Abstract
Consumers demand products of high quality, healthy, safe, and easy to manage. A freeze-dried fruit snack can meet this demand, but this kind of product may collapse rapidly, becoming highly sticky. To avoid this, adding biopolymers can improve their stability. Nevertheless, these biopolymers can negatively affect other quality characteristics such as the color, texture, and flavor. Therefore, selecting the best biopolymer or combination of biopolymers is an indispensable task to offer freeze-dried fruit. The aim of this study was to know the impact of gum Arabic, starch modified with octenyl succinic anhydrous, bamboo fiber and milk protein isolate on the color, glass transition temperature, and stability of some textural related properties of a freeze-dried mandarin snack. The study showed the convenience of incorporate mixtures of the considered biopolymers to mandarin puree as to ensure the crispy character of the snack, associated with its glassy state, without this having a significant impact on the product color. The best formulation was that obtained with a mix of gum Arabic and bamboo fiber.
Keywords: Freeze-drying, Mandarin snack, Crispness, Color, Texture
Graphical abstract
Highlights
-
•
Freeze-drying process is adequate to obtain mandarin snacks with good quality.
-
•
Biopolymers must be added to improve mandarin crispness snacks.
-
•
The Gum Arabic-bamboo fiber mix gives the best properties to the snack.
1. Introduction
Intake of fruit and vegetables is essential for a healthy diet. Recent studies recommend a daily intake of 400 g of fruits and vegetables to prevent noncommunicable diseases, including such as diabetes, heart disease, stroke and cancer. (WHO, 2020). Plant-derived foods contain nutrients (carbohydrates, proteins, fats, vitamins, and minerals) and many non-nutrient substances called phytochemicals, which function as powerful antioxidants and metabolism regulators to protect against developing chronic diseases that begin in adulthood. As they are foods that provide health benefits, they may be considered nutraceuticals (Pulvirenti and Paterna, 2022). However, the main drawback for consumption of fresh fruits and vegetables is their short shelf life, mainly due to their high water content. Because consumers are increasingly demanding for high quality, healthy, safe, and wholesome products easy to handle, fruits and vegetables are now being processed so they are less perishable. A good alternative for processing is freeze-drying, as it is a dehydration method that provides high quality, easy-to-handle, and non-perishable foods. In this way, freeze-dried fruit and vegetable “healthy snacks” are gaining great popularity (Ciurzyńska et al., 2020; Karwacka et al., 2022; Martínez-Navarrete et al., 2019; Silva-Espinoza et al., 2021). Freeze-drying involves freezing the food and then sublimating the ice in the drying stage, which is usually carried out at low pressure and temperature. In this way, water goes from a solid to gaseous state, without passing through the liquid state, so that the food preserves its maximum nutritional value and flavor (Silva-Espinoza et al., 2021). In addition to the fact that the organoleptic properties of food are preserved, microorganisms (bacteria and molds), which cause food spoilage, cannot proliferate. Furthermore, the significant loss of weight and volume that the product undergoes during dehydration makes it much easier to transport and store.
One drawback of dried fruit is related to structural changes (Telis and Martínez-Navarrete, 2009). With fruits, their sugars and organic acids composition causes the structure of their dehydrated form to collapse rapidly, becoming highly gummy and sticky and suffer a drastic decrease in porosity, which affects aroma retention and rehydration capacity (Levi and Karel, 1995). To avoid this, it is advisable to add high-molecular-weight biopolymers that limit the collapse of the product, and, at the same time, some of them act as encapsulating agents protecting from the possible loss of the compounds responsible for the nutritional and functional value of the fruits during processing and storage (Telis and Martínez-Navarrete, 2012). However, these biopolymers can negatively affect other quality characteristics such as color, texture, and flavor. Thus, selecting the best biopolymer or biopolymer combination for each product is an essential task to offer freeze-dried fruit products. Although the most commonly used materials for the encapsulation of food products are high-molecular-weight carbohydrates, these must be used in very high quantities and the films they form are easily moistened, which, although necessary to ensure a good rehydration of the product, makes them less stable. Therefore, the amphiphilic character of proteins can offer better properties to the film formed around the particles due to their preferential migration to the surface of the particles, which reduces the adhesive character between particles, avoiding their coalescence (Adhikari et al., 2007; Gharsallaoui et al., 2007; Fang and Bhandari, 2012; Bhusari et al., 2014).
Among the carbohydrates used as encapsulating materials, gum Arabic (E−414) has been widely used for its good thickening, stabilizing, and emulsifying properties. In addition, its incorporation provides benefits such as increased glass transition temperature (Tg), reduced hygroscopicity, low viscosity contribution to the product, and anti-caking properties (Cozic et al., 2009; Telis and Martínez-Navarrete, 2009; Ghosal et al., 2010). However, due to the high price and the heterogeneity of the properties, given its origin, it seems appropriate to explore this line of research to test other suitable biopolymers (Da Silva et al., 2013). Modified starches are becoming increasingly important for their low cost and their many industrial applications (Sweedman et al., 2013; Altuna et al., 2018). However, proteins show good encapsulation properties, and include sodium caseinate, soy protein isolate, and whey protein isolates and concentrates (Gharsallaoui et al., 2007; Murúa-Pagola et al., 2009; Minj and Anand, 2020).
The objective of this study was to develop different formulations of mandarin puree with different stabilizing biopolymers and to analyze their physical properties and crispness to select the snack with the highest quality and stability.
2. Materials and methods
2.1. Raw materials
Mandarin (Citrus reticulata) of the hybrid variety Ortanique was used in this study (Anecoop S. Cooperativa, Valencia). The biopolymers (Bp) gum Arabic (GA, Scharlab S.L. Sentmenat, Spain), starch modified with octenyl succinic anhydrous (OSA, Roquette, Benifaió, Spain), bamboo fiber (BF, J. Rettenamaier & Söhne, Rosenberg, Germany), and milk protein isolate (WPI, Arla Foods Ingredients Group, Denmark) were used for the different formulations studied. These biopolymers were selected to avoid structural collapse of the dehydrated product. GA and OSA for its ability to increase Tg and BF and WPI because of its steric role avoiding the formation of interparticle bridges (Silva-Espinoza et al., 2020a, Silva-Espinoza et al., 2020b).
2.2. Sample preparation
Following the conditions described by Silva-Espinoza et al., 2020a, Silva-Espinoza et al., 2020b, a puree was prepared from the previously washed and peeled mandarins, using a food processor (Thermomix TM21, Vorwek, Spain) to which the different biopolymers (Bp) were incorporated in different proportions to obtain eight snack formulations (Table 1). The ratio of biopolymers was selected based on previous studies where the color, mechanical properties, hygroscopicity, solubility, total phenols, flavonoids, vitamin C, and antioxidant activity of the obtained powder were optimized (Agudelo et al., 2017). The mixture obtained from each formulation was distributed on aluminum trays (82 × 109 mm) with a thickness of 20 mm. The samples were frozen in a chest freezer (Liebherr Mediline 7083 207–00) for 48 h, at −47 °C. After, the samples were freeze-dried (Telstar Lioalfa-6) at 0.020 Pa pressure and at −49.5 °C in the condenser for 72 h and 25 °C in the shelves. The obtained samples were placed in airtight glass containers at 4 °C until further analysis.
Table 1.
Incorporated amount of biopolymer (g Bp/100 g puree) to obtain the different mandarin snack formulations studied (FDP: mandarin puree, GA: gum Arabic, OSA: starch modified with octenyl succinic anhydrous, BF: bamboo fiber and WPI: milk protein isolate).
| Formulation | GA | OSA | BF | WPI |
|---|---|---|---|---|
| FDP | – | – | – | – |
| F(GA:BF) | 5 | – | 1 | – |
| F(GA:WPI) | 5 | – | – | 1 |
| F(OSA:BF) | – | 5 | 1 | – |
| F(OSA:WPI) | – | 5 | – | 1 |
| F(GA) | 5 | – | – | – |
| F(OSA) | – | 5 | – | – |
| F(GA:OSA:WPI) | 2.5 | 2.5 | – | 2.5 |
To study sample stability, part of the samples was carefully cut with a sharp conventional knife (25 × 25 mm) and placed in a desiccator, containing a saturated solution of K2CO3 (relative humidity, RH = 45%) at 25 °C, simulating environmental conditions for 45 and 70 min.
2.3. Analysis of soluble solids
To determine soluble solids (°Brix), a portable digital refractometer (Refracto 30PX, Mettler Toledo, Barcelona, Spain) was used. The °Brix of the eight formulations were measured in triplicate after the biopolymers were added to the mixture, before being freeze-dried.
2.4. Water activity (aw)
The water activity (aw) of the freeze-dried samples, at the three storage times, was measured in triplicate using an AquaLab Model Series 3 dew-point hygrometer (Decagon Devices, Inc, Pullman, WA, USA).
2.5. Color
The color of the snacks was studied considering the colorimetric coordinates of the CIEL*a*b* space, where L* is the lightness (with values between 0 and 100), a* the deviation toward red (+) and green (−) and b* the deviation toward yellow (+) and blue (−). A spectrocolorimeter (Minolta, CM 3600D, Japan) was used, taking the 10° observer and D65 illuminant as the reference. The readings were taken at room temperature (22 ± 1 °C) directly on the surface of cylindrical samples of 10 mm height × 20 mm diameter, with three repetitions made for each sample. From the coordinates a* and b*, the psychophysical magnitudes chroma or color purity (C*), hue (h), and color difference (ΔE*) were calculated using equations (1), (2), (3)).
| (1) |
| (2) |
| (3) |
2.6. Mechanical and acoustic properties
To analyze the texture of the snacks (25 × 25 mm cubes), a TA.XT-Plus texturometer (Stable Micro Systems, Godalming, UK) was used, performing a penetration test with a cylindrical probe of 10 mm diameter, 80% deformation, and a speed of 1 mm/s. The acoustic properties of the samples during fracture were analyzed in the same test using an acoustic detector coupled to the texturometer (Acoustic Envelope Detector, AED). The sound detector used was an 8 mm diameter Bruel and Kjaer free-field microphone, which was calibrated using an acoustic calibrator type 4231 (94 dB and 114 dB SPL-1000 Hz) and placed at a distance of 4 cm at an angle of 45° regarding the sample. This test was performed on all samples previously stored in airtight glass containers at a temperature of 25 °C and a relative humidity of 45%. Six repetitions were conducted for each sample. The area under the curve (AUC) and the number of force peaks (peaks greater than 0.049 N) were calculated from the force-time curves. From the sound curves, the number of sound peaks (peaks greater than 2.5 dB) and the sound pressure level SPL (mean of the 10 largest peaks, SPLmax10) were calculated.
2.7. Glass transition temperature (Tg)
A differential scanning calorimeter (DSC) was used to analyze the Tg (Q2000, TA-Instruments Crawley, UK), coupled to a refrigeration system (RCS 90). Before measurements, indium calibration for temperature and enthalpy was followed (Tonset = 155.74 °C and ΔH = 28.69 J/g). Triplicates of about 10 mg of each sample were placed in hermetic DSC pans with an empty pan used for a reference. The heating rate was 5 °C/min, and temperature ranged between −60 °C and 60 °C. The onset, midpoint, and endpoint of the Tg was analyzed (Tgo, Tgm, and Tge, respectively). This analysis was conducted only to the newly freeze-dried samples.
2.8. Statistical analysis
To establish the differences between the samples analyzed, a one-factor analysis of variance (ANOVA) was performed for a significance level α = 0.05 using Tukey's test. In addition, principal component analysis (PCA) was performed to evaluate the relationships among instrumental variables of texture, storage time, and formulations. Statistical analysis was performed using XLSTAT statistical software (2010.5.02, Addinsoft, Barcelona, Spain).
3. Results and discussion
3.1. Soluble solids and water activity
The mandarin puree used as a raw material gave 14.1 ± 0.3 °Brix. The incorporation of biopolymers significantly increased this value to 17.6–17.8 for the formulations F(GA:BF); F(OSA:BF); F(GA); and F(OSA), to 18.2 for F(GA:WPI) and F(OSA:WPI), and to 19.7 for F(GA:OSA:WPI) (p < 0.05). This is due to the different composition of the formulations studied as related to the different amount and solubility of the Bp used. It should be considered that BF is an insoluble fiber whereas the rest of the biopolymers are water soluble. In addition, F(GA:OSA:WPI) has a higher amount of Bp than the rest.
Once freeze-dried, the samples presented aw values between 0.224 and 0.307, with significant differences between them (p < 0.05) (Table 2). Different interactions were observed between the Bp studied and water. The sample formulated only with GA, gum characterized by its high hygroscopicity, presented the highest aw. A similar result was reported by Tonon et al., 2009, which relate this difference in water adsorption to the number of hydrophilic groups present in the structure of each biopolymer. In this respect, gum Arabic has a high number of ramifications with hydroxyl groups, so it can more easily adsorb water from the ambient air. When the sample F(GA) was mixed with BF or WPI, the aw decreases because both Bp also interact with water, which decreases the water availability. The fact that OSA showed stronger interaction with water than GA, as can be deduced from the lower aw value of sample F(OSA) than that of sample F(GA), may be related to the chemical modification carried out precisely in order to improve, among others, the interaction of starch with water (Sweedman et al., 2013). Nevertheless, when the other Bp are present, OSA interacts with them more strongly than with water (Sweedman et al., 2013), so that part of the water that was bound to OSA becomes available and therefore aw of samples F(GA:BF) and F(GA:WPI) decreases with respect to that of F(OSA:BF) and F(OSA:WPI). This effect is much more pronounced in the case of WPI, so much so that even aw of F(OSA:WPI) increases with respect to that of F(OSA). After 45 and 70 min stored at 25 °C and 45% RH, the aw significantly increased in almost all the samples, the one without Bp, the most at the end of the storage time (p < 0.05) (Table 2). The samples that presented greater stability in terms of aw was F(GA:BF), showing lower aw values at all times.
Table 2.
Mean values of water activity (aw) of the newly freeze-dried samples after 45 and 70 min maintained in an environment of 45% RH and 25 °C (FDP: mandarin puree, GA: gum Arabic, OSA: starch modified with octenyl succinic anhydrous, BF: bamboo fiber and WPI: milk protein isolate).
| Time (min) |
|||
|---|---|---|---|
| Samples | 0 | 45 | 70 |
| FDP | 0.265cdA (0.005) | 0.349 dB (0.003) | 0.387 fC (0.003) |
| F(GA:BF) | 0.244bA (0.005) | 0.311 aB (0.004) | 0.319 aC (0.002) |
| F(GA:WPI) | 0.224aA (0.005) | 0.309 aB (0.005) | 0.336 bC (0.004) |
| F(OSA:BF) | 0.260 cA (0.005) | 0.332 bB (0.006) | 0.363 dC (0.002) |
| F(OSA:WPI) | 0.283 eA (0.006) | 0.328 bB (0.003) | 0.335 bB (0.004) |
| F(GA) | 0.307 fA (0.003) | 0.339 cB (0.004) | 0.351 cC (0.002) |
| F(OSA) | 0.269 dA (0.005) | 0.352 dB (0.003) | 0.378 eC (0.002) |
| F(GA:OSA:WPI) | 0.271 dA (0.002) | 0.308 aB (0.003) | 0.322 aC (0.002) |
Values in parentheses represent the standard deviation.
a, b, c, d, e Mean values with different letters in the same column indicate the existence of significant differences (p < 0.05) between formulations at a given time according to Tukey's test.
A, B, C Mean values with different letters in the same row indicate the existence of significant differences (p < 0.05) for each formulation as a function of time according to Tukey's test..
3.2. Color
Table 3 shows the values obtained for the CIEL*a*b* coordinates that instrumentally characterize the color of the different newly freeze-dried samples. Although some significant differences in color parameters were found, all formulations presented similar optical properties. Lightness (L*) ranged from 65 to 74, color purity (C*) ranged from 43 to 56.3 and hue (h) ranged from 72.6 to 77.8, indicating a yellow-orange color in all samples. In all cases, the presence of Bp increases the lightness, decreases the chroma, and slightly masks the orange hue typical of mandarin. The smallest color difference between the formulations and the samples without Bp corresponded to formulation F(GA:BF) (Table 3). Despite the color of the samples was also measured after 45 min and 70 min of storage at 25 °C and 45% RH, no significant differences were found with storage time in the formulations (p > 0.05) (data not shown).
Table 3.
CIEL*a*b color coordinates of the newly freeze-dried products and ΔE between each formulation and the sample without Bp at time 0 (FDP: mandarin puree, GA: gum Arabic, OSA: octenyl succinic anhydrous modified starch, BF: bamboo fiber and WPI: milk protein isolate).
| L* | C* | h | ΔE* | |
|---|---|---|---|---|
| FDP | 65d (3) | 56.3 a (1.8) | 72.6 d (0.9) | – |
| F(GA:BF) | 70.2 c (0.7) | 52.1 ab (1.7) | 77.1 a (0.8) | 7.7 |
| F(GA:WPI) | 71 bc (3) | 44 cd (2) | 77.7 a (0.3) | 13.9 |
| F(OSA:BF) | 74 a (3) | 44 cd (5) | 77.5 b (1.0) | 14.8 |
| F(OSA:WPI) | 71.1abc (0.9) | 44.6 cd (0.3) | 77.1 a (1.1) | 13.7 |
| F(GA) | 69.8 c (1.5) | 46 bcd (6) | 74.2 bc (1.7) | 11.2 |
| F(OSA) | 69.8 c (1.3) | 49 bc (3) | 72.9 d (0.6) | 8.7 |
| F(GA:OSA:WPI) | 73.1 ab (0.8) | 43 d (9) | 77.8 cd (0.6) | 14.4 |
a, b, c, d Mean values with different letters in the same column indicate the existence of significant differences (p < 0.05) between formulations according to Tukey's test.
3.3. Mechanical and acoustic properties
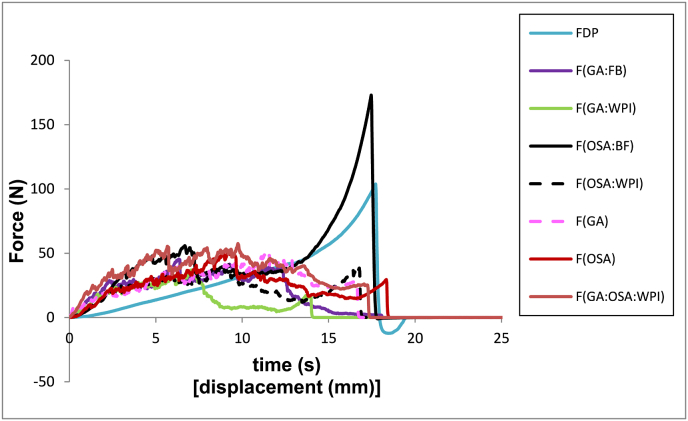
To evaluate the crispness of the samples, the force-time and sound curves of each of the newly freeze-dried samples and after 45 and 70 min stored at 25 °C and 45% RH were analyzed. Fig. 1 shows, as an example, the texture curves of one of the replicates of each of the eight newly freeze-dried formulations and Fig. 2 shows an example of the texture curves and sound profiles of two of them.
Fig. 1.
Example of the force-time curves of the newly freeze-dried mandarin formulations. (FDP: mandarin puree, GA: gum Arabic, OSA: octenyl succinic anhydrous modified starch, BF: bamboo fiber and WPI: milk protein isolate).
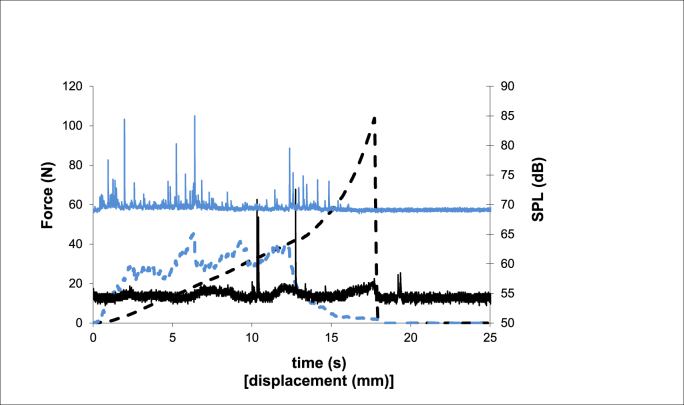
Fig. 2.
Example of the texture (dashed lines) and sound (continuous lines) profiles of the newly freeze-dried mandarin puree. (black) and mandarin puree formulated with gum Arabic and bamboo fiber (blue). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Both the FDP (without added Bp) and the F(OSA:BF) formulations showed curves different from the other six. The FDP formulation did not present fracture peaks, and the profile represents a rubbery sample, with almost no sound peaks, indicating its non-crisp character. F(OSA:BF) showed fracture peaks at the beginning of the test, although it showed an increase in strength toward the end, related to a rubberier behavior in the sample interior, like with the sound curve, where sound peaks were appreciated only at the beginning of the penetration (graph not shown). The other formulations remained crisp during the whole test, behavior that can be related to the large number of force and sound peaks shown during the whole penetration test. Notably, formulation F(GA:WPI) presented the least resistance to penetration, i.e., the most brittle sample (Fig. 1).
However, to better determine the differences between the samples, Table 4 presents the parameters calculated from the force-time and sound-time curves obtained in each formulation. First, the area under the force-time curve may be associated to the resistance of the sample to be penetrated, related to its firmness (Benkadri et al., 2018). The newly freeze-dried formulations F(GA:BF), F(GA:WPI), and F(OSA:WPI) presented the lowest area values, corresponding to samples that break at lower applied stresses. Great area values could correspond both to a rubbery sample that doesn't breaks but mostly is deformed (sample FDP), or to samples being more resistant to be fractured as they are F(GA), F(OSA), F(GA:OSA:WPI), and F(OSA:BF); with F(OSA:BF) showing the highest area value. However, the number of force and sound peaks are also related to the crispness of the samples (Salvador et al., 2009; Dias-Faceto et al., 2020). The formulations F(GA:BF), F(GA:WPI), F(OSA:WPI), and F(GA) presented higher number of fracture and sound peaks which, together with the lower firmness described above, indicates they are the crunchiest samples. The sound pressure level (SPL) values were very similar in the eight formulations, with FDP presenting the lowest value and F(OSA:WPI) the highest.
Table 4.
Values of the area under the curve (AUC), number of fracture peaks (FP), number of sound peaks (SP), and maximum sound pressure (SPL) of the newly freeze-dried samples (0 min) after 45 and 70 min storage at 25 °C and 45% RH (FDP: mandarin puree, GA: Gum Arabic, OSA: octenyl succinic anhydrous modified starch, BF: bamboo fiber and WPI: milk protein isolate).
| Time (min) | AUC (N mm) | FP | SP | SPLmax10 (dB) | |
|---|---|---|---|---|---|
| FDP | 0 | 511 a (71) | 45b (22) | 29 a (27) | 66 a (7) |
| 45 | 406 a,b (17) | 11.8a (3) | 0.25 b (1) | 18 b (4) | |
| 70 | 385 b (88) | 8c (3) | 6 a,b (1) | 58 a (1) | |
| F(GA:BF) | 0 | 393 a (77) | 245a (16) | 48 a (26) | 79 a (4) |
| 45 | 491 a (113) | 210b (20) | 28 a,b (5) | 78 a (1) | |
| 70 | 541 a (60) | 184b (10) | 15 b (8) | 75 a (2) | |
| F(GA:WPI) | 0 | 261 a (46) | 258a (29) | 59 a (35) | 80 a (4) |
| 45 | 371 a (58) | 271 a (78) | 42 a (16) | 79 a (2) | |
| 70 | 426 a (149) | 274 a (8) | 47 a (6) | 80 a (1) | |
| F(OSA:BF) | 0 | 911 b (128) | 122 a (16) | 76 a (23) | 70 b (2) |
| 45 | 669 b (46) | 143 a (7) | 16 b (7) | 77 a (3) | |
| 70 | 1694 a (150) | 25 b (19) | 26 b (14) | 69 b (4) | |
| F(OSA:WPI) | 0 | 399 a (113) | 240 a (17) | 55 a (31) | 81 a (4) |
| 45 | 496 a (136) | 199 ab (20) | 21 a (9) | 77 a (4) | |
| 70 | 610 a (109) | 175 b (26) | 31 a (12) | 77 a (2) | |
| F(GA) | 0 | 485 b (63) | 222 a (15) | 184 a (26) | 76 ab (1) |
| 45 | 516 b (152) | 225 a (14) | 41 b (9) | 79 a (2) | |
| 70 | 1179 a (103) | 66 b (23) | 45 b (21) | 71 b (7) | |
| F(OSA) | 0 | 565 b (98) | 191 a (17) | 177 a (31) | 76 a (4) |
| 45 | 457 b (61) | 179 a (12) | 17 b (11) | 75 ab (2) | |
| 70 | 1539 a (124) | 46 b (22) | 35 b (5) | 71 a (3) | |
| F(GA:OSA:WPI) | 0 | 606 b (70) | 178 a (23) | 148 a (25) | 73 ab (3) |
| 45 | 486 b (21) | 214 a (24) | 26 b (10) | 78 a (1) | |
| 70 | 1295 a (149) | 83 b (17) | 36 b (12) | 68 b (5) |
Values in parentheses represent the standard deviation.
a, b, c Mean values with different letters in the same column indicate the existence of significant differences (p < 0.05) with storage time for each formulation according to Tukey's test.
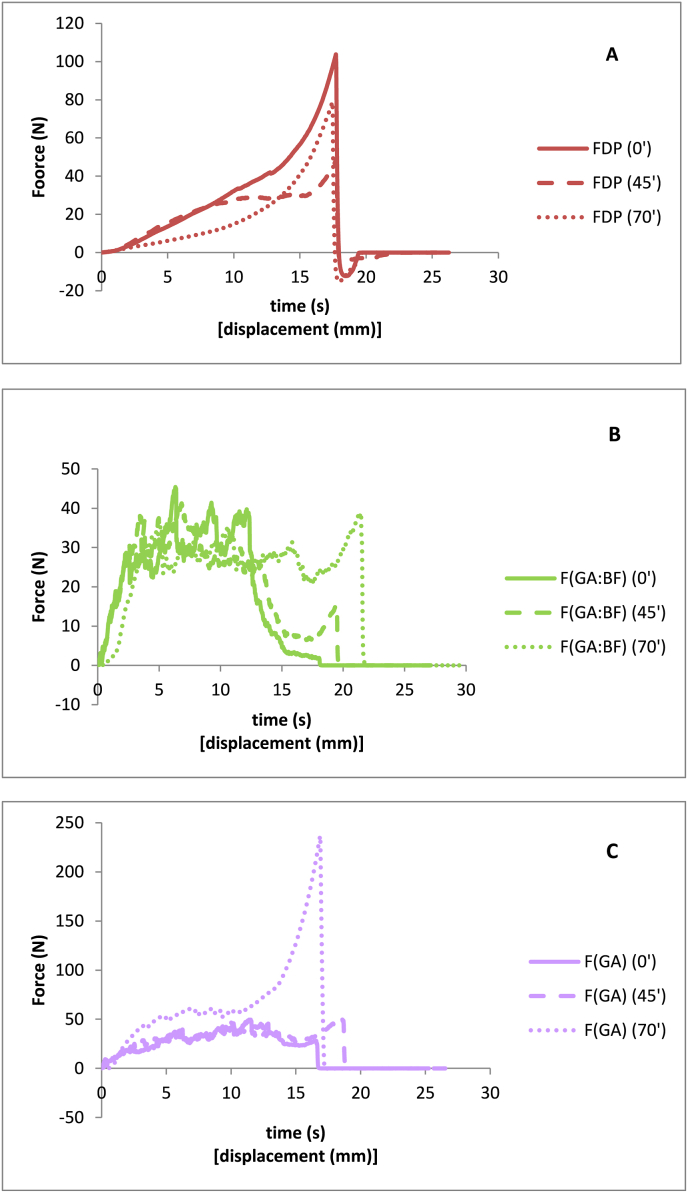
Fig. 3 shows an example of the change in texture of the samples with storage time at 45% RH and 25 °C. The 3 graphs shown reflect the 3 types of behavior shown by the samples. The absence of fracture peaks in FDP formulation, at any time (Fig. 3A), indicates its rubbery character from the beginning. The formulations F(GA:BF), F(GA:WPI), and F(OSA:WPI) behaved similarly as shown in Fig. 3B, maintaining crispness throughout the study time. The other formulations, although they seem to maintain the crisp character, show a certain rubbery behavior, as appreciated by the texture curves, because they do not present many peaks of strength but a plateau (Fig. 3C). The parameters evaluated for all samples, both force-time and sound pressure-time curves, can be seen in Table 4. In the formulations F(OSA:BF), F(GA), F(OSA) and F(GA:OSA:WPI) an increase in the AUC and a decrease in both force and SP were observed, especially at 70 min of storage. However samples F(GA:BF) (Fig. 3B), F(GA:WPI), and F(OSA:WPI) showed less increase in area and less decrease in the number of both force and SP, which indicates these samples maintain their crisp character longer.
Fig. 3.
Example of the texture and sound profiles of the formulations at different storage time. (A: FDP; B: F(GA:BF); C: F(GA)).
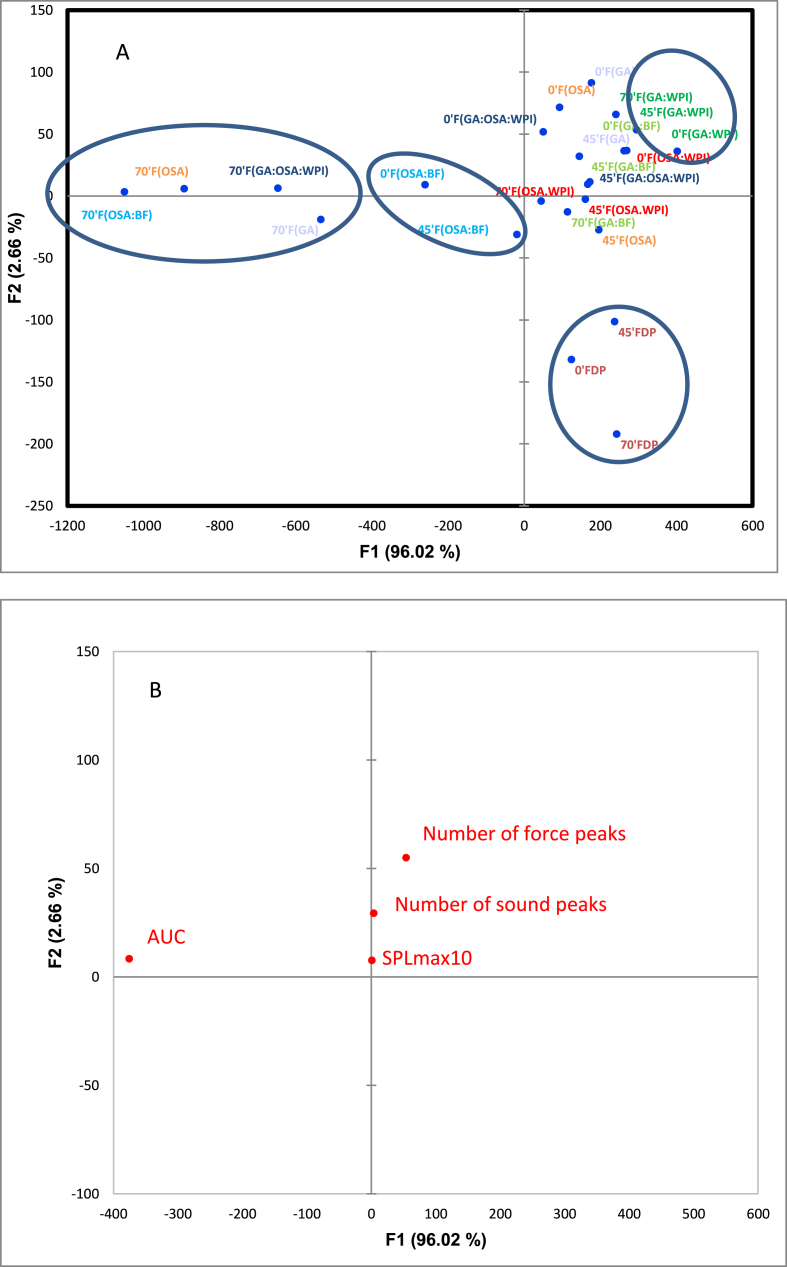
To evaluate the correlation among the different instrumental parameters and the studied samples, a PCA was carried out. The rotation method used was Varimax with Kaiser Normalisation. The first two components that explained together 98.68% of the variance are represented in Fig. 4. In Fig. 4A and B the samples distribution and the instrumental texture parameters distribution, respectively, were shown. The first component explained 96.02% of the variance and showed a positive correlation with the instrumental parameter “number of force peaks'’ and a negative correlation with AUC (area under the texture curve). On the other hand, the second component showed a positive correlation with the instrumental parameters ‘‘number of sound peaks’’ and ‘‘SPLmax10‘‘(Fig. 4B). PCA showed that all the samples except FDP and F(OSA:BF) were related to the instrumental parameters indicating crispness; however, only sample F(GA:WPI) maintains the crispness character with the storage time studied although samples F(GA:BF), F(OSA:WPI), F(GA), F(OSA), and F(GA:OSA:WPI) maintain the crispness character, but only up to 45 min, losing this character at the end of storage.
Fig. 4.
Principal component analysis of the eight formulations with storage time. A: Sample representation. B: Texture instrumental parameters representation. (FDP: mandarin puree, GA: gum Arabic, OSA: octenyl succinic anhydrous modified starch, BF: bamboo fiber and WPI: milk protein isolate).
3.4. Glass transition temperature
Table 5 shows the onset, midpoint, and endpoint of the glass transition for the different newly freeze-dried products. The amplitude of the glass transition was of the order of 15 °C, which is a usual value for foodstuffs (Roos, 1995; Silva-Espinoza et al., 2020; Telis and Martínez-Navarrete, 2010). The sample with no biopolymers added showed a Tgm of 4 °C, similar to other freeze-dried fruits, the sugar and organic acid composition of fruits being responsible for their low Tg value (Telis and Martínez-Navarrete, 2010). Table 5 shows that FDP sample is in a complete glassy state at temperatures below 0 °C while rubbery at temperatures above 10 °C. The glassy state means a higher stability of the product and is related to a crispier character. In the rubbery state, the rate of the deterioration reactions increases and the crispness is lost. In this way, the more the temperature of the sample exceeds the value of its Tg, the more pronounced the loss of crispness. (Martínez-Navarrete et al., 2004; Silva-Espinoza et al., 2020). In this study the texture test done on this sample, which was conducted at 25 °C, indicates its rubbery character (section 3.3).
Table 5.
Glass transition temperature of newly freeze-dried samples (FDP: mandarin puree, GA: gum Arabic, OSA: octenyl succinic anhydrous modified starch, BF: bamboo fiber and WPI: milk protein isolate).
| Tgo | Tgm | Tge | |
|---|---|---|---|
| FDP | 0.09 | 4.02 | 10.14 |
| F(GA:BF) | 12.08 | 18.24 | 24.85 |
| F(GA:WPI) | 16.12 | 23.00 | 38.42 |
| F(OSA:BF) | 9.02 | 16.11 | 23.79 |
| F(OSA:WPI) | 9.39 | 15.35 | 23.02 |
| F(GA) | 20.53 | 27.78 | 40.42 |
| F(OSA) | 5.61 | 13.46 | 19.95 |
| F(GA:OSA:WPI) | 6.45 | 12.96 | 21.28 |
Table 5 shows the addition of the biopolymers caused an increase in Tg, which was higher for GA than for OSA. The increase in Tg due to the effect of biopolymers also correlates with the crispness observed in these samples, although no clear differences in texture are observed due to the effect of the use of the GA or OSA biopolymer (section 3.3).
4. Conclusions
This study showed that the addition of biopolymers into a mandarin puree is necessary to ensure the crispness of a freeze-dried snack, associated with its glassy state. The low influence of the biopolymers studied on the water activity and color of the snack justifies their use. Although there seems to be no major differences in the studied properties, the formulation of the mandarin puree with GA and FB, remaining crisp throughout, showed the greater stability as related to its lower aw.
CRediT authorship contribution statement
Ana Salvador: Conceptualization, Methodology, Investigation, Formal analysis, Writing – original draft, Writing – review & editing. María del Mar Camacho: Conceptualization, Methodology, Investigation, Formal analysis, Writing – original draft, Writing – review & editing. Nuria Martínez-Navarrete: Conceptualization, Methodology, Investigation, Formal analysis, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Editor name: Xing Chen
Contributor Information
Ana Salvador, Email: asalvador@iata.csic.es.
María del Mar Camacho, Email: mdmcamvi@tal.upv.es.
Nuria Martínez-Navarrete, Email: nmartin@tal.upv.es.
References
- Adhikari B., Howes T., Shrestha A.K., Bhandari B.R. Development of stickiness of whey protein isolate and lactose droplets during convective drying. Chem. Eng. Process. 2007;46:420–428. [Google Scholar]
- Agudelo C., Igual M., Camacho M.M., Martínez-Navarrete N. Effect of process technology on the nutritional, functional, and physical quality of grapefruit powder. Food Sci. Technol. Int. 2017;23:61–74. doi: 10.1177/1082013216658368. [DOI] [PubMed] [Google Scholar]
- Altuna L., Herrera M.L., Foresti M.L. Synthesis and characterization of octenyl succinic anhydride modified starches for food applications. A review of recent literature. Food Hydrocolloids. 2018;80:97–110. doi: 10.1016/j.foodhyd.2018.01.032. [DOI] [Google Scholar]
- Benkadri S., Salvador A., Zidoune M.N., Sanz T. Gluten-free biscuits based on composite rice–chickpea flour and xanthan gum. Food Sci. Technol. Int. 2018;24(7):607–616. doi: 10.1177/1082013218779323. [DOI] [PubMed] [Google Scholar]
- Bhusari S.N., Muzaffar K., Kumar P. Effect of carrier agents on physical and microstructural properties of spray dried tamarind pulp powder. Powder Technol. 2014;266:354–364. [Google Scholar]
- Ciurzyńska A., Marczak W., Lenart A., Janowicz M. Production of innovative freeze-dried vegetable snack with hydrocolloids in terms of technological process and carbon footprint calculation. Food Hydrocolloids. 2020;108 doi: 10.1016/j.foodhyd.2020.105993. [DOI] [Google Scholar]
- Cozic C., Picton L., Garda M.-R., Marlhoux F., Le Cerf D. Analysis of Arabic gum: study of degradation and water desorption processes. Food Hydrocolloids. 2009;23:1930–1934. [Google Scholar]
- Da Silva F.C., Da Fonseca C.R., De Alencar S.M., Thomazini M., Balieiro J.C.D.C., Pittia P. Assessment of production efficiency, physicochemical properties and storage stability of spray-dried propolis, a natural food additive, using gum Arabic and OSA starch-based carrier systems. Food Bioprod. Process. 2013;91:28–36. doi: 10.1016/j.fbp.2012.08.006. [DOI] [Google Scholar]
- Dias-Faceto L.S., Salvador A., Conti-Silva A.C. Acoustic settings combination as a sensory crispness indicator of dry crispy food. J. Texture Stud. 2020;51:232–241. doi: 10.1111/jtxs.12485. [DOI] [PubMed] [Google Scholar]
- Fang Z., Bhandari B. In: Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals. Garti Nissim, McClements D.J., editors. WP Woodhead Publishing; Oxford: 2012. Spray drying, freeze drying and related processes for food ingredient and nutraceutical encapsulation; pp. 73–109. [Google Scholar]
- Gharsallaoui A., Roudaut G., Chambin O., Voilley A., Saurel R. Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Res. Int. 2007;40:1107–1121. [Google Scholar]
- Ghosal S., Indira T.N., Bhattacharya S. Agglomeration of a model food powder: effect of maltodextrin and gum Arabic dispersions on flow behavior and compacted mass. J. Food Eng. 2010;96:222–228. doi: 10.1016/j.jfoodeng.2009.07.016. [DOI] [Google Scholar]
- Karwacka M., Ciurzyńska A., Galus S., Janowicz M. Freeze-dried snacks obtained from frozen vegetable by-products and apple pomace – selected properties, energy consumption and carbon footprint. Innovat. Food Sci. Emerg. Technol. 2022;77 doi: 10.1016/j.ifset.2022.102949. [DOI] [Google Scholar]
- Levi G., Karel M. Volumetric shrinkage (collapse) in freeze-dried carbohydrates above their glass transition temperature. Food Res. Int. 1995;28(2):145–151. [Google Scholar]
- Martínez-Navarrete N., Moraga G., Talens P., Chiralt A. Water sorption and the plasticization effect in wafers. Int. J. Food Sci. Technol. 2004;39:555–562. [Google Scholar]
- Martínez-Navarrete N., Salvador A., Oliva C., Camacho M.M. Influence of biopolymers and freeze-drying shelf temperature on the quality of a Mandarin snack. LWT--Food Sci. Technol. 2019;99:57–61. doi: 10.1016/j.lwt.2018.09.040. [DOI] [Google Scholar]
- Minj S., Anand S. Whey proteins and its derivatives: bioactivity, functionality, and current applications. Dairy. 2020;1(3):233–258. doi: 10.3390/dairy1030016. [DOI] [Google Scholar]
- Murúa-Pagola B., Beristain-Guevara C.I., Martínez-Bustos F. Preparation of starch derivatives using reactive extrusion and evaluation of modified starches as shell materials for encapsulation of flavoring agents by spray drying. J. Food Eng. 2009;91:380–386. [Google Scholar]
- Pulvirenti L., Paterna A. In: Advances in Nutraceuticals and Functional Foods. Gopi S., Balakrishnan P., editors. Academic Press; New York: 2022. Introduction to functional foods and nutraceuticals. [DOI] [Google Scholar]
- Roos Y.H. Academic Press; San Diego: 1995. Phase Transitions in Foods. [Google Scholar]
- Salvador A., Varela P., Sanz T., Fiszman S.M. Understanding potato chips crispy texture by simultaneous fracture and acoustic measurements, and sensory analysis. LWT--Food Sci. Technol. 2009;42:763–767. [Google Scholar]
- Silva-Espinoza M.A., Ayed C., Foster T., Camacho M.M., Martínez-Navarrete N. The impact of freeze-drying conditions on the physico-chemical properties and bioactive compounds of a freeze-dried orange puree. Foods. 2020;9:32. doi: 10.3390/foods9010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Espinoza M.A., Camacho M.M., Martínez-Navarrete N. Use of different biopolymers as carriers for purposes of obtaining a freeze-dried orange snack. LWT--Food Sci. Technol. 2020;127 doi: 10.1016/j.lwt.2020.109415. [DOI] [Google Scholar]
- Silva-Espinoza M.A., Salvador A., Camacho M.M., Martínez-Navarrete N. Impact of freeze-drying conditions on the sensory perception of a freeze-dried orange snack. J. Sci. Food Agric. 2021;101(11):4585–4590. doi: 10.1002/jsfa.11101. [DOI] [PubMed] [Google Scholar]
- Sweedman M.C., Tizzotti M.J., Schäfer C., Gilbert R.G. Structure and physicochemical properties of octenyl succinic anhydride modified starches: a review. Carbohydr. Polym. 2013;92:905–920. doi: 10.1016/j.carbpol.2012.09.040. [DOI] [PubMed] [Google Scholar]
- Telis V.R.N., Martínez-Navarrete N. Collapse and color changes in grapefruit juice powder as affected by water activity, glass transition, and addition of carbohydrate polymers. Food Biophys. 2009;4:83–93. [Google Scholar]
- Telis V.R.N., Martínez-Navarrete N. Application of compression test in analysis of mechanical and color changes in grapefruit juice powder as related to glass transition and water activity. LWT--Food Sci. Technol. 2010;43:744–751. doi: 10.1016/j.lwt.2009.12.007. [DOI] [Google Scholar]
- Telis V.R.N., Martínez-Navarrete N. In: Biopolymer Engineering in Food Processing. Nicoletti Telis V., editor. CRC Press. Taylor & Francis Group; Boca Raton, FL: 2012. Biopolymers used as drying aids in spray drying and freeze drying of fruit juices and pulps; pp. 279–326. [Google Scholar]
- Tonon R.V., Brabet C., Pallet D., Brat P., Hubinger M.D. Physicochemical and morphological characterisation of açai (Euterpe oleraceae Mart.) powder produced with different carrier agents. Int. J. Food Sci. Technol. 2009;44:1950–1958. doi: 10.1111/j.1365-2621.2009.02012.x. [DOI] [Google Scholar]
- WHO . 2020. World Health Organization (April, 29, 2020). Healthy Diet.https://www.who.int/news-room/fact-sheets/detail/healthy-diet [Google Scholar]