Abstract
Most vertebrate sex-determining genes (SDGs) emerge as neofunctionalized genes through duplication and/or mutation of ancestral genes that are involved with sexual differentiation. We previously demonstrated dm-W to be the SDG in the African clawed frog Xenopus laevis and found that a portion of this gene emerged from the masculinization gene dmrt1 after allotetraploidization by interspecific hybridization between two ancestral species around 17–18 Ma. dm-W has four exons consisting of a noncoding exon 1, dmrt1-derived exons 2 and 3, and an orphan exon 4 (Ex4) of unknown origin that includes coding sequence (CDS). In this study, we searched for the origin of Ex4 and investigated the function of the CDS of this exon. We found that the Ex4-CDS is derived from a noncoding portion of the hAT-10 family of DNA transposon. Evolutionary analysis of transposons and determination of the Ex4 sequences from three other species indicated that Ex4 was generated before the diversification of most or all extant allotetraploid species in subgenus Xenopus, during which time we hypothesize that transposase activity of this hAT superfamily was active. Using DNA–protein binding and transfection assays, we further demonstrate that the Ex4-encoded amino acid sequence increases the DNA-binding ability and transrepression activity of DM-W. These findings suggest that the conversion of the noncoding transposon sequence to the CDS of dm-W contributed to neofunctionalization of a new chimeric SDG in the ancestor of the allotetraploid Xenopus species, offering new insights into de novo origin and functional evolution of chimerical genes.
Keywords: sex determination, transposon, chimeric gene, interspecific hybridization, frog, transcription factor
Introduction
In vertebrates, sex-determining genes (SDGs) evolved independently many times (Ito and Mawaribuchi 2013; Pan et al. 2016). Most SDGs emerged via neofunctionalization (Mawaribuchi et al. 2012); for example, mammalian Sry (Sex-determining region of Y) evolved through allelic divergence from Sox3 (Sry-box 3) in the ancestor of therian mammals (Foster and Graves 1994), and dmy/dmrt1bY and dm-W independently evolved from duplicates or partial duplicates of dmrt1 (doublesex and mab-3 related transcription factor 1) in ancestors of the teleost fish medaka and the frogs of the subgenus Xenopus, respectively (Matsuda et al. 2002; Nanda et al. 2002; Yoshimoto et al. 2008; Bewick et al. 2011). Sox3 and dmrt1 encode transcription factors with two different DNA-binding domains—HMG (high mobility group) and DM domains, respectively. The SDGs dmy, dm-W, and Sry each have higher substitution rates than their respective paralogous or gametologous genes, dmrt1, dmrt1, and Sox3 (Mawaribuchi et al. 2012). Dmrt1, which probably originated in the most recent common ancestor of vertebrates (Mawaribuchi et al. 2019), functions in testis formation to promote somatic-cell masculinization gene and germ cell development (Smith et al. 2009; Matson et al. 2010, 2011; Yoshimoto et al. 2010; Masuyama et al. 2012; Zarkower 2013; Zhao et al. 2015; Fujitani et al. 2016; Mawaribuchi, Musashijima, et al. 2017; Ge et al. 2018).
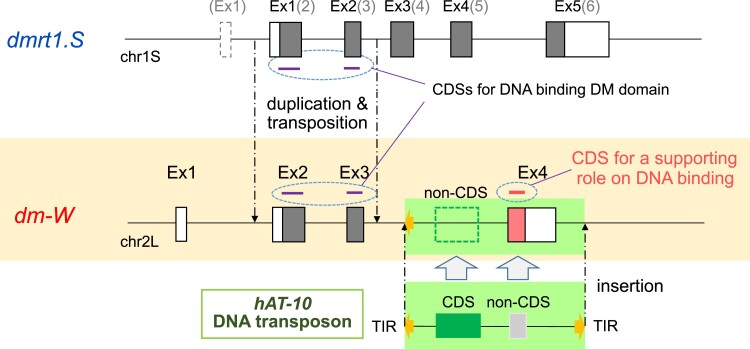
Early during evolution of the subgenus Xenopus, an allopolyploidization event between two closely related diploid species generated the tetraploid ancestor of all species with 36 chromosomes; subsequent allopolyploidization events generated octoploid (72 chromosome) and dodecaploid (108 chromosomes) species (reviewed in Evans 2008). The initial allopolyploidization event in subgenus Xenopus is estimated to have occurred around 17–18 Ma (million years ago) and resulted in two “subgenomes”—L and S—that are respectively derived from each diploid ancestor (Session et al. 2016) (see supplementary fig. S1, Supplementary Material online). Although it resides on chromosome 2 of the L subgenome, the SDG dm-W evolved from the S subgenome homeolog of dmrt1 (dmrt1.S) and is carried by several species in subgenus Xenopus including highly diverged species pairs such as Xenopus clivii and X. laevis (Bewick et al. 2011; Cauret et al. 2020). The contrast between the genealogical relationship (closer to dmrt1.S) and its genomic context (in subgenome L) coupled with its phylogenetic distribution in many species in subgenus Xenopus argues that this gene became established in its current location after allotetraploidization but before the diversification of extant allotetraploids in subgenus Xenopus (Bewick et al. 2011; Mawaribuchi, Takahashi, et al. 2017; Cauret et al. 2020; see supplementary fig. S1, Supplementary Material online). Analysis of sequence homology suggests that dm-W is a chimeric gene with Ex2 and Ex3, which encode the DM domain being derived from dmrt1.S and Ex1 and Ex4 being of unknown origin (Yoshimoto et al. 2008; Mawaribuchi, Takahashi, et al. 2017). Thus, dm-W has no homology to dmrt1 exons 4–6, which encodes a transregulation domain (Yoshimoto et al. 2010). Moreover, it remains unclear whether the distinct components of dm-W (Ex1, Ex2 + 3, and Ex4) became juxtaposed before allopolyploidization in the diploid ancestor of subgenome S and then later tandemly translocated to subgenome L after allotetraploidization, or whether juxtaposition of these components occurred after translocation of Ex2 + Ex3 to subgenome L in the allotetraploid ancestor of subgenus Xenopus.
In X. laevis, dm-W-expression or dm-W-knockdown in ZZ or ZW tadpoles induces ovarian and testicular formation, respectively (Yoshimoto et al. 2008, 2010). The sequence of the DNA-binding domain of DMRT1 is highly conserved across mammals, amphibians, and fish (Murphy et al. 2007; Yoshimoto et al. 2010; Ogita et al. 2020). In vitro assays indicate that DM-W antagonizes the transcriptional activation of downstream genes by DMRT1 (Yoshimoto et al. 2010). This is consistent with a model for sex determination in X. laevis wherein DMRT1 activates masculinizing genes in males by binding to their cis-elements in ZZ primordial gonads, whereas DM-W represses the transcriptional activity of DMRT1 in females by competing with DMRT1 for binding to cis-regulatory elements in ZW primordial gonads, leading to feminization (Yoshimoto et al. 2010; Yoshimoto and Ito 2011). In vitro assays indicate that the DNA-binding affinity to a consensus binding sequence is higher for DM-W than DMRT1 (Ogita et al. 2020), which is consistent with this model. These findings suggest that dm-W functions as a dominant-negative protein that counteracts the masculinization factor dmrt1, and that DM-W is a repressor of primary male sexual differentiation in X. laevis (Yoshimoto and Ito 2011).
Transposable elements (TEs) are able to change genomic locations or generate new copies in of themselves, and are generally considered to not benefit the host genome (i.e., “selfish DNA”). However, TEs may also influence genome architecture, gene expression, and gene content in beneficial ways (Anderson and Springer 2018; Ågren and Clark 2018; Bourque et al. 2018). For example, the generations of at least two SDGs might be linked to TE activity that influence transcription: TE-cis-regulatory modules influence expression of the SDG gsdf on the Y chromosome of sablefish, and in the fighting fish, dmrt1 on the X chromosome is subject to TE-induced epigenetic silencing (Herpin et al. 2021; Wang et al. 2022). Several eukaryotic protein-coding genes originated (partially) from TEs, such as Gary (which arose from a transposase genes) or Rag (which arose from retrotransposons and DNA transposons) (Fugmann 2010; Alzohairy et al. 2013). TEs are also the source of various long and functionally important noncoding RNAs (reviewed in Bourque et al. 2018). However, of ∼900 genes that arose de novo in an ancestor of seven closely related Caenorhabditis species including C. elegans, <1% arose from TEs (Zhang et al. 2019), and this mode of gene origin appears to also be infrequent in insects (Wissler et al. 2013). Not surprisingly, most examples of novel CDSs that arose from TEs involve coding sequence (CDS) of TEs, as opposed to from non-CDS. There are very few examples of noncoding portions of TEs becoming exon(s), resulting in the generation of different isoform proteins. Two examples include a retrotransposon-derived non-CDSs that evolved to function through alternative splicing as a CDS in bovine EP3 receptor gene and a novel TE-derived exon in mouse Sry (Shimamura et al. 1998; Miyawaki et al. 2020).
As discussed above, dm-W is a chimerical SDG that arose in an ancestor of allotetraploid Xenopus frogs that triggers female sexual differentiation in some Xenopus species; one portion of this gene arose from partial duplication of the masculinizing gene dmrt1 but the other portion is of unknown origin. The goals of this study are to determine where the orphan exon 4 of dm-W came from and what its function may be. To this end, we first deployed a bioinformatic strategy to search for sequences homologous to Ex4 and found that it was derived from a noncoding region of the DNA transposon hAT-10 family belonging to widespread eukaryotic hAT (hobo, Ac, and Tam-3) superfamily. We then performed in vitro and cotransfection assays that demonstrate that the Ex4-derived amino acid sequences both increase the DNA-binding ability of DM-W and the transrepression activity of DM-W on DMRT1. These findings indicate that the evolutionary origin and neofunctionalization of dm-W was achieved by a combination of partial gene duplication of dmrt1 coupled with the recruitment of a non-CDS transposon sequence into a functionally important component of the CDS of dm-W.
Results
dm-W Ex2 and Ex3 and Flanking Regions are Derived from dmrt1.S
To clarify the origin of the components of the chimeric gene dm-W, we first compared the genomic sequences of X. laevis dm-W, dmrt1.S, dmrt1.L, and X. tropicalis dmrt1, which were obtained from the X. laevis genome v9.2 and X. tropicalis genome v10.0 (http://www.xenbase.org/entry/). Although X. laevis has an allotetraploid genome consisting of L and S subgenomes, X. tropicalis has a diploid genome whose ancestor diverged from the ancestor of subgenus Xenopus before dm-W originated (Bewick et al. 2011; Session et al. 2016). Approximately 80 kbp nucleotide sequences in and around the four genes were aligned, and homologous regions were estimated using mVISTA (supplementary fig. S2, Supplementary material online). We found that not only Ex2 and Ex3, but also small portions of the 3′ region of intron 1, intron 2, and the 5′ region of intron 3 of dm-W shared sequence homology with the corresponding regions of the three dmrt1 genes. In contrast, Ex1, the 3′ region of intron 3, Ex4, and 5′/3′-flanking sequences of Ex1 and Ex4 have no identifiable homology with any of these three dmrt1 genomic sequences (supplementary fig. S2, Supplementary material online). These results indicate that the genomic region from the 3′ region of intron 1 to the 5′ region of intron 3 of the ancestral dmrt1.S (which resides on chromosome 1S) was duplicated into one of the two ancestral chromosome 2L. In addition, we estimated a phylogenetic tree of these genes using the homologous sequences of the dm-W’s introns 1–3 in addition to Ex2 and Ex3. We confirmed that dm-W is more closely to dmrt1.S, not dmrt1.L, with higher confidence values (supplementary fig. S3, Supplementary material online) than those reported previously (Bewick et al. 2011; Mawaribuchi, Takahashi, et al. 2017).
The CDS of dm-W Ex4 Evolved from a Non-CDS of the hAT-10 DNA Transposon Family
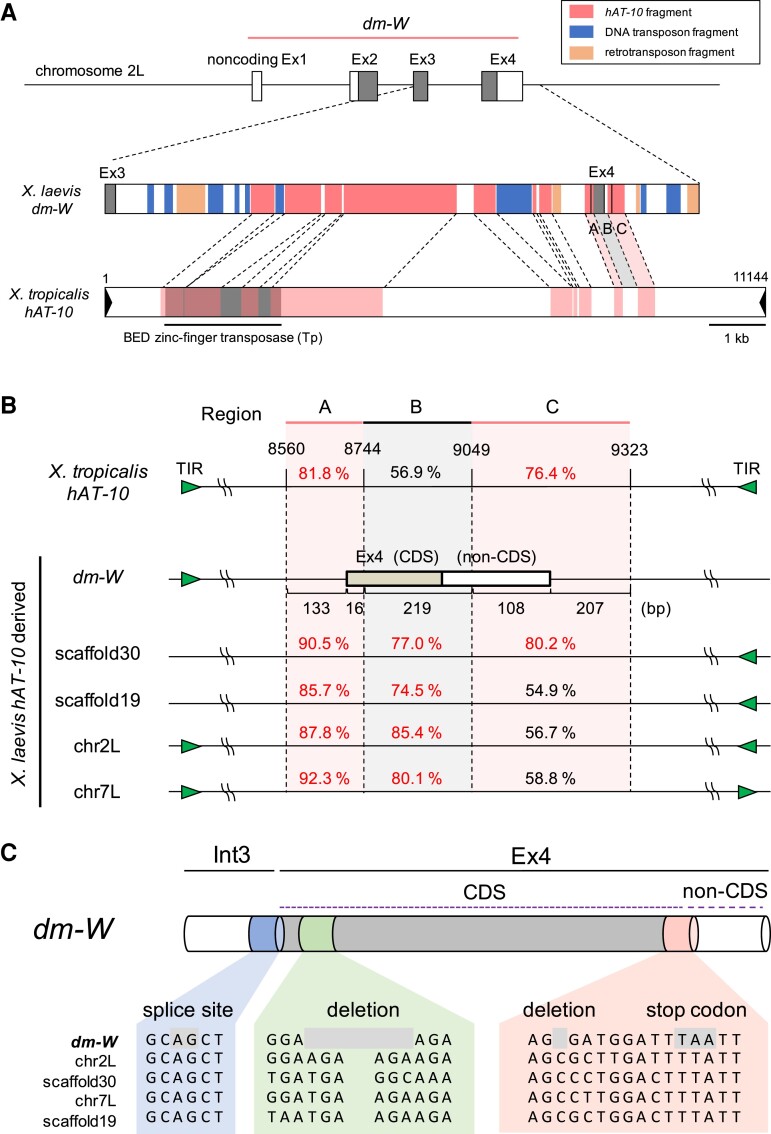
We previously reported the accumulation of TEs in W-specific regions of X. laevis (Mawaribuchi, Takahashi, et al. 2017). We examined the TE density in 80 kb region in and around dm-W using the CENSOR program (www.girinst.org/censor/index.php) (Kohany et al. 2006). There is a higher proportion of TEs in the dm-W-containing region (69.1%) than in the dmrt1.L or dmrt1.S-containing region (34.0% or 27.9%, respectively) as indicated by the red–blue gradient in supplementary fig. S4, Supplementary Material online. Interestingly, TEs were identified not only within introns 1–3, but also within Ex4 of dm-W (supplementary fig. S5, Supplementary material online). We then performed a focused search for TE-like sequences in and around Ex4. Here hAT-10 DNA transposon-like fragments were found to comprise more than half of intron 3, a portion of the 5′ region of Ex4, and about 250 bp of the 3′-flanking region of Ex4 (fig. 1A and supplementary fig. S5, Supplementary material online). In Ex4, we identified three regions designated as A, B, and C spanning a total of 683 bp with sequence similarity to X. tropicalis hAT-10: region A (133 bp of the 3′ region on intron 3 and 16 bp of the 5′ region on Ex4), region B (219 bp of the central portion of Ex4), and region C (108 bp of the 3′ portion of Ex4 and 207 bp of the 3′-flanking region downstream of Ex4) (fig. 1 and supplementary fig. S6A, Supplementary material online). The A and C regions were recognized by CENSOR, and had relatively high nucleotide identity (81.8% and 76.4%, respectively) with the corresponding sequences of X. tropicalis hAT-10, whereas the region B between A and C had a lower identity (56.9%) with X. tropicalis hAT-10 (fig. 1B). We then searched for similar nucleotide sequences to these regions containing the CDS of dm-W Ex4 in the X. laevis genome using BLAST, and found four sequences on scaffolds 19, 30, chromosome 2L, and chromosome 7L. All included hAT transposon fragments that had higher sequence similarities with the regions A and B of dm-W (85.7–92.3% and 74.5–85.4%, respectively) than with X. tropicalis hAT-10 (fig. 1B and supplementary table S1, Supplementary material online). Using the CENSOR program, we also detected 14 bp of terminal inverted repeats, which are also a feature of DNA transposons, around dm-W Ex4 and the four hAT-10-like sequences (supplementary figs. S6A and B, Supplementary material online). Together, these results indicate that these five sequences with high similarity in the X. laevis genome—including dm-W Ex4—are derived from the hAT-10 family. The hAT-10 transposon family contains one protein gene encoding a BED zinc-finger transposase consisting of 1033 amino acids (Hellsten et al. 2010). We found the corresponding region to this hAT-10 CDS in intron 3 of dm-W, but not in Ex4 of dm-W (fig. 1A). Therefore, we conclude that the CDS of dm-W Ex4 evolved from a non-CDS of a hAT-10 transposon to now encode the C-terminal region of the DM-W protein.
Fig. 1.
hAT-10 DNA transposon-derived fragments on the dm-W intron 3 and exon 4 (Ex4). (A) Distributions of transposon-derived DNA fragments in and around Ex4 of Xenopus laevis (Xl) dm-W and comparison to X. tropicalis (Xt) hAT-10 DNA transposons. Noncoding and coding portions of exons of dm-W are indicated with white and gray boxes, respectively (upper). Colored boxes in X. laevis dm-W represent TE distribution by CENSOR as indicated (middle). A box in X. tropicalis hAT-10 DNA transposon shows homologous regions to the dm-W hAT-10-derived ones (lower). (B) Schematic comparison among the dm-W Ex4 and its corresponding regions from Xt hAT-10 to Xl hAT-10-derived sequences. The hAT-10-derived sequences were classified into three regions (named A, B, and C) based on sequence similarity. Region A (133 bp of the third intron and 16 bp of Ex4 in dm-W) and C (108 bp of Ex4 and 207 bp downstream) shared high sequence identity among them, whereas region B (219 bp of the Ex4) has lower sequence identity between Xt hAT-10 and each Xl hAT-10 transposon-derived sequence. Nucleotide sequence identity (%) with dm-W is shown on each region. Noncoding and coding exons are represented by white and gray boxes, respectively. The green triangle shows a terminal inverted repeat (TIR). (C) A partial comparison of nucleotide sequences within and adjacent to the Ex4-CDS among the five hAT-10 transposon-derived sequences from X. laevis in (B). Splicing acceptor site AG, a deletion, and a sequence for a stop codon are shaded.
To further clarify how the transposon-derived non-CDS evolved into CDS of Ex4, we performed sequence alignment among the sequences around the Ex4-coding region and its corresponding sequences from the four Ex4-like sequences derived from hAT-10 using MUSCLE (fig. 1C and supplementary fig. S6A and B, Supplementary material online). The comparison identified two point mutations consisting of a deletion and substitution that eliminated a frame-shift mutation and formed a stop codon in the ancestral hAT-10-derived sequences, thereby resulting in the generation of a 71 amino acids CDS in the C-terminal of dm-W that are encoded by Ex4 (fig. 1C).
The Time of Allotetraploidization in Subgenus Xenopus Coincides with a High Rate of Replicative Transposition of dm-W Ex4-like hAT Transposons
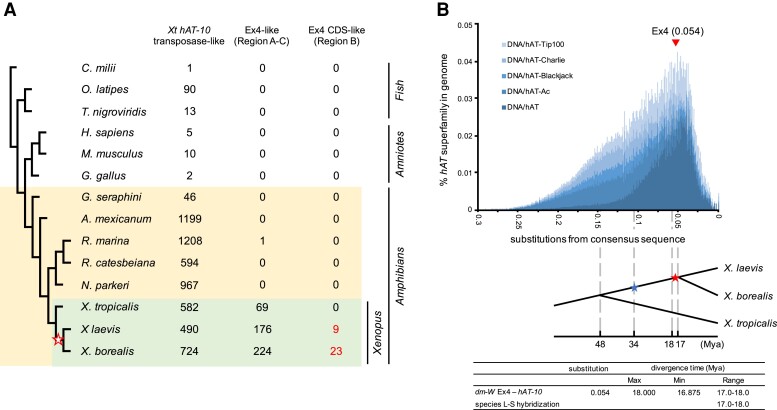
To clarify the timing of divergence of hAT-10 transposons from dm-W Ex4-related sequences, we blasted the hAT-10 sequence in 14 vertebrate genomes, including eight amphibian species (fig. 2A). All the vertebrate genomes examined had at least one copy of a X. tropicalis (Xt) hAT-10 transposase-like sequence. The caecilian and six nonamphibian species contained far fewer copies, including host–transposase fusion genes (Cosby et al. 2021), which might be derived from the hAT superfamily. However, all the amphibians except for the caecilian have hundreds of copies in their genomes. This suggests that hAT-10 transposons expanded in urodeles and anurans and/or their most recent common ancestor after divergence from caecilians. hAT-10-derived Ex4-like sequences corresponding to regions A–C were found only in anuran amphibians and appeared to actively expand only in the Xenopus genus (fig. 2A). Notably, 9 and 23 copies of the Ex4-CDS-like sequence corresponding to region B were detected in the two allotetraploid species X. laevis and X. borealis, respectively, whereas no copies were found in the diploid species X. tropicalis. As well, ∼2–3 times as many sequences similar to regions A and C were identified in X. laevis and X. borealis compared with X. tropicalis (fig. 2A). Moreover, in the other allotetraploid X. borealis, we found six sequences of the hAT-10-derived transposon fragments sharing some sequence similarities with the three regions, A, B, and C of dm-W (80.1–89.7%, 71.4–76.3%, and 74.5–84.0%, respectively) (supplementary table S2 and fig. S7, Supplementary Material online), suggesting the existence of the ancestral hAT-10 transposon with the Ex4-like sequence in the most common ancestor of X. laevis and X. borealis. Overall, this indicates that the rate of replicative transposition of this class of hAT transposons increased in African clawed frogs, and particularly so after divergence of the most common ancestor of X. laevis + X. borealis from the ancestor of X. tropicalis.
Fig. 2.
Molecular evolution of hAT superfamily, hAT-10 family, and hAT-10-derived Ex4 in Xenopus frogs. (A) Copy numbers of Xt hAT-10 transposase (Tp)-like sequences, hAT-10-derived Ex4-like sequences (A–C in fig. 1B), hAT-10-derived Ex4-CDS-like sequences [B in A] in 14 vertebrate species, including anuran amphibians (Xenopus laevis, X. borealis, X. tropicalis, Nanorana parkeri, Rana catesbeiana, and Rhinella marina), an urodele amphibian (Ambystoma mexicanum), a caecilian amphibian (Geotrypetes seraphini), a bird (Gallus gallus), mammals (Homo sapiens and Mus musculus), teleost fish (Oryzias latipes and Tetraodon nigroviridis), and a cartilaginous fish (Callorhinchus milii). GenBank assembly accession of 11 species except for the three Xenopus species used is shown in supplementary fig. S3, Supplementary Material online. (B) A repeat landscape of hAT superfamily consisting of the five families, as inferred in the X. laevis genome using RepeatMasker (upper). The y-axis and x-axis show percentages of each family on the genome and Jukes-Cantor-corrected divergence, respectively. The estimated divergence time of the hAT-10-derived regions on and around dm-W Ex4 from the hAT-10 consensus sequence is shown by a triangle on the landscape. After the ancestors of X. laevis and X. tropicalis diverged at 48 Ma, speciation and hybridization of the predicted L and S species occurred at 34 and 17–18 Ma, respectively (Session et al. 2016).
To understand evolutionary trends of hAT transposon superfamily in Xenopus, we constructed repeat landscape of the hAT superfamily consisting of hAT-Tp100, hAT-Charlie, hAT-Blackjack, hAT-Ac, and hAT, using X. laevis genome database and RepeatMasker (fig. 2B). The activation peak of the hAT superfamily was observed around 0.05 substitutions per site. The nucleotide substitution rate in Xenopus was estimated to be 3.0 × 10−9 to 3.2 × 10−9 substitutions per year (Session et al. 2016). Using this rate, we estimated the divergence time of the hAT-10-derived sequence in dm-W by comparing the sequence recognized as hAT-10 by CENSOR and its corresponding one from a consensus X. laevis hAT-10. We calculated a median divergence to consensus of 0.054, which corresponds to an estimated peak transposon activity as 17.0–18.0 Ma. Interestingly, we recently found the activation peak of total DNA transposons in the two allotetraploid Xenopus species, X. laevis and X. borealis to be about 17.0 Ma, which is around the hybridization (Suda et al. 2022). These results suggest that the dm-W-ancestral hAT-10 transposon was inserted into the ancestral chromosome 2L roughly around the time of allotetraploidization during the active peak period of DNA transposons including hAT superfamily, although we cannot definitively determine whether this was before or after this event.
Emergence and Molecular Evolution of the Chimeric Gene dm-W Before Diversification of Most Species in Subgenus Xenopus
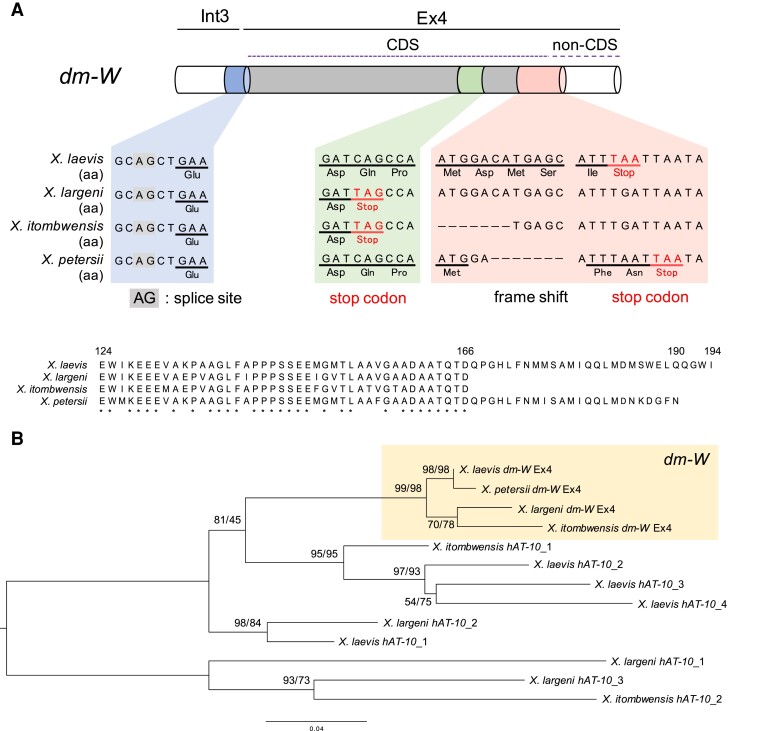
To further understand the timing of emergence of Ex4, we tried to identify dm-W Ex4-CDSs from three allotetraploid species, X. borealis, X. largeni, and X. petersii, and one allooctoploid species, X. itombwensis. We performed PCR using genomic DNA and primer pairs designed by the conserved sequences of X. laevis dm-W and the four hAT-10-like sequences (supplementary fig. S6, Supplementary material online). Ex4-like sequences were obtained from X. largeni, X. petersii, and X. itombwensis, but not from X. borealis. Multiple sequence alignment and phylogenetic analysis revealed that the sequences obtained from the three species contained the Ex4-CDSs of dm-W (fig. 3A). The Ex4-CDSs in the three species were highly conserved with that of X. laevis and had two of the same mutations consisting of one nucleotide deletion and substitution as those of X. laevis (figs. 1C and 3A), suggesting that dm-W Ex4 in these species are homologous. Interestingly, the Ex4-CDSs from X. itombwensis and X. largeni or X. petersii encoded 43 or 67 amino acids, whereas X. laevis contained 71 amino acids (fig. 3B). These differences are caused by a single nucleotide mutation that generated a UAG stop codon in the upstream region of an ancestor of X. itombwensis and X. largeni and a frameshift mutation caused by the deletion of 16 nucleotides in the ancestor of X. petersii (fig. 3A). Exons 2 and 3 of dm-W were present in the ancestor of all species in subgenus Xenopus (Cauret et al. 2020). These results demonstrate that dm-W Ex4 was also present at least as early as the most recent common ancestor of X. largeni, X. itombwensis, X. laevis, and X. petersii—this ancestor diversified after divergence from the muelleri species group (X. clivii, X. borealis, X. muelleri, X. fischbergi, and X. fraseri) (Evans et al. 2015, 2019).
Fig. 3.
The nucleotide and deduced amino acid sequence alignments of the Ex4-CDS of dm-W among four allopolyploid Xenopus species. (A) A multiple nucleotide sequence alignment within and adjacent to Ex4-CDS of X. laevis, X. largeni (MCZ-A cryogenic 333), X. itombwensis (MCZ-A A136197), and X. petersii. Red font highlights the positions of TAG and TAA stop codons. (B) A multiple alignment of the Ex4-encoded amino acid sequences from the four DNA sequences in (A) (upper) and the ML phylogenetic tree of the Ex4 sequences and/or their corresponding hAT-10-derived sequences from X. laevis, X. largeni, X. itombwensis, and X. petersii (lower). Numbers at each node denote the ML/NJ bootstrap percentages of 1000 replicates.
The 71 Amino Acid Sequence Encoded by the hAT-10 Transposon-derived Ex4 Increases the DNA-binding Affinity of DM-W
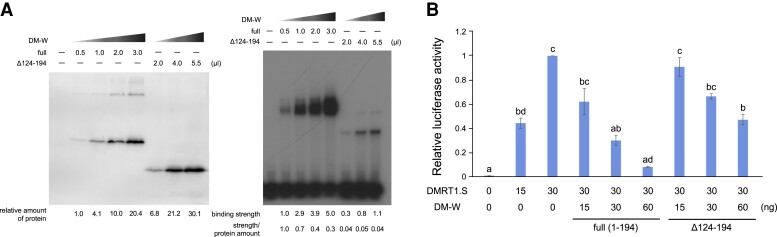
As detailed above, the hAT-10-derived Ex4 encodes the C-terminal region of DM-W, which consists of 71 amino acid residues in X. laevis. To clarify the function of this portion of the DM-W peptide, we examined the effect of the region on the DNA-binding ability of the DM domain encoded by Ex2 and Ex3. An in vitro DNA–protein binding assay by Electrophoretic Mobility Shift Assay (EMSA) using 30 bp of DM-W/DMRT1-binding DNA sequence (Yoshimoto et al. 2010) was performed for full-length DM-W and its C-terminal truncated protein DM-W (Δ124–194) carrying no Ex4-encoding 71 amino acid sequence (fig. 4A, left). The amount of each protein, which was produced by in vitro transcription/translation, was quantified using western blot analysis (fig. 4A, right). The assay showed that the full-length DM-W had a stronger binding ability to its binding sequence than the truncated DM-W peptide (Δ124–194) (fig. 4A). These results indicate that the Ex4-encoding 71 amino acid sequence increases the DNA-binding affinity of DM-W to a DM-W/DMRT1-binding DNA sequence, which is consistent with previous assays (Yoshimoto et al. 2010).
Fig. 4.
Effects of the transposon-derived Ex4 coding region in in vitro DNA binding and transrepression activities of DM-W. (A) In vitro DNA binding of DM-W (full length) and its C-terminal truncated protein, DM-W (Δ124–194), to the DMRT1-binding sequence. Flag-DM-W(full) and Flag-DM-W(Δ124–194) were produced by in vitro transcription–translation system and analyzed by Western blot analysis with an anti-FLAG antibody followed by an HRP-conjugated antimouse antibody (left). The relative intensity values were shown below. EMSA was performed using in vitro synthesized Flag-DM-W(full) (0.5, 1.0, 2.0, or 3.0 μl) or Flag-DM-W(Δ124–194) (2.0, 4.0, or 5.5 μl) and 32P-labeled double-stranded oligonucleotides containing the DMRT1-binding sequence. The relative intensity values to the protein amounts, binding strength, and ratio of strength/protein amount were shown below. (B) The luciferase reporter assay for the DM-W transrepression activity on DMRT1-driven transcription. The DMRT1-driven luciferase reporter and an expression plasmid for DMRT1.S and DM-W(full) or DM-W(Δ124–194) were transfected into HEK293T cells and posttransfection (24 h) luciferase activity was measured. Letters (a–d) indicate significant differences based on a one-way ANOVA, followed by the Tukey–Kramer HSD test (P < 0.01).
We next examined the effects of full-length and C-terminal truncated DM-W proteins on the transcriptional activity of DMRT1 using a luciferase reporter plasmid carrying four copies of the DM-W/DMRT1-binding cis-element, which were used for the in vitro DNA–protein binding assay. HEK 293 T-cells were transiently cotransfected with the reporter plasmid and expression plasmids for DMRT1.S, DM-W (full length), and/or truncated DM-W (Δ124–194). The reporter assay showed that DMRT1-driven transactivation activity was more repressed by DM-W (full length) than the truncated DM-W (Δ124–194) (fig. 4B). In other words, the hAT-10 transposon-derived C-terminal region increases the transrepression activity of DMRT1.S by DM-W, compared with a truncated version of DM-W that lacks the hAT-10 transposon-derived C-terminal region. Collectively, these findings indicate that the hAT-10 transposon-derived Ex4 may enhance the DNA-binding ability of DM-W to DM-W/DMRT1-binding cis-elements.
Discussion
Only about ten vertebrate SDGs have been identified so far; these encode various types of proteins, including transcription factors, a sex-steroid-synthesizing enzyme, and TGF-β signaling-related ligands and receptors (Mawaribuchi et al. 2012; Nagahama et al. 2021). Most SDGs were generated independently through duplication or allelic mutations in ancestral genes that functioned in gonadal differentiation. As discussed above, Xenopus dm-W and medaka fish dmy independently evolved from a broadly conserved, male-related transcription factor dmrt1 into a male repressor and male inducer, respectively (Yoshimoto and Ito 2011). Parallel amino acid substitutions enhanced DNA-binding activities in ancestral genes of both dm-W and dmy, which suggested a common mechanism for the establishment of these SDGs (Ogita et al. 2020). In this study, we demonstrate that a non-CDS derived from a hAT-10 DNA transposon evolved into the Ex4-CDS in the C-terminal region of DM-W, and that this addition strengthened the DNA-binding activity of the DM domain encoded by Ex2 and Ex3 (figs. 1, 2, and 4). These findings suggest that the molecular evolution from the non-CDS to the CDS was a fundamental event in the emergence of dm-W as a male repressor SDG.
dm-W is a chimeric gene (Yoshimoto et al. 2008), which is an unusual mode of origin among known SDGs. Although most therian orthologs of Sry have a single exon, mouse Sry encode two isoforms, single exon-derived SRY-S, and two exon-derived SRY-T (Miyawaki et al. 2020). The Ex2 gene of Sry-T encodes 15 amino acid sequence derived from a retrotransposon-derived sequence (a long interspersed nuclear element L3), which includes a degron that regulates protein degradation rates (Koren et al. 2018; Miyawaki et al. 2020). The participation of the L3 retrotransposon-derived sequence is hypothesized to maintain Sry function during rodent diversification (Miyawaki et al. 2020). Similarly, the hAT-10 transposon-derived sequence of dm-W appears to influence the DNA-binding affinity of this protein, and is thus also of functional significance. However, there is a key difference between these SDGs in terms of their maintenance and emergence: the degron-CDS of rodent Sry-T might be responsible for the continued functioning of its sex determination’s role in some rodents even though this sequence is not required in other therian mammals. In contrast, the hAT-10 transposon-derived sequence may have been essential for the ancestral neofunctionalization of dm-W into an SDG.
We previously proposed an evolutionary model for the relationship between SDGs and sex chromosomes, in which undifferentiated (homomorphic) sex chromosomes more frequently undergo sex chromosome turnover via the origin of new SDGs than do differentiated (heteromorphic) sex chromosomes (Mawaribuchi et al. 2012). Xenopus laevis has morphologically homomorphic sex chromosomes (Tymowska 1991). Recently, we demonstrated from sequence analysis of the genome that W and Z sex chromosomes, which are gametologous versions of chromosomes 2L, have ∼280 kb W-specific sequences containing dm-W and an ∼80 kb Z-specific sequence (Session et al. 2016; Mawaribuchi, Musashijima, et al. 2017). We also found that the W- and Z-specific regions have three (including dm-W) and one gametolog-specific genes, respectively, although apart from dm-W we do not know their function (Mawaribuchi, Musashijima, et al. 2017). In this study, we performed a detailed analysis of the molecular evolution of dm-W (fig. 2), indicating that dm-W arose around the time of allotetraploidization in subgenus Xenopus. It is possible that dm-W evolved as a new SDG in a newly evolved allotetraploid ancestor with an unstable sex-determining system after hybridization between two ancestral diploid species, both of which had homomorphic sex chromosomes. After the establishment of dm-W as SDG, homologous recombination suppression may have led to the accumulation of mutations and TEs.
Although most TEs are often recognized as “junk DNA” in host organisms, some TEs have important functions as long and small noncoding RNAs, and influence gene expression (Ariel and Manavella 2021). TEs can also participate in the formation of new protein-coding genes. For example, retrotransposon-derived elements play a role in signal transduction and mammalian evolution (Shimamura et al. 1998; Kaneko-Ishino and Ishino 2015). A recent comparative genomics approach identified ∼100 fusion genes with DNA transposon-derived transposase-CDSs, many of which might have evolved under functional constraints (Cosby et al. 2021). However, to our knowledge, no examples are known where DNA transposon-derived non-CDSs evolved into CDSs. In dm-W, it is possible that the conversion of a noncoding to coding region consisting of 40–70 amino acids was favored by natural selection because this sequence increased the DNA-binding affinity of DM-W.
Based on these findings, we propose further investigation to elucidate the sequence of mutation events preceding the emergence and molecular evolution of SDG dm-W (fig. 5). The mixture of two subgenomes L and S by interspecific hybridization and allotetraploidization between two closely related Xenopus species about 17–18 Ma (Session et al. 2016) may have destabilized the existing two sex-determination systems that were present in the ancestral frog species, which also may have had homomorphic sex chromosomes. In some populations, the sex ratio could have been biased, which could have favored the establishment of dm-W as the sole SDG. In the case of dm-W, the most parsimonious scenario is that at least three independent insertion events into the ancestral chromosome 2L established dm-W as a female SDG. These three events lead to the generations of noncoding Ex1, Ex2-Ex3, and Ex4 of dm-W (this study). A less parsimonious possibility is that some or all of these three components were assembled in the diploid ancestor of subgenome S (or in subgenome S after allotetraploidization) and then translocated as a unit to subgenome L, with additional components being added there if the transferred gene was partial. The noncoding Ex1 were generated for promoter/enhancer for dm-W expression in gonadal somatic cells during sex determination. Ex1 may have arisen from an insertion DNA containing a repeated sequence, and then the promoter appeared to have evolved de novo. Ex2 and Ex3 evolved from the duplicate of the region covering Ex2, intron 3, and Ex3 of the S subgenome-derived ancestral dmrt1.S, which had been inserted into the ancestral chromosome 2L of the L subgenome probably through TEs (supplementary fig. S1, Supplementary material online) (Bewick et al. 2011; Mawaribuchi, Takahashi, et al. 2017). Ex4 was generated from the hAT-10-derived sequence, which had inserted downstream of the predicted insertion position of the partial duplicate of dmrt1.S. Our analyses were unable to determine which insertion occurred first, or whether they occurred concurrently. However, divergence times of Ex4 (fig. 2) and phylogenetic relationships among species that carry Ex2 and Ex3 (Cauret et al. 2020) suggest both were in place either around the time of allotetraploidization or relatively soon thereafter (fig. 2). Xenopus borealis, which is more diverged from X. laevis than the three other species examined in this study (X. itombwensis, X. largeni, and X. petersii) has independently evolved sex chromosomes (Furman and Evans 2016), and efforts to identify dm-W in this species were unsuccessful (Furman and Evans 2016; Cauret et al. 2020). We also did not detect the Ex4-like sequences in the whole genome sequence of X. borealis or isolate it by PCR. dm-W Ex2 and Ex3 were present in the ancestor of X. clivii, which is more closely related to X. borealis than to X. laevis, which suggests that Ex2, Ex3 and Ex4 of dm-W were lost in an ancestor of X. borealis after divergence from X. clivii (this study; Cauret et al. 2020). This loss may have been associated with the origin of new extant sex chromosomes in X. borealis, occurred independently in an ancestor with the same or different sex chromosomes, or the loss could have occurred after the new sex chromosomes of X. borealis evolved.
Fig. 5.
Proposed model for the emergence of the chimeric gene dm-W as SDG. The two closely related Xenopus species having two distinct genomes named as L and S were hybridized about 17–18 Ma (Session et al. 2016). After the interspecific hybridization, at least three independent insertion events into chromosome 2L led to the establishment of dm-W as a female SDG. The three events lead to the generations of noncoding Ex1 from promoter/enhancer for expression in gonadal somatic cells during sex determination, Ex2–Ex3 from the duplication of the S subgenome-derived ancestral dmrt1.S, and Ex4 from a hAT-10 DNA transposon. A single nucleotide deletion and substitution in the non-CDS of the hAT-10 transposon-derived sequence resulted in the Ex4-CDS, which resulted in a C-terminal region that strengthens the DNA-binding ability of the DM-W protein.
In conclusion, we found that the hAT-10 transposon contributed to the birth of the chimeric SDG dm-W in the ancestor of the allotetraploid Xenopus frogs. This adds to a small number of examples where noncoding portions of TEs become CDS, and is the only known example to our knowledge of this process being a prelude to the de novo origin of an SDG. Recently, a retrotransposon-derived sequence was discovered to influence the sex-determining function of Sry-T as a splicing variant of Sry in the ancestor of mice (Miyawaki et al. 2020). However, this retrotransposon-derived sequence was not involved in the original emergence of Sry in the ancestor of therian mammals. dm-W is distinguished from this example because the hAT-10 transposon-derived sequence was involved in dm-W birth in the ancestor of allotetraploid Xenopus frogs. In this way, this study provides new insights into de novo origin of SDGs.
Materials and Methods
Bioinformatic Analyses
Genomes analyzed in this study were obtained from Xenbase (www.xenbase.org/entry/; X. laevis v9.2 and X. tropicalis v10.0). Comparative analysis of the dmrt1 subfamily was performed using mVISTA (http://genome.lbl.gov/vista) using the LAGAN alignment program (Frazer et al. 2004). Accession numbers of X. laevis dmrt1.L, dmrt1.S, dm-W, and X. tropicalis dmrt1 used in the study are NM_001096500, NM_001085483, NM_001114842, and XM_031890717, respectively.
Phylogenetic analysis was performed using the software MEGAX (www.megasoftware.net/). Nucleotide sequences were aligned using MUSCLE (Edgar 2004) and gaps (insertions/deletions) were removed. Phylogenetic trees were constructed using neighbor-joining (NJ) and maximum likelihood (ML) methods. In NJ and ML analyses, the best-fit model of nucleotide substitution was selected by model selection using a likelihood ratio test. The Tamura three-parameter (T92) + G and Kimura 2-parater (K2) + G was selected for analysis of DM domain sequences (supplementary fig. S3, Supplementary Material online) and hAT-10 sequences (fig. 3), respectively. Phylogenetic support for each node was assessed using nonparametric bootstrapping (Felsenstein 1983) with 1,000 replicates.
TEs were detected using CENSOR software (www.girinst.org/censor/index.php) (Kohany et al. 2006) and RepeatMasker (www.repeatmasker.org/) with default parameters and the Xenopus TE dataset obtained from Repbase (www.girinst.org/repbase/). DNA transposon hAT-10-derived sequences, including Xt hAT-10 transposase-like, Ex4-like, and Ex4-CDS-like sequences (supplementary table S3, Supplementary Material online), were identified using TBLASTN (<1e−6 of E-value), BLASTN (<1e−6 of E-value, >65% sequence identity), and BLASTN (<1e−6 of E-value, >65% sequence identity over >60% of query length), respectively. The values were obtained after removing duplicate fragments of 10 kb (Xt hAT-10 transposase-like), 1 kb (Ex4-like), and 150 bp (Ex4-CDS-like).
The repeat landscapes of the TEs were plotted using RepeatMasker and Python scripts (supplementary files S1–S3, Supplementary material online). Pairwise genetic distances were estimated between all sequences within each TE subfamily using MAFFT (Katoh et al. 2002), and phylogenies were constructed using FastTree (Price et al. 2010) based on the default Jukes-Cantor + CAT model. Consensus sequences of the TE subfamily were obtained from Repbase (Jurka et al. 2005) except for that of Xl hAT-10, which was determined by collecting hAT-10-like sequences among the existing transposons in the X. laevis genome sequence and constructing them using a simple majority rule based on a multiple alignment by BlastViewer. The age of TE families can be roughly estimated from the distances between each sequence and the consensus (Kapitonov and Jurkal 1996). The divergence time between the homologous regions of the most closely related hAT-10 DNA transposon and dm-W Ex4 was estimated using a Jukes-Cantor-corrected substitution rate of 3.0 or 3.2 × 10−9 substitutions per year, which was calculated from synonymous substitution levels between X. tropicalis and X. laevis orthologs, and between X. laevis L and S homeologs, respectively (Session et al. 2016).
For contig assembly and scaffolding of the genome of Xenopus borealis, contigs were assembled using ABySS (http://github.com/bvgsc/abyss) under the conditions of (k = 83, B = 30G, H = 3, kc = 3, v = −v) and female or male whole genome sequence, SRR6357672 or SRR6357673, respectively. The two resulting assemblies were merged, and reference-guided scaffolding was performed with RaGOO (Alonge et al. 2019) -T sr using X. laevis v9.2 genome assembly as described (Suda et al. 2022).
Isolation of dm-W Ex4 from Several Xenopus Species
Genomic DNA was purified from adult livers of three Xenopus species, X. largeni (MCZ-A cryogenic 333), X. itombwensis (MCZ-A A136197), and X. petersii, using the phenol–chloroform extraction method. PCR was performed using KOD FX DNA Polymerase (TOYOBO, Japan). A pair of primers, 5′-AGTTACATTACACCTCATCCTG-3′ and 5′-AGACGAGGAGTGTTATCCCTC-3′, were used for amplification of Ex4. The obtained DNA fragments were inserted into EcoRV site of pBluescript KS (+). DNA sequencing was performed using BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Bioscience, Waltham, USA). Gene bank accession numbers for the Ex4 sequences from X. largeni, X. itombwensis, and X. petersii are LC699248/LC699249, LC699247, and LC699250, respectively.
Construction of an Expression Plasmid for the C-terminal Truncated DM-W
The CDS for dm-W from 1 to 123 amino acids was amplified from cDNA by PCR using pcDNA3-FLAG-DM-W (Yoshimoto et al. 2008) as temperate and a pair of primers, 5′-GCTTATCGATACCGTCGAC-3′ and 5′-CTATGAAGTGGGTGTGCTG-3′. The amplified DNA fragment was inserted into pcDNA3-FLAG vector (Ito et al. 1999) in frame with FLAG-tag, resulting in the construction of pcDNA3-FLAG-DM-W Δ124–194.
Electrophoretic Mobility Shift Assay
The two oligonucleotides, 5′-CCATCGAGCAACAATGTATCAAATCTC-3′ and 5′-GGGGAGATTTGATACATTGTTGCATCGATGG-3′, were annealed and labeled with 32P, using the Klenow fragment. Proteins were produced using the TNT Quick Coupled Transcription/Translation System (Promega), using pcDNA3-FLAG-DMRT1, pcDNA3-FLAG-DM-W (Yoshimoto et al. 2008), and pcDNA3-FLAG-DM-WΔ124–194. The resultant labeled DNA and each protein were mixed in a reaction buffer (10 mM Tris–HCl [pH 8.0], 100 mM KCl, 10% glycerol, 5 mM MgCl2 0.075% Triton X-100, 1 mM dithiothreitol, 1% bovine serum albumin, 1 µg/µl poly (dI/dC), 1 mM spermidine) and incubated on ice for 30 min. The samples were subjected to electrophoresis through a nondenaturing 5% polyacrylamide gel containing Tris/glycine/EDTA (50 mM Tris, 380 mM glycine, 2 mM EDTA) and 2.5% glycerol in Tris/glycine/EDTA at room temperature. The dried gel was autoradiographed with a Fuji super RX film (Fujifilm) at −70°C.
Western Blot Analysis
Immunoblotting of in vitro synthesized proteins was performed using an anti-FLAG antibody, M5 (Sigma), followed by an HRP-conjugated antimouse antibody (Sigma). The reaction was developed by enhanced chemiluminescent staining using SuperSignal West Femto Maximum Sensitivity Substrate (Pierce), and the signal intensity was measured using ImageJ (NCBI, MD).
Luciferase Reporter Assay
HEK293T cells were cultured in DMEM containing 10% fetal calf serum. Twenty-four hours before transfection, cells were plated at 1 × 105 cells per well in a 24-well plate. The cells were transfected with the firefly luciferase reporter plasmid p4xDMRT1-luc (100 ng) (Yoshimoto et al. 2010), effector plasmids, and Renilla luciferase vector pRL-SV40 (20 ng) (Promega) using TransITTM-LT1 (Mirus). Total DNA was maintained at 500 ng per transfection with the pcDNA3-FLAG empty vector. After 24 h, activities from the two luciferases, which have dissimilar enzyme structures and substrate requirements, were measured in a Luminocounter 700 (NITI-ON) using the dual luciferase assay system (Promega). Firefly luciferase activity was normalized to Renilla luciferase activity.
Supplementary Material
Supplementary data are available at Molecular Biology and Evolution online.
Supplementary Material
Acknowledgements
We would like to thank Dr. Breda Zimkus, the Cryogenic Collection, Museum of Comparative Zoology, Harvard University, for providing the Xenopus samples. The Xenopus tropicalis and laevis genome browser (viewer.shigen.info/xenopus/index.php) was in part supported by Hiroshima University Amphibian Research Center through NBRP. This work was partially supported by Grant-in-Aid for Scientific Research, Japan Society for the Promotion of Science (18K06389) to M.I.
Contributor Information
Shun Hayashi, Department of Bioscience, School of Science, Kitasato University, 1-15-1 Kitasato, Minamiku Sagamihara, Kanagawa 252-0373, Japan.
Kosuke Suda, Department of Bioscience, School of Science, Kitasato University, 1-15-1 Kitasato, Minamiku Sagamihara, Kanagawa 252-0373, Japan.
Fuga Fujimura, Department of Bioscience, School of Science, Kitasato University, 1-15-1 Kitasato, Minamiku Sagamihara, Kanagawa 252-0373, Japan.
Makoto Fujikawa, Department of Bioscience, School of Science, Kitasato University, 1-15-1 Kitasato, Minamiku Sagamihara, Kanagawa 252-0373, Japan.
Kei Tamura, Department of Bioscience, School of Science, Kitasato University, 1-15-1 Kitasato, Minamiku Sagamihara, Kanagawa 252-0373, Japan.
Daisuke Tsukamoto, Department of Bioscience, School of Science, Kitasato University, 1-15-1 Kitasato, Minamiku Sagamihara, Kanagawa 252-0373, Japan.
Ben J Evans, Department of Biology, McMaster University, Life Sciences Room 328, 1280 Main Street West, Hamilton, ON, Canada L8S 4K1.
Nobuhiko Takamatsu, Department of Bioscience, School of Science, Kitasato University, 1-15-1 Kitasato, Minamiku Sagamihara, Kanagawa 252-0373, Japan.
Michihiko Ito, Department of Bioscience, School of Science, Kitasato University, 1-15-1 Kitasato, Minamiku Sagamihara, Kanagawa 252-0373, Japan.
References
- Ågren JA, Clark AG. 2018. Selfish genetic elements. PLoS Genet. 14:e1007700. 10.1371/journal.pgen.1007700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonge M, Soyk S, Ramakrishnan S, Wang X, Goodwin S, Sedlazeck FJ, Lippman ZB, Schatz MC. 2019. RaGOO: fast and accurate reference-guided scaffolding of draft genomes. Genome Biol. 20:224. 10.1186/s13059-019-1829-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzohairy AM, Gyulai G, Jansen RK, Bahieldin A. 2013. Transposable elements domesticated and neofunctionalized by eukaryotic genomes. Plasmid 69(1):1–15. 10.1016/j.plasmid.2012.08.001 [DOI] [PubMed] [Google Scholar]
- Anderson SN, Springer NM. 2018. Potential roles for transposable elements in creating imprinted expression. Curr Opin Genet Dev. 49:8–14. 10.1016/j.gde.2018.01.008 [DOI] [PubMed] [Google Scholar]
- Ariel FD, Manavella PA. 2021. When junk DNA turns functional: transposon-derived non-coding RNAs in Plants. J Exp Bot. 72(11):4132–4143. 10.1093/jxb/erab073 [DOI] [PubMed] [Google Scholar]
- Bewick AJ, Anderson DW, Evans BJ. 2011. Evolution of the closely related, sex-related genes DM-W and DMRT1 in African clawed Frogs (Xenopus). Evolution 65(3):698–712. 10.1111/j.1558-5646.2010.01163.x [DOI] [PubMed] [Google Scholar]
- Bourque G, Burns KH, Gehring M, Gorbunova V, Seluanov A, Hammell M, Imbeault M, Izsvák Z, Levin HL, Macfarlan TS, et al. 2018. Ten things you should know about transposable elements. Genome Biol. 19(1):199. 10.1186/s13059-018-1577-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauret CMS, Gansauge MT, Tupper AS, Furman BLS, Knytl M, Song XY, Greenbaum E, Meyer M, Evans BJ. 2020. Developmental systems drift and the drivers of sex chromosome evolution. Mol Biol Evol. 37(3):799–810. 10.1093/molbev/msz268 [DOI] [PubMed] [Google Scholar]
- Cosby RL, Judd J, Zhang R, Zhong A, Garry N, Pritham EJ, Feschotte C. 2021. Recurrent evolution of vertebrate transcription factors by transposase capture. Science 371:6531. 10.1126/science.abc6405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 5:113. 10.1186/1471-2105-5-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BJ. 2008. Genome evolution and speciation genetics of clawed frogs (Xenopus and Silurana). Front Biosci. 13:4687–4706. 10.2741/3033 [DOI] [PubMed] [Google Scholar]
- Evans BJ, Carter TF, Greenbaum E, Gvoždík V, Kelley DB, McLaughlin PJ, Pauwels OS, Portik DM, Stanley EL, Tinsley RC, et al. 2015. Genetics, morphology, advertisement calls, and historical records distinguish six new polyploid species of African clawed frog (Xenopus, Pipidae) from West and Central Africa. PLoS One 10(12):e0142823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BJ, Gansauge MT, Stanley EL, Furman BLS, Cauret CMS, Ofori-Boateng C, Gvoždík V, Streicher JW, Greenbaum E, Tinsley RC, et al. 2019. Xenopus fraseri: Mr. Fraser, where did your frog come from? PLoS One 14(9):e0220892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. 1983. Statistical inference of phylogenies (with discussion). J R Statist Soc A. 146(3):246–272. 10.2307/2981654 [DOI] [Google Scholar]
- Foster JW, Graves JA. 1994. An SRY-related sequence on the marsupial X chromosome: implications for the evolution of the mammalian testis-determining gene. Proc Natl Acad Sci U S A. 91(5):1927–1931. 10.1073/pnas.91.5.1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. 2004. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 32:W273–W279. 10.1093/nar/gkh458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugmann SD. 2010. The origins of the rag genes—from transposition to V(D)J recombination. Semin Immunol. 22(1):10–16. 10.1016/j.smim.2009.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujitani K, Otomo A, Wada M, Takamatsu N, Ito M. 2016. Sexually dimorphic expression of Dmrt1 and γH2AX in germ stem cells during gonadal development in Xenopus Laevis. FEBS Open Bio. 6(4):276–284. 10.1002/2211-5463.12035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman BLS, Evans BJ. 2016. Sequential turnovers of sex chromosomes in African clawed frogs (Xenopus) suggest some genomic regions are good at sex determination. G3 (Bethesda) 6(11):3625–3633. 10.1534/g3.116.033423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C, Ye J, Weber C, Sun W, Zhang H, Zhou Y, Cai C, Qian G, Capel B. 2018. The histone demethylase KDM6B regulates temperature-dependent sex determination in a turtle species. Science 360(6389):645–648. 10.1126/science.aap8328 [DOI] [PubMed] [Google Scholar]
- Hellsten U, Harland RM, Gilchrist MJ, Hendrix D, Jurka J, Kapitonov V, Ovcharenko I, Putnam NH, Shu S, Taher L, et al. 2010. The genome of the western clawed frog Xenopus Tropicalis. Science 328(5978):633–636. 10.1126/science.1183670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpin A, Schartl M, Depincé A, Guiguen Y, Bobe J, Hua-Van A, Hayman ES, Octavera A, Yoshizaki G, Nichols KM, et al. 2021. Allelic diversification after transposable element exaptation promoted GSDF as the master sex determining gene of sablefish. Genome Res. 31(8):1366–1380. 10.1101/gr.274266.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Mawaribuchi S. 2013. Molecular evolution of genes involved in vertebrate sex determination. In: eLS. Chichester: John Wiley & Sons, Ltd. p. 1–7. doi: 10.1002/9780470015902.a0024948. [DOI] [Google Scholar]
- Ito M, Yoshioka K, Akechi M, Yamashita S, Takamatsu N, Sugiyama K, Hibi M, Nakabeppu Y, Shiba T, Yamamoto KI. 1999. JSAP1, a novel Jun N-terminal protein kinase (JNK)-binding protein that functions as a scaffold factor in the JNK signaling pathway. Mol Cell Biol. 19(11):7539–7548. 10.1128/MCB.19.11.7539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurka J, Kapitonov VV, Pavlicek A, Klonowski P, Kohany O, Walichiewicz J. 2005. Repbase update, a database of eukaryotic repetitive elements. Cytogenet Genome Res. 110(1–4):462–467. 10.1159/000084979 [DOI] [PubMed] [Google Scholar]
- Kaneko-Ishino T, Ishino F. 2015. Mammalian-specific genomic functions: newly acquired traits generated by genomic imprinting and LTR retrotransposon-derived genes in mammals. Proc Jpn Acad Ser B Phys Biol Sci. 91(10):511–538. 10.2183/pjab.91.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitonov V, Jurkal J. 1996. The age of alu subfamilies. J Mol Evol. 42(1):59–65. 10.1007/BF00163212 [DOI] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30(14):3059–3066. 10.1093/nar/gkf436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohany O, Gentles AJ, Hankus L, Jurka J. 2006. Annotation, submission and screening of repetitive elements in Repbase: RepbaseSubmitter and Censor. BMC Bioinform. 7:474. 10.1186/1471-2105-7-474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren I, Timms RT, Kula T, Xu Q, Li MZ, Elledge SJ. 2018. The eukaryotic proteome is shaped by E3 ubiquitin ligases targeting c-terminal degrons. Cell 173(7):1622–1635.e14. 10.1016/j.cell.2018.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama H, Yamada M, Kamei Y, Fujiwara-Ishikawa T, Todo T, Nagahama Y, Matsuda M. 2012. Dmrt1 mutation causes a male-to-female sex reversal after the sex determination by Dmy in the medaka. Chromosome Res. 20(1):163–176. 10.1007/s10577-011-9264-x [DOI] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Griswold MD, Yoshida S, Bardwell VJ, Zarkower D. 2010. The mammalian Doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev Cell. 19(4):612–624. 10.1016/j.devcel.2010.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. 2011. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 476(7358):101–104. 10.1038/nature10239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey CE, Shibata N, Asakawa S, Shimizu N, et al. 2002. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 417(6888):559–563. 10.1038/nature751 [DOI] [PubMed] [Google Scholar]
- Mawaribuchi S, Ito Y, Ito M. 2019. Independent evolution for sex determination and differentiation in the DMRT1 family in animals. Biol Open. 8:8. 10.1242/bio.041962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawaribuchi S, Musashijima M, Wada M, Izutsu Y, Kurakata E, Park MK, Takamatsu N, Ito M. 2017. Molecular evolution of two distinct dmrt1 promoters for germ and somatic cells in vertebrate gonads. Mol Biol Evol. 34(3):724–733. [DOI] [PubMed] [Google Scholar]
- Mawaribuchi S, Takahashi S, Wada M, Uno Y, Matsuda Y, Kondo M, Fukui A, Takamatsu N, Taira M, Ito M. 2017. Sex chromosome differentiation and the W- and Z-specific loci in Xenopus laevis. Dev Biol. 426(2):393–400. 10.1016/j.ydbio.2016.06.015 [DOI] [PubMed] [Google Scholar]
- Mawaribuchi S, Yoshimoto S, Ohashi S, Takamatsu N, Ito M. 2012. Molecular evolution of vertebrate sex-determining genes. Chromosome Res. 20(1):139–151. 10.1007/s10577-011-9265-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki S, Kuroki S, Maeda R, Okashita N, Koopman P, Tachibana M. 2020. The mouse Sry locus harbors a cryptic exon that is essential for male sex determination. Science 370(6512):121–124. 10.1126/science.abb6430 [DOI] [PubMed] [Google Scholar]
- Murphy MW, Zarkower D, Bardwell VJ. 2007. Vertebrate DM domain proteins bind similar DNA sequences and can heterodimerize on DNA. BMC Mol Biol. 8:58. 10.1186/1471-2199-8-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama Y, Chakraborty T, Paul-Prasanth B, Ohta K, Nakamura M. 2021. Sex determination, gonadal sex differentiation, and plasticity in vertebrate species. Physiol Rev. 101(3):1237–1308. 10.1152/physrev.00044.2019 [DOI] [PubMed] [Google Scholar]
- Nanda I, Kondo M, Hornung U, Asakawa S, Winkler C, Shimizu A, Shan Z, Haaf T, Shimizu N, Shima A, et al. 2002. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias Latipes. Proc Natl Acad Sci U S A. 99(18):11778–11783. 10.1073/pnas.182314699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogita Y, Mawaribuchi S, Nakasako K, Tamura K, Matsuda M, Katsumura T, Oota H, Watanabe G, Yoneda S, Takamatsu N, et al. 2020. Parallel evolution of two dmrt1-derived genes, dmy and dm-W, for vertebrate sex determination. iScience 23(1):100757. 10.1016/j.isci.2019.100757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q, Anderson J, Bertho S, Herpin A, Wilson C, Postlethwait JH, Schartl M, Guiguen Y. 2016. Vertebrate sex-determining genes play musical chairs. C R Biol. 339(7–8):258–262. 10.1016/j.crvi.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price Morgan N, Dehal Paramvir S, Arkin Adam P, Poon Art FY. 2010. FastTree 2 – approximately maximum-likelihood trees for large alignments.PLoS ONE 5(3):e9490. 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Session AM, Uno Y, Kwon T, Chapman JA, Toyoda A, Takahashi S, Fukui A, Hikosaka A, Suzuki A, Kondo M, et al. 2016. Genome evolution in the allotetraploid frog Xenopus laevis. Nature 538(7625):336–343. 10.1038/nature19840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura M, Nikaido M, Ohshima K, Okada N. 1998. A SINE that acquired a role in signal transduction during evolution. Mol Biol Evol. 15(7):923–925. 10.1093/oxfordjournals.molbev.a025997 [DOI] [PubMed] [Google Scholar]
- Smith CA, Roeszler KN, Ohnesorg T, Cummins DM, Farlie PG, Doran TJ, Sinclair AH. 2009. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 461(7261):267–271. 10.1038/nature08298 [DOI] [PubMed] [Google Scholar]
- Suda K, Hayashi SR, Tamura K, Takamatsu N, Ito M. 2022. Activation of DNA transposons and evolution of piRNA genes through interspecific hybridization in Xenopus Frogs. Front Genet. 13:766424. 10.3389/fgene.2022.766424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymowska J. 1991. Polyploidy and cytogenetic variation in frogs of the genus Xenopus. In: Green DM and Sessions SK, editors. Amphibian cytogenetics and evolution. San Diego: Academic Press. p. 259–297. [Google Scholar]
- Wang L, Sun F, Wan ZY, Yang Z, Tay YX, Lee M, Ye B, Wen Y, Meng Z, Fan B, et al. 2022. Transposon-induced epigenetic silencing in The X Chromosome as a novel form of Dmrt1 expression regulation during sex determination in the fighting fish. BMC Biol. 20(1):5. 10.1186/s12915-021-01205-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissler L, Gadau J, Simola DF, Helmkampf M, Bornberg-Bauer E. 2013. Mechanisms and dynamics of orphan gene emergence in insect genomes. Genome Biol Evol. 5(2):439–455. 10.1093/gbe/evt009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto S, Ikeda N, Izutsu Y, Shiba T, Takamatsu N, Ito M. 2010. Opposite roles of DMRT1 and its W-linked paralogue, DM-W, in sexual dimorphism Of Xenopus Laevis: implications of a ZZ/ZW-type sex-determining system. Development 137(15):2519–2526. 10.1242/dev.048751 [DOI] [PubMed] [Google Scholar]
- Yoshimoto S, Ito M. 2011. A ZZ/ZW-type sex determination in Xenopus laevis. FEBS J. 278(7):1020–1026. 10.1111/j.1742-4658.2011.08031.x [DOI] [PubMed] [Google Scholar]
- Yoshimoto S, Okada E, Umemoto H, Tamura K, Uno Y, Nishida-Umehara C, Matsuda Y, Takamatsu N, Shiba T, Ito M. 2008. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus Laevis. Proc Natl Acad Sci U S A. 105(7):2469–2474. 10.1073/pnas.0712244105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkower D. 2013. DMRT genes in vertebrate gametogenesis. Curr Top Dev Biol. 102:327–356. 10.1016/B978-0-12-416024-8.00012-X [DOI] [PubMed] [Google Scholar]
- Zhang W, Gao Y, Long M, Shen B. 2019. Origination and evolution of orphan genes and de novo genes in the genome of Caenorhabditis elegans. Sci China Life Sci. 62(4):579–593. 10.1007/s11427-019-9482-0 [DOI] [PubMed] [Google Scholar]
- Zhao L, Svingen T, Ng ET, Koopman P. 2015. Female-to-male sex reversal in mice caused by transgenic overexpression of Dmrt1. Development 142(6):1083–1088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.