Summary
Background
Monoclonal antibody (mAb) and Fc-fusion protein (FcP) are highly effective therapeutic biologics. We aimed to analyse consumption and expenditure trends in 14 Asia-Pacific countries/regions (APAC) and three benchmark countries (the UK, Canada, and the US).
Methods
We analysed 440 mAb and FcP biological products using the IQVIA-MIDAS global sales database. For each year between 2010 and 2020 inclusive, we used standard units (SU) sold per 1000 population and manufacture level price (standardised in 2019 US dollars) to evaluate consumption (accessibility) and expenditure (affordability). Changes of consumption and expenditure were estimated using compound annual growth rate (CAGR). Correlations between consumption, country's economic and health performance indicators were measured using Spearman correlation coefficient.
Findings
Between 2010 and 2020, CAGRs of consumption in each region ranged from 7% to 34% and the CAGRs of expenditure ranged from 9% to 31%. The median consumption of biologics was extremely low in lower-middle-income economies (0·29 SU/1000 population) compared with upper-middle-income economies (1·20), high-income economies (40·94) and benchmark countries (109·55), although the median CAGRs of biologics consumption in lower-middle-income economies (31%) was greater than upper-middle-income (14%), high-income economies (13%) and benchmark countries (9%). Consumption was correlated with GDP per capita [Spearman's rank correlation coefficient (r) = 0·75, p < 0·001], health expenditure as a percentage of total (r = 0·83, p < 0·001) and medical doctors’ density (r = 0·85, p < 0·001).
Interpretation
There have been significant increases in mAb and FcP biologics consumption and expenditure, however accessibility of biological medicines remains unequal and is largely correlated with country's income level.
Funding
This research was funded by NHMRC Project Grant GNT1157506 and GNT1196900; Enhanced Start-up Fund for new academic staff and Internal Research Fund, Department of Medicine, LKS Faculty of Medicine, University of Hong Kong.
Keywords: Monoclonal antibodies (mAb), Fc-fusion protein, Biological medicine, Accessibility, Affordability, Unequal treatment access, Unmet needs, Healthcare systems, Healthcare professionals manpower, Asia Pacific
Research in context.
Evidence before this study
We conducted a literature search on MEDLINE and EMBASE, using the search strategies: (“consumption” OR “utilisation” OR “expenditure” OR “sale”) AND (“biologic*” OR “monoclonal antibody” OR “Fc protein”) in the title from 1 January 2010 to 13 December 2021. We found limited studies describing the consumption patterns of biologics or comparing expenditures on biologics from multiple therapeutic areas. Most of these studies described only the utilisation patterns of biologics in one type of disease (autoimmune inflammatory diseases or cancer) or described one category of biologic (antitumour necrosis factor). These studies were from European regions (France, Italy etc.), North America (Canada and USA) or high-income economies in Asia-Pacific (Japan, Taiwan, and Australia). These studies showed that the increasing use of biological therapies are significantly correlated to increasing expenditure. No study has investigated the potential unequal use of biologics amongst diverse country income levels and health systems.
Added value of this study
Our study is the first and up-to-date investigation on consumption and expenditure trends for therapeutic monoclonal antibodies and Fc-fusion proteins covering fourteen Asia-Pacific (APAC) countries/regions. Over the last 11 years, biologics consumption and expenditure increased in all the analysed countries. The annual consumption growth was greatest in middle-income economies. Large treatment access inequity existed between countries with different development levels. We found that consumption of our targeted biologics per 1000 inhabitants was highest in high-income economies – almost 1000 times the consumption in lower-middle-income economics in the same region. The accessibility of biologics is largely correlated with the country's level of economic development and health related workforce.
Implications of all the available evidence
With the development of precision immunotherapies, the consumption and healthcare expenditure of antibody biologics have increased over the last decade. There is a huge growing market in middle-income economies. In most APAC countries/regions, expenditure growth was lower than the growth rate of consumption, indicating that intensive market competition could reduce drug price and improve treatment access. There continues to be significant unmet needs for biological treatments in lower income economies and considerable inequity in biologic accessibility among APAC countries/regions. Regulating formulary listing policies, increasing healthcare budget, introduction of biosimilars to the therapeutic market and improving capacity of biological specialists could enhance accessibility and reduce inequity to biological therapies.
Alt-text: Unlabelled box
Introduction
Biologic medicines, which are produced by or derived from living organisms or their products, have revolutionised the practice of medicines that treat diseases.1 Therapeutic monoclonal antibody (mAb) and Fc-fusion protein (FcP) are new generation biological medicines and selectively block pro-inflammatory cytokines and they are used as targeted therapies for neoplasms and immunological diseases.2,3 Previous reviews and clinical studies have shown that mAb and FcP biologics have the potential to treat diseases with higher specificity and lower toxicity than conventional (non-biologic) medicines.2 FcP and mAb biologics with the highly selective targets, profound efficacy in symptom control and prognosis improvement, were developed and used in clinical practice rapidly in the last decade with growing market and drug expenditure.3,4 They have been used in a wide age range of population, covering adults, children and adolescents population.5 Research, development and production of biological medicines require significant financial investment due to complex manufacturing techniques.6 This is reflected in the higher cost to payers compared to non-biologic medicines, and casting the affordability issues for those with low incomes.
In 2020, Asia-Pacific countries/regions (APAC) made up 63% of the world's population.7 Large populations and increasing disease burden of cancer and inflammatory diseases in APAC have resulted in high demand for these therapies.8 Rapid growth of APAC economies in recent decade has led to a significant reduction in extreme poverty, however, uneven economy growth rates among these countries/regions are also accompanied by rising inequality which may result in diverse accessibility to biologic medicines.9 There are limited studies describing the consumption patterns for a variety of biologics or comparison of expenditure of biologics between countries/regions with different income levels, particularly in the APAC. In this study, using data from the IQVIA-Multinational Integrated Data Analysis System (IQVIA-MIDAS) database, we aim to investigate the market of mAb and FcP biologics in the APAC and compare consumption (accessibility) and medical expenditure (affordability) trends in APAC with Western benchmark countries. We also investigated the impact of health and economic performance indicators and considered the effects of health systems on biologics consumption to inform global medication policy on equitable access to medicines.
Methods
Data source and targeted countries
Targeted biologics’ sales data were obtained from the IQVIA-MIDAS database. IQVIA-MIDAS is a commercial database capturing medicines sold to retail and hospital pharmacies from manufacturers in different countries with standardised data to facilitate global-level analysis.10 IQVIA-MIDAS database accurately and periodically estimates product volumes, trends and market share by drug and therapy class through specifically developed algorithms.11 The dataset has been used for several drug utilisation studies with high impact.12,13
This is a cross-sectional study focused on 14 APAC countries/regions audited by IQVIA-MIDAS. Since therapeutic biologics are mainly prescribed through hospitals, we did not include APAC countries with only retail sectors coverage. A total of fourteen APAC countries/regions were included: Australia, China, Hong Kong, India, Indonesia, Japan, Korea, Malaysia, New Zealand, Philippines, Singapore, Taiwan, Thailand and Vietnam. Three high-income economies [Canada, the United States (US) and the United Kingdom (UK)], external to APAC, were selected as benchmarks to reference affordability and accessibility.
Biologics selection and classification
Our study focused on mAb and FcP biologics, which are new generation and major categories of targeted therapeutic biologics.5,14 We first generated a complete list using three-level Anatomical Therapeutic Chemical (ATC) classification system published by the World Health Organization (WHO).15 We excluded biologics used for alimentary and metabolism, genitourinary system and sex hormones, systemic hormonal preparations and anti-infectives for systemic use (e.g. insulin, analogues, hormones, immunoglobulins or vaccines etc.) to further formulate a candidate list of mAbs and FcP biologics. Next, we manually selected biologics with molecular names ending in “mab/cept”. We then classified the target biologics according to their pharmacological mechanisms of action (MOA), including tumour necrosis factor target (TNF), interleukin/interleukin receptor target (IL), lymphocyte/adhesion molecules target, check point inhibitors, growth factor/tumour cell target, and other targets. Descriptions of the selected biologics are listed in Supplementary Table 1 and Supplementary Figure 1. We further classified the biological products as bio-originators or biosimilars. We defined products as biosimilars if there were online official reports from European Medicine Agency, the US Food and Drug Administration, or local drug appoval agency. For products without an explicit report, we considered those with a specific product name but without a valid patent period as biosimilars. Other biologics were treated as bio-originators.
Data analysis
The IQVIA-MIDAS data are provided as quarterly sale volumes in standard unit (SU) and manufacturer level sales in US dollars (in the corresponding year) between 2010 and 2020 inclusive. A SU is defined as the smallest common dose of a product form, which enables comparison of biologics in different forms.13 We assumed sales volumes obtained from IQVIA-MIDAS are approximately equal to the consumption of biological medicines across countries/regions due to the high cost and storage requirements of biologics. We aggregated the quarterly data by year and calculated the number of SUs sold per thousand people in a population.16 To evaluate the expenditure of biologics, we assumed that manufacture-level sales was equal to societal medicine expenditure and used US dollars per capita as measurement. The total sales in US dollars were standardised to the value as at 2019 to avert the influence of the global pandemic in 2020, using the inflation rate obtained from International Monetary Fund (IMF).17
To estimate the increase of consumption and expenditure over time, we calculated the compound annual growth rates (CAGR) using the following equations:
where Y is the earliest market year of biologic and N is the number of years of data available.
We presented the consumption and expenditure results by country/region income level [lower-middle-income economies (LMIE), upper-middle-income economies (UMIE), and high-income economies (HIE)] according to the classification by the World Bank in 2020. Consumption and expenditure were summarised by year using mean (± standard deviation, SD) for symmetrically distributed data and median (interquartile range, IQR) for skewed/non-symmetrically distributed data.
We accessed the correlation between biologics consumption and health-social-economic development factors of the country, including 1) economic and societal development factors: gross domestic productivity (GDP) per capita, human development index (HDI), and life expectancy at birth; 2) health system performance indicators: health expenditure per capita, universal health coverage index (SCI), and medical doctors per 1000 population. Correlation of mAb/FcP biologics consumption with these indicators were assessed using Spearman's rank correlation coefficient. A correlation coefficient was deemed significant if its p-value was less than 0.05. Data on GDP per capita were collected from the IMF17 and standardised to the monetary value of US dollars in 2019. The other five indicators were collected from the WHO and the Organisation for Economic Co-operation and Development using data from the latest available year. Descriptions of these indicators are listed in Supplementary table 2.
To further investigate the impact of health systems on treatment accessibility, we classified the non-high income economics in APAC countries/regions into two different health systems [private (India, Philippines, Vietnam, and Indonesia) and public (China, Thailand and Malaysia)] according to the dominant sectors for health regulation, financing and service provision.18, 19, 20, 21, 22, 23
All data analysis and visualisation were conducted using R software version 4.3.3 (R Foundation for Statistical Computing, Vienna, Austria) and cross-checked by XT and KP.
Role of the funding source
The funding for this study supported the data acquisition, however the funding bodies had no role in study design, data analysis, interpretation nor writing of the report.
Results
We treated products with different molecule names, corporations, brand names and dosage as different products. Based on IQVIA-MIDAS data between 2010 and 2020 inclusive, we analysed 79 unique molecules in total of 440 biological products (TNF: N = 111, 25·23%; IL/ILR, N = 57, 12·95%; lymphocyte/adhesion molecules: N = 97, 22·05%; growth factor/tumour cell target: N = 115, 26·14%; check point inhibitors: N = 18, 4·09%; and other targets: N = 42, 9·55%). Bio-originators accounted for 64·32% (283 out of 440) of the total products (TNF: N = 39, 35·14%; IL/ILR, N = 56, 98·25%; lymphocyte/adhesion molecules: N = 69, 71·13%; growth factor/tumour cell target: N = 62, 53·91%; check point inhibitors: N = 18, 100%; and other targets: N = 39, 92·86%).
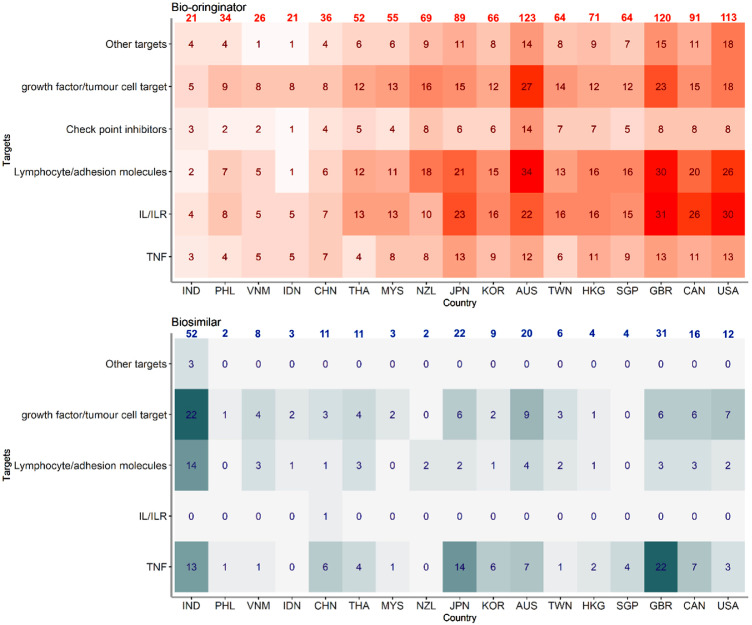
Availability of biologics in 2020
We analysed the number of available mAb and FcP products in the selected countries and observed that the number of biologics increased with economic levels in APAC (Figure 1). Available biological products ranged from 24 (N = 21 as bio-originators) in Indonesia and 143 in Australia (N = 123 as bio-originators). In terms of available biosimilars, India approved the highest number of biosimilars (N = 52, 71·23% of total available biologic products) followed by Japan (N = 22, 19·82%) and Australia (N = 20, 13·99%). In the three benchmark countries, UK approved largest number of biosimilars (N = 31, 20·53%), followed by Canada (N = 16, 14·95%) and the US (N = 12, 9·60%).
Figure 1.
Number of mAb and FcP biologics by country/region in year 2020.
Abbreviation: TNF: targeting on tumour necrosis factor; IL/ILR: targeting on interleukins/interleukin receptors; IND: India, PHL: Philippines, VNM: Vietnam, IDN: Indonesia, CHN: China, THA: Thailand, MYS: Malaysia, NZL: New Zealand, JPN: Japan, KOR: Korea, AUS: Australia, TWN: Taiwan, HKG: Hong Kong, SGP: Singapore, GBR: United Kingdom, CAN: Canada, US: United States; Countries were ordered by ascending ordered of GDP in year 2020.
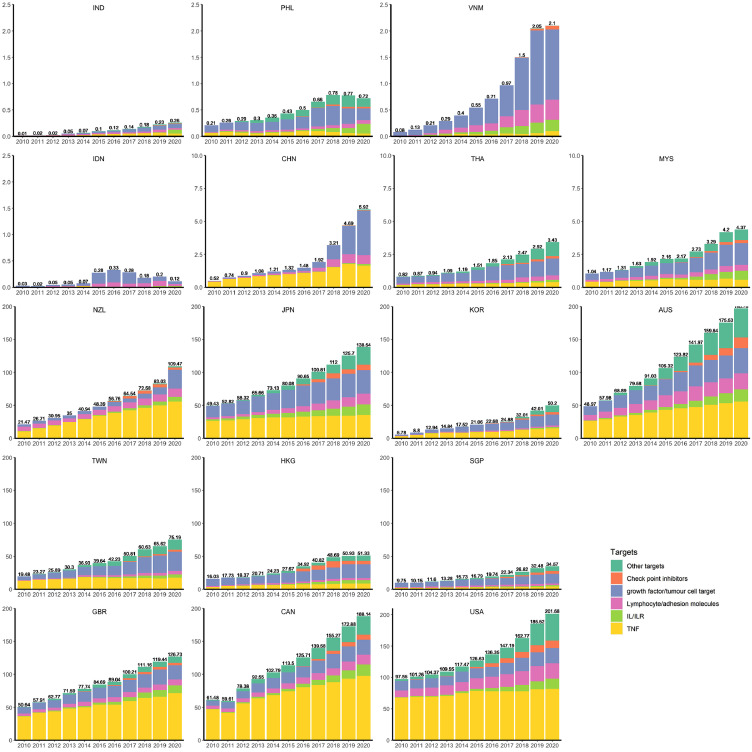
Biologics market changes in the past decade
Total consumption and compositions of biologics in different target groups changed considerably during the last 11 years (Figure 2 and Supplementary Figure 2). Compared to the higher income countries, higher uptake and earlier emergence of biosimilars are observed in middle-income countries (such as India, Vietnam, and China). (Table 1 and Supplementary Figure 3).
Figure 2.
Yearly biologics consumption (Standard Unit/1000 population) by country/region.
Abbreviation: TNF: targeting on tumour necrosis factor; IL/ILR: targeting ton interleukins/interleukin receptors; IND: India, PHL: Philippines, VNM: Vietnam, IDN: Indonesia, CHN: China, THA: Thailand, MYS: Malaysia, NZL: New Zealand, JPN: Japan, KOR: Korea, AUS: Australia, TWN: Taiwan, HKG: Hong Kong, SGP: Singapore, GBR: United Kingdom, CAN: Canada, US: United States.
Table 1.
Biologic consumption and expenditure in 2020 by countries/regions and income levels.
| Income level | Countries/regions | GDP per capita (PPP value 2019) | Consumption |
Expenditurea |
||||
|---|---|---|---|---|---|---|---|---|
| CAGR (%) | SU per 1000 population | Proportion of biosimilar (%) | CAGR (%) | US dollars per capita | Proportion of biosimilar (%) | |||
| LMIE | India | 6370 | 31 | 0·26 | 60·99 | 28 | 0·07 | 48·42 |
| Philippines | 8333 | 12 | 0·72 | 1·94 | 13 | 0·54 | 0·34 | |
| Vietnam | 10715 | 34 | 2·10 | 28·43 | 31 | 1·21 | 21·75 | |
| UMIE | Indonesia | 12049 | 14 | 0·12 | 7·05 | 10 | 0·06 | 9·18 |
| China | 16948 | 25 | 5·92 | 39·41 | 23 | 1·29 | 15·71 | |
| Thailand | 17978 | 14 | 3·43 | 7·98 | 13 | 2·48 | 4·36 | |
| Malaysia | 27015 | 14 | 4·37 | 4·18 | 10 | 2·07 | 1·85 | |
| HIE | New Zealand | 41424 | 16 | 109·47 | 6·17 | 17 | 49·67 | 1·57 |
| Japan | 41650 | 10 | 138·54 | 8·96 | 10 | 77·62 | 3·07 | |
| Korea | 43990 | 22 | 50·20 | 8·46 | 19 | 19·92 | 5·13 | |
| Australia | 50949 | 13 | 196·78 | 6·88 | 7 | 97·69 | 2·02 | |
| Taiwan | 54935 | 13 | 75·19 | 0·08 | 15 | 34·79 | 0·06 | |
| Hong Kong | 58678 | 11 | 51·33 | 1·68 | 12 | 30·71 | 0·81 | |
| Singapore | 95683 | 12 | 34·57 | 4·46 | 13 | 26·04 | 0·25 | |
| Benchmark | United Kingdom | 43493 | 9 | 126·73 | 48·10 | 11 | 79·11 | 25·29 |
| Canada | 48031 | 11 | 188·14 | 8·94 | 9 | 144·04 | 4·35 | |
| United States | 62519 | 7 | 201·68 | 4·61 | 14 | 349·68 | 2·56 | |
2020 value standardized to 2019 US dollars; CAGR: compound annual growth rate of all biological products; GDP: gross domestic productivity; PPP: purchasing power parity; SU: standard units, defined as the smallest common dose of a product form, which enables the comparison among biologics in different forms.LMIE: lower-middle income economies; UMIE: upper-middle income economies; HIE: High income economies.
The biologics that were available in all countries/regions at study inception (2010) were agents targeting lymphocyte/adhesion molecules and agents targeting TNF biologics (since 2011 for Vietnam). Check point inhibitor targeted biologics are relatively new in APAC, they were first marketed in the benchmark countries in 2011, then launched in high-income APAC economies in 2015 and recently introduced in China and Vietnam (2018). In 2020, TNF biologics accounted for the majority of the biologic market in the three-benchmark countries (USA 41%, Canada 52%, and UK 57%), New Zealand (51%) and Australia (28%) while in other APAC regions there was higher variability with biologics targeting growth factor or tumour cells representing 26 to 57% of the market.
Consumption and expenditure growth on biologics
The SU consumption per 1000 population of mAb and FcP biologic were imbalanced across the APAC and benchmark countries (Figure 2). Over the past 11 years, the median consumption of biologics in the LMIE was merely 0·29 (IQR: 0·54) SU per 1000 population, which was much lower than in that in the UMIE (median 1·20; IQR: 1·69), the high-income regions (median 40·94; IQR: 46·55) and the benchmark countries (median: 109·55; IQR: 51·67).
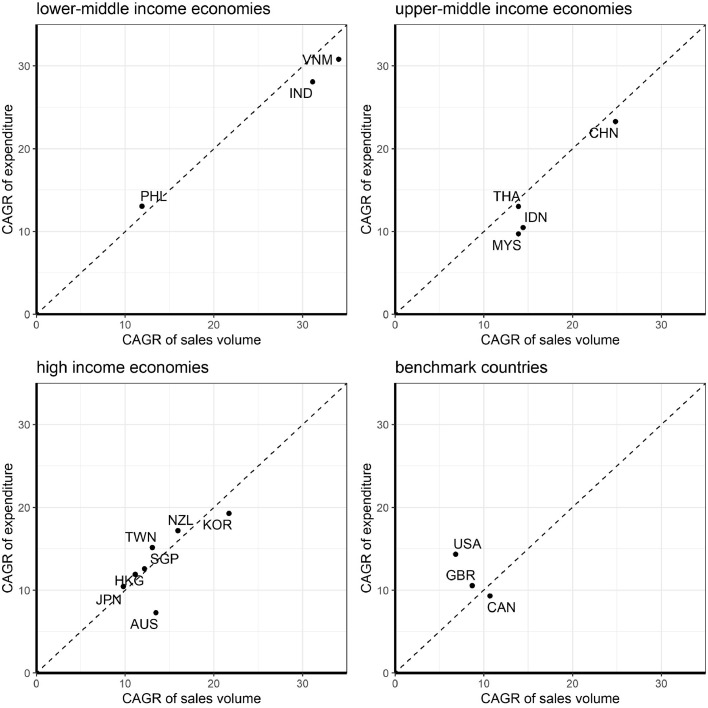
Table 1 shows the country-specific sales volume per 1000 population, expenditure of biologics per capita, proportion of biosimilar, and the corresponding CAGRs of total included biological products in each region. CAGRs of consumption ranged from 10% to 34% per year and the corresponding CAGRs of expenditure ranged from 7% to 31% per year among the APAC. The rate of increasing consumption was much higher in the LMIE than in the UMIE and the HIE in Asia Pacific. Nevertheless, in year 2020, accessibility to biologics remained considerably lower in India, Philippines, Vietnam and Indonesia than other APAC countries. The lowest consumption observed in Indonesia (0·12 SU per 1000 population) is millesimal compared to the country with the highest biologics consumption (Australia, 196·78 SU per 1000 population). We also examined the CAGRs by MOA group (Supplementary Figure 4). Overall, biologic consumption was increasing but the predominant category in each country varied. We observed that the top three growing biologics are those that target check point inhibitors followed by IL/ILR targeted or other targets. On examining the relationship between the growth rates of consumption and expenditure, we found that increase in expenditure was lower than the increase of consumption in most of the APAC countries, particularly LMIE and UMIE (Figure 3).
Figure 3.
Consumption and expenditure growths by country/region income levels.
Abbreviation: CAGR: compound annual growth rate; IND: India, PHL: Philippines, VNM: Vietnam, IDN: Indonesia, CHN: China, THA: Thailand, MYS: Malaysia, NZL: New Zealand, JPN: Japan, KOR: Korea, AUS: Australia, TWN: Taiwan, HKG: Hong Kong, SGP: Singapore, GBR: United Kingdom, CAN: Canada, US: United States.
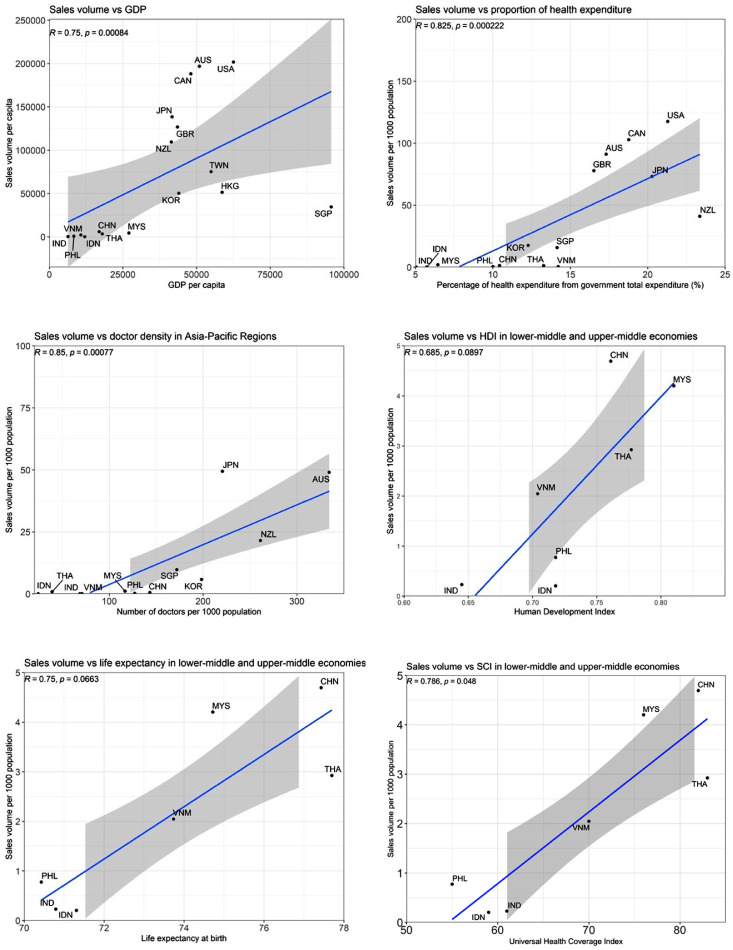
Health, economic and system factors that impact the consumption
We investigated the correlations between biologic consumption and six healthcare and economic development indicators individually (Figure 4). Among these two-by-two comparisons, we observed that consumption of biologics was significantly correlated with GDP (r = 0·75, p < 0·001), percentage of health expenditure out of total government expenditure (r = 0·83, p < 0·001) and medical doctor density (r = 0·85, p < 0·001) in all studied APAC countries. For indicators representing overall country performance (universal health coverage index, life expectancy and human development index), we observed negligible correlations within high-income APAC and benchmark countries. We further extracted LMIE and UMIE to analyse their biologics accessibility with these three factors. We found that biologics accessibility was moderately associated with life expectancy (r = 0·75, p = 0·066), human development index (r = 0·69, p = 0·090) and universal health care index (r = 0·79, p = 0·048) among these middle-income APAC countries.
Figure 4.
Correlation between consumption and health system indicators.
Abbreviation: IND: India, PHL: Philippines, VNM: Vietnam, IDN: Indonesia, CHN: China, THA: Thailand, MYS: Malaysia, NZL: New Zealand, JPN: Japan, KOR: Korea, AUS: Australia, TWN: Taiwan, HKG: Hong Kong, SGP: Singapore, GBR: United Kingdom; CAN: Canada, US: United States; GDP: gross domestic productivity; HDI: human development index; SCI: universal health coverage index.
The consumption of biologics among seven middle-income APAC economies in different health systems were compared in Supplementary Figure 5. We observed that countries/regions which were private sector-dominated had lower biologics consumption than those were public sector-dominated.
Discussion
In an era of precision medicine and population ageing, the disease and economic burden of cancer and immunological disease is rising globally.24 However, sophisticated technology used in the development and manufacture of biologics involves high drug costs and hinders equal treatment access among different income economies.24 In this study focused on 14 Asia Pacific countries, we observed an increasing trend of total biologics consumption and expenditure over the last decade with an additional increased trend of biologics focused on other targets. The pattern of biologic consumption in APAC countries/regions and benchmark countries were highly related to country development levels. In 2020, the number of available biologics in LMIE were only one-fifth compared to HIE and only a fraction of that in benchmark countries. Regarding the composition of bio-originators and biosimilars, we observed that MIE had earlier market access and higher uptake of biosimilars in the overall biologics market, such as India, Vietnam, and China in APAC. However, the high uptake of biosimilar and rapid growth of biologics consumption, suggested by CAGR, still cannot fully boost the per 1000 inhabitants’ biologics consumption in MIE, particularly for LMIE. This may be caused by several reasons: 1) high needs of biological medicines in MIE but restricted access to the bio-originators due to high cost of products;25 2) the enhanced capacity for biosimilar manufacturing and beneficial regulation for biosimilar research, development and approval in these countries cannot outweigh the great unmet needs of biologics medicine;25 and 3) large population size in LMIE which result in low access of biologics per 1000 inhibants.16 These findings suggest that governments’ regulations supports, would help improve the access to this therapeutic biologics, such as decreasing costs of experimental materials during development progress, releasing restricts on market entry of oversea products,25 improving approval pathways for biosimilars,26 and reducing monopoly protections for bio-originators.27
Our correlation analysis showed that country-specific economic, life expectancy, health system, and development levels may partially explain the discrepancies in biologic consumption. However, we acknowledge that there are other factors which could also correlate with health, social and economic factors and may have not been fully considered in the currently analysis. The in-depth mechanisms about how those factors would impact consumption would need future investigation. Considerable inequality in biologic accessibility exists among APAC countries/regions as we discovered that accessibility to biologics in MIE APAC (India and Indonesia) are only a fraction of the accessibility in several HIEs (New Zealand, Australia, and Japan). As reported in other drug consumption studies, country income measured by GDP per capita and percentage of healthcare expenditure accounting for overall governmental expenditure are the most significant drivers of novel treatment access.12,27 Potentially, the lower biologics access for people living in LMIE lies on several individual and health system aspects. First, the low individual affordability for high medical expense due to country development and economic level. Second, the life expectancy of people living in LMIE is in general lower than people living in UMIE or HIE. The former is less likely to live long enough and have lower chance of living with chronic non-communicable diseases hence the lower likelihood of biologics consumption. Third, the lack of national-level co-payment or reimbursement program for biologics further hindered the treatment accessibility in LMIE. Although the plausible mechanism of how those factors might impact the biologics access is beyond the scope of this study, further research is highly warranted to investigate the treatment access mechanism to inform health policy renovation, particularly for LMIE. We speculate that biologics consumption may be affected by a country's medical doctor density because biologics are often prescribed by medical specialists rather than being available as over-the-counter drugs.28 However, whether increasing medical doctor density will improve the patient access to biologics would need to be ascertained in future studies.
Consumption growth was faster in LMIE and UMIE than in HIE and benchmark countries. In most of the APAC countries/regions, we found that expenditure growth was lower than their corresponding consumption growth (Figure 3). The different increase rates of expenditure and consumption may be influenced by their health systems and health care policy as well as by market competition.18 For example, Australia has a government-funded Pharmaceutical Benefits Scheme which lists many biologics eligible for reimbursement for citizens. We observed that the increase in expenditure on biologics in Australia was only half of its consumption increase, thus the scheme has contributed to control drug prices, resulting in lowered expenses on biological drugs.27 In addition, the findings may be due to the bio-originator becoming off-patent and the emergence of biosimilars, which leads to intensive market competition resulting in lower prices and more product choices.29 Hence, LMIE and UMIE were able to increase their consumption of such biologics.
GDP per capita and proportion of health expenditure accounting for total government expenditure are direct economic indicators of countries’ affordability to biologics. For countries/regions with a similar economic level, HDI, life expectancy and SCI also provides a comprehensive set of indicators of countries’ development. Our results revealed that unequal biologic access in MIE was more inclined to be influenced by the level of country development, given the moderate correlation with HDI, life expectancy and SCI. However, for high-income APAC countries/regions with a similar social economic development level but different accessibilities to biologics, consumption differences cannot be explained by these three factors. The reasons are multifactorial - such as abilities of biological manufacturing and regulations regarding biosimilar medicines.
We also found that the higher proportion of private service or pricing in one health system, the lower the accessibility of new generation medicines. India, Philippines, Vietnam, and Indonesia which had inferior performances in biologic accessibility are all private sector dominated health systems. Undisputedly, countries with relatively low-income levels are unable to allocate sufficient healthcare expenditure for these new generation therapeutics. Among these four countries we observed that Vietnam, which is experiencing health system decentralization, showed the best access to therapeutic biologics.30 Our observation hints that improving the role of public sector may potentially benefit the accessibility of biological medicines in one health system, although due to the nature of study design and data availability, no causal link could be determined in this study.
This study has several limitations. Firstly, the current analysis was not able to cover all the countries and regions in APAC. For example, we did not included Pacific Island LIE who are still striving for the access to essential medicines, which would be far away for them to access the new generation therapies. We hope our findings from lower-middle income economies would serve as the reference for those countries not possibly analysed and provide some insights for future investigation in such areas. Secondly, considering the high cost and storage requirements of biologics, we assumed that biologic consumption was approximately equal to the sales volume. It was possible that we might have overestimated the consumption by using sales volume data. Thirdly, we used the manufacturer's price to assess the affordability of biologics in countries with different health systems, which may not reflect the price for the consumer. The price we used might not be equivalent to the wholesale price at institutional or government level as there will likely be a discounted price influenced by health policies. As a result, we may have overestimated expenditure in some countries with public healthcare systems. Fourthly, due to the limited available data, we could not conduct sophisticated statistics for causal inference investigation between health system indicators and biologics consumption. We acknowledge that the correlation analysis conducted does not imply causality and we cannot rule out the competing explanations for the correlation observed as common drawbacks of cross-sectional study design. Future studies are suggested to investigate the underlying causal-link using trial or quasi-experimental design with advanced statistics. Lastly, MIDAS are commercially sourced data mainly used for marketing investigation and auditing, which doesn't include clinical information, so that analysis on specific indications nor safety related events are not included in this analysis. Future studies may consider more on clinical indications.
Conclusion
In Asia-Pacific countries/regions, the uptake and healthcare spending on mAb and FcP biologics significantly increased over the last 11 years. However, accessibility to these medicines remains unequal. Apart from increasing country income and healthcare expenditure, decision-makers should also engage with the capacity building of healthcare professionals and feasible market access policies for biosimilars to reduce the inequity in biological treatment access to achieve the universal health coverage goal of the WHO sustainable development goals in the future.
Contributors
Study concept and design: X Li, ICK Wong
Data acquisition: X Li, Nicole L Pratt
Data extractions, cleaning and analysis: X Tong
Data validation and cross-check: FWT Cheng, K Peng, Jodie B Hillen, Tyman Stanford, Michael Ward
Data interpretation: all authors
Drafting of the manuscript: X Tong, X Li
Critical revision of the manuscript of significant intellectual contribution: all authors
Funding acquisition: Nicole L Pratt, ICK Wong, X Li
Study supervision: X Li
Data sharing statement
The underlying MIDAS data were provided by IQVIA under license. The terms of our agreement do not permit disclosure, sublicensing, or sharing of IQVIA MIDAS data. IQVIA will honour legitimate requests for MIDAS data from qualified researchers. Please contact IQVIA to seek approval for data access; a license fee might be applied.
Declaration of interests
XL received research grants from Food and Health Bureau of the Government of the Hong Kong SAR (HMRF, HKSAR), Research Grants Council Early Career Scheme (RGC/ECS, HKSAR), RGC Research Matching Grant Scheme, Janssen, Pfizer and internal funding from the University of Hong Kong; consultancy fee from Merck Sharp & Dohme and Pfizer, unrelated to this work. Nicole L Pratt received research grants from Australian Government. WKL received speaker fees from Ferring, Janssen and Takeda. He also participated Data Safety Advisory Board in Astra Zeneca and Pfizer. All outside the submitted work. CSL reports payment of speaker fees from AbbVie, Astra Zeneca, GSK, Janssen, Pfizer and Roche. He also has participation on Astra Zeneca Data Safety Advisory Board. All outside the submitted work. ICKW reports research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong RGC, and the Hong Kong Health and Medical Research Fund, National Institute for Health Research in England, European Commission, National Health and Medical Research Council in Australia, and also received speaker fees from Janssen and Medice the previous 3 years. He is also an independent non-executive director of Jacobson Medical in Hong Kong. All other authors have no reports on conflict of interest.
Acknowledgements
We appreciate the assistance from Dr Joseph Blais and Mr Vincent KC Yan for interpretation of the IQVIA-MIDAS dataset, Ms Miriam Tim Yin Leung on biologics selection, Mr Hin Hei Samson Yum on literature search and Miss Runqing Yang on healthcare system searching and categorisation. We also thank Ms Lisa Lam, Ms Vivien Kin Yi Chan and Ms Man Yee Leung for proofreading the manuscript.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100506.
Appendix. Supplementary materials
References
- 1.Rodgers KR, Chou RC. Therapeutic monoclonal antibodies and derivatives: Historical perspectives and future directions. Biotechnol Adv. 2016;34(6):1149–1158. doi: 10.1016/j.biotechadv.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79. doi: 10.1016/j.ejpb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Nussinov R, Jang H, Nir G, Tsai C-J, Cheng F. A new precision medicine initiative at the dawn of exascale computing. Signal Transduct Target Ther. 2021;6(1):3. doi: 10.1038/s41392-020-00420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu RM, Hwang YC, Liu IJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27(1):1. doi: 10.1186/s12929-019-0592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu XI, Dallmann A, Wang Y-M, et al. Monoclonal antibodies and fc-fusion proteins for pediatric use: dosing, immunogenicity, and modeling and simulation in data submitted to the us food and drug administration. J Clin Pharmacol. 2019;59(8):1130–1143. doi: 10.1002/jcph.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giezen T, Mantel-Teeuwisse A, Leufkens H. Pharmacovigilance of biopharmaceuticals. Drug Saf. 2009;32:811–817. doi: 10.2165/11316550-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Poot J, Roskruge M. Population Change and Impacts in Asia and the Pacific New Frontiers in Regional Science: Asian Perspectives. Springer; Singapore: 2020. Population change in the Asia-pacific region: trends, issues and models; pp. 1–26. [Google Scholar]
- 8.World Health Organization. Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000-2019.https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death. Accessed 22 June 2021
- 9.Kanbur R, Rhee C, Zhuang J. Asian Development Bank and Routledge; London and New York: 2014. Inequality in Asia and the Pacific: Trends, Drivers and Policy Implications. [Google Scholar]
- 10.Brauer R, Alfageh B, Blais JE, et al. Psychotropic medicine consumption in 65 countries and regions, 2008-19: a longitudinal study. Lancet Psychiatry. 2021;8(12):1071–1082. doi: 10.1016/S2215-0366(21)00292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein EY, Milkowska-Shibata M, Tseng KK, et al. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000-15: an analysis of pharmaceutical sales data. Lancet Infect Dis. 2021;21(1):107–115. doi: 10.1016/S1473-3099(20)30332-7. [DOI] [PubMed] [Google Scholar]
- 12.Ju C, Wei L, Man KKC, et al. Global, regional, and national trends in opioid analgesic consumption from 2015 to 2019: a longitudinal study. Lancet Public Health. 2022;7(4):e335–e346. doi: 10.1016/S2468-2667(22)00013-5. [DOI] [PubMed] [Google Scholar]
- 13.Hsia Y, Sharland M, Jackson C, Wong ICK, Magrini N, Bielicki JA. Consumption of oral antibiotic formulations for young children according to the WHO access, watch, reserve (AWaRe) antibiotic groups: an analysis of sales data from 70 middle-income and high-income countries. Lancet Infect Dis. 2019;19(1):67–75. doi: 10.1016/S1473-3099(18)30547-4. [DOI] [PubMed] [Google Scholar]
- 14.Strohl WR, Strohl LM, editors. Therapeutic antibody engineering. Woodhead Publishing; 2012. Introduction to biologics and monoclonal antibodies; pp. 1–13. [Google Scholar]
- 15.World Health Organization. WHO Collaborating Centre for DrugStatistics Methodology 2021 November 24. https://www.whocc.no/. Accessed 3 November 2020.
- 16.United Nations. Data Query, Annual Total Population at Mid-Year (thousands). https://population.un.org/wup/DataQuery/. Accessed 24 June 2021.
- 17.International Monetary Fund. World economic outlook database.https://www.imf.org/en/Publications/WEO/weo-database/2021/April/weo-report?c=111,&s=PCPIPCH,&sy=2010&ey=2020&ssm=0&scsm=1&scc=0&ssd=1&ssc=0&sic=0&sort=country&ds=.&br=1. Accessed 24 June 2021.
- 18.Takashima K, Wada K, Tra TT, Smith DR. A review of Vietnam's healthcare reform through the direction of healthcare activities (DOHA) Environ Health Prev Med. 2017;22(1):74. doi: 10.1186/s12199-017-0682-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blumenthal D, Hsiao W. Lessons from the East–China's rapidly evolving health care system. N Engl J Med. 2015;372(14):1281–1285. doi: 10.1056/NEJMp1410425. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization . 2015. Regional Office for the Western P. The Kingdom of Thailand health system review.https://apps.who.int/iris/handle/10665/208216 Accessed 21 April 2022. [Google Scholar]
- 21.World Health Organization . 2012. Regional Office for the Western P. Malaysia Health System Review.https://apps.who.int/iris/handle/10665/206911 Accessed 21 April 2022. [Google Scholar]
- 22.Dayrit MM, Lagrada LP, Picazo OF, Pons MC, Villaverde MC. The Philippines health system review. 2018. https://apps.who.int/iris/handle/10665/274579.
- 23.Mahendradhata Y, Trisnantoro L, Listyadewi S, et al. 2017. The Republic of Indonesia Health System Review.https://apps.who.int/iris/handle/10665/254716 Accessed 21 April 2022. [Google Scholar]
- 24.Coiffier B. Preparing for a new generation of biologic therapies: understanding the development and potential of biosimilar cancer therapeutics. Future Oncol. 2017;13(15s):1–3. doi: 10.2217/fon-2017-0157. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Xue S, Lv M, Wang R. Innovation, Economic Development, and Intellectual Property in India and China: Comparing Six Economic Sectors. Springer; Singapore: 2019. Pharmaceutical industry in China: policy, market and IP; pp. 215–250. [Google Scholar]
- 26.Nash L. Fast-track approval of medicines in Australia. Lancet Oncol. 2016;17(11):1487. doi: 10.1016/S1470-2045(16)30492-2. [DOI] [PubMed] [Google Scholar]
- 27.Gleeson D, Townsend B, Lopert R, Lexchin J, Moir H. Financial costs associated with monopolies on biologic medicines in Australia. Aust Health Rev. 2019;43(1):36–42. doi: 10.1071/AH17031. [DOI] [PubMed] [Google Scholar]
- 28.Hodkinson B, Blockman M. Strategies and ethics to ensure equitable access to biological medicines in the treatment of autoimmune inflammatory diseases. Curr Opin Allergy Clin Immunol. 2018;31(4):240–244. [Google Scholar]
- 29.Dutta B, Huys I, Vulto AG, Simoens S. Identifying key benefits in european off-patent biologics and biosimilar markets: it is not only about price! BioDrugs. 2020;34(2):159–170. doi: 10.1007/s40259-019-00395-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen HV, Debattista J, Pham MD, et al. Vietnam's healthcare system decentralization: how well does it respond to global health crises such as covid-19 pandemic? Asia Pac J Public Health. 2021;16(1):47–51. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.