Abstract
Background
The incidence of opioid use disorder (OUD) is increasing worldwide, and the opioid-related overdose crisis is currently a major global challenge. This study investigated the effects of adjuvant laser meridian massage (LMM) in men with OUD undergoing methadone maintenance treatment (MMT).
Methods
A case-controlled study was conducted from February 2019 to April 2020. Fourteen men with OUD on MMT were enrolled from an addiction treatment center as an experimental group. An age-matched control group comprising 13 men was also enrolled. The experimental group received LMM on the back, including over the Bladder meridian and Governor Vessel, three times weekly for 4 weeks. The control group received only MMT. Urinary morphine levels, patients’ self-reports of the number of episodes or days of heroin use, and visual analog scale scores for heroin craving/refusal to use heroin during the previous week were evaluated. Quality of life was reported using the Short Form (SF)-12v2.
Results
The experimental group showed a significant decrease in heroin use (p < 0.05), whereas the control group showed a significant increase in heroin craving (p < 0.05). The SF-12v2 Health Survey revealed a significant improvement in physical health in the experimental group (P < 0.05).
Conclusion
The results of this study suggest that laser meridian massage can be considered a safe, well-tolerated, and potentially useful adjuvant intervention for opioid use disorder.
Keywords: Laser meridian massage, Opioid use disorder, Heroin addiction, Methadone maintenance treatment, Laser acupuncture, Traditional Chinese medicine
At a glance of commentary
Scientific background on the subject
The prevalence of opioid use disorder is increasing at an alarming rate worldwide, associated with high morbidity and mortality. Pharmacological strategies remain unsatisfactory for treating withdrawal symptoms of individuals with heroin addiction; therefore, the potential advantages of complementary and alternative medicine should be actively explored.
What this study adds to the field
This study makes a significant contribution to the literature because it is the first of its kind to investigate the clinical efficacy of adjuvant laser meridian massage for opioid use disorder, and provides novel evidence that laser meridian massage can be considered a safe, well-tolerated, and potentially useful adjuvant intervention.
The incidence of opioid use disorder (OUD) is increasing at an alarming rate worldwide, and the opioid-related overdose crisis is currently recognized as a major global challenge, associated with high morbidity and mortality [1]. OUD represents the chronic use of opioids that causes clinically significant distress or impairment. It is estimated that the global opium use rate (heroin and opium) in 2017 was 0.6% (29.2 million) among individuals aged 15–64 years. In addition, the number of opium users worldwide in the past year has increased by 50% from the original estimate of 19.4 million in 2016 [2]. OUD affects 2.1 million individuals in the United States, and over 120,000 deaths worldwide are annually attributed to the use of opioids [3].
Patients with OUD show a dependence on opioid use, which leads to increased opioid tolerance over time. Addiction to opioids represents the most severe form of this disorder, and their discontinuation leads to withdrawal symptoms [4]. According to the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria, patients can be diagnosed with OUD when they express a desire to obtain and take opioids despite social and professional consequences. Methadone has been widely used for opioid replacement therapy and studied worldwide, and methadone maintenance is a well-established approach. The advantages of methadone treatment include blocking euphoric effects, decreasing narcotic cravings, and reducing the transmission of infectious diseases. Methadone maintenance is non-sedating and is medically safe, provided there is no concomitant use of other prescriptions or illicit drugs [5,6]. However, it is common to relapse into heroin use after cessation of agonist maintenance treatment. Moreover, there is lack of studies identifying the optimal time, candidate, and strategy to quit opioid agonist maintenance treatment. Complications related to opioid agonist use necessitate the need for alternative non-opioid therapies to relieve the symptoms of immediate and long-term opioid withdrawal [7,8]. There is a chronic risk of accidental overdose, trauma, suicide, and infectious diseases in patients with OUD. Pharmacological strategies remain unsatisfactory for treating withdrawal symptoms of individuals with heroin addiction; therefore, the possible advantages of complementary and alternative medicine, which has been historically used to treat diseases with limited adverse events, should be actively explored [5,6].
Since 2500 BC, a wealth of knowledge and experience has been accumulated in the practice of acupuncture. Since then, acupuncture has been used throughout the world, particularly since the 1970s [9]. According to the theory of traditional Chinese medicine (TCM), acupuncture was evolved following the principle that physiological functions are maintained through the “meridian” and “Qi and blood” systems. Along 14 meridians, 365 identified acupoints can relieve Qi stagnation for stimulating, balancing, and harmonizing the yin and yang [10]. This treatment has been used to facilitate homeostasis of human organs. Since 1972, acupuncture has been applied to heroin addicts. Dr. Wen revealed that acupuncture combined with electrical stimulation at four body acupoints and two auricular acupoints ameliorated the withdrawal symptoms in heroin addicts [11]. In 1985, the US National Acupuncture Detoxification Association reported that the relief of withdrawal syndrome, the prevention of craving, and the increase in the probability of a patient participating in a long-term treatment program could be ensured through the 5-point auricular acupuncture protocol [12]. In 1996, the World Health Organization recommended acupuncture as a therapy for drug abuse [13]. The latest modification to this therapeutic protocol was developed in 2005 by Dr. Han [14]. Nowadays, over 700 addiction treatment centers use acupuncture as an adjuvant intervention [15].
The acupuncture points on the Governor Vessel are commonly used for treating individuals with heroin addiction [16]. It is reported that electroacupuncture at the Bei-Shu acupoints can reduce the persistence of early-stage withdrawal symptoms and anxiety after heroin detoxification [17]. Laser acupuncture, a noninvasive procedure, stimulates the traditional acupuncture points with low-power, cold laser irradiation. Laser acupuncture combines the effectiveness of both acupuncture and low-intensity laser therapy [18]. Laser meridian massage (LMM) refers to the use of laser acupuncture to stimulate the meridian with low-intensity, cold laser irradiation.
With OUD cases around the world rising, novel treatment methods need to be identified to help patients suffering from this disorder. In this study, we sought to assess the efficacy of LMM in men with OUD undergoing methadone maintenance treatment (MMT) using a 4 week-long, case-controlled study. To the best of our knowledge, this study is the first to investigate the clinical efficacy of adjuvant LMM in men with OUD on MMT.
Methods
Design
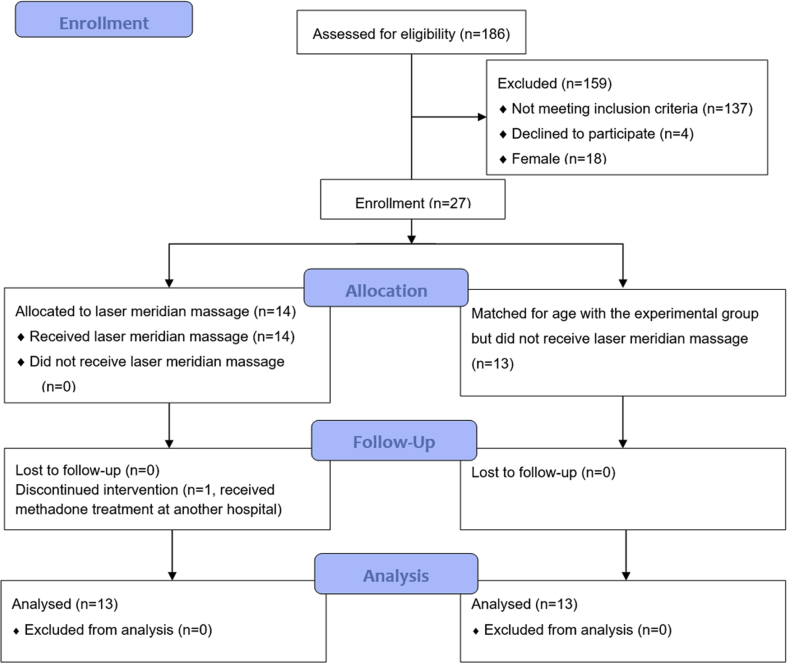
This case-controlled study was performed in the Department of Psychiatry and Chinese Medicine at Kaohsiung Chang Gung Memorial Hospital. The study began in February 2019 and continued until April 2020. Subjects recruited from our institution's addiction treatment center who were willing to receive LMM were assigned as the experimental group (LMM plus MMT). Subjects matched for age with the experimental group who did not receive LMM (MMT only) were assigned as the control group. The study design is shown in Fig. 1.
Fig. 1.
Flowchart showing the flow of subjects during the study.
Participants
A diagnosis of OUD was confirmed using the diagnostic criteria in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Male subjects aged 20–70 years with OUD, who had received MMT for at least 1 month and provided informed consent, were included in the study. Psychiatrists assessed each prospective participant's eligibility to be enrolled in the study. Subjects with a critical illness or human immunodeficiency virus infection, those on antidepressant or antipsychotic medication, those who had consumed Chinese herbs or received acupuncture treatment in the past 30 days, those who were unsuitable for recruitment according to the opinion of the attending physician, and those who were unwilling to provide informed consent were excluded from the study.
Sample size
A sample size of 28 was calculated based on a two-way repeated-measures analysis of variance with a medium effect size of 0.25, a significance level (α) of 0.05, and a desired power (1- β) of 0.80 [19].
Interventions
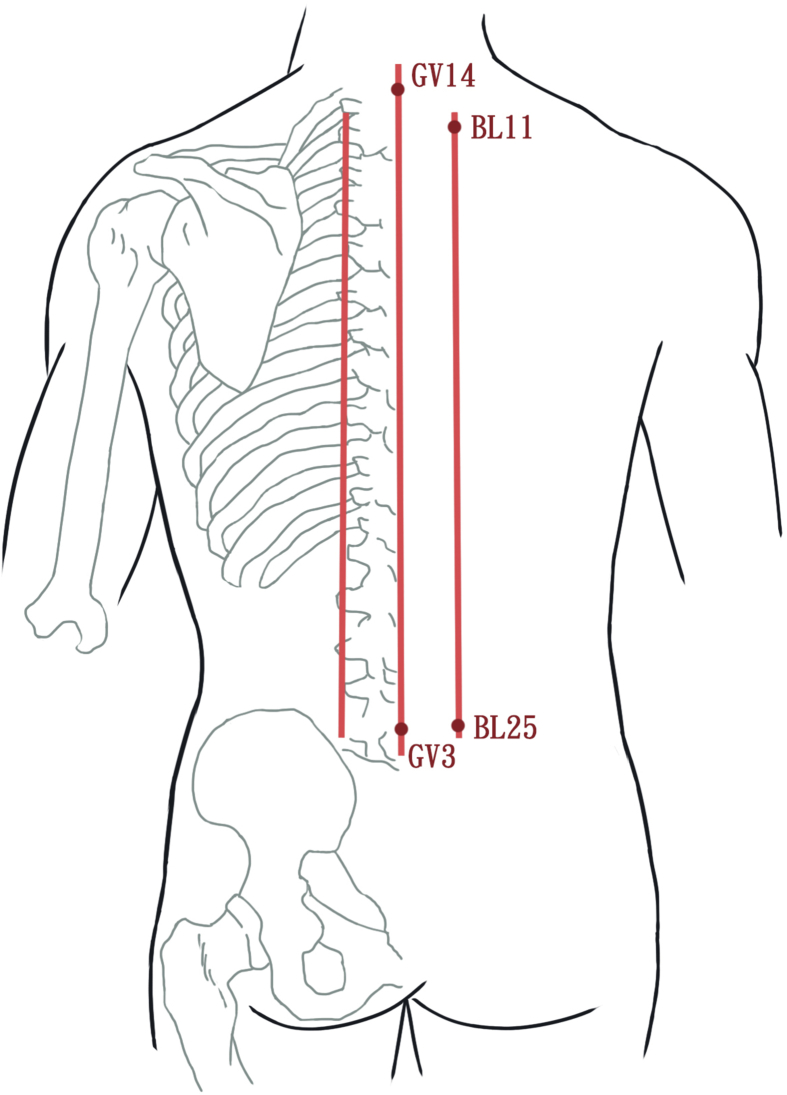
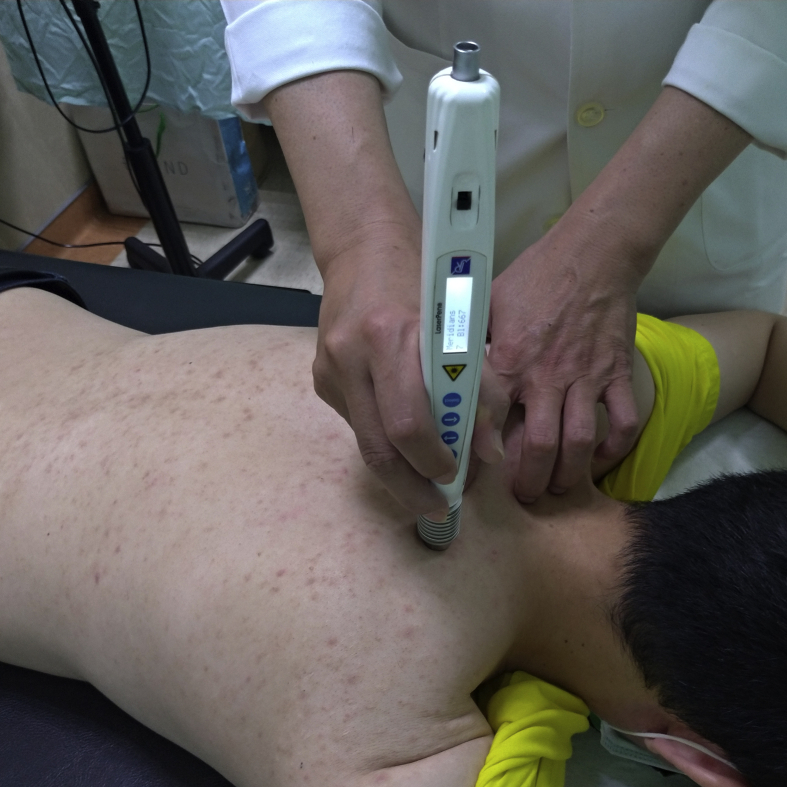
The study participants received 12 LMM sessions three times per week for 4 weeks using a gallium aluminum arsenide LaserPen (maximal power, 150 mW; wavelength, 810 nm; area of probe, 0.5 cm2; power density, 300 mW/cm2; pulsed-wave; and frequencies [Bl, 667 Hz; B4, 4796 Hz]; RJ-Laser, Reimers & Janssen GmbH, Waldkirch, Germany). Laser treatment was applied to the back, including the Bladder meridian (BL11–BL25) and Governor Vessel (GV3–GV14; Fig. 2 [20] and 3), for 15 min, to deliver a total treatment dose of 67.5 J/cm2. The laser treatment was applied by the same experienced physician who had adequate training and was a licensed Chinese medicine practitioner in Taiwan. The physician and patients were required to wear protective eye wear during the procedure.
Fig. 2.
Meridians used for heroin addiction. Bladder meridian (BL11-BL25) and Governor Vessel (GV3-GV14) [20].
Outcome measurements
Outcomes were assessed at 2 and 4 weeks after the first visit; these included subjective reporting of heroin use, quality of life, and objective urinary morphine levels. The primary outcomes were urinary morphine levels and self-reported heroin use (times or days) during the previous week. The secondary outcomes were self-reported visual analog scale (VAS) scores for heroin craving/refusal to use heroin (0–10 points) in the previous week and the quality of life, assessed using the Short Form-12v2® (SF-12v2), before and after 4 weeks of treatment. The participants’ heart rate variability before and after a single treatment session was also recorded. The evaluations were performed by one well-trained senior research assistant who was blinded to the group allocation.
A VAS score of 0 for heroin craving indicated no heroin craving, and a score of 10 indicated the strongest possible heroin craving. A VAS score of 0 for refusal to use heroin indicated no refusal, and a score of 10 indicated total refusal. The SF-12v2 Health Survey is a multipurpose, short-form health instrument containing 12 questions that yields an eight-scale profile of functional health and well-being (Physical Functioning [PF], Role-Physical [RP], Bodily Pain [BP], General Health [GH], Vitality [VT], Social Functioning [SF], Role-Emotional [RE], Mental Health [MH]), as well as two psychometrically-based physical and mental health summary measures and a preference-based health utility index. The PRO CoRE, which is part of the Smart Measurement® System suite of products and Optum's upgrade to the Quality Metric Health Outcomes™ scoring software, was used to score the SF-12v2 Health Survey.
We recorded potentially adverse events, as well as the reasons offered by the patients for not completing their follow-up visits or dropping out of the study, such as adverse events/intercurrent illnesses, suboptimal response to therapy, failure to return for follow-up, failure to meet the selection criteria at entry, other protocol violations, and refusal to receive treatment.
Statistical analysis
The data were presented as the mean ± standard deviation. The independent t-test and chi-square test were used to evaluate and compare the baseline patient characteristics between the experimental and control groups. The independent t-test was used to compare the differences between the two groups. Repeated-measures analysis of variance was used for comparisons between the two study groups. All analyses were performed using SPSS for Windows, version 22 (Statistics 22, IBM Corp., Armonk, NY, USA). A P-value of <0.05 was considered statistically significant.
Data monitoring
A data monitoring committee (DMC) was not needed because LMM is a routine and noninvasive intervention.
Ethics
This study was approved by our institutional human ethics committee (Chang Gung Medical Foundation Institutional Review Board, approval number 201801823A3) and adhered to the tenets of the Declaration of Helsinki and its amendments. The protocol was registered with ClinicalTrials.gov (identifier NCT04003077). Written informed consent was obtained from all study participants. Personal information about potential and enrolled participants were collected, shared, and maintained in an independent closet in order to protect patient confidentiality before, during, and after the trial. Fig. 3.
Fig. 3.
Laser meridian massage performed using the LaserPen device over the right Bladder meridian.
Results
The addiction treatment center assessed a total of 164 participants for study eligibility. Twenty-seven OUD patients met the inclusion criteria, and 14 subjects were placed in the experimental group. The remaining 13 subjects formed the age-matched control group. One participant from the experimental group dropped out because he had received MMT at another hospital during the study [Fig. 1]. The baseline characteristics did not reveal any important disparities between the two groups, except for the GH scale in SF-12v2 [Table 1].
Table 1.
Demographic information and characteristics of the participants.
| Variable | Experimental (n = 14) | Control (n = 13) | P-value |
|---|---|---|---|
| Age (years) | 45.64 ± 7.60 | 46.77 ± 4.25 | .642a |
| Marital status | .331b | ||
| Never married | 7 (50%) | 9 (69.2%) | |
| Married/cohabiting | 4 (28.6%) | 4 (30.8%) | |
| Divorced/widowed | 3 (21.4%) | 0 | |
| Occupation | 1.000b | ||
| Employed | 12 (85.7%) | 12 (92.3%) | |
| Unemployed | 2 (14.3%) | 1 (7.7%) | |
| Education (years) | 1.000b | ||
| 6–12 | 2 (14.3%) | 1 (7.7%) | |
| 12–15 | 9 (64.3%) | 10 (76.9%) | |
| 15–18 | 3 (21.4%) | 2 (15.4%) | |
| SF-12v2 | |||
| PF | 50.88 ± 9.35 | 53.43 ± 6.11 | .413a |
| RP | 52.63 ± 6.61 | 52.27 ± 7.57 | .899a |
| BP | 51.29 ± 9.64 | 53.57 ± 5.96 | .471a |
| GH | 54.00 ± 8.06 | 45.00 ± 10.60 | .020a,∗ |
| VT | 60.31 ± 7.57 | 54.36 ± 11.79 | .129a |
| SF | 50.55 ± 8.13 | 49.37 ± 8.78 | .722a |
| RE | 52.20 ± 5.46 | 49.08 ± 7.51 | .227a |
| MH | 53.15 ± 8.85 | 47.44 ± 11.82 | .166a |
| PCS | 52.12 ± 7.02 | 52.88 ± 6.00 | .762a |
| MCS | 54.21 ± 8.53 | 47.92 ± 8.63 | .068a |
| Heroin craving (VAS) | 3.50 ± 2.62 | 2.15 ± 1.95 | .145a |
| Refusal to use heroin (VAS) | 6.07 ± 3.69 | 4.54 ± 3.87 | .302a |
*p < 0.05.
Data are presented as mean ± SD or number (%).
Abbreviations: SF-12v2: Short Form Health Survey-12v2; PF: physical functioning; RP: role limitations due to physical problems; BP: bodily pain; GH: general health; VT: vitality; SF: social functioning; RE: role limitations due to emotional problems; MH: general mental health; PCS: physical component summary measures; MCS: mental component summary measures.
Independent t-test.
Fisher's exact test.
Urinary morphine
The positive rate of urinary morphine decreased from 0.86 to 0.64 in the experimental group, while there was no change in the control group. The adjusted differences between the two groups were 0.21 (95% CI -0.03–0.46) at 2 weeks of treatment (p = 0.082), and 0.14 (95% CI -0.07–0.35) at 4 weeks of treatment (p = 0.165) [Table 2].
Table 2.
Episodes or days of heroin use and VAS scores for heroin craving/refusal to use heroin during the previous week.
| Measurements | Experimental |
Control |
Adjusted difference (95% CI)b | ||
|---|---|---|---|---|---|
| Mean ± SD | P-valuea | Mean ± SD | P-valuea | ||
| Episodes or days of heroin use | |||||
| Baseline | 2.85 ± 2.30 | 1.46 ± 1.33 | |||
| Week 2 | 1.92 ± 2.10 | 0.008∗ | 1.23 ± 1.42 | 0.641 | 0.63 (−0.50–1.75) |
| Week 4 | 1.46 ± 1.81 | 0.030∗ | 1.23 ± 2.09 | 0.641 | 1.15 (−0.37–2.68) |
| Heroin craving (VAS) | |||||
| Baseline | 3.38 ± 2.69 | 2.15 ± 1.95 | |||
| Week 2 | 2.15 ± 2.19 | 0.147 | 3.23 ± 2.46 | 0.028∗ | 2.22 (0.42–4.02)∗ |
| Week 4 | 2.15 ± 2.34 | 0.120 | 3.15 ± 1.86 | 0.016∗ | 2.23 (0.51–3.95)∗ |
| Refusal to use heroin (VAS) | |||||
| Baseline | 6.15 ± 3.83 | 4.54 ± 3.87 | |||
| Week 2 | 5.69 ± 4.03 | 0.629 | 5.85 ± 3.21 | 0.037∗ | 1.74 (−0.42–3.89) |
| Week 4 | 6.62 ± 4.09 | 0.719 | 4.92 ± 3.25 | 0.724 | −0.77 (−3.47–3.32) |
| Urine morphine (+/−) | |||||
| Baseline | 12/2 | 6/7 | |||
| Week 2 | 9/5 | 0.082 | 6/7 | – | 0.21 (−0.03–0.46) |
| Week 4 | 9/4 | 0.165 | 6/7 | – | 0.14 (−0.07–0.35) |
∗p < 0.05.
Abbreviations: SD: standard deviation; VAS: visual analogue scale.
Repeated measures ANOVA.
Independent t-test was used for the statistical analysis of changes from baseline in each outcome between two groups.
Times or days of heroin use during the previous week
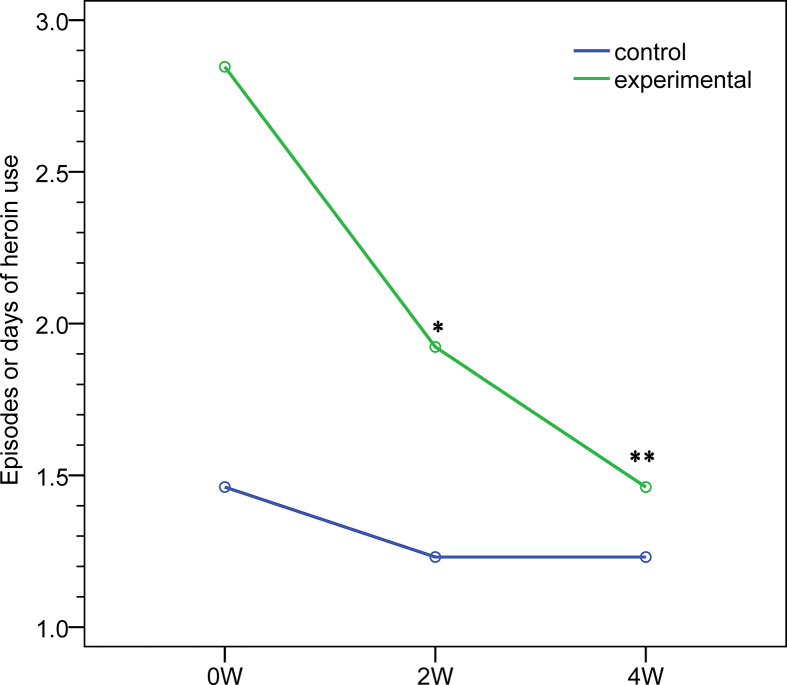
The times or days of heroin use during the previous week decreased significantly in the experimental group. At 2 weeks of treatment, the change from baseline was −0.93 and −0.23 in the experimental (p = 0.008) and control groups (p = 0.641), respectively. At 2 weeks of treatment, the change from the baseline was −1.39 and −0.23 in the experimental (p = 0.030) and control groups (p = 0.641), respectively. Adjusted differences between the two groups were 0.63 (95% CI -0.50–1.75) at 2 weeks of treatment (p = 0.261) and 1.15 (95% CI -0.37–2.68) at 4 weeks of treatment (p = 0.132) [Table 2, Fig. 4].
Fig. 4.
Times or days of heroin use over the previous week. ∗p < 0.01, ∗∗p < 0.05. Repeated measures ANOVA was used for the statistical analysis of changes from baseline in each outcome between the two groups. All outcomes were adjusted for baseline values.
Heroin craving/refusal to use heroin relative to that in the previous week
There was a marked improvement in heroin craving and refusal behavior post-LMM treatment. Heroin craving relative to that in the previous week decreased in the experimental group after 2 and 4 weeks of treatment (−1.23, p = 0.147 vs. −1.23, p = 0.120), while it increased in the control group at 2 and 4 weeks of treatment (1.08, p = 0.028 vs. 1.00, p = 0.016). Adjusted differences between the two groups at 2 and 4 weeks of treatment were 2.22 (95% CI 0.42–4.02; p = 0.018) and 2.23 (95% CI 0.51–3.95; p = 0.014), respectively [Table 2, Fig. 5].
Fig. 5.
Heroin craving over the previous week. ∗p < 0.05. Repeated measures ANOVA was used for the statistical analysis of changes from baseline in each outcome between the two groups. All outcomes were adjusted for baseline values.
Refusal to use heroin in the previous week decreased at 2 weeks of treatment and increased at 4 weeks of treatment in the experimental group (−0.46, p = 0.629 vs. 0.47, p = 0.719). However, it increased in the control group at 2 and 4 weeks of treatment (1.31, p = 0.037 vs. 0.48, p = 0.724). The adjusted differences between the two groups at 2 and 4 weeks of treatment were 1.74 (95% CI -0.42–3.89; p = 0.109) and −0.77 (95% CI -3.47–3.32; p = 0.963), respectively [Table 2].
Quality of life
The SF-12v2 Health Survey revealed a significant improvement in physical health in the experimental group (5.06, p = 0.024) at 4 weeks of treatment. The adjusted difference between the two groups was −5.85 (95% CI -11.07–0.62) (p = 0.030) [Table 3].
Table 3.
Quality of life, assessed by the SF-12v2.
| SF-12v2 | Experimental |
Control |
Adjusted difference (95% CI)b | ||
|---|---|---|---|---|---|
| Mean ± SD | P-valuea | Mean ± SD | P-valuea | ||
| PF | |||||
| Baseline | 50.40 ± 9.56 | 53.42 ± 6.12 | |||
| Week 4 | 54.64 ± 4.96 | 0.237 | 52.82 ± 6.90 | 0.673 | −4.84 (−12.65–2.97) |
| RP | |||||
| Baseline | 52.25 ± 6.72 | 52.27 ± 7.57 | |||
| Week 4 | 55.18 ± 5.07 | 0.133 | 50.30 ± 7.99 | 0.485 | −4.90 (−11.68–1.88) |
| BP | |||||
| Baseline | 51.49 ± 10.00 | 53.57 ± 5.96 | |||
| Week 4 | 55.65 ± 3.96 | 0.165 | 51.49 ± 7.71 | 0.337 | −6.24 (−13.47–0.99) |
| GH | |||||
| Baseline | 53.72 ± 8.32 | 45.00 ± 10.60 | |||
| Week 4 | 56.62 ± 4.27 | 0.193 | 43.16 ± 10.28 | 0.553 | −4.74 (−12.32–2.84) |
| VT | |||||
| Baseline | 61.17 ± 7.13 | 54.36 ± 11.79 | |||
| Week 4 | 63.44 ± 6.49 | 0.190 | 50.58 ± 10.51 | 0.337 | −6.05 (−14.78–2.67) |
| SF | |||||
| Baseline | 51.43 ± 7.74 | 49.38 ± 8.78 | |||
| Week 4 | 53.48 ± 5.78 | 0.387 | 48.01 ± 10.27 | 0.739 | −3.42 (−12.95–6.10) |
| RE | |||||
| Baseline | 52.68 ± 5.36 | 49.08 ± 7.51 | |||
| Week 4 | 49.08 ± 9.85 | 0.145 | 47.09 ± 8.26 | 0.514 | 1.60 (−6.17–9.37) |
| MH | |||||
| Baseline | 54.06 ± 8.49 | 47.44 ± 11.82 | |||
| Week 4 | 55.38 ± 9.25 | 0.598 | 43.47 ± 10.08 | 0.201 | −5.29 (−13.17–2.58) |
| PCS | |||||
| Baseline | 51.52 ± 6.93 | 52.89 ± 6.00 | |||
| Week 4 | 56.58 ± 3.20 | 0.024∗ | 52.10 ± 6.38 | 0.633 | −5.85 (-11.07∼-0.62)∗ |
| MCS | |||||
| Baseline | 55.44 ± 7.48 | 47.92 ± 8.63 | |||
| Week 4 | 55.50 ± 11.35 | 0.980 | 44.44 ± 8.25 | 0.189 | −3.54 (−10.56–3.47) |
∗p < 0.05.
Abbreviations: SD: standard deviation; SF-12v2: Short Form Health Survey-12v2; PF: physical functioning; RP: role limitations due to physical problems; BP: bodily pain; GH: general health; VT: vitality; SF: social functioning; RE: role limitations due to emotional problems; MH: general mental health; PCS: physical component summary measures; MCS: mental component summary measures.
Repeated measures ANOVA.
Independent t-test was used for the statistical analysis of changes from baseline in each outcome between two groups.
Heart rate variability
The heart rate variability analysis revealed no significant difference between the two groups after a single treatment session [Table 4].
Table 4.
HRV records before and after a single session of laser meridian massage.
| Measurements | Experimental |
Control |
Adjusted difference (95% CI)b | ||
|---|---|---|---|---|---|
| Mean ± SD | P-valuea | Mean ± SD | P-valuea | ||
| HRV | |||||
| Before | 28.54 ± 15.76 | 33.00 ± 11.69 | |||
| After | 28.62 ± 7.00 | 0.983 | 34.14 ± 6.67 | 0.670 | 1.07 (−9.71–11.84) |
| LF | |||||
| Before | 57.08 ± 25.46 | 55.57 ± 25.45 | |||
| After | 63.15 ± 16.30 | 0.164 | 54.71 ± 19.59 | 0.925 | −6.93 (−24.65–10.78) |
| HF | |||||
| Before | 42.92 ± 25.46 | 43.00 ± 26.14 | |||
| After | 36.85 ± 16.30 | 0.164 | 45.29 ± 19.59 | 0.800 | 8.36 (−9.21–25.94) |
| LF/HF | |||||
| Before | 2.55 ± 2.74 | 2.13 ± 1.69 | |||
| After | 2.29 ± 1.58 | 0.681 | 1.70 ± 1.77 | 0.455 | −0.17 (−2.14–1.80) |
Abbreviations: HRV: heart rate variability; LF: low frequency; HF: high frequency; ∗p < 0.05.
Repeated measures ANOVA.
Independent t-test was used for the statistical analysis of changes from baseline in each outcome between two groups.
Adverse events
No LMM-associated adverse events were reported.
Discussion
To our knowledge, this study is the first of its kind to investigate the clinical efficacy of adjuvant LMM in men with OUD on MMT. An improvement in physical health and decreased heroin use were observed in the experimental group, as there was a statistically significant difference that favored LMM in terms of physical health and heroin craving after 4-week treatment. There were no statistically significant differences with regard to mental health or refusal to use heroin between the two groups. The main strength of our study is that it clearly demonstrated that LMM resulted in an improvement in physical health and decreased heroin craving.
We chose the Governor Vessel and Bladder meridian for LMM, as the acupuncture points over these areas are commonly used and effective in OUD [16,17]. Electroacupuncture at GV14 and GV20 regulating the endoplasmic reticulum stress response seems to mediate the neuroprotective effect of acupuncture in heroin-addicted rats with a brain injury. The inhibition of CCAAT-enhancer-binding protein homologous protein and c-Jun N-terminal kinase upregulation, as well as the decrease in nerve cell apoptosis, may be the primary mechanisms underlying the effects of acupuncture on heroin addiction-induced brain injury [21]. Acupuncture at GV14 and GV20 significantly reduced the pathological damage in the hippocampus and frontal lobe, upregulated Bcl-2 expression, and downregulated Bax expression, thus preventing brain cell apoptosis in heroin-readdicted rats [22]. Acupuncture at the Governor Vessel points (GV20, GV14, GV11, GV10, GV9, GV4) has an auxiliary therapeutic effect on abstinence symptoms in heroin addiction, particularly with regard to symptoms such as pain in the muscle and bone, perspiration, and anxiety [23]. In our study, LMM at BL11-BL25 improved physical health in OUD. Electroacupuncture at BL23 and ST36 could suppress the expression of morphine-induced conditioned place preference (CPP) and orexin-positive nuclei in the lateral hypothalamus, relative to the effects in morphine-conditioned rats [24]. Electroacupuncture at Bei-Shu acupoints (BL13, BL15, BL23, GV20) attenuated the prolonged withdrawal symptoms following heroin detoxification, including somatic symptoms and anxiety [17]. Electroacupuncture at BL23 reduced morphine withdrawal signs and c-Fos expression in the central nucleus of the amygdala [25]. Moreover, acupuncture at GV20 plus moxibustion at BL23 can improve changes in synaptic ultrastructure in heroin-readdicted rats, which may be related to their effect in regulating the expression of some synaptic skeleton proteins and genes [26].
Electroacupuncture at LI4 and ST36 and acupuncture at ear Shenmen significantly reduced daily methadone consumption and improved sleep latency in heroin addicts. After rapid detoxification of opiate, the severity of withdrawal symptoms decreased by acupuncture at LI4, PC6, HT7, ST36, LR3, GV14, and GV20 [27], while acupuncture at ST36 quickly inhibited the activation of particular brain areas associated with craving involved in reward, learning and memory, cognition, and emotion [28]. Electroacupuncture at ST36 and SP6 reduced the active response caused by discrete signals [29], revealed synergy in the treatment of heroin-seeking behaviors in addition to extinction therapy [30], and modulated dopaminergic reward system by activating the endogenous opioid and endocannabinoid systems in rats [31]. Electroacupuncture at PC6 and ST36 could inhibit heroin's attentional bias to reduce the relapse rate [32]. Functional magnetic resonance imaging showed that hypothalamic activation related to manual acupuncture at ST36 was stronger in heroin addicts than in healthy subjects [33]. Acupuncture at HT7 significantly reduced the self-administration of morphine and modulated the enhancement of morphine by regulating gamma-aminobutyric acid (GABA) receptors [34]. In our study, LMM at GV3-GV14 and BL11-BL25 decreased heroin craving in OUD. When heroin addicts withdrew suddenly, transcutaneous electrical acupoint stimulation of 2/100 Hz at LI4, PC6, PC8, and SJ5 significantly reduced withdrawal symptoms and reduced the need for opioids [35].
A rewarding effect induced by peripheral electric stimulation (PES, 2 Hz) at ST36 and SP6 could inhibit the morphine-induced CPP and reduce craving in rats [36], and weaken morphine-induced reward memory by regulating the endogenous opioid system in the nucleus accumbens (Nac) [37]. The inhibition of opiate addiction through electroacupuncture may be related to the release of endogenous μ-, δ-, and κ-opioid agonists in the Nac shell [38] and the activation of the cannabinoid, endogenous opioid, and dopaminergic systems to induce CPP in rats [29]. PES (100 Hz) at ST36 and SP6 exerted an anti-craving effect by activating the suprasegmental δ- and κ-opioid receptors in the central nervous system in rats [39]. PES (2 Hz) raised preproenkephalin mRNA levels and PES (100 Hz) raised preprodynorphin mRNA levels in the Nac; both suppressed the expression and reinstatement of morphine-induced CPP in rats [40]. In the treatment of opiate addiction, PES (100 Hz) at ST36 and SP6 restored the function of the dopaminergic neurons in the ventral tegmental region [41], decreased p-cAMP response element binding, and increased dynorphin synthesis in the spinal cord for opioid detoxification [42]. PES (2 Hz) is a possible adjuvant treatment for restoring immune function in opiate addicts [43], and PES (2 Hz or 100 Hz) was found to improve male sexual behavior in rats during the process of morphine withdrawal by both increasing the peripheral sexual hormone concentration and regulating central neural pathways [44]. PES (2 Hz or 100 Hz) at ST36 and SP6 reversed the morphological changes caused by long-term morphine consumption [45]. By improving non-rapid eye movement (NREM) sleep, REM sleep, and total sleep times, PES (2 Hz or 100 Hz) may be a potential therapy for sleep disorders during the process of morphine withdrawal [46]. In our study, LMM at GV3-GV14 and BL11-BL25 improved physical health in OUD. Acupuncture at SI5 prevented the restoration of morphine-seeking behaviors after extinction through mediating the GABA receptor system [30,47].
Laser acupuncture at BL15 increases LF and the LF/HF ratio, and thus, activates the sympathetic nervous system [48]. Laser acupuncture at GV14 had an on/off-effect in the blood flow velocity of the cerebral arteries, induced a de qi sensation like needle acupuncture does, and significantly increased the blood flow velocity in the basilar artery [49]. Laser acupuncture combines the effectiveness of traditional acupuncture and low-intensity laser therapy [16]. LMM is the application of laser acupuncture. The Bei-Shu acupoints refer to the acupoints infusing the meridian qi of zang-fu organs into the back. Stimulation at Bei-Shu acupoints (BL13-15, BL18-23, BL25) can restore the function of zang-fu organs [50]. As such, LMM at BL11-BL25 was found to improve physical health in our study. Spinal kneading can promote homeostasis, improve appetite, free the meridians and collaterals, improve the circulation of qi and blood, and improve the function of zang-fu organs [50]. As such, LMM at GV3-GV14 was found to decrease heroin craving in our study. To improve the efficacy of LMM for OUD, the Yang Ming meridians may be added to the therapeutic protocol because ST36 and LI4 are the most common acupoints used in treating OUD [16]. The optimal frequencies applied to the meridians for LMM may be obtained by using the vascular autonomic signal. The dose energy may be properly increased.
There were several limitations of our study. First, the sample size was smaller than our expectation. Further trial and investigation with a larger number of subjects is warranted. Second, the LMM course in this study lasted 4 weeks; a longer LMM treatment period may induce more obvious effects with regard to the refusal to use heroin in the previous week as well as urine morphine levels.
Conclusion
A four-week treatment with LMM in conjunction with MMT is more effective than MMT alone in improving physical health and decreasing heroin craving in men with OUD. We suggest that LMM be utilized as adjuvant therapy in patients with OUD on MMT. Further studies should include randomization, sham control, and follow-up evaluations to strengthen this conclusion.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This study was supported by the Ministry of Health and Welfare (MOHW108-CMAP-M-113-000104; 2019/01/22 Version 2). The funder had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. We thank the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for statistics work.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Volkow N.D., Icaza M.E.M., Poznyak V., Saxena S., Gerra G., UNODC-WHO Informal Scientific Network Addressing the opioid crisis globally. World Psychiatr. 2019;18:231–232. doi: 10.1002/wps.20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations Office on Drugs and Crime . 2019. World drug report.https://wdr.unodc.org/wdr2019/ [accessed 1 February 2020]. [Google Scholar]
- 3.Chang H.Y., Kharrazi H., Bodycombe D., Weiner J.P., Alexander G.C. Healthcare costs and utilization associated with high-risk prescription opioid use: a retrospective cohort study. BMC Med. 2018;16:69. doi: 10.1186/s12916-018-1058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallersnes O.M., Jacobsen D., Ekeberg Ø., Brekke M. Mortality, morbidity and follow-up after acute poisoning by substances of abuse: a prospective observational cohort study. Scand J Publ Health. 2019;47:452–461. doi: 10.1177/1403494818779955. [DOI] [PubMed] [Google Scholar]
- 5.Faggiano F., Vigna-Taglianti F., Versino E., Lemma P. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst Rev. 2003;3:CD002208. doi: 10.1002/14651858.CD002208. [DOI] [PubMed] [Google Scholar]
- 6.Gibson A., Degenhardt L., Mattick R.P., Ali R., White J., O'Brien S. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103:462–468. doi: 10.1111/j.1360-0443.2007.02090.x. [DOI] [PubMed] [Google Scholar]
- 7.Connery H.S. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatr. 2015;23:63–75. doi: 10.1097/HRP.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . WHO; Geneva: 2009. Guidelines for the psychosocially assisted pharmacological treatment of opioid dependence; p. 7. [PubMed] [Google Scholar]
- 9.World Health Organization . 2002. WHO traditional medicine strategy 2002–2005. [Google Scholar]
- 10.Yang C.H., Lee B.H., Sohn S.H. A possible mechanism underlying the effectiveness of acupuncture in the treatment of drug addiction. Evid Based Complement Alternat Med. 2008;5:257–266. doi: 10.1093/ecam/nem081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen H.L. Fast detoxification of heroin addicts by acupuncture and electrical stimulation (AES) in combination with naloxone. Comp Med East West. 1977;5:257–263. doi: 10.1142/s0147291777000362. [DOI] [PubMed] [Google Scholar]
- 12.McLellan A.T., Grossman D.S., Blaine J.D., Haverkos H.W. Acupuncture treatment for drug abuse: a technical review. J Subst Abuse Treat. 1993;10:569–576. doi: 10.1016/0740-5472(93)90061-6. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization . World Health Organization; Geneva: 2002. Acupuncture: Review and analysis of reports on controlled clinical trials. [Google Scholar]
- 14.Cui C.L., Wu L.Z., Luo F. Acupuncture for the treatment of drug addiction. Neurochem Res. 2008;33:2013–2022. doi: 10.1007/s11064-008-9784-8. [DOI] [PubMed] [Google Scholar]
- 15.Black S., Carey E., Webber A., Neish N., Gilbert R. Determining the efficacy of auricular acupuncture for reducing anxiety in patients withdrawing from psychoactive drugs. J Subst Abuse Treat. 2011;41:279–287. doi: 10.1016/j.jsat.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Lin J.G., Chan Y.Y., Chen Y.H. Acupuncture for the treatment of opiate addiction. Evid Based Complement Alternat Med. 2012;2012:739045. doi: 10.1155/2012/739045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu S., Zhou W.H., Yang G.D. Clinical study on heroin prolonged withdrawal symptoms (early stage) treated by electroacupuncture at Bei-Shu acupoints in addicts. Chin J Drug Abuse Prev Treat. 2007;3:142–144. [Google Scholar]
- 18.Hu W.L., Chang C.H., Hung Y.C. Clinical observations on laser acupuncture in simple obesity therapy. Am J Chin Med. 2010;38:861–867. doi: 10.1142/S0192415X10008305. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J. 2nd ed. Lawrence Erlbaum Associates; New York: 1988. Statistical power analysis for the behavioral sciences; p. 286. [Google Scholar]
- 20.Hu W.L., Tsai M.C., Kuo C.E., Liu C.T., Wu S.Y., Wu T.C., et al. Adjuvant laser meridian massage in men with opioid use disorder on methadone maintenance treatment: protocol for a case-controlled study. Medicine (Baltimore) 2019;98 doi: 10.1097/MD.0000000000017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Y.L., Zhang Y., Cao J.P., Wu S.B., Cai X.H., Zhang Y.C., et al. Regulation of the endoplasmic reticulum stress response and neuroprotective effects of acupuncture on brain injury caused by heroin addiction. Acupunct Med. 2017;35:366–373. doi: 10.1136/acupmed-2016-011220. [DOI] [PubMed] [Google Scholar]
- 22.Hou X., Zhang R., Lv H., Cai X., Xie G., Song X. Acupuncture at Baihui and Dazhui reduces brain cell apoptosis in heroin readdicts. Neural Regen Res. 2014;9:164–170. doi: 10.4103/1673-5374.125345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng X., Lei L., Lu Y., Wang Z. Treatment of heroinism with acupuncture at points of the Du Channel. J Tradit Chin Med. 2005;25:166–170. [PubMed] [Google Scholar]
- 24.Wang X., Zhang B., Zhang L., Liu S. Electroacupuncture suppresses morphine reward-seeking behavior: lateral hypothalamic orexin neurons implicated. Neurosci Lett. 2017;661:84–89. doi: 10.1016/j.neulet.2017.09.057. [DOI] [PubMed] [Google Scholar]
- 25.Liu S., Zhou W., Liu H., Yang G., Zhao W. Electroacupuncture attenuates morphine withdrawal signs and c-Fos expression in the central nucleus of the amygdala in freely moving rats. Brain Res. 2005;1044:155–163. doi: 10.1016/j.brainres.2005.02.075. [DOI] [PubMed] [Google Scholar]
- 26.Zhang R.J., Hou X.R., Cai X.H., Song X.G., Wu S.B., Zhao M.J. [Influence of acupuncture plus moxibustion on changes of synaptic structure and expression of synaptic skeleton related genes and proteins in prefrontal cortex of heroin re-addicted rats] Zhen Ci Yan Jiu. 2019;44:323–328. doi: 10.13702/j.1000-0607.180437. Chinese. [DOI] [PubMed] [Google Scholar]
- 27.Montazeri K., Farahnakian M., Saghaei M. The effect of acupuncture on the acute withdrawal symptoms from rapid opiate detoxification. Acta Anaesthesiol Sin. 2002;40:173–177. [PubMed] [Google Scholar]
- 28.Cai X., Song X., Li C., Xu C., Li X., Lu Q. Acupuncture inhibits cue-induced heroin craving and brain activation. Neural Regen Res. 2012;7:2607–2616. doi: 10.3969/j.issn.1673-5374.2012.33.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S., Zhu F., Lai M., Sun L., Liu Y., Zhou W. Electroacupuncture suppresses discrete cue-evoked heroin-seeking and fos protein expression in the nucleus accumbens core in rats. Evid Based Complement Alternat Med. 2012;2012:286404. doi: 10.1155/2012/286404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu A., Lai M., Wei J., Wang L., Mao H., Zhou W., et al. The effect of electroacupuncture on extinction responding of heroin-seeking behavior and FosB expression in the nucleus accumbens core. Neurosci Lett. 2013;534:252–257. doi: 10.1016/j.neulet.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Xia W., Chu N.N., Liang J., Li Y.J., Zhang R., Han J.S., et al. Electroacupuncture of 2 Hz Has a rewarding effect: evidence from a conditioned place preference study in rats. Evid Based Complement Alternat Med. 2011;2011:730514. doi: 10.1093/ecam/nen043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Y.P., Liu H., Xu P., Wang Y., Lu G.H. Effect of electro-acupuncture intervention on cognition attention bias in heroin addiction abstinence-a dot-probe-based event-related potential study. Chin J Integr Med. 2011;17:267–271. doi: 10.1007/s11655-011-0698-y. [DOI] [PubMed] [Google Scholar]
- 33.Liu S., Zhou W., Ruan X., Li R., Lee T., Weng X., et al. Activation of the hypothalamus characterizes the response to acupuncture stimulation in heroin addicts. Neurosci Lett. 2007;421:203–208. doi: 10.1016/j.neulet.2007.04.078. [DOI] [PubMed] [Google Scholar]
- 34.Yoon S.S., Kim H., Choi K.H., Lee B.H., Lee Y.K., Lim S.C., et al. Acupuncture suppresses morphine self-administration through the GABA receptors. Brain Res Bull. 2010;81:625–630. doi: 10.1016/j.brainresbull.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Ma D., Han J.S., Diao Q.H., Deng G.F., Ping X.J., Jin W.J., et al. Transcutaneous electrical acupoint stimulation for the treatment of withdrawal syndrome in heroin addicts. Pain Med. 2015;16:839–848. doi: 10.1111/pme.12738. [DOI] [PubMed] [Google Scholar]
- 36.Chen J.H., Liang J., Wang G.B., Han J.S., Cui C.L. Repeated 2 Hz peripheral electrical stimulations suppress morphine-induced CPP and improve spatial memory ability in rats. Exp Neurol. 2005;194:550–556. doi: 10.1016/j.expneurol.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Liang J., Ping X.J., Li Y.J., Ma Y.Y., Wu L.Z., Han J.S., et al. Morphine-induced conditioned place preference in rats is inhibited by electroacupuncture at 2 Hz: role of enkephalin in the nucleus accumbens. Neuropharmacology. 2010;58:233–240. doi: 10.1016/j.neuropharm.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Liang J., Li Y., Ping X., Yu P., Zuo Y., Wu L., et al. The possible involvement of endogenous ligands for mu-, delta- and kappa-opioid receptors in modulating morphine-induced CPP expression in rats. Peptides. 2006;27:3307–3314. doi: 10.1016/j.peptides.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Shi X.D., Ren W., Wang G.B., Luo F., Han J.S., Cui C.L. Brain opioid-receptors are involved in mediating peripheral electric stimulation-induced inhibition of morphine conditioned place preference in rats. Brain Res. 2003;981:23–29. doi: 10.1016/s0006-8993(03)02798-7. [DOI] [PubMed] [Google Scholar]
- 40.Shi X.D., Wang G.B., Ma Y.Y., Ren W., Luo F., Cui C.L., et al. Repeated peripheral electrical stimulations suppress both morphine-induced CPP and reinstatement of extinguished CPP in rats: accelerated expression of PPE and PPD mRNA in NAc implicated. Brain Res Mol Brain Res. 2004;130:124–133. doi: 10.1016/j.molbrainres.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 41.Hu L., Chu N.N., Sun L.L., Zhang R., Han J.S., Cui C.L. Electroacupuncture treatment reverses morphine-induced physiological changes in dopaminergic neurons within the ventral tegmental area. Addiction Biol. 2009;14:431–437. doi: 10.1111/j.1369-1600.2009.00163.x. [DOI] [PubMed] [Google Scholar]
- 42.Wang G.B., Wu L.Z., Yu P., Li Y.J., Ping X.J., Cui C.L. Multiple 100 Hz electroacupuncture treatments produced cumulative effect on the suppression of morphine withdrawal syndrome: central preprodynorphin mRNA and p-CREB implicated. Peptides. 2011;32:713–721. doi: 10.1016/j.peptides.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Li H.Y., Zhang R., Cui C.L., Han J.S., Wu L.Z. Damage of splenic T lymphocyte proliferation and differentiation and its normalization by electroacupuncture in morphine-dependent mice mode. Evid Based Complement Alternat Med. 2011;2011:424092. doi: 10.1155/2011/424092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui G.H., Ren X.W., Wu L.Z., Han J.S., Cui C.L. Electroacupuncture facilitates recovery of male sexual behavior in morphine withdrawal rats. Neurochem Res. 2004;29:397–401. doi: 10.1023/b:nere.0000013743.53827.ad. [DOI] [PubMed] [Google Scholar]
- 45.Chu N.N., Xia W., Yu P., Hu L., Zhang R., Cui C.L. Chronic morphine-induced neuronal morphological changes in the ventral tegmental area in rats are reversed by electroacupuncture treatment. Addiction Biol. 2008;13:47–51. doi: 10.1111/j.1369-1600.2007.00092.x. [DOI] [PubMed] [Google Scholar]
- 46.Li Y.J., Zhong F., Yu P., Han J.S., Cui C.L., Wu L.Z. Electroacupuncture treatment normalized sleep disturbance in morphine withdrawal rats. Evid Based Complement Alternat Med. 2011;2011:361054. doi: 10.1093/ecam/nep133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee B.H., Ma J.H., S, Kim H.Y., Yoon S.S., Jang E.Y., et al. Acupuncture at SI5 attenuates morphine seeking behavior after extinction. Neurosci Lett. 2012;529:23–27. doi: 10.1016/j.neulet.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 48.Lee N.R., Kim S.B., Heo H., Lee Y.H. Comparison of the effects of manual acupuncture, laser acupuncture, and electromagnetic field stimulation at acupuncture point BL15 on heart rate variability. J Acupunct Meridian Stud. 2016;9:257–263. doi: 10.1016/j.jams.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Litscher G., Huang T., Wang L., Zhang W. Violet laser acupuncture--part 1: effects on brain circulation. J Acupunct Meridian Stud. 2010;3:255–259. doi: 10.1016/S2005-2901(10)60045-3. [DOI] [PubMed] [Google Scholar]
- 50.Shi D.B., editor. The encyclopedia of traditional Chinese medicine, version 1.0 for PC (e-book in Chinese) Yuan-Liou; Taipei: 2002. [Google Scholar]