Abstract
Background
Degeneration of Lumbar muscle in chronic low back pain (CLBP) is characterized by an increase in fat infiltration of paraspinal muscle, decrease in the cross-sectional area (CSA) of lumbar multifidus muscle (LMM) and increased thickness of Thoracolumbar fascia (TLF) by 25%. The study objective is to compare the effects of yoga and dynamic neuromuscular stabilization (DNS) exercise on CSA, fat infiltration of LMM with magnetic resonance imaging (MRI), and TLF thickness using musculoskeletal ultrasound imaging (MSK-USI) in CLBP.
Methods
One hundred and forty-four participants with CLBP, which persisted longer than three months, will be recruited for this trial. Both group interventions focused on LMM. The experimental group will receive structured yoga sessions, and the Control Group will receive exercise based on DNS. In each group, exercises will be performed for 3–5 days/week and progressed for 12 weeks. Baseline data will be collected, followed by the recording of primary outcome measure (MRI) and secondary outcome measures (MSK-USI, Oswestry disability index, visual analogue score, optimism, self-efficacy, mood, physical activity, fear of movement, pain catastrophizing, and coping) at baseline and the end of 12th weeks. The normality of data will be verified. Based on the data distribution, within-group analysis and between-group analysis will be performed.
Discussion
This will be the first RCT to compare the effect of yoga and DNS exercise among chronic low back pain participants. This will provide evidence of these interventions' impact on CSA, fat infiltration of LMM, and thickness of TLF in CLBP.
Registration number
CTRI/2021/08/035984 (This trial was registered prospectively).
Keywords: Chronic low back pain, Dynamic neuromuscular stabilization exercise, Multifidus, Thoracolumbar fascia, Yoga
1. Introduction
Chronic low back pain (CLBP) is defined as “pain and/or discomfort localized below the costal margin and above the gluteal folds with possible radiation to the posterior thigh not extending below the knee” [1]. Low back pain (LBP) is a common musculoskeletal condition, with prevalence rates ranging from 12 to 33%. In the Indian population, the prevalence of LBP ranges from 6.2% to 92% [2].
Lumbar paraspinal muscle degeneration is distinguished by decreased cross-sectional area (CSA) and increased fat content [3,4]. Many studies have previously compared CSA of the lumbar muscles between patients with chronic low back pain and healthy asymptomatic controls [5]. According to six studies, the multifidus (MF) muscle has a reduced cross-sectional area than healthy controls. Hides et al. found MF muscle unilateral atrophy in patients with LBP, with the atrophic alteration confined to one vertebral level [[6], [7], [8], [9], [10], [11]]. Two studies found that MF muscle size is reduced in non-specific CLBP, although CSA of ES is unaltered [[12], [13], [14]]. In people with chronic low back pain, the cross-section area of paraspinal muscles (MF and erector spinae) was smaller in two systematic reviews [15,16]. In a recent systematic review, changes in the paraspinal muscles and multifidus CSA were negatively linked with and predictive LBP for up to 12 months [17,18].
There are a variety of exercises that focus on the stability of these muscles and have shown to be helpful in the treatment of CLBP. MRI post-exercise has indicated alterations in the lumbar muscle CSA in CLBP participants [11,15,19,20]. Thoraco lumbar fascia (TLF) is densely innervated with afferent free nerve endings, including nociceptive, resulting in pain sources [21,22]. CLBP was associated with a higher average density of myofibroblasts (associated with tissue repair function) in the lumbar thoracolumbar fascia [23]. According to recent studies, the patients with CLBP have TLF thickened by 25% or more [24].
According to emerging scientific literature worldwide, Yoga has become a popular mind-body therapy for CLBP [25]. Yoga uses a multidimensional approach that includes activities for the body (postures), the breath (breathing methods), and the mind (meditation and relaxation techniques). A recent study has validated an integrated yoga module comprising yoga postures and breathing for CLBP [26]. However, scientific evidence of the effect of yoga postures on the pathology of LBP is lacking. According to a Cochrane review on Yoga for persistent low back pain, there is low-to-moderate certainty evidence that Yoga produces low to moderate improvements in back-related function after three and six months when compared to non-exercise controls. At three and six months, Yoga may be slightly more beneficial for pain. However, the effect size did not exceed predetermined clinical significance levels. It's unclear whether Yoga and other back-related exercises differ in terms of usefulness [25].
Dynamic Neuromuscular Stabilization (DNS) is a technique used to provide dynamic stability to the muscle. It is a manual and rehabilitative approach to optimize the movement system based on developmental kinesiology (DK) [27]. According to the DNS method, every joint position relies on stabilising muscle function and coordination of both local and distant muscles to ensure the neutral or centered position of joints in the kinetic chain. Its goal is to optimize the distribution of internal muscle forces acting on each spinal segment [27]. Magnetic Resonance Imaging (MRI) offers a non-invasive method that possesses outstanding spatial resolution and investigates these muscles [28]. However, no studies have shown or assessed fat infiltration of multifidus post-yoga or DNS postures. Hence, the current study aims to evaluate the effect of yoga and DNS exercise on fat infiltration of lumbar multifidus using MRI. There is a dearth of literature on the effect of yoga and DNS posture on the atrophy of MF in CLBP. This study can help us understand the effect of yoga and DNS posture on atrophic alteration using gold standard magnetic resonance imaging (MRI), thus enhancing the understanding of treatment approaches towards chronic low back pain.
2. Methods/design
2.1. Overview
We are conducting this two-arm, parallel randomized controlled trial assessing the effect of Yoga and Dynamic Neuromuscular Stabilization exercises among chronic low back pain individuals. We hypothesize that participants randomized in the yoga group will have a better outcome in terms of MRI CSA of LMM and MSK USI thickness of TLF than participants in the dynamic neuromuscular stabilization exercise group. We also hypothesize that participants in the yoga group will have lower pain intensity, disability, fear of movement, pain catastrophizing, and higher optimism, self-efficacy, physical activity, and coping strategy than DNS group participants.
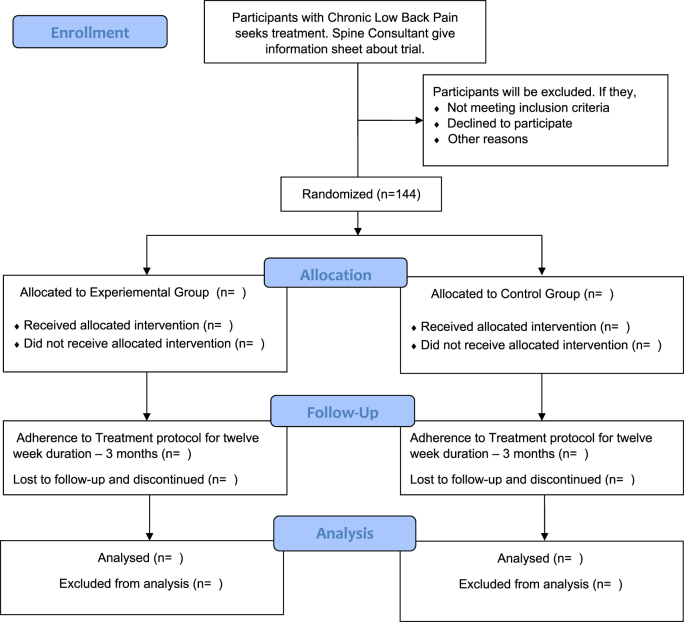
CLBP individuals who seek treatment from the Spine care center, Manipal Hospitals, Bangalore, will be screened by Spine Consultant. Spine Consultant will give trial information and refer the individual to Outpatient – Department of Physiotherapy, Manipal Hospitals, Bangalore. The Project Research Fellow will screen the eligibility of participants who are referred for trial. The Principal Investigator will explain the trial details of individuals, and written informed consent will be obtained. Once the participant signature is received in the consent form, a copy of the Informed consent form will be given to the patient for their reference. An eligible individual will first complete the demographic details and all questionnaires under the supervision of an experienced physiotherapist who is unaware of the trial. While filling the questionnaire, the therapist's supervise will not interfere or influence the participant in any way; Yet may help participants if they have any difficulty or queries in filling the form [56–63]. Once the form is filled, MRI and Musculoskeletal Ultrasound Imaging (MSK-USI) appointment will be fixed. MRI and MSK-USI will be performed by the experienced Radiologist who is unaware of the trial. Participants are reassessed at the end of the 12th week. This study trial will follow (Fig. 1) flowchart. The participants will be randomized into two groups in a 1:1 ratio. The intervention group will receive a yoga for three months, and the control group will receive a standard of care as per dynamic neuromuscular stabilization exercise alone.
Fig. 1.
Overview of trial design – CONSORT flow chart.
This protocol was approved by Institutional Review Board (IRB), Scientific Committee (SC), and Institutional Ethics Committee (IEC) of Manipal Hospital, Bangalore. The trial is registered with Clinical Trial Registry India (CTRI) before the participant's enrollment. The trial progression, Participants' safety, Documentation of form, and data entry will be reviewed, monitored every six months by the Data and Safety Monitoring Committee (DSMC).
2.2. Objectives
-
a)
To compare the effects of Yoga on fat infiltration of lumbar multifidus with DNS postures using Magnetic resonance imaging
-
b)
To compare the effects of Yoga on the thoracolumbar fascia with DNS postures using Ultrasound imaging
-
c)
To compare the effect of yoga and DNS postures on Oswestry disability index and Visual Analogue scale
-
d)
To compare the effect of yoga and DNS postures on optimism, self-efficacy, mood, physical activity, fear of movement, pain catastrophizing, and coping.
2.3. Study timeline, protocol modification
This Three-year study started on August 1, 2021, with patient recruitment initiated in September. The first enrolled participant in this trial was on September 15, 2021. No changes have been applied in study trial protocol version 1.0 Jan 2021 after funding agency, DSMC, IRC, SC, IEC, and CTRI approval. Any future study trial protocol changes/Amendments will be submitted to the funding agency, DSMC, IRC, SC, IEC, CTRI. Their review and approval will be obtained. Accordingly, the study trial protocol amendments will be done, and protocol version number, month, and year will be changed.
2.4. Study setting
Project Research Fellow will recruit the CLBP participants from Outpatient service- Department of physiotherapy, Manipal Hospitals, Bangalore. All CLBP participants will be initially assessed and referred by Spinal Consultant to the Physiotherapy department after ruling out red flags and absolute contraindication of exercises.
2.5. Study participants, eligibility criteria
The study participants are English-speaking adults who present to the spine care center with a primary complaint of low back pain for more than three months. Spine Consultant will label CLBP diagnosis based on International Classification of Diseases, Ninth Revision (ICD-9) or the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes. Participants referred to Physiotherapy OPD from Spine Care Center will be eligible for the study to fulfill the following criteria [[56], [57], [58], [59], [60], [61], [62]].
2.5.1. Inclusion criteria
Subjects diagnosed with chronic low back pain (>12 weeks).
Age between 18 and 45 years.
2.5.2. Exclusion criteria
Inflammatory pathology of the lumbar spine.
Lumbar radiculopathy.
Subjects who have recent spinal fracture or surgery.
Have lumbar pain of traumatic origin.
If they are current or past smokers or tobacco chewers.
Have participated in Yoga or lumbar exercise program in the past three months.
Pain VAS more than 4/10.
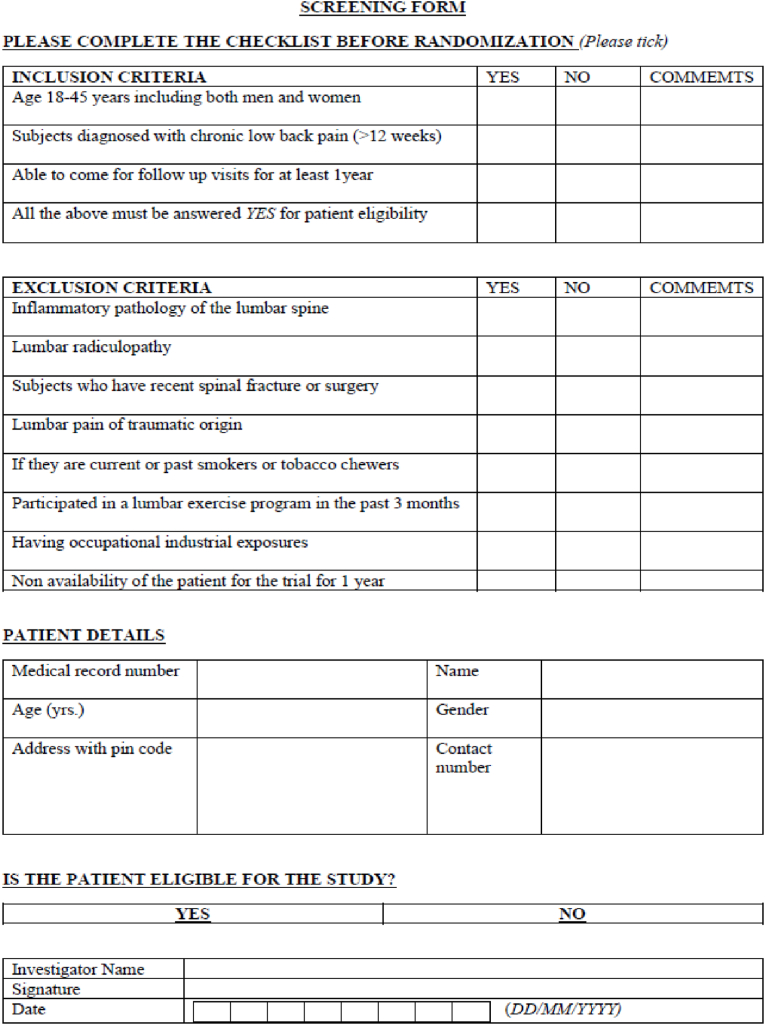
All the CLBP individuals presenting to OPD physiotherapy will be screened for eligibility using the screening form (Fig. 2). Based on the response, they will be recruited for the trial.
Fig. 2.
Screening form.
2.6. Study recruitment procedure
Participants are recruited for the study through the Spine care center and Physiotherapy OPD. The study coordinator will screen interested prospective Participants in person after they have provided verbal consent. To complete the Participants Informed Consent form, demographic information, and all baseline outcome measures, including MRI and MSK-USI, interested and eligible participants will be scheduled for an in-person appointment with the study coordinator. Once participants complete all baseline assessments will be randomized into one of the two arms intervention groups. On the morning of the MRI and MSK-USI appointment with the Radiologist, the participant will be given a reminder phone call and message by the study coordinator. Upon completion of baseline assessment appointment for exercise session cabin, timing will be fixed with study physiotherapist and informed to the participant [56–63]. Those ineligible or who decline to participate in this trial are requested to provide their basic demographic details and other clinical information (gender, age, CLBP duration, ODI, VAS). They will be provided with standard care CLBP of treatment irrespective of ineligibility or no interest in trial enrollment.
2.7. Study randomization and blinding
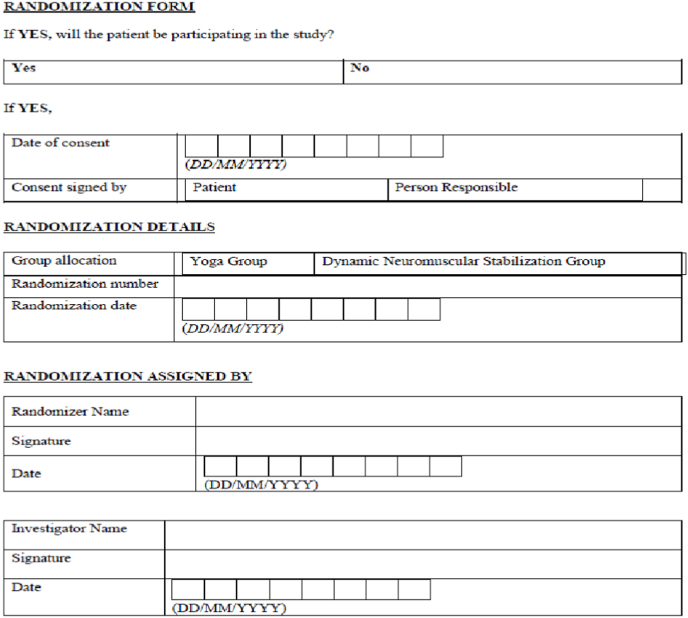
Participants are randomly assigned to one of two arms (Yoga or DNS) in a 1:1 ratio using a computer-generated random size approach developed by the study statistician. Randomization will be performed by using sealedenvelope.com. Patients will be randomized in block sizes of 8, 10 with 17 block identifiers. The unique number will also be generated along with the randomization sequence. The results of randomization will be printed on the result sheet. The result sheet consists of block identifier number, block size number, group allocation with a unique number. This result sheet will be sealed in an opaque envelope (Fig. 4). The study statistician will prepare all of these sealed envelopes, which will be handed to participants in sequential order by the study coordinator.
Fig. 4.
Randomization sealed envelope.
Once participants complete all outcome measure baseline assessment, the therapist who will treat the patient will open the opaque sealed envelope. The therapist will note the details of group allocation in the randomization register and form (Fig. 3). In the record following more information will be documented: date, time of opening, patient ID, name, randomizer with name and signature, witness name, and signature. The sealed opaque envelope will be preserved for documentation purposes. The group allocation is kept a secret from the Principal Investigator (PI), Statistical Analysts, and Qualitative Data Analysts until the final analysis is done. The primary outcome measure MRI and secondary outcome MSK-USI will be recorded by a Radiologist with 20 years of experience, blinded to the allocation of treatment groups. All other Questionnaires and baseline assessments will be documented and collected from participants by a Physiotherapist with six years of experience who is unaware of trial objectives and intervention group allocation [[56], [57], [58], [59], [60], [61], [62]]. The participants will be blinded to the group they will be allocated. It is not practically possible to blind the therapist to the intervention groups.
Fig. 3.
Randomization form.
2.8. Study interventions
Each participant will be given standard physiotherapy care for pain intensity management. Once the participant's pain scores are less than 4 out of 10 in VAS, then the participant will be randomized in any one of two intervention groups. Both therapies have the same duration, setting, and participant/therapist contact time. The choice of the standard physiotherapy pain management care will be according to the choice of the treating physiotherapist and participant requirement.
2.8.1. Yoga group – experimental group
The yoga group will receive structured sessions involving shithilikarana vyayama, pavanmukthasana, shashankasana breathing, ardhakati chakrasana, Uttita Parsvakonasana, parivritta trikosana, Vakrasana. Each Posture will be performed for 30–60 s for five reps, active movement 5–10 reps for 30 min. Pranayama involving Vibhagya pranayama, Nadi shuddi, Bhramari for 5–10 min and Shavasana: 5–10 min under supervision for two days/week and unsupervised for two days/week for 12 weeks [[29], [30], [31], [32], [33], [34], [35]].
2.8.2. Dynamic neuromuscular stabilization exercise group – control group
The dynamic neuromuscular stabilization group will receive developmental postures based on developmental kinesiology. It will involve a supine 3month Position, side-lying Position, Quadruped, oblique sitting, bear position, squat Position. Each position will be performed for 30–60 s for five reps, active exercise 5–10 reps under supervision for two days/week, and unsupervised for two days/week for 12 weeks [[36], [37], [38], [39], [40], [41], [42], [43], [44]].
For more details of Yoga and DNS exercise progression, refer to Table 1, Table 2.
Table 1.
Progression of yoga.
| Yoga Asana/Posture | Week of Follow-up |
||||||
|---|---|---|---|---|---|---|---|
| 1 & 2 | 3 & 4 | 5 & 6 | 7 & 8 | 9 & 10 | 11 & 12 | ||
| Standing | Shithilikarana Vyayama (Loosening Exercises) | S* | S* | S* | S* | S* | S* |
| Supine | Pavanamuktasana (Wind Removing Pose) | B* | B* | B* | A* | A* | A* |
| Sitting on Heel | Shashankasana(The Hare or Rabbit Pose) | B* | B* | B* | A* | A* | A* |
| Standing | Ardhakati Chakrasana (Half Lateral Wheel Posture) | B* | B* | B* | A* | A* | A* |
| Standing | Utthita Parsvakonasana (Extended Side Angle Pose) | B* | B* | B* | A* | A* | A* |
| Standing | Parivrtta Trikonasana (Revolved Triangle Pose) | B* | B* | B* | A* | A* | A* |
| Long Sitting | Vakrasana (The Twisted Pose) | B* | B* | B* | A* | A* | A* |
| Sitting on Heel | Vibhagiya Pranayama, (Sectional Breathing) | S* | S* | S* | S* | S* | S* |
| Sitting | Nadi Shuddi Pranayama (Alternate Nostril Breathing) | S* | S* | S* | S* | S* | S* |
| Sitting | Bhramari Pranayama (Humming Bee Breathing) | S* | S* | S* | S* | S* | S* |
| Supine | Shavasana (Corpse Pose) | S* | S* | S* | S* | S* | S* |
Note: S* - Standard (Maintaining correct Posture, proper breathing pattern perform limb movement as per Yoga).
B* - Basic (Maintaining normal breathing, performing Posture, limb movement depend on individual tolerance).
A* - Advance (Progress in Maintaining correct Posture, proper breathing pattern perform limb movement).
Table 2.
Progression of dynamic neuromuscular stabilization exercise.
| DNS Exercises | Week of Follow-up |
|||||
|---|---|---|---|---|---|---|
| 1 & 2 | 3 & 4 | 5 & 6 | 7 & 8 | 9 & 10 | 11 & 12 | |
| Supine position, 90° hips 90° knees (3 months old position) | With Support Breathing Ex, Limb Movement |
Without Support Breathing Ex, Limb Movement |
Without Support Breathing Ex, Resisting Limb Movement |
|||
| Prone Position (3 months old position) | Breathing Ex Lift head from Cervical spine |
Breathing Ex, Mid-thoracic movement |
Breathing Ex, Head lift from the bench, Torso support on Gym ball |
|||
| Rolling/Side-lying position (4.5 months old position) | Initial position, Breathing Ex, Arm and Trunk Movement |
Breathing Ex, Trunk rotation, Arm and Pelvis Lift |
Breathing Ex, Resisting Trunk rotation, Arm and Pelvis Lift |
|||
| Quadruped Position (5 months old position) | Initial position, Breathing Ex, Limb Movements |
Breathing Ex, Shifting the Trunk Forward and Backward | Breathing Ex, Resisting the Shift of the Trunk Forward and Backward | |||
| Side/Oblique Sitting Position (7 months old position) | Initial position, Breathing Ex, Limb and Trunk Movement |
Breathing Ex, Loading Limb, Trunk Lift |
Breathing Ex, Resisting Loading Limb, Trunk Lift |
|||
| Sitting Position (8 months old position) | Initial position, Breathing Ex |
Breathing Ex, Lower limb movement |
Breathing Ex, Resisting Lower limb movement |
|||
| Bear Position (14 months old position) | Initial position, Breathing Ex, Limb Movement |
Breathing Ex, Lift one Leg maintain Spine and Pelvis Level |
Breathing Ex, Resisting the Leg Lift maintain Spine and Pelvis Level |
|||
| Deep Squat Position (14 months old position) | Initial position, Breathing Ex |
Breathing Ex, Squatting with Back Straight | Breathing Ex, Resisting Squatting | |||
Note: All DNS Exercises performed maintaining good respiration and Intra-abdominal pressure.
2.8.3. Intervention fidelity monitoring
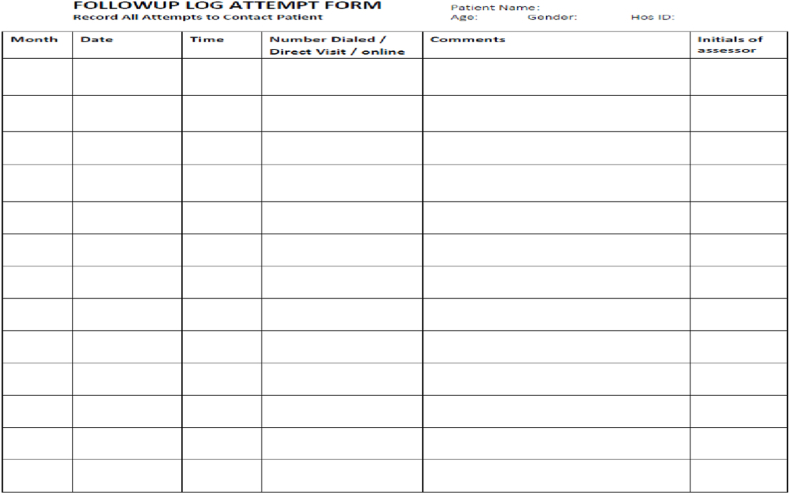
The licensed Physiotherapist will deliver interventions with a master's degree in physiotherapy and who has experience in the delivery of yoga and DNS exercise. Also, have good experience and knowledge in the treatment and management of CLBP. The study will be monitor by Study Steering Committee, Institutional Ethics Committee and Data Monitoring Committee (Table 3). The Data Safety Monitoring Board (DSMB) will monitor the therapist's adherence to the treatment protocol regimen. The main aim of DSMB is to reduce the variation or deviation in intervention delivery, limit the risk of bias, promote fidelity and optimize the treatment delivery. To maximize the treatment outcome and minimize the contamination of the treatment group (yoga and DNS group), yoga group participant's follow-up will be fixed on Monday, Wednesday, Friday, and DNS group participant's follow-up and appointments will be set on Tuesday, Thursday and Saturday. The therapist will maintain a follow-up log (Fig. 5), documenting all the details of the participant follow-up visit. Intervention sessions at the site setting will be tracked and confirmed by the study coordinator. The home practice exercise log will be maintained by the participants individually. The study coordinator will weekly check it [56,58,61].
Table 3.
Committee to monitor the study trial.
| Committee | Members | Functions |
|---|---|---|
| Study Steering Committee | Three Therapist with Ph.D. experience Prinicipal Investigator Co-Investigators |
Verify participants comfort in the trial Check for patient safety Verification of trial progress monthly Overall supervision of the trial Provide guidance and opinion on the trial progression |
| Institutional Ethics Committee | Justice, A basic medical scientist from pharmacology, Theologian, Layperson, Four experienced senior clinicians, different departments from affiliated Institutions, and the independent officer who is not affiliated to Institution. Totally ten members, which includes Chairman and member secretary. | Check participant informed consent and protocol Review amendments to protocol and approve the amendments Monitor trial collected data Advice on adverse events reports |
| Data Monitoring Committee | Prinicipal Investigator Co-Investigators Statisticians have experience in the clinical trial |
Estimate sample size Prepare statistical analysis plan Data checking and verification To check the recruitment figure, data quality, and completeness Evaluate the datasheet |
Fig. 5.
Follow-up log form.
2.9. Study participants protocol adherence and retention
To encourage adherence to the protocol therapy and follow-up visits for exercise progression, various tactics will be used. Commitment to the treatment group will be checked using an Exercise log, Text messaging, Video/Audio Call. Two days once, the study coordinator will call participants and enquire about their protocol adherence, any adverse event, and general health. Participants are also encouraged, permitted to contact the study coordinator if they have any concerns, questions. The appointment of the participants will be fixed on flexible timing of the participants to promote the participant engagement in trial and adherence. The exercise pamphlet and videos will be shared with participants weekly upon the follow-up visit [56,58].
2.10. Outcome measures
2.10.1. Primary outcome measures
The multifidus muscle's atrophy will be measured using magnetic resonance imaging as the major outcome measure. A 0.2 T resistive Open imaging system was used to obtain the magnetic resonance images (Magnetom Open Viva, Siemens, Erlangen, Germany). Two fast spin-echo sequences with varied T2 weighting will be used in the MR procedure. The sagittal and axial planes were used to create the images. Four images per spinal level will be displayed in the MRI scans. To compute the muscle CSA, an axial slice at the level of the L3-L4, L4-L5, L5-S1 will be chosen. The participants will be positioned in a supine position with a pillow beneath their knees. The hips and knees will be flexed. To avoid lumbar lordosis, the patients' neutral postures will be maintained [45]. The outcome measure will be recorded by a Radiologist with 20 years of experience, blinded to the allocation of treatment groups.
2.10.2. Secondary outcome measures
2.10.2.1. Ultrasound imaging
The musculoskeletal ultrasound will help to measure the thickness of the thoracolumbar fascia. The Terason 3000 scanner (Terason, Burlington MA) with a 4 cm, 10 MHz linear array transducer will be used to perform ultrasound B-mode imaging of the lumbar area on each individual during a single testing session. The transducer will be centered on a spot 2 cm lateral to the midpoint of the L2-L3 and L4-L5 interspinous ligaments to get a parasagittal image bilaterally [46]. The outcome measure will be recorded by a Radiologist with 20 years of experience, blinded to the allocation of treatment groups.
2.10.2.2. Visual analogue scale
A 10-cm visual analogue scale was used to assess participants' pain perception. On a 100 mm pain scale, participants were asked to rate the degree of their pain. Subjects were asked to rate their pain on a scale of zero (0 mm) to one hundred (100 mm), with zero (100 mm) being no pain and one hundred (100 mm) representing the worst agony they could imagine. The inter-rater reliability The interclass correlation (ICC – 0.97) indicates that VAS is reliable. In chronic musculoskeletal pain, a difference of 13 mm on the VAS reflects the smallest detectable change in pain intensity that is clinically significant [47]. All other outcome measures will be recorded by a Physiotherapist with six years of experience, who will be blinded to treatment groups.
2.10.2.3. Oswestry disability index
ODI is a questionnaire based on patients’ disabilities. The test is widely regarded as the gold standard for determining low-back functional outcomes. It has ten components, and each component scored 0 to 5. Scores are associated with the degree of disability ranging from minimal to bedbound. Studies have shown that ODI has excellent Reliability (ICC 0.877) [48].
2.10.2.4. The Life Orientation Test (LOT)
Scheier, Carver, and Bridges developed the Life Orientation Test (LOT-R). The test examines a person's broad expectations for the future. Dispositional optimism is a personality trait that encompasses these expectations. Cronbach's alfa co-efficient value of 0.76 was used to compute LOT-R reliability [49].
2.10.2.5. Pain catastrophizing was measured using the pain catastrophizing scale
The Pain Catastrophizing Scale is a 13-item questionnaire that assesses how often people have catastrophic thoughts and sentiments about pain, on a scale of 0–4, with 0 equaling “not at all” and 4 equaling “always.” The scale runs from 0 to 52, with higher scores indicating more pain catastrophizing [[50], [51]].
2.10.2.6. Fear of movement was measured using the Tampa scale for Kinesiophobia (TSK)
The TSK is a 17-item Likert scale that assesses fear of movement and re-injury concerning various physical activities. With scores ranging from 17 to 68, the TSK total score is the sum of all components. Greater fear of movement or re-injury is associated with higher ratings [52].
2.10.2.7. Positive and negative affect schedule (PANAS)
It's a popular scale with excellent psychometric features. Positive (e.g., “excited,” “strong,” “interesting”) and negative (e.g., “nervous,” “irritable,” “upset”) affect are scored separately on this twenty-item scale [53].
2.10.2.8. The pain coping strategies questionnaire (CSQ)
Rosenstiel and Keefe created it. The questionnaire is used to assess the effectiveness of pain management measures that have been used to cope with the pain. It consists of 42 statements that describe various coping mechanisms and two questions that assess one's pain-control abilities. Six cognitive strategies (reassessment of pain sensations, sensations ignoring, one's declaration of coping with pain, diverting attention, catastrophizing, praying/pinning) and one behavioral strategy (reassessment of pain sensations, sensations ignoring, one's declaration of coping with pain, diverting attention, catastrophizing, praying/pinning) are represented in the ways to cope with pain (increased behavioral activity). For the entire questionnaire, Cronbach's alpha coefficient is 0.80. We examined the links between dispositional optimism and pain coping strategies such as shifting attention, increasing behavioral activity, catastrophizing, and an individual's assertion of pain coping [54].
2.10.2.9. Self-efficacy for pain scale
It was created in 1995 and consists of 22 questions divided into three domains: self-efficacy for pain control (how the patient manages physical symptoms such as fatigue and pain), self-efficacy for functionality (how the patient performs some daily activities), and self-efficacy to deal with other signs (addresses how pain affects the individual). A maximum of 100 points and a minimum of 10 points are available in each domain. This scale has a maximum score of 300 and a minimum score of 30; the greater the score, the better the self-efficacy [55].
2.11. Safety monitoring
Data and safety of the study participants will be monitored by applying several strategies.
All the materials, discussions, proceedings, events happening during the trial period are entirely confidential. Members and other participants of all these above committees are expected to maintain confidentiality [56,58,60,61].
When a participant exhibits any serious physical or mental health symptoms as noted by the study therapist, a participant or attendee, the study coordinator, or anyone else, the participant safety protocol will be triggered. The participant is contacted and assessed by a study clinician before being cleared for safety or referred for additional care in all cases of Safety protocol beginning. All adverse events are recorded, reported, and categorized as per funding agency, IEC, and other regulatory requirements. The study team reviews the adverse event reports regularly.
2.12. Study participants withdrawal
Participants who choose to leave the trial are contacted to address their concerns and any issues they encountered during the study. The study coordinator will try to resolve their issue or problem. Try to give a solution to participant concerns. If participants are convinced with the explanation, they can continue in the trial. After resolving the issue, if a Participant wants to withdraw from the study, they are eligible to withdraw from the trial as per the Clinical Trial Practice Guideline. The study coordinator will ensure that Participants receive standard treatment care after withdrawal from the study [56,58,60,61]. The revoke of the consent form will be obtained from the participant.
2.13. Data management
Data collection from the participants will be the direct entry into the MS Excel sheet. Participants will be given a printed copy of all surveys during the in-person assessment sessions and asked to complete all forms. A study coordinator collects data on participants' daily opioid use (medication type and dose), probable adverse events, and home practice. Participants can also conduct their surveys over the phone using a Google form or on paper, depending on their preference and availability. Study coordinators collect data and enter it into MS Excel sheets, which are then double-checked for accuracy. Participants who do not complete their planned follow-up assessment will be contacted once a week until their information is collected. During the data collection phase, the study coordinator conducts audits, supervises, and guarantees compliance with the study protocol and data integrity [56,58,61].
2.14. Sample size estimation
The sample size calculation is done using magnetic resonance imaging (MRI) as a primary outcome measure. Assuming a change in multifidus muscle thickness to 40% in the yoga group and 20% in the DNS group, an estimated sample size of n = 36 each group will be required to produce >80% power for a two-sided 5% statistical test, considering 10% drop out. The total sample size is 72 for each group and a total of 144 CLBP participants.
2.15. Analysis approach
The data will be analyzed with the statistics software. The descriptive statistics, percentage analysis, will be used for categorical and continuous variables. Intention to treat (ITT) will be considered during the analysis of data. All events will be reported, and percentage analysis will be used. The normative data will be verified using the Kolmogorov-Smirnov test. For within-group analysis: if normally distributed data - paired t-test or Skewed data - Wilcoxon signed-rank test will be used. For between-group analysis: Normally distributed data - Independent samples t-test or Skewed data - Mann-Whitney U test will be preferred.
3. Discussion
According to recent studies, the application of Yoga is an effective method in the treatment of CLBP. However, the yoga effect on the LMM fat infiltration, CSA, and thickness of TLF remains unclear. This is the first randomized, double-blind clinical trial to look at the effects of yoga and DNS exercise on fat infiltration in the lumbar multifidus and thoracolumbar regions using magnetic resonance imaging and ultrasonic imaging, respectively. The findings of this study could help determine the impact of yoga and DNS exercise on the lumbar multifidus and thoracolumbar fascia in CLBP treatment.
Funding
This study trial is funded by the DST-funded project (Reference No- DST/SATYAM/2018/156) under Science and Technology for Yoga and Meditation (SATYAM).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors express their gratitude to the Government of India Ministry of Science and Technology, Department of Science and Technology (DST) under Science and Technology for Yoga and Meditation (SATYAM) and Manipal College of Health Professionals (MCHP) Bangalore Campus- Manipal Academy of Higher Education (MAHE), Manipal Hospital Bangalore (MHB) which supported and facilitated this study.
Data availability
No data was used for the research described in the article.
References
- 1.Hayden J.A., van Tulder M.W., Malmivaara A., Koes B.W. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst. Rev. 2005 July 20;(3):CD000335. doi: 10.1002/14651858.CD000335.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bindra S., Sinha A.G., Benjamin A.I. Epidemiology of low back pain in Indian population: a review. Int J Basic Appl Med Sci. 2015;5:166–179. https://www.cibtech.org/JMEDICALSCIENCES/PUBLICATIONS/2015/Vol_5_No_1/29-JMS-029-BINDRA-EPIDEMIOLOGY-REVIEW.pdf [Google Scholar]
- 3.Hildebrandt M., Fankhauser G., Meichtry A., Luomajoki H. Correlation between lumbar dysfunction and fat infiltration in lumbar multifidus muscles in patients with low back pain. BMC Muscoskel. Disord. 2017;18(1) doi: 10.1186/s12891-016-1376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sions J., Elliott J., Pohlig R., Hicks G. Trunk muscle characteristics of the multifidi, erector spinae, psoas, and quadratus lumborum in older adults with and without chronic low back pain. J. Orthop. Sports Phys. Ther. 2017;47(3):173–179. doi: 10.2519/jospt.2017.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh R., Yadav S., Sood S., Yadav R., Rohilla R. Magnetic resonance imaging of lumbar trunk parameters in chronic low backache patients and healthy population: a comparative study. Eur. Spine J. 2016;25(9):2864–2872. doi: 10.1007/s00586-016-4698-7. [DOI] [PubMed] [Google Scholar]
- 6.Hides J., Gilmore C., Stanton W., Bohlscheid E. Multifidus size and symmetry among chronic LBP and healthy asymptomatic subjects. Man. Ther. 2008;13:43–49. doi: 10.1016/j.math.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Chan S.T., Fung P.K., Ng N.Y., Ngan T.L., Chong M.Y., Tang C.N., He J.F., Zheng Y.P. Dynamic changes of elasticity, cross-sectional area, and fat infiltration of multifidus at different postures in men with chronic low back pain. Spine J. 2012;12:381–388. doi: 10.1016/j.spinee.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Barker K.L., Shamley D.R., Jackson D. Changes in the cross-sectional area of multifidus and psoas in patients with unilateral back pain: the relationship to pain and disability. Spine. 2004;29:E515–E519. doi: 10.1097/01.brs.0000144405.11661.eb. [DOI] [PubMed] [Google Scholar]
- 9.Lee S., Chan C.K., Lam T., Lam C., Lau N., Lau R.W., Chan S.T. Relationship between low back pain and lumbar multifidus size at different postures. Spine. 2006;31:2258–2262. doi: 10.1097/01.brs.0000232807.76033.33. [DOI] [PubMed] [Google Scholar]
- 10.Wallwork T.L., Stanton W.R., Freke M., Hides J. The effect of chronic low back pain on size and contraction of the lumbar multifidus muscle. Man. Ther. 2009;14:496–500. doi: 10.1016/j.math.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Mayer J., Graves J., Clark B., Formikell M., Ploutz-Snyder L. The use of magnetic resonance imaging to evaluate lumbar muscle activity during trunk extension exercise at varying intensities. Spine. 2005;30(22):2556–2563. doi: 10.1097/01.brs.0000186321.24370.4b. [DOI] [PubMed] [Google Scholar]
- 12.D'Hooge R., Cagnie B., Crombez G., Vanderstraeten G., Achten E., Danneels L. Lumbar muscle dysfunction during remission of unilateral recurrent non-specific low-back pain. Clin. J. Pain. 2012;29:187–194. doi: 10.1097/AJP.0b013e31824ed170. http://www.issls.org/wp-content/uploads/2015/01/00002508-201303000-00001.pdf [DOI] [PubMed] [Google Scholar]
- 13.Lee S., Chan C.K., Lam T., Lam C., Lau N., Lau R.W., et al. Relationship between low back pain and lumbar multifidus size at different postures. Spine. 2006;31:2258–2262. doi: 10.1097/01.brs.0000232807.76033.33. [DOI] [PubMed] [Google Scholar]
- 14.Goubert D., De Pauw R., Meeus M., Willems T., Cagnie B., Schouppe S., et al. Lumbar muscle structure and function in chronic versus recurrent low back pain: a cross-sectional study. Spine J. 2017 Sep;17(9):1285–1296. doi: 10.1016/j.spinee.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Imai A., Okubo Y., Kaneoka K. Evaluation of psoas major and quadratus lumborum recruitment using diffusion-weighted imaging before and after 5 trunk exercises. J. Orthop. Sports Phys. Ther. 2017;47(2):108–114. doi: 10.2519/jospt.2017.6730. [DOI] [PubMed] [Google Scholar]
- 16.Fortin M., Macedo L.G. Multifidus and paraspinal muscle group cross-sectional areas of patients with low back pain and control patients: a systematic review with a focus on blinding. Phys. Ther. 2013 doi: 10.2522/ptj.20120457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goubert D., Oosterwijck J., Meeus M., Danneels L. Structural changes of lumbar muscles in non-specific low back pain. Pain Physician. 2016;19:E985–E1000. https://www.painphysicianjournal.com/current/pdf?article=MzAwNw%3D%3D&journal=99 September/October 2016. [PubMed] [Google Scholar]
- 18.Ranger T., Cicuttini F., Jensen T., Peiris W., Hussain S., Fairley J., et al. Are the size and composition of the paraspinal muscles associated with low back pain? A systematic review. Spine J. 2017;17(11):1729–1748. doi: 10.1016/j.spinee.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Yanagisawa O., Matsunaga N., Okubo Y., Kaneoka K. Noninvasive evaluation of trunk muscle recruitment after trunk exercises using diffusion-weighted MR imaging. Magn. Reson. Med. Sci. 2015;14(3):173–181. doi: 10.2463/mrms.2014-0073. [DOI] [PubMed] [Google Scholar]
- 20.De Ridder E.M., Van Oosterwijck J.O., Vleeming A., Vanderstraeten G.G., Danneels L.A. Muscle functional MRI analysis of trunk muscle recruitment during extension exercises in asymptomatic individuals. Scand. J. Med. Sci. Sports. 2015;25:196–204. doi: 10.1111/sms.12190. [DOI] [PubMed] [Google Scholar]
- 21.Levine J.D., Fields H.L., Basbaum A.I. Peptides and the primary afferent nociceptor. J. Neurosci. 1993;13(6):2273e2286. doi: 10.1523/JNEUROSCI.13-06-02273.1993. Jun. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tesarz J., Hoheisel U., Wiedenhofer B., et al. Sensory innervation of the thoracolumbar fascia in rats and humans. Neuroscience. 2011;194:302e308. doi: 10.1016/j.neuroscience.2011.07.066. [DOI] [PubMed] [Google Scholar]
- 23.Bednar D.A., Orr F.W., Simon G.T. Observations on the pathomorphology of the thoracolumbar fascia in chronic mechanical back pain. A microscopic study. Spine. 1995;20:1161–1164. doi: 10.1097/00007632-199505150-00010. [DOI] [PubMed] [Google Scholar]
- 24.Langevin H.M., Stevens-Tuttle D., Fox J.R., Badger G.J., Bouffard N.A., Krag M.H., et al. Ultrasound evidence of altered lumbar connective tissue structure in human subjects with chronic low back pain. BMC Muscoskel. Disord. 2009;10:151. doi: 10.1186/1471-2474-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cramer H., Lauche R., Dobos G. Characteristics of randomized controlled trials of Yoga: a bibliometric analysis. BMC Compl. Alternative Med. 2014;14:328. doi: 10.1186/1472-6882-14-328. authors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patil N.J., Nagarathna R., Tekur P., Patil D.N., Nagendra H.R., Subramanya P. Designing, validation, and feasibility of integrated yoga therapy module for chronic low back pain. Int. J. Yoga. 2015 Jul-Dec;8(2):103–108. doi: 10.4103/0973-6131.158470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frank C., Kobesova A., Kolar P. Dynamic neuromuscular stabilization & sports rehabilitation. The International Journal of Sports Physical Therapy. 2013;8:62. https://pubmed.ncbi.nlm.nih.gov/23439921/ Number 1 February. [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher M., Meyer R., Adams G., et al. Direct relationship between proton T2 and exercise intensity in skeletal muscle MR images. Invest. Radiol. 1990;25:480–485. doi: 10.1097/00004424-199005000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Büssing A., Poier D., Ostermann T., Kröz M., Michalsen A. Treatment of chronic lower back pain: study protocol of a comparative effectiveness study on Yoga, eurythmy therapy, and physiotherapeutic exercises. Compl. Med. Res. 2018;25(1):24–29. doi: 10.1159/000471801. [DOI] [PubMed] [Google Scholar]
- 30.Cramer H., Lauche R., Dobos G. Characteristics of randomized controlled trials of Yoga: a bibliometric analysis. BMC Compl. Alternative Med. 2014;14:328. doi: 10.1186/1472-6882-14-328. authors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patil N.J., Nagarathna R., Tekur P., Patil D.N., Nagendra H.R., Subramanya P. Designing, validation, and feasibility of integrated yoga therapy module for chronic low back pain. Int. J. Yoga. 2015 Jul-Dec;8(2):103–108. doi: 10.4103/0973-6131.158470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qaseem A., Wilt T.J., McLean R.M., Forciea M.A. Non-invasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 2017 April 4;166(7):514–530. doi: 10.7326/M16-2367. [DOI] [PubMed] [Google Scholar]
- 33.Kreiner D.S., Matz P., Bono C.M., Cho C.H., Easa J.E., Ghiselli G., Ghogawala Z., Reitman C.A., Resnick D.K., Watters W.C., 3rd, Annaswamy T.M., Baisden J., Bartynski W.S., Bess S., Brewer R.P., Cassidy R.C., Cheng D.S., Christie S.D., Chutkan N.B., Cohen B.A., Yahiro A.M. Guideline summary review: an evidence-based clinical guideline for the diagnosis and treatment of low back pain. Spine J.: official journal of the North American Spine Society. 2020;20(7):998–1024. doi: 10.1016/j.spinee.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Zhu F., Zhang M., Wang D., Hong Q., Zeng C., Chen W. Yoga compared to non-exercise or physical therapy exercise on pain, disability, and quality of life for patients with chronic low back pain: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2020 Sep 1;15(9) doi: 10.1371/journal.pone.0238544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wieland L.S., Skoetz N., Pilkington K., Vempati R., D'Adamo C.R., Berman B.M. Yoga treatment for chronic non‐specific low back pain. Cochrane Database Syst. Rev. 2017;(1) doi: 10.1002/14651858.CD010671.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park C., Yoon S., Yoon H., Kim K., Cha Y., Park I. Effects of core stabilization exercise on muscle activity during horizontal shoulder adduction with loads in healthy adults: a randomized controlled study. J. Mech. Med. Biol. 2021 Jul 30 doi: 10.1142/S0219519421400492. [DOI] [Google Scholar]
- 37.Bokarius V. Long-term efficacy of dynamic neuromuscular stabilization in treatment of chronic musculoskeletal pain. Age. 2008;18(25):3. https://www.ijhsr.org/IJHSR_Vol.10_Issue.9_Sep2020/29.pdf [Google Scholar]
- 38.a Park I., Park C., Kim K., Cha Y. The effects of dynamic neuromuscular stability exercise on the scoliosis and pain control in the youth baseball players. J. Mech. Med. Biol. 2021 Aug 5 [Google Scholar]; b Sharma K., Yadav A. Dynamic neuromuscular stabilization- a narrative review. Int. J. Health Sci. Res. 2020;10(9):221–231. doi: 10.1142/S0219519421400303. [DOI] [Google Scholar]
- 39.Lim Y.L., Lepsikova M., Singh D.K. Regional Conference on Science, Technology and Social Sciences (RCSTSS 2016) Springer; Singapore: 2018. Effects of dynamic neuromuscular stabilization on lumbar flexion kinematics and Posture among adults with chronic non-specific low back pain: a study protocol; pp. 715–724.https://www.springerprofessional.de/effects-of-dynamic-neuromuscular-stabilization-on-lumbar-flexion/17629510 [Google Scholar]
- 40.Son M.S., Jung D.H., You J.S., Yi C.H., Jeon H.S., Cha Y.J. Effects of dynamic neuromuscular stabilization on diaphragm movement, postural control, balance and gait performance in cerebral palsy. NeuroRehabilitation. 2017 January 1;41(4):739–746. doi: 10.3233/NRE-172155. [DOI] [PubMed] [Google Scholar]
- 41.Yoon H.S., You J.S. Reflex-mediated dynamic neuromuscular stabilization in stroke patients: EMG processing and ultrasound imaging. Technol. Health Care. 2017 January 1;25(S1):99–106. doi: 10.3233/THC-171311. [DOI] [PubMed] [Google Scholar]
- 42.Novak J., Jacisko J., Busch A., Cerny P., Stribrny M., Kovari M., Podskalska P., Kolar P., Kobesova A. Intra-abdominal pressure correlates with abdominal wall tension during clinical evaluation tests. Clin. BioMech. 2021 Aug 1;88 doi: 10.1016/j.clinbiomech.2021.105426. [DOI] [PubMed] [Google Scholar]
- 43.Bae W.S., Lee K.C., Lee D.Y. The effects of dynamic neuromuscular stabilization exercise on forward head posture and spine posture. Med. Leg. Update. 2019 August 8;19(2):670–675. doi: 10.21203/rs.3.rs-384623/v1. [DOI] [Google Scholar]
- 44.Kader D., Wardlaw D., Smith F. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin. Radiol. 2000;55(2):145–149. doi: 10.1053/crad.1999.0340. [DOI] [PubMed] [Google Scholar]
- 45.Langevin H., Stevens-Tuttle D., Fox J., Badger G., Bouffard N., Krag M., et al. Ultrasound evidence of altered lumbar connective tissue structure in human subjects with chronic low back pain. BMC Muscoskel. Disord. 2009;10(1) doi: 10.1186/1471-2474-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boonstra A.M., Schiphorst P., Henrica R., Michiel F., Jitze B., Roy E. Reliability and validity of the Visual Analogue Scale for Disabilty in patients with chronic musculoskeletal pain. J. Int. Res. 2008;2:165–169. doi: 10.1097/MRR.0b013e3282fc0f93. [DOI] [PubMed] [Google Scholar]
- 47.Fairbank J.C., Pynsent P.B. The Oswestry disability index. Spine. 2000;25(22):2940–2952. doi: 10.1093/occmed/kqw051. [DOI] [PubMed] [Google Scholar]
- 48.Scheier M.F., Carver C.S., Bridges M. Distinguishing Optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J. Pers. Soc. Psychol. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 49.Merriwether E.N., Rakel B.A., Zimmerman M.B., Dailey D.L., Vance C.G.T., Darghosian L., Golchha M., Geasland K.M., Chimenti R., Crofford L.J., et al. Reliability and construct validity of the Patient-Reported Outcomes Measurement Information System (PROMIS) instruments in women with fibromyalgia. Pain Med. 2017;18(8):1485–1495. doi: 10.1186/s13075-018-1671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan M.J.L., Bishop S.R., Pivik J. The pain catastrophizing scale: development and validation. Psychol. Assess. 1995;7:524–532. doi: 10.1037/1040-3590.7.4.524. [DOI] [Google Scholar]
- 51.Roelofs J., Goubert L., Peters M.L., Vlaeyen J.W., Crombez G. The Tampa Scale for Kinesiophobia: further examination of psychometric properties in patients with chronic low back pain and fibromyalgia. Eur. J. Pain. 2004;8(5):495–502. doi: 10.1016/j.ejpain.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 52.Watson D., Clark L.A., Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 53.Rosenstiel A., Keefe F.J. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 54.Anderson K.O., Dowds B.N., Pelletz R.E., Edwards W.T., Peeters- Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain [Internet] 1995;63(1):77–84. doi: 10.1016/0304-3959(95)00021-J. [cited 2016 mar 31] [DOI] [PubMed] [Google Scholar]
- 55.Zgierska A.E., Burzinski C.A., Garland E.L., Lennon R.P., Jamison R., Nakamura Y., Barrett B., Sehgal N., Mirgain S.A., Singles J.M., Cowan P. Mindfulness-based therapy compared to cognitive behavioral therapy for opioid-treated chronic low back pain: protocol for a pragmatic randomized controlled trial. Contemp. Clin. Trials. 2021 Nov 1;110 doi: 10.1016/j.cct.2021.106548. [DOI] [PubMed] [Google Scholar]
- 56.Traeger A.C., Moseley G.L., Hübscher M., Lee H., Skinner I.W., Nicholas M.K., Henschke N., Refshauge K.M., Blyth F.M., Main C.J., Hush J.M. Pain education to prevent chronic low back pain: a study protocol for a randomised controlled trial. BMJ Open. 2014 May 1;4(6) doi: 10.1136/bmjopen-2014-005505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delitto A., Patterson C.G., Stevans J.M., Brennan G.P., Wegener S.T., Morrisette D.C., Beneciuk J.M., Freel J.A., Minick K.I., Hunter S.J., Ephraim P.L. Study protocol for targeted interventions to prevent chronic low back pain in high-risk patients: a multi-site pragmatic cluster randomized controlled trial (TARGET Trial) Contemp. Clin. Trials. 2019 Jul 1;82:66–76. doi: 10.1016/j.cct.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 58.Cavalcante P.G., Baptista A.F., Cardoso V.S., Filgueiras M.D., Hasue R.H., João S.M., Hazime F.A. Transcranial direct current stimulation combined with therapeutic exercise in chronic low back pain: protocol of a randomized controlled trial. Phys. Ther. 2020 Aug 31;100(9):1595–1602. doi: 10.1093/ptj/pzaa105. [DOI] [PubMed] [Google Scholar]
- 59.Iversen V.M., Vasseljen O., Mork P.J., Berthelsen I.R., Børke J.B., Berheussen G.F., Tveter A.T., Salvesen Ø., Fimland M.S. Resistance training in addition to multidisciplinary rehabilitation for patients with chronic pain in the low back: study protocol. Contemp. Clin. Trials Commun. 2017 Jun 1;6:115–121. doi: 10.1016/j.conctc.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.D O'Halloran P., Holden J., Breckon J., Davidson M., Rahayu W., Monfries M., Taylor N.F. Embedded Motivational Interviewing combined with a smartphone app to increase physical activity in people with sub-acute low back pain: study protocol of a cluster randomised control trial. Contemp. Clin. Trials Commun. 2020 Mar 1;17 doi: 10.1016/j.conctc.2019.100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clark B.C., Russ D.W., Nakazawa M., France C.R., Walkowski S., Law T.D., Applegate M., Mahato N., Lietkam S., Odenthal J., Corcos D. A randomized control trial to determine the effectiveness and physiological effects of spinal manipulation and spinal mobilization compared to each other and a sham condition in patients with chronic low back pain: study protocol for the RELIEF Study. Contemp. Clin. Trials. 2018 Jul 1;70:41–52. doi: 10.1016/j.cct.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Groessl E.J., Schmalzl L., Maiya M., Liu L., Goodman D., Chang D.G., Wetherell J.L., Bormann J.E., Atkinson J.H., Baxi S. Yoga for veterans with chronic low back pain: Design and methods of a randomized clinical trial. Contemp. Clin. Trials. 2016 May 1;48:110–118. doi: 10.1016/j.cct.2016.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.