Abstract
Crimean-Congo hemorrhagic fever virus (CCHFV) causes mild to severe and fatal disease in humans. Person-to-person transmission is common, necessitating the availability of rapidly deliverable therapeutic and prophylactic interventions to mitigate CCHFV spread. Previously, we showed complete protection using one dose of a viral replicon particle (VRP) vaccine administered 28 days before CCHFV challenge. In order to determine the utility of the VRP vaccine for rapid vaccination protocols, we assessed the efficacy of such vaccination administered at various intervals relative to challenge in IFNAR−/− mice. Unvaccinated mice uniformly succumbed to disease by 8 days post infection (dpi). All mice vaccinated 14, 7, or 3 days prior to CCHFV challenge survived infection. Mice vaccinated −14 or −7 dpi were fully protected from clinical disease, whereas mice inoculated −3 dpi developed signs of disease prior to recovering to baseline values 5–9 dpi. These data support the utility of the VRP vaccine for modified short course vaccination protocols to protect against disease and severe outcomes.
Keywords: Crimean-Congo hemorrhagic fever, viral replicon particle, vaccine, IFNAR−/− mice
Crimean-Congo hemorrhagic fever virus (CCHFV; family Nairoviridae; genus Orthonairovirus) causes a range of clinical signs, from asymptomatic infection to acute and fatal hemorrhagic disease. The virus is maintained in nature by Hyalomma spp. ticks over a wide geographical area, including the Mediterranean countries, China, central Asia, Africa, the Middle East, and the Indian subcontinent (Bente et al., 2013). CCHFV causes disease in humans, but other mammalian species are widely susceptible to infection without developing clinical signs of disease. Humans become infected through tick bites or direct contact with infected animal blood or tissue. Person-to-person transmission via contact with infectious blood or body fluids is well documented. Nosocomial spread of CCHFV has also occurred due to inadequate barrier protections, improper sterilization of medical equipment, and reuse or contamination of medical supplies. Additionally, possible sexual transmission has been reported (Pshenichnaya et al., 2016). Outbreaks of disease are generally restricted in size and can result in high case fatality rates (up to 50%); the exception occurred in Turkey, which experienced the largest recorded Crimean-Congo hemorrhagic fever (CCHF) outbreak with more than 4,400 confirmed cases, most of which presented with milder disease (Yilmaz et al., 2009).
To date, no vaccines are commercially licensed to prevent CCHF. We previously described the development of a CCHFV viral replicon particle (VRP) vaccine (Scholte et al., 2019). VRPs contain the complete S and L genome segments, but lack the M genome segment entirely. While VRPs are able to enter target cells and subsequently perform authentic viral transcriptional and translational processes, they are unable to produce the required proteins essential for nascent particle formation due to the absence of the GPC gene, found on the M segment. Thus, VRPs are defined as single-cycle and non-spreading. VRPs are generated using reverse genetics based on the S and L segments of the IbAr10200 CCHFV strain (Bergeron et al., 2015) and a plasmid encoding the codon-optimized glycoprotein open reading frame of the Oman-98 strain (Scholte et al., 2019).
Using IFNAR−/− mice, which develop acute lethal disease within a week of CCHFV infection, we demonstrated single-dose protection of VRP vaccination given 28 d prior to homologous (IbAr10200) and heterologous (strain Turkey-200406546 and Oman-199723179) virus challenge (Scholte et al., 2019; Spengler et al., 2019). Here, we sought to determine rapidity of protection of VRP vaccination. Groups of 8 B6.129S2-Ifnar1tm1Agt/Mmjax mice (4 male and 4 female) were VRP-vaccinated subcutaneously (SC) in the inter-scapular region (target dose: 1 × 105 TCID50; back-titer dose: 2 × 104 TCID50) at various timepoints either before (−14, −7, −3 or −1 days post infection [dpi]) or after (+1 dpi) virus challenge. Unvaccinated controls and VRP-vaccinated mice were challenged with CCHFV strain Turkey-200406546 (target dose: 100 TCID50; back-titer dose: 24 TCID50). As the lethal dose of the Turkey-200406546 strain is less well characterized than that of other strains like IbAr10200, we included an unvaccinated control group that received a 10-fold dilution of the same inoculum of virus received by the other mice (target dose: 10 TCID50; back-titer dose: 2.4 TCID50), to provide additional data on dose-dependent lethality of this strain.
All animal procedures were approved by the CDC Institutional Animal Care and Use Committee and conducted in accordance with the Guide for the Care and Use of Laboratory Animals at an AAALAC-International accredited facility. All work with infectious virus or infected animals was conducted in a biosafety level 4 (BSL-4) laboratory at the Centers for Disease Control and Prevention (CDC) following established BSL-4 standard operating procedures approved by the Institutional Biosafety Committee. All recombinant virus work was approved by the Centers for Disease Control and Prevention Institutional Biosafety Committee.
Mice were housed in a climate-controlled laboratory with a 12 h day/night cycle; provided sterilized commercially available mouse chow and water ad libitum; and group-housed on autoclaved corn cob bedding (Bed-o’Cobs ¼”, Anderson Lab Bedding) with cotton nestlets in an isolator-caging system (Thoren Caging, Inc., Hazleton, PA, USA) with a HEPA-filtered inlet and exhaust air supply. During the vaccination period, health checks were performed daily and mice were weighed periodically (every 1–4 days). Baseline weights for the challenge period were obtained at −1 dpi, and mice were monitored daily for 21 dpi. Clinical signs in mice were scored based on 14 parameters: 2 points each for quiet, dull, responsive (QDR) disposition, hunched back or ruffled coat; 3 points each for dehydration or abnormal huddling/hypoactivity; 5 points each for ataxia/circling/tremors/paresis, abnormal breathing, or anemia; 7 points for weight loss of >20%; 10 points each for inability to bear weight, paralysis, frank hemorrhage or bleeding, moribund state, or weight loss of >25%. Animals were humanely euthanized when end-point criteria were reached (clinical score ≥10) or at study completion (21 dpi). Mean clinical scores were calculated by dividing the daily sum of all scores in an experimental group by the total by the number of animals remaining. To quantify water consumption, water bottles were weighed daily; the total volume consumed was divided by the number of animals remaining per cage. Values for each group are presented as the mean amount of water consumed per mouse (male and female cages; in mL/mouse).
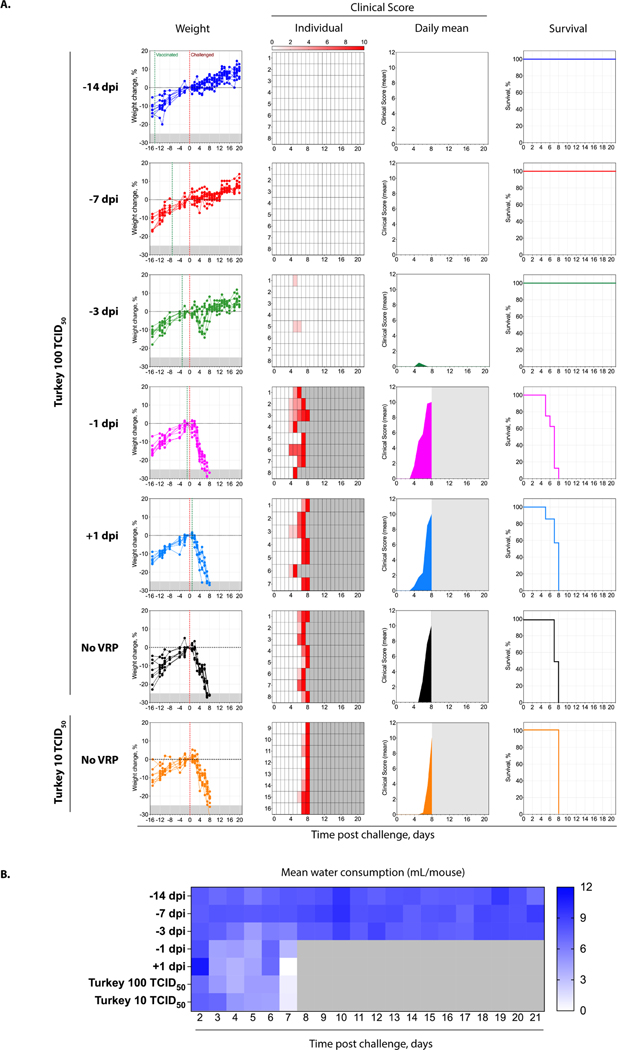
Comparable to previous reports, unvaccinated control mice all succumbed to disease by 8 dpi (Fig 1A; Supplementary Table 1). Further supporting the use of low-passage clinically derived strains in therapeutic studies, infection was uniformly lethal and end-point criteria were only delayed by 1 day in unvaccinated mice receiving 2.4 TCID50 of Turkey-200406546 compared to unvaccinated mice receiving a 10-fold higher dose. Mice that were vaccinated either 1 day before or 1 day after challenge also uniformly succumbed to disease, following similar clinical trends as unvaccinated mice (Supplementary Table 2). Compared to control mice, survival was statistically significant for mice vaccinated 1 day before challenge but with a p-value of only 0.0417, and was not significant for mice vaccinated 1 day after challenge. A decrease in mean water consumption coincided with clinical signs and was most pronounced in experimental groups in which all animals reached end-point criteria (Fig 1B). In contrast, mice vaccinated 14 or 7 days prior to challenge were uniformly protected from clinical disease and lethal outcome; they exhibited no notable clinical signs and gained expected amounts of weight over the study period. Mice vaccinated 3 days before challenge showed progressive weight loss starting 3 dpi; however, 6–7 dpi, these mice began recovering weight and returned to levels above baseline by the end of the study period. Despite the transient yet uniform weight loss seen in this group, clinical signs were mild and observed in only two animals (5–6 dpi). Within experimental groups, there was no significance difference (p > 0 .9999) in survival between male and female mice.
Fig 1. Clinical signs and survival in VRP-vaccinated IFNAR−/− mice.
Groups of 8 mice (4 male and 4 female) were vaccinated subcutaneously (SC) with viral replicon particles (VRP) (back-titer dose: 2 × 104 TCID50) at various intervals relative to SC challenge (−14, −7, −3, −1, or +1 days post infection [dpi]) with CCHFV Turkey-200406546 (target dose: 100 TCID50; backtiter dose: 24 TCID50). One mouse was removed prior to challenge from the +1 dpi group due to severe malocclusion resulting in a failure to maintain weight. Two unvaccinated control groups were challenged in parallel, one with the same dose of inoculum (24 TCID50) as the vaccinated groups, and the other with a 10-fold dilution of the inoculum (target dose: 10 TCID50; back-titer dose: 2.4 TCID50). (A) Weight change from baseline at −1 dpi (−25% weight loss criteria for euthanasia indicated in grey); time of vaccination and challenge, indicated by green and red vertical dotted lines, respectively; individual clinical scores (0–10); mean clinical scores; and survival. Grey shading in individual or mean clinical score graphs indicate animals no longer in study (i.e., met euthanasia criteria). (B) Mean water intake (mL) per mouse in the experimental group.
To assess viral levels in tissues of unvaccinated and vaccinated animals that survived or succumbed to infection, samples were obtained when mice met euthanasia criteria (5–8 dpi) or at study completion (21 dpi). RNA was extracted from blood and homogenized tissue using the MagMAX-96 Total RNA Isolation Kit (Thermo-Fisher Scientific) on a 96-well ABI MagMAX extraction platform with a DNaseI treatment step according to manufacturer’s instructions. Independently of vaccination status, all animals that succumbed to disease had comparable levels of viral RNA throughout the tissues (Fig 2). Two mice with the longest period of clinical signs (i.e., weight loss) of those vaccinated at −3 dpi were the only surviving mice in that group with detectable viral RNA at study completion (21 dpi); viral RNA was found in the spleen of one, and in liver and lung of the other. Viral RNA was undetectable in all other mice in this group. No viral RNA was detected in mice vaccinated −7 or −14 dpi.
Fig 2. CCHFV RNA levels in blood and tissues of VRP-vaccinated IFNAR−/− mice.
Mice were vaccinated SC with VRP (back-titer dose: 2 × 104 TCID50) and subsequently challenged SC at the indicated timepoints with CCHFV Turkey-200406546 (back-titer dose: 24 TCID50). Two unvaccinated control groups were challenged in parallel, one with the same dose of inoculum (24 TCID50) as vaccinated groups, and the other with a 10-fold dilution of the inoculum (2.4 TCID50). CCHFV S genome RNA levels were assessed in blood and tissues collected when mice reached end-point criteria (fatal; 5–8 dpi) or at completion of the study (survivors; 21 dpi). ND, not detected. Bars indicate mean levels.
Here, using IFNAR−/− mice, an animal model highly susceptible to CCHFV, we demonstrate that VRP vaccination provides rapid protection from lethal disease as early as 3 days post vaccination. When vaccinated 7 or more days before CCHFV challenge, mice were also protected against clinical disease. Rapid efficacy following VRP vaccination provides critical options for use in the field. Ring vaccination, targeting of the exposed contacts of infected individuals, has been used successfully as a disease-controlling strategy for a variety of viral pathogens and is considered a key approach for response to natural outbreaks or bioterrorism events. These data support considering VRP use in ring vaccination, offering a novel short-course prophylactic treatment for CCHFV.
Protective immune responses that arise shortly after vaccination may be mediated by the innate or adaptive immune response or a combination of both. Innate immunity is critical in protection against CCHFV, as disruptions in the innate immune response are a determinant of susceptibility in animal species refractory to disease (Garrison et al., 2019). Furthermore, in humans, both T cell and humoral responses appear to contribute to protection against CCHF. Humoral responses are seen as early as 4–5 days post symptom onset and the appearance of antibody responses coincides with declining viremia (Kaya et al., 2014). However, virus titers decrease in survivors during the first week independently of antibody levels, suggesting that cellular immunity contributes to CCHFV elimination (Duh et al., 2007).
Given the impaired interferon (IFN) response function in IFNAR−/− mice, we were encouraged to see that complete protection from lethal outcome could be achieved with vaccination at −3 dpi. While VRPs do not spread due to the absence of the GPC gene, they are capable of replicating in the first cells they encounter and can induce innate immune responses (Kainulainen et al., 2018). Although innate immunity does not provide immunological memory, it can protect against infection; for example, pre-treatment with IFN 24 h prior to infection reduces CCHFV replication in culture (Andersson et al., 2006; Bordi et al., 2015). Though IFNAR−/− mice lack the ability to respond to type I IFN, they maintain other innate immune functions that might contribute to VRP-induced protection. Indeed, in response to CCHFV infection, IFNAR−/− mice express a number of cytokines, including IFN-, IL-6, IL-22, MCP-1, MIP-2, and MIP-1β (Welch et al., 2019). Future studies to assess levels of innate immune cells, cytokines, and interferon-stimulated gene expression in blood and tissues after vaccination will aid in determining which IFNAR-independent responses contribute to rapid protection against CCHFV.
In previous studies, we assessed immunological responses 24–28 days after VRP vaccination in IFNAR−/− mice fully protected from disease, demonstrating absent or low IgM and robust IgG responses predominantly targeting CCHFV NP (Scholte et al., 2019; Spengler et al., 2019). Adaptive responses can also develop very shortly after immunization; in mice infected with Ebola virus and treated with virus-like particles (VLPs) 24 h post exposure, innate immunity facilitated the adaptive immune response (Ayithan et al., 2015), but increased antibody production and generation of cytotoxic T cells were required for survival (Bradfute et al., 2015). This suggests the potential for contributions of adaptive immunity to VRP-mediated protection against CCHFV even in animals challenged within days of vaccination.
Altogether, our data provide key support for further investigations of alternate dosing schemes for VRP vaccination. Further studies of the properties and kinetics of these responses, examining both arms of the immune response, will allow informed and targeted delivery of VRP vaccination based on public health needs, including use in outbreak response when rapid induction of protection either before or after virus exposure is needed.
Supplementary Material
Highlights.
Low-dose subcutaneous infection with a low passage human isolate from Turkey results in uniform lethality in IFNAR−/− mice.
Viral replicon particle (VRP) vaccination was evaluated at different intervals to challenge.
Single-dose VRP vaccination protects against clinical disease in mice challenged at 7 days post-vaccination.
Single-dose VRP vaccination protects against lethal outcome in mice challenged at 3 days post-vaccination.
Acknowledgements
We thank Tatyana Klimova for assistance with editing the manuscript and members of the Centers for Disease Control and Prevention’s Comparative Medicine Branch for providing care for the animals.
Financial support.
This work was partially supported by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC (S.R.W.), an NIAID grant (1R01AI151006) and by CDC Emerging Infectious Disease Research Core Funds.
Footnotes
Potential conflicts of interest. All authors: no reported conflicts.
Declaration of interests statement. Provisional US Patent Application No. 62/780,098
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson I, Lundkvist Å, Haller O, Mirazimi A, 2006. Type I Interferon Inhibits Crimean-Congo Hemorrhagic Fever Virus in Human Target Cells 222, 216–22. [DOI] [PubMed] [Google Scholar]
- Ayithan N, Bradfute SB, Anthony SM, Stuthman KS, Bavari S, Bray M, Ozato K, 2015. Virus-like particles activate type I interferon pathways to facilitate post-exposure protection against Ebola virus infection. PLoS One 10, e0118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M, 2013. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 100, 159–89. [DOI] [PubMed] [Google Scholar]
- Bergeron É, Zivcec M, Chakrabarti AK, Nichol ST, Albariño CG, Spiropoulou CF, 2015. Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin. PLoS Pathog. 11, e1004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi L, Lalle E, Caglioti C, Travaglini D, Lapa D, Marsella P, Quartu S, Kis Z, Arien KK., Huemer HP., Meschi S., Ippolito G., Di Caro A., Capobianchi MR., Castilletti C., 2015. Antagonistic antiviral activity between IFN-lambda and IFN-alpha against lethal Crimean-Congo hemorrhagic fever virus in vitro. PLoS One 10, e0116816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfute SB, Anthony SM, Stuthman KS, Ayithan N, Tailor P, Shaia CI, Bray M, Ozato K, Bavari S, 2015. Mechanisms of immunity in post-exposure vaccination against Ebola virus infection. PLoS One 10, e0118434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duh D, Saksida A, Petrovec M, Ahmeti S, Dedushaj I, Panning M, Drosten C, Avsic-Zupanc T, 2007. Viral load as predictor of Crimean-Congo hemorrhagic fever outcome. Emerg. Infect. Dis. 13, 1769–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison AR, Smith DR, Golden JW, 2019. Animal Models for Crimean-Congo Hemorrhagic Fever Human Disease. Viruses 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainulainen MH, Spengler JR, Welch SR, Coleman-McCray JD, Harmon JR, Klena JD, Nichol ST, Albariño CG, Spiropoulou CF, 2018. Use of a Scalable Replicon-Particle Vaccine to Protect Against Lethal Lassa Virus Infection in the Guinea Pig Model. J. Infect. Dis. 217, 1957–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya S, Elaldi N, Kubar A, Gursoy N, Yilmaz M, Karakus G, Gunes T, Polat Z, Gozel MG, Engin A, Dokmetas I, Bakir M, Yilmaz N, Sencan M, 2014. Sequential determination of serum viral titers, virus-specific IgG antibodies, and TNF-α, IL-6, IL-10, and IFN-γ levels in patients with Crimean-Congo hemorrhagic fever. BMC Infect. Dis. 14, 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pshenichnaya NY, Sydenko IS, Klinovaya EP, Romanova EB, Zhuravlev AS, 2016. Possible sexual transmission of Crimean-Congo hemorrhagic fever. Int. J. Infect. Dis. 45, 109–111. [DOI] [PubMed] [Google Scholar]
- Scholte FEM, Spengler JR, Welch SR, Harmon JR, Coleman-McCray JD, Freitas BT, Kainulainen MH, Pegan SD, Nichol ST, Bergeron É, Spiropoulou CF, 2019. Single-dose replicon particle vaccine provides complete protection against Crimean-Congo hemorrhagic fever virus in mice. Emerg. Microbes Infect. 8, 575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler JR., Welch SR., Scholte FEM., Coleman-McCray JD., Harmon JR., Nichol ST., Bergeron É., Spiropoulou CF., 2019. Heterologous protection against Crimean-Congo hemorrhagic fever in mice after a single dose of replicon particle vaccine. Antiviral Res. 170, 104573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch SR, Ritter JM, McElroy AK, Harmon JR, Coleman-McCray JD, Scholte FEM, Kobinger GP, Bergeron É, Zaki SR, Nichol ST, Spengler JR, Spiropoulou CF, 2019. Fluorescent Crimean-Congo hemorrhagic fever virus illuminates tissue tropism patterns and identifies early mononuclear phagocytic cell targets in IFNAR−/− mice. PLoS Pathog. 15, e1008183–e1008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz GR, Buzgan T, Irmak H, Safran A, Uzun R, Cevik MA, Torunoglu MA, 2009. The epidemiology of Crimean-Congo hemorrhagic fever in Turkey, 2002–2007. Int. J. Infect. Dis. 13, 380–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.