Abstract
Mechanisms underlying physical inactivity-induced insulin resistance are not well understood. In addition to a role in muscle repair, immune cell populations such as macrophages may regulate insulin sensitivity.
Aim.
To examine if the dynamic changes in insulin sensitivity during and after recovery from reduced physical activity corresponded to changes in skeletal muscle macrophages.
Methods.
In this prospective clinical study we collected muscle biopsies from healthy older adults (70±2y, n=12) before and during a hyperinsulinemic-euglycemic clamp were assessed before (PRE) and after 2-weeks reduced physical activity (RA), and following 2-weeks of recovery (REC). Insulin sensitivity (hyperinsulinemic-euglycemic clamp), skeletal muscle mRNA expression of inflammatory markers, and immunofluorescent quantification of skeletal muscle macrophages, myofiber-specific satellite cell and capillary content.
Results.
Insulin sensitivity was decreased following reduced activity and rebounded following recovery above PRE levels. We observed an increase (p<0.01) in muscle macrophages (CD68+CD206+: 190 (55, 324); CD11b+CD206+: 117 (28, 205) % change from PRE) and CD68 [2.4 (1.4, 3.4) fold] and CCL2 [1.9 (1.3, 2.5) fold] mRNA following RA concurrent with increased (p<0.03) satellite cells [55 (6,104) %] in slow twitch myofibers. Moreover, the distance of satellite cells to the nearest capillary was increased 7.7 (1.7,13.7) μm in fast twitch myofibers at RA (p=0.007). Changes in macrophages was positively associated with increased insulin sensitivity following RA (R>0.57, P<0.05).
Conclusion.
These findings suggested that a dynamic response of skeletal muscle macrophages following acute changes in physical activity in healthy older adults are related to insulin sensitivity.
Keywords: capillaries, immune, injury, insulin resistance, step-reduction
Introduction
Older adults are rapidly representing a greater proportion of the general population [1] and are the most sedentary age group both in the United States [2] and worldwide [3]. Within the aging population, there is higher prevalence of disabilities, with mobility deficits being the most frequently reported disability type [4]. Diminished physical function and greater risk of chronic conditions leads to a higher likelihood of injury, disease-related symptoms, rates of hospitalization and surgery which further impairs functional status. Increased physical inactivity in older adults represents a major contributing factor [5] to metabolic dysfunction and insulin resistance [6, 7]. Prior studies suggest, that the insulin resistance of aging may share a similar mechanism to IR that accompanies physical inactivity from muscle disuse [8–11]. This further signifies the importance between aging and physical inactivity [12].
Macrophages are a class of myeloid cells critical for immune regulation, inflammation, and regeneration of skeletal muscle tissues. In rodents, macrophages are important in the process of repair after exercise, muscle disuse and cytotoxic injury. Macrophages exist in a spectrum of polarized states yet they are commonly classified as either a M1 and M2 macrophages for simplicity [13]. M1 macrophages produce pro-inflammatory cytokines to clear cellular debris and M2 macrophages produce anti-inflammatory cytokines that aid in regeneration. However, these macrophage sub-types also have separate and distinct roles in the regulation of insulin sensitivity [14–16]. For example, during a high-fat diet in mice, adipose tissue macrophages contributed to lipid-induced insulin resistance through inflammatory responses [17]. Moreover, pharmacological inhibition of macrophages/monocytes blunted the insulin sensitizing effects of exercise [18]. Since macrophages have been observed to accumulate in skeletal muscle during disuse in rodents [19–23] it remains unclear whether skeletal muscle macrophages also play a role in inactivity-induced insulin resistance, especially in humans.
The delivery of macrophages to muscle (via monocyte migration and adhesion) is partially dependent on the capillarization of skeletal muscle [24]. The capillarization of skeletal muscle is important for the transport of mononuclear cells, may even be directly related to insulin sensitivity [25, 26] and has involvement in maintenance of other supporting cell types, such as satellite cells [27]. Thus, as a secondary objective, we also examined skeletal muscle capillarization and its relationships to these supporting mononuclear cells (macrophages and satellite cells) in skeletal muscle during, and following recovery from, physical inactivity in older adults.
The purpose of the current study was to quantify skeletal muscle M1 and M2 macrophages and capillarization during a two-week period of physical inactivity in older adults, and to examine the potential relationship between macrophage mediation and the development of inactivity-induced insulin resistance. Moreover, we also sought to examine the relationship between skeletal muscle macrophages, capillaries, and satellite cells in this context. The main hypothesis tested was that reduced physical inactivity in older adults would increase macrophage abundance and the distance of satellite cells to the nearest capillary and decrease capillarization and satellite cell content. Secondly, we hypothesized that these cellular events would be reversed following recovery. Third, that changes at baseline in macrophage counts and capillarization would be correlated with the change in insulin sensitivity (glucose infusion rate at after reduced activity versus PRE).
Materials and Methods
General experimental design
Healthy older adults from a previous study [28] were assessed for muscle and metabolic endpoints at 4-periods: before (PRE), at 7 (RA7) and 14 days of step reduction (RA14: <75% of normal activity), and following 14 days of recovery to baseline physical activity level (REC). At PRE, RA and REC we assessed insulin sensitivity (hyperinsulinemic-euglycemic clamp) coupled with muscle biopsies for immunofluorescent detection of macrophages, satellite cells and capillaries and mRNA expression of markers of inflammation. At RA7, there was a single muscle biopsy instead of a clamp study. Measurements at each of these periods included lean mass and mid-plantar flexor muscle area (DXA and pQCT scan) before the clamp and biopsies and assessment of muscle function (isometric knee strength and 6MW test) after those procedures. The above functional and muscle size assessments were conducted in the same order during all visits, with the exception of PRE, where the functional tests were conducted several days before the clamp.
Subjects
We recruited 12 (7 male, 5 female) healthy, community dwelling, glucose tolerant subjects from the Salt Lake City area between the ages of 60–85 years and with a BMI <30 kg·m−2. Healthy subjects were recruited and screened similarly as we have previously reported [28]. All subjects read and signed the informed consent form. The current study was approved by the University of Utah Institutional Review Board (no. 00084354) and conformed to the Declaration of Helsinki and Title 45, US Code of Federal Regulations, Part 46, “Protection of Human Subjects”. This study was registered at the clinical trials registry at ClinicalTrials.org (NCT02971098).
Reduced activity and recovery period
After completion of the first hyperinsulinemic-euglycemic clamp (Day 1- PRE), participants were instructed to adhere to 14 consecutive days of reduced physical activity at their home as described previously [28–30]. The goal was for the participants to reduce their daily step counts by 75% from their baseline (PRE) activity levels as determined by the accelerometer given to the subjects. The participants were also informed that their normal diet should remain unaltered during the RA and REC periods. Following the insulin clamp study (Day 14 - RA14), all participants were asked to return to their day-to-day habitual activities (e.g., walking). After the 14d recovery period (Day 28 – REC), the subjects underwent a final hyperinsulinemic-euglycemic clamp study coupled with muscle biopsies. Muscle function (strength and 6MW) was re-assessed similarly afterwards.
Physical activity monitoring
Subjects were given a step activity monitor (Omron) to measure their step counts at PRE and used as feedback during RA and REC periods.
Lean mass and mid-plantar flexor muscle area and muscle function
Total and leg lean mass and mid-plantar flexor muscle area were assessed via dual-energy X-ray absorptiometry (DXA) and peripheral quantitative computed tomography (pQCT), respectively, and were conducted by a trained technician at the Clinical Research Unit. University of Utah Center for Clinical and Translational Sciences (CCTS). These tests were performed prior to the hyperinsulinemic-euglycemic clamp studies but after the baseline muscle biopsy. Muscle function tests were performed after the biopsies for RA and REC, but not after PRE. Mid-plantar flexor muscle area was conducted according to previous methods [31] with modifications. Muscle function was assessed via knee extension strength and then the 6-minute walk test (6MW) [32]. These tests occurred before the reduced activity protocol and were repeated within 24h after completing the RA and REC period. Isometric strength was assessed with maximal voluntary isometric (at a 60° knee angle) contractions produced by the knee extensors and plantar and dorsi flexor muscles (at a 0° angle) on a Humac Norm dynamometer (CSMi Solutions, Stoughton, MA). For lower extremity extension power testing, a Nottingham power rig (Queen’s Medical Centre, Nottingham, UK) was used. Mid-plantar flexor muscle area was conducted according to previous methods [31], with modifications. Briefly, only two cross-sectional slices were measured at the 4 and 66% sites, relative distances were measured from the tibial end plate, rather than the growth plate. Tibial length was measured in duplicate and repeated length measures were confirmed to match that of the original measurement, in order to ensure the same slice was scanned at each time point). The left leg was scanned in all instances.
Hyperinsulinemic-euglycemic clamp and muscle biopsies
For measurements of insulin sensitivity, subjects arrived in the morning at the CCTS after an overnight fast, completed a DXA and pQCT scan, underwent a baseline thigh muscle biopsy (below) then participated in a ~3h hyperinsulinemic-euglycemic clamp [33]. To assess insulin sensitivity during the clamp, insulin was infused at a constant rate of 80 mU/m2/min as we have previously described [28]. Vastus lateralis muscle biopsies (5mm Bergström needle with manual suction) [28] were sampled (~150 mg) on the right thigh before and then on the left thigh after the clamp for measurements of fasting and insulin-stimulated muscle samples for immunofluorescent detection of macrophages, satellite cells and capillaries and mRNA expression of markers of inflammation. A portion of the muscle sample was prepared for immunohistological examination and frozen in liquid nitrogen cooled isopentane mounted in cork with Tissue-Tek® O.C.T. Compound (Sakura Finetek, Torrance, CA). The remaining muscle sample was quickly dissected into visible connective tissue and fat, rinsed with saline, frozen with liquid nitrogen and then stored at −80°C for RNA isolation. The DXA and pQCT scans and hyperinsulinemic-euglycemic clamp with muscle biopsies were repeated similarly at RA14 and REC. Insulin sensitivity data are reported percent change from PRE.
Serum glucose and insulin
Serum collected during the clamp was assessed for glucose levels using a standard glucose analyzer (YSI, Yellow Springs, OH). Serum insulin levels (EMD Millipore, Billerica, MA) were determined at baseline and the final time point of the clamp according to manufacturers’ instructions.
mRNA Expression
mRNA expression of vastus lateralis was conducted as follows [34]. Total RNA was isolated by homogenizing 10–15 mg tissue with a hand-held homogenizer in a solution containing Tri reagent LS (Molecular Research Centre, Cincinnati, OH, USA). The RNA was separated and precipitated using chloroform and isopropanol. Extracted RNA was washed with ethanol then suspended in nuclease-free water with EDTA. RNA concentration was determined using the EPOCH (Take3; BioTek) spectrophotometer. cDNA was synthesized using a commercially available kit (iScript, BioRad, Hercules, CA, USA). All isolated RNA and cDNA samples were stored at −80°C until analysis. Real-time PCR was carried out with a CFX Connect real-time PCR cycler (BioRad) under similar protocol as reported previously [35, 36] using SYBR green custom designed primers for beta 2-microglobulin (β2M), cluster of differentiation 68 (CD68), cluster of differentiation 45 (CD45), interleukin 6 (IL-6) and C-C motif chemokine ligand 2 (CCL2) which have been previously described [37] in addition to myoD and myogenin [38]. Cycle threshold values of target genes were normalized to β2M then fold change values were calculated (ΔΔCt). β2M remained stable across the interventions.
Immunofluorescence
Samples were removed from the cork at −25°C in a Microtome PLUS (Triangle Biomedical Sciences) where they were individually cut in 8 μm cross-sections. Baseline and insulin-stimulated muscle samples at PRE, RA7 (only baseline), RA14, and REC for the same subject were placed on the same slide (Fisherbrand Superfrost®/Plus microscope slides; Fisher Scientific, USA). Several slides were generated per subject, for 1) fiber-type-specific satellite cell content and capillaries, 2) fiber-type-specific CD68 content and capillaries, 3) CD68/CD206 co-staining and, 4) CD11b/CD206 co-staining. Following cutting, a hydrophobic marker (Vector, H-4000, Burlingame, CA) separated the sections, which were dried at room temperature (RT) and then stored at −20°C until analysis.
Fiber-type-specific satellite cell content and capillaries, CD68 content and capillaries and distance of satellite cells and macrophages to the nearest capillary were conducted as previously demonstrated with modifications [27, 34, 39]. Example images with Pax7 (Supplemental Figure 1A) and CD68 (Supplemental Figure 1B) on a muscle cross-section are shown in Supplemental Figure 1. Briefly, MHC I (BA.D5, Developmental Studies Hybridoma Bank, Iowa, USA) and laminin (L9393, Sigma-Aldrich, St. Louis, MO, USA) were both resolved in Alexa Fluor 647 secondary (Goat anti-Mouse IgG2b and Goat anti-Rabbit IgG, Alexa Fluor 647 conjugated 2°Ab (1:500) (Invitrogen, Cat#A21242 and A21245), and rhodamine labeled Ulex Europaeus Agglutinin I (UEA I) (1:50) (Vector Labs cat #RL-1062) was used to mark capillaries. All capillaries were traced in Image J and cutoffs of > 5 μm2 and < 500 μm2. Because the tissue for one subject was exhausted, we only have n=11 for satellite cell analyses. For immunofluorescence of macrophages and capillaries, CD68 was swapped with Pax7 since they are of the same isotype and host. Centrally-located myonuclei [40] and myonuclei per fiber [41] were analyzed as previously conducted.
Skeletal muscle macrophage markers for CD68/CD206 and CD11b/CD206 co-labeling were performed as follows: Sections were fixed in −20°C acetone at RT followed by 3×3 min rinses in PBS. Sections were incubated for 8 min with 3% H₂O₂ in PBS treatment at RT to block endogenous peroxidases. Following 3×3 min washes in PBS, sections were blocked for 60 min in 2.5% normal horse serum (NHS, Vector, S-2012) with vector avidin D solution (4 drops per 1 mL solution) at RT and subsequently incubated for 60 min at 4°C with primary antibody mouse anti-human CD68 (Dako #M0814, 1:200) or mouse anti-human CD11b (Cell Sciences, #MON1019-1, 1:100) diluted in 2.5% NHS with biotin solution block (4 drops per 1 mL solution). After 4×5 min rinses in PBS, slides were incubated for 30 min in secondary antibody donkey α mouse IgG biotin –SP-conjugated (Jackson Immuno, #715-065-150, 1:500) diluted in 2.5% NHS. Sections were washed 4×5 min in PBS, and then incubated for 30 min in SA-HRP (Invitrogen, S-911, 1:500) diluted in PBS. After 3×5 min washes in PBS, sections were incubated for 10 min in Alexa flour 488 (1:500) in TSA kit solution (Invitrogen). Sections were washed 3×3 min in PBS, and blocked for 10 min in 2.5% NHS with vector avidin D solution at RT. Subsequent to a quick wash, the sections were incubated overnight in goat anti-human CD206 (R&D, #AF2534, 1:200) diluted in 2.5% NHS with biotin solution block at 4°C. On day 2 of staining, sections were washed 4×5 min, and then incubated for 60 min with a secondary antibody bovine anti-goat IgG (H+L) Cy3 (Jackson, #805-165-180, 1:250). Following 3×5 min rinses in PBS, sections were incubated for 30 min in primary antibody mouse IgG2b anti-human IgG Laminin (DSHB, 2EB, 1:250). After 3×5 min washes in PBS, the slides were incubated for 30 min in secondary antibody donkey-goat IgG (H+L) AF647 (Jackson Immuno research, #715-605-151, 1:500). Sections were washed for 3×5 min in PBS, rinsed once with distilled water, and mounted with vector shield mounting media with DAPI (Vector, #H-1200). Slides were drained of excess mounting media, allowed to air dry at RT, and were subsequently stored at 4°C. This staining protocol for CD68/CD206 or CD11b/CD206 resulted in DAPI positive nuclei (blue), dystrophin (purple- changed to white during image processing), CD68 or CD11b (green) and CD206 (red). Validation of these stains was conducted with secondary only controls (Supplemental Figure 2). Example images of baseline CD11b/CD206 co-staining (Supplemental Figure 3) immunofluorescence at PRE, RA7, RA14 and REC for each subject are shown at 200x magnification. Example images of baseline CD68 (Figure 1G) and CD11b (Figure 1H) immunofluorescence at PRE, RA7, RA14 and REC from the same subject in the same image are shown at 40x magnification. An example image of CD11b/CD206 immunofluorescence on a muscle cross-section close up was taken at 200x to demonstrate the skeletal muscle macrophage infiltration at RA7 (Figure 1I). Immunofluorescent stained sections were imaged on a fully automated Nikon Ti-E inverted wide field microscope using a high sensitivity Hamastu camera at 200X magnification. Image processing and analysis was completed using Image J (FIJI). Muscle macrophage content was quantified via two methods; 1) per fiber (range: 0.05–1.90 cells/myofiber) and 2) density per unit area (range: 17–440 cells/mm2). Macrophages were counted by determining overlap at CD68+ DAPI+ (green-blue). A recent protocol published by the Petersen laboratory confirms the validity of our chosen skeletal muscle macrophage protocols [42]. Also, we also counted the skeletal muscle perimysium macrophages expressed as density per unit area because there were high concentrations of cells in these areas (range: 100–2000 cells/mm2) as they may play a physiologically important role [43].
Figure 1.
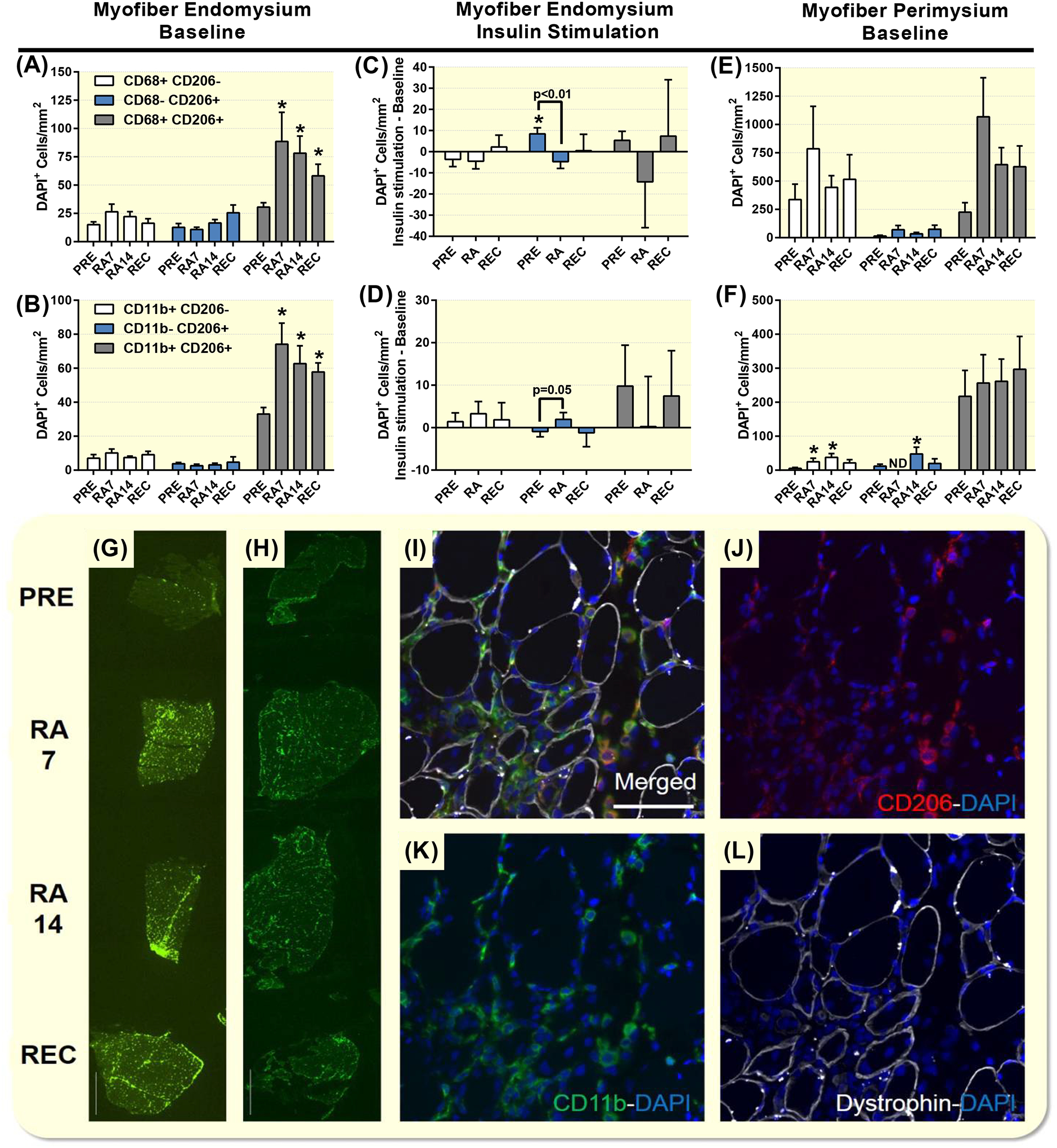
Increased immunofluorescence of CD11b/CD206 and CD68/CD206 macrophages on skeletal muscle cross-sections with reduced activity and recovery. Skeletal muscle endomysium baseline CD68/CD206 (A), CD11b (B) and insulin-stimulated CD68/CD206 (C), CD11b (D) expressed as mm2 at PRE during reduced activity (RA7 and RA14) and after recovery (REC) in healthy older adults. Baseline muscle perimysium CD68/CD206 (E), CD11b (F) expressed as mm2 at PRE during reduced activity (RA7 and RA14) and after recovery (REC) in healthy older adults. Example images of baseline CD68 (G) and CD11b (H) immunofluorescence at PRE, RA7, RA14 and REC from the same subject captured at 40x magnification. An example image of CD11b/CD206 immunofluorescence was taken at 200x to demonstrate the skeletal muscle macrophage infiltration at RA7 (I,J,K,L). Data are Mean±SEM. * P<0.05 vs PRE (A,B,F) or baseline value (C). Scale bar set to 1000 (G,H) or 100 (I) microns. ND, not detectable.
Statistical analysis.
Values in tables and figures are raw values or change scores expressed as Mean ± SEM or Mean ± 95% CI. Primary outcome data were evaluated for equal variances and normality. A priori, we set the primary comparisons of interest; the changes from PRE to RA, RA to REC and PRE to REC to test for these effects using individual t-tests when analyzing change data. These specific comparisons enabled us to test our specific hypotheses. When examining the baseline values, we utilized a one-way ANOVA with Holm-Sidak’s multiple comparisons tests. When examining the effect of insulin stimulation or myofiber-specific effects we utilized a two-way ANOVA with Sidak’s multiple comparisons tests. Centrally-located myonuclei data was nonparametric and thus analyzed with a Kruskal-Wallis test and multiple comparisons were examined with Dunn’s tests. Significance was set as P≤0.05 and trends were noted as 0.05 < p < 0.10. All analyses and figures were conducted with Graph Pad Prism 6.0f (La Jolla, CA).
Results
Subject characteristics, step counts and baseline dietary intake
Subject characteristics have been outlined previously [28]. Healthy older adults (5F/7M, 70±2y, BMI of 26±1, 92±2mg/dl resting glucose, HbA1c % 5.5±0.1, 31±2% fat and 49±3kg of total lean mass) participated in the study. Baseline steps were 9009 (7149,10868) and that they were decreased to 3099 (2088,4109) in the 1st week of RA, and to 2888 (2036,3741) steps in the 2nd week of RA. Although, participants were instructed to return to their baseline activity level steps were only partially recovered to 7402 (5909,8896) in the 1st week of REC, and to 7834 (6050,9618) steps in the 2nd week of REC. Step counts (per day) decreased from PRE to RA by 66 (59,73)% and was not recovered to PRE after REC by 12 (2,23)%. Baseline dietary intake was 2125 ± 61 kcals, with 1.17 ± 0.04 g/kg protein, 3.49 ± 0.11 g/kg carbohydrate and 1.17 ± 0.03 g/kg fat with no differences between men and women.
Muscle macrophages
Because of the stability of myofiber CSA, the data was similar when expressed as per myofiber or mm2 and the latter was reported. CD68+CD206−DAPI+ and CD11b+CD206−DAPI+ cells (per myofiber and mm2) were unchanged at baseline across the study and following insulin stimulation (Figure 1A and 1B). CD68−CD206+DAPI+ and CD11B−CD206+DAPI+ cells (per myofiber and mm2) were unchanged at baseline across the study (Figure 1A and 1B). Following insulin stimulation, CD68−CD206+DAPI+ cells (per myofiber (not shown) and mm2) were increased at PRE and the change at PRE was greater than at RA (Figure 1C). A similar pattern was seen for total CD206+DAPI+ cells (data not shown). Following insulin stimulation, CD11b−CD206+DAPI+ cells (per myofiber and mm2) were different than at RA14 vs PRE (per myofiber: P=0.033 and mm2: p=0.050) (Figure 1D).
Baseline CD68+CD206+DAPI+ and CD11B+CD206+DAPI+ cells (per myofiber and mm2) were increased from PRE at RA7, while RA14 and REC and were unchanged following insulin stimulation (Figure 1A and 1B). A similar pattern was observed with total CD68+ DAPI+, total CD11B+ DAPI+, and total CD206+DAPI+ cells (data not shown).
Skeletal muscle perimysium CD68+CD206−DAPI+, CD68−CD206+DAPI+, CD11b+CD206+DAPI+ and CD68+CD206+DAPI+ macrophages were unchanged at baseline across the study (Figure 1E and 1F) and following insulin stimulation (data not shown). Skeletal muscle perimysium CD11b+CD206−DAPI+ and CD11b−CD206+DAPI+ macrophages were unchanged following insulin stimulation (data not shown). Baseline increases in CD11b+CD206−DAPI+ macrophages were seen at RA7 and RA14. CD11b−CD206+DAPI+ macrophages were not detectable at RA7, yet increased above PRE levels at RA14 (Figure 1E and 1F). There were no differences in the macrophage responses between men and women, as we were not powered to detect differences.
Muscle characteristics, leg lean mass, mid-plantar flexor muscle area, insulin sensitivity and function
Myofiber CSA and MHC fiber type percentage (52±3% MHC I) was unchanged at baseline across the study and following insulin stimulation (data not shown). Leg lean mass (Figure 2A) displayed patterns to increase from PRE to RA7 and decrease from PRE to RA14 that did not reach statistical significance. The lean mass change from RA7 to RA14 (−0.92±0.80%) and RA14 to REC (0.52±0.72%) was unchanged. Mid-plantar flexor muscle area (Figure 2B) increased from PRE to RA7 and decrease from PRE to RA14. There was decrease in muscle area from RA7 to RA14 (−4.36±0.97%) and no change from RA14 to REC (1.64±1.72%). Knee extension leg strength (Figure 2C) decreased from PRE to RA14 and REC. The change from RA14 to REC (2.19±4.36%) was unchanged. The 6MW test (Figure 2D) did not change across the intervention. The change from RA14 to REC (−0.02±3.88%) was insignificant. Insulin sensitivity assessed as glucose infusion rate (per kg body weight) was 9.2±0.8 (PRE), 7.8±0.9 (RA) and 10.6±1.2 (REC). Insulin sensitivity decreased from PRE to at RA14 and recovered at REC with supercompensation above PRE levels. Individual change responses are seen in Figure 2E.
Figure 2.
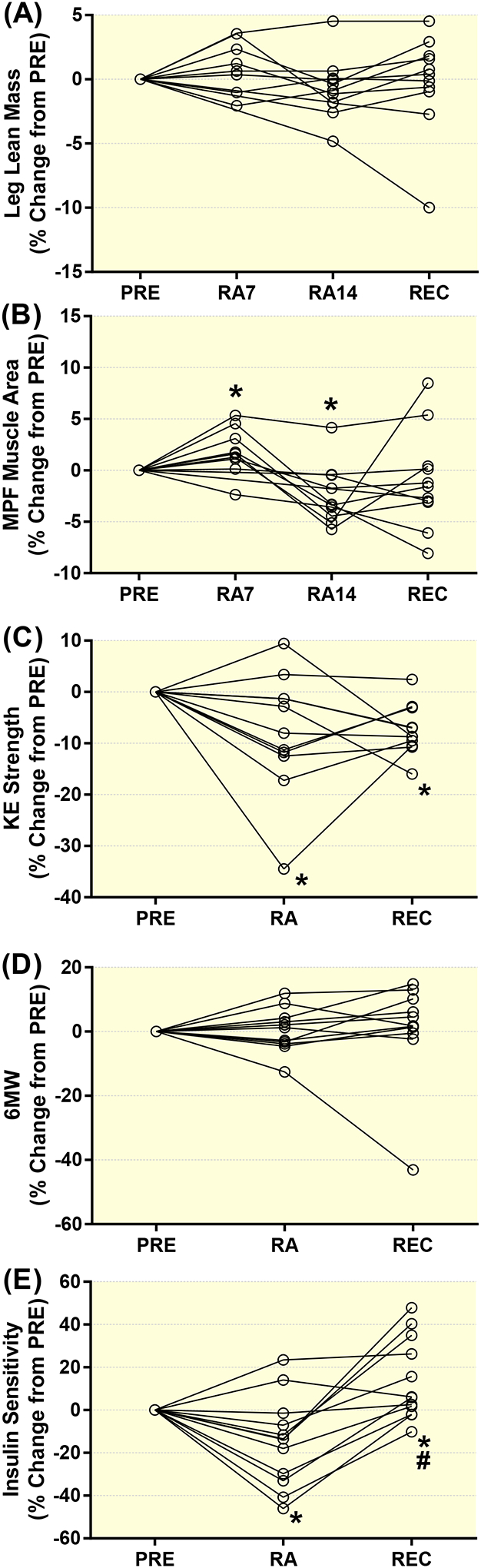
Individual responses for the percent change from PRE during reduced activity (RA7 and RA14) and after recovery (REC) in healthy older adults for leg lean mass (A), mid-plantar flexor (MPF) muscle area (B), knee extension strength (C), 6 minute walk test (D) and glucose infusion rate (insulin sensitivity, E). Data are Mean±SEM. Mid-plantar flexor, MPF; knee extension, KE; 6 minute walk test, 6WT. * P<0.05 vs PRE. # P<0.05 vs RA.
mRNA expression
Baseline CD45, IL-6 and TLR4 mRNA expression did not change over the course of the experiment, but CD68 and CCL2 mRNA expression increased from PRE to RA14. Following insulin stimulation, there were no increases from baseline for CD45, IL-6 or CD68 mRNA expression at PRE, RA14 or REC. TLR4 mRNA increased during RA14 and CCL2 mRNA increased at PRE. There was a trend (p=0.064) for the increase in TLR4 mRNA at RA14 to be greater than following REC, and the increase in CCL2 mRNA at PRE was greater than following REC after insulin stimulation. The CD68 mRNA at PRE was also greater than following RA14. Furthermore, the IL-6 mRNA expression at RA14 was greater than following REC. MyoD and Myogenin mRNA expression was unchanged at baseline, but was increased in response to insulin stimulation. However, the MyoD mRNA response to insulin stimulation was attenuated at REC vs PRE and RA14. Likewise, the Myogenin mRNA response to insulin stimulation was attenuated at REC vs. PRE and there was a trend (p=0.091) at RA vs. PRE. Skeletal muscle mRNA expression is shown in Figure 3. There were no differences in the mRNA responses between men and women, with the exception of IL-6, where significant interaction, time and sex effects were observed. Baseline IL-6 mRNA expression (delta delta CT) was (1.10±0.20, 1.10±0.20) at PRE, (0.90±0.18, 4.39±1.69) at RA and (0.66±0.11, 1.46±0.27) at REC for men and women, respectively. Women increased IL-6 mRNA expression after RA, where it was significantly greater than with men. The women restored their IL-6 mRNA levels at recovery.
Figure 3.
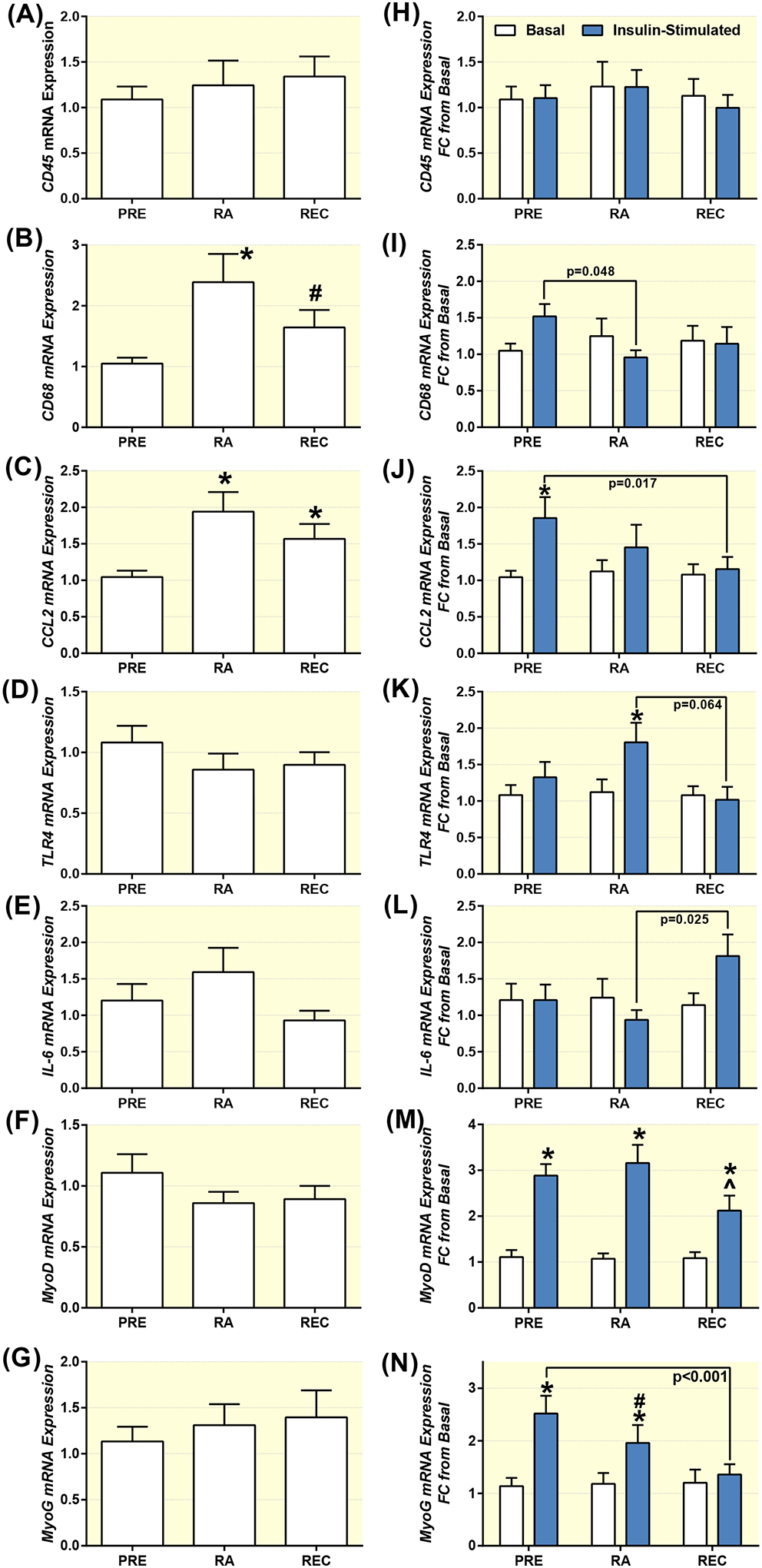
Baseline and insulin-stimulated mRNA expression in skeletal muscle. Baseline (A,B,C,D,E,F,G), and insulin-stimulated (H,I,J,K,L,M,N) CD45 (A,H), CD68 (B,I), CCL2 (C,J), TLR4 (D,K), IL-6 (E,L), MyoD (F,M) and Myogenin (G,N) mRNA expression at PRE and after reduced activity (RA) and recovery (REC) in healthy older adults. Data are Mean±SEM. * P<0.05 vs Pre (A-G) or baseline (H-N), # P<0.10 vs PRE., ^ P<0.05 vs PRE and RA.
Muscle capillarization
Mean myofiber (Table 1) and myofiber-type specific (Figure 4A–C) CFi, CFPE, capillary contacts, sharing factor and capillary area were unchanged at baseline across the study and following insulin stimulation.
Table 1 –
Mean myofiber capillarization in healthy older adults at PRE, following RA and REC with and without insulin stimulation.
| PRE | RA7 | RA14 | REC | PRE | RA14 | REC | |
|---|---|---|---|---|---|---|---|
| −Insulin | +Insulin | ||||||
| CFi | 1.54 ± 0.08 | 1.67 ± 0.18 | 1.5 ± 0.11 | 1.6 ± 0.15 | 1.52 ± 0.12 | 1.55 ± 0.14 | 1.49 ± 0.11 |
| CFPE | 5.59 ± 0.19 | 5.86 ± 0.36 | 5.37 ± 0.2 | 5.57 ± 0.3 | 5.4 ± 0.23 | 5.75 ± 0.24 | 5.6 ± 0.18 |
| Fiber Perimeter, μm | 274.3 ± 12.2 | 279.9 ± 14.6 | 278.5 ± 18.6 | 284.1 ± 21.3 | 278.2 ± 18.4 | 265.8 ± 17.1 | 292.6 ± 40.6 |
| CC | 3.88 ± 0.18 | 4.05 ± 0.4 | 3.71 ± 0.24 | 3.92 ± 0.32 | 3.7 ± 0.25 | 3.8 ± 0.31 | 3.7 ± 0.2 |
| Sharing Factor | 2.57 ± 0.04 | 2.5 ± 0.04 | 2.53 ± 0.04 | 2.53 ± 0.04 | 2.56 ± 0.04 | 2.51 ± 0.05 | 2.57 ± 0.07 |
| Capillary Area, μm2 | 24.07 ± 2.82 | 24.15 ± 4.24 | 25.71 ± 4.02 | 25.6 ± 2.72 | 21.96 ± 2.98 | 17.09 ± 1.35 | 26.32 ± 4.15 |
Data are Mean ± SEM. capillary-to-fiber perimeter exchange (CPFE) index, individual capillary-to-fiber ratio (CFi), the number of fibres sharing each capillary (sharing factor) and the number of capillaries around a fibre - capillary contacts (CC)). Baseline (PRE), 7 (RA7) and 14 (RA14) days of reduced activity and recovery (REC).
Figure 4.
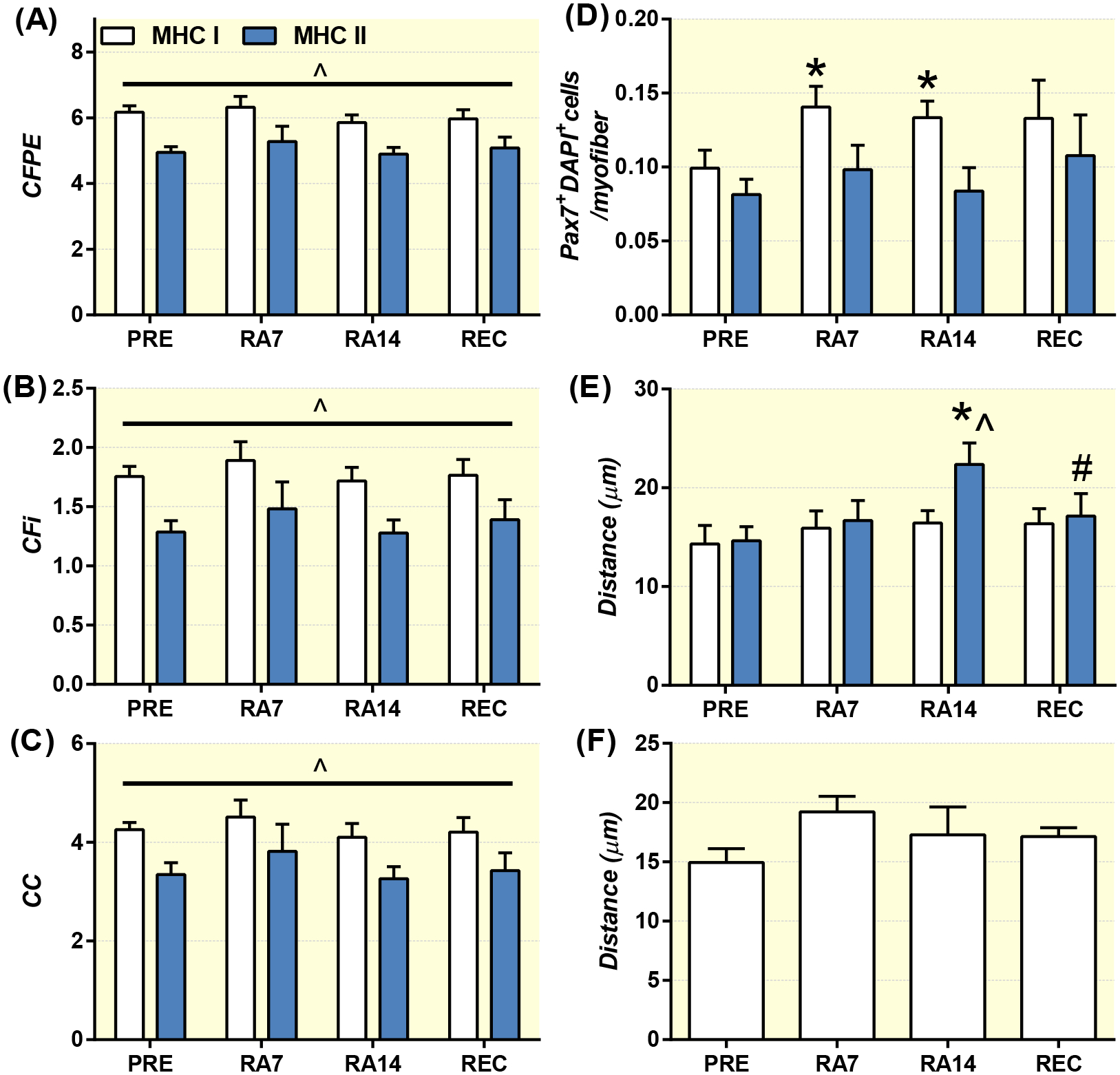
Baseline and myofiber-type specific measures of skeletal muscle capillarization (A,B,C), satellite cell content (D), distance of satellite cells to the nearest capillary (E) and mean myofiber distance of macrophages to the nearest capillary (F) at PRE, during reduced activity (RA7 and RA14) and after recovery (REC) in healthy older adults. Data are Mean±SEM. * P<0.05 PRE, ^ P<0.05 myofiber type difference, # P<0.05 vs RA14.
Muscle satellite cells
Pax7+DAPI+ cells (per MHC I myofibers) at baseline increased at RA7 and RA14 compared to PRE and were unaltered following insulin stimulation (Figure 4D) with an exception at RA14 where a trend (p=0.059) for a decrease was observed following insulin stimulation (82.8% (64.8,100.8% of RA14 baseline – data not shown). MHC II myofiber (Figure 4D) Pax7+DAPI+ cells (per myofiber and mm2) were unchanged at baseline across the study and following insulin stimulation.
Distance to the nearest capillary
Distance of Pax7+DAPI+ cells to the nearest capillary (Figure 4E) was unaltered at baseline and following insulin stimulation in MHC I myofibers, but was increased from baseline PRE to baseline RA14 and returned to PRE values at baseline REC in MHC II myofibers. Additionally, the distance of Pax7+DAPI+ cells to the nearest capillary was greater in MHC II vs MHC I myofibers at RA14. Distance of CD68+DAPI+ cells to the nearest capillary was unaltered at baseline and following insulin stimulation, yet there was a trend (p=0.09) for a greater distance at baseline RA7 vs baseline PRE (Figure 4F).
Myonuclei
Myonuclei per myofiber were unchanged across the intervention regardless of myofiber type (data not shown). Percentage centrally-located myonuclei out of total myonuclei were 0.19% (0.04–0.33%) at PRE, displayed a trended (p=0.062) to increase at RA7 0.53% (0.28–0.78%), and significantly increased at RA14 0.89% (0.41, 1.38%) but not at REC 0.57% (0.13, 1.01%).
Correlations
As we have previously demonstrated [34], CD68+DAPI+ cells per myofiber were positively associated with Pax7+DAPI+ cells per myofiber (R=0.415, p=0.016) and Pax7+DAPI+ cells per MHC I myofibers (R=0.411, p=0.018). Insulin sensitivity at baseline (PRE, RA14 or REC) was not correlated with mean skeletal muscle capillarity (R<±0.5, p>0.07). CD68 mRNA was correlated with CD11b+CD206+DAPI+ cells (R=0.721, p=0.0001) and CD68+CD206+DAPI+ cells (R=0.485, p=0.003). CCL2 mRNA was correlated with CD11b+CD206+DAPI+ cells (R=0.482, p=0.003) and CD68+CD206+DAPI+ cells (R=0.415, p=0.012). CCL2 mRNA was positively associated with CD68 mRNA at RA14 (R=0.606, p=0.037) and REC (R=0.693, p=0.012).
The change in insulin sensitivity from PRE to RA14 was positively associated with the change in CD68 mRNA (R=0.604, p=0.038; (Figure 5A)), CD68+CD206−DAPI+ cells (R=0.743, p=0.006; (Figure 5B)), CD68+CD206+DAPI+ cells (R=0.793, p=0.002; (Figure 5C)) and CD11b+CD206+DAPI+ cells (R=0.578, p=0.048; (Figure 5D)). The change in the distance of CD68+DAPI+ cells to the nearest capillary from PRE to RA14 was positively correlated with the change in CD68+DAPI+ cells (R=0.597, p=0.041)
Figure 5.
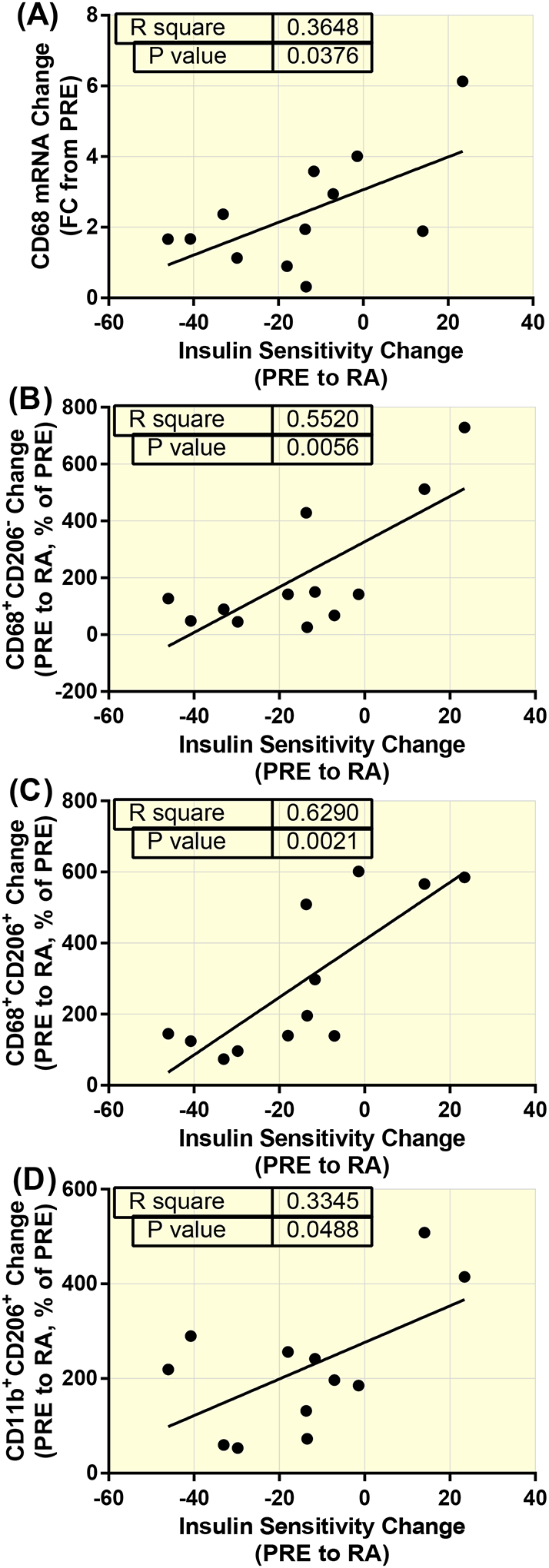
Positive associations between the accumulation of macrophages and insulin sensitivity change in healthy older adults following reduced activity (RA). CD68 mRNA (R=0.604, p=0.038; (A)), CD68+CD206-DAPI+ cells (R=0.743, p=0.006; (B)), CD68+CD206+DAPI+ cells (R=0.793, p=0.002; (C)) and CD11b+CD206+DAPI+ cells (R=0.578, p=0.048; (D)).
The change in the distance of CD68+DAPI+ cells to the nearest capillary from RA14 to REC was negatively correlated with the change in insulin sensitivity (R=−0.775, p=0.005) (Figure 6A). Also, the change in insulin sensitivity from RA14 to REC was positively correlated with the change in CD11b+CD206+DAPI+ cells (R=0.581, p=0.048; (Figure 6B)), and the CFPE (R=0.634, p=0.027; (Figure 6C)) and capillary density (R=0.698, p=0.012; (Figure 6D)) of MHC II myofibers.
Figure 6.
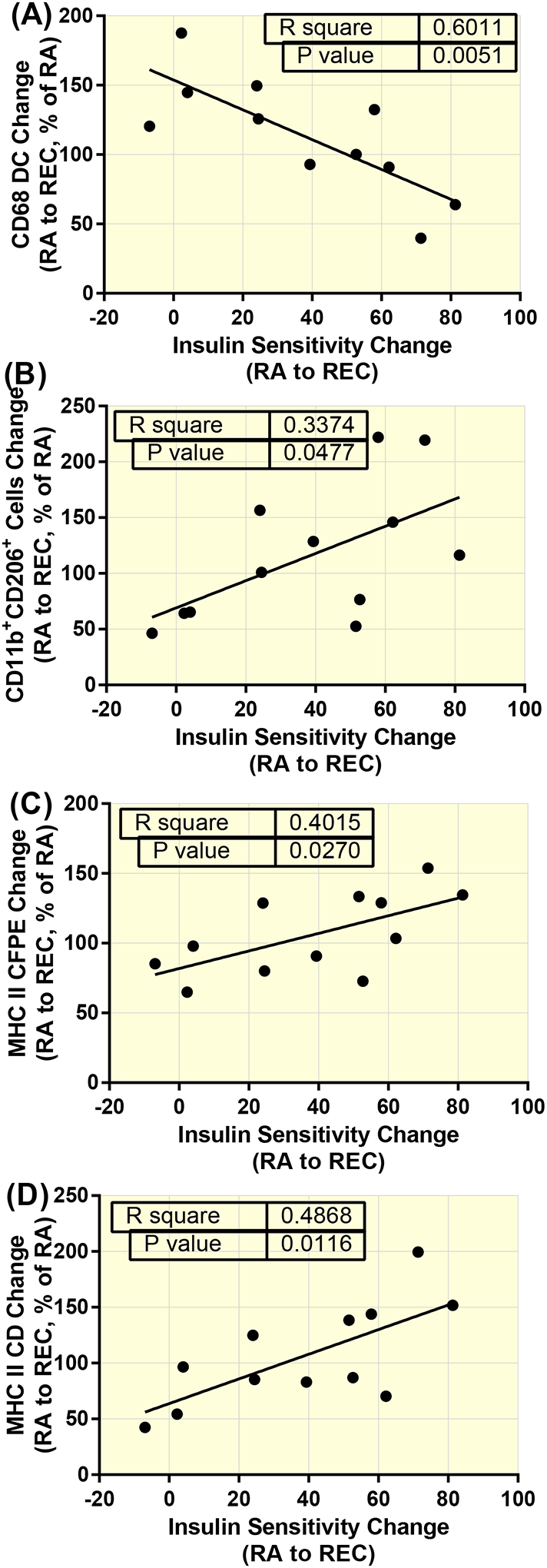
Associations between, macrophages, the proximity of macrophages to capillaries, and fast-twitch myofiber capillarity and the insulin sensitivity rebound during recovery (REC) from reduced activity (RA) in healthy older adults. The change in the distance of CD68+DAPI+ cells to the nearest capillary (R=−0.775, p=0.005) (A). The change in CD11b+CD206+DAPI+ cells (R=0.581, p=0.048; (B)), and the CFPE (R=0.634, p=0.027; (C)) and capillary density (R=0.698, p=0.012; (C)) of MHC II myofibers.
Discussion
Our primary finding was that skeletal macrophages robustly increased in skeletal muscle of healthy older adults subjected to reduced activity. The change in these macrophages were positively correlated with the change in insulin sensitivity during inactivity and the rebound of insulin sensitivity during recovery above baseline values. We speculate that the immune cell infiltration was conceivably due to increased chemotaxis and monocyte adhesion/migration theoretically initially supported by 1) an increase in transient muscle edema and/or 2) minor myofiber damage as indicated by increased percentage of centrally located myonuclei. Additionally, the presence of macrophages and minor myofiber damage following reduced activity may have prompted satellite cell proliferation in skeletal muscle as evidenced by increased Pax7+ cell content in slow twitch myofibers. Finally, Pax7+ cells in fast-twitch myofibers increased their distance to the nearest capillary following reduced activity.
Skeletal muscle macrophages in humans have been demonstrated to increase in response to exercise, injury and high fat diet [42, 44]. We are the first to demonstrate macrophage accumulation in skeletal muscle (with both immunofluorescence and mRNA proxies) after a two week period of reduced physical activity in humans. We also demonstrated that skeletal muscle CCL2 mRNA expression (which was correlated with CD68 mRNA) was increased during reduced activity and remained elevated following 2-weeks of recovery suggesting that reduced muscle contraction may have initiated a signal for macrophage chemotaxis that persisted even after 2-weeks of re-ambulation.
The sparse collection of literature using M1 and M2 co-immunostaining of human skeletal muscle sections to date suggests that the vast majority of human macrophages co-express both M1 and M2 markers, while few macrophages purely express either M1 or M2 markers alone in skeletal muscle [34, 42, 45]. The macrophages in this study that co-expressed CD11b and CD206 or CD68 and CD206 are the most abundant type (~70–85% of the pool) and we report that they increased in response to inactivity. Changes in the CD11b+CD206−DAPI+ populations, considered to be M1 pro-inflammatory macrophages in human skeletal muscle [42], represent a very small proportion (~10–15%) of the muscle macrophage pool and thus possibly the dominant macrophage population in these samples may be more representative of a M2-like macrophage. A preponderance of pro-inflammatory macrophages in human skeletal muscle may only be noticeable in severe muscle injury and myopathy [46] thus a low abundance of these macrophages may be expected in healthy, physical active older adults. As recently discussed [34, 42], due to tissue limitations, the phenotyping of macrophages is limited to immunohistochemical characterization and double-labeling at most, which even then is rarely conducted [42]. One additional revelation from this study was the alterations in CD11b+CD206+DAPI+ cells and CD11b−CD206+DAPI+ cells located in the skeletal muscle perimysium following the intervention. CD11b+CD206+DAPI+ cells increased during RA. CD11b−CD206+DAPI+ cells were undetectable at RA7 but much more apparent during RA14. This indicates a possible dynamic response of these small skeletal muscle perimysium populations during reduced activity and recovery, which requires validation with a larger sample size.
One of the main goals of this project was to examine an alternate function of macrophages beyond the canonical role of myofiber repair. Macrophages are most commonly implicated with insulin resistance in the context of overfeeding where they respond in tissues such as necrotic adipocytes in a pro-inflammatory manner with effects that antagonize insulin signaling [24]. We originally hypothesized that macrophages, particularly the pro-inflammatory M1 macrophages, would be related (inversely) to insulin sensitivity as previously suggested in rodent models [24]. However, in opposition to what has been shown in other tissue types (e.g., fat) [16] (i.e., an inverse relationship), we demonstrated several positive associations with CD68 mRNA expression and macrophage immunofluorescent quantification (Figure 8). In agreement with our findings, a recent article with a much larger sample size in lean and obese subjects measured at pre and post-exercise training showed that mRNA of muscle macrophage markers (CD68, CCL2, CD40, CD163, CD206, CD11b) were positively associated with insulin sensitivity [45]. Interestingly, these authors reported a divergent role in adipose tissue such that macrophage markers inversely correlated with insulin sensitivity [45]. Together, our data supports that muscle macrophages may positively regulate insulin sensitivity. This relationship may be different in other tissue types (i.e. fat) and is unknown in the context of physical inactivity.
The majority of the attention have focused on anti-inflammatory, M1 macrophages which are well linked to insulin resistance. However, much less attention has been directed to the “alternatively activated” or M2 macrophages in skeletal muscle, which may actually be required to help maintain normal insulin sensitivity in the context of exercise [18, 47, 48] and now from these study’s results, involved with modulations of insulin sensitivity with physical inactivity. We also examined the distance of CD68+ macrophages to the nearest capillary with the thought that macrophage trafficking in capillary networks would limit muscle remodeling and metabolic cellular function. Though the distance of macrophages to capillaries did not significantly change across the experimental conditions, we did observe an inverse relationship for the change from reduced activity to recovery between the distance of macrophages (pan CD68+) to the nearest capillary and insulin sensitivity (R=0.601, p=0.005). This suggests that there was a more robust improvement in insulin sensitivity when macrophages stayed closer to the capillaries. A concurrent observation was that this rebound of insulin sensitivity was proportional to increases in capillarity of fast-twitch myofibers during this time frame. Therefore, these data suggest a linkage between macrophage location and myofiber-specific capillarity in the change in insulin sensitivity during recovery from physical inactivity.
Alterations with blood flow and capillarization as a result of physical inactivity have been considered as a candidate mechanism for insulin resistance [25, 49] in humans. Bed rest reduces muscle blood flow [50], but various studies have demonstrated a decrease [39, 51–53], no change [49, 50, 54, 55] and, in rare cases, increases skeletal muscle capillarization [56]. These seeming paradoxical results can be made clearer with the realization that the decrease in capillarity is mostly accompanied by decreased muscle volume during physical inactivity and that these two factors do not occur on the same time course, with myofiber atrophy typically occurring at a faster rate than loss of capillarity [54, 57]. Although capillarization has been shown to be reduced following two weeks of immobilization in old and young men [58] and two weeks of bed rest in middle aged men [39] some indices of capillarization are unchanged in a similar model of two week step-reduction in older adults [59] as we report here. The differences in capillarity across human inactivity studies could be a result of the severity of the level in inactivity employed especially since muscle capillarization is relatively stable (~2–4 weeks) following detraining [26, 60] which is likely why 4d of bed rest in young adults similarly did not affect muscle capillarization [56]. Altogether, this data may suggest divergence in inactivity-induced effects with vascular flow versus microvascular perfusion in skeletal muscle.
An influx in macrophages during physical inactivity may have been initiated because of reduced blood flow and venous return resulting in edema. One previous report of hindlimb unloading suggested that muscle macrophages accumulated as a result of reduced blood flow [22]. Decreased velocity of blood flow may increase the incidence of leukocyte contact (i.e. rolling) to promote adhesion and migration [61]. Venous pooling (or possibly edema) would explain the transient muscle hypertrophy that was observed at 7d of reduced activity and especially in the lower limb muscle plantar flexor area, where this event would be most obvious. Indeed, hindlimb immobilization [62] and hindlimb unloading with intermittent weight bearing [63] resulted in greater muscle water content reflective of edema. Unfortunately, we did not utilize methods to directly confirm venous pooling, edema or alterations in the vasculature with reduced physical activity as others have indicated [64, 65]. Although we did not measure blood flow directly, reduced muscle blood flow in humans in response to physical inactivity has been observed [50, 66, 67].
We cannot rule out that macrophages also increased in response to muscle damage caused by the intermittent reloading during this period of reduced activity. In support, muscle sections had an increased presence of centrally-located myonuclei suggesting minor muscle damage during reduced activity in these older adults. Myofiber degeneration occurs in both unloaded [23, 68–70] and reloaded myofibers [68, 69] while muscle disuse is well known for increasing the propensity of injury to exercise [71, 72] and minimal contractile insults [73]. Additionally, aging skeletal muscle is more susceptible to contraction-induced myofiber damage [74]. It appears that myofiber damage during disuse and intermittent weight bearing is most prominent with weight bearing of shorter durations [73] (like that of the current reduced activity model).
Finally, we were surprised that MHC I Pax7+ satellite cells increased at 7 and 14d of reduced activity. This is in contrast to a cross-sectional study by Moore et al. in which Pax7+ satellite cell content decreased between older healthy male controls and older healthy males after reduced activity [59]. The response of satellite cells to physical inactivity is currently debated [75, 76] and inconsistent across human inactivity studies. Satellite cells have been shown to decline with bed rest [34, 39, 77], yet with immobilization they either tend to increase [75] or accumulate [78]. Given the robust level of macrophage accumulation observed in the current study, the presence of minor myofiber damage and the well described influence of macrophages on satellite cells [79, 80], the fact that MHC I Pax7+ satellite cell content increased in this study should not be unanticipated. One intriguing speculation is that macrophages may promote satellite cell proliferation [80]. Although we did not observe changes in baseline levels of MyoD and Myogenin across the intervention, these mRNAs robustly increased after insulin stimulation with an attenuated response following recovery; the latter may partially explain the return of satellite cell abundances back to baseline levels during recovery.
In conclusion, the current study suggests the existence of a dynamic response of skeletal muscle macrophages and satellite cells following reduced activity and return to recovery in healthy older adults. Macrophages may have accumulated during physical inactivity due to local edema and/or minor myofiber damage and their local presence may have influenced a change in insulin sensitivity.
Supplementary Material
Acknowledgments
We greatly appreciate the CCTS for their nursing staff assistance on the clinical study days.
Funding was provided by NIA R01AG AG050781, NCATS UL1TR001067, University of Utah Fluorescence Microscopy Core Facility, and PTR fellowship support from the Jeane B. Kempner Award and NIAMS F32 AR072481.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
References
- [1].Rowe JW, Fulmer T, Fried L. Preparing for Better Health and Health Care for an Aging Population. JAMA 2016; 316(16): 1643–1644. [DOI] [PubMed] [Google Scholar]
- [2].Watson KB, Carlson SA, Gunn JP, Galuska DA, O’Connor A, Greenlund KJ, Fulton JE. Physical Inactivity Among Adults Aged 50 Years and Older - United States, 2014. MMWR Morb Mortal Wkly Rep 2016; 65(36): 954–958. [DOI] [PubMed] [Google Scholar]
- [3].WHO | Prevalence of insufficient physical activity. 2015.
- [4].Courtney-Long EA, Carroll DD, Zhang QC, Stevens AC, Griffin-Blake S, Armour BS, Campbell VA. Prevalence of Disability and Disability Type Among Adults -- United States, 2013. 2015; 64(29): 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hamburg NM, McMackin CJ, Huang AL, Shenouda SM, Widlansky ME, Schulz E, Gokce N, Ruderman NB, Keaney JF, Vita JA. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol 2007; 27(12): 2650–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Thyfault JP, Krogh-Madsen R. Metabolic disruptions induced by reduced ambulatory activity in free-living humans. J Appl Physiol (1985) 2011; 111(4): 1218–1224. [DOI] [PubMed] [Google Scholar]
- [7].Thyfault JP, Booth FW. Lack of regular physical exercise or too much inactivity. Curr Opin Clin Nutr Metab Care 2011; 14(4): 374–378. [DOI] [PubMed] [Google Scholar]
- [8].Fink RI, Kolterman OG, Griffin J, Olefsky JM. Mechanisms of insulin resistance in aging. J Clin Invest 1983; 71(6): 1523–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Burant CF, Lemmon SK, Treutelaar MK, Buse MG. Insulin resistance of denervated rat muscle: a model for impaired receptor-function coupling. American Journal of Physiology-Endocrinology and Metabolism 1984; 247(5): E657–E666. [DOI] [PubMed] [Google Scholar]
- [10].Robinson KA, Boggs KP, Buse MG. Okadaic acid, insulin, and denervation effects on glucose and amino acid transport and glycogen synthesis in muscle. American Journal of Physiology-Endocrinology and Metabolism 1993; 265(1): E36–E43. [DOI] [PubMed] [Google Scholar]
- [11].Sowell MO, Boggs KP, Robinson KA, Dutton SL, Buse MG. Effects of insulin and phospholipase C in control and denervated rat skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism 1991; 260(2): E247–E256. [DOI] [PubMed] [Google Scholar]
- [12].Amati F, Dube JJ, Coen PM, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care 2009; 32(8): 1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol 2007; 578(Pt 1): 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Olefsky JM, Glass CK. Macrophages, Inflammation, and Insulin Resistance. Annual Review of Physiology 2010; 72(1): 219–246. [DOI] [PubMed] [Google Scholar]
- [15].Meshkani R, Vakili S. Tissue resident macrophages: Key players in the pathogenesis of type 2 diabetes and its complications. Clinica Chimica Acta 2016; 462: 77–89. [DOI] [PubMed] [Google Scholar]
- [16].McNelis Joanne C, Olefsky Jerrold M. Macrophages, Immunity, and Metabolic Disease. Immunity 2014; 41(1): 36–48. [DOI] [PubMed] [Google Scholar]
- [17].Fink LN, Costford SR, Lee YS, Jensen TE, Bilan PJ, Oberbach A, Bluher M, Olefsky JM, Sams A, Klip A. Pro-Inflammatory macrophages increase in skeletal muscle of high fat-Fed mice and correlate with metabolic risk markers in humans. Obesity (Silver Spring) 2014; 22(3): 747–757. [DOI] [PubMed] [Google Scholar]
- [18].Ikeda S, Tamura Y, Kakehi S, Takeno K, Kawaguchi M, Watanabe T, Sato F, Ogihara T, Kanazawa A, Fujitani Y, Kawamori R, Watada H. Exercise-induced enhancement of insulin sensitivity is associated with accumulation of M2-polarized macrophages in mouse skeletal muscle. Biochem Biophys Res Commun 2013; 441(1): 36–41. [DOI] [PubMed] [Google Scholar]
- [19].Noriaki K, Tomoko F, Shuichi M. Time‐course study of macrophage infiltration and inflammation in cast immobilization–induced atrophied muscle of mice. Muscle & Nerve 2018; 57(6): 1006–1013. [DOI] [PubMed] [Google Scholar]
- [20].Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol 2012; 189(7): 3669–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Frenette J, St-Pierre M, Côté CH, Mylona E, Pizza FX. Muscle impairment occurs rapidly and precedes inflammatory cell accumulation after mechanical loading. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology 2002; 282(2): R351–R357. [DOI] [PubMed] [Google Scholar]
- [22].Tidball JG, Berchenko E, Frenette J. Macrophage invasion does not contribute to muscle membrane injury during inflammation. Journal of Leukocyte Biology 1999; 65(4): 492–498. [PubMed] [Google Scholar]
- [23].Haida N, Fowler WM Jr., Abresch RT, Larson DB, Sharman RB, Taylor RG, Entrikin RK. Effect of hind-limb suspension on young and adult skeletal muscle. I. Normal mice. Exp Neurol 1989; 103(1): 68–76. [DOI] [PubMed] [Google Scholar]
- [24].Chan KL, Boroumand P, Milanski M, Pillon NJ, Bilan PJ, Klip A. Deconstructing metabolic inflammation using cellular systems. Am J Physiol Endocrinol Metab 2017; 312(4): E339–e347. [DOI] [PubMed] [Google Scholar]
- [25].Snijders T, Nederveen JP, Verdijk LB, Houben A, Goossens GH, Parise G, van Loon LJC. Muscle fiber capillarization as determining factor on indices of insulin sensitivity in humans. Physiol Rep 2017; 5(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Prior SJ, Goldberg AP, Ortmeyer HK, Chin ER, Chen D, Blumenthal JB, Ryan AS. Increased Skeletal Muscle Capillarization Independently Enhances Insulin Sensitivity in Older Adults After Exercise Training and Detraining. Diabetes 2015; 64(10): 3386–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nederveen JP, Joanisse S, Snijders T, Ivankovic V, Baker SK, Phillips SM, Parise G. Skeletal muscle satellite cells are located at a closer proximity to capillaries in healthy young compared with older men. J Cachexia Sarcopenia Muscle 2016; 7(5): 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Reidy PT, McKenzie AI, Mahmassani Z, Morrow VR, Yonemura NM, Hopkins PN, Marcus RL, Rondina MT, Lin YK, Drummond MJ. Skeletal muscle ceramides and relationship with insulin sensitivity after 2 weeks of simulated sedentary behaviour and recovery in healthy older adults. J Physiol 2018; 596(21): 5217–5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Breen L, Stokes KA, Churchward-Venne TA, Moore DR, Baker SK, Smith K, Atherton PJ, Phillips SM. Two Weeks of Reduced Activity Decreases Leg Lean Mass and Induces “Anabolic Resistance” of Myofibrillar Protein Synthesis in Healthy Elderly. The Journal of Clinical Endocrinology & Metabolism 2013; 98(6): 2604–2612. [DOI] [PubMed] [Google Scholar]
- [30].Krogh-Madsen R, Thyfault JP, Broholm C, Mortensen OH, Olsen RH, Mounier R, Plomgaard P, van Hall G, Booth FW, Pedersen BK. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J Appl Physiol (1985) 2010; 108(5): 1034–1040. [DOI] [PubMed] [Google Scholar]
- [31].Stevenson DA, Viskochil DH, Carey JC, Slater H, Murray M, Sheng X, D’Astous J, Hanson H, Schorry E, Moyer-Mileur LJ. Tibial geometry in individuals with neurofibromatosis type 1 without anterolateral bowing of the lower leg using peripheral quantitative computed tomography. Bone 2009; 44(4): 585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Addison O, Drummond MJ, LaStayo PC, Dibble LE, Wende AR, McClain DA, Marcus RL. Intramuscular fat and inflammation differ in older adults: the impact of frailty and inactivity. J Nutr Health Aging 2014; 18(5): 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Marcus RL, Addison O, LaStayo PC, Hungerford R, Wende AR, Hoffman JM, Abel ED, McClain DA. Regional muscle glucose uptake remains elevated one week after cessation of resistance training independent of altered insulin sensitivity response in older adults with type 2 diabetes. J Endocrinol Invest 2013; 36(2): 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Reidy PT, Lindsay CC, McKenzie AI, Fry CS, Supiano MA, Marcus RL, LaStayo PC, Drummond MJ. Aging-related effects of bed rest followed by eccentric exercise rehabilitation on skeletal muscle macrophages and insulin sensitivity. Exp Gerontol 2018; 107: 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Drummond MJ, Addison O, Brunker L, Hopkins PN, McClain DA, Lastayo PC, Marcus RL. Downregulation of E3 Ubiquitin Ligases and Mitophagy-Related Genes in Skeletal Muscle of Physically Inactive, Frail Older Women: A Cross-Sectional Comparison. J Gerontol A Biol Sci Med Sci 2014; 69(8): 1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Exercise training following bed rest in older adults modulates skeletal muscle anti-inflammatory (M2) macrophage polarization Proceedings of the Advances in Skeletal Muscle Biology in Health and Disease; 2016, Gainesville, FL. [Google Scholar]
- [37].Kwon OS, Tanner RE, Barrows KM, Runtsch M, Symons JD, Jalili T, Bikman BT, McClain DA, O’Connell RM, Drummond MJ. MyD88 regulates physical inactivity-induced skeletal muscle inflammation, ceramide biosynthesis signaling, and glucose intolerance. American Journal of Physiology - Endocrinology and Metabolism 2015; 309(1): E11–E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Drummond MJ, Miyazaki M, Dreyer HC, Pennings B, Dhanani S, Volpi E, Esser KA, Rasmussen BB. Expression of growth-related genes in young and older human skeletal muscle following an acute stimulation of protein synthesis. J Appl Physiol (1985) 2009; 106(4): 1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Arentson-Lantz EJ, English KL, Paddon-Jones D, Fry CS. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. J Appl Physiol (1985) 2016; 120(8): 965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Reidy PT, Fry CS, Dickinson JM, Drummond MJ, Rasmussen BB. Postexercise essential amino acid supplementation amplifies skeletal muscle satellite cell proliferation in older men 24 hours postexercise. Physiological Reports 2017; 5(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Reidy PT, Fry CS, Igbinigie S, Deer RR, Jennings K, Cope MB, Mukherjea R, Volpi E, Rasmussen BB. Protein Supplementation Does Not Affect Myogenic Adaptations to Resistance Training. Medicine & Science in Sports & Exercise 2017; 49(6): 1197–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kosmac K, Peck BD, Walton RG, Mula J, Kern PA, Bamman MM, Dennis RA, Jacobs CA, Lattermann C, Johnson DL, Peterson CA. Immunohistochemical Identification of Human Skeletal Muscle Macrophages. Bio Protoc 2018; 8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liu D, Gordon PM. Low macrophage content in diabetic and aging human skeletal muscle. Obesity 2013; 21(1): 2–2. [DOI] [PubMed] [Google Scholar]
- [44].Boon Mariëtte R, Bakker Leontine EH, Haks Mariëlle C, Quinten E, Schaart G, Van Beek L, Wang Y, Van Schinkel L, Van Harmelen V, Meinders AE, Ottenhoff Tom HM, Van Dijk Ko W, Guigas B, Jazet Ingrid M, Rensen Patrick CN. Short-term high-fat diet increases macrophage markers in skeletal muscle accompanied by impaired insulin signalling in healthy male subjects. Clinical Science 2015; 128(2): 143–151. [DOI] [PubMed] [Google Scholar]
- [45].Liu D, Morales FE, IglayReger HB, Treutelaar MK, Rothberg AE, Hubal MJ, Nadler EP, Robidoux J, Barakat H, Horowitz JF, Hoffman EP, Burant CF, Gordon PM. Expression of macrophage genes within skeletal muscle correlates inversely with adiposity and insulin resistance in humans. Appl Physiol Nutr Metab 2018; 43(2): 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wang Q, Li Y, Ji S, Feng F, Bu B. Immunopathological Characterization of Muscle Biopsy Samples from Immune-Mediated Necrotizing Myopathy Patients. Med Sci Monit 2018; 24: 2189–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ruffino JS, Davies NA, Morris K, Ludgate M, Zhang L, Webb R, Thomas AW. Moderate-intensity exercise alters markers of alternative activation in circulating monocytes in females: a putative role for PPARgamma. Eur J Appl Physiol 2016; 116(9): 1671–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fink LN, Oberbach A, Costford SR, Chan KL, Sams A, Bluher M, Klip A. Expression of anti-inflammatory macrophage genes within skeletal muscle correlates with insulin sensitivity in human obesity and type 2 diabetes. Diabetologia 2013; 56(7): 1623–1628. [DOI] [PubMed] [Google Scholar]
- [49].Dirks ML, Wall BT, van de Valk B, Holloway TM, Holloway GP, Chabowski A, Goossens GH, van Loon LJ. One Week of Bed Rest Leads to Substantial Muscle Atrophy and Induces Whole-Body Insulin Resistance in the Absence of Skeletal Muscle Lipid Accumulation. Diabetes 2016; 65(10): 2862–2875. [DOI] [PubMed] [Google Scholar]
- [50].Mikines KJ, Richter EA, Dela F, Galbo H. Seven days of bed rest decrease insulin action on glucose uptake in leg and whole body. Journal of Applied Physiology 1991; 70(3): 1245–1254. [DOI] [PubMed] [Google Scholar]
- [51].Hikida RS, Gollnick PD, Dudley GA, Convertino VA, Buchanan P. Structural and metabolic characteristics of human skeletal muscle following 30 days of simulated microgravity. Aviat Space Environ Med 1989; 60(7): 664–670. [PubMed] [Google Scholar]
- [52].Rudnick J, Puttmann B, Tesch PA, Alkner B, Schoser BG, Salanova M, Kirsch K, Gunga HC, Schiffl G, Luck G, Blottner D. Differential expression of nitric oxide synthases (NOS 1–3) in human skeletal muscle following exercise countermeasure during 12 weeks of bed rest. Faseb j 2004; 18(11): 1228–1230. [DOI] [PubMed] [Google Scholar]
- [53].Salanova M, Schiffl G, Puttmann B, Schoser BG, Blottner D. Molecular biomarkers monitoring human skeletal muscle fibres and microvasculature following long-term bed rest with and without countermeasures. J Anat 2008; 212(3): 306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Degens H, Alway SE. Control of Muscle Size During Disuse, Disease, and Aging. Int J Sports Med 2006; 27(02): 94–99. [DOI] [PubMed] [Google Scholar]
- [55].Ringholm S, Biensø RS, Kiilerich K, Guadalupe-Grau A, Aachmann-Andersen NJ, Saltin B, Plomgaard P, Lundby C, Wojtaszewski JFP, Calbet JA, Pilegaard H. Bed rest reduces metabolic protein content and abolishes exercise-induced mRNA responses in human skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism 2011; 301(4): E649–E658. [DOI] [PubMed] [Google Scholar]
- [56].Montero D, Oberholzer L, Haider T, Breenfeldt Andersen A, Dandanell S, Meinild-Lundby AK, Maconochie H, Lundby C. Increased capillary density in skeletal muscle is not associated with impaired insulin sensitivity induced by bed rest in healthy young men. Appl Physiol Nutr Metab 2018. [DOI] [PubMed] [Google Scholar]
- [57].Roudier E, Gineste C, Wazna A, Dehghan K, Desplanches D, Birot O. Angio-adaptation in unloaded skeletal muscle: new insights into an early and muscle type-specific dynamic process. The Journal of Physiology 2010; 588(Pt 22): 4579–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Vigelso A, Gram M, Wiuff C, Andersen JL, Helge JW, Dela F. Six weeks’ aerobic retraining after two weeks’ immobilization restores leg lean mass and aerobic capacity but does not fully rehabilitate leg strength in young and older men. J Rehabil Med 2015; 47(6): 552–560. [DOI] [PubMed] [Google Scholar]
- [59].Moore DR, Kelly RP, Devries MC, Churchward-Venne TA, Phillips SM, Parise G, Johnston AP. Low-load resistance exercise during inactivity is associated with greater fibre area and satellite cell expression in older skeletal muscle. J Cachexia Sarcopenia Muscle 2018; 9(4): 747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mujika I, Padilla S. Detraining: Loss of Training-Induced Physiological and Performance Adaptations. Part II. Sports Medicine 2000; 30(3): 145–154. [DOI] [PubMed] [Google Scholar]
- [61].Jung U, Bullard DC, Tedder TF, Ley K. Velocity differences between L- and P-selectin-dependent neutrophil rolling in venules of mouse cremaster muscle in vivo. Am J Physiol 1996; 271(6 Pt 2): H2740–2747. [DOI] [PubMed] [Google Scholar]
- [62].Frimel TN, Walter GA, Gibbs JD, Gaidosh GS, Vandenborne K. Noninvasive monitoring of muscle damage during reloading following limb disuse. Muscle Nerve 2005; 32(5): 605–612. [DOI] [PubMed] [Google Scholar]
- [63].Yamazaki T Effects of Intermittent Weight-Bearing and Clenbuterol on Disuse Atrophy of Rat Hindlimb Muscles. Journal of the Japanese Physical Therapy Association 2005; 8(1): 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Credeur DP, Reynolds LJ, Holwerda SW, Vranish JR, Young BE, Wang J, Thyfault JP, Fadel PJ. Influence of physical inactivity on arterial compliance during a glucose challenge. Experimental Physiology 2018; 103(4): 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Boyle LJ, Credeur DP, Jenkins NT, Padilla J, Leidy HJ, Thyfault JP, Fadel PJ. Impact of reduced daily physical activity on conduit artery flow-mediated dilation and circulating endothelial microparticles. Journal of Applied Physiology 2013; 115(10): 1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Convertino VA, Doerr DF, Mathes KL, Stein SL, Buchanan P. Changes in volume, muscle compartment, and compliance of the lower extremities in man following 30 days of exposure to simulated microgravity. Aviat Space Environ Med 1989; 60(7): 653–658. [PubMed] [Google Scholar]
- [67].Kroese AJ. The effect of inactivity on reactive hyperaemia in the human calf: a study with strain gauge plethysmography. Scand J Clin Lab Invest 1977; 37(1): 53–58. [PubMed] [Google Scholar]
- [68].Bigard AX, Merino D, Lienhard F, Serrurier B, Guezennec CY. Quantitative assessment of degenerative changes in soleus muscle after hindlimb suspension and recovery. Eur J Appl Physiol Occup Physiol 1997; 75(5): 380–387. [DOI] [PubMed] [Google Scholar]
- [69].Krippendorf BB, Riley DA. Distinguishing unloading- versus reloading-induced changes in rat soleus muscle. Muscle Nerve 1993; 16(1): 99–108. [DOI] [PubMed] [Google Scholar]
- [70].Brown M, Hasser EM. Weight-bearing effects on skeletal muscle during and after simulated bed rest. Arch Phys Med Rehabil 1995; 76(6): 541–546. [DOI] [PubMed] [Google Scholar]
- [71].Clarke MSF, Bamman MM, Feeback DL. Bed rest decreases mechanically induced myofiber wounding and consequent wound-mediated FGF release. Journal of Applied Physiology 1998; 85(2): 593–600. [DOI] [PubMed] [Google Scholar]
- [72].Kasper CE, White TP, Maxwell LC. Running during recovery from hindlimb suspension induces transient muscle injury. J Appl Physiol (1985) 1990; 68(2): 533–539. [DOI] [PubMed] [Google Scholar]
- [73].Someya F, Tachino K. Effects of Various Daily Weight-Bearing Periods on Rat Soleus Muscle during Hindlimb Suspension Histochemical and Mechanical Properties. The Japanese Journal of Rehabilitation Medicine 1997; 34(6): 410–417. [Google Scholar]
- [74].Choi S-J. Age-related functional changes and susceptibility to eccentric contraction-induced damage in skeletal muscle cell. Integrative Medicine Research 2016; 5(3): 171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Snijders T, Nederveen JP, Parise G. Are satellite cells lost during short-term disuse-induced muscle fiber atrophy? J Appl Physiol (1985) 2016; 120(12): 1490. [DOI] [PubMed] [Google Scholar]
- [76].Arentson-Lantz EJ, Paddon-Jones D, Fry CS. The intersection of disuse-induced muscle atrophy and satellite cell content: reply to Snijders, Nederveen, and Parise. J Appl Physiol (1985) 2016; 120(12): 1491. [DOI] [PubMed] [Google Scholar]
- [77].Reidy PT, McKenzie AI, Brunker P, Nelson DS, Barrows KM, Supiano M, LaStayo PC, Drummond MJ. Neuromuscular Electrical Stimulation Combined with Protein Ingestion Preserves Thigh Muscle Mass But Not Muscle Function in Healthy Older Adults During 5 Days of Bed Rest. Rejuvenation Res 2017; 20(6): 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Suetta C, Frandsen U, Mackey AL, Jensen L, Hvid LG, Bayer ML, Petersson SJ, Schroder HD, Andersen JL, Aagaard P, Schjerling P, Kjaer M. Ageing is associated with diminished muscle re-growth and myogenic precursor cell expansion early after immobility-induced atrophy in human skeletal muscle. J Physiol 2013; 591(15): 3789–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Chazaud B Macrophages: supportive cells for tissue repair and regeneration. Immunobiology 2014; 219(3): 172–178. [DOI] [PubMed] [Google Scholar]
- [80].Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, Kjaer M, Chazaud B. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 2013; 31(2): 384–396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


