Abstract
SARS-CoV-2 infection manifestation has great diversity and it becomes even greater while co-infection occurs or there is a serious underlying disease in an affected patient. In this case report, we present a case of a 71-year-old man who underwent a chest CT scan following the development of fever, weakness, and pulmonary symptoms. Chest CT scan showed segmental consolidation with centrilobular nodular infiltration, ground glass opacifications in the inferior segment of the left upper and lower lobes, and left lung pleural thickening which was atypical for either COVID-19 infection or pneumocystis carinii pneumonia but his SARS-CoV-2 PCR result was positive and he received COVID-19 treatment. His symptoms recurred after a few months with the same chest CT findings and subsequent bronchoalveolar lavage revealed the presence of pneumocystis carinii infection. Consequently, he received cotrimoxazole which caused improvement in symptoms, nonetheless splenomegaly and anemia remained in his clinical and laboratory investigation. Accordingly, bone marrow study and flow cytometry was done and confirmed the previously undiagnosed hairy cell leukemia. This case accentuates the fact that when we face atypical clinical or paraclinical features in a COVID-19 patient, we should explore for coinfection or unknown underlying diseases.
Keywords: COVID-19, SARS-CoV-2, Pneumocystis carinii, Pneumocystis Jirovecii, Infection, Hairy cell leukemia, Pneumonia, Chest CT scan, Bone marrow, Flow cytometry
Introduction
The consequences of the rapidly evolving COVID-19 pandemic do not require to be highlighted [1,2]. SARS-CoV-2 infection has various clinical pictures and its presentation could be more complex when there is co-infection or background of serious illness. Moreover, given the uncertainty in the presentation of COVID-19 and the varying sensitivities of laboratory tests for SARS-CoV-2, other etiologies may be overlooked when making a diagnosis of COVID-19 without a high degree of suspicion [3].
Despite the high focus on COVID-19 in the clinical setting, reports are emerging of missed or delayed treatments for diseases that mimic COVID-19, such as pneumonia caused by the fungus pneumocystis carinii [3]. Clinically, COVID-19 and pneumocystis carinii pneumonia (PCP) may exhibit similar features of dry cough and relatively normal chest auscultation. Common chest CT findings in both conditions include thickening of the interlobular septa with ground-glass opacities [3,4].
On the other hand, malignancy is among the risk factors which intensify the COVID-19 severity with higher rates of intensive care, artificial mechanical ventilation, and even death [2,5,6]. Hairy cell leukemia (HCL) is a rare and indolent tumor of variable cytology and immunophenotype consisting of oval nuclei and abundant cytoplasm with so-called hairy protrusions involving peripheral blood, bone marrow, and spleen [6].
Herein, we report a complicated case of coexisting COVID-19 and pneumocystis carinii infection with an atypical chest CT scan finding in a patient who was finally diagnosed with underlying HCL.
Case report
A previously healthy 71-year-old man presented to a primary care physician with fever, cough, and weakness during the COVID-19 pandemic. The patient had been in his usual state of health until 14 days before presentation when fever and cough developed. During an evaluation by his physician, he was reportedly told that he had had acute sinusitis and had been prescribed amoxicillin and acetaminophen which did not result in symptoms abatement. After 10 days, he noted the development of dyspnea at rest. Accordingly, the patient was referred to the emergency department of our hospital for further assessment. He was an ex-smoker but he did not use illicit drugs or alcohol and he lived in an apartment with his wife. The patient reported that he did not receive any type of COVID-19 vaccine prior to his admission and had no known close contact with COVID-19-infected patients.
On examination, the temperature was 38.5°C, the blood pressure 110/75 mm Hg, the pulse 98 beats per minute, the respiratory rate 25 breaths per minute, and the oxygen saturation 92%, while the patient was breathing ambient air. The respiratory rate decreased to 22 breaths per minute and the oxygen saturation improved to 96% with the administration of supplemental oxygen through a nasal cannula at a rate of 4 liters per minute. The body-mass index (the weight in kilograms divided by the square of the height in meters) was 23. On chest examination, mild retractions were noted in the supraclavicular areas, inspiratory coarse crackles could be heard in the left lung, the heart sounds were regular, with tachycardia but no murmur, and spleen was palpable on abdominal examination. The rest of the systemic physical examinations were otherwise unremarkable.
In laboratory data, the white cell count was 1300 per cubic millimeter (reference range: 4500-11,000, with 82% neutrophils, and 16% lymphocytes). Hemoglobin level was 11.1 gr per deciliter (reference range: 13-17) and the platelet count was 148,000 per cubic millimeter (reference range: 150,000-400,000). The reverse transcription-polymerase chain reaction (RT-PCR) test was positive, qualitative C-reactive protein titer was high (3+), erythrocyte sedimentation rate was 84 millimeter per hour (reference range: 0-13), serum lactate dehydrogenase was1478 unit per liter (reference range: 140-280) and serum ferritin was 850 microgram per liter (reference range: 20-300). Other laboratory results were otherwise unremarkable.
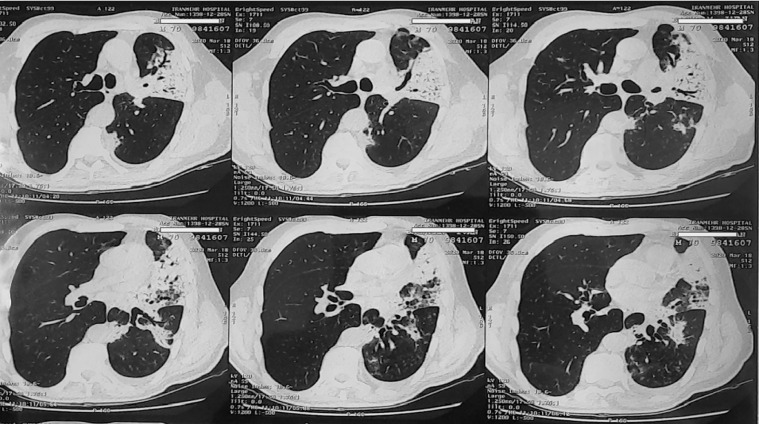
Chest CT scan showed segmental consolidation with centrilobular nodular infiltration, ground-glass opacifications in the inferior segment of the left upper and lower lobes and left lung pleural thickening (Fig. 1).
Fig. 1.
Segmental consolidation with centrilobular nodular infiltration and ground glass opacifications in the posterior segment of left lower lobes.
With the diagnosis of COVID-19 infection and superimposed bacterial pneumonia, the patient received intravenous remdesivir and oral levofloxacin. He was discharged in relatively stable condition 6 days after admission.
After 4 months of discharge, he was brought to the emergency department with persistent symptoms of dyspnea on exertion, fatigue, cough, and lethargy. On arrival during the current presentation, he had a respiratory rate of 24 breaths per minute, and an oxygen saturation of 93% while he was breathing ambient air. Auscultation of the lungs was notable for coarse crackles in the middle of the left lung. Laboratory studies showed a leukocyte count of 1300 per cubic millimeter (reference range, 4500-11,000 with 87% neutrophils), hemoglobin 10 (g/dl) (reference range: 13-17), and platelet count of 127,000 (reference range: 150,000-400,000).
Repeated chest CT revealed the same finding as the previous imaging. Considering the persistence of the pathology, the patient underwent bronchoscopy and bronchoalveolar lavage (BAL), and the result for PCP PCR was positive. The HIV test resulted in a negative. Subsequently, the patient received oral co-trimoxazole for 14 days with a dramatic response to treatment. The patient's general condition improved, exertional dyspnea resolved and oxygen saturation reached 95% while the patient was breathing ambient air.
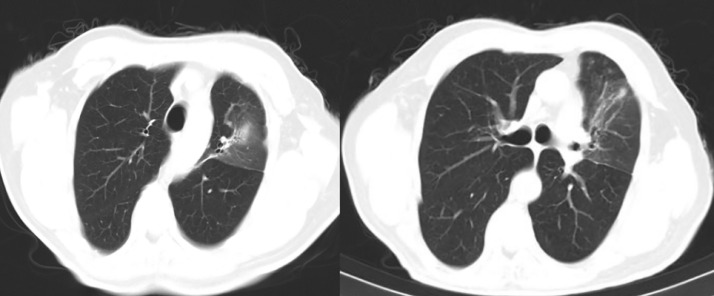
The chest CT after treatment demonstrated consolidation had been resolved and only fibrotic bands remained at the site of the lesion (Fig. 2).
Fig. 2.
Resolving pattern of left upper lobe consolidation following treatment with co-trimoxazole.
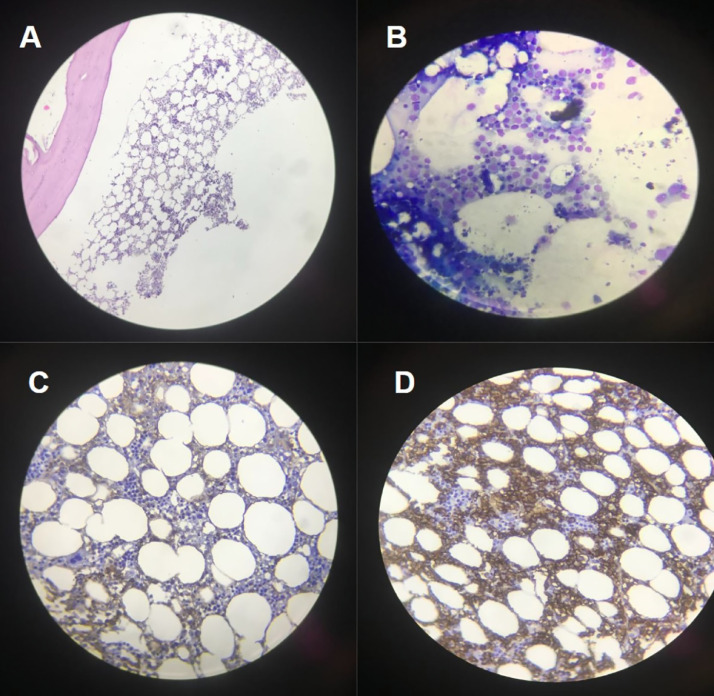
Despite the resolution of symptoms and chest CT findings with the treatment of COVID-19 and PCP, his anemia and splenomegaly (which was confirmed by ultrasonography) persisted. To investigate the cause of these 2 phenomena, bone marrow aspiration and biopsy (BM A/B), and flow cytometry analysis were performed. BMA/B are shown in Fig. 3 below and flow cytometry-reported immunophenotyping of bone marrow with gating mononuclear region revealed cells which are positive for CD11c, CD19, CD20, CD103, CD200, KAPPA, & FMC7. According to these results, the diagnosis of an underlying HCL was confirmed. Given hematology-oncology consult, there was no therapeutic indication for the patient's HCL and he currently follows routine follow-up visits by hemato-oncologist and is doing well without recurrence of symptoms.
Fig. 3.
(A and B) Bone marrow biopsy and aspiration show some rather small atypical lymphoid like cells with open chromatin which consist of 25% of nucleated bone marrow cells (Hairy cell index: 25%), (C and D) immunohistochemical staining (IHC) indicates the aforementioned atypical cells are positive for CD10 and Annexin-A1, respectively, and also for CD20 and patchy DBA44 (not shown).
Discussion
COVID-19 infection solitarily has myriad manifestations and complications which can make its diagnosis challenging and this challenge will be more difficult when co-infection occurs or there is an underlying disease that can affect the COVID-19 infection disease course [7,8]. PCP infection in a COVID-19-infected patient occurred either in the context of immunosuppression due to HIV infection or using long-term immunosuppressives like high-dose corticosteroids but we reported the SARS-CoV-2 and PCP infection in an individual with a background of HCL which is for the first time to the best of our knowledge [9].
Two pathophysiological pathways were presumed for the development of PCP infection in this patient; one due to COVID-19 infection itself and the other owing to HCL.
Previous studies appeared that as numerous as 19% of patients with COVID-19 have co-infections [10]. In some cases, a dysfunctional immune response occurs, leaving COVID-19 patients vulnerable to lung coinfection. This dysregulated immune response represents an immunosuppressive stage after the pro-inflammatory stage characterized by a sustained and significant reduction in peripheral lymphocyte counts [11]. Severe lymphocytopenia (<1000 cells/mm3) is one of the most important risk factors in most patients with COVID-19 and PCP coinfection [12]. Our patient had an absolute lymphocyte count of 200 at the beginning of the visit, and despite COVID-19 treatment, the patient's lymphocyte count remained low. However, this reduction in lymphocytes may pertain to COVID-19 or the patient's underlying malignancy.
Infection is the most common cause of death in patients with HCL. Bacterial, viral, fungal, and opportunistic infections should be anticipated in all patients. Although the underlying defect in HCL is usually a decrease in neutrophils and monocytes, Patients with HCL have compromised immune systems due to the disease's effect on immune function [6,13]. The resulting immunodeficiency could provide the basis for co-infection with opportunistic infections in our case.
Given the chest CT findings of COVID-19 and PCP, the most common ones are bilateral, multifocal rounded, and peripheral ground-glass opacity [14,15].
Nevertheless, the findings of the chest CT in our patient were in favor of unilateral lobar pneumonia, which is neither typical for COVID-19 nor PCP. For this reason and due to the persistence of clinical symptoms and radiological abnormalities despite treatment and considering negative COVID-19 PCR, bronchoscopy was planned to further evaluate the patient. Bronchoalveolar lavage fluid is still the gold standard for PCP diagnosis but bronchoscopy to collect bronchoalveolar lavage samples is an invasive procedure that is not always easy in patients with severe hypoxia. Additional attention should be paid to the procedure due to the risk of aerosolization of SARS-CoV-2 [16]. In our case, obtaining a bronchial alveolar lavage specimen led to the diagnosis of co-infection with PCP, which was clinically improved following effective treatment with cotrimoxazole. Eventually remaining anemia, high level of ferritin, and the presence of unexplained splenomegaly despite clinical improvement and CT imaging led to bone marrow biopsy which established the diagnosis of HCL.
Finally, it merits consideration that there is increasing recognition of PCP co-occurring with COVID-19, primarily in immunocompromised individuals therefore, it is recommended to continue follow-up of COVID-19 patients after discharge, especially in patients who do not have a favorable clinical response or have abnormal imaging and laboratory findings [10,15,16].
Conclusion
This case presentation underscores the fact that when we confront a patient affected by SARS-CoV-2 infection but with associated unusual features in either chest CT or laboratory data, investigate for coinfection (like PCP in our case) or unknown underlying diseases (such as HCL in our case). As a result, with earlier detection of these associated diseases and their management, the physicians could reduce disease sequels and provide more favorable outcomes for COVID-19 patients.
Author contribution
All authors contributed to the preparation and finalization of this article.
Acknowledgments
Acknowledgment
The authors would like to express their gratitude to the staff of the Rasool Akram Medical Complex Clinical Research Development Center (RCRDC) for their technical and editorial assistance.
Patient consent
Written informed consent was obtained from the patient for publishing the images for scientific and educational purposes.
Footnotes
Funding: We received no funding for this article.
Competing Interests: All authors declare no conflict of interest.
References
- 1.Seirafianpour F, Pourriyahi H, Gholizadeh Mesgarha M, Pour Mohammad A, Shaka Z, Goodarzi A. A systematic review on mucocutaneous presentations after COVID-19 vaccination and expert recommendations about vaccination of important immune-mediated dermatologic disorders. Dermatol Ther. 2022:e15461. doi: 10.1111/dth.15461. Epub ahead of printPMID: 35316551; PMCID: PMC9111423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bidari A, Hassanzadeh M, Naderkhani M, Gholizadeh Mesgarha M, Pour Mohammad A, Azadeh A, et al. Predictors of critical COVID-19 in an Iranian population: age and disabilities play a special role. Med J Islam Repub Iran. 2021;35:94. doi: 10.47176/mjiri.35.94. PMID: 34956940; PMCID: PMC8683779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly S, Waters L, Cevik M, Collins S, Lewis J, Wu MS, et al. Pneumocystis pneumonia, a COVID-19 mimic, reminds us of the importance of HIV testing in COVID-19. Clin Med (Lond) 2020;20(6):590–592. doi: 10.7861/clinmed.2020-0565. PMID: 33199326; PMCID: PMC7687333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman H, Snell LB, Simons R, Douthwaite ST, Lee MJ. Coronavirus disease 2019 and Pneumocystis jirovecii pneumonia: a diagnostic dilemma in HIV. AIDS. 2020;34(8):1258–1260. doi: 10.1097/QAD.0000000000002571. PMID: 32501852; PMCID: PMC7309642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tassone D, Thompson A, Connell W, Lee T, Ungaro R, An P, et al. Immunosuppression as a risk factor for COVID-19: a meta-analysis. Intern Med J. 2021;51(2):199–205. doi: 10.1111/imj.15142. PMID: 33631862; PMCID: PMC8014211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohla S, Ibrahim FA, Aldapt MB, ELSabah H, Mohamed S, Youssef R. A rare case of hairy cell leukemia with unusual loss of CD123 associated with COVID-19 at the time of presentation. Case Rep Oncol. 2020;13(3):1430–1440. doi: 10.1159/000512830. Published 2020 Dec 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pour Mohammad A, Mashayekhi F, Seirafianpour F, Gholizadeh Mesgarha M, Goodarzi A. COVID-19 and COVID-19 vaccine-related dermatological reactions: an interesting case series with a narrative review of the potential critical and non-critical mucocutaneous adverse effects related to virus, therapy, and the vaccination. Clin Case Rep. 2022;10(4):e05775. doi: 10.1002/ccr3.5775. PMID: 35498347; PMCID: PMC9040420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bahadorizadeh L, Emamikhah M, Pour Mohammad A, Gholizadeh Mesgarha M. Simultaneous occurrence of cerebral venous sinus thrombosis and immune thrombocytopenic purpura in a patient with a history of COVID-19 infection. Neurol Ther. 2022;11(1):491–497. doi: 10.1007/s40120-021-00294-9. Epub 2021 Oct 29. PMID: 34714517; PMCID: PMC8554500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdoli A, Falahi S, Kenarkoohi A. COVID-19-associated opportunistic infections: a snapshot on the current reports. Clin Exp Med. 2021:1–20. doi: 10.1007/s10238-021-00751-7. Epub ahead of printPMID: 34424451; PMCID: PMC8381864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musuuza JS, Watson L, Parmasad V, Putman-Buehler N, Christensen L, Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: a systematic review and meta-analysis. PLoS One. 2021;16(5) doi: 10.1371/journal.pone.0251170. PMID: 33956882; PMCID: PMC8101968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong WH, Saha BK, Ramani A, Chopra A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection. 2021;49(4):591–605. doi: 10.1007/s15010-021-01602-z. doi: 10.1007/s15010-021-01602-z. Epub 2021 Mar 11. PMID: 33709380; PMCID: PMC7951131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raffaelli F, Tanzarella ES, De Pascale G, Tumbarello M. Invasive respiratory fungal infections in COVID-19 critically ill patients. J Fungi (Basel) 2022;8(4):415. doi: 10.3390/jof8040415. Published 2022 Apr 17. doi:10.3390/jof8040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraut E. Infectious complications in hairy cell leukemia. Leuk Lymphoma. 2011;52(suppl 2):50–52. doi: 10.3109/10428194.2011.570819. Epub 2011 Apr 19. PMID: 21504285. [DOI] [PubMed] [Google Scholar]
- 14.Barben J, Quipourt V, Vovelle J, Putot A, Manckoundia P. Not COVID-19, don't overlook pneumocystis in patients on Gefitinib! Curr Oncol. 2021;28(1):961–964. doi: 10.3390/curroncol28010094. Published 2021 Feb 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong WH, Saha BK, Chopra A. Narrative review of the relationship between COVID-19 and PJP: does it represent coinfection or colonization? Infection. 2021;49(6):1079–1090. doi: 10.1007/s15010-021-01630-9. Epub 2021 May 31. PMID: 34059997; PMCID: PMC8166366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubiano C, Tompkins K, Sellers SA, Bramson B, Eron J, Parr JB, et al. Pneumocystis and severe acute respiratory syndrome coronavirus 2 coinfection: a case report and review of an emerging diagnostic dilemma. Open For um Infect Dis. 2020;8(1):ofaa633. doi: 10.1093/ofid/ofaa633. Published 2020 Dec 18. [DOI] [PMC free article] [PubMed] [Google Scholar]