Abstract
Yawn contagion (YC) is, compared to spontaneous yawning, an evolutionary recent phenomenon probably linked to behavioral synchronization in highly social species that is more likely when it involves familiar subjects. Here, we investigate for the first time in monkeys which factors modulate intra- and interspecific YC. Through an experimental approach, we exposed 17 red-capped mangabeys to video stimuli (Yawn vs Control) depicting familiar/unfamiliar red-capped mangabeys and humans, and unfamiliar hamadryas. We found that mangabeys yawned more often in response to Yawn than Control videos independently from the species depicted, demonstrating both intra- and interspecific YC in the tested species. Moreover, both mangabey and human familiar yawning stimuli evoked a stronger yawning response in the subjects compared to the unfamiliar counterparts. Neither the amount of time spent looking frontally at the screen (probability of stimulus perception) nor the levels of self-directed behaviors (a proxy of anxiety) accounted for the results. In conclusion, we provide the first evidence that in non-human primate familiarity modulates both intra- and inter-specific YC. Stimuli emitted by familiar faces somehow ease the mechanisms underlying YC, and this modulation can also apply to heterospecific subjects when previous shared experiences provide the prerequisites for the development of social bonds.
Subject terms: Neuroscience, Psychology
Introduction
Spontaneous yawning is an evolutionary ancient trait that is consistent in its presence and expression across several groups of vertebrates1,2. Yawning is a multi-functional phenomenon whose functions are probably context-dependent and linked to both physiological as well as behavioral aspects of life3. Yawn contagion (YC), the ability to respond with a yawn to others’ yawns, is instead an evolutionary more recent phenomenon4,5. Recent findings suggest that YC can have an adaptive social role in bodily coordination, mood alignment and alertness increase6,7. Both experimental and observational evidence indicate sociality and cooperation as triggering factors for YC to evolve in different taxa6,8–15. YC can also be present between subjects belonging to different species (i.e., interspecific YC16–18) thus indicating a certain level of flexibility of its underlying mechanisms.
Familiarity shared between the interacting subjects seems to play a role in the modulation of stimuli contagiousness, and an animated debate exists on the proximate causes at the basis of such modulation (emotional closeness vs attentional bias,19,20). Relevant clues supporting the linkage between yawn and emotional contagion (a building-block of empathy,21) come from psychological studies. People showing higher susceptibility to contagious yawning do better at making inferences about mental states22 and exhibit fewer schizotypal traits23. Moreover, when exposed to yawn vs control stimuli, higher activity in empathy-related neuronal areas was recorded in people scoring highly on empathy (24 but note that the authors did not measure the contagion itself25). The emotional bias hypothesis predicts that the degree of emotional involvement with a first yawner reflects the observer’s susceptibility to respond with a yawn4,21. Instead, the attentional bias hypothesis predicts that YC is merely linked to the higher levels of social attention that observers devote to more relevant subjects (i.e., familiar or dominant)25. More recently, Gallup et al.26 found that those people scoring high levels of psychopathic traits, which are associated with reduced affective empathy, also showed low levels of YC, with the yawn response not depending on the attentional level. Although this dualistic conceptual approach is present in literature, the two aspects are difficult to disentangle since probably both attentional and affective processes play a role in the modulation of YC20. Whatever the proximate factors at the basis of YC modulation, several studies show how affiliation and kinship increase the susceptibility to respond to others’ yawns in several species10–12,14,16,18,27. Concurrently, the available data do not always go in the same direction, with different examples of social closeness not influencing YC28–31, thus challenging the view about the positive effect of familiarity on the phenomenon. Species scoring low affiliation and/or high degrees of ingroup competition do not seem to show YC32,33 and, moreover, while xenophilic species show similar levels of YC towards known and unknown individuals34, species classified as xenophobic show contagion strictly towards ingroup subjects18. Another factor that has been found to influence the YC distribution is the sex of the interacting subjects, especially in those species where bonding is sex-biased (e.g., bonobos, 10; wolves12). Specifically, wolf females showed a faster yawning response when sharing a strong bond with the first yawner, such difference was not found in males12.
Here, we aim at testing the effect of familiarity on both intra- and interspecific YC in monkeys due to the absence of data covering these intermingled aspects in non-ape species. Filling the gap is necessary if we want to properly understand the evolutionary aspects of the phenomenon. Our model is the red-capped mangabey (Cercocebus torquatus), a highly sexually dimorphic species living in multi-male multi-female groups variable in size and characterized by fission–fusion dynamics35. Although clear hierarchies exist in social groups, the dominance relationships are generally relaxed thus predicting a certain level of tolerance36. C. torquatus further possesses a rich repertoire of visual signals with a large variety of yawning types37. The peculiarity of their social life and the variability of yawn performance make the species a valuable candidate to test hypotheses on the proximate factors leading to YC. To accomplish the goal, we showed red-capped mangabeys video stimuli from familiar and unfamiliar individuals belonging to three species (Cercocebus torquatus, Papio hamadryas, Homo sapiens). In our protocol, we also took into consideration the probability of stimulus perception (i.e., how long the subjects were frontally looking at the screen) as well as the anxiety level of the subjects when exposed to the different stimuli (i.e., self-directed behaviours38), as both factors can act as confounding factors for YC and its modulation25,39.
If YC is sensitive to the phylogenetic closeness between the interacting subjects (hypothesis 1), we expect red-capped mangabeys to be more susceptible to yawns produced by conspecifics and hamadryas baboons than to yawns produced by humans (prediction 1).
If YC is modulated by previous experiences (i.e., familiarity) between the interacting subjects (hypothesis 2), we expect red-capped mangabeys to be more susceptible to yawns produced by familiar red-capped mangabeys and humans than to those produced by the unfamiliar counterparts (prediction 2).
Methods
Experimental procedures
17 adult captive-born red-capped mangabeys (10 males, 7 females) hosted at the Station Biologique de Paimpont (University of Rennes 1) were involved in the study. The animals occupy enclosures with indoor and outdoor spaces and live in groups with different compositions ranging from one-male to all-male groups. The experimental sessions took place in the indoor enclosure (where the temperature is kept at 22 °C), while non-tested subjects remained outside. The animals, well-habituated to be separated, were tested only when they spontaneously entered the test area (see “Supplementary Information” for further details).
The video stimuli showed three different species (red-capped mangabeys, humans, and hamadryas baboons). We generated both Yawn and Control videos depicting unfamiliar hamadryas and unfamiliar/familiar mangabeys and humans, for a total of 10 video stimuli per tested subject (i.e., unfamiliar and familiar mangabeys Control/Yawn, unfamiliar and familiar humans Control/Yawn, hamadryas Control/Yawn). Indeed, hamadryas baboons are unknown to our tested subjects and to the species in general (no geographical range overlap), thus only unfamiliar stimuli depicting baboons were prepared. The videos were mute 5-min videos, composed by 2 alternating videos depicting 2 different individuals. The two videos alternated each other along the whole duration of the stimulus (i.e., 5 min) and were separated each time by a black blank screen (random duration, 1–3 s). Each 5-min video included 55 yawning events. Mangabey videos were collected at the Station Biologique de Paimpont (Paimpont, France) and hamadryas videos were recorded at the Hellabrunn Zoo (Munich, Germany). To prepare the human stimuli, videos were recorded ad hoc from nine volunteers. To assess the spontaneity level of human yawns, we asked seven other volunteers to score each yawn (1 = fake yawn; 10 = real yawn). Only 65 out of the 85 yawns scoring higher than 5 were used. Since hamadryas were completely unfamiliar to the tested subjects only for mangabeys and humans we designed video stimuli depicting familiar or unfamiliar individuals ad hoc for each subject. The mangabeys in the video were considered as familiar if they have lived together or in adjacent cages with the tested subject within the last 5 years. Human familiar subjects were the caretakers who have been daily interacting with animals for more than 1 year18. The Yawn videos included male yawns from different age classes, both adults and juveniles, emitted with different mouth opening degrees, face orientations and backgrounds. The choice of male yawns for the three species derives from the fact that it was not possible to have unfamiliar mangabey females for each of the subjects (specifically, all the females lived in the same enclosure for at least a period within the last 5 years). Then, to make the stimuli from the different species comparable, we also included male yawns for humans and hamadryas. All the yawns were performed under relaxed conditions (e.g., excluding feeding times, in the absence of aggression preceding the yawn stimulus). For all the three species used as stimuli, each Control video stimulus included two alternated videos of the same 2 individuals depicted in the Yawn video stimulus. The videos used for the two conditions (Yawn/Control) were extracted from the same original video. Hence, in both of them the individuals had identical body orientation, context and background, with the only difference being that in the Control videos they chewed or opened/closed their mouth without yawning. The video stimuli depicting the three different species were prepared to be as much standardized as possible. Mangabeys and hamadryas were recorded in their different enclosures (outdoor and indoor areas). Human videos were thus recorded in variable areas, with variable lighting, head orientation and proportion of the body filmed, so as to reproduce the conditions of the other two species and to make the stimuli comparable in terms of quality of depicted yawns. We prepared the video stimuli ad hoc for each tested subject depending on the research question (i.e., species, degree of familiarity). The two filmed individuals were randomly extracted from recorded videos including 22 red-capped mangabeys, 9 humans, 10 hamadryas baboons.
We introduced a two-step habituation phase before the experimental sessions to i) reduce neophobia towards the equipment (5-h presence of the screen kept off in the indoor enclosure) and ii) make animals more comfortable in watching images in the screen (5-h presence to the screen showing neutral images of the environment surrounding the animals’ enclosures—with no humans or animals depicted). Each experimental session lasted 10 min: 2-min blank screen (pre-stimulus), 5-min of video stimulus, and 3-min blank screen (post-stimulus, to account for latency in the response40). The 10 stimuli (i.e., unfamiliar and familiar mangabeys Control/Yawn, unfamiliar and familiar humans Control/Yawn, hamadryas Control/Yawn) were randomly presented in two time slots (i.e., morning and afternoon). Each subject had 5 sessions in the morning and 5 in the afternoon (random division, no more than a session per day per subject). Within each time slot the order in which the 5 stimuli were presented to each subject across different days was also randomized. Since yawns performed by the subjects outside of the enclosure could have been possibly visible to the tested subject, all these yawns and their exact time of occurrence were collected (Outside Yawns). Further details on the experimental protocol are provided as “Supplementary Information”.
Video analysis and statistics
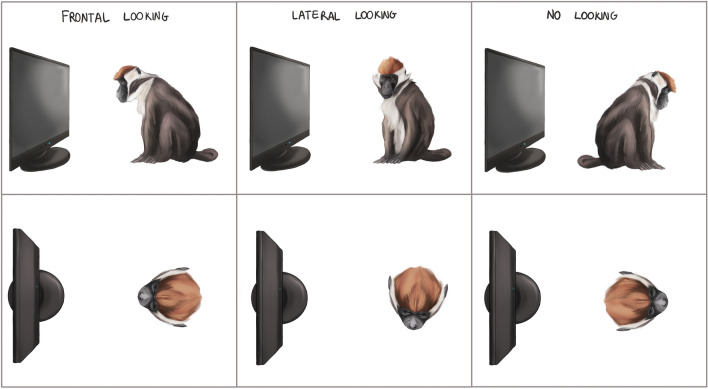
When analyzing the videos of the sessions, we coded for the presence/absence of yawns (Yawn Response, YR) and the amount of time the tested subject looked frontally at the screen when showing the video stimulus (Frontal Looking, FL; Fig. 1). The other conditions shown in Fig. 1 and those in between them (in terms of head orientation) were parsimoniously not coded as FL. We also recorded self-directed behaviors (SDB, scratching, self-grooming, head-shaking) during the sessions, considered as good indicators of anxiety in primates38. We ran two Linear Mixed Models (glmmTMB 1.2.5042 package on R) to evaluate which variables affect the rates of FL and of SDBs. Then, we ran a Generalized Linear Mixed Model (glmmTMB 1.2.5042) to evaluate which variables affected the YR. According to the hypotheses tested, we split the analysis into two steps. Firstly, we focused on the effect of the species in the video (unfamiliar individuals of the three different species—Model set1). Secondly, we focused on the effect of familiarity (video stimuli of unfamiliar and familiar mangabeys and humans—Model set2). In all the models the identity of the tested subject (i.e., ID) was included as a random factor. See “Supplementary Information” for further details on statistics used.
Figure 1.
Codification of stimuli perception by means of looking directions during the experimental sessions. Under the FL condition the head of the subject had to be directed towards the screen. Credits Fosca Mastrandea.
Model set1—YC is sensitive to the phylogenetic closeness between unfamiliar subjects (hp 1)
Model1a—FL (response variable, Gaussian error distribution)
The fixed factors considered were: Sex of the tested subject, Species in the video (Mangabey/Human/Hamadryas), Condition (Yawn/Control), Time of the session (Morning/Afternoon), Order of the stimuli presentation.
Model1b—SDB (response variable, Gaussian error distribution)
The fixed factors included were those considered in Model1a.
Model1c—YR (response variable, binomial error distribution)
The fixed factors were the same of Model1a with the addition of Outside Yawns and FL.
Model set2—YC is modulated by familiarity between subjects (hp 2)
Model2a—FL (response variable, Gaussian error distribution)
The fixed factors considered were: Sex of the tested subject, Species in the video (Mangabey/Human), Condition (Yawn/Control), Time (Morning/Afternoon), Order of the stimuli presentation, Familiarity of the stimulus (Familiar/Unfamiliar).
Model2b—SDB (response variable, Gaussian error distribution)
The fixed effects were the same considered in Model2a.
Model2c—YR (response variable, binomial error distribution)
The fixed factors were the same considered in Model2a, with the addition of Outside Yawns and FL.
We first included in the models meaningful interactions between the predictors: Sex*Condition, Sex*Species (Model set1) and Sex*Condition, Sex*Species, Familiarity*Species, Familiarity*Sex (Model set2). As discussed in the “Supplementary Information”, the interactions were included in each final model only if they were significant.
Ethics
The present study consisted in a non-invasive protocol conducted with captive mangabeys housed at the Station Biologique de Paimpont (University Rennes 1, France), where animal facilities and animal care procedures are regularly monitored by the responsible local authorities (Housing agreement for research D35-211-18, delivered by the “Direction Départementale de la Cohésion Sociale et de la Protection des Populations” (DDCSPP)). The ethical authorisations for conducting research studies in this facility are given by the CREEA Ethic committee (“Comité Rennais d’Ethique en matière d’expérimentation animale”), which is the legal representative for all animal experimentations from institutions located within this geographical area. This committee is registered with the "Comité National de Réflexion Ethique sur l'Expérimentation Animale" under the number 07. The current research protocol has been approved by the CREEA Ethic committee under the reference APAFIS#24418-2020012319192625 v2. This study is reported in accordance with ARRIVE guidelines. All human volunteers filmed to constitute the video stimuli gave an informed consent to the use of their images for this research project and were informed about their rights concerning the treatment of their personal data, in accordance with the General Data Protection Regulation (RGPD) compliances. The treatment and storage of these personal data were declared and approved by the Data Protection Officer (DPO) of the University of Rennes 1. Human ethic committees equivalent to IRBs exist at national level but no such approval was required for our study design according to the French regulations since we just used video recordings of humans.
Results
Model set1—YC is sensitive to the phylogenetic closeness between unfamiliar subjects (hp1)
Model1a
The full model (fixed variables and interactions) significantly differed from the null one (X29 = 22.233, P = 0.008, R2marginal = 0.231, R2conditional = 0.511). Since both the interactions were not significant (PSex*Species = 0.939; PSex*Condition = 0.057), we ran the final LMM excluding them. In Model1a, the full model significantly differed from the null one (X26 = 18.495, P = 0.005; R2marginal = 0.214, R2conditional = 0.490). The variables Sex and Species had a significant effect on FL (Table 1a). The Tukey test revealed that the subjects looked more at the videos depicting conspecifics than at those showing humans (t-ratio = − 2.847; df = 93; p = 0.015) and baboons (t-ratio = -2.320; df = 93; p = 0.058) (Fig. S1a). Males looked significantly more at the screen than females (mean ± SE: 0.420 ± 0.028 vs 0.254 ± 0.028) (Fig. S1b).
Table 1.
(a) Estimated parameters (Estimate), Standard Error (SE), and results of the likelihood ratio tests (χ2) of the LMM (gaussian distribution) with Frontal Looking as response variable (Model1a). (b) Estimated parameters (Estimate), Standard Error (SE), and results of the likelihood ratio tests (χ2) of the GLMM (binomial error distribution) with Yawn Response as response variable (Model1c). For both the models the sessions were 102. Significant values are in bold.
| Fixed factors | Estimate | SE | df | X2 | p value |
|---|---|---|---|---|---|
| (a) Frontal looking. Variance for the random factor (tested individual) = 0.010, SD = 0.102 | |||||
| Intercept | 0.609 | 0.058 | – | – | – |
| Species | 2 | 8.825 | 0.012 | ||
| Species (human)a,b | − 0.017 | 0.035 | |||
| Species (mangabey)a,b | 0.079 | 0.034 | |||
| Condition (yawn)a,b | 0.049 | 0.028 | 1 | 3.086 | 0.079 |
| Sex (female)a,b | − 0.198 | 0.075 | 1 | 5.692 | 0.017 |
| Time (afternoon)a,b | − 0.030 | 0.029 | 1 | 1.056 | 0.304 |
| Order of the session | − 0.005 | 0.008 | 1 | 0.330 | 0.566 |
| (b) Yawn response. Variance for the random factor (tested individual) = 0.518, SD = 0.720 | |||||
| Intercept | − 0.040 | 1.074 | – | – | – |
| Species | 2 | 0.780 | 0.677 | ||
| Species (human)a,b | − 0.379 | 0.593 | |||
| Species (mangabey)a,b | 0.123 | 0.593 | |||
| Condition (yawn)a,b | 1.287 | 0.507 | 1 | 7.182 | 0.007 |
| Sex (female)a,b | − 1.683 | 0.700 | 1 | 6.527 | 0.011 |
| Time (afternoon)a,b | − 0.857 | 0.518 | 1 | 2.951 | 0.086 |
| Order of the session | 0.207 | 0.142 | 1 | 2.199 | 0.138 |
| Frontal looking | 0.177 | 1.364 | 1 | 0.017 | 0.897 |
| Number of outside yawns | − 0.056 | 0.164 | 1 | 0.117 | 0.733 |
aEstimate ± SE refers to the difference of the response between the reported level of this categorical predictor and the reference category of the same predictor.
bThese predictors were dummy coded, with the “Species (Hamadryas)”, “Condition (Control)”, “Sex (Male)”, “Time (Morning)” being the reference categories.
Model1b
There was no difference between the full (fixed variables and interactions) and null model (X29 = 15.012, P = 0.091). Similarly, no significant difference was found between the final full and null model (X26 = 3.484, P = 0.746). During the entire 8-min block of each session the time spent performing SDBs scored low values (mean ± SE: 21.181 ± 2.891 s). This allowed us not to include SDB in Model1c.
Model1c
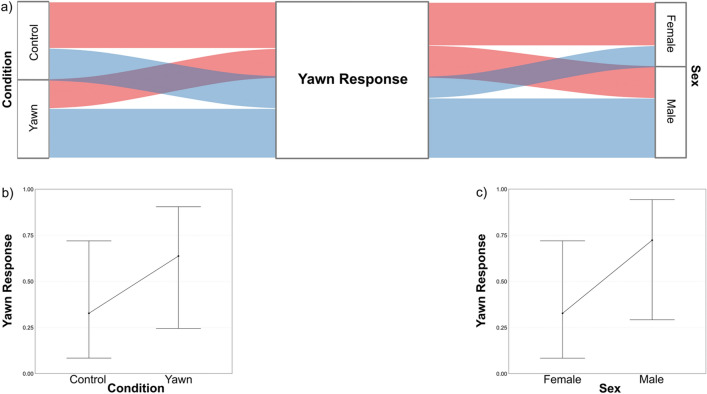
The full model (fixed variables and interactions) significantly differed from the null one (X211 = 19.731, P = 0.049, R2marginal = 0.278, R2conditional = 0.377). Since both the interactions were not significant (PSex*Species = 0.734; PSex*Condition = 0.392), we ran the final GLMM excluding them. In Model1c, the full and the null model significantly differed (X28 = 18.435, P = 0.018; R2marginal = 0.258, R2conditional = 0.359). The YR was significantly influenced by the Condition and Sex variables (Table 1b, Fig. 2a). Under the Yawn condition, the likelihood of YR was about three times higher (odds ratio = 3.62) compared to Control condition (Fig. 2b). Overall males yawned more than females (Fig. 2c). Neither the Species (P = 0.677) nor the rate of FL (P = 0.897) affected the likelihood of YR.
Figure 2.
Results of Model1c (Yawn Response as response variable) showing the effect of the significant predictors. (a) Alluvial plot showing the occurrence of Yawn Response (Blue streams = presence; Pink streams = absence) for each level of the factors “Condition” (Yawn vs Control) and “Sex” (Male vs Female). (b) Effect of the variable Condition on the Yawn response. (c) Effect of the variable Sex on the Yawn Response. Bands represent the confidence interval.
Model set2—YC is modulated by familiarity between subjects (hp 2)
Model2a
The full model (fixed variables and interactions) significantly differed from the null one (X211 = 45.804, P < 0.0001, R2marginal = 0.293, R2conditional = 0.571). Since the interactions were not significant (PSex*Species = 0.686; PSex*Condition = 0.282, PFamiliarity*Species = 0.178; PFamiliarity*Sex = 0.574), we ran the final LMM excluding them. In Model2a, the full model significantly differed from the null one (X26 = 42.566, P < 0.0001; R2marginal = 0.283, R2conditional = 0.558). Familiarity, Condition, Sex, and Species significantly affected FL rates (Table 2a). The tested subjects looked more at videos depicting unfamiliar than familiar subjects (mean ± SE: 0.367 ± 0.027 vs 0.246 ± 0.018) (Fig. S2a) and at Yawn compared to Control videos (Fig. S2b). Males looked at the screen for longer than females (Fig. S2c), and conspecifics elicited a higher FL than humans (Fig. S2d).
Table 2.
(a) Estimated parameters (Estimate), Standard Error (SE), and results of the likelihood ratio tests (χ2) of the LMM (gaussian distribution) with Frontal Looking as response variable (Model2a). (b) Estimated parameters (Estimate), Standard Error (SE), and results of the likelihood ratio tests (χ2) of the GLMM (binomial error distribution) with Yawn Response as response variable (Model2c). For both the models the sessions were 136. Significant values are in bold.
| Fixed factors | Estimate | SE | df | X2 | p value |
|---|---|---|---|---|---|
| (a) Frontal looking. Variance for the random factor (Tested individual) = 0.009, SD = 0.096 | |||||
| Intercept | 0.550 | 0.052 | – | – | – |
| Species (mangabey)a,b | 0.068 | 0.021 | 1 | 10.073 | 0.002 |
| Condition (yawn)a,b | 0.045 | 0.021 | 1 | 4.518 | 0.034 |
| Sex (female)a,b | − 0.140 | 0.052 | 1 | 6.056 | 0.014 |
| Familiarity (familiar)a,b | − 0.100 | 0.021 | 1 | 21.012 | 0.000 |
| Time (afternoon)a,b | − 0.027 | 0.022 | 1 | 1.523 | 0.217 |
| Order of the session | − 0.007 | 0.005 | 1 | 2.262 | 0.133 |
| (b) Yawn response. Variance for the random factor (Tested individual) = 0.172, SD = 0.415 | |||||
| Intercept | − 1.190 | 0.930 | – | – | – |
| Species (mangabey)a,b | 0.156 | 0.455 | 1 | 0.118 | 0.731 |
| Condition (yawn)a,b | 2.809 | 0.529 | 1 | 41.232 | 0.000 |
| Sex (female)a,b | − 1.231 | 0.579 | 1 | 4.863 | 0.027 |
| Familiarity (familiar)a,b | 0.973 | 0.481 | 1 | 4.260 | 0.039 |
| Time (afternoon)a,b | − 0.734 | 0.455 | 1 | 2.680 | 0.102 |
| Order of the session | 0.094 | 0.096 | 1 | 0.971 | 0.324 |
| Frontal looking | 0.212 | 1.443 | 1 | 0.021 | 0.884 |
| Number of outside yawns | 0.045 | 0.111 | 1 | 0.165 | 0.685 |
aEstimate ± SE refers to the difference of the response between the reported level of this categorical predictor and the reference category of the same predictor.
bThese predictors were dummy coded, with the “Species (Human)”, “Condition (Control)”, “Sex (Male)”, “Time (Morning)”, “Familiarity (Unfamiliar)” being the reference categories.
Model2b
There was no difference between the full (fixed variables and interactions) and null model (X211 = 14.617, P = 0.201). Similarly, no significant difference was found between the final full and null model (X27 = 9.711, P = 0.206). During the 8-min block of each session the time spent performing SDBs scored low values (mean ± SE: 18.974 ± 2.130 s). This allowed us not to include SDB in Model2c.
Model2c
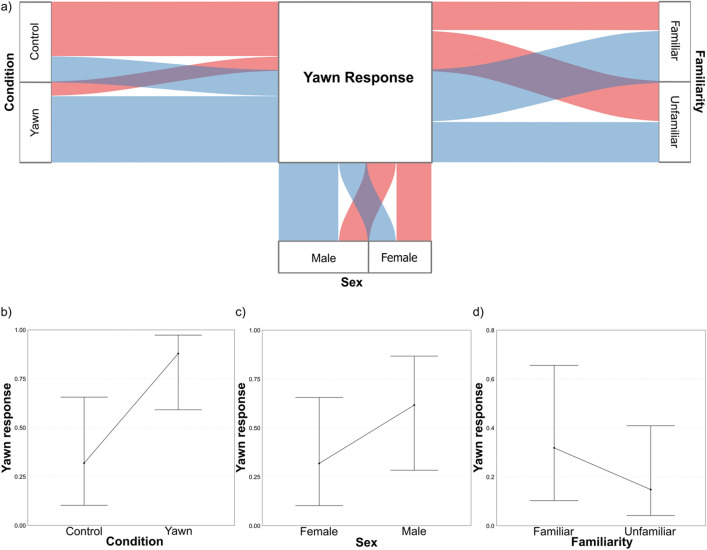
The full model (fixed variables and interactions) significantly differed from the null one (X211 = 55.098, P < 0.0001, R2marginal = 0.454, R2conditional = 0.491). Since all interactions were not significant (PSex*Species = 0.832; PSex*Condition = 0.329, PFamiliarity*Species = 0.462; PFamiliarity*Sex = 0.529), we ran the final GLMM excluding them. In Model2c, the full model strongly differed from the null one (X28 = 53.516, P < 0.0001; R2marginal = 0.444, R2conditional = 0.471). Sex, Condition, and Familiarity significantly affected the YR (Table 2b; Fig. 3a). The likelihood of YR was more than 16 times higher in Yawn than Control condition (odds ratio = 16.60) (Fig. 3b). Overall males yawned more than females (Fig. 3c). The likelihood of yawning was more than twice higher (odds ratio = 2.64) when exposed to familiar rather than unfamiliar subjects (Fig. 3d). All the tested mangabeys emitted at least a yawn in response to familiar subjects’ yawns. Neither FL (p = 0.884) nor Species (p = 0.731) affected the likelihood of YR.
Figure 3.
Results of Model2c (Yawn Response as response variable) showing the effect of the significant predictors. (a) Alluvial plot showing the occurrence of Yawn Response (Blue streams = presence; Pink streams = absence) for each level of the factors “Condition” (Yawn vs Control), “Sex” (Male vs Female), and “Familiarity” (Familiar vs Unfamiliar). (b) Effect of the variable Condition on the Yawn Response; (c) Effect of the variable Sex on the Yawn Response; (d) Effect of the variable Familiarity on the Yawn Response. Bands represent the confidence interval.
Discussion
Here, we found that red-capped mangabeys yawned more often to videos depicting yawning individuals than to control videos, thus demonstrating yawn contagion (YC) in this species. Since self-directed behaviors (SDB, a proxy of anxiety) were not affected by neither of the factors considered, this rules out the possibility that yawning response (YR) was a by-product of anxiety. This result contrasts with those from other studies where the perception of yawn stimuli produced an increase of both YR and SDB39,41. Our finding also seems to suggest that mangabeys do not perceive yawning behavior as a negative stimulus, differently from what has been found in humans42.
The YR was not affected by the amount of frontal looking (FL), thus suggesting that in the species the probability of responding to a yawn cannot be merely explained by the quantitative perception of the stimulus (e.g., for how long you perceive it). Male YR was higher than female YR independently from the species depicted and from the condition of the video stimulus. The higher male tendency to spontaneously yawn is not surprising in those primate species showing remarkable sexual dimorphism in canine size37,43. Here, males yawned more often than females both when exposed to Control and Yawn stimuli; importantly, the effect of the Yawn condition in increasing the likelihood of yawning did not differ between the two sexes (i.e., the interaction term Sex*Condition was not significant in the models), with yawn stimuli producing a comparable YC effect in both males and females. Our animals showed similar levels of susceptibility to yawns emitted by conspecific and heterospecific subjects thus demonstrating both intra- and inter-specific YC (prediction 1 not supported). Mangabeys frontally looked for longer the videos depicting conspecific than heterospecific subjects, probably related to the greater socio-ecological relevance for mother-reared primates to strictly monitor members of their own species42,44,45. A possible limit of the study is that we could not use eye-tracking techniques, preventing us from actually making conclusions on the attentional state of the subjects. However, we can confidently assume that the longer the animals looked with their head frontally towards the screen, the higher the probability for them actually looking at the individual depicted in the video (which occupied a good portion of the screen and was easily visible). Moreover, since YC does not require an active and conscious attentional and perceptive state of the yawn stimulus46, we can affirm that when the mangabey looked frontally at the screen the stimulus was at least passively perceived.
The literature does not provide data on inter-specific YC deriving exclusively in response to unfamiliar subjects/species. Although direct comparisons are difficult, our data are consistent with the at least partial automaticity of motor mimicry phenomena4,47, which allows a basal likelihood of contagion independently from the identity and the social features of the trigger subject. Our data can be discussed by two not mutually exclusive interpretations. The consistency of yawning motor patterns (i.e., stereotypy) across different primate species48 might explain the similar efficacy in stimulating a yawning response in the receiver. It is also possible that monkeys, as it occurs in humans8, might be able to automatically generalize the yawning stimulus whatever its origins.
Our second set of data adds an important piece of information about the phenomenon in mangabeys. When familiarity comes into play, it scores the highest YC rates (prediction 2 supported). Indeed, compared to the unfamiliar counterparts, both familiar mangabey and human yawning stimuli evoked a higher YR in the tested subjects and this held for both males and females. This higher susceptibility towards familiar yawns was not clearly due to a higher probability of familiar stimulus perception, because mangabeys spent more time frontally looking unfamiliar rather than familiar individuals, possibly due to the potential adaptive implications involved when facing novel stimuli18,49,50. Similar results have been recently found in great apes, with chimpanzees and gorillas being more attracted by novel than familiar human faces51. The familiarity bias in FL that we found is not in contrast with the higher level of FL devoted to conspecific than to heterospecific subjects; indeed, competition for resources is expected to be higher between subjects belonging to the same species (complete ecological niche overlap) but belonging to different social groups, and thus stimuli from unfamiliar conspecifics should be more attentively monitored. During YC, one can share the state of the other based on a perceived motor pattern, through an automatic distributive associative process, which may get easier when the stimuli come from familiar faces52. Hence familiarity with the yawner, independently from the species it belongs to, may potentiate the Perception Action Mechanism at the basis of motor resonance phenomena21.
In chimpanzees, Campbell and de Waal18 reported a familiarity-biased contagiousness only towards conspecifics, but not towards humans, a species to which chimpanzees were well habituated. Conversely, in our study, not only were red-capped mangabeys susceptible to yawns produced by conspecifics and humans, but they also showed a comparable familiarity bias towards both species. Similar hypotheses have been so far rarely tested in primates18 and a modulation in the contagiousness of heterospecific yawns was only found in domesticated dogs (16, but see also 28) where, on the other hand, no study investigated intraspecific YC. Our data thus represent the first evidence in mammals of familiar modulation on YC operating at both intra- and interspecific level. Unfamiliar humans seem to be perceived by mangabeys as something equivalent to the ‘outgroup’ conspecifics, both in terms of frontal looking at the screen (FL) and contagiousness (YR). Conversely, since caretakers daily spend a considerable amount of time with animals, their faces and yawns are probably processed as those of ‘ingroup’ companions. Stimuli produced by extensively known humans might be easier to be processed and thus might be more contagious than those emitted by stranger faces. Our results also highlight the ability of mangabeys to discriminate between familiar and unfamiliar human faces. Although the capacity to process universal face prototypes is thought to be present at birth, it is highly adaptive that face recognition abilities narrow in the course of ontogeny and reach a high specificity later in life53. This can lead to a scarce competence in discriminating familiar vs unfamiliar heterospecific faces. Yet, early experience can maintain a certain degree of plasticity in the primate face recognition system54. The daily experience that our mangabeys have with caretakers are probably at the basis of their ability to properly process human faces.
Recent data suggests that the propensity to mimic ingroup subjects has been favored by natural selection to increase behavioral synchronization, fundamental for survivorship and improvement of individual fitness55. For example, YC in lions translates into a higher probability to align social activities that are at the basis of cooperative hunting, offspring care, and territorial defense6. Other forms of behavioral synchronization have been reported to be extremely adaptive (vigilance against predators56). It is possible that the natural propensity to be behaviorally infected by group mates could expand to familiar subjects of different species under contexts allowing interspecies interactions (e.g., captivity). Experimental data demonstrate that capuchin monkeys preferentially engaged in objects sharing with experimenters who previously imitated them compared to experimenters who performed non-imitative gestures57. This finding is in line with our results on the importance of familiarity in modulating YC despite the inter-specific context. Here, the adaptive value might reside in the possible linkage between motor resonance phenomena (YC) and the resource benefits (e.g., food provisioning) gathered by monkeys from their caretakers.
In conclusion, in mangabeys YC is socially modulated not only at intra- but also at interspecific level, suggesting that the phylogenetic closeness of the interacting subjects is not enough to explain the phenomenon. Other factors such as the social feature of a species (e.g., social system, preferential relationships, ingroup competition) and the ontogenetic pathways of each individual (e.g., rearing conditions, more or less opportunities of early experiences, degree of plasticity in forming bonds) should be taken into account to fully explain the propensity to YC and its modulation from an adaptive perspective.
Supplementary Information
Acknowledgements
We thank Marine Mergen and Philippe Bec for the help given during the experiments and Dimitri Giunchi for his helpful suggestions on statistical analysis. Thanks also to Filippo Bigozzi and Marco Germain Riccobono for providing us with the videos of hamadryas, and to the human volunteers for their participation. Many thanks to the talented Fosca Mastrandea for Fig. 1 illustration.
Author contributions
A.L. and E.P. conceived the study; L.P. performed the experiment; A.L., J.A., L.L.V., L.P. prepared the video stimuli; A.R. provided technical support during the experiment; L.P., V.M., A.L., E.P. performed statistical analysis; L.P., A.L., and E.P. drafted the manuscript; all the authors revised the final version of the manuscript.
Data availability
The raw data supporting the conclusions of this article are provided as supporting information to the article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Alban Lemasson and Elisabetta Palagi.
Contributor Information
Alban Lemasson, Email: alban.lemasson@univ-rennes1.fr.
Elisabetta Palagi, Email: elisabetta.palagi@unipi.it.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-15395-0.
References
- 1.Baenninger R. On yawning and its functions. Psychon. Bull. Rev. 1997;4:198–207. doi: 10.3758/BF03209394. [DOI] [PubMed] [Google Scholar]
- 2.Bakkegard KA. Yawning by Red Hills salamanders (Phaeognathus hubrichti) at their burrow entrance. Herpetol. Rev. 2017;48(1):32–36. [Google Scholar]
- 3.Gallup AC. The causes and consequences of yawning in animal groups. Anim. Behav. 2022;187:209–219. doi: 10.1016/j.anbehav.2022.03.011. [DOI] [Google Scholar]
- 4.Palagi E, Celeghin A, Tamietto M, Winkielman P, Norscia I. The neuroethology of spontaneous mimicry and emotional contagion in human and non-human animals. Neurosci. Biobehav. Rev. 2020;111(1):149–165. doi: 10.1016/j.neubiorev.2020.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Gallup AC. Why do we yawn? Primitive versus derived features. Neurosci. Biobehav. Rev. 2011;35(3):765–769. doi: 10.1016/j.neubiorev.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Casetta G, Nolfo AP, Palagi E. Yawn contagion promotes motor synchrony in wild lions, Panthera leo. Anim. Behav. 2021;174:149–159. doi: 10.1016/j.anbehav.2021.02.010. [DOI] [Google Scholar]
- 7.Gallup AC, Meyers K. Seeing others yawn selectively enhances vigilance: An eye-tracking study of snake detection. Anim. Cogn. 2021;24:583–592. doi: 10.1007/s10071-020-01462-4. [DOI] [PubMed] [Google Scholar]
- 8.Provine RR. Yawning as a stereotyped action pattern and releasing stimulus. Ethology. 1986;72:109–122. doi: 10.1111/j.1439-0310.1986.tb00611.x. [DOI] [Google Scholar]
- 9.Campbell MW, Cox CR. Observational data reveal evidence and parameters of contagious yawning in the behavioral repertoire of captive-reared chimpanzees (Pan troglodytes) Sci. Rep. 2019;9(1):1–13. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demuru E, Palagi E. In bonobos yawn contagion is higher among kin and friends. PLoS ONE. 2012;7(11):e49613. doi: 10.1371/journal.pone.0049613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palagi E, Leone A, Mancini G, Ferrari PF. Contagious yawning in gelada baboons as a possible expression of empathy. Proc. Natl. Acad. Sci. USA. 2009;106(46):19262–19267. doi: 10.1073/pnas.0910891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero T, Ito M, Saito A, Hasegawa T. Social modulation of contagious yawning in wolves. PLoS ONE. 2014;9(8):e105963. doi: 10.1371/journal.pone.0105963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yonezawa T, Sato K, Uchida M, Matsuki N, Yamazaki A. Presence of contagious yawning in sheep. J. Anim. Sci. 2017;88(1):195–200. doi: 10.1111/asj.12681. [DOI] [PubMed] [Google Scholar]
- 14.Norscia I, Coco E, Robino C, Chierto C, Cordoni G. Yawn contagion in domestic pigs (Sus scrofa) Sci. Rep. 2021;11(1):1–12. doi: 10.1038/s41598-020-80545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller ML, Gallup AC, Vogel AR, Vicario SM, Clark AB. Evidence for contagious behaviors in budgerigars (Melopsittacus undulatus): An observational study of yawning and stretching. Behav. Process. 2012;89(3):264–270. doi: 10.1016/j.beproc.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Romero T, Konno A, Hasegawa T. Familiarity bias and physiological responses in contagious yawning by dogs support link to empathy. PLoS ONE. 2013;8(8):e71365. doi: 10.1371/journal.pone.0071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossman ZT, Padfield C, Young D, Hart BL, Hart LA. Contagious yawning in African elephants (Loxodonta africana): responses to other elephants and familiar humans. Front. Vet. Sci. 2020;7(5):1–8. doi: 10.3389/fvets.2020.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell MW, de Waal FBM. Chimpanzees empathize with group mates and humans, but not with baboons or unfamiliar chimpanzees. Proc. R. Soc. B. 2014;281:20140013. doi: 10.1098/rspb.2014.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallup AC. On the link between emotional contagion and contagious yawning. Neurosci. Biobehav. Rev. 2021;121:18–19. doi: 10.1016/j.neubiorev.2020.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Palagi, E., Celeghin, A., Tamietto, M., Winkielman, P., Norscia, I. Disentangling attentional and affective contribution to contagious yawning. Neurosci. Biobehav. Rev. S0149–7634 (2021) [DOI] [PubMed]
- 21.de Waal FBM, Preston SD. Mammalian empathy: Behavioural manifestations and neural basis. Nat. Rev. Neurosci. 2017;18(8):498–509. doi: 10.1038/nrn.2017.72. [DOI] [PubMed] [Google Scholar]
- 22.Franzen A, Mader S, Winter F. Contagious yawning, empathy, and their relation to prosocial behavior. J. Exp. Psychol. Gen. 2018;147(12):1950–1958. doi: 10.1037/xge0000422. [DOI] [PubMed] [Google Scholar]
- 23.Helt MS, Sorensen TM, Scheub RJ, Nakhle MB, Luddy AC. Patterns of contagious yawning and itching differ amongst adults with autistic traits vs psychopathic traits. Front. Psychol. 2021;12:645310. doi: 10.3389/fpsyg.2021.645310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper NR, Puzzo I, Pawley AD, Bowes-Mulligan RA, Kirkpatrick EV, Antoniou PA, Kennett S. Bridging a yawning chasm: EEG investigations into the debate concerning the role of the human mirror neuron system in contagious yawning. Cogn. Affect. Behav. Neurosci. 2012;12(2):393–405. doi: 10.3758/s13415-011-0081-7. [DOI] [PubMed] [Google Scholar]
- 25.Massen JJM, Gallup AC. Why contagious yawning does not (yet) equate to empathy. Neurosci. Biobehav. Rev. 2017;80(5):573–585. doi: 10.1016/j.neubiorev.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Gallup AC, Kret ME, Eldakar OT, Folz J, Massen JJM. People that score high on psychopathic traits are less likely to yawn contagiously. Sci. Rep. 2021;11:23779. doi: 10.1038/s41598-021-03159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norscia I, Zanoli A, Gamba M, Palagi E. Auditory contagious yawning is highest between friends and family members: Support to the emotional bias hypothesis. Front. Psychol. 2020;11(4):1–8. doi: 10.3389/fpsyg.2020.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neilands P, et al. Contagious yawning is not a signal of empathy: No evidence of familiarity, gender or prosociality biases in dogs. Proc. R. Soc. B. 2020;287:20192236. doi: 10.1098/rspb.2019.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Berlo E, Díaz-Loyo AP, Juárez-Mora OE, Kret ME, Massen JJM. Experimental evidence for yawn contagion in orangutans (Pongo pygmaeus) Sci. Rep. 2020;10(1):1–11. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallup AC, Swartwood L, Militello J, Sackett S. Experimental evidence of contagious yawning in budgerigars (Melopsittacus undulatus) Anim. Cogn. 2015;18(5):1051–1058. doi: 10.1007/s10071-015-0873-1. [DOI] [PubMed] [Google Scholar]
- 31.Bartholomew AJ, Cirulli ET. Individual variation in contagious yawning susceptibility is highly stable and largely unexplained by empathy or other known factors. PLoS ONE. 2014;9(3):e91773. doi: 10.1371/journal.pone.0091773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palagi E, Norscia I, Cordoni G. Lowland gorillas (Gorilla gorilla gorilla) failed to respond to others’ yawn: Experimental and naturalistic evidence. J. Comp. Psychol. 2019;133(3):406–416. doi: 10.1037/com0000175. [DOI] [PubMed] [Google Scholar]
- 33.Reddy RB, Krupenye C, MacLean EL, Hare B. No evidence for contagious yawning in lemurs. Anim. Cogn. 2016;19(5):889–898. doi: 10.1007/s10071-016-0986-1. [DOI] [PubMed] [Google Scholar]
- 34.Tan J, Ariely D, Hare B. Bonobos respond prosocially toward members of other groups. Sci. Rep. 2017;7(1):1–11. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolado R, Gimeno E, Beltran FS. Modeling the emergence of seasonal fission-fusion dynamics in red-capped mangabeys (Cercocebus torquatus) Behav. Ecol. Sociobiol. 2017;71(7):1–12. doi: 10.1007/s00265-017-2331-3. [DOI] [Google Scholar]
- 36.Dolado R, Beltran FS. Dominance hierarchy and spatial distribution in captive red-capped mangabeys (Cercocebus torquatus torquatus) Interact. Stud. 2011;12(3):461–473. doi: 10.1075/is.12.3.05dol. [DOI] [Google Scholar]
- 37.Aychet J, Blois-Heulin C, Palagi E, Lemasson A. Facial displays in red-capped mangabeys (Cercocebus torquatus): Repertoire, social context, and potential intentionality. J. Comp. Psychol. 2021;135(1):98–113. doi: 10.1037/com0000252. [DOI] [PubMed] [Google Scholar]
- 38.Maestripieri D, Schino G, Aureli F, Troisi A. A modest proposal: Displacement activities as an indicator of emotions in primates. Anim. Behav. 1992;44:967–979. doi: 10.1016/S0003-3472(05)80592-5. [DOI] [Google Scholar]
- 39.Paukner A, Anderson JR. Video-induced yawning in stumptail macaques (Macaca arctoides) Biol. Lett. 2006;2(1):36–38. doi: 10.1098/rsbl.2005.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell, M. W., de Waal, F. B. M. Methodological problems in the study of contagious yawning. in The Mystery of Yawning in Physiology and Disease, Vol. 28, 120–127. (Karger Publishers, 2010) [DOI] [PubMed]
- 41.Buttner AP, Strasser R. Contagious yawning, social cognition, and arousal: An investigation of the processes underlying shelter dogs’ responses to human yawns. Anim. Cogn. 2014;17(1):95–104. doi: 10.1007/s10071-013-0641-z. [DOI] [PubMed] [Google Scholar]
- 42.Kret ME, van Berlo E. Attentional bias in humans toward human and bonobo expressions of emotion. Evol. Psychol. 2021;19(3):14747049211032816. doi: 10.1177/14747049211032816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leone A, Ferrari PFF, Palagi E. Different yawns, different functions? Testing social hypotheses on spontaneous yawning in Theropithecus gelada. Sci. Rep. 2014;4:32–35. doi: 10.1038/srep04010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adams LC, MacDonald SE. Spontaneous preference for primate photographs in Sumatran orangutans (Pongo abelii) Int. J. Comp. Psychol. 2018;31:16. [Google Scholar]
- 45.Dufour V, Pascalis O, Petit O. Face processing limitation to own species in primates: A comparative study in brown capuchins, Tonkean macaques and humans. Behav. Process. 2006;73(1):107–113. doi: 10.1016/j.beproc.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 46.De Waal FB, Ferrari PF. Towards a bottom-up perspective on animal and human cognition. Trends Cogn. Sci. 2010;14(5):201–207. doi: 10.1016/j.tics.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Yoon JMD, Tennie C. Contagious yawning: A reflection of empathy, mimicry, or contagion? Anim. Behav. 2010;79(5):2007–2009. doi: 10.1016/j.anbehav.2010.02.011. [DOI] [Google Scholar]
- 48.Walusinski O, Le Deputte BL. Bâillement: Phylogenèse, éthologie, nosogénie. Revue Neurologique. 2004;160(11):1011–1021. doi: 10.1016/S0035-3787(04)71138-8. [DOI] [PubMed] [Google Scholar]
- 49.Candiotti A, Zuberbühler K, Lemasson A. Voice discrimination in four primates. Behav. Process. 2013;99:67–72. doi: 10.1016/j.beproc.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 50.Damon F, Quinn PC, Pascalis O. When novelty prevails on familiarity: Visual biases for child versus infant faces in 3.5- to 12-month-olds. J. Exp. Child Psychol. 2021;210:105174. doi: 10.1016/J.JECP.2021.105174. [DOI] [PubMed] [Google Scholar]
- 51.Leinwand JG, Fidino M, Ross SR, Hopper LM. Familiarity mediates apes' attentional biases toward human faces. Proc. R. Soc. B Biol. Sci. 2022;289(1973):20212599. doi: 10.1098/rspb.2021.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haker H, Kawohl W, Herwig U, Rössler W. Mirror neuron activity during contagious yawning-an fMRI study. Brain Imaging Behav. 2013;7(1):28–34. doi: 10.1007/s11682-012-9189-9. [DOI] [PubMed] [Google Scholar]
- 53.Dufour V, Coleman M, Campbell R, Petit O, Pascalis O. On the species-specificity of face recognition in human adults. Cah. de Psychol. Cogn. 2004;22(3):315–333. [Google Scholar]
- 54.Sliwa J, Duhamel JR, Pascalis O, Wirth S. Spontaneous voice-face identity matching by rhesus monkeys for familiar conspecifics and humans. Proc. Natl. Acad. Sci. USA. 2011;108(4):1735–1740. doi: 10.1073/pnas.1008169108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duranton C, Gaunet F. Behavioural synchronization from an ethological perspective: Overview of its adaptive value. Adapt. Behav. 2016;24(3):181–191. doi: 10.1177/1059712316644966. [DOI] [Google Scholar]
- 56.Iki S, Kutsukake N. Social bias affects vigilance contagion in Japanese macaques. Anim. Behav. 2021;178:67–76. doi: 10.1016/j.anbehav.2021.05.019. [DOI] [Google Scholar]
- 57.Paukner A, Suomi SJ, Visalberghi E, Ferrari PF. Capuchin monkeys display affiliation toward humans who imitate them. Science. 2009;325(5942):880–883. doi: 10.1126/science.1176269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article are provided as supporting information to the article.