Abstract
Background
Comprehensive Geriatric Assessment (CGA) is the gold standard for detecting frailty in elderly patients with cancer. Since CGA is time- and resource-consuming, many alternative frailty screening tools have been developed; however, it remains unknown whether these tools are suitable for older and adult patients with cancer. Therefore, we used the data collected for a large longitudinal study to compare the diagnostic performances of two frailty screening tools (Geriatric 8 [G8] and Flemish version of the Triage Risk Screening Tool [fTRST]) to identify frailty risk profile among patients with cancer.
Methods
Patients aged ≥20 years with newly diagnosed cancer were enrolled. Frailty screening with G8, fTRST, and CGA were performed before anti-cancer treatment. Diagnostic characteristics obtained using G8 and fTRST were analyzed by C-index, and the validity of G8 and fTRST was also determined.
Results
40.9% of the 755 patients with cancer displayed frailty on CGA. Both G8 and fTRST showed high sensitivity (80.6–88.4%) and negative predictive value (81.0–81.2%). The C-index of G8 was higher than that of fTRST (0.77 vs 0.71, p = .01). Moreover, the best G8 and fTRST cut-off points were ≤13 and ≥ 2, respectively. The validities of G8 and fTRST were also confirmed; however, frailty age differences were not observed in our study.
Conclusion
Frailty is a common problem for patients with cancer, and routine frailty screening is essential for both older and adult patients. G8 and fTRST are simple and useful frailty screening tools, while G8 is more suitable than fTRST for Taiwanese patients with cancer.
Keywords: Cancer, Frailty, Performance, Screening tools
At a glance commentary
Scientific background on the subject
Frailty refers to the decline in reserve capacities of multiple systems (physiology, cognition, nutrition, social support, and psychology), which makes individuals fail to maintain homeostasis in the face of stressors. The early assessment of frailty can serve
What this study adds to the field
Previous studies on frailty and screening tools were mainly designed for the elderly population, and screening tool recommendations have been inconsistent. However, non-elderly populations have also experienced frailty. Therefore, our study is the first to applyG8 and fTRST to both elderly and non-elderly patient with various cancer types.
In 2012, the World Health Organization reported that 14.1 million patients were newly diagnosed with cancer, with an increase of 1.4 million patients from 2008. The prevalence of cancer has increased yearly, and the number of patients with newly diagnosed cancer is predicted to increase by 70% in 20 years [1,2]. Therefore, an increasing number of patients require anti-cancer treatments. However, not all patients are able to bear the physical burdens that are caused by cancer treatments. Previous studies have indicated that frailty can be used to predict the prevalence of postoperative complications [3], mortality [4], and quality of life [4] of patients with cancer. The early assessment of frailty can also serve as a reference for treatment-related decision-making.
Frailty refers to the decline in reserve capacities of multiple systems (physiology, cognition, nutrition, social support, and psychology), which makes individuals fail to maintain homeostasis in the face of stressors [[5], [6], [7], [8]]. Many international academic associations (e.g., National Comprehensive Cancer Network [NCCN] and International Society of Geriatric Oncology [SIOG]) have indicated that the Comprehensive Geriatric Assessment (CGA) is the gold standard for assessing frailty and helps us to objectively understand the health condition of various systems in elderly patients with cancer [9,10]. Our previous study indicated frailty was common in patients with primary head and neck cancer and was independently associated with poor survival, high treatment-related complications, and severe adverse events of concurrent chemoradiotherapy [11]. Nevertheless, it is time- and resource-consuming to perform CGA, and those that assess CGA must receive professional geriatric training as well. Therefore, in order to strengthen the clinical use of frailty indicators, many frailty screening tools have been developed, including Geriatric 8 (G8) [12], Flemish version of the Triage Risk Screening Tool (fTRST) [13], and Abbreviated Comprehensive Geriatric Assessment (aCGA) [14]. Hopefully, clinicians will be able to efficiently use conventional screening tools to assess frailty in the future.
Many studies have compared the diagnostic characteristic of frailty screening tools [8,[15], [16], [17], [18]]. However, screening tool recommendations have been inconsistent. Previous studies on frailty and screening tools have mainly been designed for the elderly population. However, non-elderly populations have also experienced frailty [19,20]. To date, no study has investigated frailty in both elderly and non-elderly patients with various cancer types. Therefore, our study aimed to test the performance and validity of G8 and fTRST for frailty screening in elderly and non-elderly patients with cancer in Taiwan.
Materials and methods
Patient population and data collection
Our study is part of a large longitudinal study that investigated factors affecting the frailty of patients with cancer in Taiwan and the correlation of these factors with the occurrence of severe complications. Data were collected from three hospitals in Taiwan, with a total of four data collection points. For the purpose of our study, we used only baseline data to investigate the performance of G8 and fTRST in patients with cancer. We used convenience sampling, and the inclusion criteria were as follows: (1) patients ≥20 years of age; (2) outpatients or inpatients who were diagnosed with cancer by clinical physicians and scheduled for high-intensity anti-cancer treatments within two weeks (radical surgery, adjuvant chemotherapy, or concurrent chemoradiotherapy [CCRT]); (3) patients who are conscious and can communicate in Mandarin or Taiwanese. The exclusion criteria were as follows: (1) patients with cognitive impairments, who are unable to complete the questionnaires; (2) patients who did not receive treatment with a curative purpose (e.g., palliative chemotherapy or radiotherapy) or had distant metastases. Our study was approved by the institutional review board of the study site (No: 1608080002). We obtained informed consent from all patients before the interviews. All patients with cancer completed the frailty questionnaires (G8, fTRST, and CGA) with the assistances of the first author (SY Chen) and a trained research assistant before receiving anti-cancer treatment. The average assessment time was 30 min (2 min for G8, 2 min for fTRST, and 26 min for CGA).
Frailty screening tools (G8 and fTRST)
The G8 was developed by Soubeyran et al. [12] and has been used to assess the risk of frailty in elderly patients with cancer [18,21]. This screening tool contains seven items and the total score is 0 (heavily impaired) to 17 (not at all impaired) points. The score ≤14 is considered abnormal and indicates a frailty risk profile [18,21].
The TRST was developed by Meldon et al. and has been used to assess the risk of being unable to be discharged from the emergency room [13,22]. The research team of the University Hospitals Leuven partially modified the TRST to the fTRST, which evaluates the frailty risk profile of elderly patients [23]. This screening tool includes a total of five items, and the total score is 0–6 points. Within the oncologic population, the score ≥1 is considered to represent a frailty risk profile [23,24]. Both G8 and fTRST cut-off scores were taken into account in our study.
CGA
CGA is the gold standard tool for assessing frailty in elderly patients [9,10]. Since no comprehensive measurement tool was developed to assess frailty in the non-elderly population, CGA has been chosen to assess frailty in non-elderly population [11]. Therefore, we used CGA as the measurement tool for assessing frailty in both elderly and non-elderly patients in our study. In past studies, CGA mainly included five dimensions. Patients with ≥2 impaired dimensions were considered as patients with frailty [25,26]. Specifically, the CGA was used to define which patients had a frailty risk profile, over five dimensions (functional status, nutrition, comorbidity, mobility/falls, and polypharmacy), and patients who exhibited impairments in ≥2 domains within the CGA were defined as patients with frailty. The assessment tools and cut-off standards of various dimensions are shown in [Table 1].
Table 1.
Measures of CGA.
| Frailty domain | Measures | No items | Score range | Cut-off value |
|---|---|---|---|---|
| Functional status | Barthel index (ADL) [27] | 10 | 0–100 | ≤100 |
| Lawton scale (IADL) [28] | 8 | 0–8 | ≥7 | |
| Nutrition | MNA-SF [29] | 6 | 0–14 | ≤11 |
| Comorbidity | CCI-Q [30] | 17 | 0–33 | >3 |
| Mobility/Falls | Number of falls [14] | 1 | 0-∞ | ≥2 |
| Polypharmacy | Number of medications [9,31] | 1 | 0-∞ | ≥5 |
Abbreviations: ADL: activities of daily living; CCI-Q: Charlson comorbidity Index-Quan; IADL: instrumental activities of daily living; MNA-SF: mini nutritional assessment-short form.
Statistical analysis
Data analyses were performed using SPSS 22.0 statistical software package (SPSS, Armonk, NY: IBM Corp) and a p-value <0.05 was considered statistically significant. The ROC curve was used to detect diagnostic characteristics of G8 and fTRST. CGA was the comprehensive tool for diagnosing frailty and for comparing the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), Youden index [32], and C-index [33] of G8 and fTRST. In general, a C-index ≥0.7 was deemed acceptable for discriminating frailty [34].
Our study used logistic regression and multiple regression to establish the validity of screening tools. Construct validity was used to investigate the effects of ECOG and age on frailty. Previous studies indicated that impaired functional status and elderly always with high level of frailty [35,36], thus we divided ECOG (0 vs ≥ 1 points) and age (<65 and ≥ 65 year) into two groups for known groups comparison. The significant variables in the univariate analysis were set as control variables for multiple regression analysis, and we subsequently detected the effects of ECOG and age on frailty.
Results
Analysis of patient demographic and clinical characteristics
Seven hundred and fifty-five patients with cancer were enrolled in our study. The mean age was 63.3 ± 12.2 years, and 418 (55.4%) patients were over the age of 65. Most of the patients were male (71%) and married (75.4%). Head and neck cancer was the most general diagnosis (50.3%), followed by colorectal cancer (20.0%). Moreover, 28.5% of the patients were diagnosed with stage III cancer, and 33.0% of the patients were of stage IV. For ECOG, 59.6% of the patients had a score of 0 point. Most of the patients had chronic comorbidities (59.1%). Relevant demographic and clinical characteristics are shown in [Table 2].
Table 2.
Patient demographic and clinical characteristic (N = 755).
| Demographic and clinical characteristic | N | % | t/F |
|
|---|---|---|---|---|
| G8 | fTRST | |||
| Age (mean ± SD) | 63.3 ± 12.2 | |||
| ≥ 65 y | 418 | 55.4 | −2.41∗ | −4.84∗∗∗ |
| <65 y | 337 | 44.6 | ||
| Gender | ||||
| Male | 536 | 71.0 | −0.29 | −2.31∗ |
| Female | 219 | 29.0 | ||
| Marital status | ||||
| Married | 569 | 75.4 | 2.40∗ | −2.75∗∗ |
| Other | 186 | 24.6 | ||
| Education | ||||
| Primary | 297 | 39.3 | 7.33∗∗ | 2.83 |
| Junior/Senior high | 419 | 55.5 | ||
| College/Master | 39 | 5.2 | ||
| Cancer type | ||||
| Colorectal | 151 | 20.0 | 4.96∗∗∗ | 4.35∗∗∗ |
| Head & Neck | 380 | 50.3 | ||
| Liver | 37 | 4.9 | ||
| Breast | 48 | 6.4 | ||
| Gastric | 50 | 6.6 | ||
| Non-Hodgkin Lymphoma | 32 | 4.2 | ||
| Other | 57 | 7.5 | ||
| Cancer stage | ||||
| Stage I | 97 | 12.8 | 8.02∗∗∗ | 1.76 |
| Stage II | 194 | 25.7 | ||
| Stage III | 215 | 28.5 | ||
| Stage IV | 249 | 33.0 | ||
| ECOG | ||||
| Score = 0 | 450 | 59.6 | 9.94∗∗∗ | −4.24∗∗∗ |
| Score≥1 | 305 | 40.4 | ||
| Comorbidity | ||||
| Yes | 446 | 59.1 | −0.44 | −5.09∗∗∗ |
| No | 309 | 40.9 | ||
∗p < .05 ∗∗p < .01 ∗∗∗p < .001.
Prevalence of frailty in overall, elderly, and non-elderly patients as per CGA and two other screening tools
A frailty risk profile, determined by CGA, was present in 40.9% of the overall patient population, and 47.6% and 32.6% of the elderly (≥65) and non-elderly (<65) patients, respectively. With respect to the different screening tools, the prevalence of frailty, as determined by G8 (≤14), was 57.7% in the patient population, and 55.5% and 60.5% in elderly and non-elderly patients, respectively. The prevalence of frailty, as determined by fTRST (≥1), was 75.0% in the overall patient population, and 78.1% and 71.2% in elderly and non-elderly patients, respectively.
Diagnostic characteristics and C-index of G8 and fTRST
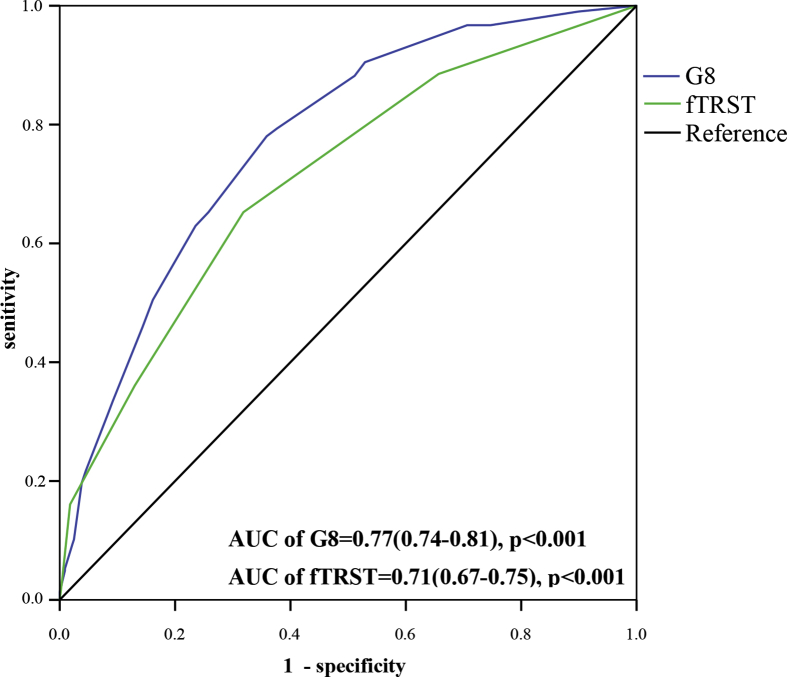
High sensitivities (80.6% and 88.4%) and NPVs (81.2% and 81.0%) were found for both G8 (≤14) and fTRST (≥1). However, the C-index of G8 was higher than that of fTRST (0.77 vs 0.71, p = .01). According to the Youden index, our study found that the best cut-off for G8 was ≤13 points (Youden index = 0.404), which had a sensitivity of 68.4% and NPV of 78.6%, and the best cut-off for fTRST was ≥2 points (Youden index = 0.337), which had a sensitivity of 65.5% and NPV of 74.2% [Table 3] and [Fig. 1].
Table 3.
Diagnostic Characteristics of G8 and fTRST for Patients with Cancer (N = 755).
| Measure, cut-off point | Prevalence of frailty (%) (G8/fTRST) |
Prevalence of frailty (%) (CGA) |
Sensitivity (%) (95% CI) |
Specificity (%) (95% CI) |
PPV (%) (95% CI) |
NPV (%) (95% CI) |
YI | C-index (95% CI) |
|---|---|---|---|---|---|---|---|---|
| G8 | ||||||||
| ≤14 | 57.7 | 40.9 | 80.6 (75.7–84.8) | 58.1 (53.3–62.7) | 57.1 (52.3–61.8) | 81.2 (76.5–85.3) | 0.39 | 0.77 (0.74–0.81)∗∗∗ |
| ≤13 | 44.5 | 40.9 | 68.4 (62.9–73.6) | 72.0 (67.6–76.1) | 62.7 (57.3–67.9) | 76.8 (72.5–80.8) | 0.40 | |
| ≤12 | 35.5 | 40.9 | 56.7 (50.9–62.3) | 79.2 (75.1–82.8) | 65.2 (59.1–70.9) | 72.6 (68.4–76.6) | 0.36 | |
| fTRST | ||||||||
| ≥1 | 75.0 | 40.9 | 88.4 (84.2–91.7) | 34.3 (29.9–38.9) | 48.2 (44.1–52.4) | 81.0 (74.6–86.3) | 0.23 | 0.71 (0.67–0.75)∗∗∗ |
| ≥2 | 45.6 | 40.9 | 65.5 (59.9–70.8) | 68.2 (63.6–72.5) | 58.6 (53.2–63.9) | 74.2 (69.6–78.3) | 0.34 | |
| ≥3 | 22.6 | 40.9 | 36.5 (31.1–42.1) | 87.0 (83.5–90.0) | 65.9 (58.2–73.0) | 66.6 (62.6–70.4) | 0.24 | |
∗∗∗p < .001.
NPV, negative predictive value; PPV, positive predictive value; YI, Youden Index.
Fig. 1.
Area under the Curve (C-index) of Receiver Operating Characteristic Analysis of G8 and fTRST.
Validity of G8 and fTRST
Our study used CGA as the comprehensive tool for frailty assessment, and found that both G8 (Wald’ sχ2 = 127.80, p < .001) and fTRST (Wald’ sχ2 = 94.22, p < .001) significantly correlated with CGA, which supports the convergent validity of G8 and fTRST. By using known groups comparison, we found that there were significant differences in G8 (ΔR2 = 0.06, p < .001) and fTRST (ΔR2 = 0.02, p < .001) scores among different ECOG scores (≥1 vs 0 point). A higher ECOG score (poorer physical activity) indicates a higher frailty [Table 4]; however, age did not have a significant influence on both G8 (ΔR2 = 0.00, p = .999) and fTRST (ΔR2 = 0.00, p = .397) scores [Table 5].
Table 4.
The Effect of ECOG on G8 and fTRST (Construct Validity) (N = 755).
| Predictor | Unstandardized coefficient |
95% CI for β |
p | |||
|---|---|---|---|---|---|---|
| β | SE | Lower limit | Upper limit | |||
| G8 | ||||||
| ECOG | Score = 0a | – | ||||
| Score≥1 | −1.61 | 0.21 | −2.02 | −1.20 | 0.000 | |
| F | 11.78∗∗∗ | |||||
| Adjusted R2 | 0.20 | |||||
| ΔF | 59.55∗∗∗ | |||||
| ΔR2 | 0.06 | |||||
| fTRST | ||||||
| ECOG | Score = 0a | – | ||||
| Score≥ 1 | 0.40 | 0.10 | 0.22 | 0.59 | 0.000 | |
| F | 5.02∗∗∗ | |||||
| Adjusted R2 | 0.08 | |||||
| ΔF | 17.94∗∗∗ | |||||
| ΔR2 | 0.02 | |||||
∗∗∗p < .001.
Adjust (G8): age, marital status, education, cancer type, cancer stage. Adjust (fTRST): age, gender, marital status, cancer type, comorbidity. a: reference group.
Abbreviation: SE: standard error.
Table 5.
The Effects of Age on G8 and fTRST (Construct Validity) (N = 755).
| Predictor | Unstandardized coefficient |
95% CI for β |
p | |||
|---|---|---|---|---|---|---|
| β | SE | Lower limit | Upper limit | |||
| G8 | ||||||
| age | Non-elderlya | – | ||||
| Elderly | 0.00 | 0.35 | −0.68 | 0.68 | 0.999 | |
| F | 11.78∗∗∗ | |||||
| Adjusted R2 | 0.20 | |||||
| ΔF | 0.00 | |||||
| ΔR2 | 0.00 | |||||
| fTRST | ||||||
| age | Non-elderlya | – | ||||
| Elderly | 0.14 | 0.16 | −0.18 | 0.46 | 0.397 | |
| F | 5.02∗∗∗ | |||||
| Adjusted R2 | 0.08 | |||||
| ΔF | 0.72 | |||||
| ΔR2 | 0.00 | |||||
∗∗∗p < .001.
Adjust (G8): marital status, education, cancer type, cancer stage, ECOG. Adjust (fTRST): gender, marital status, cancer type, ECOG, comorbidity. a: reference group.
Abbreviation: SE: standard error.
Comparison of G8 and fTRST performance between patients with or without head and neck cancer (HNC)
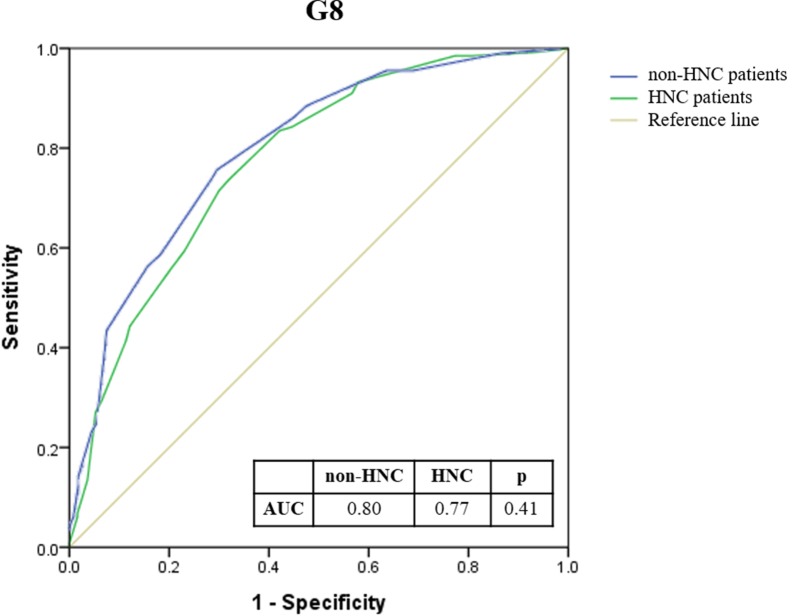
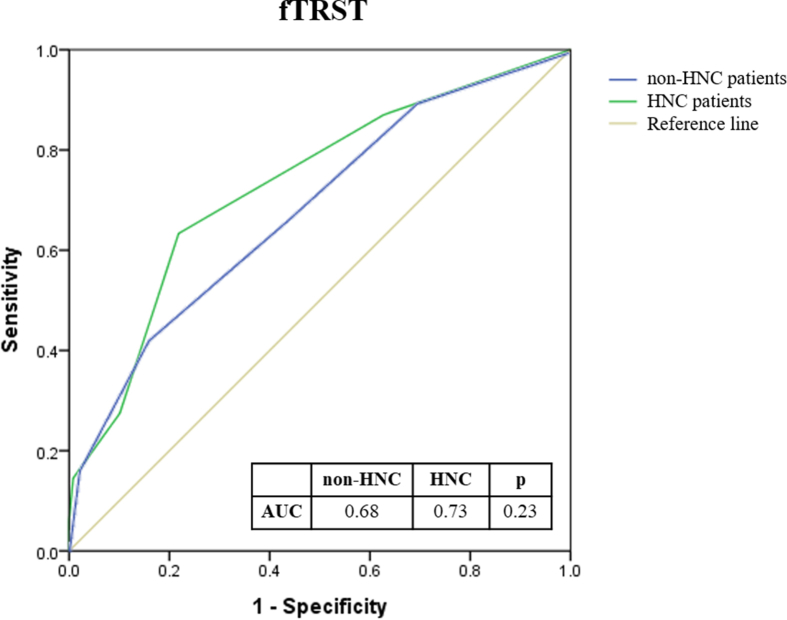
As patients with HNC accounted for more than 50% of our subjects, we divided the entire patient cohort into HNC and non-HNC groups to evaluate the performance of G8 and fTRST. No significant differences were found in the C-index of G8 and fTRST between HNC and non-HNC groups (G8: 0.77 vs. 0.80, p = 0.41; fTRST: 0.73 vs. 0.68, p = 0.23) (Supplementary Fig. 1 & Fig. 2).
Discussion
Our study investigated the diagnostic characteristics and validities of G8 and fTRST in patients with cancer and compared them to a frailty risk profile according to the CGA. The prevalence of frailty in the patients with cancer who were enrolled in our study was 40.9% (CGA). Handforth et al. [26] reviewed 16 studies of frailty in elderly patients and discovered that the mean prevalence of frailty was 43%, which is similar to that in our study. Apart from the elderly population, our study also enrolled a non-elderly population, to accentuate the fact that frailty is also universal in non-elderly patients with cancer (32.6%) and to remind our readers that clinical frailty assessments should be performed on both elderly and non-elderly patients. Our study is the first to apply G8 and fTRST to both elderly and non-elderly patients with cancer. Previous studies have mainly focused on elderly patients with cancer and have rarely investigated frailty in both elderly and non-elderly patients with cancer. Two studies have analyzed frailty in healthy elderly and non-elderly population, and these investigations found that the mean prevalence of frailty was 7.4% [19,20], which is significantly lower than that reported in our study. This difference might be caused by the disease, since the physical status of patients with cancer is weaker than that of generally healthy individuals, and thus, the prevalence of frailty is higher for patients with cancer.
The sensitivity of G8 (≤14) and fTRST (≥1) in our study were 80.6% and 88.4%, respectively. We reviewed previous studies of frailty in elderly patients with cancer and found that the mean sensitivity of G8 and fTRST was 86% [[15], [16], [17], [18],21,[37], [38], [39]] and 92% [16,23,38], respectively, which are higher than the values that were observed in our study. The high sensitivity can be explained by the fact that G8 and fTRST were developed for elderly patients. Previous studies have also applied these tools to elderly patients (≥70 years old) [[16], [17], [18],21,37], and most of these patients were in advanced stages or received palliative care [16,21,[37], [38], [39]]. In addition, our study excluded patients with cognitive impairment, and there have been no discriminations in cognition-related items in the screening tools that were used for patients in our study. This lessened the sensitivity of our study, in comparison to the results of past studies. Although frailty in both elderly and non-elderly patients with cancer has rarely been assessed in previous studies, our study verified that both G8 and fTRST can effectively screen frailty risk profiles in patients with cancer who are of different ages, and the sensitivity of these tools is not inferior to that observed in prior frailty studies in elderly patient with cancer.
Many past studies have applied G8 and fTRST to clinical practices and have verified that these screening tools are highly sensitive and effective screening tools [15,16,18,21,37,39]. Our study also found that both G8 and fTRST can effectively predict frailty in elderly and non-elderly patients with cancer (C-index = 0.71–0.77). Although the G8 cut-off point that has been recommended by scholars was ≤14 [18,21], and that of fTRST was ≥1 [16,23,24], our study found that the best cut-off point of G8 for patients with cancer in Taiwan should be decreased to ≤13, and that of fTRST should be increased to ≥2. Such differences may be caused by the fact that the cut-off points that were recommended by past studies were for elderly patients with cancer [16,18,21,23], most of whom were in advanced stages or received palliative care [16,21] and had a high prevalence of frailty (71–83%). Therefore, the cut-off points for both tools should be modified to be stricter than the currently used standards. In addition to elderly patients with cancer, our study also enrolled non-elderly patients with cancer and excluded those receiving palliative care. Therefore, the prevalence of frailty in our investigation is lower than that in past studies, and the best cut-off point that we recommend based on our study also differs from the recommendations of prior studies. For the assessments of screening tools, sensitivity and NPV are the most important indicators [37]. If the best cut-off points for G8 and fTRST that were recommended by our study are used (G8 ≤13; fTRST ≥2), both the sensitivity (68.4% vs 65.5%) and the NPV (76.8% vs 74.2%) of G8 are higher than those of fTRST. Furthermore, the C-index of G8 is also significantly higher than that of fTRST. Therefore, compared with fTRST, G8 is more suitable for patients with cancer in Taiwan. The reason for the differences in the two screening tools may be because G8 was developed based on the Mini Nutritional Assessment and 50.3% of the participants in our study had head and neck cancer and more likely had a malnourished status. However, G8 was originally designed for elderly patients with cancer. To expand the use of G8 to non-elderly populations, we advise for the scoring standards of some of the items to be modified. Taking item 8 for example, the score of both patients’ ≤65 years of age and patients 65–79 years of age was 2, and as such, it was impossible to distinguish the impact of age from this data. According to our results, we found that the frequencies of patients with cancer who are 40–64 years of age are 41.3%, while that of patients who are 65–74 years of age is 36.8%. Therefore, we suggest patient ages to be divided into <65 and ≥ 65 years cohorts, to distinguish the impact of age on frailty.
Our study found that both G8 and fTRST were significantly correlated with CGA, which supports the convergent validity of G8 and fTRST. Our study also verified the construct validity of G8 and fTRST, through comparing different ECOG scores (ECOG = 0 vs ≥ 1). Our result showed that patients with higher ECOG scores were more likely to be screened for frailty risk profiles using the G8 and fTRST, which supported the construct validity of G8 and fTRST. This result is also consistent with that of other past studies, which have found that the poorer the physical functional status of patients, the higher the associated frailty level [7,35,40]. Unfortunately, most of the studies on frailty have primarily analyzed the diagnostic characteristics of screening tools, and although the diagnostic characteristic of screening tools is important, testing their construct validity is also extremely important [41]. Therefore, we advise for future studies to include a validity test, while assessing the effectiveness of frailty screening tools, to better complete the assessment of screening tools.
Moreover, prior studies have also indicated that the prevalence of frailty increases with age [19,20,35]; however, the results of our study showed that age did not have a significant influence on G8 and fTRST. Such differences might be caused by the sample populations that had been enrolled in different studies. Previous studies had not concurrently analyzed frailty in both elderly and non-elderly patients with cancer. Only two studies have analyzed the prevalence of frailty in healthy populations, which included both elderly and non-elderly patients, and found that the prevalence of frailty increased with age [19,20]. However, frailty in patients with cancer is affected by more complicated factors than those in healthy populations; in the elderly population, age is a factor that affects frailty. In patients with cancer, the main cause of frailty is the decline of multiple physiological systems, caused by disease [5,6]. Although the construct validity of G8 and fTRST could not be verified in patients of different ages, our results revealed a critical message: age is an important factor; however, it may not be the only factor for distinguishing frailty in patients with cancer. Past studies have never investigated this issue, which reflects that it is necessary to screen frailty in non-elderly patients with cancer and conduct more studies to confirm our findings.
Our study has some limitations that merit further discussion. First, our study utilized CGA as the tool to identify the frailty of all adult patients with cancer. The CGA was developed for frailty assessment in geriatric populations, and some dimensions, such as number of falls, included in CGA may not be applicable in younger individuals. However, no comprehensive measurement tool for frailty assessment has been developed in non-elderly population till date and the aspects and instruments of frailty assessment in non-elderly patients with cancer are still debated and not validated [42]. Our data highlights that frailty is a common symptom among patients with cancer in various age groups. Thus, we suggest that researchers in the future could develop a comprehensive measurement tool to assess frailty in the non-geriatric population through sound research. Second, past studies of frailty have mainly focused on elderly patients and have rarely focused on both elderly and non-elderly patients with cancer. Therefore, it is difficult to compare the results of our study with those of other relevant studies. However, it reflects an advantage of our study that will enable experts and scholars to understand the importance of frailty assessments in non-elderly patients with cancer. Moreover, the pre-treatment screening of frailty in patients with cancer can serve as reference for physicians to discuss treatment plans with patients [40]. We also advise future studies to test more frailty screening tools to determine which screening tools are most suitable for patients with cancer in Taiwan. Third, the higher proportion of patients with HNC with high risk for malnutrition might bring bias to frailty assessment. However, our study showed similar performance of G8 and fTRST between patients with and without HNC. This suggests that our study results represent both HNC and non-HNC groups. To avoid such concern of bias, we recommend that future studies should, on average, enroll patients with different cancer types to balance the number of study participants with different cancer types.
Conclusions
Frailty is common in both elderly and non-elderly patients with cancer, and it may cause severe adverse outcomes. Thus, we advise clinicians to use G8 or fTRST to routinely screen for frailty risk profiles in patients with cancer before the commencement of cancer treatment. For patients with cancer in Taiwan, G8 is superior to fTRST in frailty screening tool performance. Furthermore, the optimal cut-off point recommended by our study can serve as reference point for clinicians to assess frailty and assist in treatment-related decision-making.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors would like to thank all members of the Chang Gung Memorial Hospital Cancer Center for their help in data collection. Special thanks to all patients who participated in this project.
Footnotes
Peer review under responsibility of Chang Gung University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bj.2021.03.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
References
- 1.Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Tan K.Y., Kawamura Y.J., Tokomitsu A., Tang T. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. Am J Surg. 2012;204:139–143. doi: 10.1016/j.amjsurg.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Green P., Arnold S.V., Cohen D.J., Kirtane A.J., Kodali S.K., Brown D.L., et al. Relation of frailty to outcomes after transcatheter aortic valve replacement (from the PARTNER trial) Am J Cardiol. 2015;116:264–269. doi: 10.1016/j.amjcard.2015.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clegg A., Young J., Iliffe S., Rikkert M.O., Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. Erratum in: Lancet 2013;382:1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ethun C.G., Bilen M.A., Jani A.B., Maithel S.K., Ogan K., Master V.A. Frailty and cancer: implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J Clin. 2017;67:362–377. doi: 10.3322/caac.21406. [DOI] [PubMed] [Google Scholar]
- 7.Huisingh-Scheetz M., Walston J. How should older adults with cancer be evaluated for frailty? J Geriatr Oncol. 2017;8:8–15. doi: 10.1016/j.jgo.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenig J., Zychiewicz B., Olszewska U., Barczynski M., Nowak W. Six screening instruments for frailty in older patients qualified for emergency abdominal surgery. Arch Gerontol Geriatr. 2015;61:437–442. doi: 10.1016/j.archger.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network . 2017. NCCN Clinical Practice Guidelines in Oncology. Older adult oncology.https://oncolife.com.ua/doc/nccn/Older_Adult_Oncology.pdf Version 2, 2017. [cited 12 December 2019] Available from: [Google Scholar]
- 10.International Society of Geriatric Oncology . 2011. Practice guideline: comprehensive geriatric assessment (CGA) in oncological patients 2010.http://siog.org/files/public/cga_practice_guideline_wildiers_jul2011.pdf [cited 12 December 2019] Available from: [Google Scholar]
- 11.Chou W.C., Chang P.H., Chen P.T., Wang H.M., Yeh K.Y., Lu C.H., et al. Clinical significance of vulnerability assessment in patients with primary head and neck cancer undergoing definitive concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2020;108:602–611. doi: 10.1016/j.ijrobp.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Soubeyran P., Rainfray M., Mathoulin-Pélissier S., Blanc-Bisson C., Mertens C., Blanc J., et al. Prediction of early death risk in the elderly with cancer: results of a prospective multicentric study of 364 patients under chemotherapy. J Clin Oncol. 2007;25:9040. [Google Scholar]
- 13.Hustey F.M., Mion L.C., Connor J.T., Emerman C.L., Campbell J., Palmer R.M. A brief risk stratification tool to predict functional decline in older adults discharged from emergency departments. J Am Geriatr Soc. 2007;55:1269–1274. doi: 10.1111/j.1532-5415.2007.01272.x. [DOI] [PubMed] [Google Scholar]
- 14.Overcash J.A., Beckstead J., Moody L., Extermann M., Cobb S. The abbreviated comprehensive geriatric assessment (aCGA) for use in the older cancer patient as a prescreen: scoring and interpretation. Crit Rev Oncol Hematol. 2006;59:205–210. doi: 10.1016/j.critrevonc.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Kenig J., Zychiewicz B., Olszewska U., Richter P. Screening for frailty among older patients with cancer that qualify for abdominal surgery. J Geriatr Oncol. 2015;6:52–59. doi: 10.1016/j.jgo.2014.09.179. [DOI] [PubMed] [Google Scholar]
- 16.Kenis C., Decoster L., Van Puyvelde K., De Greve J., Conings G., Milisen K., et al. Performance of two geriatric screening tools in older patients with cancer. J Clin Oncol. 2014;32:19–26. doi: 10.1200/JCO.2013.51.1345. [DOI] [PubMed] [Google Scholar]
- 17.Smets I.H., Kempen G.I., Janssen-Heijnen M.L., Deckx L., Buntinx F.J., van den Akker M. Four screening instruments for frailty in older patients with and without cancer: a diagnostic study. BMC Geriatr. 2014;14:26. doi: 10.1186/1471-2318-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soubeyran P., Bellera C., Goyard J., Heitz D., Cure H., Rousselot H., et al. Screening for vulnerability in older cancer patients: the ONCODAGE prospective multicenter cohort study. PloS One. 2014;9 doi: 10.1371/journal.pone.0115060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kehler D.S., Ferguson T., Stammers A.N., Bohm C., Arora R.C., Duhamel T.A., et al. Prevalence of frailty in Canadians 18–79 years old in the Canadian health measures survey. BMC Geriatr. 2017;17:28. doi: 10.1186/s12877-017-0423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rockwood K., Song X., Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ. 2011;183:E487–E494. doi: 10.1503/cmaj.101271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellera C.A., Rainfray M., Mathoulin-Pelissier S., Mertens C., Delva F., Fonck M., et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23:2166–2172. doi: 10.1093/annonc/mdr587. [DOI] [PubMed] [Google Scholar]
- 22.Meldon S.W., Mion L.C., Palmer R.M., Drew B.L., Connor J.T., Lewicki L.J., et al. A brief risk-stratification tool to predict repeat emergency department visits and hospitalizations in older patients discharged from the emergency department. Acad Emerg Med. 2003;10:224–232. doi: 10.1111/j.1553-2712.2003.tb01996.x. [DOI] [PubMed] [Google Scholar]
- 23.Kenis C., Geeraerts A., Braes T., Milisen K., Flamaing J., Wildiers H. 19 the Flemish version of the Triage Risk Screening Tool (TRST): a multidimensional short screening tool for the assessment of elderly patients. Crit Rev Oncol Hematol. 2006;60:S31. [Google Scholar]
- 24.Lee J.S., Schwindt G., Langevin M., Moghabghab R., Alibhai S.M., Kiss A., et al. Validation of the triage risk stratification tool to identify older persons at risk for hospital admission and returning to the emergency department. J Am Geriatr Soc. 2008;56:2112–2117. doi: 10.1111/j.1532-5415.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- 25.Hamaker M.E., Jonker J.M., de Rooij S.E., Vos A.G., Smorenburg C.H., van Munster B.C. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol. 2012;13:e437–e444. doi: 10.1016/S1470-2045(12)70259-0. [DOI] [PubMed] [Google Scholar]
- 26.Handforth C., Clegg A., Young C., Simpkins S., Seymour M.T., Selby P.J., et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26:1091–1101. doi: 10.1093/annonc/mdu540. [DOI] [PubMed] [Google Scholar]
- 27.Mahoney F.I., Barthel D.W. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 28.Lawton M.P., Brody E.M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontol. 1969;9:179–186. [PubMed] [Google Scholar]
- 29.Rubenstein L.Z., Harker J.O., Salva A., Guigoz Y., Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF) J Gerontol A Biol Sci Med Sci. 2001;56:M366–M372. doi: 10.1093/gerona/56.6.m366. [DOI] [PubMed] [Google Scholar]
- 30.Quan H., Sundararajan V., Halfon P., Fong A., Burnand B., Luthi J.C., et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 31.Owusu C., Berger N.A. Comprehensive geriatric assessment in the older cancer patient: coming of age in clinical cancer care. Clin Pract (Lond) 2014;11:749–762. doi: 10.2217/cpr.14.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruopp M.D., Perkins N.J., Whitcomb B.W., Schisterman E.F. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom J. 2008;50:419–430. doi: 10.1002/bimj.200710415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bamber D. The area above the ordinal dominance graph and the area below the receiver operating characteristic graph. J Math Psychol. 1975;12:387–415. [Google Scholar]
- 34.Baesens B. John Wiley & Sons; New York: 2014. Analytics in a big data world: the essential guide to data science and its applications. [Google Scholar]
- 35.Shamliyan T., Talley K.M., Ramakrishnan R., Kane R.L. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2013;12:719–736. doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Perna S., Francis M.D.A., Bologna C., Moncaglieri F., Riva A., Morazzoni P., et al. Performance of Edmonton Frail Scale on frailty assessment: its association with multi-dimensional geriatric conditions assessed with specific screening tools. BMC Geriatr. 2017;17:2. doi: 10.1186/s12877-016-0382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baitar A., Van Fraeyenhove F., Vandebroek A., De Droogh E., Galdermans D., Mebis J., et al. Geriatric screening results and the association with severe treatment toxicity after the first cycle of (radio)chemotherapy. J Geriatr Oncol. 2014;5:179–184. doi: 10.1016/j.jgo.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Kenis C., Schuermans H., Van Cutsem E., Verhoef G., Vansteenkiste J., Vergote I., et al. P8 Screening for a geriatric risk profile in older cancer patients: a comparative study of the predictive validity of three screening tools. Crit Rev Oncol Hematol. 2009;72:S22. [Google Scholar]
- 39.Pottel L., Boterberg T., Pottel H., Goethals L., Van Den Noortgate N., Duprez F., et al. Determination of an adequate screening tool for identification of vulnerable elderly head and neck cancer patients treated with radio (chemo) therapy. J Geriatr Oncol. 2012;3:24–32. [Google Scholar]
- 40.Wildiers H., Heeren P., Puts M., Topinkova E., Janssen-Heijnen M.L., Extermann M., et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32:2595–2603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolarinwa O.A. Principles and methods of validity and reliability testing of questionnaires used in social and health science researches. Niger Postgrad Med J. 2015;22:195–201. doi: 10.4103/1117-1936.173959. [DOI] [PubMed] [Google Scholar]
- 42.Dharmarajan K.V., Mohile S.G. Can geriatric assessment measures be used to determine cancer treatment vulnerability in nongeriatric patients? Int J Radiat Oncol Biol Phys. 2020;108:612–614. doi: 10.1016/j.ijrobp.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]