Abstract
Tourette syndrome (TS) is a frequently observed developmental neuropsychological disorder occurring in children. The pathophysiology involves both genetic and environmental factors. In this review, clinical characteristics, pathophysiology, and treatment approaches based on the pathophysiology of TS are presented. The pathophysiology is the acceleration of developmental decrement of dopamine (DA) activity at the terminal of nigro-striatal (NS)-DA system causing DA D2 receptor up-ward regulation. Serotonergic neurons involving in development of the biphasic sleep-wake-rhythm, and locomotion may be involved. Pharmacological treatments constitute an important part in managing TS. Small dose of levodopa and aripiprazole showed the good effect controlling the tics, without side effects. Intervention with enhancing the day time activity and keeping the regular sleep-wake-rhythm, and encouraging locomotion are important. The data from Yoshiko Nomura Neurological Clinic for Children regarding the clinical features and outcomes, medication effects, and OCD and outcomes are shown. To discuss about the environmental factor, how the COVID-19 pandemic affected the TS patients is also presented.
Keywords: Tourette syndrome (TS), Managements of TS, Pharmacological treatment, Small dose of levodopa, Aripiprazole
Tourette syndrome (TS) is a frequently observed neuropsychiatric disorder in children of all ethnic backgrounds [[1], [2], [3], [4], [5]]. Tics and tic disorders are familiar to most pediatricians, neurologists, and psychiatrists. The underlying neuronal mechanisms should be considered when treating TS, as the underlying neuronal systems of TS involve both systems controlled by environmental and genetic factors [[6], [7], [8]]. Thus, for the latter factor pharmacological therapy is essential. Here, I review the clinical characteristics, pathophysiological considerations, and treatment approaches based on the pathophysiological neuronal mechanism.
The following subjects are summarized.
-
I.
Clinical characteristics of TS
-
II.
General approach of treating TS
-
III.
Pathophysiological considerations of TS
-
IV.
The treatment of TS based on the pathophysiology
-
V.Data from Yoshiko Nomura Neurological Clinic for Children
-
1.Clinical features and outcomes
-
2.Medication effects
-
3.Obsessive compulsive disorder (OCD) and outcomes
-
4.How the COVID-19 pandemic has affected TS patients
-
1.
-
VI.
Conclusion
Clinical characteristics of TS
Tic disorders are movement disorders associated with the wide repertoire of psycho-behavioral disorders [8,9]. Among the various movement disorders in children, tics are most frequently observed. Emilio Fernandez-Alvarez and Jean Aicardi reported that about 40% of almost 700 cases had tics [10].
Tics are classified as being motor or vocal (phonic) tics, and each are further classified into simple and complex tics. According to the type of tics and the duration from the onset of tics, tic disorders are classified as being transient tic disorder, chronic motor tic disorder, chronic phonic disorder, and chronic multiple tic disorder associated with phonic tics (TS) [1].
The clinical characteristics of tics are age related. Tics occur at any age in childhood, but in general around 2–18 years of age, with a peak at around 6 years [[1], [2], [3], [4],11,12]. Motor tics tend to start the earliest, from simple then complex, and phonic tics tend to start later than motor tics, also from simple to complex [4,8,[11], [12], [13]]. The peak severity of tics is often in the second decade, around 10–13 years in my experience, and then they decline or cease altogether toward the late teens [4,8,[11], [12], [13]]. However, tics may last through late teens and even into adulthood [14,15]. Natural course shows the characteristic wax and wane changes in the types and severities of tics.
Premonitory urge or antecedent sensory phenomena are often observed. Tics are more common in males than females, and they are often associated with comorbidities such as attention deficit hyperactivity disorder (ADHD) and OCD, of which ADHD starts earlier and OCD tends to start later [4,8,[11], [12], [13]].
TS is also associated with other psychiatric, behavioral, and neurological disorders, including aggressiveness, self-mutilation, panic disorder, depression, autism spectrum disorder, restless legs syndrome, and migraine [8,9]. The age-related occurrence and clinical course are important, and suggest age-dependent changes in the underlying pathophysiology [7].
General approach of treating TS
Therapeutic or experimental interventions for TS include pharmacological and non-pharmacological approaches [[16], [17], [18], [19]]. The various types of intervention suggest the complexity of the pathophysiology of TS [7,20]. There are currently no clear guidelines regarding the type and when to start treatment [[21], [22], [23], [24]]. In a clinical setting, the following processes will help the patient and family to understand the treatment.
-
1)
Discussion and education of the family and child on TS and comorbidities. On some occasions, the information needs to be provided by school personnel.
-
2)
Initial supportive psychoeducational interventions.
-
3)
Advice regarding daily life; such as sleep-wake rhythm, exercise, attending school, etc.
-
4)
Pharmacological therapy.
-
5)
Behavioral therapy and other nonpharmacological therapy.
With regards to when to start taking medication, factors including the severity of the tics and comorbidities, the age at onset, current age, and duration from the onset are helpful. Factors such as the symptoms causing significant distress to the patient, those having an adverse effect on self-esteem, difficulty in forming friendships and interactions with peers, and those interfering with school or work tasks are also important and should be taken into consideration. In addition, with regards to factors affecting the family, symptoms causing distress to the family or impairing the parents' or siblings’ ability to function in their roles effectively, and with adult patients the thoughts of the spouse and other family members should also be discussed [25].
Pharmacological treatment was first reported by Seignot in 1961 who used haloperidol [26]. A similar dopamine (DA) blocker, pimozide, was introduced later [27,28], and the use of clonidine was proposed by the Yale group [[29], [30], [31]]. However, a double-blind study failed to show its effectiveness [32]. The often used pharmacological treatments for tics include typical neuroleptics (haloperidol, pimozide, and other classical neuroleptics), alpha-adrenergic receptor agonists (clonidine, guanfacine), atypical neuroleptics and other agents that block D2 DA receptors (risperidone [33,34], clozapine, sulpiride and tiapride), and both full or partial DA agonists at dopaminergic receptors (levodopa, aripiprazole, pergolide-mixedD2/D1 agonist-, talipexole, pramipexole, and terguride-partial D2 agonist), and others. However, the effectiveness of each medication is not consistent.
With any pharmacological treatment, caution is necessary with regards to the possibility of side effects, and the dose of the medication should be titrated according to the severity of symptoms. The possibility of the medicine causing underlying problems, such as ADHD and susceptibility to addiction, should also be taken into consideration [35,36].
Nonpharmacological approaches include psycho-education, habit reversal therapy [37,38], comprehensive behavioral intervention for tics [39], and others. As for surgical treatment, deep brain stimulation has been reported to be effective for very severe cases [40,41].
A recent paper entitled “Comprehensive systematic review summary; Treatment of tics in people with TS and chronic tic disorder”, reported the following four findings, highlighting the complexity of practical approaches for TS [42].
-
(1)
High confidence in evidence was observed for comprehensive behavioral intervention than supportive psychotherapy.
-
(2)
Moderate confidence in evidence was observed for haloperidol, risperidone, aripiprazole and others.
-
(3)
Low confidence in evidence was observed for pimozide and others.
-
(4)
Very low confidence in evidence was observed for others.
The authors concluded that there is evidence to support the efficacy of various medical, behavioral, and neurostimulation interventions for the treatment of tics, and that the efficacy and harms associated with these interventions must be considered when making treatment recommendations.
Pathophysiological considerations of TS
The report of the effectiveness of haloperidol on TS by Seignot in 1961 [26] was a breakthrough in the research of the pathophysiology of TS relating to the involvement of DA [4,7,8,[43], [44], [45]]. Further extensive research of clinical characteristics, neurophysiological, neurochemical and neuroradiological evaluations have explored the pathophysiology of TS [7]. Studies on the clinical characteristics have suggested that genetic susceptibility [6,46], age, and gender are involved in the pathophysiology of TS [[1], [2], [3], [4],7,8], i.e. that TS is a genetically influenced developmental disorder involving males more than females, and primarily affecting the DA and serotonin (5HT) neuronal systems [7,8]. Neurophysiological evaluations have postulated that the main lesions involve the basal ganglia and functionally related neuro-limbic structures, as well as areas of the sensorimotor cortex and non-motor cortex, which are targets of the projection of the basal ganglia through the thalamo-cortical pathways.
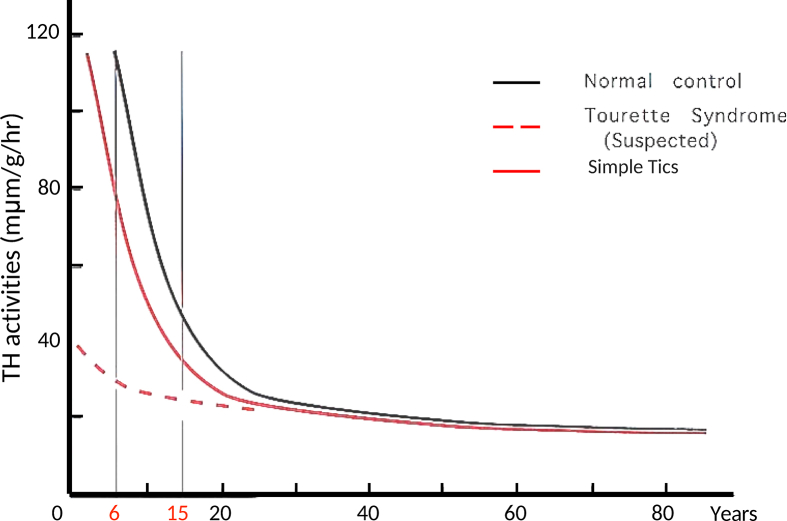
Polysomnographic and voluntary saccade evaluations have suggested hypofunction of nigro-striatal (NS)-DA neurons and association with D2 receptor super-sensitivity [7,8]. Analysis of the sleep factors indicated that activity of the NS-DA neurons in patients with TS decreases with age faster than normal, but that it follows a normal age decline with low levels [Fig. 1] [7,8].
Fig. 1.
Age variation in tyrosine hydroxylase (TH) activity in the caudate nucleus, and suspected changes in Tourette syndrome and simple tics.
Tyrosine hydroxylase activity at the substantia nigra has been shown to gradually increase with age. This is in contrast to activity at the striatum, the terminal end of the NS-DA system, which has been shown to exponentially decrease, most rapidly in the first decade, moderately in the second decade, and plateauing thereafter, i.e. high activity in early childhood (over 6 times that in adulthood), decreasing rapidly until 10 years of age, and then decreasing at a slower rate until reaching an adult level in the early 20s [47]. The high activity of tyrosine hydroxylase in early childhood suggests that DA neurons act not only as neurotransmitter, but also influence the morphological formation and functional expression of their innervation area, i.e. the striatum and cerebral cortex. Thus, the postulated decrease in DA activity in TS patients may be an accentuation or early occurrence of the normal decline in DA activity, and that this is a self-limited process but without progressive consequences [7,8,45]. The age-related features and self-limited process without progressive consequences seen in TS may reflect these changes. Therefore, in adolescence, when the age-related decreases become slower, the differences in DA activity become much less compared to normal. This would be consistent with the natural remission or disappearance of tics after adolescence.
In response to the dramatic decrease in the DA system, the brain compensates through the upregulation of D2 receptors. Thus, this upregulation of the D2 receptors in TS is a compensatory or developmental mechanism to maintain the development of the cerebral cortex. This process is different from the denervation upward regulation observed in degenerative neuronal diseases [7,8].
Polysomnographic studies have also suggested the involvement of 5HT neurons as well as DA neurons. Dysfunction or hypofunction of 5HT neurons may affect orbitofrontal and anterior cingulate non-motor basal ganglia-thalamo-cortical circuits through projection to the striatum involving these circuits [7].
Insufficient function of the circuits can then lead to the development of sympathy and sociality disorders and OCD [7]. Substantial neurophysiological evidence has shown that abnormalities in these cortical areas are related to comorbid conditions associated with TS patients, and may also be related to complex tics [7].
These two non-motor circuities receive 5HT innervation at the striatum, indicating that they are affected by environmental factors and are related to the clinical features of TS [7,8]. Male predominance in TS is due to DA under the influence of genetically controlled sex differences [48]. Thus, the activities of DA and 5HT neurons, which have critical ages of development from late infancy to early childhood, are key elements in the pathophysiology of TS [7,8]. The clinical characteristics and evidence reported in neurophysiological investigations suggest that DA neurons which innervate both motor and non-motor basal ganglia-thalamo-cortical circuits, and 5HT neurons which modulate postural tone and locomotion and innervate the striatum of the non-motor circuits, are primarily affected in TS [7]. These developmental processes of the striatal projections and efferent pathways of the basal ganglia also underlie the characteristic clinical course of TS, that is, the early occurrence of simple tics and late occurrence of complex tics and OCD. Thus, simple tics appear through descending efferent pathways which mature earlier, while complex tics and OCD are provoked later by dysfunctional cortical areas, which are targets of the basal ganglia-thalamo-cortical circuits after functional maturation of ascending pathways of the basal ganglia [7]. Some dysfunction can be restored by appropriate environmental stimulation, as the activities of 5HT neurons are influenced by environmental factors. Thus, if the activity of 5HT neuron is preserved or improved, the comorbidities of TS may be milder, even though tics remain prominent. Taken together, these findings suggest that improvement or accentuation of environmental factors in early childhood is important for the long-term outcomes of TS patients [7,8,45].
The treatment of TS based on the pathophysiology
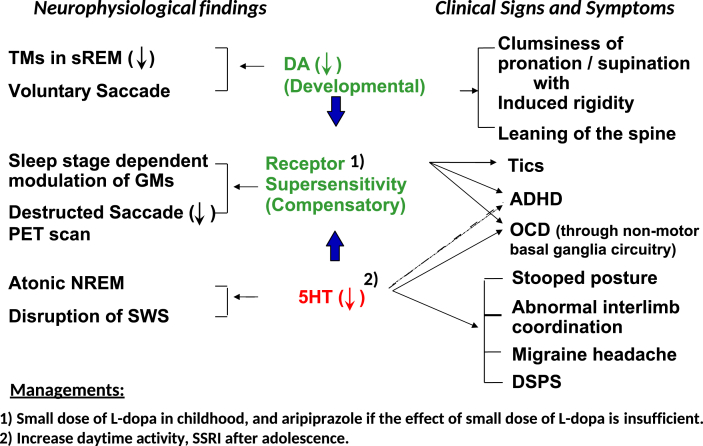
As shown in section “III. Pathophysiological considerations of TS”, the pathophysiology of TS may be attributable to developmental dopamine disorders based on the clinical and neurophysiological findings [Fig. 2] [7,8,45]. Neurophysiological findings shown on the left column of Fig. 2 and clinical signs and symptoms shown on the right column of Fig. 2 suggest that enhanced developmental decreases in DA activity lead to compensatory DA receptor upregulation. Decreased 5HT activity further enhances receptor upregulation.
Fig. 2.
Pathophysiology of Tourette Syndrome. Abbreviations: sREM; rapid eye movement sleep. NREM; non-rapid eye movement sleep. SWS; slow wave sleep. TMs; twitch movements. GMs; gross movements. PET; positron emission tomography. DA; dopamine. 5HT; 5-hydroxytryptamine. ADHD; attention deficit hyperactivity disorder. OCD; obsessive compulsive disorder. DSPS; delayed sleep phase syndrome. SSRI; selective serotonin reuptake inhibitor.
Furthermore, we previously showed that the developmental DA receptor upregulation could be corrected by a very small dose of levodopa based on extensive sleep studies [49]. Accordingly, we have been using very small doses of levodopa as pharmacological treatment for TS [45]. We also found that the effectiveness of a small dose of levodopa on TS was age related. In that report, the patients were divided into three age groups, 6–12 years, 13–20 years, and above 21 years, and the results showed that the youngest group had the best response to a small dose of levodopa [45].
Recent reports have indicated the effectiveness of aripiprazole in patients with TS [50,51]. Aripiprazole is a partial DA D2 receptor agonist, and it is a potential new class of medication for the treatment of TS. Aripiprazole suppresses the upregulation of DA receptors and normalizes DA transmission. That is, it can act as a DA system stabilizer.
Data from Yoshiko Nomura Neurological Clinic for Children
For the five years (2015–2020) since I opened my own clinic, about 1200 TS patients have visited for treatment. The clinical management and care of TS in my clinic is based on the pathophysiology underlying TS.
Analysis of the effects of treatment on TS patients in my clinic are summarized below. We also discuss how certain environmental changes affect TS.
-
1)
Clinical features and outcomes
-
2)
Medication effects (small dose of levodopa and aripiprazole)
-
3)
OCD and outcomes
-
4)
How the COVID-19 pandemic has affected TS patients
Material and Method
Subject
A total of 303 children with TS who were followed for more than 1 year at Yoshiko Nomura Neurological Clinic for Children were evaluated. This number did not include preschool children (younger than 5 years of age) or adults (above 19 years of age). According to the type of tics at the initial visit, the cases were divided into simple tics (146 cases) and complex tics (157 cases) groups. The cases were also divided into four groups according to their age at the initial visit. Group I; 5–8 years of age (lower grade of primary school) Group II; 9–12 years of age (higher grade of primary school) Group III; 13–15 years of age (middle school) Group IV; 16–18 years of age (high school).
Severity
The overall severity of complex and simple tics was rated by the author as follows;
Most severe (rated 5)
Tics are present almost all the time, throughout the day.
Severe (rated 4)
Tics are present about half the day.
Moderate (rated 3)
Tics are present more than half a week, there may be days without any tics.
Mild (rated 2)
Tics are seen occasionally.
Good (rated 1)
Almost no tics observed.
Evaluation of improvement
The improvement rate was calculated as A-B/A (A; the severity at the initial visit, B; the severity at the end of 2019).
Medications used
In my clinic, for the management of upregulated DA receptor sensitivity, a small dose of l- Dopa (levodopa) for younger children, and aripiprazole for older children and adults are used. Increasing daytime general activity and locomotion (walking), and keeping good sleep hygiene are strongly encouraged. These practices will enhance brainstem 5HT activity. After adolescence, medications such as selective serotonin reuptake inhibitor (SSRI) may be indicated.
Results
-
1)Clinical features and outcomes
- (1)
-
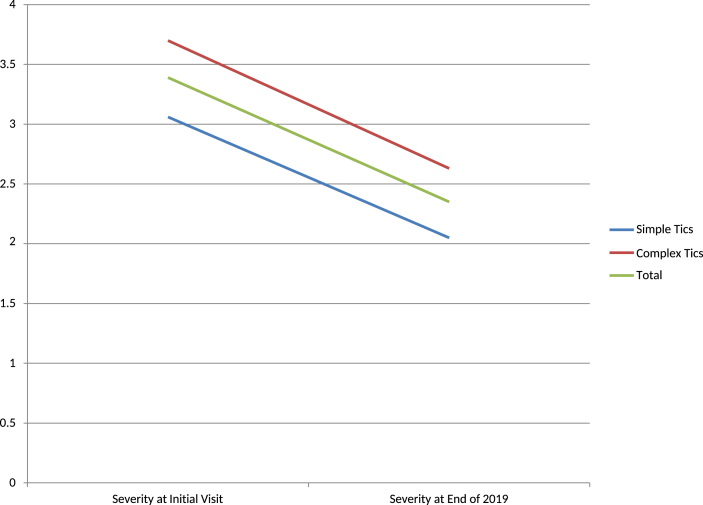
(2)All cases were divided into simple tics (motor and phonic) and complex tics (motor and phonic) groups. The improvement rate according to the type of tics showed that the simple tics group (33.0%) had better improvement than the complex tics group (28.9%). The follow-up durations were 1.89 years and 2.06 years, respectively [Table 2, Fig. 4].
-
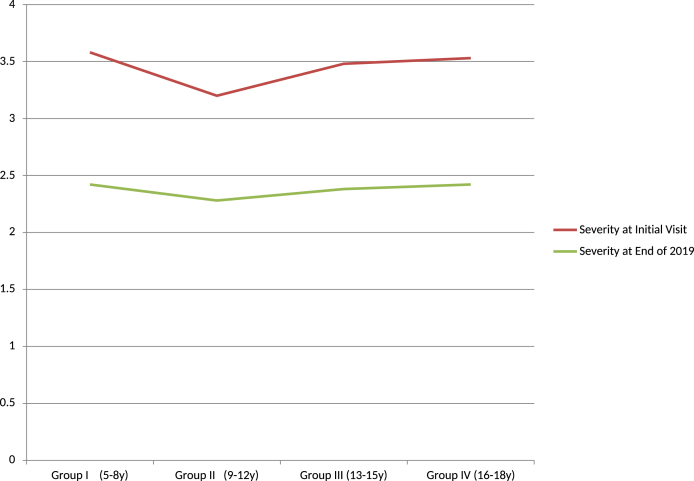
(3)The improvement rates according to the age at the initial visit were compared. The severity score at the initial visit ranged from 3.2 to 3.58, and at the end of 2019 ranged from 2.28 to 2.42, with a follow-up period ranging from 1.74 to 2.06 years. All four groups showed improvement rates of about 30%, with slightly higher rates in group I, III and IV, and a slightly lower rate in group II (9–12 years; 28.7%) [Table 3, Fig. 5]. In addition, the simple tics subgroup had a better improvement rate than the complex tics subgroup in groups II and IV.
-
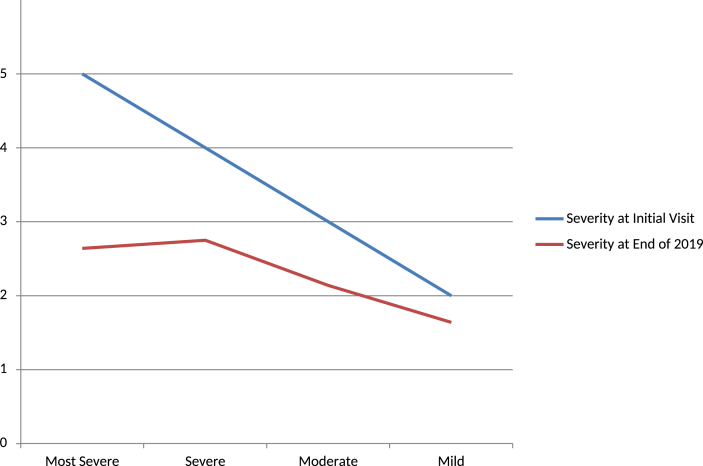
(4)With regards to the severity at the initial visit, the most severe cases had the best improvement. However, the end-point severity was still higher in the most severe and severe groups, suggesting a limitation in the improvement. The follow-up duration was longest in the most severe group, suggesting that a longer duration was needed to obtain improvements [Fig. 6].
-
(5)With regards to follow-up duration, there was a gradual increase in improvement rate for up to four years. Those with simple tics showed the best improvement.
-
(6)Improvement rates were compared between age, severity and follow-up duration groups. Those in the youngest group (group I) rated 3 and 4 had a gradual improvement for up to 4 years, and those rated 3 had better improvement than those rated 4. The children in group II (age 9–12 years) and group III (13–15 years) rated 3 and 4 had a gradual improvement for up to 2 years, and then decreased by 3 years. The children in group IV (16–18 years) rated 3 and 4 had the worst results.
-
2)Medication effects
-
(1)With or without medication
- Cases with a follow-up duration of 1 year were excluded.
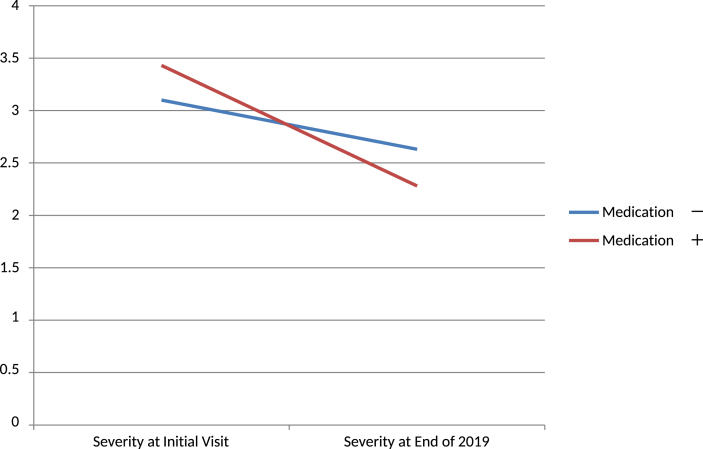
- Thirty cases did not use medications and 251 cases used medications. The improvement rate of the cases with medications was higher (−33.4%) than in those without medications (−15.1%). The follow-up duration was slightly shorter in the group without medication (1.43 years) compared to the group with medications (2.13 years) [Table 4, Fig. 7]. In the group with medication, those with simple tics had better improvement.
-
(2)The medication and follow-up duration analysis revealed that the group without medications had less improvement up to 2 years. The group with medications showed improvements with the follow-up duration. They also had a higher improvement rate at 1 year and 2 years compared to the group without medications.
- (3)
-
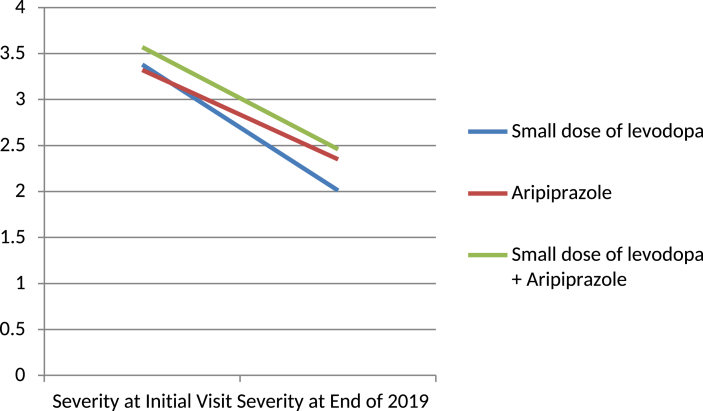
(4)The effects of medications were compared among the different age groups. In my clinic, aripiprazole is usually used for older age groups. However, if the symptoms are severe, aripiprazole is used even in younger cases. A small dose of levodopa was used in group I and group II. Both groups showed improvement, with group II (−45.3%) being better than group I (−36.6%). Those who received aripiprazole showed a fair improvement in all ages, and the end-point rate were higher than in the small dose of levodopa group. The small dose of levodopa and aripiprazole group showed better results than aripiprazole group except in group II.
-
(5)Medications and follow-up duration analysis revealed that the small dose of levodopa group had a gradual improvement, the aripiprazole group had good improvement up to 1 year and then a similar effect up to 3 years, and the small dose of levodopa and aripiprazole group also had a gradual improvement, but less than the small dose of levodopa group.
-
(6)The effects of medication were compared against the duration of follow-up and age. The cases who did not receive any medication were in the younger groups (group I and group II). The effect of a small dose of levodopa in group I and II showed a gradual increase. Aripiprazole had a good effect at 1 year in group I and IV. In group II, the effect of aripiprazole remained the same. The small dose of levodopa and aripiprazole group had an increasing effect up to 4 years in group I and III, and in group IV the effect decreased as the follow-up duration increased.
-
(1)
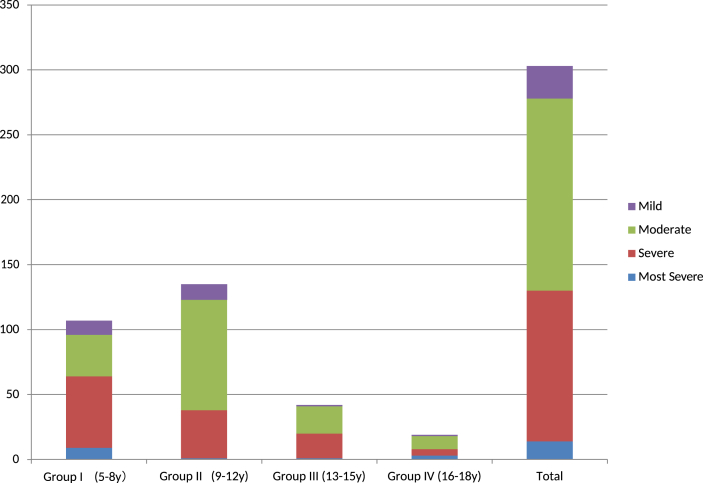
Table 1.
Severities at initial visit; numbers of the patients.
| Most Severe | Severe | Moderate | Mild | Total | |
|---|---|---|---|---|---|
| Group I (5–8 y) | 9 | 55 | 32 | 11 | 107 |
| Group II (9–12 y) | 1 | 37 | 85 | 12 | 135 |
| Group III (13–15 y) | 1 | 19 | 21 | 1 | 42 |
| Group IV (16–18 y) | 3 | 5 | 10 | 1 | 19 |
| Total | 14 | 116 | 148 | 25 | 303 |
Fig. 3.
Severity at initial visit; number of patients (total 303). ‘Ordinate; number of the patients.’
Table 2.
Improvement Rate; Simple tics vs Complex tics.
| No of Patients | Severity at Initial Visit | Severity at End of 2019 | Improvement Rate | Average Follow up years | |
|---|---|---|---|---|---|
| Simple Tics | 146 | 3.06 | 2.05 | −33.00% | 1.89 |
| Complex Tics | 157 | 3.7 | 2.63 | −28.90% | 2.06 |
| Total | 303 | 3.39 | 2.35 | −30.60% | 1.98 |
Fig. 4.
Improvement Rate; Simple Tics vs Complex Tics. ‘Ordinate is the severity rate.’
Table 3.
Improvement Rate According to the Ages at Initial Visit (Group I∼IV).
| No of Patients | Severity at Initial Visit | Severity at End of 2019 | Improvement Rate | Average Follow up years | |||
|---|---|---|---|---|---|---|---|
| Group I (5-8 y) | Simple Tics | 52 | 3.19 | 2.19 | −31.30% | 1.88 | |
| ComplexTics | 55 | 3.95 | 2.64 | −33.20% | 1.93 | ||
| Total |
107 |
3.58 |
2.42 |
−32.40% |
1.91 |
||
| Group II (9-12 y) | Simple Tics | 65 | 2.92 | 1.95 | −33.20% | 1.91 | |
| ComplexTics | 70 | 3.46 | 2.59 | −25.20% | 2.2 | ||
| Total |
135 |
3.2 |
2.28 |
−28.70% |
2.06 |
||
| Group III (13-15 y) | Simple Tics | 22 | 3.7 | 2.7 | −27.00% | 2 | |
| ComplexTics | 20 | 3.8 | 2.7 | −28.90% | 2.1 | ||
| Total |
42 |
3.48 |
2.38 |
−31.50% |
2.02 |
||
| Group IV (16-18 y) | Simple Tics | 7 | 3 | 1.86 | −38.10% | 1.57 | |
| ComplexTics | 12 | 3.83 | 2.75 | −28.30% | 1.83 | ||
| Total | 19 | 3.53 | 2.42 | −31.30% | 1.74 | ||
Fig. 5.
Improvement rate according to the age at initial visit (group I ∼ IV). ‘Ordinate is the severity rate.’
Fig. 6.
Improvement rate according to the severity at initial visit. ‘Ordinate is the severity rate.’
Table 4.
Improvement rate of the cases with or without medication.
| Severity at Initial Visit | Severity at End of 2019 | Improvement Point | Improvement Rate (%) | |
|---|---|---|---|---|
| Medication - | 3.1 | 2.63 | −0.47 | −15.10% |
| Medication + | 3.43 | 2.28 | −1.15 | −33.40% |
Fig. 7.
Improvement rate of the cases with or without medication. ‘Ordinate is the severity rate.’
Table 5.
Effects of the medication.
| Severity at Initial Visit | Severity at End of 2019 | Improvement Point | Improvement Rate (%) | |
|---|---|---|---|---|
| Small dose of levodopa | 3.38 | 2.01 | −1.37 | −39.00% |
| Aripiprazole | 3.32 | 2.35 | −0.98 | −29.40% |
| Small dose of levodopa + Aripiprazole | 3.57 | 2.46 | −1.11 | −31.10% |
Fig. 8.
Effects of medication. ‘Ordinate is the severity rate.’
In summary, the severities of the patients with TS ranged from moderate to severe 1-(1). The results in 1-(2–6) showed overall improvements in both who did and did not receive medications, indicating that both pharmacological and non-pharmacological treatments, such as advice regarding improvements in environmental factors, were beneficial in improving TS. These findings were correlated with overall severity at the initial visit to the clinic 1-(4), types of tics 1-(2), age at initial visit 1-(3), follow-up duration 1-(5), and follow-up duration and age 1-(6).
With regards to the relationship with medications, the medication group showed better results than the without medication group 2-(1). The effects of the medication increased as the follow-up duration increased 2-(2). The small dose of levodopa group had better improvement than the aripiprazole and small dose of levodopa and aripiprazole groups 2-(3). There were some differences in the effects of medication among the age groups 2-(4), and some between follow-up duration 2-(5) and age 2-(6).
-
3)
OCD and outcomes
OCD was observed in 39% of all cases, and more often in those with complex tics (49%) than in those with simple tics (28%). The most severe (rated 5) and severe (rated 4) cases (48%) had a higher frequency of OCD than the moderate (rated 3) cases (32%). The effect of medication was less in the cases with OCD in group I, II, and III. There was no difference in group IV. The effect of medication was less in the group with OCD.
-
4)
How the COVID-19 pandemic has affected TS patients
It is known the environmental changes can affect tic severity. During the months of April and May 2020, an emergency condition was declared in Japan under the COVID-19 pandemic. To evaluate how this particular drastic and unexpected environmental change affected tic severity, we asked the patients (and their guardians if the patients were children) how the severity of tics changed during the COVID-19 pandemic. During the two months of the emergency declaration, everyone was required to stay at home. Of the 247 TS subjects who were asked about changes in tic severity [Table 6], 39–63% stated that they had improved. Furthermore, the improvement was greatest in the preschool age group. Tics worsened in only a few patients. Factors which may have influenced the changes in tic severity were both positive and negative. The former included an increase in free time, and the latter included isolation from friends and co-workers, fewer motor activities, disruption of sleep-wake rhythm, and increased screen time.
Table 6.
How the severity of tics changed during the COVID-19 pandemic.
| Pre- school (N:9) |
Primary school 7–9 yrs (N:48) |
Primary School 10–12 yrs (N:83) |
Middle school 13–15 yrs (N:59) |
High school 16–18 yrs (N:19) |
Adult (N:29) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | V | M | V | M | V | M | V | M | V | M | V | |
| Subject Numbers | 9 | 8 | 48 | 42 | 81 | 74 | 58 | 54 | 18 | 13 | 28 | 23 |
| Improved (%) | 56 | 63 | 46 | 57 | 44 | 46 | 50 | 46 | 56 | 46 | 39 | 57 |
| Worsened (%) | 11 | 13 | 31 | 19 | 23 | 19 | 12 | 17 | 28 | 23 | 14 | 9 |
| Unchanged (%) | 33 | 24 | 23 | 24 | 33 | 35 | 38 | 37 | 16 | 31 | 47 | 34 |
M; motor tics, V; vocal (phonic) tics.
Conclusion
Tic disorders are neurodevelopmental disorders involving both genetic and environmental factors, which may be caused by the acceleration of developmental decreases in DA activity at the terminal of the NS-DA system causing DA D2 receptor upregulation. DA receptor upregulation may be compensatory mechanism. High DA activity in early childhood plays an important role in the development of the frontal cortex. Serotonergic neurons involved in the development of the biphasic sleep-wake rhythm and locomotion may be involved. Serotonergic neurons play important roles in the associated co-morbidity, OCD, by activating the non-motor basal ganglia-thalamo-cortical circuit.
Treatments should be targeted to the involved neuronal circuits. Pharmacological treatments plays an important role in managing TS. A small dose of levodopa and aripiprazole had a good effect in controlling tics without side effects. We suggest that DA blockers should not be used before 10 years of age, as this may exacerbate DA receptor upregulation. Interventions that enhance daytime activities, keep a regular sleep-wake rhythm, and encourage locomotion are important. Individualized psycho-behavioral support is helpful. Education and encouragement are essential to achieve individual goals in patients with TS.
Funding support
Yoshiko Nomura Neurological Clinic for Children supported this work.
Conflicts of interest
There are no conflicts of interest associated with this work.
Acknowledgements
The author expresses sincere appreciation to late Dr. Masaya Segawa, who initiated and extensively performed the research on TS, and cared for the patients and their families. The author also thanks Katsufumi Nomura and Syoko Yukishita, who helped to analyze the data from Yoshiko Nomura Neurological Clinic for Children.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Shapiro A.K., Shapiro E.S., Bruun R.D., Sweet R., Wayne H., Solomon G., et al. Gilles de la Tourette's syndrome: summary of clinical experience with 250 patients and suggested nomenclature for tic syndromes. Adv Neurol. 1976;14:277–283. [PubMed] [Google Scholar]
- 2.Bruun R.D., Shapiro A.K., Shapiro E., Sweet R., Wayne H., Solomon G.E. A follow-up of 78 patients with Gilles de la Tourette's syndrome. Am J Psychiatr. 1976;133:944–947. doi: 10.1176/ajp.133.8.944. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro A.K., Shapiro E.S., Bruun R.D., Sweet R.D. Gilles de la Tourette syndrome. Raven Press; New York: 1978. Signs, symptoms, and clinical course; pp. 119–149. [Google Scholar]
- 4.Nomura Y., Segawa M. Gilles de la Tourette syndrome in Oriental children. Brain Dev. 1979;1:103–111. doi: 10.1016/s0387-7604(79)80018-2. [DOI] [PubMed] [Google Scholar]
- 5.Wang H.S., Kuo M.F. Tourette's syndrome in Taiwan: an epidemiological study of tic disorders in an elementary school at Taipei County. Brain Dev. 2003;25:S29–S31. doi: 10.1016/s0387-7604(03)90005-2. [DOI] [PubMed] [Google Scholar]
- 6.Pauls D.L., Alsobrook J.P., II, Gelernter J., Leckman J.F. In: Tourette's syndrome – tics, obsessions, compulsion, developmental psychopathology and clinical care. Leckman J.F., Cohen D.J., editors. John Wiley & Sons, Inc; 1999. Genetic vulnerability; pp. 194–211. [Google Scholar]
- 7.Segawa M. Neurophysiology of Tourette's syndrome: pathophysiological considerations. Brain Dev. 2003;25:S62–S69. doi: 10.1016/s0387-7604(03)90011-8. [DOI] [PubMed] [Google Scholar]
- 8.Nomura Y. Tourette syndrome: clinical features and pathophysiology. Brain Nerve. 2017;69:1373–1385. doi: 10.11477/mf.1416200922. Japanese. [DOI] [PubMed] [Google Scholar]
- 9.Robertson M.M. Tourette syndrome, associated conditions and the complexities of treatment. Brain. 2000;123:425–462. doi: 10.1093/brain/123.3.425. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Alvarez E., Aicardi J. Mac Keith Press; London England: 2001. Movement disorders in children international review of child neurology series; pp. 1–23. [Google Scholar]
- 11.Leckman J.F., King R.A., Cohen D.J. In: Tourette's syndrome – tics, obsessions, compulsion, developmental psychopathology and clinical care. Leckman J.F., Cohen D.J., editors. John Wiley & Sons, Inc; 1999. Tics and tic disorder; pp. 23–42. [Google Scholar]
- 12.Leckman J.F., Zhang H., Vitale A., Lahnin F., Lynch K., Bondi C., et al. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. 1998;102:14–19. doi: 10.1542/peds.102.1.14. [DOI] [PubMed] [Google Scholar]
- 13.Jagger J., Prusoff B.A., Cohen D.J., Kidd K.K., Carbonari C.M., John K. The epidemiology of Tourette's syndrome: a pilot study. Schizophr Bull. 1982;8:267–278. doi: 10.1093/schbul/8.2.267. [DOI] [PubMed] [Google Scholar]
- 14.Bruun R.D., Budman C.L. In: Adv neurol. Chase T.N., Friedhoff A.J., Cohen D.J., editors. vol. 58. 1992. The natural history of Tourette syndrome; pp. 1–6. [PubMed] [Google Scholar]
- 15.Nomura Y. Tic Disorders – ageing changes of clinical features and long term prognosis. Clin Neurosci. 1998;16:73–75. Japanese. [Google Scholar]
- 16.Sweet R.D., Bruun R.D., Shapiro A.K., Shapiro E.S. The pharmacology of gilles de la Tourette's syndrome (chronic multiple tic) Clin Neuropharmacol. 1976;1:81–106. [Google Scholar]
- 17.Singer H.S., Walkup J.T. Tourette syndrome and other tic disorders. Diagnosis, pathophysiology, and treatment. Medicine (Baltim) 1991;70:15–32. doi: 10.1097/00005792-199101000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter L.L., Leckman J.F., Scahill L., Mcdougle C.J. In: Tourette syndrome. Leckman J.F., Cohen D.J., editors. John Wiley & Sons, Inc; 1999. Pharmacological and other somatic approaches to treatment; pp. 370–398. [Google Scholar]
- 19.Wang H.S. Clinical diagnosis and management of Tourette syndrome. Acta Neurol Taiwan. 2001;10:219–227. [Google Scholar]
- 20.Albin R.L., Mink J.W. Recent advances in Tourette syndrome research. Trends Neurosci. 2006;29:175–182. doi: 10.1016/j.tins.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Singer H.S. The treatment of tics. Curr Neurol Neurosci Rep. 2001;1:195–202. doi: 10.1007/s11910-001-0016-8. [DOI] [PubMed] [Google Scholar]
- 22.Singer H.S. Treatment of tics and Tourette syndrome. Curr Treat Options Neurol. 2010;12:539–561. doi: 10.1007/s11940-010-0095-4. [DOI] [PubMed] [Google Scholar]
- 23.Pringsheim T., Okun M.S., Müller-Vahl K., Martino D., Jankovic J., Cavanna A.E., et al. Practice guideline recommendations summary: treatment of tics in people with Tourette syndrome and chronic tic disorders. 2019;92:896–906. doi: 10.1212/WNL.0000000000007466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Essoe J.K., Grados M.A., Singer H.S., Myers N.S., McGuire J.F. Evidence-based treatment of Tourette's disorder and chronic tic disorders. Expert Rev Neurother. 2019;19:1103–1115. doi: 10.1080/14737175.2019.1643236. [DOI] [PubMed] [Google Scholar]
- 25.Nomura Y., Kita M., Segawa M. Social adaptation of Tourette syndrome families in Japan. Adv Neurol. 1992;58:323–332. [PubMed] [Google Scholar]
- 26.Seignot J.N. Un cas de maladie des tics de Gilles de la Tourette gueri par le R-1625. Ann Med-Psychol. 1961;119:578–579. [PubMed] [Google Scholar]
- 27.Ross M.S., Moldofsky H. Comparison of pimozide with haloperidol in Gilles de la Tourette synndrome. Lancet. 1977;1:103. doi: 10.1016/s0140-6736(77)91126-6. [DOI] [PubMed] [Google Scholar]
- 28.Nomura Y., Segawa M. Presented at Vth world congress of psychiatry, Honolulu. 1977. Gilles de la Tourette syndrome in oriental children. [Google Scholar]
- 29.Leckman J.F., Cohen D.J., Detlor J., Young J.G., Harcherik D., Shaywitz B.A. Clonidine in the treatment of Tourette syndrome: a review of data. Adv Neurol. 1982;35:391–401. [PubMed] [Google Scholar]
- 30.Leckman J.F., Detlor J., Harcherik D.F., Ort S., Shaywitz B.A., Cohen D.J. Short and long-term treatment of Tourette syndrome with clonidine: a clinical perspective. Neurology. 1985;35:343–351. doi: 10.1212/wnl.35.3.343. [DOI] [PubMed] [Google Scholar]
- 31.Leckman J.F., Hardin M.T., Riddle M.A., Stevenson J., Ort S.I., Cohen D.J. Clonidine treatment of Gilles de la Tourette's syndrome. Arch Gen Psychiatr. 1991;48:324–328. doi: 10.1001/archpsyc.1991.01810280040006. [DOI] [PubMed] [Google Scholar]
- 32.Goetz C.G., Tanner C.M., Wilson R.S., Carroll V.S., Como P.G., Shannon K.M. Clonidine and Gilles de la Tourette syndrome: double-blind study using objective rating method. Ann Neurol. 1987;21:307. doi: 10.1002/ana.410210313. [DOI] [PubMed] [Google Scholar]
- 33.Stamenkovic M., Aschauer H., Kasper S. Risperidone for Tourette's syndrome. Lancet. 1994;344:1577–1578. doi: 10.1016/s0140-6736(94)90389-1. [DOI] [PubMed] [Google Scholar]
- 34.Bruun R.D., Budman C.L. Risperidone as a treatment for Tourette's syndrome. J Clin Psychiatr. 1996;57:29–31. [PubMed] [Google Scholar]
- 35.Golden G.S. Gilles de la Tourette's syndrome following methylphenidate administration. Dev Med Child Neurol. 1974;16:76–78. doi: 10.1111/j.1469-8749.1974.tb02715.x. [DOI] [PubMed] [Google Scholar]
- 36.Golden G.S. The relationship between stimulant medication and tics. Pediatr Ann. 1988;17:405–408. doi: 10.3928/0090-4481-19880601-08. [DOI] [PubMed] [Google Scholar]
- 37.Wilhelm S., Deckersbach T., Coffey B.J., Bohne A., Peterson A.L., Baer L. Habit reversal versus supportive psychotherapy for Tourette's disorder: a randomized controlled trial. Am J Psychiatr. 2003;160:1175–1177. doi: 10.1176/appi.ajp.160.6.1175. [DOI] [PubMed] [Google Scholar]
- 38.Hwang G.C., Tillberg C.S., Scahill L. Habit reversal training for children with Tourette syndrome: update and review. J Child Adolesc Psychiatr Nurs. 2012;25:178–183. doi: 10.1111/jcap.12002. [DOI] [PubMed] [Google Scholar]
- 39.Piacentini J., Woods D.W., Scahill L., Wilhelm S., Peterson A.L., Chang S., et al. Behavior therapy for children with Tourette disorder: a randomized controlled trial. J Am Med Assoc. 2010;303(19):1929–1937. doi: 10.1001/jama.2010.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Visser-Vandewalle V., Temel Y., van der Linden C.H., Ackermans L., Beuls E. Deep brain stimulation in movement disorders. The applications reconsidered. Acta Neurol Belg. 2004;104:33–36. [PubMed] [Google Scholar]
- 41.Visser-Vandewalle V., Ackermans L., van der Linden C., Temel Y., Tijssen M.A.J., Schruers K., et al. Deep brain stimulation in Gilles de la Tourette's syndrome. Neurosurgery. 2006;58:E590. doi: 10.1227/01.NEU.0000207959.53198.D6. [DOI] [PubMed] [Google Scholar]
- 42.Pringsheim T., Holler-Managan Y., Okun M.S., Jankovic J., Piacentini J., Cavanna A.E., et al. Comprehensive systematic review summary: treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurol. 2019;92:907–915. doi: 10.1212/WNL.0000000000007467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singer H.S., Butler I.J., Tune L.E., Seifert W.E., Jr., Coyle J.T. Dopaminergic dysfunction in Tourette syndrome. Ann Neurol. 1982;12:361–366. doi: 10.1002/ana.410120408. [DOI] [PubMed] [Google Scholar]
- 44.Mink J.W. Basal ganglia dysfunction in Tourette's syndrome: a new hypothesis. Pediatr Neurol. 2001;25:190–198. doi: 10.1016/s0887-8994(01)00262-4. [DOI] [PubMed] [Google Scholar]
- 45.Nomura Y., Segawa M. Neurology of Tourette's syndrome (TS) TS as a developmental dopamine disorder: a hypothesis. Brain Dev. 2003;25:S37–S42. doi: 10.1016/s0387-7604(03)90007-6. [DOI] [PubMed] [Google Scholar]
- 46.McMahon W.M., Carter A.S., Fredine N., Pauls D.L. Children at familial risk for Tourette's disorder: child and parent diagnoses. Am J Med Genet B Neuropsychiatr Genet. 2003;121B:105–111. doi: 10.1002/ajmg.b.20065. [DOI] [PubMed] [Google Scholar]
- 47.McGeer E.G., McGeer P.L. In: New concept in neurotransmitter regulation. Mandel J., editor. Prenum Press; New York: 1973. Some characteristics of brain tyrosine hydroxylase; pp. 53–68. [Google Scholar]
- 48.Reisert I., Pilgrim C. In: Age-related dopamine-dependent disorders, monographs in neural sciences. Segawa M., Nomura Y., editors. vol. 14. Karger; Basel: 1995. Catecholaminergic systems and the sexual differentiation of the brain; pp. 216–224. [Google Scholar]
- 49.Tanaka S., Nomura Y., Segawa M. Rotational seizures in tuberous sclerosis. Jutendo-Igaku (Tokyo) 1989;34:520–527. Japanese. [Google Scholar]
- 50.Zheng W., Li X.B., Xiang Y.Q., Zhong B.L., Chiu H.F., Ungvari G.S., et al. Aripiprazole for Tourette's syndrome: a systematic review and meta-analysis. Hum Psychopharmacol. 2016;31:11–18. doi: 10.1002/hup.2498. [DOI] [PubMed] [Google Scholar]
- 51.Sallee F., Kohegyi E., Zhao J., McQuade R., Cox K., Sanchez R., et al. Randomized, double-blind, placebo-controlled trial demonstrates the efficacy and safety of oral aripiprazole for the treatment of tourette's disorder in children and adolescents. J Child Adolesc Psychopharmacol. 2017;27:771–781. doi: 10.1089/cap.2016.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]