Abstract
Surgical techniques and technology are steadily improving, thereby expanding the pool of patients amenable for spine surgery. The growing and aging population in the United States further contributes to the increase in spine surgery cases. Traditionally, spine surgery is performed under general anesthesia. However, awake spinal surgery has recently gained traction due to evidence of decreased perioperative risks, postoperative opioid consumption, and costs, specifically in lumbar spine procedures. Despite the potential for improving outcomes, awake spine surgery has received resistance and has yet to become adopted at many healthcare systems. We aim to provide the fundamental steps in facilitating the initiation of awake spine surgery programs. We also present case reports of two patients who underwent awake spine surgery and reported improved clinical outcomes.
Keywords: Awake spine surgery, Awake protocol, Spinal anesthesia, Awake spine cases, Awake Spinal Fusion, ERAS
Highlights
-
•
Starting an Awake Spine program is feasible and may improve clinical outcomes.
-
•
Awake Spine Surgery is associated with reduced cardiopulmonary complications and opioid consumption.
-
•
Awake Spine Surgery is effective at reducing LOS, HAC, and cost of surgery.
-
•
Awake Spine Surgery increases the pool of patients eligible for spinal procedures.
Introduction
Lumbar degenerative disease is a leading cause of disability in the United States, resulting in a substantial burden on both patient quality of life and healthcare costs. As the United States’ population continues to age, the need for operative treatment of lumbar pathologies will grow exponentially. In fact, the proportion of the US population over age 65 has increased from 12% in 2000 to a projected 20% by 2030 (O’Lynnger et al., 2015). This is associated with an increase in prevalence of degenerative spinal disorders, leading to an increase for surgical treatment of these conditions (O’Lynnger et al., 2015). Despite increasing evidence recommending avoidance of general anesthesia in older patients (Berthoud and Reilly, 1992, Xie et al., 2018, Patel et al., 2015, Fiani et al., 2021; Meng et al., 2017), surgical management using traditional general anesthesia remains the standard of care for several surgeons.
Multiple novel techniques have been employed in recent years in order to adequately treat lumbar disease while mitigating perioperative morbidity associated with traditional spine surgery. Some of these techniques include minimally invasive (MIS) and endoscopic procedures, percutaneous fixation, osteobiologic use, and expandable bone grafts. In recent years, Enhanced Recovery After Surgery (ERAS) protocols have been developed and applied in the field of spine surgery with the goal of reducing complications, readmissions and improving functional recovery (Dietz et al., 2019, Elsarrag et al., 2019, Wang et al., 2019). As such, ERAS protocols use a multidisciplinary evidence-based approach to perioperative counseling and alternative approaches to anesthesia and analgesia (Dietz et al., 2019). Awake spine surgery (ASPS) with regional anesthesia has gained attention in the field of spine surgery, as it has demonstrated improved perioperative outcomes and reduced surgical costs in comparison to traditional general anesthesia (GA) (Wang and Grossman, 2016, Lessing et al., 2017, Jellish et al., 1996; McLain et al., 2005; Chen et al., 2011; Kahveci et al., 2014; Soh et al., 2020). Indeed, some retrospective studies have found improved outcomes with the use of local anesthesia for various surgical procedures in older adults suggesting an advantage over general anesthesia for this age group (Meier et al., 2021, Meier et al., 2021; Balentine et al., 2021, Balentine et al., 2021).
Despite the clinical and financial benefits of avoiding general anesthesia, awake spine surgery has not been readily adopted among healthcare systems. Some of this resistance has been attributed to decreased patient acceptance and anesthesiologist preference for GA as it allows for a secure airway prior to placing a patient in a prone position. Due to these concerns, starting an awake surgery program can be a challenging process as it requires a multi-disciplinary effort, as well as sufficient patient and healthcare provider education in order to improve its acceptance.
Although fewer studies have described the steps undertaken to perform awake spine surgery (De Biase et al., 2021) and patient selection algorithm (Letchuman et al., 2021), very little has been published with practical instruction on starting an awake spine surgery program. Thus, here we provide a comprehensive template for healthcare providers and leaders for starting an awake spine surgery program.
Advances in the field of awake spine surgery
There has been significant increase in the occurrence of ASPS over the past years (Martin et al., 2019). Many factors have led to this increase, and it is largely due to advances in administration of anesthesia as well as advances in surgical techniques.
Advances in anesthesia
Regarding anesthesia in spine surgery, general anesthesia (GA) is still the most prevalent anesthetic technique. GA is not without drawbacks as it is associated with cognitive, renal, and, most notably, cardiopulmonary complications as well as unpleasant postoperative nausea and vomiting (Berthoud et al., 1992). Spinal anesthesia (SA) has emerged as an alternative to GA and has been increasingly used in lumbar spine procedures of limited duration (<3–4 h). SA allows the patient to avoid tracheal intubation, mechanical ventilation, and the cardiopulmonary morbidity associated with GA (Xie et al., 2018, Patel et al., 2015; Miller et al., 2020). In addition, there is a favorable recovery profile for spine surgery performed under spinal anesthesia, with significantly reduced rates of postoperative nausea and vomiting, shorter length of stay (LOS), and lower intraoperative blood loss compared to those under general anesthesia (Wang and Grossman, 2016).
Recently, some centers have begun to employ targeted nerve block techniques to further improve the comfort of patients undergoing spine surgery and accelerate recovery and time to discharge. Examples of such are the erector spinae plane (ESP) block and thoracolumbar interfascial plane (TLIP) block (Wang and Grossman, 2016, Kolcun et al., 2019, Kai-Hong Chan et al., 2019, Kolcun et al., 2019). These ultrasound-guided techniques block the dorsal rami of relevant spinal roots by depositing local anesthetic posterior to the transverse process or within the paraspinal musculature itself (Xie et al., 2018).
Advances in surgical technique
The development of minimally invasive and endoscopic spinal surgery techniques have been critical in enabling the development of ASPS. Minimally invasive spine surgery not only facilitates ASPS through reducing the psychological burden on the patient, but also is associated with reduced blood loss, reduced length of stay, less postoperative disability, and fewer complications such as dural injuries and wound infections (Xie et al., 2018, Patel et al., 2015; Miller et al., 2020).
Common applications of awake spine surgery
ASPS has been used for a wide variety of spinal procedures and commonly performed awake spinal surgeries include, but are not limited to laminectomy, discectomy, anterior cervical discectomy and fusion, lumbar fusion, and dorsal column stimulator placement (Fiani et al., 2021).
Other instances of awake surgery
It is also worth noting that spine surgery under spinal anesthesia is not the only current application of awake surgery. The field of orthopedic surgery has long utilized awake anesthesia, namely with wide-awake local anesthesia with no tourniquet (WALANT) techniques for hand and upper extremity procedures (Lalonde, 2019; Ayhan and Akaslan, 2020). The WALANT technique has been commonly applied to tendon repair, tendon transfer, simple bony procedures, and more. More recently, it has been applied to extensive soft-tissue repair, bony manipulation, and more. The WALANT technique not only negates post-operative concerns associated with GA, but also reduces cost, eliminates the need for clearance, and reduces the number of required staff (Kurtzman et al., 2021).
Awake surgery and COVID 19
This cost and resource effectiveness has been taken advantage of by surgeons during the COVID-19 pandemic, allowing for surgeries to be performed in the setting of supply and provider shortages (Turcotte et al., 2021). During the COVID-19 pandemic, the CMS (Center of Medicare and Medicaid Services) guidelines regarding spine surgery recommended that physicians consider postponing surgery for non-urgent and elective spine procedures, allowing for conservation of beds, ventilators, PPE, and workforce (CMS 2020). It is feasible, then, that implementation of awake anesthesia could facilitate the responsible execution of spine surgery in future times of medical scarcity. This is particularly relevant for those practicing in low to middle income countries (LMIC), where these scarcities are even more pronounced (Khattab et al., 2021).
Protocol
Patient selection
Although no direct studies have identified the ideal candidate for ASPS, there are relative contraindications to regional anesthesia for spine surgery. The indications and contraindication for awake spine surgery are summarized in Table 1. Despite these general considerations, the decision to utilize local and regional anesthesia should be left to the discretion and comfort of the surgical and anesthesia teams.
Table 1.
Indications and contraindications for awake spine surgery (Fiani et al., 2021).
| Indications | Contraindications |
|---|---|
|
|
Anesthesia protocol
Preoperative
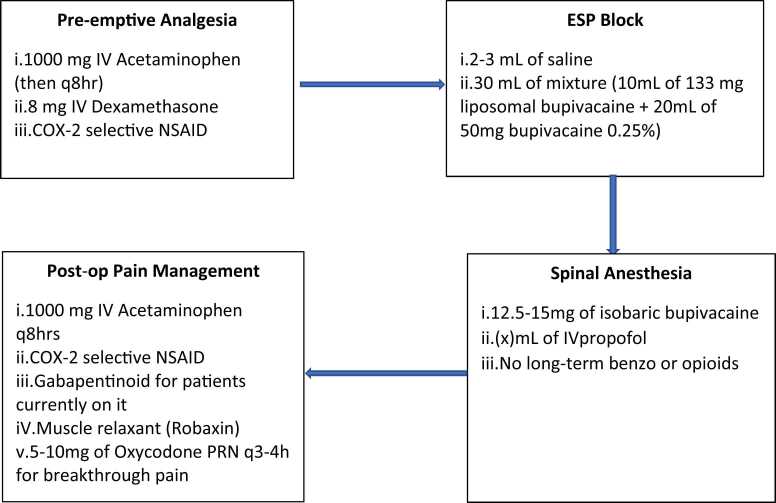
Preemptive analgesia and regional anesthesia are paramount to ASPS. Pre-emptive analgesia involves preoperative analgesia administration. This technique combines nonopioid agents with opioids in order to reduce narcotics consumptions while improving patient satisfaction after spinal surgery (Eckman et al., 2014). To date, the most commonly reported nonopioids analgesic used are gabapentin, pregabalin, acetaminophen, dexamethasone and NSAIDs (Rivkin et al., 2014). Our recommendation for preemptive anesthesia includes acetaminophen 1000 mg in preop (and q8h thereafter), dexamethasone 8 mg IV, and COX-2 selective NSAIDs such as meloxicam.
The anesthesia team performs an erector spinae plane (ESP) block in the preoperative holding area using light sedation. This workflow allows time for the block to be fully functioning when incision is made, and also improves efficiency by taking this step out of the operating room. With the patient sitting, lateral or prone position (operator’s preference), the skin over the lumbar spine is prepped with chlorhexidine and a low-frequency curvilinear ultrasound probe is placed in the parasagittal orientation. The transverse process of either L2 or L3 is identified (whichever is most clearly visualized), and a 21 G 100 mm nerve block needle advanced in-plane under direct guidance through the erector spinae group of muscles until contact is made with the dorsal surface of the transverse process. Following negative aspiration, 2–3 mL of saline are administered to confirm the correct submuscular plane, followed by 30 mL of a mixture containing 133 mg of liposomal bupivacaine (10 mL) and 50 mg of bupivacaine 0.25% (20 mL). The needle is then removed, and the procedure repeated on the contralateral side.
Intraoperative
There are a variety of choices for the ideal spinal anesthetic; at our institution, bupivacaine is often used, together with intravenous propofol for sedation. Once in the operating room, our patients receive a spinal anesthetic at the L3–4 or L4–5 level, using 12.5–15 mg of isobaric bupivacaine. This provides a dense block of the lower thoracic and entire lumbar region for greater than four hours. Our sedation protocol includes intravenous propofol infusion titrated to effect. No long-term benzodiazepines or opioids are used, in order to promote a rapid recovery.
Postoperative
With the combination of a spinal anesthetic and the erector spinae plane blocks, patients typically enjoy a “soft landing” upon emergence in the recovery room, compared to patients that receive a general anesthetic and no blocks. Every effort is made to maximize non-opioid analgesia in order to prevent opioid-related adverse events such as nausea/vomiting, ileus, constipation, pruritis and respiratory depression. Patients receive acetaminophen and NSAIDs as standing medications (not prn) as the foundation of their pain control regimen. Patients who are already taking gabapentinoids are prescribed these, but these are not administered routinely to those who have not been taking these medications due to concerns for respiratory depression. A muscle relaxant, such as methocarbamol, as needed can be a very effective analgesic tool in those patients who exhibit a degree of spasm postoperatively. Oxycodone 5–10 mg q3–4 h prn is prescribed for breakthrough pain, and patients are encouraged to use this if needed (but not routinely) after physical therapy or other activity.
The anesthesia protocol is summarized in Fig. 1.
Fig. 1.
Summary: Anesthesia Protocol.
Positioning protocol
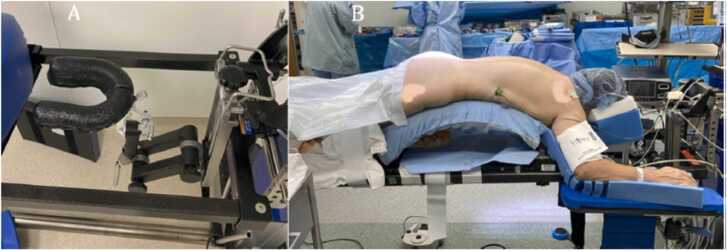
Once spinal anesthesia is injected, the patient is instantly placed in a supine position. For some cases, urinary catheter and neuromonitoring leads are placed. The patient is placed in prone position in preparation of the surgical intervention. Patient is provided adequate support for headrest with face foam (Fig. 8). The patient is allowed to adjust to a comfortable position before surgical preparation of the surgical site. Patient may be provided with active noise canceling headphone or with music previously selected by the patient (De Biase et al., 2021).
Fig. 8.
A) Horseshoe headrest mounted on a Jackson table. B) Patient positioned on a Jackson table. Credit from (De Biase et al., 2021).
Airway management
Patient airway is managed in the prone position, and patient should maintain spontaneous breathing as he/she is mildly sedated. Nasal canula is provided for oxygen delivery. Once airway is stabilized in the prone position, mild sedation is induced with propofol infusion per the anesthesiologist preference. We recommend having a laryngeal mask airway (LMA) and video laryngoscopy equipment ready in case transition to general anesthesia or emergent airway management are necessary (De Biase et al., 2021).
Surgical protocol
Minimally invasive (MIS) or endoscopic surgical technique is the preferred technique for awake spine surgery. MIS is associated with decreased length of stay, shorter surgical duration, reduced blood loss and better patient outcomes when compared to open surgical techniques (Eckman et al., 2014). Though our exact surgical technique varies on the pathology; we generally use tubular or endoscopic methods for decompression. We also utilize tubular or endoscopic methods for our fusion surgeries. We have used ultra-minimally invasive techniques such as percutaneous interbody fusion to minimize tissue destruction (Wang and Grossman, 2016). We have also utilized the robot for placement of percutaneous screws, as the robot and drill obviate the use of malleating screws, which is more comfortable for the patient (Wang et al., 2020; Dalton et al., 2021). As such, we recommend percutaneous transforaminal interbody fusion with robotic navigation and instrumentation as surgical procedure of choice as it is more comfortable for the patient (Wang et al., 2020).
Nursing and physical therapist (PT) roles
Nurses and PT roles are summarized in Table 2.
Table 2.
Nurses and Physical Therapists Roles.
| Preoperative Course | RN
|
| Postoperative Course | RN
|
Preoperative course
The preoperative nurse role is essentially the same in procedures performed under awake vs GA. The nurse role consists of reinforcing the teaching given to the patient by the providers, making sure the patient has a good understanding of the treatment plan, the postoperative expectations and the educational goals. Additionally, a thorough neurological assessment should be performed and documented to monitor patient progress postoperatively.
Postoperative course
Neurologic assessment
Assessment of the lower extremity strength and sensation remains a priority (Strayer, 2005). It is important to compare these findings with baseline preoperative baseline documented in the EMR by the preoperative nurse.
Mobilization
Patients, under the supervision of the PT, will mobilize with assistance shortly after returning from the post-anesthesia unit (PACU). A patient should be instructed to roll onto his/her side, bring the legs down the side of the bed while simultaneously raising the torso up using the arms; this method decreases pain by limiting the amount of twisting/bending (Strayer, 2005).
Pain control
The nurse should follow the pharmacological postoperative anesthesia regimen and should also implement nonpharmaceutical measures to improve comfort. Heat and Cold alternative therapy may be used no more than 20 min per hour for up to 4 consecutive hours (Strayer, 2005). This helps reduce muscle discomfort and speed up recovery.
Urination
Bladder function is assessed with a bladder scanner to ensure adequate emptying. Intake and output monitoring should be carefully monitored as well.
Discharge planning
The nurse should stress the importance of limitations; in fact, patient should be counsel to avoid bending, twisting or lifting more than 10 lbs the first two weeks postoperatively (Strayer, 2005).
Patients should be encouraged to walk as much as tolerated and to increase activity as recommended by the physician plan of care.
Illustrative cases
The following cases demonstrate two examples of ASPS, highlighting the benefits of awake surgery. Case 1 involves a 70-year-male who underwent a L4–5 instrumented percutaneous lumbar interbody fusion, and Case 2 presents a 63-year-old female who underwent a L3-L5 minimally invasive decompression.
Case 1
Preoperative course
A 70-year-old male with a history of a cerebrovascular accident (CVA), on systemic anticoagulation and type 2 diabetes presented with back pain radiating bilaterally to the lower extremities. Pain had been ongoing for years, worsening during periods of standing and walking. Conservative measures (physical therapy and injections) did not provide significant relief.
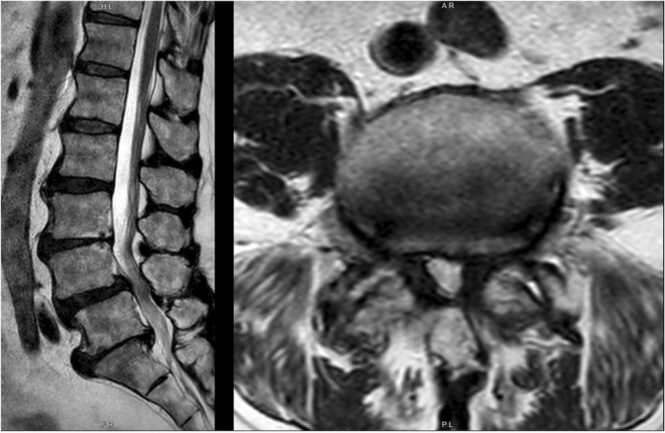
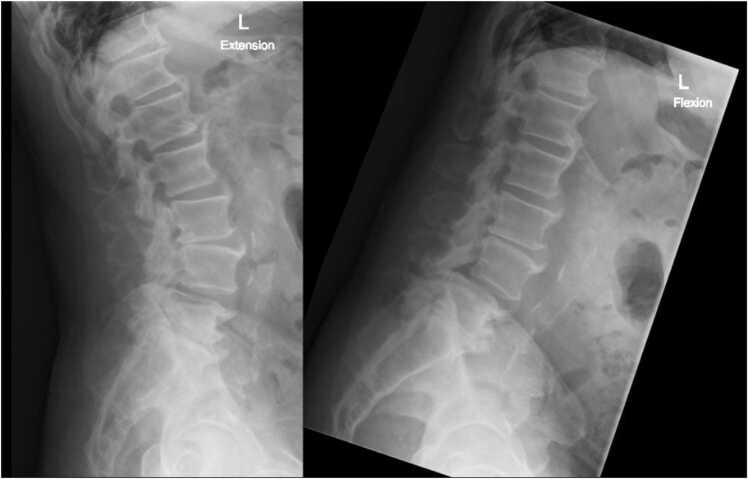
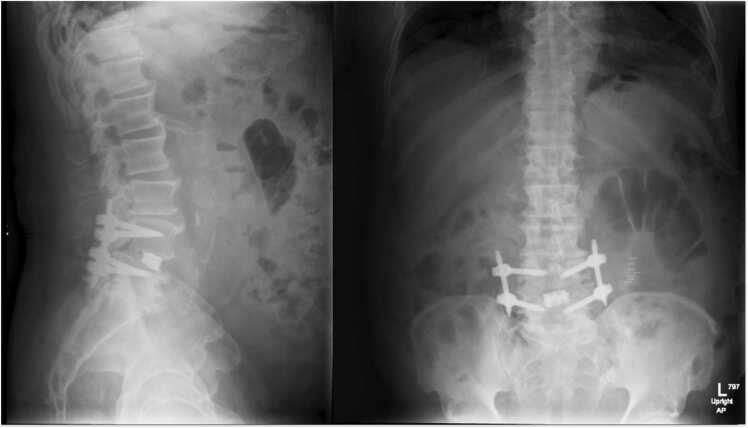
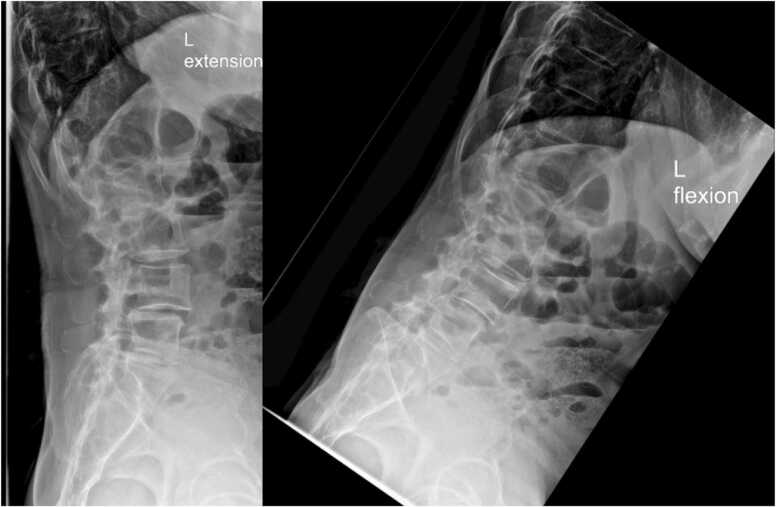
Imaging showed multilevel degenerative disc disease and bilateral facet arthrosis. MRI imaging showed multilevel lumbar spondylolisthesis and facet arthropathy, as well as spinal stenosis at L4-L5 (Fig. 2). X-ray imaging showed L4 and L5 anterolisthesis with little reduction on extension (Fig. 3).
Fig. 2.
Patient one, preoperative T2 weighted MRI. Mid-sagittal (left) and L4-L5 axial (right) view showing L4-L5 spondylolisthesis, facet arthropathy, and spinal stenosis at L4-L5.
Fig. 3.
Patient one, preoperative standing flexion (left) and extension (right) radiographs of the lumbar spine, demonstrating L4 and L5 anterolisthesis with reduction on extension.
Due to failure of more conservative measures and evidence of mobile spondylolisthesis at L4-L5, patient was felt to benefit from L4–5 instrumented lumbar interbody fusion. Due to his significant comorbidities such as history of CVA, it was felt that the patient would be a candidate for ‘awake’ spine surgery.
Operative details
In the preoperative holding area, an erector spinae plane (ESP) block was used to prevent postoperative pain with liposomal bupivacaine 1.3% and liquid bupivacaine (concentration 0.25%) at L2. In the operating room, the neuro-anesthesia team began by administering a spinal block at L3–4 using a single shot of 0.5% bupivacaine resulting in sensory loss and paresis below approximately T4 level.
The patient was then positioned prone on a Jackson bed. Percutaneous screws were placed bilaterally at L4 and L5 with robotic-assistance. Using robotic-assistance, a dilator was placed into the L4–5 disc space through Kambin’s triangle (Dalton et al., 2021), followed by a kirschner-wire (k-wire) placement into the disc, which was widened until an 8 mm portal could be placed. Discectomy was then done using a variety of instruments and then finally, an expandable cage was placed. After posterolateral fusion and bilateral rod placement, proper placement was confirmed with fluoroscopy.
The wound was then cleaned, closed, and covered. Blood loss was estimated to be less than 100 mL. There were no intraoperative complications. During the procedure, electromyography (EMG) monitoring of both lower extremities was performed. EMG activity was quiet throughout the procedure without any spontaneous or neurotonic activity observed.
Post-operative course
In the post anesthesia care unit (PACU), the patient had good pain control with oral pain medications, requiring only one dose of acetaminophen (975 mg). He tolerated regular diet and was cleared by physical and occupational therapy for same day discharge. Immediate postoperative x-rays revealed proper placement of all instrumentation and reduction of L4–5 spondylolisthesis (Fig. 4).
Fig. 4.
Patient one, post-operative radiograph demonstrating reduction of L4–5 spondylolisthesis.
At his six week follow up, he reported cessation of preoperative symptoms and began physical therapy. He continued to be doing well and to have symptom relief at his three months follow up clinic visit.
Case 2
Preoperative course
A 63-year-old female presented with bilateral lower extremity pain and paresthesia, worse in the right leg. This pain was significantly impairing her ability to stand and walk and had been worsening for six years. Conservative management did not provide adequate relief.
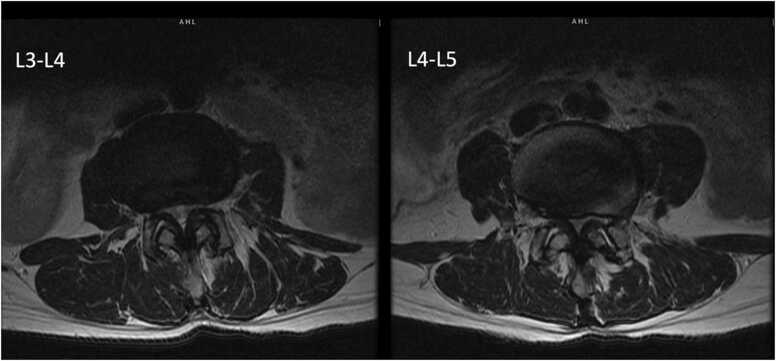
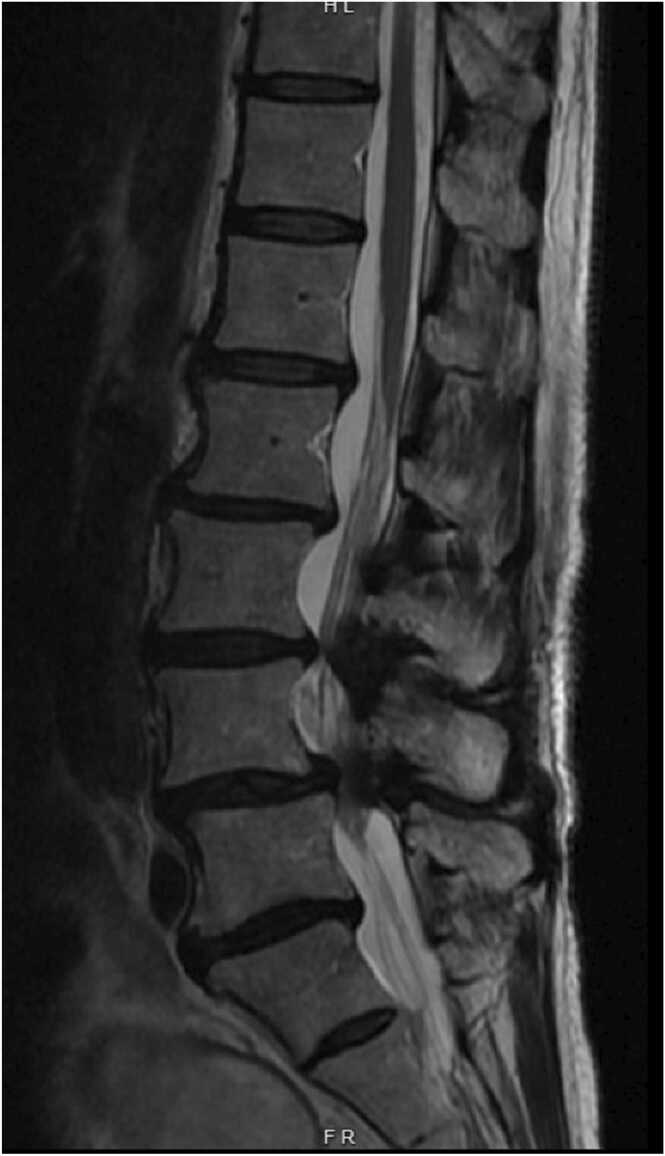
MRI imaging showed multi-level spinal stenosis in the lumbar spine, most prominently at the L3-L4 and L4-L5 levels (Fig. 5, Fig. 6). X-ray imaging showed anterolisthesis of L3 on L4 and multilevel degenerative disc disease throughout the cervical, thoracic, and lumbar spine (Fig. 7).
Fig. 5.
Patient two, preoperative axial T2 weighted MRI showing multi-level spinal stenosis most prominently at the L3-L4 and L4–5 levels.
Fig. 6.
Patient two, preoperative mid-sagittal T2 weighted MRI.
Fig. 7.
Patient two, preoperative extension (left) and flexion (right) radiograph showing anterolisthesis of L3 on L4 with no dynamic component and multilevel degenerative disc disease.
Given inadequate relief from conservative measures, we thought that the patient could benefit from surgery. Though she had radiographic evidence of spondylolisthesis, due to the fact that her major complaints were neurogenic claudication in nature, we thought she would benefit from a minimally invasive L3–5 decompression. Due to her history of anesthetic complications, we also thought she would benefit from ‘awake surgery’.
Operative details
The patient underwent a L3-L5 minimally invasive decompression in an ambulatory surgical center. In the preoperative holding area, an ESP block was done using a 0.375% bupivacaine injection at L3 to provide post-operative pain management. A spinal block using 0.5% bupivacaine at the L2–3 level was performed that resulted in sensory loss and paresis below approximately the T4 level.
The patient was positioned prone on a Wilson frame. After an incision was made slightly off midline, a dilator was placed near the inferior lamina of L4 and dilated. The ligamentum flavum was then identified and dissected. The dura and theca were visualized, and by rolling the patient away, the contralateral decompression was completed. This was repeated at the L3 level. The wound was then cleaned, closed, and covered. Minimal blood loss was noted and there were no intraoperative complications.
Post-operative course
The patient was monitored after the surgery and required no oral pain medications. She was discharged home the same day. Six weeks post-operatively she reported no pain, complete abatement of symptoms, and was doing well.
Discussion
The development of an awake spine surgery program (ASPS) is a challenging one but one that has many advantages to patients and healthcare systems.
ASPS offers several benefits to the patients. The greatest advantage of awake spine surgery is the avoidance of general anesthesia and its associated risks and negative patient outcomes (Kolcun et al., 2019, Fiani et al., 2021, Kolcun et al., 2019). There is an increasing appreciation of the side effects of general anesthesia such as opioid use and cardiopulmonary complications (Fiani et al., 2021, Meng et al., 2017). These complications of anesthesia have been reported to be worse in elderly patients (Fiani et al., 2021, Meng et al., 2017). With the awake alternative, these side effects are significantly reduced, making elderly patients’ prime candidates for awake spine surgery (Fiani et al., 2021). In our two illustrative case examples, neither patient experienced side effects or complications. Patients had a good pain control with no opioid use postoperatively.
Awake spine surgeries have been associated with reduced LOS and healthcare costs, and reduced healthcare associated complications (HAC) such as surgical site infections (SSI) (Meng et al., 2017). Consistent with this, the two patients presented here were discharged home on the day of surgery.
Another advantage of ASPS is its ability to increase the pool of patients eligible for spinal procedures. In fact, it has been shown that patients with multiples comorbidities, as described by an American Society of Anesthesiologist physical status (ASA ≥3), were at higher risk of postoperative complications for spinal procedures under general anesthesia (Khan et al., 2014). However, (Khan et al., 2014) reported that patients with multiple comorbidities (ASA≥3) were low risk candidates for ASPS with outcomes as good as those with an ASA< 3 and undergoing general anesthesia. In our study, the patients were high risk candidates for GA due to their multiple comorbidities including cardiovascular disease and history of anesthetic complications. The combination of ASPS and ERAS protocols is particularly helpful, as it allows for the best preoperative, intraoperative and postoperative care for patients.
Limitations
The use of spinal anesthesia expands the patient population eligible for spinal surgery; however, it is still limited by the same contraindications of any surgical procedure (Fiani et al., 2021, Meng et al., 2017). The main exclusions to spinal anesthesia are patient refusal, coagulopathy or anticoagulation that would preclude safe neuraxial procedures (Horlocker et al., 2010), infection near the surgical site, or cardiovascular contraindications (ie patients who cannot withstand spinal anesthesia-induced reductions in systemic vascular resistance and cardiac preload). Morbidly obese patients, patients with COPD, and patients with obstructive sleep apnea are at risk for pulmonary complications during the procedure, and spinal anesthesia may be contraindicated due to risks associated with the potential need for emergent airway management in these patients (Fiani et al., 2021). Additionally, general anesthesia is preferred over local anesthesia in patients under 15 years of age or in individuals who may become restless or agitated over the course of a long procedure (over 90 min) (Fiani et al., 2021). Since ASPS requires the operation to be performed in a short amount of time, the number of surgical procedures available is reduced. Chronic alcohol consumption and administering spinal anesthesia in an acute setting increases the rate of a hypotensive episode by 200% compared to that seen in patients with no risk factors (Fiani et al., 2021). General anesthesia is also preferred over local anesthesia when the patient has a low pain tolerance, high tolerance to the anesthesia, or a high level of anxiety (Fiani et al., 2021).
There have been a few studies trying to compare the results of GA vs SA in spine surgery; most have been meta-analyses (Perez et al., 2021). It will be important to have more robust studies such as matched cohort studies or randomized control studies. Importantly, one of the main aspects that may be lost in the more traditional methods of clinical statistical analyses is the concept of “survival bias” where subjects will not be analyzed unless they “survive” an initial selection phase. In the case of spinal anesthesia, it allows surgeons to offer spine surgery to patients that are not candidates for general anesthesia.
Conclusion
Our preliminary experience demonstrated that starting an ASPS program is feasible, and may improve clinical outcomes. Here we have provided a template for starting an awake spine program and shows its potential effects on clinical outcomes following spine surgery. The patients described in the cases were not good candidates for surgery under GA, and they benefited from ASPS. ASPS is a promising new technique which is effective at reducing LOS, HAC, cost of surgery and faster recovery and rehabilitation. Additionally, ASPS is an important tool in the hands of surgeons in their fight against the “opioid epidemic.” Despite its various advantages, randomized clinical trials studies with appropriately large sample sizes are necessary to provide high quality evidence for the safety, efficacy, and cost effectiveness of ASPS.
Ethical statement
The authors declare that the work described has not involved experimentation on humans or animals. Additionally, the authors declare that this report does not contain personal information that could lead to the identification of the patients.
Conflicts of Interest
Romaric Waguia, Elisabeth Kakmou, David Sykes, Margot Kelly-Hedrick, Hiji Fady, Norah Foster: None. Muhammad Abd-El-Barr: Consultant for Spineology, Depuy Synthes.
References
- Ayhan E., Akaslan F. Patients’ perspective on carpal tunnel release with WALANT or intravenous regional anesthesia. Plast. Reconstr. Surg. 2020;145(5):1197–1203. doi: 10.1097/PRS.0000000000006741. [DOI] [PubMed] [Google Scholar]
- Balentine C.J., Meier J., Berger M., et al. Using local rather than general anesthesia for inguinal hernia repair is associated with shorter operative time and enhanced postoperative recovery. Am. J. Surg. 2021;221(5):902–907. doi: 10.1016/j.amjsurg.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balentine C.J., Meier J., Berger M. Using local anesthesia for inguinal hernia repair reduces complications in older patients. J. Surg. Res. 2021;258:64–72. doi: 10.1016/j.jss.2020.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud M.C., Reilly C.S. Adverse effects of general anaesthetics. Drug Saf. 1992;7(6):434–459. doi: 10.2165/00002018-199207060-00005. [DOI] [PubMed] [Google Scholar]
- Chen H.T., Tsai C.H., Chao S.C., Kao T.H., Chen Y.J., Hsu H.C., Shen C.C., Tsou H.K. Endoscopic discectomy of L5-S1 disc herniation via an interlaminar approach: prospective controlled study under local and general anesthesia. Surg. Neurol. Int. 2011;2:93. doi: 10.4103/2152-7806.82570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CMS Adult Elective Surgery and Procedures Recommendations: limit all non-essential planned surgeries and procedures, including dental, until further notice. March 18 2020. 〈https://www.cms.gov/files/document/31820-cms-adult-elective-surgery-and-procedures-recommendations.pdf〉. Accessed April 1, 2020.
- Dalton T., Sykes D., Wang T.Y., et al. Robotic-assisted trajectory into Kambin’s triangle during percutaneous transforaminal lumbar interbody fusion-initial case series investigating safety and efficacy. Oper. Neurosurg. 2021 doi: 10.1093/ons/opab325. [DOI] [PubMed] [Google Scholar]
- De Biase G., Bechtle P., Leone B., Quinones-Hinojosa A., Abode-Iyamah K. Awake minimally invasive transforaminal lumbar interbody fusion with a pedicle-based retraction system. Clin. Neurol. Neurosurg. 2021;200 doi: 10.1016/j.clineuro.2020.106313. Epub 2020 Oct 23. [DOI] [PubMed] [Google Scholar]
- Dietz N., Sharma M., Adams S. Enhanced Recovery After Surgery (ERAS) for spine surgery: a systematic review. World Neurosurg. 2019;130:415–426. doi: 10.1016/j.wneu.2019.06.181. [DOI] [PubMed] [Google Scholar]
- Eckman W.W., Hester L., McMillen M. Same-day discharge after minimally invasive transforaminal lumbar interbody fusion: a series of 808 cases. Clin. Orthop. Relat. Res. 2014;472(6):1806–1812. doi: 10.1007/s11999-013-3366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsarrag M., Soldozy S., Patel P. Enhanced recovery after spine surgery: a systematic review. Neurosurg. Focus. 2019;46(4) doi: 10.3171/2019.1.FOCUS18700. [DOI] [PubMed] [Google Scholar]
- Fiani B., Reardon T., Selvage J., Dahan A., El-Farra M.H., Endres P., Taka T., Suliman Y., Rose A. Awake spine surgery: an eye-opening movement. Surg. Neurol. Int. 2021;12:222. doi: 10.25259/SNI_153_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlocker, T.T., Wedel, D.J., Rowlingson, J.C., et al., 2010. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg. Anesth. Pain Med. 35 (1) 64–101. doi: 10.1097/aap.0b013e3181c15c70. [DOI] [PubMed]
- Jellish W.S., Thalji Z., Stevenson K., Shea J. A prospective randomized study comparing short- and intermediate-term perioperative outcome variables after spinal or general anesthesia for lumbar disk and laminectomy surgery. Anesth. Analg. 1996;83:559–564. doi: 10.1097/00000539-199609000-00021. [DOI] [PubMed] [Google Scholar]
- Kahveci K. Perioperative outcome and cost-effectiveness of spinal versus general anesthesia for lumbar spine surgery. Neurol. Neurochir. Pol. 2014 doi: 10.1016/j.pjnns.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Kai-Hong Chan A., Choy W., Miller C.A., Robinson L.C., Mummaneni P.V. A novel tech- nique for awake, minimally invasive transfor- aminal lumbar interbody fusion: technical note. Neurosurg. Focus. 2019;46(4) doi: 10.3171/2019.1.FOCUS18510. [DOI] [PubMed] [Google Scholar]
- Khan M.B., Kumar R., Enam S.A. Thoracic and lumbar spinal surgery under local anesthesia for patients with multiple comorbidities: a consecutive case series. Surg. Neurol. Int. 2014;5(Suppl 3):S62–S65. doi: 10.4103/2152-7806.130669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattab M.F.M., Sykes D.A.W., Abd-El-Barr M.M., Waguia R., Montaser A., Ghamry S.E., Elhawary Y. Spine surgery under awake spinal anesthesia: an Egyptian experience during the COVID-19 pandemic. Neurosurg. Focus. 2021;51(6) doi: 10.3171/2021.9.FOCUS21456. [DOI] [PubMed] [Google Scholar]
- Kolcun J.P.G., Brusko G.D., Basil G.W., Epstein R., Wang M.Y. Endoscopic transforaminal lumbar interbody fusion without general anesthesia: operative and clinical outcomes in 100 con- secutive patients with a minimum 1-year fol- low-up. Neurosurg. Focus. 2019;46(4) doi: 10.3171/2018.12.FOCUS18701. [DOI] [PubMed] [Google Scholar]
- Kolcun J.P.G., Brusko G.D., Basil G.W., Epstein R., Wang M.Y. Endoscopic transforaminal lumbar interbody fusion without general anesthesia: operative and clinical outcomes in 100 consecutive patients with a minimum 1-year follow-up. Neurosurg. Focus. 2019;46(4) doi: 10.3171/2018.12.FOCUS18701. [DOI] [PubMed] [Google Scholar]
- Kurtzman J.S., Etcheson J.I., Koehler S.M. Wide-awake local anesthesia with no tourniquet: an updated review. Plast. Reconstr. Surg. Glob. Open. 2021;9(3) doi: 10.1097/GOX.0000000000003507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde D.H. Latest advances in wide awake hand surgery. Hand Clin. 2019;35(1):1–6. doi: 10.1016/j.hcl.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Lessing N.L., Edwards C., Brown C.H., Ledford E.C., Dean C.L., Lin C., Edwards C. Spinal anesthesia in elderly patients undergoing lumbar spine surgery. Orthopedics. 2017;40(2):e317–e322. doi: 10.3928/01477447-20161219-01. [DOI] [PubMed] [Google Scholar]
- Letchuman V., Agarwal N., Mummaneni V.P. Awake spinal surgery: simplifying the learning curve with a patient selection algorithm. Neurosurg. Focus. 2021;51(6) doi: 10.3171/2021.9.FOCUS21433. [DOI] [PubMed] [Google Scholar]
- Martin B.I., Mirza S.K., Spina N., Spiker W.R., Lawrence B., Brodke D.S. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine. 2019;44(5):369–376. doi: 10.1097/BRS.0000000000002822. [DOI] [PubMed] [Google Scholar]
- McLain R.F., Kalfas I., Bell G.R., Tetzlaffj E., Yoon H.J., Rana M. Comparison of spinal and general anesthesia in lumbar laminectomy surgery: a case- controlled analysis of 400 patients. J. Neurosurg. Spine. 2005;2:17–22. doi: 10.3171/spi.2005.2.1.0017. [DOI] [PubMed] [Google Scholar]
- Meier J., Berger M., Hogan T., et al. Using local rather than general anesthesia for inguinal hernia repair may significantly reduce complications for frail Veterans. Am. J. Surg. 2021;222(3):619–624. doi: 10.1016/j.amjsurg.2021.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J., Berger M., Hogan T.P., et al. Local anesthesia is associated with fewer complications in umbilical hernia repair in frail veterans. J. Surg. Res. 2021;266:88–95. doi: 10.1016/j.jss.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng T., Zhong Z., Meng L. Impact of spinal anaesthesia vs. general anaesthesia on peri-operative outcome in lumbar spine surgery: a systematic review and meta-analysis of randomised, controlled trials. Anaesthesia. 2017;72(3):391–401. doi: 10.1111/anae.13702. Epub 2016 Oct 22. [DOI] [PubMed] [Google Scholar]
- Miller L.E., Bhattacharyya S., Pracyk J. Minimally invasive versus open transforaminal lumbar interbody fusion for single-level degenerative disease: a systematic review and meta-analysis of randomized controlled trials. World Neurosurg. 2020;133:358–365.e4. doi: 10.1016/j.wneu.2019.08.162. [DOI] [PubMed] [Google Scholar]
- O’Lynnger T.M., Zuckerman S.L., Morone P.J., Dewan M.C., Vasquez-Castellanos R.A., Cheng J.S. Trends for spine surgery for the elderly: implications for access to healthcare in North America. Neurosurgery. 2015;77(Suppl 4):S136–S141. doi: 10.1227/NEU.0000000000000945. [DOI] [PubMed] [Google Scholar]
- Patel A.A., Zfass-Mendez M., Lebwohl N.H., Wang M.Y., Green B.A., Levi A.D., Vanni S., Williams S.K. Minimally invasive versus open lumbar fusion: a comparison of blood loss, surgical complications, and hospital course. Iowa Orthop. J. 2015;35:130–134. [PMC free article] [PubMed] [Google Scholar]
- Perez-Roman R.J., Govindarajan V., Bryant J.P., Wang M.Y. Spinal anesthesia in awake surgical procedures of the lumbar spine: a systematic review and meta-analysis of 3709 patients. Neurosurg. Focus. 2021;51(6) doi: 10.3171/2021.9.FOCUS21464. [DOI] [PubMed] [Google Scholar]
- Rivkin A., Rivkin M.A. Perioperative nonopioid agents for pain control in spinal surgery. Am. J. Health Syst. Pharm. 2014;71(21):1845–1857. doi: 10.2146/ajhp130688. [DOI] [PubMed] [Google Scholar]
- Soh T., Ho S., Yap W., Oh J.Y. Spine surgery and COVID-19: challenges and strategies from the front lines. J. Bone Jt. Surg. Am. 2020;102(12) doi: 10.2106/JBJS.20.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer A. Lumbar spine: common pathology and interventions. J. Neurosci. Nurs. 2005;37(4):181–193. doi: 10.1097/01376517-200508000-00003. 〈http://proxy.campbell.edu/login?url=https://www.proquest.com/scholarly-journals/lumbar-spine-common-pathology-interventions/docview/219186681/se-2?accountid=9858〉 [DOI] [PubMed] [Google Scholar]
- Turcotte J.J., Gelfand J.M., Jones C.M., Jackson R.S. Development of a low-resource operating room and a wide-awake orthopedic surgery program during the COVID-19 pandemic. Surg. Innov. 2021;28(2):183–188. doi: 10.1177/15533506211003530. [DOI] [PubMed] [Google Scholar]
- Wang M.Y., Grossman J. Endoscopic mini- mally invasive transforaminal interbody fusion without general anesthesia: initial clinical experience with 1-year follow-up. Neurosurg. Focus. 2016;40(2) doi: 10.3171/2015.11.FOCUS15435. [DOI] [PubMed] [Google Scholar]
- Wang M.Y., Grossman J. Endoscopic minimally invasive transforaminal interbody fusion without general anesthesia: initial clinical experience with 1-year follow-up. Neurosurg. Focus. 2016;40(2) doi: 10.3171/2015.11.FOCUS15435. [DOI] [PubMed] [Google Scholar]
- Wang M.Y., Tessitore E., Berrington N., Dailey A. Introduction. Enhanced recovery after surgery (ERAS) in spine. Neurosurg. Focus. 2019;46(4) doi: 10.3171/2019.1.FOCUS1957. [DOI] [PubMed] [Google Scholar]
- Wang Timothy Y., Mehta Vikram A., Sankey Eric W., Than Khoi D., Goodwin C.Rory, Karikari Isaac O., Gupta Dhanesh K., Abd-El-Barr Muhammad M. Awake percutaneous transforaminal lumbar interbody fusion with expandable cage and robotic-assisted navigation and instrumentation: case report and review of literature, Interdisciplinary. Neurosurgery. 2020;20 [Google Scholar]
- Xie Q., Zhang J., Lu F., Wu H., Chen Z., Jian F. Minimally invasive versus open transforaminal lumbar interbody fusion in obese patients: a meta-analysis. BMC Musculoskelet. Disord. 2018;19(1):15. doi: 10.1186/s12891-018-1937-6. [DOI] [PMC free article] [PubMed] [Google Scholar]