Abstract
Aim
To report the final results of the 5-year follow-up of the non-randomized SafeHER Phase III study (NCT01566721) describing the safety, tolerability, and efficacy of subcutaneous (SC) trastuzumab alone and in combination with concurrent or sequential chemotherapy.
Methods
Patients with human epidermal growth factor receptor 2 (HER2)-positive early breast cancer (EBC) with no prior anti-HER2 therapy were included. SC trastuzumab was administered every 3 weeks for 18 cycles as adjuvant therapy with or without chemotherapy (concurrent or sequential). The primary objective was overall safety and tolerability of SC trastuzumab; efficacy was a secondary objective.
Results
No new safety signals were observed during the final evaluation. The majority of adverse events (AEs) were grade 1 or 2 across the chemotherapy subgroups. Treatment discontinuation due to AEs was 5.1% for the intent-to-treat (ITT) population and similar for all chemotherapy subgroups. The overall disease-free survival (DFS) 5-year event-free rate in the ITT population (n = 2573) was 86.6% (95% CI, 85.2%–87.9%) with a median follow-up of 72 months. Based on chemotherapy timing, the no (n = 235), concurrent (n = 1533), and sequential (n = 805) chemotherapy subgroups had DFS 5-year event-free rates (95% CI) of 88.5% (83.4%–92.2%), 88.4% (86.6%–89.9%), and 82.6 (79.7%–85.2%), respectively.
Conclusions
The 5-year follow-up analysis of the SafeHER trial demonstrating that SC trastuzumab has an acceptable safety profile, including cardiac toxicity, and efficacy for the treatment of HER2-positive EBC with and without chemotherapy, corresponding with historical data with trastuzumab.
Keywords: Breast neoplasms, Disease-free survival, Follow-up studies, Receptor ErbB2, Safety, Trastuzumab
Highlights
-
•
Final analysis of SafeHER showed the long-term safety of subcutaneous trastuzumab.
-
•
Cardiac safety was maintained, with no late signs of cardiac toxicity discovered.
-
•
The 5-year disease-free survival rate was 87% for patients with early breast cancer.
-
•
The no chemotherapy subgroup also had a similar 5-year disease-free survival rate.
-
•
Subcutaneous trastuzumab alone may reduce relapse for select older/low-risk patients.
1. Introduction
Trastuzumab (Herceptin® [H]) is a humanized monoclonal antibody directed against the extracellular domain of the human epidermal growth factor receptor 2 (HER2) indicated for treatment of patients with HER2-positive metastatic breast cancer (MBC; first approved 1998), HER2-positive early breast cancer (EBC; approved 2005), and HER2-positive metastatic gastric cancer (approved 2010) [[1], [2], [3], [4]]. Patients with HER2-positive EBC frequently require extensive treatment including surgery, neoadjuvant/adjuvant chemotherapy, hormonal therapy, radiotherapy, and HER2-targeted therapy [4].
A subcutaneous (SC) formulation (H SC) comprising 600 mg of trastuzumab plus recombinant human hyaluronidase (rHuPH20) [5] is currently approved in >100 countries for HER2-positive breast cancer and is given every 3 weeks (q3w) for 18 cycles to treat HER2-positive EBC [6]. The H SC dose was based on a pharmacokinetic bridging approach that aimed to achieve noninferior trastuzumab serum trough concentration compared with trastuzumab administered intravenously (H IV) [7]. Population modeling and simulation showed that a fixed trastuzumab SC 600-mg dose q3w would be comparable with H IV q3w administration. The HannaH study, a Phase III, randomized, open-label study for women with HER2-positive EBC, validated that the pharmacokinetic profile and efficacy of H SC was noninferior to those of H IV and had a similar safety profile to H IV [8,9].
The SafeHER trial (Safety and Tolerability Study of Assisted and Self-Administered Subcutaneous [SC] Herceptin [Trastuzumab] as Adjuvant Therapy in Early Human Epidermal Growth Factor Receptor 2 [HER2]-Positive Breast Cancer; NCT01566721) was a Phase III, prospective, 2-cohort, nonrandomized, multicenter, multinational, open-label study, and the largest study (>2500 treated patients) to investigate H SC to date [10]. H SC was administered q3w for 18 cycles as adjuvant therapy with (concurrent or sequential) or without chemotherapy for HER2-positive EBC [10]. Results from the SafeHER primary analysis provided no new safety signals, and the H SC adjuvant profile was consistent with the known trastuzumab adjuvant profile for patients who had completed the safety follow-up [10].
The SafeHER trial included a subgroup of patients with HER2-positive EBC who received H SC without chemotherapy. Emerging evidence has suggested that some patients do not receive sufficient benefit from additional chemotherapy necessary to offset toxicities. Therefore, the development of approaches to distinguish patients who are likely to benefit from less or no chemotherapy is necessary [11]. Here the final results of the 5-year follow-up of the SafeHER Phase III study describing the safety, tolerability, and efficacy of H SC alone and in combination with concurrent or sequential chemotherapy are reported.
2. Methods
2.1. Patients
The inclusion criteria and study design were previously reported by Gligorov et al. [10]; however, key points are summarized. Key eligibility criteria included HER2-positive EBC (clinical stage I−IIIC), Eastern Cooperative Oncology Group performance status of 0 or 1, intact thigh skin, baseline left ventricular ejection fraction (LVEF) ≥55%, and no prior anti-HER2 therapy. Key exclusion criteria are included in the supplemental methods.
2.2. Study design
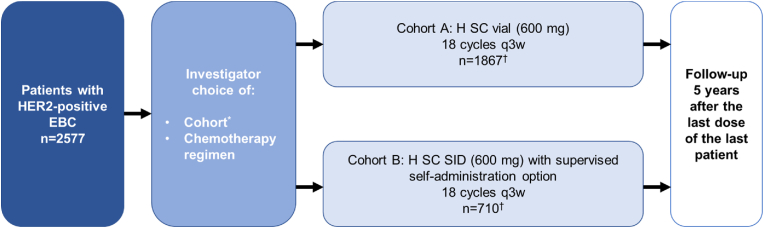
Eligible patients were allocated to Cohort A or B at the investigator's discretion, depending on availability of the cohort's recruitment (Fig. 1). Patients in Cohort A received H SC 600 mg via assisted administration using conventional handheld syringes with hypodermic needles. Patients in Cohort B received H SC 600 mg presented in a single-use injection device (SID). Patients were followed up for cancer recurrence and survival until the study end, with a duration of follow-up of ≥5 years after last study treatment. Supplemental methods include additional study design information.
Fig. 1.
Study design of SafeHER (ITT population). EBC, early breast cancer; H, trastuzumab; ITT, intent-to-treat; q3w, every 3 weeks; SC, subcutaneous; SID, single-use injection device. * Depending on availability of cohorts. † H SC was administered in the neoadjuvant phase for 44 patients (Cohort A, n = 20; Cohort B, n = 24).
2.3. Study objectives
The primary objective was to assess the overall safety and tolerability of H SC in patients with HER2-positive EBC. Secondary objectives included efficacy (disease-free survival [DFS] and overall survival [OS]) for both cohorts and patient satisfaction with H SC administration using the SID (patients in Cohort B who opted for self-administration of the study drug).
2.4. Assessments
Adverse events (AEs) and serious AEs (SAEs) were recorded/graded according to National Cancer Institute Common Terminology criteria for Adverse Events 4.0 (NCI-CTCAE 4.0) and congestive heart failure, according to the NCI-CTCAE 4.0 and the New York Heart Association functional classification (supplemental methods). The final analyses that included updated safety, DFS, and OS were performed when the last patient was followed for ≥5 years (last patient last visit: February 19, 2020). DFS and OS were analyzed as time-to-event variables. Analyses by timing of chemotherapy were also explored for safety and efficacy.
3. Results
3.1. Patient demographics and disposition
All patients were included in the intent-to-treat (ITT) population (n = 2573) and received H SC with no chemotherapy (n = 235) or H SC with chemotherapy concurrently (n = 1533) or sequentially (n = 805) at the enrolling investigators’ discretion (Table 1). Patients who received ≥1 dose of H SC were included in the safety population (n = 2569) and received no (n = 233), concurrent (n = 1533), or sequential (n = 803) chemotherapy.
Table 1.
Patient disposition from treatment during follow-up of SafeHER (ITT population)a.
| Patient follow-up | Overall N = 2573 | No chemotherapy n = 235 | Concurrent chemotherapy n = 1533 | Sequential chemotherapy n = 805 |
|---|---|---|---|---|
| Completed treatment according to protocol, n (%) | ||||
| Yes | 2319 (90.1) | 207 (88.1) | 1394 (90.9) | 718 (89.2) |
| No | 254 (9.9) | 28 (11.9) | 139 (9.1) | 87 (10.8) |
| Reason patient discontinued treatment, n (%) | ||||
| AEs | 130 (5.1) | 13 (5.1) | 73 (4.8) | 44 (5.5) |
| Withdrew consent | 36 (1.4) | 8 (3.4) | 15 (1.0) | 13 (1.6) |
| Disease progression/disease recurrence | 50 (1.9) | 3 (1.3) | 27 (1.8) | 20 (2.5) |
| Lack of compliance | 5 (0.2) | 0 | 2 (0.1) | 3 (0.4) |
| Otherb | 33 (1.3) | 4 (1.7) | 22 (1.4) | 7 (0.9) |
AE, adverse event; ITT, intent-to-treat.
Percentages are based on the overall number of patients in each group.
Other includes pregnancy, investigator/sponsor decision, protocol violations, failure of inclusion criteria, and immobility.
Of the patients in the ITT population, 90.1% of patients completed treatment according to protocol (Table 1). For patients receiving no, concurrent, or sequential chemotherapy, 88.1%, 90.9%, and 89.2% completed treatment according to protocol, respectively (Table 1). AEs (5.1%) were the most common reason for treatment discontinuation in the ITT population.
Patients in the ITT population were more likely to be < 70 years old (89.9%) and White (76.8%; Table 2). The majority of patients had invasive ductal carcinoma (92.3%), and tumors that were negative for the estrogen receptor (61.2%) and positive for the progesterone receptor (50.1%). The no chemotherapy subgroup included a higher proportion of patients ≥70 years of age (34.5%) compared with the concurrent and sequential chemotherapy subgroups (7.6% and 7.8%, respectively). Overall, 11.7% of patients included in the study were considered low risk, defined as patients with a tumor size ≤1 cm and no nodal involvement at baseline. An increased percentage of patients who did not receive chemotherapy (36.6%) were considered low risk compared with patients who received concurrent or sequential chemotherapy (8.5% and 10.6%, respectively). Patients who did not receive chemotherapy had tumors that were considered lower grade based on tumor size and nodal involvement (Table 2). Patients in the no chemotherapy subgroup had lower rates of estrogen receptor– and progesterone receptor–positive tumors (24.3% and 38.3%) compared with the chemotherapy subgroups (concurrent: 37.8% and 51.0%; sequential: 39.8% and 51.7%). The no chemotherapy subgroup had the greatest percentage of patients with an active medical history (76.6%), defined as patients with ongoing medical conditions at the start of the study, compared with patients who received chemotherapy (concurrent: 67.5%; sequential: 60.1%).
Table 2.
Patient demographics and tumor characteristics of SafeHER (ITT population).
| Characteristics, n (%) | Overall N = 2573 | No chemotherapy n = 235 | Concurrent chemotherapy n = 1533 | Sequential chemotherapy n = 805 |
|---|---|---|---|---|
| Age, years | ||||
| <70 years | 2312 (89.9) | 154 (65.5) | 1416 (92.4) | 742 (92.2) |
| ≥70 years | 261 (10.1) | 81 (34.5) | 117 (7.6) | 63 (7.8) |
| Race, n (%) | ||||
| White | 1977 (76.8) | 199 (84.7) | 1179 (76.9) | 599 (74.4) |
| Black | 31 (1.2) | 0 | 17 (1.1) | 14 (1.7) |
| Asian | 378 (14.7) | 25 (10.6) | 183 (11.9) | 170 (21.1) |
| Other | 89 (3.5) | 5 (2.1) | 69 (4.5) | 15 (1.9) |
| N/A | 89 (3.5) | 6 (2.6) | 76 (5.0) | 7 (0.9) |
| Unknown | 9 (0.3) | 0 | 9 (0.6) | 0 |
| Breast cancer type, n (%) | ||||
| Ductal | 2376 (92.3) | 212 (90.2) | 1424 (92.9) | 740 (91.9) |
| Lobular | 94 (3.7) | 12 (5.1) | 50 (3.3) | 32 (4.0) |
| Inflammatory | 5 (0.2) | 0 | 4 (0.3) | 1 (0.1) |
| Other | 96 (3.7) | 10 (4.3) | 54 (3.5) | 32 (4.0) |
| Unknown | 2 (0.1) | 1 (0.4) | 1 (0.1) | 0 |
| ER status, n (%) | ||||
| Negative | 1574 (61.2) | 171 (72.8) | 940 (61.3) | 463 (57.5) |
| Positive | 956 (37.2) | 57 (24.3) | 579 (37.8) | 320 (39.8) |
| Missing | 43 (1.6) | 7 (3.0) | 14 (0.9) | 22 (2.7) |
| PR status, n (%) | ||||
| Negative | 1153 (44.8) | 123 (52.3) | 697 (45.5) | 333 (41.4) |
| Positive | 1288 (50.1) | 90 (38.3) | 782 (51.0) | 416 (51.7) |
| Missing | 132 (5.1) | 22 (9.4) | 54 (3.5) | 56 (7.0) |
| TNM classification at diagnosis (primary tumor), n (%) | ||||
| T0 | 0 | 0 | 0 | 0 |
| T1 | 1254 (48.7) | 166 (70.6) | 724 (47.2) | 364 (45.2) |
| T2 | 1037 (40.3) | 60 (25.5) | 638 (41.6) | 339 (42.1) |
| T3 | 191 (7.4) | 5 (2.1) | 118 (7.7) | 68 (8.4) |
| T4 | 76 (3.0) | 3 (1.3) | 45 (2.9) | 28 (3.5) |
| TX | 7 (0.3) | 0 | 4 (0.3) | 3 (0.4) |
| Unknown | 8 (0.3) | 1 (0.4) | 4 (0.3) | 3 (0.4) |
| Lymph node status, n (%) | ||||
| N0 | 1416 (55.0) | 188 (80.0) | 759 (49.5) | 469 (58.3) |
| N1 | 742 (28.8) | 35 (14.9) | 500 (32.6) | 207 (25.7) |
| N2 | 271 (10.5) | 6 (2.6) | 181 (11.8) | 84 (10.4) |
| N3 | 124 (4.8) | 2 (0.9) | 82 (5.3) | 40 (5.0) |
| NX | 12 (0.5) | 3 (1.3) | 7 (0.5) | 2 (0.2) |
| Unknown | 8 (0.3) | 1 (0.4) | 4 (0.3) | 3 (0.4) |
| Distant metastasis, n (%) | ||||
| M0 | 2565 (99.7) | 234 (99.6) | 1529 (99.7) | 802 (99.6) |
| M1 | 0 | 0 | 0 | 0 |
| MX | 0 | 0 | 0 | 0 |
| Unknown | 8 (0.3) | 1 (0.4) | 4 (0.3) | 3 (0.4) |
| Low-risk populationa | ||||
| Yes | 301 (11.7) | 86 (36.6) | 130 (8.5) | 85 (10.6) |
| No | 2272 (88.3) | 149 (63.4) | 1403 (91.5) | 720 (89.4) |
| Active medical history, n (%)b | 1699 (66.0) | 180 (76.6) | 1035 (67.5) | 484 (60.1) |
ER, estrogen receptor; ITT, intent-to-treat; M, distant metastasis; N, regional lymph nodes; N/A, not available; PR, progesterone receptor; T, primary tumor.
Defined as patients with a tumor size ≤1 cm and no nodal involvement at baseline.
Defined as patients with ongoing medical conditions at the start of the study.
3.2. Safety
Drug exposure was comparable across all chemotherapy subgroups in the safety population, with a planned 18-cycle treatment regimen. The overall median (range) number of cycles was 18 (1–18) for the no chemotherapy subgroups and 18 (1–19) for both chemotherapy subgroups. Multiple events of the same CTC grade were counted only once in that CTC grade. However, patients could be counted more than once overall. The majority of AEs were Grade 1 or 2 for the overall safety population and across the chemotherapy subgroups (Table 3), and no new safety signals were observed for H SC. Grade 1 or 2 AEs occurred in 85.7% and 66.9% of patients in the safety population. When assessed by timing of chemotherapy, Grade 1 or 2 AEs occurred in 85.8% and 64.4% of patients in the safety population who did not receive chemotherapy, 91.7% and 74.8% of patients who received concurrent chemotherapy, and 74.3% and 52.7% of patients who received sequential chemotherapy. Despite an increased proportion of Grade ≤3 AEs for patients who received no vs sequential chemotherapy, AEs did not result in increased discontinuation (Table 1). Grade ≥3 AEs occurred in 33.0% (none), 38.5% (concurrent), and 21.0% (sequential) of patients, respectively (Table 3). Overall, incidences of Grade 4 or 5 AEs were low (4.8%).
Table 3.
Summary of AEs (safety population).
| AEs, n (%) | Overall N = 2569 | No chemotherapy n = 233 | Concurrent chemotherapy n = 1533 | Sequential chemotherapy n = 803 |
|---|---|---|---|---|
| AEs (any grade)a | 2334 (90.9) | 214 (91.8) | 1458 (95.1) | 662 (82.4) |
| Grade 1 | 2202 (85.7) | 200 (85.8) | 1405 (91.7) | 597 (74.3) |
| Grade 2 | 1719 (66.9) | 150 (64.4) | 1146 (74.8) | 423 (52.7) |
| Grade 3 | 713 (27.8) | 66 (28.3) | 506 (33.0) | 141 (17.6) |
| Grade 4 | 85 (3.3) | 4 (1.7) | 66 (4.3) | 15 (1.9) |
| Grade 5 | 38 (1.5) | 7 (3.0) | 19 (1.2) | 12 (1.5) |
| SAEs | 542 (21.1) | 65 (27.9) | 354 (23.1) | 123 (15.3) |
| Cardiac AEs | 529 (20.6) | 41 (17.6) | 337 (22.0) | 151 (18.8) |
| Significant cardiac AEsb | 136 (5.3) | 10 (4.3) | 79 (5.2) | 47 (5.9) |
AE, adverse event; CTC, common terminology criteria; SAE, serious adverse event.
If a patient had multiple events of the same CTC grade, relationship or outcome, then they were counted only once in that CTC grade relationship or outcome. However, patients could be counted more than once overall.
Significant cardiac AEs were defined as all cardiac events that were serious, Grade ≥3, New York Heart Association Class ≥ III, or leading to discontinuation of study medication.
SAEs occurred in 542 (20.6%) patients in the safety population (Table 3). When evaluated by timing of chemotherapy, 65 (27.9%) patients receiving no chemotherapy, 354 (23.1%) patients receiving concurrent chemotherapy, and 123 (15.3%) patients receiving sequential chemotherapy experienced SAEs. Cardiac AEs were reported by 20.6% of patients overall, with 5.3% of patients experiencing significant cardiac AEs. Significant cardiac AEs occurred in 4.3% (none), 5.2% (concurrent), and 5.9% (sequential) of patients in the safety population. The event-free rate at 5 years for LVEF in the safety population based on rates of clinical indication was 95.0% for patients who received no chemotherapy, and 92.7% and 92.6% for patients who received concurrent or sequential chemotherapy, respectively. Deaths due to AEs occurred in 1.5% of patients in the safety population, where 3.0% of patients received no chemotherapy, 1.2% of patients received concurrent chemotherapy, and 1.5% of patients received sequential chemotherapy. These deaths occurred during the follow-up period and were unrelated to the study drug.
3.3. Efficacy
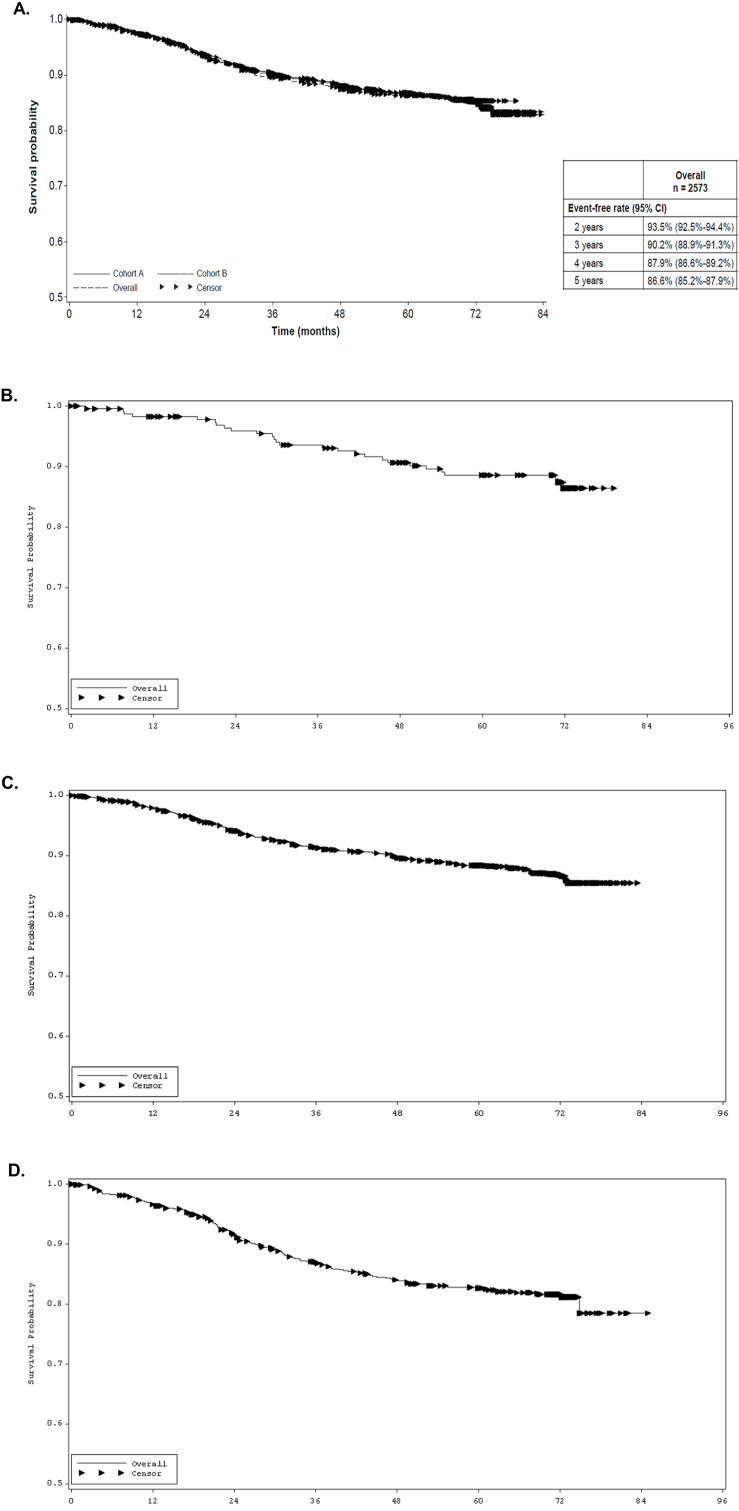
The overall median duration of follow-up was 71.7 months (95% CI, 71.7–71.8 months). For DFS, the overall 5-year event-free rate in the ITT population was 86.6% (95% CI, 85.2%–87.9%; Fig. 2A; Table 4). The most commonly reported events were distant (8.9%) and local (2.7%) recurrences. When DFS was assessed based on timing of chemotherapy in the ITT population, the 5-year event-free rates (95% CI) were 88.5% (83.4%–92.2%) for the no chemotherapy subgroup, 88.4% (86.6%–89.9%) for the concurrent chemotherapy subgroup, and 82.6% (79.7%–85.2%) for the sequential chemotherapy subgroup (Fig. 2; Table 4). Assessment of DFS in patients ≥70 years of age resulted in numerically lower DFS (95% CI) for patients in all 3 chemotherapy subgroups (none: 82.0% [71.0%–89.2%], concurrent: 86.1% [77.7%–91.6%] and sequential: 72.6% [59.1%–82.3%]; Table 4).
Fig. 2.
Final analysis of DFS of SafeHER for the (A) entire ITT population, (B) no chemotherapy subgroup, (C) concurrent chemotherapy subgroup, and (D) sequential chemotherapy subgroup. DFS, disease-free survival; ITT, intent-to-treat.
Table 4.
Disease-free survival of SafeHER (ITT population) and by timing of chemotherapy.
| Disease-free survival | Patients with events, n (%) | Event-free rate at 5 years (95% CI) |
|---|---|---|
| ITT population, N = 2573 | 2213 (86.0) | 86.6% (85.2%–87.9%) |
| Overall | ||
| No chemotherapy, n = 235 | 27 (11.5) | 88.5% (83.4%–92.2%) |
| Concurrent chemotherapy, n = 1533 | 193 (12.6) | 88.4% (86.6%–89.9%) |
| Sequential chemotherapy, n = 805 | 140 (17.4) | 82.6% (79.7%–85.2%) |
| Including SPM | ||
| No chemotherapy, n = 235 | 43 (18.3) | 82.1% (76.2%–86.6%) |
| Concurrent chemotherapy, n = 1533 | 246 (16.0) | 85.3% (83.4%–87.0%) |
| Sequential chemotherapy, n = 805 | 166 (20.6) | 79.6% (76.5%–82.3%) |
| Low-risk populationa, n = 301 | ||
| No chemotherapy, n = 86 | 6 (7.0) | 93.7% (85.4%–97.3%) |
| Concurrent chemotherapy, n = 130 | 2 (1.5) | 98.3% (93.6%–99.6%) |
| Sequential chemotherapy, n = 85 | 10 (11.8) | 90.1% (81.2%–94.9%) |
| ≥70 years of age, n = 261 | ||
| No chemotherapy, n = 81 | 15 (18.5) | 82.0% (71.0%–89.2%) |
| Concurrent chemotherapy, n = 117 | 18 (15.4) | 86.1% (77.7%–91.6%) |
| Sequential chemotherapy, n = 63 | 17 (27.0) | 72.6% (59.1%–82.3%) |
ITT, intent-to-treat; SPM, secondary primary malignancy.
Defined as patients with a tumor size ≤1 cm and no nodal involvement at baseline.
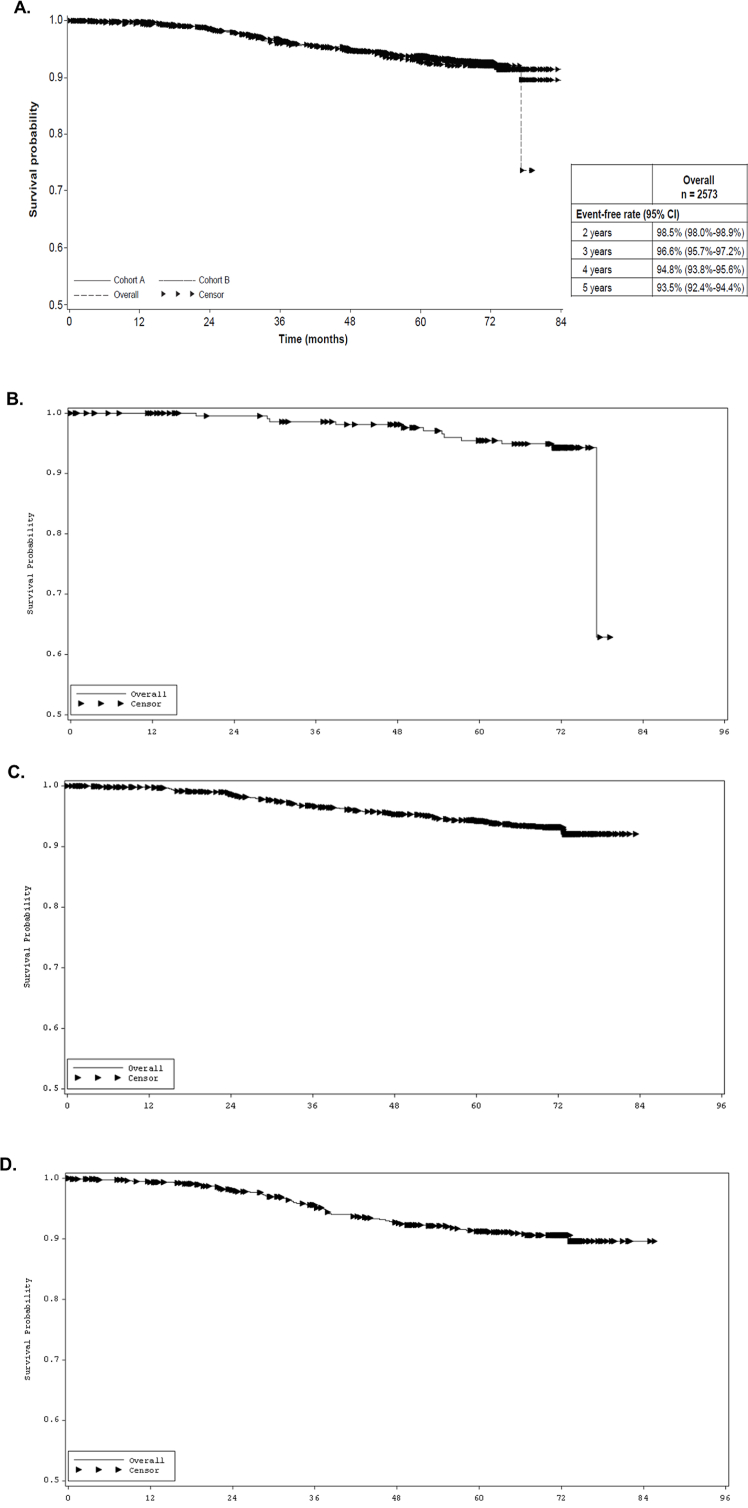
The 5-year OS rate was 93.5% (95% CI, 92.4%–94.4%) in the ITT population (Fig. 3A; Table 5). Twelve (5.1%), 98 (6.4%), and 68 (8.4%) deaths occurred in the survival analysis in the no, concurrent, and subsequent chemotherapy subgroups, respectively (Fig. 3; Table 5).
Fig. 3.
Final analysis of OS of SafeHER for the (A) entire ITT population, (B) no chemotherapy subgroup, (C) concurrent chemotherapy subgroup, and (D) sequential chemotherapy subgroup. ITT, intent-to-treat; OS, overall survival.
Table 5.
Overall survival of SafeHER (ITT population) and by timing of chemotherapy.
| Overall survival | Patients with events, n (%) | Event-free rate at 5 years (95% CI) |
|---|---|---|
| Overall population, N = 2573 | 2395 (93.1) | 93.5% (92.4%– 94.4%) |
| No chemotherapy, n = 235 | 12 (5.1) | 95.5% (91.5%–97.6%) |
| Concurrent chemotherapy, n = 1533 | 98 (6.4) | 94.4% (93.0%–95.5%) |
| Sequential chemotherapy, n = 805 | 68 (8.4) | 91.2% (88.9%–93.1%) |
ITT, intent-to-treat.
4. Discussion
The final analysis of the SafeHER Phase III study with a median follow-up of 72 months demonstrated that the safety and tolerability of H SC 600 mg q3w for patients with HER2-positive EBC was maintained and remained consistent with those reported in the primary analysis [10] and with H IV [9,12]. The 5-year follow-up established the reliability of H SC and provided the framework for the development of other SC formulations (i.e., fixed-dose formulation of pertuzumab and trastuzumab with recombinant human hyaluronidase for SC use; approved in June 2020) [13,14].
For Grade ≤3 AEs, H SC administered without chemotherapy exhibited a safety profile in between that of H SC administered with chemotherapy concurrently and sequentially. However, patients who received no chemotherapy exhibited the lowest proportion of discontinuations due to AEs, which suggests that the AEs were manageable. Patients who received H SC with no chemotherapy experienced a higher 5-year event-free rate of LVEF events (95.0%) compared with patients who received concurrent (92.7%) or sequential (92.6%) chemotherapy, which suggests that this regimen may offer some advantage for patients at risk for cardiac toxicity. Low proportions (<6%) of significant cardiac AEs were observed for all chemotherapy subgroups. The final safety results demonstrated that cardiac safety was maintained over time, and no late signs of cardiac toxicity were discovered.
Although the main objective of the study was to further evaluate the safety of the SC formulation of trastuzumab, the final survival outcomes were immature when the previous interim analyses were published. Therefore, the final efficacy results of the SafeHER trial indicated a survival benefit of H SC over 5 years, which is in accordance with the totality of evidence with H in this setting. Previous studies have shown that some patients may not receive substantial benefits from the addition of chemotherapy, and the de-escalation of chemotherapy is an ongoing topic of discussion and evaluation [11]. While the SafeHER trial included patients who did not receive chemotherapy, the number of patients was small (n = 235), and this subgroup had a higher percentage of patients considered low risk compared with the overall ITT population (36.6% vs 11.7%). When DFS was analyzed for low-risk patients, the 5-year DFS (95% CI) for patients who received H SC with no chemotherapy was 93.7% (85.4%–97.3%). The DFS for those who received H SC with concurrent and sequential chemotherapy was 98.3% (93.6%–99.6%) and 90.1% (81.2%–94.9%), respectively. A single-group study of the less-toxic paclitaxel and trastuzumab regimen by Tolaney et al. in patients with small, node-negative, HER2-positive breast cancer, showed a 5-year DFS of 96.3% (95% CI, 44.4%–98.2%) [15,16]. These results demonstrated a low risk of recurrence with a less toxic regimen; however, a trastuzumab monotherapy arm was not included. Nonetheless, this study supports low toxicity-based chemotherapy regimens for patients with small tumors and no nodal involvement. Interestingly, DFS seemed comparable to the SafeHER low-risk population treated with H SC monotherapy. This may support the need for further investigation via a prospective clinical study with trastuzumab H SC monotherapy as a treatment option.
The RESPECT trial evaluated DFS in patients 70–80 years of age with surgically treated HER2-positive invasive breast cancer who received trastuzumab monotherapy vs trastuzumab plus chemotherapy. Secondary endpoints included AEs and health-related quality of life [17]. The 3-year DFS was 89.5% with trastuzumab monotherapy vs 93.8% with trastuzumab and chemotherapy (hazard ratio, 1.36; 95% CI, 0.72–2.58; P = 0.56) [17]. Although the primary objective of noninferiority for trastuzumab monotherapy was not met, trastuzumab monotherapy demonstrated a favorable 3-year DFS with fewer AEs and a more favorable health-related quality of life. Studies evaluating trastuzumab monotherapy in older populations [18] and/or those with low-grade tumors [19] showed favorable DFS, suggesting that additional assessments of chemotherapy de-escalation strategies, including HER2-targeted therapies only, for the treatment of HER2-positive EBC are warranted to identify candidates who may benefit from a chemotherapy-free regimen.
There were limitations of the SafeHER trial. Patients were not randomly allocated to the chemotherapy subgroups. Instead, chemotherapy regimens were left to the discretion of treating physicians, and physicians often preferred the no chemotherapy option for patients with tumors with a better prognosis. The no chemotherapy subgroup contained a small number of patients (n = 235) and was limited to ≤10% of the total study population per protocol. For all chemotherapy subgroups, the percentages of patients who experienced an event were small, resulting in wide CIs. This was a single-arm safety study that lacked a traditional control arm.
The 5-year follow-up analysis of the SafeHER Phase III trial demonstrated that H SC has an acceptable safety profile, including cardiac toxicity, and efficacy for the treatment of HER2-positive EBC with and without chemotherapy in keeping with historical trastuzumab data. Additional studies are warranted to further evaluate the efficacy of a chemotherapy-free regimen and dual HER2 blockade with a fixed dose combination of pertuzumab and trastuzumab to increase the DFS to >90% in an older or low-risk population of patients with HER2-positive EBC.
Data sharing statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Author contributions
JG provided conceptualization, methodology, investigation, resources, writing/editing, visualization, supervision, and project administration. BA provided conceptualization, resources, and writing/editing. KHJ and MDL provided resources and writing/editing. LHB provided conceptualization, investigation, data curation, writing/editing, and project administration. KG provided validation, formal analysis, and writing/editing. HAA provided investigation, resources, writing, and supervision. XP provided methodology, investigation, and writing/editing. ER provided conceptualization, investigation, writing/editing, and supervision. NT provided data analysis/interpretation and writing/editing. AA and AM provided writing/editing.
Declaration of competing interest
The study was funded by F. Hoffmann-La Roche Ltd. AA, LHB, NT, and ER are employees of and hold stock in F. Hoffmann-La Roche Ltd. JG reports grants, personal fees and non-financial support from Roche-Genentech, personal fees and non-financial support from Novartis, personal fees from Onxeo, personal fees from Daiichi Sankyo, personal fees from MSD, grants, personal fees and non-financial support from Eisai, grants, personal fees and non-financial support from Genomic Health, personal fees from Ipsen, personal fees from Macrogenics, grants, personal fees and non-financial support from Pfizer, outside the submitted work. KG is an employee of IQVIA RDS India Pvt. Ltd., Bangalore, India and external business partner consultant for F. Hoffmann-La Roche Ltd. BA reports personal fees and nonfinancial support from Roche, personal fees from Amgen, personal fees from AstraZeneca, personal fees and non-financial support from Tesaro/GSK, personal fees from Clovis, personal fees from Celgene, nonfinancial support from PharmaMar, personal fees and nonfinancial support from MSD, and personal fees from Novartis, outside the submitted work. KHJ reports personal fees from AstraZeneca, Celgene, Novartis, Roche, Pfizer, and Takeda. MDL reports personal fees from Pfizer, Novartis, Roche, Celgene, AstraZeneca, Eisai, Eli Lilly, Amgen, and Pierre Fabre, MSD, Genomic Health, Daiichi-Sankyo, Gilead, and Seagen, outside the submitted work. XP and AM report no disclosures. HAA reports research funds from Pfizer, MSD, and ASZ; serves on the speaker bureau of Roche, MSD, BMS, Bayer, Lilly, Novartis, Pfizer, ASZ, and Amgen.
Acknowledgments
We thank the patients and their families who participated in this study. This manuscript was sponsored by F. Hoffmann-La Roche Ltd. and Genentech, Inc. Support for third-party writing assistance, furnished by Jessica Swanner, PhD, of Health Interactions, Inc., was provided by F. Hoffmann-La Roche Ltd.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.03.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ross J.S., Slodkowska E.A., Symmans W.F., Pusztai L., Ravdin P.M., Hortobagyi G.N. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 2.Gnant M., Harbeck N., Thomssen C. St. Gallen 2011: summary of the consensus discussion. Breast Care. 2011;6:136–141. doi: 10.1159/000328054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aebi S., Davidson T., Gruber G., Cardoso F., ESMO Guidelines Working Group Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(suppl 6) doi: 10.1093/annonc/mdr371. vi12–24. [DOI] [PubMed] [Google Scholar]
- 4.Batoo S., Bayraktar S., Al-Hattab E., Basu S., Okuno S., Glück S. Recent advances and optimal management of human epidermal growth factor receptor-2-positive early-stage breast cancer. J Carcinog. 2019;18:5. doi: 10.4103/jcar.JCar_14_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bookbinder L.H., Hofer A., Haller M.F., Zepeda M.L., Keller G.A., Lim J.E., et al. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Contr Release. 2006;114:230–241. doi: 10.1016/j.jconrel.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 6.European Medicines Agency . 2016. Herceptin summary of product characteristics. November 2015. [Google Scholar]
- 7.Hourcade-Potelleret F., Lemenuel-Diot A., McIntyre C., Brewster M., Lum B., Bittner B. Use of a population pharmacokinetic approach for the clinical development of a fixed-dose subcutaneous formulation of trastuzumab. CPT Pharmacometrics Syst Pharmacol. 2014;3:e87. doi: 10.1038/psp.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ismael G., Hegg R., Muehlbauer S., Heinzmann D., Lum B., Kim S.B., et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13:869–878. doi: 10.1016/S1470-2045(12)70329-7. [DOI] [PubMed] [Google Scholar]
- 9.Jackisch C., Stroyakovskiy D., Pivot X., Ahn J.S., Melichar B., Chen S.C., et al. Subcutaneous vs intravenous trastuzumab for patients with ERBB2-positive early breast cancer: final analysis of the HannaH phase 3 randomized clinical trial. JAMA Oncol. 2019;5 doi: 10.1001/jamaoncol.2019.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gligorov J., Ataseven B., Verrill M., De Laurentiis M., Jung K.H., Azim H.A., et al. Safety and tolerability of subcutaneous trastuzumab for the adjuvant treatment of human epidermal growth factor receptor 2-positive early breast cancer: SafeHer phase III study's primary analysis of 2573 patients. Eur J Cancer. 2017;82:237–246. doi: 10.1016/j.ejca.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Goutsouliak K., Veeraraghavan J., Sethunath V., De Angelis C., Osborne C.K., Rimawi M.F., et al. Towards personalized treatment for early stage HER2-positive breast cancer. Nat Rev Clin Oncol. 2020;17:233–250. doi: 10.1038/s41571-019-0299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pivot X., Gligorov J., Müller V., Barrett-Lee P., Verma S., Knoop A., et al. Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013;14:962–970. doi: 10.1016/S1470-2045(13)70383-8. [DOI] [PubMed] [Google Scholar]
- 13.Gao J.J., Osgood C.L., Gong Y., Zhang H., Bloomquist E.W., Jiang X., et al. FDA approval summary: pertuzumab, trastuzumab, and hyaluronidase-zzxf injection for subcutaneous use in patients with HER2-positive breast cancer. Clin Cancer Res. 2020;27:2126–2129. doi: 10.1158/1078-0432.CCR-20-3474. [DOI] [PubMed] [Google Scholar]
- 14.Tan A.R., Im S.A., Mattar A., Colomer R., Stroyakovskii D., Nowecki Z., et al. Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection plus chemotherapy in HER2-positive early breast cancer (FeDeriCa): a randomised, open-label, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2021;22:85–97. doi: 10.1016/S1470-2045(20)30536-2. [DOI] [PubMed] [Google Scholar]
- 15.Tolaney S.M., Barry W.T., Dang C.T., Yardley D.A., Moy B., Marcom P.K., et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372:134–141. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tolaney S.M., Guo H., Pernas S., Barry W.T., Dillon D.A., Ritterhouse L., et al. Seven-year follow-up analysis of adjuvant paclitaxel and trastuzumab trial for node-negative, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2019;37:1868–1875. doi: 10.1200/JCO.19.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawaki M., Taira N., Uemura Y., Saito T., Baba S., Kobayashi K., et al. Randomized controlled trial of trastuzumab with or without chemotherapy for HER2-positive early breast cancer in older patients. J Clin Oncol. 2020;38:3743–3752. doi: 10.1200/JCO.20.00184. [DOI] [PubMed] [Google Scholar]
- 18.Owusu C., Margevicius S.P., Klepin H.D., Vogel C.L., Alahmadi A., Vuyyala S., et al. Safety and efficacy of single-agent adjuvant trastuzumab in older women with early-stage breast cancer. J Clin Oncol. 2020;38(15 suppl):528. [Google Scholar]
- 19.Dall P., Koch T., Göhler T., Selbach J., Ammon A., Eggert J., et al. Trastuzumab without chemotherapy in the adjuvant treatment of breast cancer: subgroup results from a large observational study. BMC Cancer. 2018;18:51. doi: 10.1186/s12885-017-3857-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.