Highlights
-
•
Mainstream PNA achieved excellent effluent quality (TIN < 2 mgN/L).
-
•
Good performance was maintained even at wastewater temperatures < 15 °C.
-
•
An average N2O emission factor of 1.2% was measured over a full year.
-
•
Natural isotope abundance measurements indicate het. denitrification as N2O source.
Keywords: Two-stage, Pilot-scale, PNA, Isotopes, Municipal wastewater
Abstract
For two decades now, partial nitritation anammox (PNA) systems were suggested to more efficiently remove nitrogen (N) from mainstream municipal wastewater. Yet to date, only a few pilot-scale systems and even fewer full-scale implementations of this technology have been described. Process instability continues to restrict the broad application of PNA. Especially problematic are insufficient anammox biomass retention, the growth of undesired aerobic nitrite-oxidizers, and nitrous oxide (N2O) emissions. In this study, a two-stage mainstream pilot-scale PNA system, consisting of three reactors (carbon pre-treatment, nitritation, anammox - 8 m3 each), was operated over a year, treating municipal wastewater. The aim was to test whether both, robust autotrophic N removal and high effluent quality, can be achieved throughout the year. A second aim was to better understand rate limiting processes, potentially affecting the overall performance of PNA systems. In this pilot study, excellent effluent quality, in terms of inorganic nitrogen, was accomplished (average effluent concentrations: 0.4 mgNH4-N/L, 0.1 mgNO2-N/L, 0.9 mgNO3-N/L) even at wastewater temperatures previously considered problematic (as low as 8 °C). N removal was limited by nitritation rates (84 ± 43 mgNH4-N/L/d), while surplus anammox activity was observed at all times (178 ± 43 mgN/L/d). Throughout the study, nitrite-oxidation was maintained at a low level (<2.5% of ammonium consumption rate). Unfortunately, high N2O emissions from the nitritation stage (1.2% of total nitrogen in the influent) were observed, and, based on natural isotope abundance measurements, could be attributed to heterotrophic denitrification. In situ batch experiments were conducted to identify the role of dissolved oxygen (DO) and organic substrate availability in N2O emission-mitigation. The addition of organic substrate, to promote complete denitrification, was not successful in decreasing N2O emission, but increasing the DO from 0.3 to 2.9 mgO2/L decreased N2O emissions by a factor of 3.4.
Graphical abstract
1. Introduction
Anammox-based mainstream N removal has multiple advantages over conventional nitrification/denitrification (N/D) systems commonly used in wastewater treatment. Firstly, the anammox metabolism produces only 0.07–0.11 mgNO3-N per mg NH4-N oxidized, thus less organic substrate is needed for its denitrification to N2 (Lotti et al., 2014; Strous et al., 1998). In turn, a greater fraction of organic substrate can be valorized, e.g., used for bio-plastic production or bio-gas generation (Siegrist et al., 2008). In addition, to reach low N effluent concentrations (<8 mgTN/L), no additional organic substrate is required, reducing the operation costs (EPA, 2013). Furthermore, energy consumption for additional aeration can be lowered, if the organic substrate is treated anaerobically (Daigger, 2014; Shin et al., 2021).
The first step in many mainstream PNA systems is to capture most organic matter (carbon depletion), since the organic substrate requirements (for nitrate denitrification) are reduced. Carbon capture is achieved with various approaches, such as high rate activated sludge (HRAS) systems (Rahman et al., 2019; Wett et al., 2013), chemically enhanced precipitation (Taboada-Santos et al., 2020), or micro-sieving (Paulsrud et al., 2014; Rusten et al., 2016).
After carbon depletion, N removal by PNA can be performed in a single combined PNA stage or in a two-stage process (i.e., partial nitritation followed by anammox). In recent years, there has been significant debate regarding the preference of one- versus two-stage systems (one stage: (Han et al., 2018; Li et al., 2016; Pérez et al., 2014; Schoepp et al., 2018; Vlaeminck et al., 2009); two-stage: (Jung et al., 2021; Kouba et al., 2017; Kowalski et al., 2019; Poot et al., 2016)). Single stage PNA results in lower peak nitrite concentrations because nitrite is, continuously or intermittently, reduced by anammox bacteria (AMX). Low nitrite concentrations have two distinct advantages. Firstly, they reduce the growth rate of unwanted nitrite oxidizing bacteria (NOB). Secondly, they help to keep nitrous oxide (N2O) production low (Chen et al., 2020; Ma et al., 2017). On the other hand, reaching low effluent ammonium concentrations (<5 mgNH4-N/L) can be problematic in single stage systems. At low ammonium concentrations, the growth rate of ammonium oxidizing bacteria (AOB) slows down, which complicates the out-competition of NOB (Corbalá-Robles et al., 2016; Poot et al., 2016). Additionally, two trade-offs need to be addressed in single-stage systems. On the one hand, a long solids retention time (SRT) is required to retain the slow growing AMX while on the other hand NOB need to be washed out. The second trade-off is related to the need for dissolved oxygen (DO) by AOB and the inhibiting effect of DO on AMX (Agrawal et al., 2018). In two-stage PNA systems, NOB suppression and AOB enhancement (stage one) as well as anammox retention (stage two) can be addressed separately.
Over the last 15 years, many publications have reported on working PNA systems in lab-scale reactors and a plethora of strategies to limit NOB growth have been proposed (reviewed in Agrawal et al., 2018; Cao et al., 2017; Li et al., 2018). Yet, only sporadic reports of pilot- or full-scale mainstream anammox installations can be found.
Four pilot- or full-scale mainstream anammox systems reported in the literature (Strass WWTP, Marlisborg WWTP, Dokhaven WWTP and Sjölunda WWTP) are briefly discussed here in light of the potential challenges of full-scale PNA (Gustavsson et al., 2020; Hoekstra et al., 2019; Kamp et al., 2019; Weissenbacher et al., 2013; Wett et al., 2013). During the reported study periods, they were operated as single stage PNA systems and three of them (St, D, Sj) received pretreated wastewater (carbon removal in an HRAS reactor). Anammox biomass retention was achieved through hydrocyclones (St), gravitational settling of granulated biomass (D) or biofilm formation on carriers (Sj). N removal rates were on the order of 50–600 mgN/L/d. However, in (St, D, Sj) high ammonium effluent concentrations were measured (4–10 mgNH4-N/L). Furthermore, significant unwanted nitrate production by NOB was observed in some of the plants (St, D, Sj), and occasionally, problematic nitrite concentrations of 0.2–1 and 1–6 mgNO2-N/L were measured in the effluent of Sj and St, respectively. In D and Sj, nitritation instability due to high organic substrate loading was observed. For M it was demonstrated that the addition of sidestream anammox granules to a nitrification/denitrification basin contributed only 1% to the observed N removal. Lastly, in St, the switch from N/D to deammonification led to an increase in N2O emissions.
As mentioned above, the unifying feature of all four WWTPs presented, is that they were operated as single stage PNAs. Refurbishing old WWTPs to single stage PNA systems is simpler and cheaper, since no new basins are needed. Moreover, immediate nitrite consumption by AMX should limit N2O emissions, which is critical for the greenhouse gas footprint of WWTPs, and reduces NOB growth rates. However, two common issues prevailed in these single stage PNA trials. (1) High ammonium concentrations in the effluent. Strict ammonium effluent requirements of <2 mgNH4-N/L are becoming more common (in Switzerland, ammonium effluent concentrations are not allowed to exceed 2 mgNH4-N/L in a 24 h average effluent sample (GeSchV, 2021, Art. 6 Abs. (1)). Achieving low effluent ammonium concentration will thus be a basic requirement for future PNA systems. (2) Unwanted NOB activity. The imminent risk of re-occurring NOB growth, and the consequent demand for operational changes, jeopardizes the long-term stability of the nitritation stage. In addition, as has been documented in D and Sj, increased organic loading can pose a challenge. For example, organic substrate may be used by denitrifying bacteria, thus consuming nitrite that would otherwise be available for AMX. Moreover, AOB performance can deteriorate in response to increased organic loading, possibly because of inhibiting effects of certain organic compounds, or due to competition for DO with heterotrophs. N2O emissions were only quantified in St, where they increased from 0.3 to 1% of total nitrogen (TN) in the influent after the switch from N/D to mainstream anammox-based N removal. Given the 298 fold greater greenhouse gas potential of N2O with respect to CO2, even a small increase in N2O emissions can drastically alter the climate impact of wastewater treatment, and thus negate any sustainability gains achieved through reduced aeration or decreased organic substrate dosing (Liao et al., 2020).
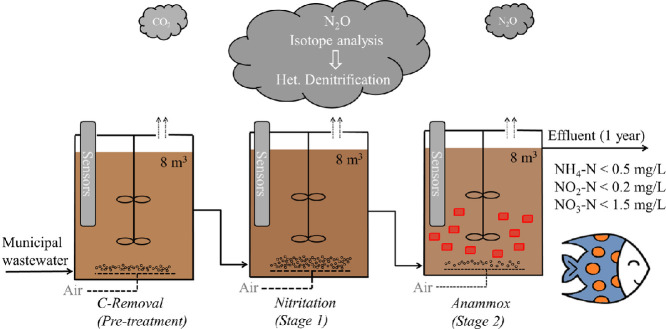
In this study, we established a pilot-scale mainstream two-stage PNA system with prior C-removal and monitored its performance over one year. The long-term observational data presented here, allowed a clear attribution of the rate limiting steps, process disruptions and N2O emissions to either the nitritation or anammox stage. In addition, N2O production pathways were studied using stable isotopes ratio measurements, and strategies to mitigate N2O emissions were investigated experimentally. Moreover, this study explored whether limitations observed in previously published pilot-scale mainstream anammox studies (see above) can be overcome in a two-stage PNA system. Can low ammonium effluent concentrations be reached (<1 mgNH4-N/L)? Is a higher degree of NOB suppression achievable? Is organic matter that escapes from the pretreatment stage as harmful to two-stage systems, as observed for one-stage systems?
2. Material and methods
2.1. Pilot-scale wastewater treatment plant
The pilot-scale WWTP is directly fed from the sewer system of the municipality of Dübendorf, Switzerland. Raw wastewater is pumped from the sewer, and mechanically pretreated (5 mm punched-hole sieve followed by oil and sand trap) before it enters a primary settler (PS), which has a hydraulic retention time of 0.5–1 h. Wastewater composition after the PS is provided in Table 1 (taken from Hausherr et al., 2022). From the PS, wastewater is fed to the first reactor (R1-Carb).
Table 1.
Municipal wastewater characterization after the primary settler (PS) and after pretreatment (R-Carb). Chemical oxygen demand (COD), soluble COD (sCOD), NH4+, NO2−, NO3−, PO43−, total nitrogen (TN) and total phosphorus (TP) average values (n = 8) and standard deviations are presented. TN and TP were only measured in the effluent of the PS.
| Effluent | COD[mg/L] | sCOD[mg/L] | TN[mg/L] | NH4-N[mg/L] | NO2-N[mg/L] | NO3-N[mg/L] | PO4-P[mg/L] | TP[mg/L] |
|---|---|---|---|---|---|---|---|---|
| PS | 469 ± 235 | 277 ± 189 | 47 ± 12 | 25 ± 7 | 0.3 ± 0.03 | 0.6 ± 0.5 | 1.7 ± 1.2 | 5.2 ± 1.6 |
| R-Carb | 137 ± 81 | 51 ± 22 | - | 20 ± 8 | 0.3 ± 0.1 | 0.4 ± 0.5 | 1.2 ± 0.5 | - |
Table 2 presents a summary of the SBR cycles of the three sequential reactors (R1-Carb, R2-PN and R3-AMX). They are all operated as SBRs with linked cycles. Step 8, the idle phase, was used to synchronize the reactors. A volume exchange of ∼90% was carried out for each SBR cycle. To achieve such a high volume exchange, the reactors were bottom-fed (i.e., fresh wastewater was pumped in at the bottom of the reactor and treated wastewater was displaced at the top). A brief illustration of the sequential feeding process is shown in the supporting information (SI, Fig. S1). The SBR cycles were automated and controlled in a supervisory control and data acquisition software (Citect, Australia). All SBR steps with a fixed length were terminated after the specified amount of time had elapsed (Table 2). The control for the variable steps are described for each reactor below.
Table 2.
SBR cycle steps for R1, R2 and R3. All reactors were operated as bottom-fed SBRs. The idle phase was used to synchronize the reactors for the next cycle.
| Step | 1Sedimentation[min] | 2Decant 8 m3→4.5 m3[min] | 3Decant and plug flow[min] | 4Fill4.5m3→8 m3[min] | 5Mixing[min] | 6Aeration[min] | 7Sludge wasting0.75–1.5 m3[min] | 8Idle mixing(Synchronization)[min] | |
|---|---|---|---|---|---|---|---|---|---|
| R1-Carb | 45 | 45 | 45 | 45 (aerobic, DO = 0.8 mg/L) |
60 (aerobic, DO = 0.8 mg/L) |
- | 2–5 | 0–300 | |
| R2-PN | 45 | 45 | 45 | 45 | 30 or 60 (anaerobic) |
45–300 (nitritation, DO = 1–5 mg/L) |
- | 0–35 | |
| R3-AMX | 15 | 45 | 45 | 45 | 20 (anoxic) |
30–120 (ammonium polish, DO = 0.8–1.2 mg/L) |
- | 0–340 (NOx polish) |
|
C-Removal (R1-Carb): The reactor contained floccular sludge. During aerated steps a DO of 0.8 mgO2/L was targeted although over-aeration occasionally occurred due to biofilm formation on the DO sensor. Sludge wasting (Step 7) was manually adjusted every few weeks to reach an SRT of 1.5–2.5 d (aerobic SRT = 0.5–1 d). The idle step was terminated when R2-PN entered its decant step. The reactor was equipped with a temperature sensor (Endress+Hauser, CTS1-A2GSA) and DO electrode (Endress+Hauser, Oxymax COS61). The wastewater temperature in R1-Carb ranged from 7.4 to 22.6 °C, Fig. S2 in the SI shows the online temperature measurements. The wastewater warmed up by 1–2 °C from the time it entered R1-Carb to the time it left R3-AMX.
Nitritation (R2-PN): To reach an adequate NO2−:NH4+ ratio in R2-PN for subsequent anammox treatment, a control algorithm was implemented for the aeration step. This algorithm calculated, for each batch, a target ammonium concentration (35% of the value measured at the end of the filling step). When this target concentration was reached, aeration was stopped (Table 2, R2-PN: Aeration). The idle step was terminated when R3-PN entered its decantation step. The sludge in R2-PN was floccular and SRT was not controlled. The SRT depended on the sludge lost during the decantation step and plug flow step and varied between 10–30 d (SI, Fig. S3). The resulting total suspended solids (TSS) concentration was stable around 1.5–2 gTSS/L. The reactor was equipped with the same electrodes as R1-Carb and, in addition, had an ion selective ammonium sensor (Endress+Hauser, 71109938). More information on the start-up of R2-PN and the establishment of nitritation is found in Hausherr et al. (2022).
Anammox (R3-PN): The anammox reaction occurred mainly during the reactor filling, and all nitrite was already consumed after the filling step (Table 2). Around 2–4.5 mgNH4-N/L remained (i.e., no more nitrite was available for anaerobic ammonium oxidation). Thus, after nitrite depletion, R3-AMX was aerated until ammonium was <1 mgNH4-N/L (Table 2, R3-AMX: Aeration). A schematic of the process is depicted in Fig. 1. The idle step was terminated when both R1-Carb and R2-PN had entered their idle step. Biomass grew in biofilms on carriers (FLUOPUR, Wabag) and in suspension (mostly washed in from R2-PN, 0–1.5 gTSS/L), no active sludge wasting was performed. The reactor contained 167 m2/m3 carrier surface (≈10% volume fill). R3-AMX had the same electrodes as R2-PN (DO, temperature and ammonium). R3-AMX has been running continuously since 2017; for more information on the start-up phase and anammox characterization of R3-AMX the reader is referred to Hausherr et al. (2021).
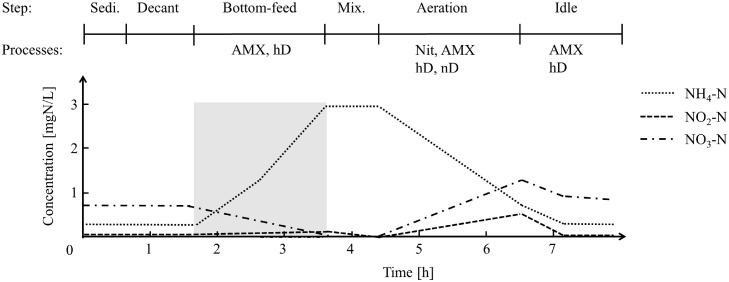
Fig. 1.
Schematic profile of dissolved nitrogen species in the anammox reactor (R3-AMX). Grey box: Concentrations are not homogenous in the reactor due to the plug flow. The drawn lines indicate the mathematically averaged concentrations. Cycle duration and absolute concentrations vary depending on the influent composition. Abbreviations: anammox (AMX), heterotrophic denitrification (hD), nitrification (Nit), nitrifier denitrification (nD).
2.2. . Analytical methods
Wastewater samples were centrifuged (3200 g, 2 min) and filtered (0.45 µm, Macherey–Nagel) prior to analysis. Ammonium, nitrite, nitrate, and phosphate concentrations were determined using ion chromatography (Metrohm AG, 761 Compact IC and 881 Compact IC pro, Switzerland). Soluble chemical oxygen demand (sCOD), COD, total nitrogen, and total phosphorus were quantified photometrically with test kits (Hach Lange, LCK 314, 114, 338, and 348, respectively, Germany). Total suspended solids (TSS), volatile suspended solids (VSS), and the sludge volume index (SVI) were measured according to APHA et al., 2012 standard protocols.
The floc size distribution was determined by static light scattering using a Beckman Coulter (Pasadena, CA, USA) LS 13 320 particle size analyzer with a universal liquid module for the volume percentile distributions.
N2O was measured for 1 min every 12 min in the off-gas from the reactors with a non-dispersive infrared spectrometer (X-stream, Emerson, St. Louis MO, USA) in parts per million (ppmv). N2O is assumed to be an ideal gas, where 1 mole (44 gN2O/mol) occupies 22.7 L under standard conditions. The total amount of N2O-N [g] emitted per SBR cycle is calculated by multiplying the N2O concentration with the air flow rate and the length of aeration.
N2O emission are expressed as an emission factor (EF), which is a percentage of the total nitrogen load in the influent.
| (1) |
Where N2O-Nemitted,D is the cumulative N2O emissions per day [gN/d], TNInfluent is the total nitrogen concentration in the influent [gN/m3] and Q is the influent flow rate [m3/d].
2.3. Activity measurements
2.3.1. AOB and NOB activity
Every 1–3 days two grab samples were taken during the aerated phase of R2-PN. The first sample was taken 5–20 min after aeration had started and the second sample was taken towards the end of the aeration phase. NH4+, NO2− and NO3− concentration were then measured to determine ammonium oxidation-, nitrite accumulation- and nitrate production-rates. The same procedure was applied for the measurement of nitrifier activity during the aerated phase of R3-AMX (Fig. 1, aeration).
2.3.2. Anammox activity
During regular reactor operation, the anammox reaction occurred mainly during the bottom-feed period, in which the wastewater from the nitritation reactor flowed upward through the carriers in R3-AMX (Fig. 1, grey box). Nitrogen species could not be measured during this feeding step (due to the inhomogeneity during the plug flow). By the end of the feeding step, nitrite was usually already consumed (Fig. 1, mix.). Therefore, to measure the anammox activity, ex-situ batch experiments were performed.
For ex-situ batch experiments 750 g (wet weight) of carriers were removed from R3-AMX and resuspended in 12 L (169 m2/m3) of effluent from R3-AMX (containing < 2 mgTN/L and <30 mg soluble COD). The batch reactor was stirred until oxygen was low (DO < 0.1 mg/L). Then, 15–30 mgNH4-N/L as NH4Cl and 15 mgNO2-N/L as NaNO2 were added to the reactor. Sampling was started 15 min after NH4+ and NO2− addition. Two to three samples were taken at 20–30 min intervals and the NH4+, NO2−, and NO3− were analyzed, and rates determined from concentration changes.
2.3.3. Inorganic nitrogen calculations
Inorganic nitrogen mass balances showed that nitrogen removal occurred during the aerated phase of R3-AMX (Fig. 1). The difference in total inorganic nitrogen concentration (ΔTIN, mgN/L) was calculated between the start and end of the aeration phase (Eq. (2)) and normalized to the consumed ammonium (Eq. (3)).
| (2) |
| (3) |
2.4. N2O experiments and isotope analysis
Six experiments were performed in R2-PN (in situ) in March 2020 to assess the impact of DO and organic substrate availability on N2O emissions.
2.4.1. Experimental setup
The experiments were conducted during a regular SBR cycle of R2-PN, i.e., the reactor was filled, stirred anaerobically, and then aerated. The wastewater temperature was not controlled and ranged from 12.5 to 14.9 °C. At the beginning of the experiments, the pH was 7.5–7.6 and declined to 7.2–7.3 at the end of the aerated phase. At the beginning of each of the experiments NH4Cl was added to R2-PN to reach an ammonium concentration of 25 ± 2 mgNH4-N/L. In experiments 1–3 the blower frequency was set to 60, 50, and 40 Hz, respectively, resulting in an aeration rate of 30.5, 23.6 and 13.2 m3 air/h. In experiments 4–6 the blower frequency was always at 60 Hz (i.e., 30.5 m3 air/h). In experiment 4 and 5, 1 m3 effluent from the primary settler was pumped into R2-PN before (Exp. 4) or just after (Exp. 5) the anaerobic phase. In experiment 6, four times 0.25 m3 effluent from the primary settler was pumped into R2-PN during the aerated phase (SI Fig. S3, numbered rectangles).
2.4.2. N2O quantification
N2O concentrations [ppm] were analyzed continuously in the headspace of R2-PN with a non-dispersive infrared spectrometer (X-stream, Emerson, St. Louis MO, USA). For these experiments the emitted N2O was normalized to the total ammonium consumed (Eq. (4)) during nitritation (since all experiments were started with the same ammonium concentration) and not to the total nitrogen in the influent of the WWTP (see, Eq. (1)).
| (4) |
Where N2O-Nemitted is the total N2O emitted during one SBR cycle [gN], NH4,Start and NH4,End are the ammonium concentrations at the start and the end of the batch [gN/L] and VR the reactor volume (8000 L).
2.4.3. Sampling and isotope analysis
For each experiment two off-gas samples were collected in 24 liter aluminum coated gas bags (model GSB-P/44, Wohlgroth AG). One bag was filled during the first half of the aeration phase, and a second bag during the second half (SI, Fig. S4, braces). The collected gas was later analyzed by quantum cascade laser absorption spectroscopy (QCLAS) for N2O isotopocules (isotopically substituted molecules, e.g., 15N-14N-16O, 14N-15N-16O, and 14N-14N-18O); more details in (Ibraim et al., 2018). The abundance of the different isotopocules is expressed on an international scale, i.e., for 14N/15N atmospheric air N2 (AIR-N2) and for 16O/18O Vienna Standard Mean Ocean Water (VSMOW) (Mohn et al., 2016; Toyoda and Yoshida, 1999) in the conventional delta notation as follows:
| (5) |
Where Rsample and Rstandard are the ratio of the isotopically substitute and the most abundant (14N-14N-16O) species in the sample and standard, respectively.
Different N2O production and consumption pathways leave specific fingerprints on the O atom or the N atoms in the central (α) and terminal (β) position of the N2O molecule. The average 15N content of N2O is reported as δ15NbulkN2O, the preference for central over terminal position is termed site preference (SP) and are calculated as follows:
| (6) |
3. Results
3.1. Effluent quality
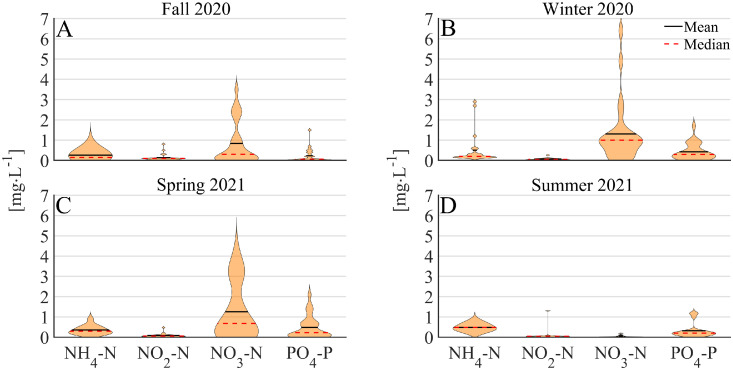
The effluent quality, in terms of dissolved inorganic nitrogen and phosphate concentrations, of R3-AMX was assessed over a year (Sept. 2020-July 2021) and seasonal averages are depicted in Fig. 2. Ammonium concentrations in the effluent exceeded 1 mgNH4-N/L only during winter (3/97 measurements). Elevated nitrite concentrations of 0.5–1 mgNO2-N were only measured in 4/97 samples. Nitrate concentrations in the effluent were highest during winter 2020 and spring in 2021, yet, never exceeded 6.4 mgNO3-N/L. Anaerobic phosphate release and aerobic phosphate re-uptake, i.e., enhanced biological phosphorus removal (EBPR) took place in R2-PN (SI, Fig. S5). The effluent contained therefore generally less than 0.5 mgPO4-P/L. The averages and third quartile (Q3, i.e., 75% of values are lower than Q3) effluent concentrations, over the whole year, of NH4+, NO2−, NO3− and PO43− are shown in Table 3.
Fig. 2.
Violin plots for ammonium, nitrite, nitrate, and phosphate concentrations in the effluent from R3-AMX are shown for each season (n = 27, 20, 26 and 24, for A, B, C, and D, respectively).
Table 3.
Ammonium (NH4+), nitrite (NO2−), nitrate (NO3−), and phosphate (PO43−) concentrations in the effluent of R3-AMX are provided as average ± standard deviation (SD) and third quartile (Q3) over the complete study period (n = 97).
| NH4-N[mg/L] | NO2-N[mg/L] | NO3-N[mg/L] | PO4-N[mg/L] | |
|---|---|---|---|---|
| Average±SD | 0.4 ± 0.4 | 0.1 ± 0.2 | 0.9 ± 1 | 0.4 ± 0.4 |
| Q3 | 0.5 | 0.1 | 1.1 | 0.5 |
3.2. Mainstream partial nitritation anammox
3.2.1. Nitritation: AOB and NOB activity
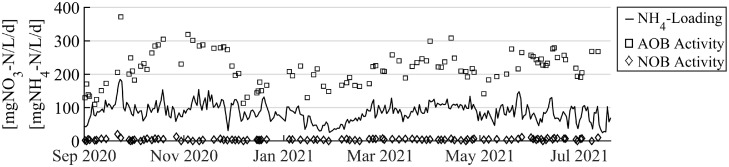
The ammonium oxidation rates in the aerobic phase of R2-PN averaged 219 ± 74 mgNH4-N/L/d (Fig. 3, AOB Activity). Average ammonium loading, defined as mass of ammonia per maximum reactor volume and overall cycle time, was 84 ± 43 mgNH4-N/L/d (Fig. 3). The AOB activity is often significantly higher than the ammonium loading rate because the aerated time is small fraction of overall SBR cycle time. Changes in NH4-loading were driven by the influent ammonium concentration. Especially during rain events NH4-loading decreased since the plug flow feeding phase and anaerobic stirring phase had fixed lengths and thus limited how much HRT could be reduced. NOB activity was successfully suppressed throughout the year, i.e., nitrite oxidation to nitrate was 40 times lower than AOB activity.
Fig. 3.
AOB activity (squares, quantified as ammonium consumption rate) and NOB activity (diamonds, quantified as nitrate production rate) during the aerated phase of the SBR cycle and ammonium loading of R2-PN are shown.
3.2.2. Nitritation: ammonium conversion efficiency and the NO2−:NH4+ ratio
An average ammonium to nitrite conversion efficiency (i.e., how much of the consumed ammonium is found as nitrite) of 89% was achieved in the nitritation reactor, over the full study period (SI, Fig. S6). The 11% deficit in conversion efficiency cannot solely be attributed to nitrite oxidation to nitrate by NOB (∼2.5%; Fig. 4), but is also due to denitrification (emission of N2 and N2O) and assimilative ammonium usage for cell growth.
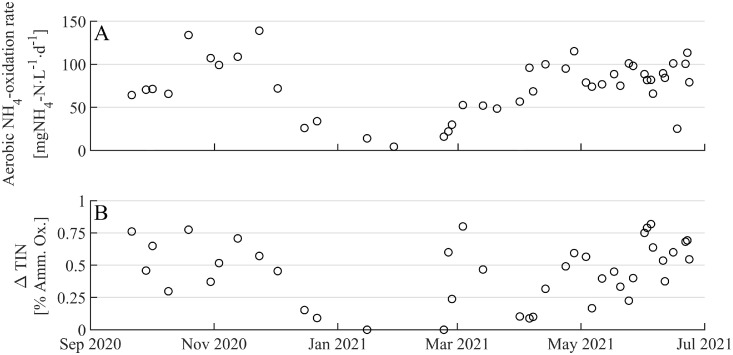
Fig. 4.
(A) Ammonium oxidation rates [mgNH4-N/L/d] in R3-AMX during the aerated phase. (B) The amount of total inorganic nitrogen removed during aeration (ΔTIN) (Eq. (2)) as a fraction of the ammonium oxidized (Eq. (3)).
Ammonium oxidation was stopped (i.e., aeration ceased), when 65% of the initial ammonium concentration, quantified at the onset of the aeration cycle, was consumed. Taking into account the 89% conversion efficiency of NH4+ → NO2− an NO2−:NH4+ ratio of 1.7 is obtained in R2-PN at the end of the aeration phase (SI, Fig. S6). Limited mixing of inflow and outflow, i.e. imperfect plug flow, during the bottom feeding of the nitritation reactor and transfer to R3-AMX resulted in an estimated NO2−:NH4+ ratio in the anammox reactor of 0.8–1.4 (SI, Fig. S6).
3.2.3. Anammox activity and nitrogen removal
R3-AMX had been operating, with external nitrite addition, at a N removal rate of 150–300 mgN/L/d for three years prior to this study (Hausherr et al., 2021). Ex-situ batch activity measurements during this study (with nitrite coming only from the nitritation reactor) showed that R3-AMX still supported high N removal rates (178 ± 43 mgN/L/d) during 2020–2021 (SI, Table S1). However, due to the imperfect plug flow the NO2−:NH4+ ratio supplied to R3-AMX was 0.8–1.4, which usually resulted in 1–3 mgNH4-N/L remaining after nitrite had been exhausted. This incomplete ammonium removal and the lack of nitrate accumulation (as a by-product of the anammox anabolism), clearly indicate that anammox and denitrification were co-occurring. COD for denitrification is available in R3-AMX due to the imperfect plug flow conditions in R1-Carb and R2-PN (SI, Fig. S10). The remaining ammonium was oxidized during an aeration step (4–139 mgNH4-N/L/d, Fig. 4A). Washout of nitritation-biomass from R2-PN into R3-AMX led to nitrite accumulation during the aeration step (average = 0.7 mgNO2-N/L, Fig. 1). Moreover, nitrogen mass balances indicated that the low DO (0.8–1.2 mgO2/L) allowed N removal in the deeper layers of the biofilm (SI, Table S2). The inorganic nitrogen removed during aeration (ΔTIN, Eq. (2)) was between 0–75% of the consumed ammonium (Eq. (3), Fig. 4B). Washout of suspended biomass from R3-AMX (SI, Fig. S7), led to the decrease of ammonium oxidation rates and TIN removal from December 2020 to February 2021 (Fig. 4).
3.3. N2O production and emission
3.3.1. N2O emissions from R1-Carb, R2-PN and R3-AMX
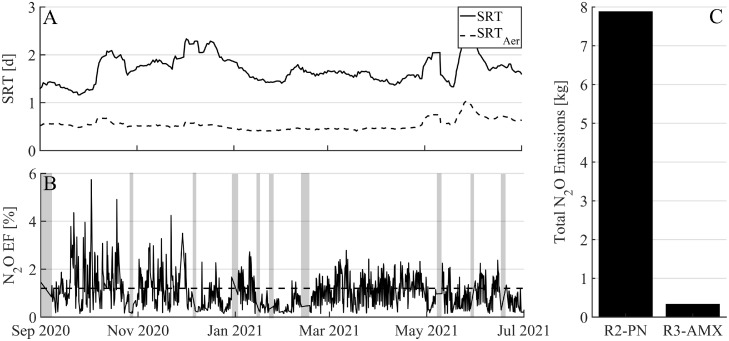
N2O in the off-gas was analyzed for each reactor for 1 min every 12 min throughout the year. No N2O emissions were recorded in R1-Carb, where, due to the short aerobic SRT (0.5–1 d, Fig. 5A), ammonium oxidation did not take place, i.e., no nitrite or nitrate was available to be denitrified and possibly emitted as N2O. In contrast, for R2-PN high N2O emission factors from 0.2–6% (Eq. (1)) were measured (Fig. 5B). During regular reactor operation N2O emissions averaged 1.2% of total nitrogen in the influent (Fig. 5B, dashed line). In R3-AMX N2O was emitted during the aerated phase (ammonium polish), but cumulative emission (over the study period) from R3-AMX were only a small fraction (4%) of cumulative emissions from R2-PN (Fig. 5C).
Fig. 5.
(A) Solids retention time (SRT) and aerobic SRT (SRTAer) in R1-Carb. (B) N2O emission factor (EF) of R2-PN in [%] of total nitrogen in the influent of the pilot WWTP. Grey boxes: data could not be used or is not available due to N2O sensor failure, ammonium sensor drift or maintenance shutdown. Dashed-line: average EF from Sep. 2020 to Jul. 2021. (C) Cumulative N2O emissions [kg] during the aerated phase of R2-PN and R3-AMX over the complete study period.
3.3.2. Influence of DO and organic substrate on N2O emissions
Six experimental conditions were tested (Table 4) to identify whether N2O emissions in the nitritation reactor (R2-PN) are affected by DO and organic substrate availability (Material and methods: N2O experiments). Decreasing the aeration rate significantly impacted N2O emission. More precisely, emissions increased by a factor of 3.4 (from 5 to 17.1% of the consumed ammonium, Eq. (4)), when the aeration rate was decreased by a factor of 2.3 (30.5 to 13.2 m3 air/h). In contrast, dosing of carbon-rich wastewater (primary settler effluent), in Exp. 4–6, did not markedly reduce N2O emissions. It has to be noted, that before the “Pre-Dosing” experiment, a rain-event diluted the wastewater resulting in an increased DO (5.4 mgO2/L), which presumably lowered N2O emissions (3.4%). The effect of intermittent dosing of carbon-rich wastewater was clearly indicated by sequential drops in DO (SI, Fig. S8, Exp. 6), but did not lead to an increase in complete denitrification to N2. In all experiments N2O emissions continuously rose over the course of the experiment (SI, Fig. S8) .
Table 4.
N2O emissions were assessed under six different experimental conditions. N2O emission are provided as percentage of ammonium consumed in R2-PN. In addition the average dissolved oxygen concentrations and the nitritation efficiency are reported. Abbreviations: Dissolved oxygen (DO).
| Experiment | Conditions:Aeration rate 1–3Organic substrate 4–6 | Average DO[mgO2/L] | Nitritationefficiency[%] | N2O emission[%NH4 consumed] |
|---|---|---|---|---|
| 1 | 30.5 m3 air/h | 2.9 | 90 | 5 |
| 2 | 23.6 m3 air/h | 2.1 | 84 | 6.9 |
| 3 | 13.2 m3 air/h | 0.3 | 45 | 17.1 |
| 4 | Pre-Dosing | 5.4 | 94 | 3.4 |
| 5 | Post-Dosing | 2.9 | 92 | 5.3 |
| 6 | Intermittent-Dosing | 2.5 | 87 | 5.2 |
3.3.3. Identification of N2O production pathways by isotope analysis
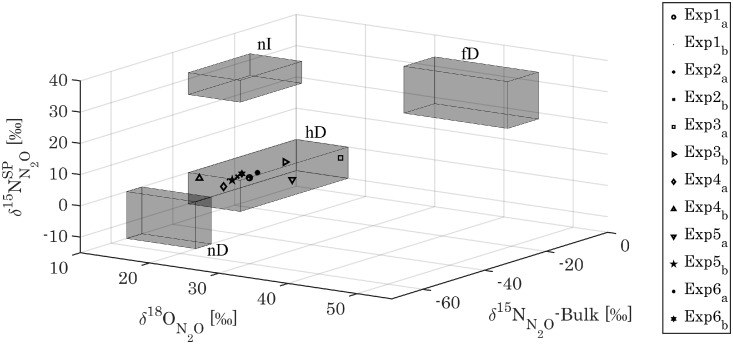
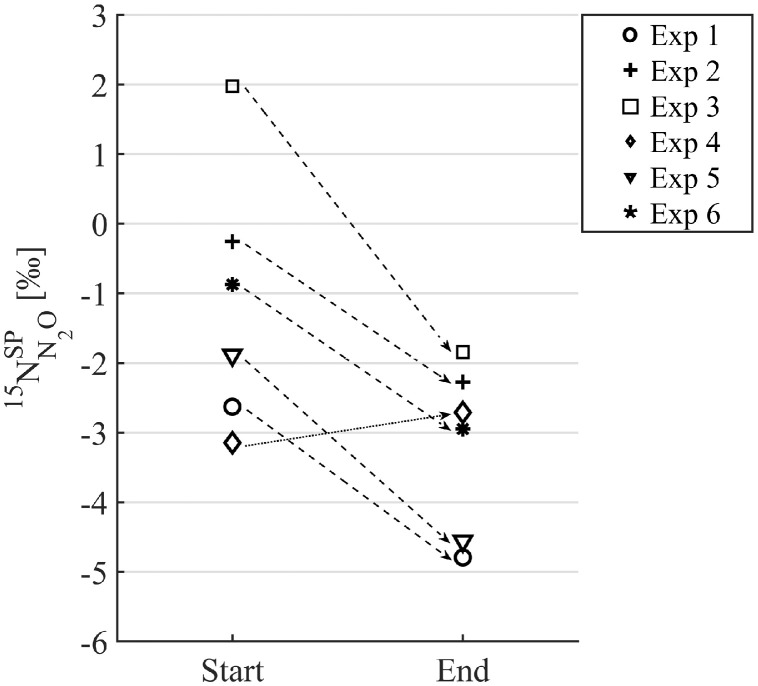
Natural abundance isotope signatures of N2O (δ15Nbulk, δ18O, and δ15NSP), indicate that heterotrophic denitrification is the main process responsible for N2O production (Fig. 6). Low δ15NSP reveal that the contribution of N2O production through the hydroxylamine oxidation pathway are minor, even in the presence of relatively high ammonium concentration (5–25 mgNH4-N/L) and despite high ammonium oxidation activity (i.e., compared to a mainstream flow-through N/D system). A consistent decline in δ15NSP over the course of almost all experiments (except for experiment 4), however, might point to a minor contribution of hydroxylamine oxidation or N2O reduction at the onset of experiments (Fig. 7).
Fig. 6.
Natural isotope abundance signatures of N2O (δ15Nbulk, δ18O and δ15NSP) of the two off-gas samples (a and b in the legend) collected for each experiment in R2-PN. Grey boxes denote expected ranges for: nitrifier denitrification (nD), heterotrophic denitrification (hD), nitrification (nI, hydroxylamine oxidation pathway), fungal denitrification (fD). Figure adapted from (Yu et al., 2020).
Fig. 7.
15N site preference (SP) of N2O is shown for the start and end off-gas sample during the N2O experiments. SP declined over the course of the experiments (dashed arrows), except for Exp. 4 (black arrow).
4. Discussion
The main goals of this study were to use a two-stage PNA system to clearly identify performance limitations (treatment rates and N2O emissions), and to attribute them to either the nitritation or the anammox stage. In contrast to other pilot-scale studies, effluent polishing was targeted.
4.1. Reactor characteristics and changes in N-polishing
The aerobic ammonium oxidation rates in R3-AMX decreased from December to February (Fig. 4A). This was linked to an observed decrease in suspended solids in R3-AMX. As a result of the decreasing biomass in suspension, ammonium polishing (ammonium < 1 mgNH4-N/L) took place only through AOB and NOB residing in the biofilm on the carriers and was not supported anymore through nitrifiers in suspension, i.e., the nitrification rate decreased. To increase the nitrification rate the aeration rate was increased. This ensured low ammonium concentrations in the effluent, but inhibited anoxic processes, i.e., denitrification and anammox. The enhanced aeration resulted in a 1:1 ratio of the oxidation of ammonium to nitrate without simultaneous TIN removal (Fig. 4B, January-February). Effluent quality was restored (i.e., low nitrate concentrations in the effluent) as suspended biomass accumulated again in R3-AMX (SI, Fig. S7). Higher rates of ammonium oxidation were possible at lower aeration rates, which re-enabled simultaneous nitrification, denitrification and anammox (Fig. 4B).
As reported in the supplementary material (Fig. S5), there was significant mixing of fresh influent and treated wastewater in R2-PN during the bottom-feed step (imperfect plug flow conditions). This would be detrimental to the effluent quality in R3-AMX since ammonium- and nitrite-rich influent wastewater would mix with the effluent. Interestingly, such mixing was not observed in R3-AMX (SI, Fig. S10). The carrier-bed likely dissipated wastewater currents and helped to distribute the influent evenly across the reactor footprint, enabling much better plug flow filling. A similar result was observed in a study were granular sludge led to better plug flow conditions (Yang et al., 2015). Imperfect plug flow conditions in R1-Carb are likely responsible for the EBPR in R2-PN. Phosphate release during the anaerobic phase of R2-PN was observed throughout the year except for January and February (sparse data, SI, Fig. S9). However, nitrogen removal and not phosphate removal was the focus of this study, therefore, the EBPR was not investigated thoroughly. Futures studies should assess which process is best suited for phosphate removal in PNA systems, e.g., EBPR in the nitritation stage or iron or aluminum salt addition in the carbon removal stage.
The combination of high ammonium polishing capacity in the suspended biomass and N removal capacity in the biofilm, enabled excellent effluent quality (<1 mgNH4-N/L and <2 mgTIN/L). However, the impact of very high influent flow rates, which need to be handled by WWTPs during rain periods, on effluent quality were not investigated in this study.
4.2. Nitritation rather than anammox constrained overall PNA performance
In the PNA system presented here, the wastewater is treated sequentially (C-removal, nitritation, anammox, polish). The rate-limiting step in the sequence will thus constrain the overall N-elimination performance. In this study, treatment rates were limited by the ammonium loading rates of R2-PN (84 ± 43 mgNH4-N/L/d, Fig. 3) rather than the N removal rates of R3-AMX (178 ± 43 mgN/L/d). Two parameters were primarily responsible for limiting nitritation. First, biomass accumulation could not be increased successfully to concentrations above 2 gTSS/L (SI, Fig. S11), at which point biomass lost in the effluent balanced biomass generation. Even though only a short settling phase (15 min) and upflow selection pressure was applied, no large particles (i.e., granules) were observed in the reactor. On the contrary, small flocs (<125 µm diameter) dominated the reactor (SI, Fig. S12), and SVI30 was 100–200 mL/gTSS (SI, Fig. S13), which is typical for floccular sludge. Limited biomass accumulation in R2-PN resulted in an AOB activity of 219 ± 74 mgNH4-N/L/d. However, in previous work, in bench-scale reactors with additional biomass retention, it was shown that rates up to 3000 mgNH4-N/L/d and ammonium loading rates of 400 mgN/L/d were possible (Hausherr et al., 2022). Second, long anoxic and anaerobic SBR steps prevented AOB activity throughout extended periods of the total SBR cycle. In particular, the 90% plug flow volume exchange took close to two hours (at an upflow velocity of 1 m/h) during which AOB are not active. Hence, to increase treatment rates, better biomass retention (granular sludge, an intermediary clarifier, or a membrane bio-reactor) and faster volume exchange is required. To achieve fast volume exchange, while maintaining plug flow conditions, necessitates a pipe system, which effectively distributes the wastewater across the bottom of the reactor without creating turbulence. The high-volume exchange is necessary, since it ensures high ammonium and low nitrite concentration at the start of the SBR cycle, which fosters AOB growth. In addition, lower volume exchange would result in denitrification of the produced nitrite during the anaerobic cycle phase, deteriorating the EBPR.
4.3. N2O emission patterns and scope for emission reduction
The highest N2O emissions were observed from the nitritation reactor, while emissions from the carbon removal stage and the anammox reactor were absent or small. N2O emissions were mainly modulated by nitrite concentrations, as often observed in wastewater treatment (Blum et al., 2018; Castro-Barros et al., 2016; Kuokkanen et al., 2021). This was most obvious for targeted experiments, where the nitrite concentrations rose steadily over the course of the aeration phase, and N2O emissions increased concomitantly (SI, Fig. S8). However, in the nitritation-system used for this study, the nitrite concentrations cannot be influenced because they are constrained by the ammonium level in the influent (each cycle 65% of ammonium needs to be oxidized to nitrite for subsequent anammox treatment). Thus, other parameters (e.g., DO and organic substrate availability), which can be influenced, were further investigated for their potential to reduce the overall N2O emissions.
Natural abundance N2O isotopes, in particular low SP values, indicate that N2O emissions stemmed mostly from denitrification, and not from the hydroxylamine pathway (Fig. 6) (Koba et al., 2009; Wunderlin et al., 2013). The decrease of SP over the course of most experiments, while DO steadily increased, could be related to a decreasing share of N2O reduction (Fig. 7), as the N2O molecule with the heavier 15N atom in the central position is slightly more resistant towards N-O-bond cleavage (Butterbach-Bahl et al., 2013). Correspondingly, in Exp. 4, where a rain event diluted the wastewater resulting in very high DO values from the beginning of the experiment (SI, Fig. S8, Exp. 4), constantly low δ15NSP values were measured (Fig. 7).
Given that N2O production is primarily due to denitrification, increasing the aeration rate should decrease overall N2O production (i.e., inhibit denitrification enzymes in general). Indeed, in N2O experiments with high aeration rate and high DO, both N2O emissions and δ15NSPN2O (N2O reduction) were minimal, indicating that reductive N2O production associated to partial denitrification, the prime source of N2O, was strongly reduced. While increasing the DO from 0.3 to 3 mgO2/L had a strong N2O mitigation effect (17.1 to 5% of ammonium oxidized in R2-PN), increasing it further to 5.4 mgO2/L only reduced N2O emissions by an additional 1.6%. To achieve high DO concentrations intensive, energy consuming aeration is required, which increases indirect CO2 emission from the WWTP. Yet, because N2O has a 298 times higher GHG potential than CO2, increasing electricity consumption to reduce N2O emissions at higher DO levels would nevertheless help in most cases to reduce the net carbon footprint of WWTPs (Liao et al., 2020). Increasing the DO is, however, not a feasible option in many nitritation system, because DO limitation is often the chosen strategy to minimize NOB activity (Isanta et al., 2015; Laureni et al., 2019). In the nitritation reactor used here, in contrast, DO limitation is not an important factor (Hausherr et al., 2022), and increased aeration could be used to reduce N2O emissions.
Fostering N2O to N2 reduction by increasing organic carbon availability, similar to Wan et al. (2021) did not affect N2O emissions nor δ15NSPN2O (i.e. N2O reduction). However, if such an organic-substrate dosing strategy were conducted over longer periods, and not just once, as in the case of this study, N2O reduction specialists might establish in the sludge helping to reduce the net N2O emissions (Orschler et al., 2021; Qi et al., 2022).
With an average DO of 3–4 mgO2/L during regular reactor operation, an average of 1.2±1 % of total nitrogen in the influent (Table 1, 47 ± 12 mgTN/L) were emitted as N2O. This is close to the average N2O emission factor of 0.9% for N/D WWTP in Switzerland (Gruber et al., 2021). As demonstrated here, N2O stable isotope analyses may provide a useful analytical tool to gain a deeper understanding of biological and, possibly, abiotic N2O production and consumption pathways. This knowledge, in turn, will support the design and establishment of new N2O mitigation strategies. In addition, investigations into catalytic off-gas treatment of N2O are essential (Duan et al., 2021; Scherson et al., 2013). Such treatment will further reduce GHG emissions, and is even evaluated for its potential to generate energy, as for example in the coupled aerobic-anoxic nitrous decomposition operation (CANDO) process (Scherson et al., 2014).
4.4. Implications for PNA design
A clear consensus with regards to whether a one- or a two-stage PNA system is to be favored is still missing. The right choice of the PNA design may depend on the prioritized target (e.g., high N removal, low GHG emissions, stability) and the given framework and constraints (operator knowledge, costs or space availability), and will likely involve compromises.
To achieve high effluent quality (in particular, low effluent ammonium concentrations), one-stage PNA system currently require an additional polishing reactor, since NOB out-competition is often not stable under low ammonium conditions. In contrast, in two-stage PNA systems (operated as SBRs), ammonium polishing can be performed in the anammox stage, where NOB growth is less problematic. Indeed, as shown in this study, in the anammox stage, at low DO concentrations simultaneous TIN removal is possible, which further increases the net N-removal.
Organic shock loadings have repeatedly caused problems in one-stage PNA systems. Generally, HRAS reactors should attenuate shock loadings (Böhnke et al., 1997). But in Dokhaven WWTP and Sjölunda WWTPs, for example, organic shock loading led to PNA process disruption, even though a HRAS pretreatment was performed. Also in this study, similar to D and Sj, organic loading peaks were not completely mitigated during the C-removal stage. In the nitritation stage, they led to an increase in assimilative ammonium consumption by heterotrophic bacteria, which negatively affects the NO2−:NH4+ ratio (since ammonium assimilation lowers the nitritation conversion efficiency). Moreover, heterotrophic activity led to lower DO concentrations, which reduced AOB activity and increased N2O emissions (SI, Figs. S8 and S14). Thus, high organic loading resulted in reduced treatment rates for one or two SBR cycles, but very importantly, without any noticeable long-term effects.
N2O emissions may make up a significant fraction of the net GHG emissions of PNA-based wastewater treatment. Two-stage system may offer more flexibility for operational adjustments, such as higher aeration to reduce N2O emissions without affecting anammox activity. On the other hand, such adjustments might not be needed in one-stage systems, if their lower nitrite levels in general lead to lower N2O emission. For pilot- or full-scale mainstream PNA system little information is available regarding N2O emissions. Here we demonstrate that in two-stage mainstream PNA the N2O emission factor was similar (1%) to a one-stage system (Strass WWTP). Further reduction of N2O emissions is required to clearly decrease the carbon footprint of PNA systems compared to N/D systems. However, the N2O production and emission dynamics are still not well understood. Thus, future tasks will include the characterization of N2O production under various operating regimes, and the optimization of strategies to mitigate GHG emissions without compromising the N-eliminating performance of PNA systems. Once efficient off-gas treatment is viable, and routine applications is feasible, and/or more efficient strategies to reduce N2O emissions are discovered, two-stage PNA is a promising tool to achieve high effluent quality, and to bring WWTPs closer to energy autarky.
5. Conclusion
From a year-long monitoring campaign of a two-stage pilot-scale PNA system, which treated real municipal wastewater, the following conclusions are drawn:
-
•
Suspended biomass washed out from the nitritation stage to the anammox stage allowed for PNA-based effluent polishing, resulting in very low inorganic nitrogen concentrations (average: NH4+ < 0.4 mgNH4-N/L and TIN <2–3 mgN/L).
-
•
Faster plug flow filling (>1 m/h) of the nitritation stage without mixing of fresh and treated wastewater would allow for higher treatment rates than currently achieved (84 ± 43 mgNH4-N/L/d) and simplify reactor operation.
-
•
N2O emissions averaged 1.2% of total nitrogen in the influent, higher than advanced N/D systems. Increasing the DO concentration reduced N2O production. But, further reduction of N2O emissions are required to clearly decrease the carbon footprint of PNA systems compared to N/D systems.
N2O isotopocule analysis showed that:
-
•
Heterotrophic denitrification was the main process responsible for N2O production.
-
•
N2O reduction to N2 was limited, and not increased significantly through the addition of organic substrate.
-
•
Even at high ammonium concentrations (25 mgNH4-N/L) and high AOB activities (219±74 mgNH4-N/L/d) N2O production through the hydroxylamine pathway was negligible.
Data availability
Data used for this study is available at the Eawag Research Data Institutional Collection (ERIC) at DOI: 10.25678/0006AH
CRediT authorship contribution statement
D. Hausherr: Conceptualization, Methodology, Investigation, Data curation, Writing – original draft, Visualization. R. Niederdorfer: Conceptualization, Writing – review & editing. H. Bürgmann: Conceptualization, Writing – review & editing, Funding acquisition. M.F. Lehmann: Conceptualization, Writing – review & editing, Funding acquisition. P. Magyar: Conceptualization, Methodology, Writing – review & editing. J. Mohn: Conceptualization, Methodology, Writing – review & editing, Funding acquisition. E. Morgenroth: Conceptualization, Writing – review & editing, Supervision. A. Joss: Conceptualization, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was financed by the Swiss national science foundation (SNSF) under the IsoMol initiative (Project Number 170876). We thank Wenzel Gruber for the discussions on N2O experimental design and Kerstin Zeyer (Empa, Emissions & Isotopes group) for N2O isotope analyses by QCLAS. We are also obliged to Marco Kipf and Richard Fankhauser for maintaining the reactor infrastructure. Sylvia Richter, Karin Rottermann and Brian Sinnet are thanked for their help with analytical methods. The graphical abstract has been designed with elements from Flaticon.com. Fish (Image name “Fish”, by Made by Made Lineal).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.wroa.2022.100145.
Appendix. Supplementary materials
Bibliography
- Agrawal S., Seuntjens D., Cocker P.De, Lackner S., Vlaeminck S.E. Success of mainstream partial nitritation/anammox demands integration of engineering, microbiome and modeling insights. Curr. Opin. Biotechnol. 2018;50:214–221. doi: 10.1016/j.copbio.2018.01.013. [DOI] [PubMed] [Google Scholar]
- APHA/AWWA/WEF . Standard Methods for the Examination of Water and Wastewater, Standard Methods. American Public Health Association; 2012. Standard Methods for the Examination of Water and Wastewater, Standard Methods. [Google Scholar]
- Blum J., Jensen M.M., Smets B.F. Nitrous oxide production in intermittently aerated partial nitritation-anammox reactor: oxic N2O production dominates and relates with ammonia removal rate. Chem. Eng. J. 2018;335:458–466. doi: 10.1016/j.cej.2017.10.146. [DOI] [Google Scholar]
- Böhnke B., Diering B., Zuckut S.W. AB process removes organics and nutrients. Water Environ. Technol. 1997;9:23–27. https://www.jstor.org/stable/24666275 [Google Scholar]
- Butterbach-Bahl K., Baggs E.M., Dannenmann M., Kiese R., Zechmeister-Boltenstern S. Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013;368 doi: 10.1098/rstb.2013.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., van Loosdrecht M.C.M., Daigger G.T. Mainstream partial nitritation–anammox in municipal wastewater treatment: status, bottlenecks, and further studies. Appl. Microbiol. Biotechnol. 2017;101:1365–1383. doi: 10.1007/s00253-016-8058-7. [DOI] [PubMed] [Google Scholar]
- Castro-Barros C.M., Rodríguez-Caballero A., Volcke E.I.P., Pijuan M. Effect of nitrite on the N2O and NO production on the nitrification of low-strength ammonium wastewater. Chem. Eng. J. 2016;287:269–276. doi: 10.1016/j.cej.2015.10.121. [DOI] [Google Scholar]
- Chen H., Zeng L., Wang D., Zhou Y., Yang X. Recent advances in nitrous oxide production and mitigation in wastewater treatment. Water Res. 2020;184 doi: 10.1016/j.watres.2020.116168. [DOI] [PubMed] [Google Scholar]
- Corbalá-Robles L., Picioreanu C., van Loosdrecht M.C.M., Pérez J. Analysing the effects of the aeration pattern and residual ammonium concentration in a partial nitritation-anammox process. Environ. Technol. 2016;37:694–702. doi: 10.1080/09593330.2015.1077895. [DOI] [PubMed] [Google Scholar]
- Daigger G.T. Oxygen and carbon requirements for biological nitrogen removal processes accomplishing nitrification, nitritation, and anammox. Water Environ. Res. 2014;86:204–209. doi: 10.2175/106143013X13807328849459. [DOI] [PubMed] [Google Scholar]
- Duan H., Zhao Y., Koch K., Wells G.F., Weißbach M., Yuan Z., Ye L. Recovery of nitrous oxide from wastewater treatment: current status and perspectives. ACS ES&T Water. 2021;1:240–250. doi: 10.1021/acsestwater.0c00140. [DOI] [Google Scholar]
- EPA . EPA; 2013. Wastewater Treatment Fact Sheet : External Carbon Sources for Nitrogen Removal 5. [Google Scholar]
- GeSchV, 2021. Gewässerschutzverordnung 1998, 1–72.
- Gruber W., von Känel L., Vogt L., Luck M., Biolley L., Feller K., Moosmann A., Krähenbühl N., Kipf M., Loosli R., Vogel M., Morgenroth E., Braun D., Joss A. Estimation of countrywide N2O emissions from wastewater treatment in Switzerland using long-term monitoring data. Water Res. X. 2021;13 doi: 10.1016/j.wroa.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson D.J.I., Suarez C., Wilén B.M., Hermansson M., Persson F. Long-term stability of partial nitritation-anammox for treatment of municipal wastewater in a moving bed biofilm reactor pilot system. Sci. Total Environ. 2020;714 doi: 10.1016/j.scitotenv.2019.136342. [DOI] [PubMed] [Google Scholar]
- Han Y., Liu F., Xu X., Yan Z., Liu Z. Nitrogen removal via a single-stage PN–anammox process in a novel combined biofilm reactor. Water Sci. Technol. 2018;77:1483–1492. doi: 10.2166/wst.2017.572. [DOI] [PubMed] [Google Scholar]
- Hausherr D., Niederdorfer R., Bürgmann H., Lehmann M.F., Magyar P., Mohn J., Morgenroth E., Joss A. Successful mainstream nitritation through NOB inactivation. Sci. Total Environ. 2022;822 doi: 10.1016/j.scitotenv.2022.153546. [DOI] [PubMed] [Google Scholar]
- Hausherr D., Niederdorfer R., Morgenroth E., Joss A. Robustness of mainstream anammox activity at bench and pilot scale. Sci. Total Environ. 2021;796 doi: 10.1016/j.scitotenv.2021.148920. [DOI] [PubMed] [Google Scholar]
- Hoekstra M., Geilvoet S.P., Hendrickx T.L.G., van Erp Taalman Kip C.S., Kleerebezem R., van Loosdrecht M.C.M. Towards mainstream anammox: lessons learned from pilot-scale research at WWTP Dokhaven. Environ. Technol. 2019;40:1721–1733. doi: 10.1080/09593330.2018.1470204. [DOI] [PubMed] [Google Scholar]
- Ibraim E., Harris E., Eyer S., Tuzson B., Emmenegger L., Six J., Mohn J. Development of a field-deployable method for simultaneous, real-time measurements of the four most abundant N2O isotopocules. Isot. Environ. Health Stud. 2018;54:1–15. doi: 10.1080/10256016.2017.1345902. [DOI] [PubMed] [Google Scholar]
- Isanta E., Reino C., Carrera J., Pérez J. Stable partial nitritation for low-strength wastewater at low temperature in an aerobic granular reactor. Water Res. 2015;80:149–158. doi: 10.1016/j.watres.2015.04.028. [DOI] [PubMed] [Google Scholar]
- Jung M., Oh T., Kim S., Rhu D., Liberzon J., Kang S.J., Daigger G.T. A high-rate and stable nitrogen removal from reject water in a full-scale two-stage AMX® system. Water Sci. Technol. 2021;83:652–663. doi: 10.2166/wst.2021.002. [DOI] [PubMed] [Google Scholar]
- Kamp A., Ottosen L.D.M., Thøgersen N.B., Revsbech N.P., Thamdrup B., Andersen M.H. Anammox and partial nitritation in the mainstream of a wastewater treatment plant in a temperate region (Denmark) Water Sci. Technol. 2019;79:1397–1405. doi: 10.2166/wst.2019.141. [DOI] [PubMed] [Google Scholar]
- Koba K., Osaka K., Tobari Y., Toyoda S., Ohte N., Katsuyama M., Suzuki N., Itoh M., Yamagishi H., Kawasaki M., Kim S.J., Yoshida N., Nakajima T. Biogeochemistry of nitrous oxide in groundwater in a forested ecosystem elucidated by nitrous oxide isotopomer measurements. Geochim. Cosmochim. Acta. 2009;73:3115–3133. doi: 10.1016/j.gca.2009.03.022. [DOI] [Google Scholar]
- Kouba V., Vejmelkova D., Proksova E., Wiesinger H., Concha M., Dolejs P., Hejnic J., Jenicek P., Bartacek J. High-rate partial nitritation of municipal wastewater after psychrophilic anaerobic pretreatment. Environ. Sci. Technol. 2017;51:11029–11038. doi: 10.1021/acs.est.7b02078. [DOI] [PubMed] [Google Scholar]
- Kowalski M.S., Devlin T.R., di Biase A., Oleszkiewicz J.A. Effective nitrogen removal in a two-stage partial nitritation-anammox reactor treating municipal wastewater – piloting PN-MBBR/AMX-IFAS configuration. Bioresour. Technol. 2019;289 doi: 10.1016/j.biortech.2019.121742. [DOI] [PubMed] [Google Scholar]
- Kuokkanen A., Blomberg K., Mikola A., Heinonen M. Unwanted mainstream nitritation–denitritation causing massive N2O emissions in a continuous activated sludge process. Water Sci. Technol. 2021;83:2207–2217. doi: 10.2166/wst.2021.127. [DOI] [PubMed] [Google Scholar]
- Laureni M., Weissbrodt D.G., Villez K., Robin O., de Jonge N., Rosenthal A., Wells G., Nielsen J.L., Morgenroth E., Joss A. Biomass segregation between biofilm and flocs improves the control of nitrite-oxidizing bacteria in mainstream partial nitritation and anammox processes. Water Res. 2019;154:104–116. doi: 10.1016/j.watres.2018.12.051. [DOI] [PubMed] [Google Scholar]
- Li X., Klaus S., Bott C., He Z. Status, challenges, and perspectives of mainstream nitritation-anammox for wastewater treatment. Water Environ. Res. 2018;90:634–649. doi: 10.2175/106143017x15131012153112. [DOI] [PubMed] [Google Scholar]
- Li X., Sun S., Badgley B.D., Sung S., Zhang H., He Z. Nitrogen removal by granular nitritation–anammox in an upflow membrane-aerated biofilm reactor. Water Res. 2016;94:23–31. doi: 10.1016/j.watres.2016.02.031. [DOI] [PubMed] [Google Scholar]
- Liao X., Tian Y., Gan Y., Ji J. Quantifying urban wastewater treatment sector's greenhouse gas emissions using a hybrid life cycle analysis method – an application on Shenzhen city in China. Sci. Total Environ. 2020;745 doi: 10.1016/j.scitotenv.2020.141176. [DOI] [PubMed] [Google Scholar]
- Lotti T., Kleerebezem R., Lubello C., van Loosdrecht M.C.M. Physiological and kinetic characterization of a suspended cell anammox culture. Water Res. 2014;60:1–14. doi: 10.1016/j.watres.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Ma C., Jensen M.M., Smets B.F., Thamdrup B. Pathways and controls of N2O production in nitritation–anammox biomass. Environ. Sci. Technol. 2017;51:8981–8991. doi: 10.1021/acs.est.7b01225. [DOI] [PubMed] [Google Scholar]
- Mohn J., Gutjahr W., Toyoda S., Harris E., Ibraim E., Geilmann H., Schleppi P., Kuhn T., Lehmann M.F., Decock C., Werner R.A., Yoshida N., Brand W.A. Reassessment of the NH4NO3 thermal decomposition technique for calibration of the N2O isotopic composition. Rapid Commun. Mass Spectrom. 2016;30:2487–2496. doi: 10.1002/rcm.7736. [DOI] [PubMed] [Google Scholar]
- Orschler L., Agrawal S., Lackner S. Targeted metagenomics reveals extensive diversity of the denitrifying community in partial nitritation anammox and activated sludge systems. Biotechnol. Bioeng. 2021;118:433–441. doi: 10.1002/bit.27581. [DOI] [PubMed] [Google Scholar]
- Paulsrud B., Rusten B., Aas B. Increasing the sludge energy potential of wastewater treatment plants by introducing fine mesh sieves for primary treatment. Water Sci. Technol. 2014;69:560–565. doi: 10.2166/wst.2013.737. [DOI] [PubMed] [Google Scholar]
- Pérez J., Lotti T., Kleerebezem R., Picioreanu C., van Loosdrecht M.C.M. Outcompeting nitrite-oxidizing bacteria in single-stage nitrogen removal in sewage treatment plants: a model-based study. Water Res. 2014;66:208–218. doi: 10.1016/j.watres.2014.08.028. [DOI] [PubMed] [Google Scholar]
- Poot V., Hoekstra M., Geleijnse M.A.A., van Loosdrecht M.C.M., Pérez J. Effects of the residual ammonium concentration on NOB repression during partial nitritation with granular sludge. Water Res. 2016;106:518–530. doi: 10.1016/j.watres.2016.10.028. [DOI] [PubMed] [Google Scholar]
- Qi C., Zhou Y., Suenaga T., Oba K., Lu J., Wang G., Zhang L., Yoon S., Terada A. Organic carbon determines nitrous oxide consumption activity of clade I and II nosZ bacteria: genomic and biokinetic insights. Water Res. 2022;209 doi: 10.1016/j.watres.2021.117910. [DOI] [PubMed] [Google Scholar]
- Rahman A., De Clippeleir H., Thomas W., Jimenez J.A., Wett B., Al-Omari A., Murthy S., Riffat R., Bott C. A-stage and high-rate contact-stabilization performance comparison for carbon and nutrient redirection from high-strength municipal wastewater. Chem. Eng. J. 2019;357:737–749. doi: 10.1016/j.cej.2018.09.206. [DOI] [Google Scholar]
- Rusten B., Razafimanantsoa V.A., Andriamiarinjaka M.A., Otis C.L., Sahu A.K., Bilstad T. Impact of fine mesh sieve primary treatment on nitrogen removal in moving bed biofilm reactors. Water Sci. Technol. 2016;73:337–344. doi: 10.2166/wst.2015.498. [DOI] [PubMed] [Google Scholar]
- Scherson Y.D., Wells G.F., Woo S.G., Lee J., Park J., Cantwell B.J., Criddle C.S. Nitrogen removal with energy recovery through N2O decomposition. Energy Environ. Sci. 2013;6:241–248. doi: 10.1039/C2EE22487A. [DOI] [Google Scholar]
- Scherson Y.D., Woo S.G., Criddle C.S. Production of nitrous oxide from anaerobic digester centrate and its use as a co-oxidant of biogas to enhance energy recovery. Environ. Sci. Technol. 2014;48:5612–5619. doi: 10.1021/es501009j. [DOI] [PubMed] [Google Scholar]
- Schoepp T., Bousek J., Beqaj A., Fiedler C., Wett B., Fuchs W., Ertl T., Weissenbacher N. Nitrous oxide emissions of a mesh separated single stage deammonification reactor. Water Sci. Technol. 2018;78:2239–2246. doi: 10.2166/wst.2018.500. [DOI] [PubMed] [Google Scholar]
- Shin C., Tilmans S.H., Chen F., McCarty P.L., Criddle C.S. Temperate climate energy-positive anaerobic secondary treatment of domestic wastewater at pilot-scale. Water Res. 2021;204 doi: 10.1016/j.watres.2021.117598. [DOI] [PubMed] [Google Scholar]
- Siegrist H., Salzgeber D., Eugster J., Joss A. Anammox brings WWTP closer to energy autarky due to increased biogas production and reduced aeration energy for N-removal. Water Sci. Technol. 2008;57:383–388. doi: 10.2166/wst.2008.048. [DOI] [PubMed] [Google Scholar]
- Strous M., Heijnen J.J., Kuenen J.G., Jetten M.S.M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol. 1998;50:589–596. doi: 10.1007/s002530051340. [DOI] [Google Scholar]
- Taboada-Santos A., Rivadulla E., Paredes L., Carballa M., Romalde J., Lema J.M. Comprehensive comparison of chemically enhanced primary treatment and high-rate activated sludge in novel wastewater treatment plant configurations. Water Res. 2020;169 doi: 10.1016/j.watres.2019.115258. [DOI] [PubMed] [Google Scholar]
- Toyoda S., Yoshida N. Determination of nitrogen isotopomers of nitrous oxide on a modified isotope ratio mass spectrometer. Anal. Chem. 1999;71:4711–4718. doi: 10.1021/ac9904563. [DOI] [Google Scholar]
- Vlaeminck S.E., Terada A., Smets B.F., Linden D.V, Boon N., Verstraete W., Carballa M. Nitrogen removal from digested black water by one-stage partial nitritation and anammox. Environ. Sci. Technol. 2009;43:5035–5041. doi: 10.1021/es803284y. [DOI] [PubMed] [Google Scholar]
- Wan X., Laureni M., Jia M., Volcke E.I.P. Impact of organics, aeration and flocs on N2O emissions during granular-based partial nitritation-anammox. Sci. Total Environ. 2021;797 doi: 10.1016/j.scitotenv.2021.149092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher N., Wett B., de Clippeleir H., Hell M. Bundesministerium für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft; 2013. Hauptstromdeammonifikation in Kläranlagen; pp. 1–44. [Google Scholar]
- Wett B., Omari A., Podmirseg S.M., Han M., Akintayo O., Gómez Brandón M., Murthy S., Bott C., Hell M., Takács I., Nyhuis G., O'Shaughnessy M. Going for mainstream deammonification from bench to full scale for maximized resource efficiency. Water Sci. Technol. 2013;68:283–289. doi: 10.2166/wst.2013.150. [DOI] [PubMed] [Google Scholar]
- Wunderlin P., Lehmann M.F., Siegrist H., Tuzson B., Joss A., Emmenegger L., Mohn J. Isotope signatures of N2O in a mixed microbial population system: constraints on N2O producing pathways in wastewater treatment. Environ. Sci. Technol. 2013;47 doi: 10.1021/es303174x. 130118101927005. [DOI] [PubMed] [Google Scholar]
- Yang J., Yang Y., Ji X., Chen Y., Guo J., Fang F. Three-dimensional modeling of hydrodynamics and biokinetics in EGSB reactor. J. Chem. 2015;2015:1–7. doi: 10.1155/2015/635281. [DOI] [Google Scholar]
- Yu L., Harris E., Lewicka-Szczebak D., Barthel M., Blomberg M.R.A., Harris S.J., Johnson M.S., Lehmann M.F., Liisberg J., Müller C., Ostrom N.E., Six J., Toyoda S., Yoshida N., Mohn J. What can we learn from N2O isotope data? Analytics, processes and modelling. Rapid Commun. Mass Spectrom. 2020;34:1–14. doi: 10.1002/rcm.8858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used for this study is available at the Eawag Research Data Institutional Collection (ERIC) at DOI: 10.25678/0006AH