.50 ± 2.12 | 11.50 ± 3.54 | 8.50 ± 0.71 | 7.00 ± 0.00 | |
| 125 | 9.50 ± 0.70 | 9.00 ± 0.00 | 12.00 ± 5.66 | 10.00 ± 0.00 | 11.00 ± 2.83 | |
| 62.5 | 7.50 ± 0.70 | 10.00 ± 2.82 | 10.50 ± 3.54 | 8.50 ± 0.71 | 8.50 ± 0.71 | |
| Ctrl | 13.50 ± 0.70 | 29.00 ± 1.41 | 24.00 ± 5.66 | 19.00 ± 1.41 | 34.00 ± 7.00 | |
|
| ||||||
| Bay leaves | 500 | 9.00 ± 0.00 | NZI | 10.00 ± 0.00 | 9.00 ± 2.82 | NZI |
| 250 | 7.00 ± 0.00 | NZI | 7.50 ± 0.71 | 9.00 ± 2.82 | NZI | |
| 125 | 6.00 ± 0.00 | NZI | 6.00 ± 0.00 | 9.00 ± 4.24 | NZI | |
| 62.5 | 6.00 ± 0.00 | NZI | 6.00 ± 0.00 | NZI | NZI | |
| Ctrl | 20.00 ± 7.07 | 28.50 ± 0.71 | 26.50 ± 0.71 | 29.50 ± 0.71 | 33.00 ± 0.00 | |
|
| ||||||
| Gboma | 500 | 13.00 ± 1.41 | 9.50 ± 0.71 | 8.50 ± 2.12 | 11.00 ± 0.00 | NZI |
| 250 | 8.00 ± 1.41 | 8.00 ± 1.41 | 7.50 ± 0.71 | 7.00 ± 0.00 | NZI | |
| 125 | 7.00 ± 0.00 | 8.00 ± 0.00 | 7.50 ± 0.71 | NZI | NZI | |
| 62.5 | 7.50 ± 0.71 | NZI | NZI | NZI | NZI | |
| Ctrl | 10.50 ± 0.71 | 29.00 ± 1.41 | 25.00 ± 0.00 | 15.00 ± 0.00 | NZI | |
|
| ||||||
| Dawadawa | 500 | 15.00 ± 0.00 | NZI | 16.50 ± 0.71 | 23.50 ± 0.71 | NZI |
| 250 | 10.00 ± 0.00 | NZI | 9.50 ± 0.71 | 14.00 ± 0.00 | NZI | |
| 125 | NZI | NZI | NZI | NZI | NZI | |
| 62.5 | NZI | NZI | NZI | NZI | NZI | |
| Ctrl | 22.50 ± 3.54 | 33.5 ± 4.95 | 25.00 ± 0.00 | 15.00 ± 0.00 | 27.5.0 ± 3.54 | |
Mean zone of inhibition of extracts from four spices and herbs against bacterial species. PA = Pseudomonas aeruginosa; KP = Klebsiella pneumoniae; SA = Staphylococcus aureus; SM = Streptococcus mutans; ST = Salmonella typhi; NZI = no zone of inhibition; control (Ctrl) = chloramphenicol 30 μg.
Figure 4.
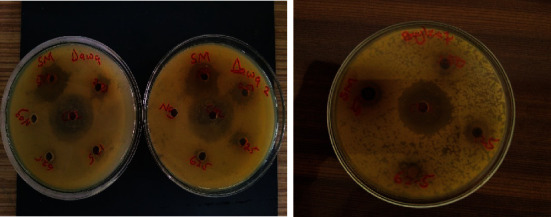
Zone of inhibition exhibited by (a) dawadawa and (b) bay leaf extracts against Streptococcus mutans.
At 500 mg/ml, gboma extract exhibited an inhibitory zone of 13 ± 1.41 mm and 7.5 ± 0.71 mm at 62.5 mg/ml against Pseudomonas aeruginosa and there was a range of no zone of inhibition.
Gboma extract performed fairly against Pseudomonas aeruginosa with a range of 7 ± 0.00 mm to 13 ± 1.41 mm zone of inhibition where there was a statistically significant difference among the means of the various concentrations (p = 0.008). Extracts from the four samples showed poor antibacterial susceptibility to Klebsiella pneumoniae; gboma extract inhibited the growth of Klebsiella pneumoniae at 500 mg/ml (9.5 ± 0.71 mm), 250 mg/ml (8 ± 1.41 mm), and 125 mg/ml (8 ± 0.00 mm); there was a statistically significant difference (p < 0.001) among mean zone of inhibition for the various concentrations of gboma extracts. For Salmonella typhi, only ginger extract produced zones of inhibition which ranged from 7 ± 0.00 mm to 11 ± 2.83 mm diameter. There was a statistically significant difference (p = 0.001) between the zones of inhibition for the various concentrations.
3.4. Mean Aflatoxin Counts
Aflatoxin counts investigated in the various spice and herb samples are represented in Figure 5. Bay leaf and ginger (fresh and powdered) samples had no aflatoxins detected in them. Gboma samples tested recorded a mean aflatoxin count of 2.06 ± 0.50 μg/kg, whereas dawadawa had a mean aflatoxin concentration of 2.13 ± 0.61 μg/kg (Figure 5).
Figure 5.
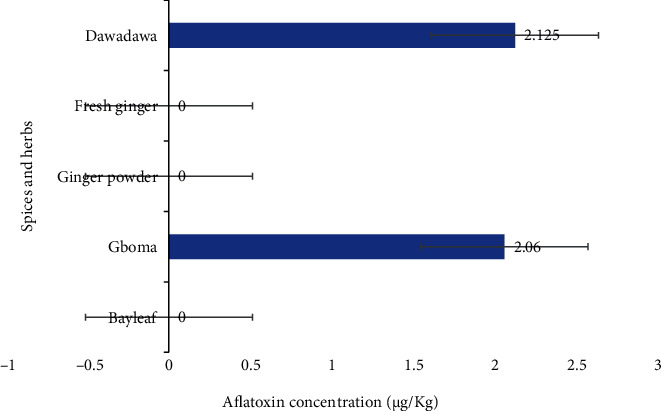
Mean aflatoxin concentrations in spices and herbs of samples from the Ho municipality.
3.5. pH of Samples
The pH readings of the four spice and herb samples are presented in Table 5. Values of 6.56, 5.81, 6.20, and 6.76 were recorded for ginger, gboma, bay leaf, and dawadawa, respectively. The pH value for gboma (5.81) was significantly lower (p < 0.05) than that for the other samples.
Table 5.
pH of spices and herbs from the Ho municipality.
| Sample | Mean ± std |
|---|---|
| Ginger powder | 6.56 ± 0.01b |
| Fresh ginger | 6.50 ± 0.00b |
| Gboma | 5.81 ± 0.00a |
| Bay leaf | 6.20 ± 0.01b |
| Dawadawa | 6.76 ± 0.04b |
Means with the same superscripts in a column are not significantly different (p > 0.05).
4. Discussion
4.1. Fungal Counts
The count of the fungal species in the spices and herbs that were studied which ranged from 3.71 log10 CFU/g (5129) in fresh ginger to 1.83 log10 CFU/g in bay leaf is comparable to fungal counts of common spices studied in Saudi Arabia market (2325–6800 CFU/g) with the highest fungal counts also reported in ginger samples [23]. Similar studies reported 1120-1580 CFU/g (log10 3.049-3.198 CFU/g) in some herbs and spices in Bahrain [24] and 1 × 103–6 × 103 CFU/g (log10 3.0-3.778 CFU/g) in herbs and medicinal plants in Iraq [25]. However, other studies have reported much lower resident mycoflora ranged between 1.22 and 1.88 log10 CFU/g samples in some spices in Ghana [9, 26, 27]. Variation between fungal counts is to be expected because of sample and sampling variability, country of origin, and differences in processing and storage practices. For example, all spices in the present study manifest comparable species percentage occurrence and fungal counts to those reported by Chourasia [28]. The source of these foodstuff contaminants may be the result of natural extraneous pollution with dust particles containing spores from the field or storage [24, 29–31].
The observed higher fungal counts in ginger could be attributed to the soil origin of the rhizome, which is known to be a principal reservoir of all microorganisms. Zhang et al. [32] highlighted that variation in fungal counts could also be attributed to differences in compliance with good agricultural practices (GAP) as well as good manufacturing practice (GMP) conditions during the growing, processing, or storage of the raw material. Furthermore, the effect of storage on the viability of fungal propagules could also be worth considering.
Interestingly, another research work in Qatar found no fungal contamination in ginger, curry, and garlic samples but high fungal inoculum in chili powder [33]. Comparing fresh ginger with powdered ginger samples, fungal counts were higher in fresh ginger.
4.2. Fungal Species
Twelve distinct fungal species belonging to six genera were isolated from the four spices tested. Aspergillus species were the most isolated genus, and Fusarium oxysporum was the most isolated fungal species identified as it occurred in eleven out of a total of fifteen individual samples investigated. Fungal species occurrence in this study was sporadic with respect to the spice and herb. Ahene et al. [9] isolated similar species of fungi from raw spices and the spice products which belonged to eight (8) genera, Absidia, Aspergillus, Cladosporium, Fusarium, Rhizopus, Penicillium, Neurospora, Eurotium, but Aspergillus species (A. flavus, A. fumigatus, A. alutaceus, A. niger, and A. sulphureus) were the predominant species isolated. Mandeel [24] isolated some fungal species, and the most predominant fungal genera encountered were Aspergillus, Penicillium, Rhizopus, Cladosporium, and Trichoderma. In their work, yeasts were also frequently recovered, but not identified. Painstil [27] examined mycoflora in stored fresh ginger and powdered ginger. They recorded the occurrence of Aspergillus flavus, Aspergillus fumigatus, Aspergillus nidulans, Aspergillus ochraceus, Aspergillus glaucus, Aspergillus niger, Cladosporium herbarum, Fusarium, Penicillium digitatum, and Trichoderma viride. These fungal species showed five patterns of infection. In Indonesia, Nurtjahja et al. [34] reported that all tested spices were contaminated by storage fungi of which species of Aspergillus were the most commonly isolated molds followed by species of Fusarium, Mucor, Penicillium, and Rhizopus. The greatest number of Aspergillus flavus isolates was found on white pepper, followed by nutmeg, cardamom, and black pepper.
Toma and Abdulla [25] recorded ten different fungal genera, and 16 species were isolated and identified as Alternaria alternata, Aspergillus spp., Gliocladium sp., Hyalodendron diddeus, Memnoniella sp., Penicillium spp., Rhizopus spp., Syncephalastrum sp., Cladosporium lignicolum, and Ulocladium botrytis in spices and herbal medicinal plants in Iraq. Hashem and Alamri [23] reported Aspergillus, Penicillium, and Rhizopus as the most predominant fungal genera encountered in their research on spices. Samples obtained from sumac encountered very unusual colony counts indicating its antifungal affluences. Alternaria was characterized by Alternaria alternate. Ath-Har et al. [35] reported that A. flavus, A. niger, Aspergillus nidulans, A. sydowii, A. ochraceus, Penicillium, and Rhizopus spp. were most frequently isolated from spices and drug plants. Bugno et al. [36] showed that the predominant mycoflora obtained was distributed in 10 genera. The genus Aspergillus was the most dominant genus recovered (179 isolates) followed by Penicillium (44 isolates). The presence of a wide range of storage fungi indicates that considerable improvements could be made during postharvest storage. The dominance of Aspergillus and Penicillium spp. in all examined medicinal plant samples and spices was in accord with the results of Takatori et al. [37], Aziz et al. [29], and Hashem and Alamri [23], who stated that Aspergillus and Penicillium spp. were the main components of cardamom, cinnamon, fennel, coriander, cumin, black cumin, and white pepper, all of which are common in the food industry. They found a high degree of contamination in all samples.
Fresh ginger also outweighed powdered ginger samples for the occurrence of fungal species. Nine individual fungal species were isolated from fresh ginger, whereas four fungal species were isolated from powdered ginger samples. This is because moisture facilitates the growth of fungi [38]; powdered ginger has a low moisture content as is not with fresh ginger. Bay leaf samples had the lowest plate counts compared to the other three spices and herbs (Figure 1). This is attributed to the fact that the bay leaf samples were dry and thus had low moisture content, thereby reducing the chances of survival of microorganisms (fungi) on the herb spice. This notwithstanding, there was a total number of nine fungal species isolated from bay leaf samples tested with low percentage occurrence levels (Figure 2).
Presently, the prime challenge of toxicogenic fungi and mycotoxin proliferation in our foods is the link with climate change. There is now a widespread consensus that the world is warming at an unparalleled rate, and this is expected to seriously affect our crop production as well as the phyllosphere microflora of these crops. The irrepressible growth of A. flavus under extreme heat and dry conditions is an expected and emerging dilemma mainly in many parts of the world (Serbia, Hungary, etc.) where there were very low reports of mycotoxin contamination [39]. However, surges in maize contamination were observed after prolonged hot and dry weather. Due to this, the world's largest agricultural food exporters such as Brazil, Argentina, and some parts of Asia including China and India have been identified as hot spots for the impacts of climate change. From a food security viewpoint, a more precise forecast of the impacts of climate change on mycotoxins needs to be addressed to prevent conceded food sustainability, which possibly results in negative social consequences.
4.3. Aflatoxin Contamination
Aflatoxin concentrations recorded in this present work were 2.06 ± 0.50 μg/kg and 2.125 ± 0.61 μg/kg, respectively, for gboma and dawadawa and were found to be below regulatory limits for both Ghana Standards Authority (10 μg/kg) and the European Food Safety Authority (4 μg/kg) [40]. Seemingly not harmful, it is worthy to note that no amount of aflatoxin is considered safe no matter how minute it is in public health. The Ghanaian public health authorities as well as the populace have to monitor ceaselessly to detect and reduce AFB1 contamination in various foods and make conscious efforts to suppress to an ALARA (as low as reasonably achievable) level.
The main mycotoxins that contaminate spices are aflatoxins and ochratoxins. Furthermore, aflatoxin is the most common mycotoxin in spices, and aflatoxin contamination in spices has been studied widely by several researches [41–43].
AFB1 was found in 8 of the 10 nutmeg samples tested in Portugal [44], with levels ranging from 1.25 to 58 g/kg, whereas AFB1 was found in only one of the three nutmeg products in Italy [6, 44] which disagreed with results of this study as mycotoxins in nutmeg and aniseed were below detection limits. In five nutmeg samples from Egypt, El-Kady et al. [45] found no aflatoxins. Ochratoxins A (OTA) was also not found in nutmeg purchased in Belgium, the Netherlands, or Russia from retail stores [46]. Yarru et al. [47] found that curcumin has partial protective effects on alterations in antioxidant biotransformation and immune system gene expression in the livers of chicks fed AFB1. Turmeric powder recorded values 100-200 mg kg−1 bw were found to protect mice from AFB1 (2 g kg−1 bw)-induced toxicity by regulating lipid peroxidation and enhancing the defense system in a comparable investigation [48]. Another study contrasted with Garcia et al. [49] who did similar work on various fungal and bacteria species with different samples recorded fungal contamination and its negative effect on mankind, ocimum, clove, and turmeric aflatoxin analysis, they were below the limit of quantification (LOQ = 2 μg/kg). However, there were little or no detections of aflatoxin in the samples from Ho except for dawadawa. Several studies have been done concerning these spices and others in different countries like India and Portugal, where aflatoxins were detected, which implies that we made a careful move on how to handle our spices since mostly contamination occurs right from poor agricultural, manufacturing, and storage processes. Azzoune et al. [1] reported that of the 36 spice samples analyzed, 23 (63.9%) were contaminated with AFB1 at concentrations ranging from 0.2 to 26.50 μg/kg. High levels of AFB1 (26.50, 24.34, 19.07, and 14.65 μg/kg) were found in saffron and sweet cumin, respectively. These levels of AFB1 are lower than the maximum limits set by Ghanaian regulations (10 μg/kg). Although the residue limit of aflatoxin levels in spices is not well established in Ghana, a limit of 10 μg/kg has been proposed by Ghana Standards Authority [40]. In the European Union, aflatoxin levels in several spices are regulated to maximum residue levels that cannot be greater than 5 μg/kg for AFB1 and 10 μg/kg for aflatoxins (AFs) (Commission Regulation (EC) No. 472/2002).
In black pepper, caraway, cinnamon, coriander, cumin, ginger, red pepper, and sweet pepper, AFB1 was detected with levels ranging from 0.10 to 3.44 μg/kg, lower than the limit as recognized in Ghana [50]. The occurrence of mycotoxins in spices differs geographically and depending on the climatic conditions. The presence of AFB1 in widely varying amounts in spices has been reported by many authors [43, 51, 52]. In agreement with our findings in Ghana, Ahene et al. [9] reported that aflatoxin analysis conducted on the set of spices investigated showed that aflatoxins B1, B2, G1, and G2 were not formed at all.
Toxicogenic species are fungal species that produce potentially harmful natural toxic compounds known as mycotoxins [53, 54]. These cause health hazards and even death in humans and animals when they occur in large quantities in food. It is worthy to note that regarding the mycotoxins produced by toxicogenic species, no amount of toxin above the zero level is regarded as safe. “Reduction to as low as reasonably achievable” is the endorsement of JECFA concerning the safe level in foods following the significant genotoxic, carcinogenic, etc. probabilities of these toxins [53].
Results from the present study confirmed the presence of Aspergillus species as well as a natural occurrence of aflatoxins. Nonetheless, there is a high probability of the occurrence of diverse types of mycotoxins produced by this species of fungi in the spice and herb samples. Aspergillus (niger, parasiticus, fumigatus, terreus, and ochraceus) species isolated from these spices and herbs in this work confirmed the contamination of aflatoxins (produced chiefly by A. parasiticus, A. flavus) which is categorized as a class 1 carcinogen [55]. When ingested even at the tiniest quantities via the skin, aflatoxins have teratogenic, carcinogenic, hepatotoxic, and mutagenic outcomes on human health [56], due to their accumulative potential. Persistent intake of foods contaminated with aflatoxins leads to the unadorned and unimaginable conditions listed herein. When food contaminated with aflatoxins is ingested, the aflatoxins are then transformed into aflatoxin-8,9-epoxide metabolites in the liver, which has been implicated in numerous unsafe consequences in the body [57, 58]. Epidemiological studies of human populations exposed to diets naturally contaminated with aflatoxins revealed a connection between the high incidence of liver cancer in Africa and elsewhere and dietary intake of aflatoxins [59]. Aflatoxin assimilation surges the risk of liver cancer by exceeding tenfold compared to either exposure unaccompanied [60].
4.4. Antimicrobial Activity
The antibiotic used as a control against the sample extracts exhibited effectiveness against the bacteria. There was a relatively magnificent antimicrobial sensitivity of Streptococcus mutans to dawadawa 500 mg/ml as it cleared a zone of 23.50 ± 0.71 mm being the highest inhibition zone recorded. Ginger is well known for its excellent antioxidant and phytochemical composition. In a study by Hasan et al. [61] investigating the effects of different drying methods of ginger on its nutritional composition and antimicrobial properties, ginger produced relatively similar antimicrobial properties with Salmonella spp., S. aureus, and Staphylococcus epidermis. According to the findings, ginger contains monoterpenoids, sesquiterpenoids, phenolic compounds, and their derivatives, as well as aldehydes, ketones, alcohols, and esters, all of which have a broad antibacterial spectrum against various microbes [62]. In another study, Chand [63] reported the effectiveness of ginger against E. coli, Salmonella sp., Staphylococci, and Streptococci. Ginger has been reported to be effective on the control of many microorganisms including L. monocytogenes [64–66], Escherichia coli [65], Salmonella enteritidis [67], Aspergillus niger [68, 69], and varied fungal species [70–72].
Dawadawa illustrated a fair antimicrobial sensitivity against Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus mutans at 500 mg/ml and 250 mg/ml concentrations only, but revealed no antimicrobial potential against Salmonella typhi and Klebsiella pneumoniae for all concentrations. Olukunle et al. [73] also found that effluent of West African locust beans (P. biglobosa) without its chaff inhibited growth on S. aureus, S. typhi, E. coli, and S. pyogenes, but P. aeruginosa and K. pneumoniae were resistant to the treatment. The antimicrobial activity of P. biglobosa bark extract was investigated against some microbial isolates. The extract at a concentration of 20 mg/ml was found to inhibit the growth of all fifteen bacterial isolates comprising both Gram-positive and Gram-negative organisms. The zones of inhibition exhibited by the extract ranged between 14 ± 0.00 mm for E. coli and 28 ± 0.71 mm for P. aeruginosa. The antibacterial properties of alkaloids from P. biglobosa have been reported to have the ability to intercalate with DNA [74]. Cardiac glycosides are an important class of naturally occurring drugs whose actions help in the treatment of congestive health failure [75]. This class of phytochemical compounds was detected in P. biglobosa extract and thus supports the usefulness of this plant for the treatment of cardiac infections along with other P. biglobosa to treat ailments such as dental caries and cough among the Yoruba tribe of southwestern Nigeria because of its antimicrobial activity [75].
Although scanty data exists on the antimicrobial properties of S. macrocarpon leaves, the ethanolic extract has been reported to demonstrate increased antimicrobial sensitivity against S. aureus from 50 g/100 ml through 75 g/100 ml, 100 g/100 ml, to 150 g/100 ml; their study confirmed the antimicrobial potential of methanolic extract of Gboma leaves against S. aureus [76, 77]. Results from this study were in agreement with our findings. Additionally, leaf extracts of some species of Solanum spp. have been reported to be effective against some fungal species such as Aspergillus flavus [78] and Penicillium notatum [78, 79] and bacteria species Staphylococci and Klebsiella pneumoniae [79].
Laurus nobilis L. (bay leaf) is also reported to be effective against several microorganisms. Xu et al. [80] and Peixoto et al. [81] found it to be effective against Alternaria alternate and E.coli as well as Candida spp., respectively. Again, Fidan et al. [82] reported its effectiveness against 4 Gram-positive bacteria and 12 Gram-negative bacteria. Furthermore, Ramos et al. [83] reported its antimicrobial activity against B. thermosphacta, E. coli, L. innocua, L. monocytogenes, P. putida, S. typhimurium, and Shewanella putrefaciens. Ceyhan et al. [84] confirmed an inhibitory strength against B. cereus, S. aureus, E. coli, K. pneumoniae, and C. albicans.
The variation of the rate of resistance can be related to the difference in time and place. Another reason for the difference in resistance rates might be a rapid change in antibiotic sensitivity patterns of bacteria within a short period [85]. The prevalence of antibiotic-resistant bacteria is growing due to the random use of antibiotics in human therapy, animal farming, and other prophylactic usages [86]. Resistance enables bacteria to escape from being killed by antibiotics and reduces the ability to treat infections [87]. Therefore, antibiotic resistance has been considered one of the greatest threats to medicine [87, 88]. The growing concern about food safety has recently led to the development of natural antimicrobials to control foodborne pathogens and spoilage bacteria. Many of the spices and herbs used today have been valued for their antibacterial effects and medicinal powers in addition to their flavor and fragrance qualities [89].
4.5. pH of Samples
According to data recorded by the Scientific Sper and the Centre for Food Safety and Applied Nutrition, the pH range of ginger root is 5.60 and an upper pH value of 5.90 (cabbage, 6.00; broccoli, 6.41 when cooked and 6.58 in its raw frozen state) [90]. Most plant-based foods, except for a few, have pH values from 3 to 6, being acidic to slightly acidic. All samples tested in this study had a slightly acidic pH between 5.80 ± 0.00 and 6.76 ± 0.35. Recently, Nieva et al. [91] highlighted that vegetables with slightly acidic pH tend to have a longer shelf life as this property provides microbe- and spoilage-resistant nature.
5. Conclusion
From our study, it can be surmised that the local herbs and spices ginger, bay leaf, gboma, and dawadawa harbored a total of 12 species belonging to 7 genera; Aspergillus (niger, flavus, fumigatus, and ochraceus), Fusarium (oxysporum, verticillioides), Mucor (racemosus), Penicillium (digitatum, expansum), Rhizopus (stolonifer), Rhodotorula sp., and Trichoderma harzianum were identified as fungal contaminants. Fusarium oxysporum was the most predominant species identified. Mycobiota encountered with mycotoxigenic potential and human health importance were A. niger, A. flavus, A. fumigatus, F. verticillioides, and Penicillium verrucosum. The presence of some of these toxigenic fungi and the subsequent detection of aflatoxins in these herbs and species have the potential of causing adverse health effects, especially hepatocellular carcinoma on the consumer and supply chain populace of the Ho municipality. These herbs and spices investigated also showed sufficient effectiveness against many of the tested microorganisms. Their pH also played an important role in the multiplication of molds of spices and herbs.
Although aflatoxin levels recorded were in small quantities in dawadawa and gboma, it is noteworthy that no minute amount is considered safe in public health since they can accumulate and cause some adverse effects for consumers. Therefore, for the contamination levels to reduce, marginally intensive public awareness should be carried out by FDA on food hygiene practices for local market retailers and food vendors in the local markets for farmers to indulge in good agricultural practices, and proper storage conditions should also be established in order not to expose spices and some herbs to mold infection.
Acknowledgments
The authors are very grateful to the technical staff of the Microbiology Laboratory of the School of Allied Health Sciences, University of Health and Allied Sciences, for culturing and enumeration of fungi and to all technical staff of the Microbiology Laboratory, Department of Biomedical Sciences, School of Basic and Biomedical Sciences, University of Health and Allied Sciences, for antimicrobial studies.
Data Availability
The datasets used during the current study are available from the corresponding author on request.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
NKK, BTD, and CO designed the study. NKK, BTD, CO, EA, and TA carried out the experiment, data collection, and analysis and wrote the first version of the manuscript. NKK, EA, AOA, AAB, EKE, and TA contributed to the interpretation of the data and critically revised the manuscript. All authors read and approved the final manuscript.
References
- 1.Azzoune N., Mokrane S., Riba A., et al. Contamination of common spices by aflatoxigenic fungi and aflatoxin B1in Algeria. Quality Assurance and Safety of Crops & Foods . 2016;8(1):137–144. doi: 10.3920/QAS2014.0426. [DOI] [Google Scholar]
- 2.Si-Yuan P., Gerhard L., Si-Hua G., et al. Historical perspective of traditional indigenous medical practices: the current renaissance and conservation of herbal resources. Evidence-Based Complementary and Alternative Medicine . 2014;2014:20. doi: 10.1155/2014/525340.525340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakatani N. Phenolic antioxidants from herbs and spices. BioFactors . 2000;13(1-4):141–146. doi: 10.1002/biof.5520130123. [DOI] [PubMed] [Google Scholar]
- 4.Kabak B., Dobson A. D. Mycotoxins in spices and herbs–an update. Critical Reviews in Food Science and Nutrition . 2017;57(1):18–34. doi: 10.1080/10408398.2013.772891. [DOI] [PubMed] [Google Scholar]
- 5.Dimić G. R., Kocić-Tanackov S. D., Tepić A. N., Vujičić B. L., Šumić Z. M. Mycopopulation of spices. Acta Periodica Technologica . 2008;39:1–9. [Google Scholar]
- 6.Romagnoli B., Menna V., Gruppioni N., Bergamini C. Aflatoxins in spices, aromatic herbs, herb-teas and medicinal plants marketed in Italy. Food Control . 2007;18(6):697–701. doi: 10.1016/j.foodcont.2006.02.020. [DOI] [Google Scholar]
- 7.Adegbenu P. S., Aboagye G., Amenya P., Tuah B. Susceptibility of bacterial and fungal isolates to spices commonly used in Ghana. Scientific African . 2020;9, article e00530 doi: 10.1016/j.sciaf.2020.e00530. [DOI] [Google Scholar]
- 8.Xu D.-P., Li Y., Meng X., et al. Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. International Journal of Molecular Sciences . 2017;18(1):p. 96. doi: 10.3390/ijms18010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahene R., Odamtten G., Owusu E. Fungal and bacterial contaminants of six spices and spice products in Ghana. African Journal of Environmental Science and Technology . 2011;5(9):633–640. [Google Scholar]
- 10.Borquaye L. S., Darko G., Laryea M. K., Gasu E. N., Amponsah N. A. A., Appiah E. N. Nutritional and anti-nutrient profiles of some Ghanaian spices. Cogent Food & Agriculture . 2017;3(1, article 1348185) doi: 10.1080/23311932.2017.1348185. [DOI] [Google Scholar]
- 11.Osei-Djarbeng S. N., Abubakari A., Okai C. A., Boateng R., Pesewu G. A. Antimicrobial potential of some local spices used in Ghanaian meals. International Journal of Current Microbiology and Applied Sciences . 2016;5(5):692–700. doi: 10.20546/ijcmas.2016.505.070. [DOI] [Google Scholar]
- 12.Aiko V., Mehta A. Prevalence of toxigenic fungi in common medicinal herbs and spices in India. Biotech . 2016;6(2):1–10. doi: 10.1007/s13205-016-0476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottardi D., Bukvicki D., Prasad S., Tyagi A. K. Beneficial effects of spices in food preservation and safety. Frontiers in Microbiology . 2016;7:p. 1394. doi: 10.3389/fmicb.2016.01394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kortei N. K., Annan T., Quansah L., Aboagye G., Akonor P., Tettey C. Microbiological quality evaluation of ready-to-eat mixed vegetable salad, food ingredients and some water samples from a restaurant in Accra: a case study. African Journal of Food, Agriculture, Nutrition and Development . 2020;20(6):16669–16688. doi: 10.18697/ajfand.94.18805. [DOI] [Google Scholar]
- 15.Kortei N. K., Annan T., Akonor P. T., et al. The occurrence of aflatoxins and human health risk estimations in randomly obtained maize from some markets in Ghana. Scientific Reports . 2021;11(1):1–13. doi: 10.1038/s41598-021-83751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odamtten G., Nartey L., Wiafe-Kwagyan M., Anyebuno G., Kyei-Baffour V. Resident microbial load, toxigenic potential and possible quality control measures of six imported seasoning powders on the Ghanaian market. Journal of Nutritional Health and Food Engineering . 2018;8(1):24–35. [Google Scholar]
- 17.Moss M. Samson RA, Van Reenen-Hoekstra ES, Introduction to food-borne fungi, edit. 3, Centraalbureau voor Schimmelcultures, PO Box 273, 3740 AG Baarn, the Netherlands (1988) Elsevier; 1989. [Google Scholar]
- 18.Samson R. A., Hoekstra E. S., Frisvad J. S., Filtenborg O. Introduction to Foodborne Fungi . Netherlands: Centraal Bureau voor Schimmel cultures; 1995. Methods for the detection and isolation of food-borne fungi; pp. 235–242. [Google Scholar]
- 19.Stroka J., Anklam E. New strategies for the screening and determination of aflatoxins and the detection of aflatoxin-producing moulds in food and feed. TrAC Trends in Analytical Chemistry . 2002;21(2):90–95. doi: 10.1016/S0165-9936(01)00133-9. [DOI] [Google Scholar]
- 20.Kortei N. K., Asiedu P., Annan T., Deku J. G., Boakye A. A. Fungal diversity of “Solom” a Ghanaian traditional beverage of millet (Pennisetum glaucum) Food Science & Nutrition . 2021;9(2):811–821. doi: 10.1002/fsn3.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez C. Antibiotic assay by agar-well diffusion method. Acta Biologiae et Medicinae Experimentalis . 1990;15:113–115. [Google Scholar]
- 22.Ahmad I., Beg A. Z. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. Journal of Ethnopharmacology . 2001;74(2):113–123. doi: 10.1016/S0378-8741(00)00335-4. [DOI] [PubMed] [Google Scholar]
- 23.Hashem M., Alamri S. Contamination of common spices in Saudi Arabia markets with potential mycotoxin-producing fungi. Saudi Journal of Biological Sciences . 2010;17(2):167–175. doi: 10.1016/j.sjbs.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandeel Q. A. Fungal contamination of some imported spices. Mycopathologia . 2005;159(2):291–298. doi: 10.1007/s11046-004-5496-z. [DOI] [PubMed] [Google Scholar]
- 25.Toma F. M., Abdulla N. F. Isolation and identification of fungi from spices and medicinal plants. Research Journal of Environmental and Earth Sciences . 2013;5(3):131–138. doi: 10.19026/rjees.5.5648. [DOI] [Google Scholar]
- 26.Addo A. Premilinary Studies on the Microbiological and Nutrient Quality of Three Local Spices on the Ghanaian Market and the Control of Resident Microflora by Gamma Irradiation: BSc, [Ph.D. Thesis] Department of Botany, University of Ghana, Legon; 2005. [Google Scholar]
- 27.Painstil D. Studies on the Phenology of Air Mycoflora and Spoilage of Storage Ginger by Fungi, [Ph.D. Thesis] Department of Botany, University of Ghana, Legon; 1996. [Google Scholar]
- 28.Chourasia H. K. Mycobiota and mycotoxins in herbal drugs of Indian pharmaceutical industries. Mycological Research . 1995;99(6):697–703. doi: 10.1016/S0953-7562(09)80531-5. [DOI] [Google Scholar]
- 29.Aziz N. H., Youssef Y. A., El-Fouly M. Z., Moussa L. A. Contamination of some common medicinal plant samples and spices by fungi and their mycotoxins. Botanical Bulletin of Academia Sinica . 1998;39 [Google Scholar]
- 30.Gnonlonfin G., Adjovi Y., Tokpo A., et al. Mycobiota and identification of aflatoxin gene cluster in marketed spices in West Africa. Food Control . 2013;34(1):115–120. doi: 10.1016/j.foodcont.2013.04.021. [DOI] [Google Scholar]
- 31.Hell K., Gnonlonfin B., Kodjogbe G., Lamboni Y., Abdourhamane I. Mycoflora and occurrence of aflatoxin in dried vegetables in Benin, Mali and Togo, West Africa. International Journal of Food Microbiology . 2009;135(2):99–104. doi: 10.1016/j.ijfoodmicro.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J., Wider B., Shang H., Li X., Ernst E. Quality of herbal medicines: challenges and solutions. Complementary Therapies in Medicine . 2012;20(1-2):100–106. doi: 10.1016/j.ctim.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Hammami W., Fiori S., Al Thani R., et al. Fungal and aflatoxin contamination of marketed spices. Food Control . 2014;37:177–181. doi: 10.1016/j.foodcont.2013.09.027. [DOI] [Google Scholar]
- 34.Nurtjahja K., Zuhra C. F., Sembiring H., et al. Fungal contamination spices from Indonesia with emphasis on Aspergillus flavus. Czech Journal of Food Sciences . 2019;37(5):338–344. doi: 10.17221/18/2019-CJFS. [DOI] [Google Scholar]
- 35.Ath-Har M., Prakash H., Shetty H. Mycoflora of Indian spices with special reference to aflatoxin producing isolates of Aspergillus flavus. Indian Journal of Microbiology . 1988;28(1-2):125–127. [Google Scholar]
- 36.Bugno A., Almodovar A. A. B., Pereira T. C., Pinto T. J. A., Sabino M. Occurrence of toxigenic fungi in herbal drugs. Brazilian Journal of Microbiology . 2006;37(1):47–51. doi: 10.1590/S1517-83822006000100009. [DOI] [Google Scholar]
- 37.Takatori K., Watanabe K., Udagawa S., Kurata H. Studies on the contamination of fungi and mycotoxins in spices. JSM Mycotoxins . 1977;1977(5-6):36–38. doi: 10.2520/myco1975.1977.36. [DOI] [Google Scholar]
- 38.Dedesko S., Siegel J. A. Moisture parameters and fungal communities associated with gypsum drywall in buildings. Microbiome . 2015;3(1):1–15. doi: 10.1186/s40168-015-0137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leggieri M. C., Toscano P., Battilani P. Predicted aflatoxin B1 increase in Europe due to climate change: actions and reactions at global level. Toxins . 2021;13(4):p. 292. doi: 10.3390/toxins13040292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kortei N. K., Annan T., Kyei-Baffour V., Essuman E. K., Okyere H., Tettey C. O. Exposure and risk characterizations of ochratoxins A and aflatoxins through maize (Zea mays) consumed in different agro-ecological zones of Ghana. Scientific Reports . 2021;11(1, article 23339) doi: 10.1038/s41598-021-02822-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akhtar S., Riaz M., Naeem I., et al. Risk assessment of aflatoxins and selected heavy metals through intake of branded and non-branded spices collected from the markets of Multan city of Pakistan. Food Control . 2020;112, article 107132 doi: 10.1016/j.foodcont.2020.107132. [DOI] [Google Scholar]
- 42.Kang Y.-W., Cho T.-Y., Park H.-R., Oh K.-S., Kim D.-S. Analysis of total aflatoxins in spices and dried fruits. Journal of Food Hygiene and Safety . 2010;25(1):65–72. [Google Scholar]
- 43.Ozbey F., Kabak B. Natural co-occurrence of aflatoxins and ochratoxin A in spices. Food Control . 2012;28(2):354–361. doi: 10.1016/j.foodcont.2012.05.039. [DOI] [Google Scholar]
- 44.Martins M. L., Martins H. M., Bernardo F. Aflatoxins in spices marketed in Portugal. Food Additives & Contaminants . 2001;18(4):315–319. doi: 10.1080/02652030120041. [DOI] [PubMed] [Google Scholar]
- 45.El-Kady I., El-Maraghy S., Mostafa M. E. Natural occurrence of mycotoxins in different spices in Egypt. Folia Microbiologica . 1995;40(3):297–300. doi: 10.1007/BF02814212. [DOI] [PubMed] [Google Scholar]
- 46.Goryacheva I. Y., De Saeger S., Lobeau M., Eremin S., Barna-Vetró I., Van Peteghem C. Approach for ochratoxin A fast screening in spices using clean-up tandem immunoassay columns with confirmation by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) Analytica Chimica Acta . 2006;577(1):38–45. doi: 10.1016/j.aca.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 47.Yarru L., Settivari R., Gowda N., Antoniou E., Ledoux D., Rottinghaus G. Effects of turmeric (Curcuma longa) on the expression of hepatic genes associated with biotransformation, antioxidant, and immune systems in broiler chicks fed aflatoxin. Poultry Science . 2009;88(12):2620–2627. doi: 10.3382/ps.2009-00204. [DOI] [PubMed] [Google Scholar]
- 48.Sharma V., Sharma C., Pracheta R. P., Sharma S. Protective potential of Curcuma longa and curcumin on aflatoxin B1 induced hepatotoxicity in Swiss albino mice. Asian Journal of Pharmaceutical and Health Sciences . 2011;1:116–122. [Google Scholar]
- 49.Garcia M. V., Parussolo G., Moro C. B., Bernardi A. O., Copetti M. V. Fungi in spices and mycotoxigenic potential of some _Aspergilli_ isolated. Food Microbiology . 2018;73:93–98. doi: 10.1016/j.fm.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 50.Food, & Nations. Assuring Food Safety and Quality: Guidelines for Strengthening National Food Control Systems . FAO; 2003. [Google Scholar]
- 51.El Mahgubi A., Puel O., Bailly S., et al. Distribution and toxigenicity of Aspergillus section Flavi in spices marketed in Morocco. Food Control . 2013;32(1):143–148. doi: 10.1016/j.foodcont.2012.11.013. [DOI] [Google Scholar]
- 52.Prelle A., Spadaro D., Garibaldi A., Gullino M. L. Co-occurrence of aflatoxins and ochratoxin A in spices commercialized in Italy. Food Control . 2014;39:192–197. doi: 10.1016/j.foodcont.2013.11.013. [DOI] [Google Scholar]
- 53.Hathout A. S., Abel-Fattah S. M., Abou-Sree Y. H., Fouzy A. S. Incidence and exposure assessment of aflatoxins and ochratoxin A in Egyptian wheat. Toxicology Reports . 2020;7:867–873. doi: 10.1016/j.toxrep.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reddy K., Nurdijati S., Salleh B. An overview of plant-derived products on control of mycotoxigenic fungi and mycotoxins. Asian Journal of Plant Sciences . 2010;9(3):126–133. doi: 10.3923/ajps.2010.126.133. [DOI] [Google Scholar]
- 55.World Health Organization. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins (Vol. 56) World Health Organization; 1993. [Google Scholar]
- 56.Pleadin J., Frece J., Markov K. Mycotoxins in food and feed. Advances in Food and Nutrition Research . 2019;89:297–345. doi: 10.1016/bs.afnr.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 57.Bbosa G. S., Kitya D., Lubega A., Ogwal-Okeng J., Anokbonggo W. W., Kyegombe D. B. Review of the biological and health effects of aflatoxins on body organs and body systems. Aflatoxins-Recent Advances and Future Prospects . 2013;12:239–265. [Google Scholar]
- 58.Kew M. C. Aflatoxins as a cause of hepatocellular carcinoma. Journal of Gastrointestinal & Liver Diseases . 2013;22(3):305–310. [PubMed] [Google Scholar]
- 59.Wild C. P., Montesano R. A model of interaction: aflatoxins and hepatitis viruses in liver cancer aetiology and prevention. Cancer Letters . 2009;286(1):22–28. doi: 10.1016/j.canlet.2009.02.053. [DOI] [PubMed] [Google Scholar]
- 60.Turner P. C., Moore S. E., Hall A. J., Prentice A. M., Wild C. P. Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environmental Health Perspectives . 2003;111(2):217–220. doi: 10.1289/ehp.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hasan H. A., Raauf A. M. R., Razik B., Hassan B. A. R. Chemical composition and antimicrobial activity of the crude extracts isolated from Zingiber officinale by different solvents. Pharmaceut Anal Acta . 2012;3(9):1–5. [Google Scholar]
- 62.Beristain-Bauza S. D. C., Hernández-Carranza P., Cid-Pérez T. S., Ávila-Sosa R., Ruiz-López I. I., Ochoa-Velasco C. E. Antimicrobial activity of ginger (Zingiber officinale) and its application in food products. Food Reviews International . 2019;35(5):407–426. doi: 10.1080/87559129.2019.1573829. [DOI] [Google Scholar]
- 63.Chand B. Antibacterial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against Staphylococcus aureus, Salmonella typhi, Escherichia coli and Bacillus cereus. Journal of Microbiology, Biotechnology and Food Sciences . 2021;2021:2481–2491. [Google Scholar]
- 64.Bagheri Darvish H., Bahrami A., Jafari S. M., Williams L. Micro/nanoencapsulation strategy to improve the efficiency of natural antimicrobials againstListeria monocytogenesin food products. Critical Reviews in Food Science and Nutrition . 2021;61(8):1241–1259. doi: 10.1080/10408398.2020.1755950. [DOI] [PubMed] [Google Scholar]
- 65.Indu M., Hatha A., Abirosh C., Harsha U., Vivekanandan G. Antimicrobial activity of some of the south-Indian spices against serotypes of Escherichia coli, Salmonella, Listeria monocytogenes and Aeromonas hydrophila. Brazilian Journal of Microbiology . 2006;37(2):153–158. doi: 10.1590/S1517-83822006000200011. [DOI] [Google Scholar]
- 66.Thongson C., Davidson P. M., Mahakarnchanakul W., Vibulsresth P. Antimicrobial effect of Thai spices against Listeria monocytogenes and Salmonella typhimurium DT104. Journal of Food Protection . 2005;68(10):2054–2058. doi: 10.4315/0362-028X-68.10.2054. [DOI] [PubMed] [Google Scholar]
- 67.Sa-Nguanpuag K., Kanlayanarat S., Srilaong V., Tanprasert K., Techavuthiporn C. Ginger (Zingiber officinale) oil as an antimicrobial agent for minimally processed produce: a case study in shredded green papaya. International Journal of Agriculture & Biology . 2011;13(6) [Google Scholar]
- 68.Mvuemba H., Green S., Tsopmo A., Avis T. Antimicrobial efficacy of cinnamon, ginger, horseradish and nutmeg extracts against spoilage pathogens. Phytoprotection . 2009;90(2):65–70. doi: 10.7202/044024ar. [DOI] [Google Scholar]
- 69.Singh G., Maurya S., Catalan C., De Lampasona M. Studies on essential oils, part 42: chemical, antifungal, antioxidant and sprout suppressant studies on ginger essential oil and its oleoresin. Flavour and Fragrance Journal . 2005;20(1):1–6. doi: 10.1002/ffj.1373. [DOI] [Google Scholar]
- 70.Abdullahi A., Khairulmazmi A., Yasmeen S., et al. Phytochemical profiling and antimicrobial activity of ginger (Zingiber officinale) essential oils against important phytopathogens. Arabian Journal of Chemistry . 2020;13(11):8012–8025. doi: 10.1016/j.arabjc.2020.09.031. [DOI] [Google Scholar]
- 71.Ali B. H., Blunden G., Tanira M. O., Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food and Chemical Toxicology . 2008;46(2):409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 72.Riaz H., Begum A., Raza S. A., Khan Z. M.-U.-D., Yousaf H., Tariq A. Antimicrobial property and phytochemical study of ginger found in local area of Punjab, Pakistan. International Current Pharmaceutical Journal . 2015;4(7):405–409. doi: 10.3329/icpj.v4i7.23591. [DOI] [Google Scholar]
- 73.Olukunle O. F., Umar O. K., Oriola O. B. Antimicrobial properties of cooked African locust beans (Parkia biglobosa) effluent with and without its chaff. Microbiology Research Journal International . 2019;27(4):1–9. [Google Scholar]
- 74.Phillipson J., ONeill M. New leads to the treatment of protozoal infections based on natural product molecules. Acta Pharmaceutica Nordica . 1989;1(3):131–144. [Google Scholar]
- 75.Ikeda Y., Fujii Y., Nakaya I., Yamazaki M. Quantitative HPLC analysis of cardiac glycosides in Digitalis purpurea leaves. Journal of Natural Products . 1995;58(6):897–901. doi: 10.1021/np50120a012. [DOI] [PubMed] [Google Scholar]
- 76.Ilodibia C., Akachukwu E., Chukwuma M., Igboabuchi N., Adimonyemma R., Okeke N. Proximate, phytochemical and antimicrobial studies on Solanum macrocarpon L. Journal of Advances in Biology & Biotechnology . 2016;9(2):1–7. doi: 10.9734/JABB/2016/27922. [DOI] [Google Scholar]
- 77.Senizza B., Rocchetti G., Sinan K. I., et al. The phenolic and alkaloid profiles of Solanum erianthum and Solanum torvum modulated their biological properties. Food Bioscience . 2021;41, article 100974 doi: 10.1016/j.fbio.2021.100974. [DOI] [Google Scholar]
- 78.Koduru S., Grierson D., Afolayan A. Antimicrobial activity ofSolanum aculeastrum. Pharmaceutical Biology . 2006;44(4):283–286. doi: 10.1080/13880200600714145. [DOI] [Google Scholar]
- 79.Aliero A., Afolayan A. Antimicrobial activity of Solanum tomentosum. African Journal of Biotechnology . 2006;5(4):369–372. [Google Scholar]
- 80.Xu S., Yan F., Ni Z., Chen Q., Zhang H., Zheng X. In vitro and in vivo control of Alternaria alternata in cherry tomato by essential oil from Laurus nobilis of Chinese origin. Journal of the Science of Food and Agriculture . 2014;94(7):1403–1408. doi: 10.1002/jsfa.6428. [DOI] [PubMed] [Google Scholar]
- 81.Peixoto L. R., Rosalen P. L., Ferreira G. L. S., et al. Antifungal activity, mode of action and anti-biofilm effects of Laurus nobilis Linnaeus essential oil against Candidaspp. Archives of Oral Biology . 2017;73:179–185. doi: 10.1016/j.archoralbio.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 82.Fidan H., Stefanova G., Kostova I., et al. Chemical composition and antimicrobial activity of Laurus nobilis L. essential oils from Bulgaria. Molecules . 2019;24(4):p. 804. doi: 10.3390/molecules24040804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramos C., Teixeira B., Batista I., et al. Antioxidant and antibacterial activity of essential oil and extracts of bay laurel Laurus nobilis Linnaeus (Lauraceae) from Portugal. Natural Product Research . 2012;26(6):518–529. doi: 10.1080/14786419.2010.531478. [DOI] [PubMed] [Google Scholar]
- 84.Ceyhan N., Keskin D., Ugur A. Antimicrobial activities of different extracts of eight plant species from four different family against some pathogenic microoorganisms. Journal of Food, Agriculture and Environment . 2012;10:193–197. [Google Scholar]
- 85.Bantawa K., Rai K., Limbu D. S., Khanal H. Food-borne bacterial pathogens in marketed raw meat of Dharan, eastern Nepal. BMC Research Notes . 2018;11(1):1–5. doi: 10.1186/s13104-018-3722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Addis Z., Kebede N., Sisay Z., Alemayehu H., Wubetie A., Kassa T. Prevalence and antimicrobial resistance of salmonella isolated from lactating cows and in contact humans in dairy farms of Addis Ababa: a cross sectional study. BMC Infectious Diseases . 2011;11(1):1–7. doi: 10.1186/1471-2334-11-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spellberg B., Guidos R., Gilbert D., et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clinical Infectious Diseases . 2008;46(2):155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 88.Broughton E. I., Walker D. G. Prevalence of antibiotic-resistantSalmonellain fish in Guangdong, China. Foodborne Pathogens and Disease . 2009;6(4):519–521. doi: 10.1089/fpd.2008.0196. [DOI] [PubMed] [Google Scholar]
- 89.El-Sayed S. M., Youssef A. M. Potential application of herbs and spices and their effects in functional dairy products. Heliyon . 2019;5(6, article e01989) doi: 10.1016/j.heliyon.2019.e01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sper Scientific. The Importance of pH in Food Quality and Production . Sperdirect; 2015. [Google Scholar]
- 91.Nieva S. G., Jagus R. J., Aguero M. V. Fruit and vegetable smoothies preservation with natural antimicrobials for the assurance of safety and quality. LWT . 2022;154, article 112663 doi: 10.1016/j.lwt.2021.112663. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on request.


