Abstract
Introduction
BRAF variants were reported resistant mechanisms to EGFR tyrosine kinase inhibitors (TKIs) in EGFR-mutant NSCLC. Nevertheless, characteristics and subsequent treatment strategies of such patients remain unclear.
Methods
From October 2016 to May 2020, patients with advanced NSCLC for whom next-generation sequencing detected mutations of both BRAF and EGFR were retrospectively included. From June 2020 to January 2021, patients with EGFR-mutant NSCLC who acquired the BRAF V600E mutation after progression on osimertinib were prospectively enrolled to explore the efficacy and safety of EGFR plus BARF co-inhibition.
Results
A total of 58 patients were retrospectively identified and five prospectively included. BRAF variants were acquired after a median time of 22.7 months from initial diagnosis. The frequency of variations in TP53, PIK3CA, RB1, MET, LRP1B, APC, CDKN2A, MYC, ERBB2, and SMAD4 was all more than 10%; these mutations affected the cell cycle or p53 pathway and the EGFR downstream and bypass pathways. The median progression-free survival was 5.0 months for patients on chemotherapy and 2.1 months for those on TKIs not targeting both of EGFR and BRAF (p = 0.019). The median PFS was 7.8 months in five patients who received EGFR plus BRAF co-inhibitory drugs. RAS signaling was activated on disease progression.
Conclusions
Variations in the EGFR downstream and bypass pathways were frequent in patients with dual mutations of EGFR and BRAF. The efficacies of TKIs not targeting both EGFR and BRAF were inferior to chemotherapy. EGFR plus BRAF co-inhibition improved efficacy. Such treatment strategies should be further explored.
Keywords: BRAF, EGFR, NSCLC, Characteristics, Treatment strategy
Introduction
EGFR tyrosine kinase inhibitors (TKIs) are routinely given to patients with advanced EGFR-mutant NSCLC. Nevertheless, acquired resistance to such drugs is inevitable, and treatment remains challenging. Resistance mechanisms include activation of EGFR-dependent or -independent pathways and histologic transformation.1 The mechanisms of EGFR-dependent drug resistance have been well studied and include the acquired T790M mutation for first- or second-generation EGFR TKIs and the acquired C797S mutation for the third-generation EGFR TKI.2,3 EGFR-independent resistance is less common, and more complicated, including amplification of ERBB2 and MET and mutation of PI3KCA, KRAS, and BRAF.4,5
Acquired BRAF mutations or fusions develop in 1% to 3% patients with NSCLC who receive EGFR TKIs.4,5 The RAS-RAF-MEK-ERK signaling pathway is a key regulator of cell growth; BRAF (a serine/threonine kinase) operates downstream of RAS. Usually, three classes of BRAF mutations are recognized based on the different activation mechanisms, which are as follows: RAS-independent active monomers (class 1), constitutively active dimers (class 2), and kinase-dead or -impaired mutations (class 3).6,7 BRAF V600 encodes an active monomer; most other BRAF mutants are constitutively active RAS-independent dimers. In clinical trials, vemurafenib or dabrafenib plus trametinib effectively treated patients with NSCLC with BRAF V600E mutation but not those with BRAF non-V600E mutations.8,9 Given the increasing accessibility of next-generation sequencing (NGS) in clinical practice, both BRAF V600E and non-V600E mutations are now frequently identified in tumors. Nevertheless, the clinical significance of acquired BRAF variants remains poorly understood.
In vitro, an acquired BRAF V600E mutation rendered some EGFR-mutant cell lines insensitive to EGFR TKIs; such insensitivity was eliminated by BRAF inhibitors.10,11 Several case studies have reported that combinations of EGFR TKIs and BRAF inhibitors were effective (mediating EGFR + BRAF co-inhibition).12,13 Nevertheless, no clinical trial on the safety and efficacy of such co-inhibition has been performed. Aboubakar Nana and Ocak14 reviewed case reports on the efficacies and toxicities of EGFR plus BRAF co-inhibition and suggested that combination strategies might be appropriate.14,15 Here, we retrospectively studied the clinical and genetic characteristics of patients with EGFR plus BRAF-mutant NSCLC and their prognoses. We also prospectively investigated the efficacy and safety of EGFR plus BRAF co-inhibition.
Materials and Methods
Study Patients
From October 2016 to May 2020, patients with advanced NSCLC with EGFR and BRAF co-mutations were retrospectively included. Clinical information was extracted from the electronic medical records of Guangdong Provincial People’s Hospital. From June 2020 to January 2021, patients with acquired BRAF V600E mutation after failure of osimertinib were recommended to accept an EGFR plus BRAF co-inhibition therapy. This study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital (approval number GDREC2019304H). Written informed consent was provided by all participants. The study adhered to the principles of the Declaration of Helsinki.
Next-Generation Sequencing
At initial diagnosis or disease progression, tumor tissues or body fluids (pleural effusion, plasma, cerebrospinal fluid) were collected from all patients and subjected to panel NGSs exploring the status of 168, 196, 425, or 520 cancer-relevant genes.16,17 A total of 75 genes evaluated by all four panels were analyzed in the present study. The clinical significance of BRAF mutations or fusions was that of the annotations of OncoKB (https://www.oncokb.org/) (a Food and Drug Administration–recognized, public, human genetic variant database).18 Tier Ⅰ and Ⅱ variants are considered clinically significant, but tier Ⅲ and Ⅳ variants are insignificant.19
Treatment and Efficacy
Patients initially received oral osimertinib 80 mg once daily plus vemurafenib 480 mg twice daily or osimertinib 80 mg once daily plus dabrafenib 150 mg twice daily plus trametinib 2 mg once daily until disease progression or the development of unacceptable adverse events. Computed tomography and brain magnetic resonance imaging (if needed) were performed 4 weeks after treatment initiation and then every 8 weeks. Responses were evaluated based on the Response Evaluation Criteria in Solid Tumors version 1.1 criteria. Progression-free survival (PFS) was defined as the time from the start of treatment to disease progression or death. Adverse events were recorded. Overall survival (OS) was defined as the time from identification of BRAF variants to death from any cause. Global OS was defined as the time from the initial diagnosis to death from any cause.
Statistical Analysis
Categorical variables were compared using the chi-square or Fisher’s exact test. The nonparametric test used was the ranked sum test. In terms of survival analyses, Kaplan-Meier curves were compared using the log-rank test and hazard ratios (HRs) calculated using the Cox’s proportional hazards model. A two-sided p value less than 0.05 was considered significant. All statistical analyses were performed by IBM SPSS version 22.0 software.
Results
Patient Characteristics
A total of 58 patients were retrospectively identified and five patients were prospectively included (Supplementary Fig. 1). In total, 63 patients with EGFR-mutant NSCLC with concomitant BRAF mutations (n = 53) or fusions (n = 10) were included. Clinical and demographic characteristics are listed in Table 1. The BRAF variants were V600E (44.4%, 28 of 63), non-V600E (39.7%, 25 of 63), and fusions (15.9%, 10 of 63) (Fig. 1A); 52.4% (33 of 63) of BRAF mutations occurred in the tyrosine kinase domain (Fig. 1B); and 90.5% patients (57 of 63) developed BRAF variants on disease progression. A total of 49 patients exhibited clinically significant BRAF mutations or fusions (Supplementary Table 1).
Table 1.
Demographic and Clinical Characteristics of Cohort Patients
| Characteristics | Total (N = 63) | V600E (n = 28) | Non-V600E (n = 25) | Fusion (n = 10) | p |
|---|---|---|---|---|---|
| Age (y), median | 0.412 | ||||
| ≤60 | 39 (61.9) | 17 (60.7) | 14 (56.0) | 8 (80.0) | |
| >60 | 24 (38.1) | 11(39.3) | 11 (44.0) | 2(20.0) | |
| Sex, n (%) | 0.218 | ||||
| Female | 39 (61.9) | 14 (50.0) | 18 (72.0) | 7 (70.0) | |
| Male | 24 (38.1) | 14 (50.0) | 7 (28.0) | 3 (30.0) | |
| Smoking, n (%) | 0.215 | ||||
| Yes | 10 (15.9) | 5 (17.9) | 2 (8.0) | 3 (30.0) | |
| No | 53 (84.1) | 23 (82.1) | 23 (92.0) | 7 (70.0) | |
| Pathology, n (%) | 1 | ||||
| ADC | 62 (98.4) | 27 (96.4) | 25 (100.0) | 10 (100.0) | |
| ASC | 1 (1.6) | 1 (3.6) | 0 (0.0) | 0 (0.0) | |
| Stage, n (%) | 0.691 | ||||
| ⅢB–ⅣA | 12 (19.0) | 4 (14.3) | 2 (8.0) | 0 (0.0) | |
| ⅣB | 51 (81.0) | 24 (85.7) | 23 (92.0) | 10 (100.0) | |
| Brain metastasis, n (%) | 0.59 | ||||
| Yes | 33 (52.4) | 16 (57.1) | 11 (44.0) | 6 (60.0) | |
| No | 30 (47.6) | 12 (42.9) | 14 (56.0) | 4 (40.0) | |
| PS, n (%) | 1 | ||||
| 0–2 | 61 (93.7) | 27 (96.4) | 24 (96.0) | 10 (100.0) | |
| 3 | 2 (6.3) | 1 (3.6) | 1 (4.0) | 0 (0.0) | |
| EGFR subtype, n (%) | 0.374 | ||||
| L858R | 30 (47.6) | 13 (46.4) | 9 (36.0) | 3 (30.0) | |
| 19Del | 33 (52.4) | 15 (53.6) | 16 (64.0) | 7 (70.0) | |
| EGFR T790M status, n (%) | 0.037 | ||||
| Yes | 37 (58.7) | 19 (67.9) | 10 (40.0) | 8 (80.0) | |
| No | 26 (41.3) | 9(32.1) | 15 (60.0) | 2 (20.0) | |
ADC, adenocarcinoma; ASC, adenosquamous carcinoma; PS, performance status.
Figure 1.
(A) The distribution of BRAF variants; (B) the distribution of BRAF mutations; and (C) the oncoprints of the clinical information and the gene profiles of 63 patients; genes that were altered at rates greater than 5% are illustrated. amp, amplification; del, deletion; SV, structural variation.
Clinical Characteristics of EGFR-Mutant NSCLC With Concomitant BRAF Variations
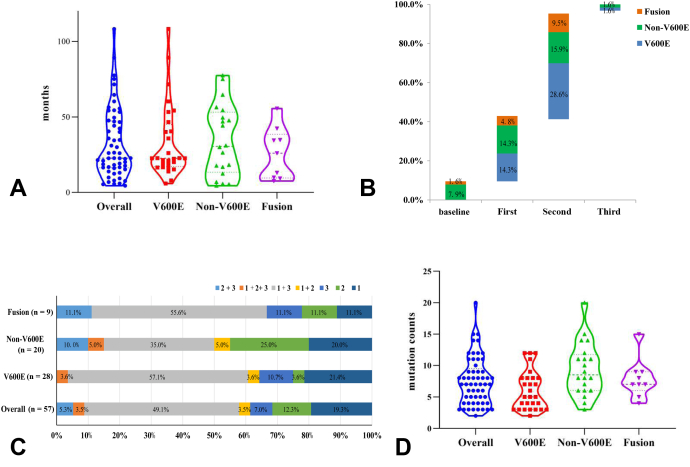
The median time from initial diagnosis of advanced NSCLC to the detection of acquired BRAF variations was 22.7 months (95% confidence interval [CI]: 26.4–38.7 mo); the times were similar for the three BRAF subtypes (V600E versus non-V600E versus fusion: 22.6 versus 30.5 versus 26.0 mo, p = 0.639; Fig. 2A). Most patients (61.4%, 35 of 57) acquired BRAF variants after failure of second-line EGFR TKI treatment (Fig. 2B). Before detection of the BRAF variants, gefitinib/icotinib/erlotinib followed by osimertinib was the most common treatment (49.1%, Fig. 2C). Of all patients, 61.4% (35 of 57) developed BRAF variants after osimertinib, including three who received osimertinib as first-line treatment. The global median OS of all patients was 53.6 months (95% CI: 42.3–85.2 mo). The median OS of the patient cohort was 11.6 months (95% CI 9.7 mo–not applicable) from the time of detection of BRAF variants. A somewhat longer OS was achieved by patients with BRAF V600E (V600E versus non-V600E versus fusion: 16.8 versus 15.2 versus 10.7 mo, p = 0.72).
Figure 2.
(A) Time from initial diagnosis to acquisition of BRAF variants. (B) The lines of TKI treatments to the times of acquisition of BRAF variants. (C) The TKI sequences before acquisition of BRAF variants: 1, 2, and 3 indicate the first-, second-, and third-generation EGFR TKIs, respectively. (D) The mutation counts in the overall population and patients of different BRAF subtypes. TKI, tyrosine kinase inhibitor.
Molecular Characteristics of EGFR-Mutant NSCLC With Concomitant BRAF Variations
The median mutation count was seven in all patients (Fig. 2D) and was significantly higher in patients with non-V600E than V600E mutations (5 versus 8.5, p = 0.004). Apart from the BRAF variants, variations with frequencies more than 10% were noted in TP53, PIK3CA, RB1, MET, LRP1B, APC, CDKN2A, MYC, ERBB2, and SMAD4 (Fig. 1C), thus in genes involved in the cell cycle/p53 pathway, EGFR downstream pathways (the PI3K and RAS pathways), and other receptor tyrosine kinases. On multivariate analysis, RB1 mutations were significantly enriched in patients with non-V600E mutations (Supplementary Table 2, p = 0.04), as were TP53 mutations (the latter marginally) (p = 0.09). Of 11 patients with RB1 alterations, nine underwent tumor rebiopsy; no histologic transformation was evident.
A total of 33 patients underwent NGS before the development of BRAF variants (Supplementary Fig. 2). As BRAF variants were acquired, the frequencies of variations in PIK3CA, APC, MYC, LRP1B, CDNK2A, KRAS, and SMAD4 also increased. BRAF V600E and BRAF fusions were uncommon in patients with preexisting PIK3CA alterations, but common in patients receiving osimertinib (Supplementary Table 3).
Potential Impact of De Novo BRAF Variants on EGFR-Mutant NSCLC
Concomitant BRAF variants were detected in six treatment-naive patients with NSCLC and included five BRAF non-V600E mutations (P422L, G466R, E533K, D555Y, and E611Q) and one BRAF fusion (BRAF∼IGR) (B8: upstream MRPS33). BRAF G466R is a tier Ⅱ variant; the other five variants have not been reported. The impacts of mutations P422L, E533K, D555Y, and E611Q on protein structure were predicted to be benign by PolyPhen-220 (Supplementary Table 4). The BRAF∼IGR (B8: upstream MRPS33) fusion did not include the BRAF kinase domain. All patients received first-line EGFR TKIs and achieved an objective response rate (ORR) of 83.3% (five of six) with a median PFS of more than 12 months.
Efficacies of Following Treatments in Patients With Clinically Significant BRAF Variants
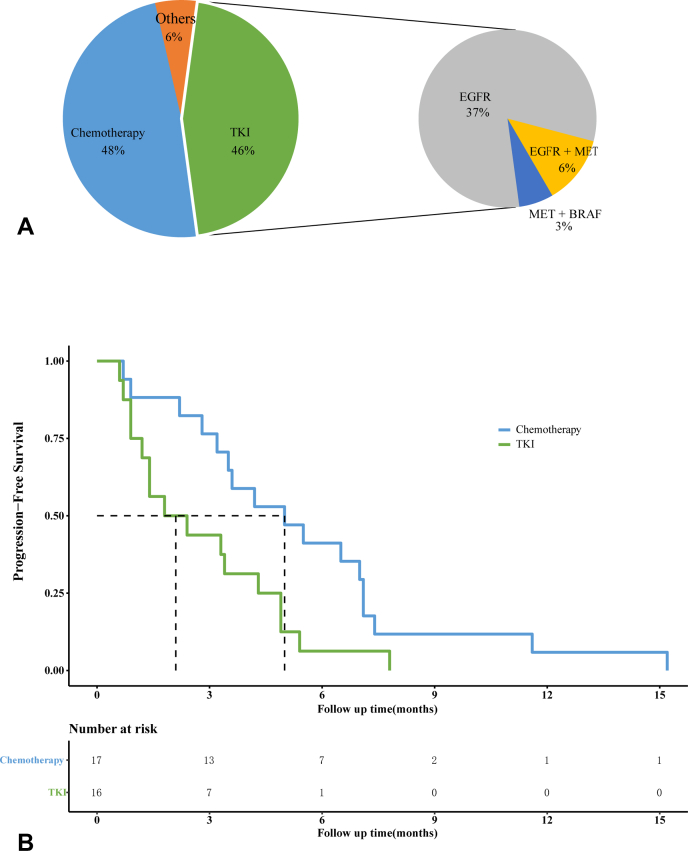
A total of 35 patients with acquired BRAF variants of clinical significance have received further treatment; most underwent chemotherapy (48.6%, 17 of 35: single nab-paclitaxel/docetaxel, n = 2; nab-paclitaxel/pemetrexed + cisplatin/carboplatin, n = 6; taxol/pemetrexed + cisplatin/carboplatin + bevacizumab, n = 9) or received TKIs (45.7%, 16/35) (Fig. 3A). Overall, the ORR and disease control rate were 14.3% (5 of 35) and 57.1% (20 of 35); the median PFS was 3.5 months. Of the TKI group, most patients (75.0%, 12 of 16) solely targeted EGFR only. The ORR and disease control rates were 6.3% (1 of 16) and 43.8% (7 of 16) in the TKI group but 23.5% (4 of 17) and 64.7% (11 of 17) in the chemotherapy group. The PFS was significantly longer in the chemotherapy than in the TKI group (5.0 versus 2.1 mo, log-rank test: p = 0.019, Fig. 3B). Among the 35 patients, 18 were positive for T790M before detection of BRAF variants, but eight lost T790M when BRAF variants were detected; seven patients acquired T790M and BRAF variants concurrently. Thus, the T790M positivity rate was 48.6% (17 of 35) when BRAF variants were detected. On multivariate Cox regression analysis, both the number of lines of previous treatment (1 versus ≥2: HR = 0.61, 95% CI: 0.38–0.99, p = 0.044) and treatment (TKI versus chemotherapy: HR = 3.61, 95% CI: 1.54–8.45, p = 0.003) were independently associated with PFS, whereas T790M status and the previous use of osimertinib were not (Supplementary Table 5).
Figure 3.
(A) Treatments after acquisition of clinically significant BRAF variants and the targets of TKIs; others indicated that one patient received chemotherapy plus PD-L1 inhibitors, another one received anlotinib. (B) Kaplan-Meier curves of patients who received chemotherapies and TKIs. PD-L1, programmed death-ligand 1; TKI, tyrosine kinase inhibitor.
We identified patients who had been naive for osimertinib or chemotherapy after acquiring BRAF variants and explored the potential impacts of the BRAF variants (Fig. 4). Five patients acquired BRAF V600E (n = 4) or G466E (n = 1) and concurrent T790M, after failure of the first- or second-generation EGFR TKIs. All received osimertinib as the next treatment. No patient achieved an objective response; the median PFS was 3.4 months. Four patients acquired BRAF fusions of the kinase domain-containing 3' region (Fig. 4). Two patients developed progressive disease after icotinib or afatinib and acquired BRAF fusions and T790M. One patient achieved a partial response but the PFS was short (4.8 mo); another experienced progressive disease at 1 month. Ten chemo-naive patients received chemotherapies as their next treatments after acquiring BRAF mutations (Fig. 4). Four achieved a partial response (40.0%) with a disease control rate of 80%. The median PFS was 6.0 months (range: 0.7–11.6 mo).
Figure 4.
The efficacies of chemotherapy for chemo-naive patients and osimertinib for patients with both BRAF variants and T790M after failure of previous EGFR TKIs. Chemo is shown by the green bar; the chemo-regimens are indicated. Osimertinib is indicated by the blue bar. ∗These two patients experienced disease progression after 1 month and BRAF fusions were subsequently detected. Chemo, chemotherapy; NGS, next-generation sequencing; P, patient; PD, progressive disease; PR, partial response; SD, stable disease; TKI, tyrosine kinase inhibitor.
Efficacy and Safety of Combinations of Osimertinib and BRAF Inhibitors
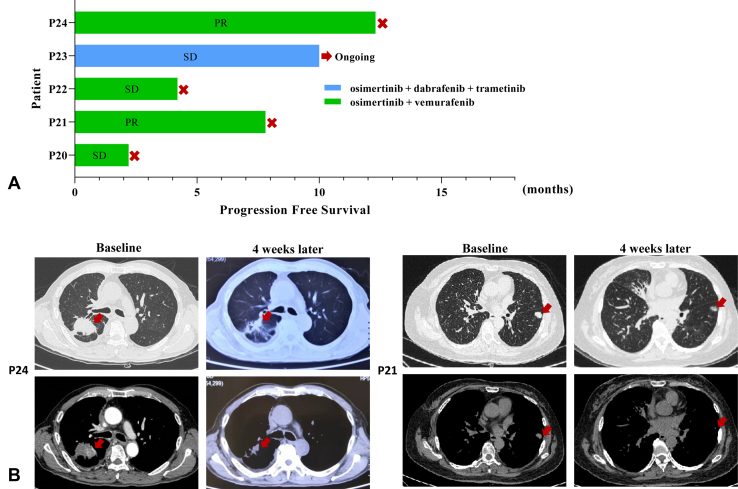
Osimertinib plus vemurafenib (n = 4) or osimertinib plus dabrafenib plus trametinib (n = 1) was prospectively given to five patients. The median PFS was 7.8 months (range: 2.2–12.3 mo) (Fig. 5A). The disease control rate was 100%. Two patients achieved an objective response (Fig. 5B). Grade 3 rash was observed in one patient; other grade 1 or 2 adverse events included fatigue, arthralgia, fever, diarrhea, and anorexia (Supplementary Table 6).
Figure 5.
(A) Efficacy of EGFR plus BRAF co-inhibition in five patients with EGFR-mutant NSCLC who acquired BRAF V600E after progression on osimertinib. (B) Computed tomographic images of P21 and P24 before and 4 weeks after EGFR plus BRAF co-inhibition. P, patient; PR, partial response; SD, stable disease.
Resistance Profiling of Patients on Combinations of Osimertinib and BRAF Inhibitors
Two patients (P20 and P21) underwent plasma or tissue-based NGS testing on failure of EGFR plus BRAF co-inhibition (Supplementary Table 7). P20 exhibited stable disease (with a PFS of 7.8 mo) but developed progressive disease. Given the difficulty of rebiopsy, plasma NGS was performed instead. Multiple mutations including KRAS G12R, HRAS Q61K, and PIK3CA E542K had been acquired. P21 seemed to be primarily resistant to treatment; she experienced pleural effusion and enlargement of the left supraclavicular lymph node at a short PFS of 2.2 months. Compared with previous pleural effusion-based NGS, NARS Q61K and an MYC amplification were newly identified in the enlarged lymph node.
Discussion
To the best of our knowledge, this is the first study to explore patient characteristics and treatment strategies in those with both EGFR-mutant NSCLC and concomitant BRAF variants. The BRAF variants developed in subclones and acquired late during disease progression of EGFR-mutant NSCLC. Acquired BRAF variants may weaken the efficacies of subsequent EGFR TKIs; chemotherapy was superior to TKIs that did not target both EGFR and BRAF. Our pilot study revealed that EGFR plus BRAF co-inhibition might afford clinical benefits (with manageable toxicity) for such patients.
In a preclinical study, class 1 BRAF variants (BRAF V600) were sensitive to Food and Drug Administration–approved BRAF inhibitors (dabrafenib or vemurafenib) but class 2 or 3 BRAF variants were not.7 This was replicated in a clinical trial of vemurafenib.9 BRAF V600E was the most common acquired BRAF variant in our present patient cohort. Schrock et al. reported that BRAF kinase fusions were associated with acquired resistance to EGFR TKIs.21 In our present study, we identified a new BRAF kinase fusion partner (RELCH),5 also known as KIAA1468. The encoded protein is involved in intracellular cholesterol transport and was a fusion partner of RET.22 Vojnic et al.23 introduced an AGK~BRAF fusion into the H1975 cell line (L858R+ and T790M+); growth inhibition by osimertinib was impaired. One of our patients acquired an AKG~BRAF fusion and T790M after progression on afatinib. Although this patient evidenced a partial response to single-agent osimertinib, the PFS was short (4.9 mo). Combined inhibition using osimertinib and trametinib, or a pan-RAF inhibitor, may be optimal for such patients. Apart from BRAF variants, a high frequency of tumor-promoting variants was observed in our cohort, especially PI3KCA mutation and MET amplification. The genetic profiles of those who acquired BRAF V600E and non-V600 mutations differed. Although statistical significance was not attained, the genetic background seemed to be “cleaner” in the BRAF V600E group. Thus, BRAF V600E may confer high-level resistance to EGFR TKIs, emphasizing that EGFR plus BRAF co-inhibition may be useful.
Peng et al.24 comprehensively reported co-mutations of different BRAF subtypes with EGFR, including BRAF V600E and non-V600E mutations and fusions/rearrangements. Nevertheless, the impacts of those variants on later treatments were unclear.24 Although we found concomitant BRAF variants in treatment-naive patients with EGFR-mutant NSCLC, these seemed to have minimal impact on subsequent EGFR TKI treatments (Supplementary Table 4). The variants may not have affected protein structure. In other words, de novo BRAF variants may be benign in EGFR-mutant patients, in line with the observation that an EGFR-activating mutation is seldom co-mutated with other driver genes in treatment-naive patients.25 BRAF V600E and BRAF fusions conferred resistance to osimertinib in first-line settings.26 Given the fact that most of the patients in our study developed BRAF variants after osimertinib indicates the importance of NGS to find resistant mechanisms for patients after failure of osimertinib. In addition, osimertinib is now the standard first-line treatment for advanced EGFR-mutant NSCLC, whether acquired BRAF variants more frequently occurred in patients who received osimertinib than other EGFR TKIs remained unknown. In our cohort, TKI treatment was of limited efficacy in patients with acquired BRAF variants. Nevertheless, chemotherapy remains an option and rather effective as in previous reports.27,28 Notably, BRAF V600E was concurrently acquired with T790M after first- or second-generation EGFR TKIs. Moreover, subsequent osimertinib was not very effective. This may part explain why some patients with acquired T790M do not respond to osimertinib.
In a clinical trial of patients with NSCLC with BRAF mutations, the discontinuation rate of vemurafenib at 960 mg twice daily attained 24%, and 60% of patients experienced dose reductions/treatment delays.9 Although no phase 1 trial has explored co-inhibition by EGFR and BRAF, case reports and a literature review support the efficacy of a combined strategy but also indicate that full doses of vemurafenib and osimertinib are usually followed by dose reductions or treatment cessation.15,29 In the present study, although the vemurafenib dose was only 480 mg twice daily, two of four patients required dose reductions. In a clinical trial, the ORR and PFS of vemurafenib were 44.8% and 5.2 months for patients with NSCLC with BRAF V600, respectively.9 The efficacy of osimertinib plus vemurafenib in our study was similar. Our pilot study also indicated that clinical benefits improved by co-inhibition of EGFR and BRAF, although vemurafenib was delivered at half the regular dose. The optimal doses, safety, and efficacy of EGFR plus BRAF co-inhibition should be further investigated in a clinical trial. The genetic profiles of two patients were explored after both developed progressive disease on a combination of osimertinib and BRAF inhibitors. Both had acquired functional mutations in RAS genes. RAS mutations reportedly conferred resistance to osimertinib.4 Activation of RAS signaling in patients with melanoma also conferred resistance to BRAF inhibitors.30 On activation of RAS signaling, RAS-driven heterodimerization of BRAF and CRAF increases, enhancing drug resistance.31 Together, the data suggest that RAS mutations may mediate resistance to EGFR plus BRAF co-inhibition.
Our work had several limitations. First, given the difficulties to rebiopsy, NGS was performed on tumor tissue, plasma, cerebral fluid, and pleural effusions. Second, approximately 30% of patients had BRAF variants after first-line EGFR TKI treatments, but some lacked baseline NGS panel data. Thus, such patients may have had preexisting BRAF variants. Third, the sample size of the pilot study on EGFR plus BRAF co-inhibition was small.
In conclusion, we found that acquired BRAF variants may reduce EGFR TKI efficacies. A combination of osimertinib with BRAF inhibitors improved efficacy in patients acquiring BRAF V600E mutations after failure of osimertinib. EGFR plus BRAF co-inhibition should be investigated in a clinical trial.
CRediT Authorship Contribution Statement
Xue-Wu Wei: Investigation, Formal analysis, Data curation, Writing—original draft, Visualization.
Jia-Yi Deng, Chong-Rui Xu: Resources, Data curation.
Zhi-Hong Chen: Project administration.
Dong-Qin Zhu, Qian Wu: Visualization.
Xu-Chao Zhang: Resources.
Yi-Long Wu: Supervision.
Qing Zhou: Conceptualization, Methodology, Writing—review and editing, Funding acquisition.
Acknowledgments
The study was supported by the National Natural Science Foundation of China (grant number: 82072562 to Dr. Zhou), the High-level Hospital Construction Project (grant number: DFJH201810 to Dr. Zhou), and the GDPH Scientific Research Funds for Leading Medical Talents in Guangdong Province (grant number: KJ012019428 to Dr. Zhou). The authors thank the patients and their families for participating in this study.
Footnotes
Disclosure: Prof. Zhou reports receiving honoraria from AstraZeneca, United Kingdom, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme, United Kingdom, Pfizer, Roche, and Sanofi, outside the submitted work. Prof. Wu reports receiving advisory services for AstraZeneca, Boehringer Ingelheim, Novartis, Switzerland, and Takeda; personal fees from AstraZeneca, BeiGene, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Pfizer, Roche, and Sanofi; and grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Hengrui, and Roche outside of the submitted work. The remaining authors declare no conflict of interest.
Cite this article as: Wei XW, Deng JY, Xu CR, et al. Characteristics of and treatment strategies for advanced EGFR-mutant NSCLC with concomitant BRAF variations. JTO Clin Res Rep. 2022;3:100348.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2022.100348.
Supplementary Data
References
- 1.Lim S.M., Syn N.L., Cho B.C., Soo R.A. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: mechanisms and therapeutic strategies. Cancer Treat Rev. 2018;65:1–10. doi: 10.1016/j.ctrv.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen K.S., Kobayashi S., Costa D.B. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer. 2009;10:281–289. doi: 10.3816/CLC.2009.n.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jia Y., Yun C.H., Park E., et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature. 2016;534:129–132. doi: 10.1038/nature17960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westover D., Zugazagoitia J., Cho B.C., Lovly C.M., Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29:i10–i19. doi: 10.1093/annonc/mdx703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricordel C., Friboulet L., Facchinetti F., Soria J.C. Molecular mechanisms of acquired resistance to third-generation EGFR-TKIs in EGFR T790M-mutant lung cancer. Ann Oncol. 2018;29:i28–i37. doi: 10.1093/annonc/mdx705. [DOI] [PubMed] [Google Scholar]
- 6.Dankner M., Rose A.A.N., Rajkumar S., Siegel P.M., Watson I.R. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene. 2018;37:3183–3199. doi: 10.1038/s41388-018-0171-x. [DOI] [PubMed] [Google Scholar]
- 7.Yao Z., Yaeger R., Rodrik-Outmezguine V.S., et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature. 2017;548:234–238. doi: 10.1038/nature23291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Planchard D., Smit E.F., Groen H.J.M., et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18:1307–1316. doi: 10.1016/S1470-2045(17)30679-4. [DOI] [PubMed] [Google Scholar]
- 9.Mazieres J., Cropet C., Montane L., et al. Vemurafenib in non-small-cell lung cancer patients with BRAF(V600) and BRAF(nonV600) mutations. Ann Oncol. 2020;31:289–294. doi: 10.1016/j.annonc.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Ho C.C., Liao W.Y., Lin C.A., Shih J.Y., Yu C.J., Yang J.C. Acquired BRAF V600E mutation as resistant mechanism after treatment with osimertinib. J Thorac Oncol. 2017;12:567–572. doi: 10.1016/j.jtho.2016.11.2231. [DOI] [PubMed] [Google Scholar]
- 11.La Monica S., Minari R., Cretella D., et al. Acquired BRAF G469A mutation as a resistance mechanism to first-line osimertinib treatment in NSCLC cell lines harboring an EGFR exon 19 deletion. Target Oncol. 2019;14:619–626. doi: 10.1007/s11523-019-00669-x. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro M., Knebel F.H., Bettoni F., et al. Impressive response to dabrafenib, trametinib, and osimertinib in a metastatic EGFR-mutant/BRAF V600E lung adenocarcinoma patient. NPJ Precis Oncol. 2021;5:5. doi: 10.1038/s41698-021-00149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaufler D., Ast D.F., Tumbrink H.L., et al. Clonal dynamics of BRAF-driven drug resistance in EGFR-mutant lung cancer. NPJ Precis Oncol. 2021;5:102. doi: 10.1038/s41698-021-00241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aboubakar Nana F., Ocak S. Targeting BRAF activation as acquired resistance mechanism to EGFR tyrosine kinase inhibitors in EGFR-mutant non-small-cell lung cancer. Pharmaceutics. 2021;13:1478. doi: 10.3390/pharmaceutics13091478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mauclet C., Collard P., Ghaye B., Hoton D., Nana F.A. Tumor response to EGFR/BRAF/MEK co-inhibition in a patient with EGFR mutated lung adenocarcinoma developing a BRAF(V600) mutation as an acquired resistance mechanism. Lung Cancer. 2021;159:42–44. doi: 10.1016/j.lungcan.2021.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y.C., Chen Z.H., Zhang X.C., et al. Analysis of resistance mechanisms to abivertinib, a third-generation EGFR tyrosine kinase inhibitor, in patients with EGFR T790M-positive non-small cell lung cancer from a phase I trial. EBiomedicine. 2019;43:180–187. doi: 10.1016/j.ebiom.2019.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y.S., Jiang B.Y., Yang J.J., et al. Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: a new medium of liquid biopsy. Ann Oncol. 2018;29:945–952. doi: 10.1093/annonc/mdy009. [DOI] [PubMed] [Google Scholar]
- 18.Chakravarty D., Gao J., Phillips S.M., et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol. 2017;2017 doi: 10.1200/PO.17.00011. PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M.M., Datto M., Duncavage E.J., et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19:4–23. doi: 10.1016/j.jmoldx.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adzhubei I.A., Schmidt S., Peshkin L., et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schrock A.B., Zhu V.W., Hsieh W.S., et al. Receptor tyrosine kinase fusions and BRAF kinase fusions are rare but actionable resistance mechanisms to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2018;13:1312–1323. doi: 10.1016/j.jtho.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 22.Nakaoku T., Tsuta K., Ichikawa H., et al. Druggable oncogene fusions in invasive mucinous lung adenocarcinoma. Clin Cancer Res. 2014;20:3087–3093. doi: 10.1158/1078-0432.CCR-14-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vojnic M., Kubota D., Kurzatkowski C., et al. Acquired BRAF rearrangements induce secondary resistance to EGFR therapy in EGFR-mutated lung cancers. J Thorac Oncol. 2019;14:802–815. doi: 10.1016/j.jtho.2018.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng P., Lv G., Hu J., Wang K., Lv J., Guo G. Co-mutations of epidermal growth factor receptor and BRAF in Chinese non-small cell lung cancer patients. Ann Transl Med. 2021;9:1321. doi: 10.21037/atm-21-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Y., Song J., Wang Y., et al. Concurrent genetic alterations and other biomarkers predict treatment efficacy of EGFR-TKIs in EGFR-mutant non-small cell lung cancer: a review. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.610923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ríos-Hoyo A., Moliner L., Arriola E. Acquired mechanisms of resistance to osimertinib—the next challenge. Cancers (Basel) 2022;14:1931. doi: 10.3390/cancers14081931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou C., Wu Y.L., Chen G., et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y.L., Zhou C., Hu C.P., et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 29.Sun M., Wang X., Xu Y., et al. Combined targeting of EGFR and BRAF triggers regression of osimertinib resistance by using osimertinib and vemurafenib concurrently in a patient with heterogeneity between different lesions. Thorac Cancer. 2022;13:514–516. doi: 10.1111/1759-7714.14295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizos H., Menzies A.M., Pupo G.M., et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res. 2014;20:1965–1977. doi: 10.1158/1078-0432.CCR-13-3122. [DOI] [PubMed] [Google Scholar]
- 31.Yao Z., Torres N.M., Tao A., et al. BRAF mutants evade ERK-dependent feedback by different mechanisms that determine their sensitivity to pharmacologic inhibition. Cancer Cell. 2015;28:370–383. doi: 10.1016/j.ccell.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.