Abstract
Background
Nitrite stimulates insulin secretion from pancreatic β-cells; however, the underlying mechanisms have not been completely addressed. The aim of this study is to determine effect of nitrite on gene expression of SNARE proteins involved in insulin secretion from isolated pancreatic islets in Type 2 diabetic Wistar rats.
Methods
Three groups of rats were studied (n = 10/group): Control, diabetes, and diabetes + nitrite, which treated with sodium nitrite (50 mg/L) for 8 weeks. Type 2 diabetes was induced using a low-dose of streptozotocin (25 mg/kg) combined with high-fat diet. At the end of the study, pancreatic islets were isolated and mRNA expressions of interested genes were measured; in addition, protein expression of proinsulin and C-peptide in pancreatic tissue was assessed using immunofluorescence staining.
Results
Compared with controls, in the isolated pancreatic islets of Type 2 diabetic rats, mRNA expression of glucokinase (59%), syntaxin1A (49%), SNAP25 (70%), Munc18b (48%), insulin1 (56%), and insulin2 (52%) as well as protein expression of proinsulin and C-peptide were lower. In diabetic rats, nitrite administration significantly increased gene expression of glucokinase, synaptotagmin III, syntaxin1A, SNAP25, Munc18b, and insulin genes as well as increased protein expression of proinsulin and C-peptide.
Conclusion
Stimulatory effect of nitrite on insulin secretion in Type 2 diabetic rats is at least in part due to increased gene expression of molecules involved in glucose sensing (glucokinase), calcium sensing (synaptotagmin III), and exocytosis of insulin vesicles (syntaxin1A, SNAP25, and Munc18b) as well as increased expression of insulin genes.
Keywords: Nitrite, Type 2 diabetes, Insulin secretion, Gene expression, Rat
At a glance of commentary
Scientific background on the subject
Type 2 diabetes (T2D) is associated with decreased nitric oxide (NO) availability. Animal studies have shown that nitrite has beneficial metabolic effects in T2D by decreasing insulin resistance and increasing insulin secretion. However, mechanisms by which nitrite increases insulin secretion have not been fully understood.
What this study adds to the field
This study shows that nitrite stimulates insulin secretion in rats with T2D by increasing gene expression of molecules involved in glucose sensing (glucokinase), calcium sensing (synaptotagmin III), and exocytosis of insulin vesicles (syntaxin1A, SNAP25, and Munc18b) in pancreatic islets.
Diabetes, the largest epidemic in human history [1], is the ninth cause of death [2] with one death every eight seconds in 2019 [3]. Despite available treatments, Type 2 diabetes is poorly controlled in many afflicted subjects, an issue that warrants development of new therapeutic approaches. Type 2 diabetes is associated with decreased nitric oxide (NO) bioavailability [4] and impaired NO homeostasis contributes in the development of Type 2 diabetes [5]. It has been proposed that new strategies that increase NO bioavailability potentially have therapeutic application in Type 2 diabetes [6]. Favorable metabolic effects of nitrite, a NO releasing agent, has been well documented in animal studies and recently reviewed by Kapil et al. who conclude that nitrite/nitrate supplementation is associated with improved glucose tolerance, suggesting nitrite/nitrate-based therapeutics to be added to the management of Type 2 diabetes [7].
Mechanisms underlying favorable metabolic effects of nitrite/nitrate have been mostly investigated at the level of insulin action and include browning of white adipose tissue [8] and increased translocation of glucose transporter 4 (GLUT4) from cytoplasm to plasma membrane in the skeletal muscle [9,10]. Few studies have reported stimulatory effects of nitrite on insulin secretion from pancreatic β-cells [11,12] indicating that nitrite increases insulin secretion by increasing pancreatic islet blood flow [11] or insulin content of pancreatic islets [12].
Decreased expression of proteins involved in exocytosis of insulin in islets of Type 2 diabetic GK rats has been previously reported [13]. Regarding proteins involved in insulin secretion, glucose enters β-cell via GLUT2, which is the predominant carrier in rodent β-cells [14] and may have a role in glucose sensing [15]; glucokinase phosphorylates glucose to glucpse-6-phosphate and acts as glucose sensor [14]. Calcium ion is essential for exocytosis and synaptotagmins act as calcium sensor [16]. Synaptosome-associated protein of 25 kDa (SNAP25) and syntaxin-1A are soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins in plasma membrane involved in vesicle exocytosis [17]. Mammalian homologue of the unc (uncoordinated mutant)-18 gene (Munc18) or syntaxin-binding protein (STXBP) helps bridging between membrane of insulin-containing vesicles and plasma membrane of β-cells [17]. Although favorable metabolic effects of nitrite in Type 2 diabetes have been reported, effect of nitrite on insulin exocytosis from pancreatic β-cells has not been investigated; therefore, the aim of this study is to determine effect of nitrite administration on gene expression of GLUT2, glucokinase, synaptotagmin III, SNAP25, syntaxin1A, Munc18b (also called STXBP2 or Munc18-2), and insulin in isolated pancreatic islets as well as protein expression of proinsulin and C-peptide in pancreatic tissue of Type 2 diabetic male Wistar rats.
Materials and methods
Animals and induction of Type 2 diabetes
Male Wistar rats (weight range: 190–210 g, n = 30) were kept in standard conditions. Type 2 diabetes was induced using a low-dose of streptozotocin combined with high-fat diet (HFD) as described previously [18]. In brief, after two-week HFD feeding, streptozotocin (25 mg/kg) was intraperitoneally injected and one week later, animals with fasting serum glucose ≥ 150 mg/dL were considered to be diabetic; diabetic rats were on HFD throughout the study. All experimental procedures were approved by the Ethics committee of the Research Institute for Endocrine Sciences of Shahid Beheshti University of Medical Sciences (9ECRIES 94/02/15 Approval Date: 2015-05-05 and IR.SBMU.ENDOCRINE.REC.1399.107; Approval Date: 2020-10-20).
Experimental design
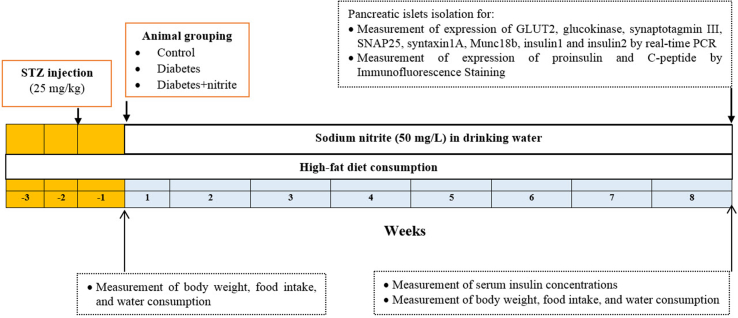
The experimental design is presented in Fig. 1. In this study, three groups of rats were studied (n = 10/group): Control, diabetes, and diabetes + nitrite. Rats in the control and diabetic groups received tape water, whereas in the diabetes + nitrite group, sodium nitrite (50 mg/L) was added to the drinking water of animals for 8 weeks. Body weight was measured weekly from the first day of nitrite administration (week 0) until the end of week 8, using a A&D Scale, EK-300i, Japan; sensitivity 0.01 g. Water consumption (mL/day), and food intake (g/day) were measured weekly and calorie intake was calculated (kcal/day). At the end of the study, pancreatic islets were isolated using the method of Lacy & Kostianovsky [19] as described elsewhere [12] and mRNA levels of GLUT2, glucokinase, synaptotagmin III, SNAP25, syntaxin1A, Munc18b and insulin genes were measured using real-time PCR. Rat β-cells have two different non-allelic insulin genes named insulin1 and insulin2 that are localized in chromosome 1 [20]. In a separate set of experiments, expression of protein levels of proinsulin and C-peptide were measured by immunofluorescence staining in isolated pancreatic tissues.
Fig. 1.
Experimental design of the study. Abbreviations: STZ: streptozotocin; GLUT2: glucose transporter 2; Munc18: mammalian homologue of the unc (uncoordinated mutant)-18; SNAP-25: synaptosome associated protein-25.
Measurement of insulin in serum
Serum concentrations of insulin were measured in overnight-fasted rats at week 8. Insulin in serum was measured using a rat ELISA kit (Rat insulin ELISA; Mercodia, Uppsala, Sweden, sensitivity of the assay was ≤26.1 pmol/L); Intra-assay coefficient of variation was 8%.
RNA extraction, cDNA synthesis, and real-time PCR
Isolated pancreatic islets were frozen in liquid nitrogen and stored at −80 °C; total RNA was extracted using RNX-Plus solution kit (Cinagen Co., Tehran, Iran) according to the manufacturer instruction. A nanodrop spectrophotometer (NanoDrop-1000, Thermo Scientific, USA) was used for measuring quantity and purity of RNA samples. Thermo Scientific RevertAid Reverse Transcriptase was used for cDNA synthesis according to the manufacturer instruction. Primers were designed with primer3 and Gene Runner programs [Table 1]. Total volume of PCR reaction was 15 μL containing cDNA, 1 μL; each forward and reverse primer, 0.5 μL; SYBR Green PCR Master Mix 2X (Thermo Fisher, USA), 7.5 μL; and nuclease-free water, 5.5 μL. For PCR reactions, the following cycling profile was used: Initial denaturation (10 min at 95 °C) followed by 40 cycles with 45 s at 94 °C, 45 s at 58 °C, and 1 min at 72 °C; final extension for 5 min at 72 °C. All samples were run in duplicate and in negative control reactions, nuclease-free water was used instead of templates. Relative mRNA levels of interested genes were normalized using β-actin as reference gene.
Table 1.
Primers for real time PCR.
| (Target) Gene Name | Accession No. | Primer sequence (5′→3′) | Product length (bp) |
|---|---|---|---|
| GLUT2 | NM_012879.2 | F: GCAACATGTCAGAAGAGAAGATCA R: AGGAGCATTGATCACACCGA |
107 |
| Glucokinase | NM_001270849.1 | F: GGTCAGCAGCTGTACGAGAAG R: CCGTGTAACAGAAGGTTCTCG |
101 |
| Synaptotagmin III | NM_019122.1 | F: CGGAACCGAGATGCTGATACC R: AGGCTCACGGAAATGTCTGC |
101 |
| Syntaxin1A | NM_053788.2 | F: AAGACGCAGCATTCCACGC R: TGCAGCGTTCTCGGTAGTCT |
88 |
| SNAP25 | NM_001270576.1 | F: GGCTTCATCCGCAGGGTAAC R: TGACGCAGGTTTCCGATGATG |
92 |
| Munc18b | NM_031126.1 | F: GAAACTGATTGTCCCGGTGC R: TTTCCTCACTCACACCGTTCC |
104 |
| Insulin1 | NM_019129.3 | F: GACCTTGGCACTGGAGGTT R: CCAGTTGGTAGAGGGAGCAG |
80 |
| Insulin2 | NM_019130.2 | F: CGAAGTGGAGGACCCACA R: TGCTGGTGCAGCACTGAT |
128 |
| β-actin | NM_031144.3 | F: GCGTCCACCTGCTAGTACAAC R: CGACGACTAGCTCAGCGATA |
100 |
Abbreviations: GLUT2: glucose transporter 2; Munc18b: mammalian homologue of the unc (uncoordinated mutant)-18 gene; SNAP-25: synaptosome associated protein-25.
Immunofluorescence staining for proinsulin and C-peptide
At the end of the study (week 8 of the intervention), rats were anesthetized with sodium pentobarbital (60 mg/kg; Sigma Aldrich, Hamburg, Germany) and pancreatic tissues were dissected and fixed in 10% formalin. Following fixation, samples were embedded in paraffin and then sectioned (5-μm thickness), deparaffinized, and rehydrated using a series of 10-min washes in xylene (×2) and graded alcohol solutions (100% ethanol × 2, 90% ethanol × 1, 80% ethanol × 1, and 70% ethanol × 1), followed by washing four times in phosphate-buffered saline (PBS, 100 mM, pH 7.4) for 5 min per wash [21].
For immunofluorescence staining, sections were incubated in 2N HCl for 30 min for antigen retrieval. Borate buffer was added for 5 min to neutralize acid and the section was washed with PBS. For permeabilization of cells, sections were incubated with 0.3% Triton X-100 for 30 min. After washing with PBS, sections were incubated in 10% goat serum for 30 min to block unspecific binding of the antibodies. After that, sections were incubated overnight at 4 °C with primary antibodies (Proinsulin: Bio-Rad, Kidlington, UK, Cat#5330-3369 and C-peptide: Cell Signaling Technology, Danvers, USA, Cat# 5330-3369) diluted in PBS (1:100). The next day, sections were washed four times in PBS for about 5 min per wash. Subsequently, secondary antibodies (Abcam, USA, Cat# ab6785) were added to the samples at a dilution of 1:150 and then incubated in room temperature in a dark humid chamber for 1 h. Finally, sections were counterstained with a nuclear staining DAPI (Merck, Darmstadt, Germany, Cat#D9542). The sections were then photographed using an fluorescence microscope (Labome, USA) equipped with digital imaging accessories and the percentage of positive labeled cells was calculated using imageJ analysis software [21].
Statistical analyses
Relative mRNA expressions of target genes were calculated using formula [22] in which (cycle threshold) = of target gene – of reference gene and = of the target genes in the interested group – of the target gene in the corresponding control group. GraphPad Prism software version 8.0.2 was used for statistical analysis. The Kruskal Wallis test with Dunn's test as pos-hoc was used for comparing fold changes in mRNA expression between groups. One-way analysis of variance (ANOVA) followed by Bonferroni post-hoc test was used for comparing water consumption, food intake, calorie intake, body weight, serum insulin concentrations, as well as proinsulin and C-peptide in isolated pancreatic tissues. Two-sided p values < 0.05 were considered statistically significant.
Results
Body weight, food intake and water consumption
As shown in Table 2, all groups had comparable body weight at the start of study. Compared to controls, diabetic rats had significantly higher final body weight and body weight gain at the end of study. Nitrite administration significantly decreased final body weight and body weight gain in diabetic rats. Compared to controls, diabetic rats had higher calorie intake and water consumption but lower food intake; nitrite administration had no effect on these parameters.
Table 2.
Effects of sodium nitrite on body weight, water consumption, food intake, and calorie intake.
| Parameters | Groups |
||
|---|---|---|---|
| Control | Diabetes | Diabetes + Nitrite | |
| Initial body weight (g) | 191.1 ± 3.2 | 189.0 ± 4.9 | 185.3 ± 3.4 |
| Final body weight (g) | 363.7 ± 5.9 | 382.1 ± 9.3a | 351.0 ± 18.4b |
| Body weight gain (g) | 116.3 ± 1.0 | 150.6 ± 3.3a | 129.5 ± 7.7b |
| Initial food intake (g/day/rat) | 17.2 ± 0.4 | 14.3 ± 0.2v | 14.6 ± 0.5a |
| Final food intake (g/day/rat) | 18.2 ± 1.4 | 13.9 ± 1.0a | 14.2 ± 1.0 |
| Initial calorie intake (kcal/day/rat) | 52.6 ± 1.1 | 70.0 ± 1.1a | 71.8 ± 2.6a |
| Final calorie intake (kcal/day/rat) | 55.5 ± 0.7 | 68.1 ± 0.8a | 69.6 ± 5.1a |
| Initial water consumption (ml/day/rat) | 27.9 ± 0.8 | 34.2 ± 0.7a | 34.3 ± 1.1a |
| Final water consumption (ml/day/rat) | 25.8 ± 0.5 | 38.3 ± 0.7a | 39.3 ± 3.4a |
Statistically significant difference compared to the control and diabetes groups, respectively. Values are mean ± SEM (n = 10/group).
Statistically significant difference compared to the diabetes group. Values are mean ± SEM (n = 10/group). This Table is modified from our previously published paper [12].
Serum insulin concentrations
Compared to the control group, diabetic rats had higher insulin concentration in serum (158.1 ± 8.7 vs. 75.0 ± 12.2 pmol/L, p < 0.0001). Nitrite administration for 8 weeks decreased serum insulin concentration by 23% (p = 0.0596) in diabetic rats.
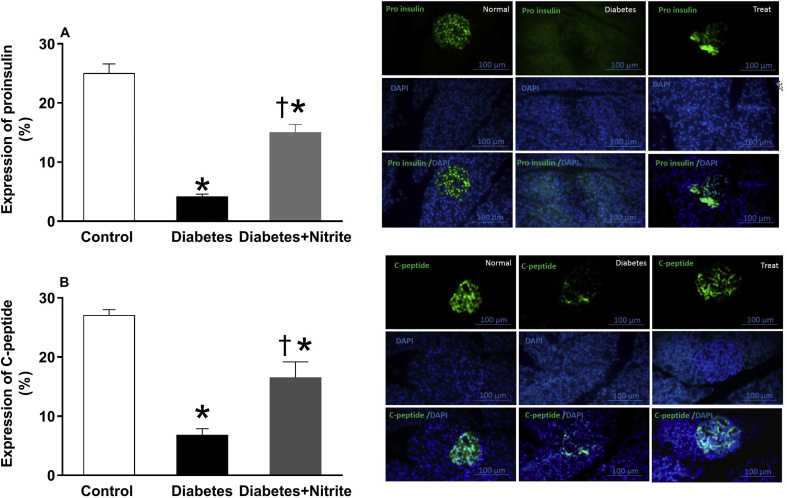
Protein expression of proinsulin and C-peptide
As shown in the Fig. 2, compared with controls, expression of proinsulin and C-peptide were significantly lower in isolated pancreatic tissues of rats with Type 2 diabetes. Nitrite administration for 8 weeks increased expression of proinsulin and C-peptide in rats with Type 2 diabetes.
Fig. 2.
Effect of nitrite on expression of proinsulin (A) and C-peptide (B) in the pancreatic tissues of Type 2 diabetic rats. Representative images of pancreatic tissue immune fluorescence stained with DAPI (blue, DNA stain), proinsulin, and C-peptide (green) as well as quantification of immune fluorescence staining by imageJ analysis are shown. Scale bar = 100 mm. Values are mean ± SEM (n = 6/group). ∗, † significant difference (p values < 0.05) compared with control and diabetes groups, respectively.
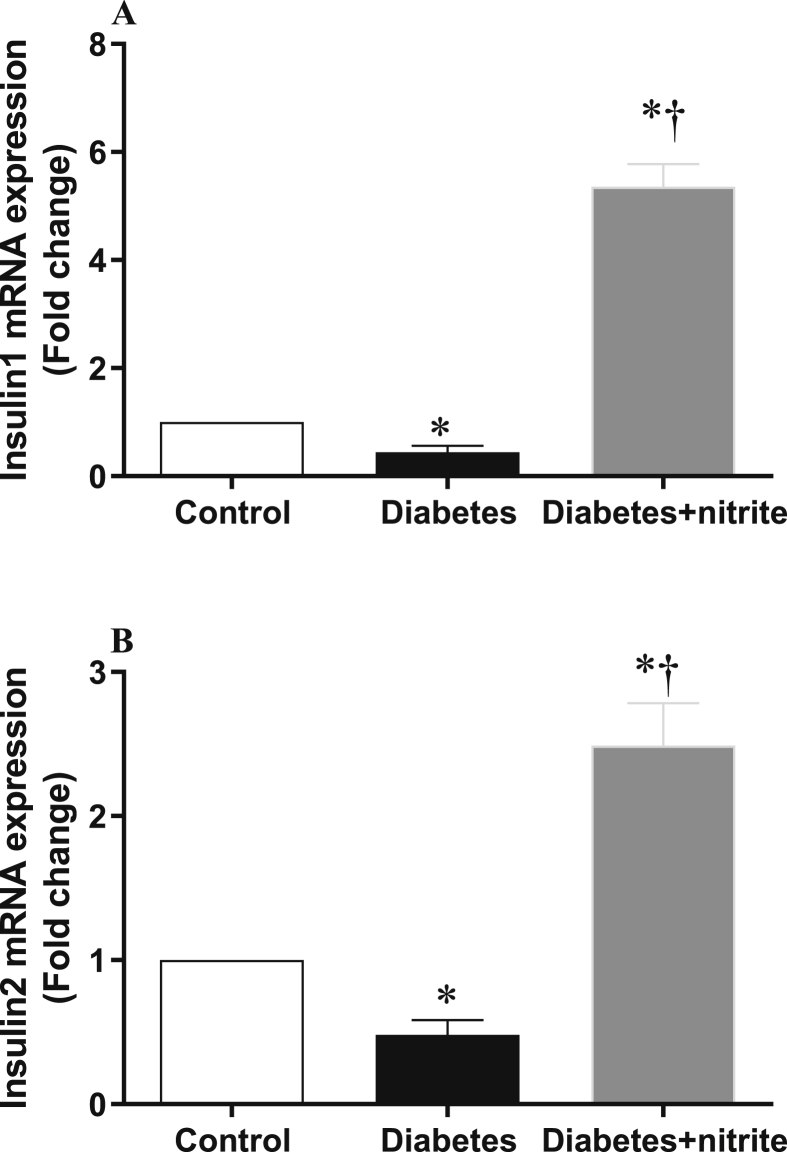
Gene expression of insulin1 and insulin2
As shown in the Fig. 3, compared with controls, rats with Type 2 diabetes had lower mRNA expression of insulin1 (56%) and insulin2 (52%) in their islets and nitrite administration for 8 weeks increased expression of both genes.
Fig. 3.
Effect of nitrite administration on mRNA expression of insulin1 (A) and insulin2 (B) in pancreatic isolated islets in Type 2 diabetic rats. ∗, † significant difference compared with control and diabetes, respectively. n = 10/group.
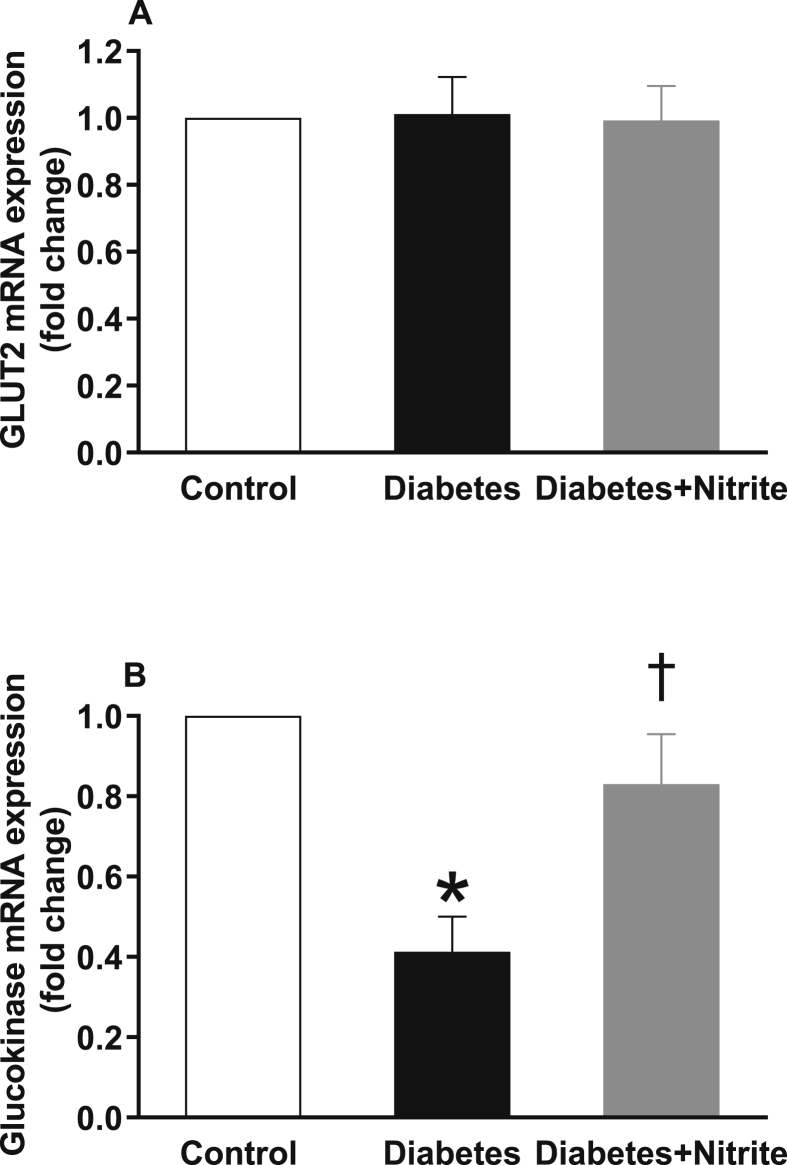
Gene expression of SNARE proteins involved in insulin secretion
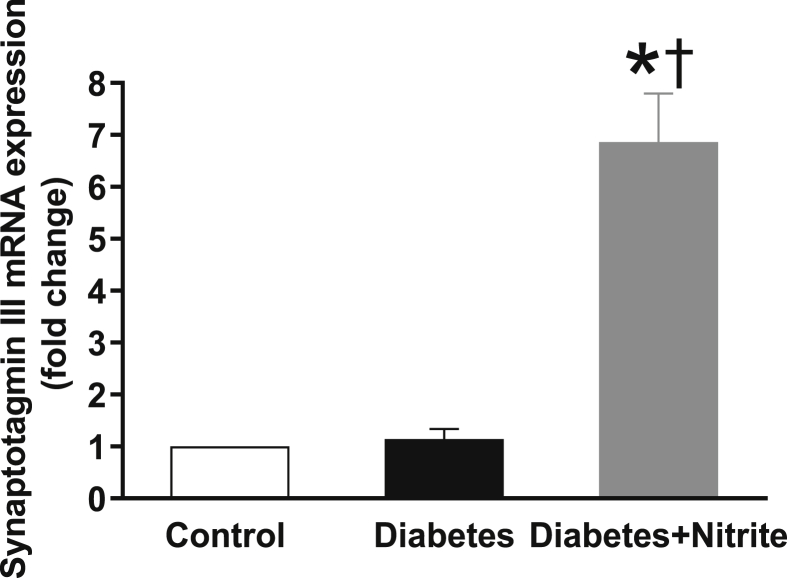
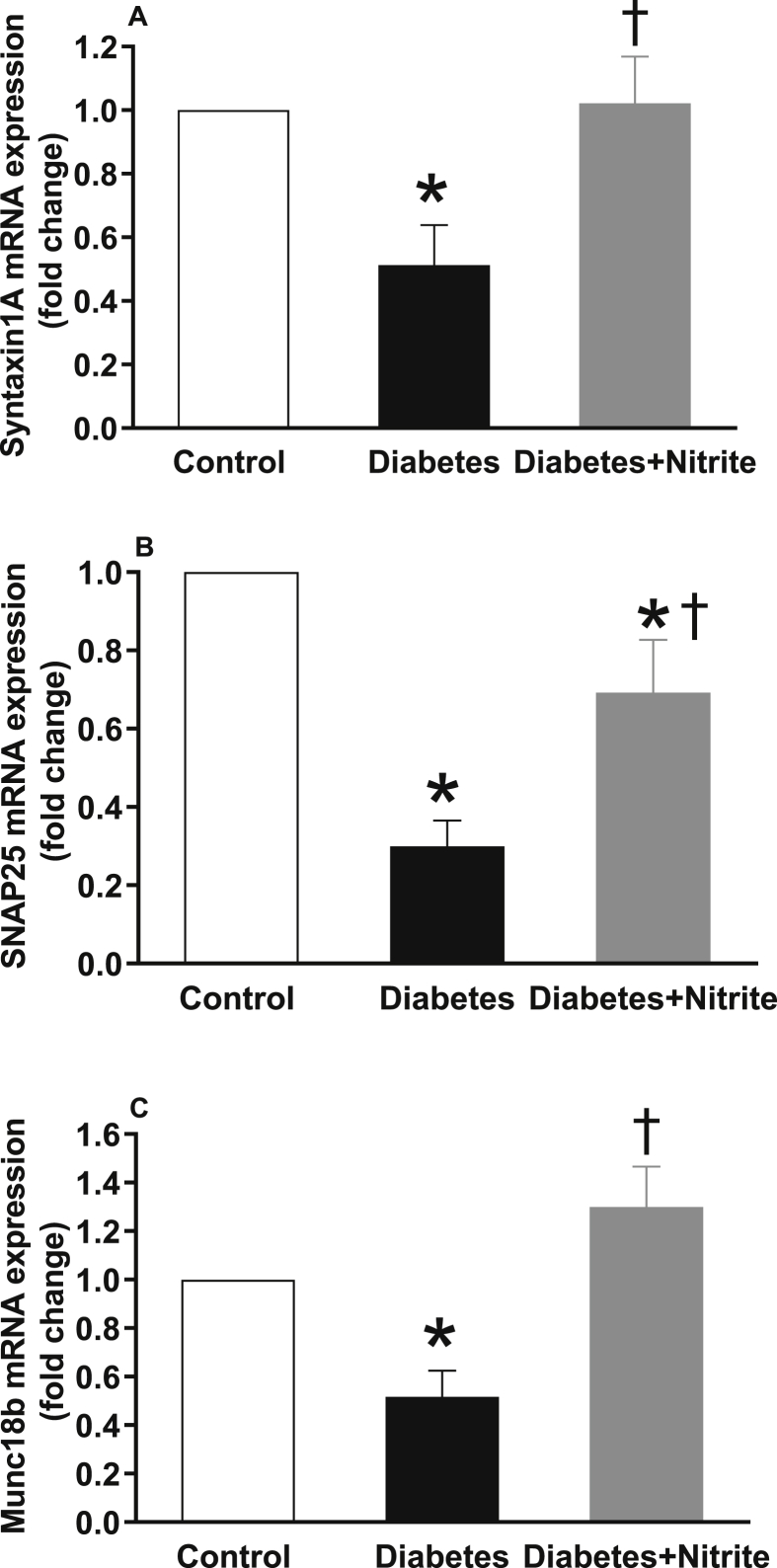
As shown in the Fig. 4, compared with controls, expression of glucokinase but not GLUT2 was significantly lower in isolated islets of rats with Type 2 diabetes. Nitrite administration significantly increased glucokinase expression in diabetic rats but had no effect on expression of GLUT2. Expression of calcium sensor synaptotagmin III remained unchanged in the isolated islets of Type 2 diabetic rats; however, administration of nitrite significantly increased mRNA expression of synaptotagmin III by 6.9 folds [Fig. 5]. As shown in Fig. 6, compared with controls, mRNA expression of syntaxin1A, SNAP25, and Munc18b were significantly lower in the isolated islets of rats with Type 2 diabetes by 49%, 70%, and 48%, respectively. Administration of nitrite, significantly increased expression of these genes in diabetic rats to almost near control values.
Fig. 4.
Effect of nitrite administration of mRNA expression of glucose transporter 2 (GLUT2) and glucokinase in pancreatic isolated islets in Type 2 diabetic rats. ∗, † significant difference compared with control and diabetes, respectively. n = 10/group.
Fig. 5.
Effect of nitrite administration of mRNA expression of calcium sensor synaptotagmin III in pancreatic isolated islets in Type 2 diabetic rats. ∗, † significant difference compared with control and diabetes, respectively. n = 10/group.
Fig. 6.
Effect of nitrite administration of mRNA expression of syntaxin1A, synaptosome-associated protein of 25 kDa (SNAP25), and mammalian homologue of the unc (uncoordinated mutant)-18 gene (Munc18b) in pancreatic isolated islets in Type 2 diabetic rats. ∗, † significant difference compared with control and diabetes, respectively. n = 10/group.
Discussion
Results of this study indicated that in isolated pancreatic islets of Type 2 diabetic rats, nitrite increases expression of genes involved in glucose sensing (glucokinase), calcium sensing (synaptotagmin III), and exocytosis of insulin-containing vesicles (syntaxin1A, SNAP25, and Munc18b); in addition nitrite increases mRNA expression of both insulin 1 and insulin 2 genes and therefore insulin synthesis. To our knowledge, these data for the first time present a new mechanism for explaining nitrite-stimulated insulin secretion and therefore for metabolic favorable effects of nitrite in Type 2 diabetes.
Data presented in this study are in continuation of our previous report that nitrite increases glucose-stimulated insulin secretion (GSIS) by ∼35%, and improves glucose tolerance in Type 2 diabetic rats [12]. In the current study, Type 2 diabetic rats showed insulin resistance as reflected by higher serum concentrations of insulin and glucose than controls and nitrite administration restored these increased values to their near normal levels. The mechanisms by which nitrite decreases insulin resistance include insulin-independent translocation of GLUT4 to plasma membrane [9] and therefore increasing glucose uptake in skeletal muscle and adipose tissue [9,10], decreasing body weight [23], and increasing browning of white adipose tissue [24]. Through these effects, nitrite decreases circulating concentrations of both glucose and insulin and therefore insulin resistance in animal models of Type 2 diabetes.
In this study, compared to the control group, rats with Type 2 diabetes had lower gene expression of insulin1 and insulin2 as well as lower protein expression of proinsulin and C-peptide, indicating impaired insulin production. Nitrite increased mRNA expression of insulin1 and insulin2 in isolated pancreatic islets as well as expression of proinsulin and C-peptide in pancreatic tissue; these data indicate that nitrite increases insulin synthesis in Type 2 diabetic rats. Similar to our results, it has been shown that nitrite increases insulin content in pancreatic islets in Type 2 diabetic rats [12] and NO increases insulin mRNA levels in isolated islets from male Wistar rats and in Min6 β-cell line; in fact NO stimulates insulin gene promoter via phosphoinositide 3-kinase signaling pathway [25]. In addition, C-peptide, an indicator of β-cell function [26], increases NO production [27] and blood flow in pancreatic β-cells [28] that may be involved in higher insulin production after nitrite administration.
In line with our results decreased expressions of SNARE proteins involved in insulin secretion including glucokinase [29], syntaxin1A [[30], [31], [32]], SNAP25 [[30], [31], [32]], and Munc18b [30] have been reported in islets of Type 2 diabetic animals or patients. In our study, mRNA expression of synaptotagmin III in pancreatic islets was comparable between control and diabetic rats; nitrite administration however markedly increased expression of synaptotagmin III by 6.9 folds. Synaptotagmins act as calcium sensors in β-cells [33] and among different isoforms, synaptotagmin III is one the most sensitive to calcium [34] and an important candidate that have physiological role in insulin secretion in β-cells [35]. Unlike our results, weaker immunoreactivity for synaptotagmin III has however been observed in islets of GK Type 2 diabetic rats compared to controls [13]; possible explanation for this difference may be related to the animal models used.
In the current study, mRNA expression of syntaxin1A and SNAP25 were 49% and 70% lower in islets of Type 2 diabetic rats compared with controls and nitrite administration restored these decreased values to their near normal levels. In syntaxin1A-knockout mice, first phase of GSIS is decreased and animals have impaired glucose tolerance [36]. It has been shown that in β-cell-specific syntaxin1A-knockout mice, both first and second phases of insulin secretion are impaired that resulted in hyperglycemia and glucose intolerance [37]. In addition, restoration of decreased expression of syntaxin1A and SNAP25 in GK Type 2 diabetic rats increases GISIS [32].
Our results indicated that in diabetic rats, expression of Munc18b was 48% lower than controls; nitrite administration increased Munc18b expression and restored it to normal values. Munc18b is a major mediator of insulin secretion in isolated islets of rats and accounts for one-half of the physiologic biphasic GSIS [38]. Overexpression of Munc18b increases insulin secretion of isolated islets in rats [38] and humans [30] and improves glucose homeostasis in GK Type 2 diabetic rats [30].
Nitrite is mostly converted to NO and exerts its metabolic effects [6], however NO-independent effects of nitrite cannot be ruled out. Contribution of NO in insulin secretion is not straightforward and may be a summation of positive and negative effects [39]. The dual effect of NO on insulin secretion has been reviewed recently [5,40] and can be attributed to NOS enzyme isoforms participating in NO production [5], low or high NO concentration [41], and the variability of the experimental conditions in different studies [40]. High amount of iNOS-derived NO (micro molar range) decreases insulin secretion, whereas cNOS-derived NO (low-nano molar range) mostly increases insulin secretion by inhibition of KATP channels in β-cells and activation of the cGMP pathway [5,41]. NO can stimulate insulin release by interaction with glucokinase, which is the main glucose sensor in the pancreatic β-cells [42]; similarly, in our study, nitrite increased glucokinase mRNA expression in isolated pancreatic islets. In neurons, it has been shown that SNAP25 and syntaxin1A are required for NO-stimulated neurotransmitter release and that NO potentiates binding of syntaxin1A to SNAP25 [43]. In addition, S-nitrosylation of syntaxin1A facilitates vesicle exocytosis in the brain [44]. SNARE proteins involved in insulin secretion are potential targets for enhancing β-cell function [15,38] and our data indicate that nitrite supplementation is a potential ligand for this purpose.
As a strength, this is the first study reporting stimulatory effect of long-term in vivo nitrite administration on SNAREs involved in insulin secretion from isolated pancreatic islets of Type 2 diabetic rats; these data may have implications considering potential effects of dietary nitrite interventions as a cost-effective approach for prevention and treatment of Type 2 diabetes [6]. This study has some limitations that should be taken into account when interpreting results. First, we did not measure effect of nitrite on expression of proteins involved in exocytosis of insulin and also S-nitrosylation of related proteins. Second, we only assessed effect of nitrite on gene expression of SNARE proteins involved in insulin secretion in male rats. However, the National Institute of Health recommends balancing sex in animal studies [45]. Finally, we did not measure effect of nitrite on pancreatic islet survival; however, we previously reported that following 8-week consumption of 50 mg/L nitrite in the drinking water, GSIS (in presence of 16.7 mM glucose) was 29% and 34% higher in isolated islets of control and Type 2 diabetic rats while no effect was observed in response to basal glucose level (in presence of 5.6 mM glucose) [12]. This stimulation index is used for assessing functional viability of islets [46]. In addition, the dose of nitrite used in this study (50 mg/Lin drinking water, equates to 5.6–9.2 mg/kg) is translated to a human equivalent dose of 0.9–1.5 mg/kg [47], which is a safe and low-dose of nitrite in human [48]; it has been reported that low NO concentration can increases proliferation and survival of pancreatic β-cells [49] by providing protection against DNA fragmentation and apoptosis [50,51]. Therefore, it is possible that nitrite increased survival of pancreatic β-cell in our study.
Conclusion
Our results indicate that stimulatory effect of nitrite on insulin secretion in Type 2 diabetic rats is at least in part due to increased gene expression of molecules involved in glucose sensing (glucokinase), calcium sensing (synaptotagmin III), exocytosis of insulin vesicles (syntaxin1A, SNAP25, and Munc18b) as well as increased insulin production in pancreatic islets.
Conflicts of interest
The authors declare that they have no competing interest.
Acknowledgement
This study was supported by Shahid Beheshti University of Medical Sciences [grant No. 24844-5], Tehran, Iran. Authors would like to thank Dr. Sevda Gheibi for her helps in rat handling and islet isolation and Ms. Hanieh Gholami for her assistance in RT-PCR experiments.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Zimmet P.Z. Diabetes and its drivers: the largest epidemic in human history? Clin Diabetes Endocrinol. 2017;3:1. doi: 10.1186/s40842-016-0039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Y., Ley S.H., Hu F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 3.International diabetes federation. IDF diabetes atlas. 9th ed, https://www.diabetesatlas.org/en/; 2019. [accessed 14 November 2019].
- 4.Ghasemi A., Jeddi S. Anti-obesity and anti-diabetic effects of nitrate and nitrite. Nitric Oxide. 2017;70:9–24. doi: 10.1016/j.niox.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Bahadoran Z., Mirmiran P., Ghasemi A. Role of nitric oxide in insulin secretion and glucose metabolism. Trends Endocrinol Metab. 2020;31:118–130. doi: 10.1016/j.tem.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Lundberg J.O., Carlström M., Weitzberg E. Metabolic effects of dietary nitrate in Health and disease. Cell Metab. 2018;28:9–22. doi: 10.1016/j.cmet.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Kapil V., Khambata R.S., Jones D.A., Rathod K., Primus C., Massimo G., et al. The noncanonical pathway for in vivo nitric oxide generation: the nitrate-nitrite-nitric oxide pathway. Pharmacol Rev. 2020;72:692–766. doi: 10.1124/pr.120.019240. [DOI] [PubMed] [Google Scholar]
- 8.Roberts L.D., Ashmore T., Kotwica A.O., Murfitt S.A., Fernandez B.O., Feelisch M., et al. Inorganic nitrate promotes the browning of white adipose tissue through the nitrate-nitrite-nitric oxide pathway. Diabetes. 2015;64:471–484. doi: 10.2337/db14-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohtake K., Nakano G., Ehara N., Sonoda K., Ito J., Uchida H., et al. Dietary nitrite supplementation improves insulin resistance in type 2 diabetic KKA(y) mice. Nitric Oxide. 2015;44:31–38. doi: 10.1016/j.niox.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Jiang H., Torregrossa A.C., Potts A., Pierini D., Aranke M., Garg H.K., et al. Dietary nitrite improves insulin signaling through GLUT4 translocation. Free Radic Biol Med. 2014;67:51–57. doi: 10.1016/j.freeradbiomed.2013.10.809. [DOI] [PubMed] [Google Scholar]
- 11.Nyström T., Ortsäter H., Huang Z., Zhang F., Larsen F.J., Weitzberg E., et al. Inorganic nitrite stimulates pancreatic islet blood flow and insulin secretion. Free Radic Biol Med. 2012;53:1017–1023. doi: 10.1016/j.freeradbiomed.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Gheibi S., Bakhtiarzadeh F., Jeddi S., Farrokhfall K., Zardooz H., Ghasemi A. Nitrite increases glucose-stimulated insulin secretion and islet insulin content in obese type 2 diabetic male rats. Nitric Oxide. 2017;64:39–51. doi: 10.1016/j.niox.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W., Khan A., Ostenson C.G., Berggren P.O., Efendic S., Meister B. Down-regulated expression of exocytotic proteins in pancreatic islets of diabetic GK rats. Biochem Biophys Res Commun. 2002;291:1038–1044. doi: 10.1006/bbrc.2002.6555. [DOI] [PubMed] [Google Scholar]
- 14.Matschinsky F.M. Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45:223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- 15.Thurmond D.C., Gaisano H.Y. Recent insights into beta-cell exocytosis in type 2 diabetes. J Mol Biol. 2020;432:1310–1325. doi: 10.1016/j.jmb.2019.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Söllner T.H. Regulated exocytosis and SNARE function (Review) Mol Membr Biol. 2003;20:209–220. doi: 10.1080/0968768031000104953. [DOI] [PubMed] [Google Scholar]
- 17.Martin-Urdiroz M., Deeks M.J., Horton C.G., Dawe H.R., Jourdain I. The exocyst complex in Health and disease. Front Cell Dev Biol. 2016;4:24. doi: 10.3389/fcell.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gheibi S., Kashfi K., Ghasemi A. A practical guide for induction of type-2 diabetes in rat: incorporating a high-fat diet and streptozotocin. Biomed Pharmacother. 2017;95:605–613. doi: 10.1016/j.biopha.2017.08.098. [DOI] [PubMed] [Google Scholar]
- 19.Lacy P.E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- 20.Roderigo-Milne H., Hauge-Evans A.C., Persaud S.J., Jones P.M. Differential expression of insulin genes 1 and 2 in MIN6 cells and pseudoislets. Biochem Biophys Res Commun. 2002;296:589–595. doi: 10.1016/s0006-291x(02)00913-0. [DOI] [PubMed] [Google Scholar]
- 21.Asadi A., Bruin J.E., Kieffer T.J. Characterization of antibodies to products of proinsulin processing using immunofluorescence staining of pancreas in multiple species. J Histochem Cytochem. 2015;63:646–662. doi: 10.1369/0022155415576541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gholami H., Jeddi S., Zadeh-Vakili A., Farrokhfall K., Rouhollah F., Zarkesh M., et al. Transient congenital hypothyroidism alters gene expression of glucose transporters and impairs glucose sensing apparatus in young and aged offspring rats. Cell Physiol Biochem. 2017;43:2338–2352. doi: 10.1159/000484386. [DOI] [PubMed] [Google Scholar]
- 23.Khorasani V., Jeddi S., Yaghmaei P., Tohidi M., Ghasemi A. Effect of long-term sodium nitrate administration on diabetes-induced anemia and glucose homeostasis in obese type 2 diabetic male rats. Nitric Oxide. 2019;86:21–30. doi: 10.1016/j.niox.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Jeddi S., Yousefzadeh N., Afzali H., Ghasemi A. Long-term nitrate administration increases expression of browning genes in epididymal adipose tissue of male type 2 diabetic rats. Gene. 2021;766:145155. doi: 10.1016/j.gene.2020.145155. [DOI] [PubMed] [Google Scholar]
- 25.Campbell S.C., Richardson H., Ferris W.F., Butler C.S., Macfarlane W.M. Nitric oxide stimulates insulin gene transcription in pancreatic β-cells. Biochem Biophys Res Commun. 2007;353:1011–1016. doi: 10.1016/j.bbrc.2006.12.127. [DOI] [PubMed] [Google Scholar]
- 26.Leighton E., Sainsbury C.A., Jones G.C. A practical review of C-peptide testing in diabetes. Diabetes Ther. 2017;8:475–487. doi: 10.1007/s13300-017-0265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallerath T., Kunt T., Forst T., Closs E.I., Lehmann R., Flohr T., et al. Stimulation of endothelial nitric oxide synthase by proinsulin C-peptide. Nitric Oxide. 2003;9:95–102. doi: 10.1016/j.niox.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Wu L., Olverling A., Huang Z., Jansson L., Chao H., Gao X., et al. GLP-1, exendin-4 and C-peptide regulate pancreatic islet microcirculation, insulin secretion and glucose tolerance in rats. Clin Sci (London) 2012;122:375–384. doi: 10.1042/CS20090464. [DOI] [PubMed] [Google Scholar]
- 29.Jörns A., Tiedge M., Ziv E., Shafrir E., Lenzen S. Gradual loss of pancreatic beta-cell insulin, glucokinase and GLUT2 glucose transporter immunoreactivities during the time course of nutritionally induced type-2 diabetes in Psammomys obesus (sand rat) Virchows Arch. 2002;440:63–69. doi: 10.1007/s004280100490. [DOI] [PubMed] [Google Scholar]
- 30.Qin T., Liang T., Zhu D., Kang Y., Xie L., Dolai S., et al. Munc18b increases insulin granule fusion, restoring deficient insulin secretion in type-2 diabetes human and goto-kakizaki rat islets with improvement in glucose homeostasis. EBioMedicine. 2017;16:262–274. doi: 10.1016/j.ebiom.2017.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostenson C.G., Gaisano H., Sheu L., Tibell A., Bartfai T. Impaired gene and protein expression of exocytotic soluble N-ethylmaleimide attachment protein receptor complex proteins in pancreatic islets of type 2 diabetic patients. Diabetes. 2006;55:435–440. doi: 10.2337/diabetes.55.02.06.db04-1575. [DOI] [PubMed] [Google Scholar]
- 32.Nagamatsu S., Nakamichi Y., Yamamura C., Matsushima S., Watanabe T., Ozawa S., et al. Decreased expression of t-SNARE, syntaxin 1, and SNAP-25 in pancreatic beta-cells is involved in impaired insulin secretion from diabetic GK rat islets: restoration of decreased t-SNARE proteins improves impaired insulin secretion. Diabetes. 1999;48:2367–2373. doi: 10.2337/diabetes.48.12.2367. [DOI] [PubMed] [Google Scholar]
- 33.Eliasson L., Abdulkader F., Braun M., Galvanovskis J., Hoppa M.B., Rorsman P. Novel aspects of the molecular mechanisms controlling insulin secretion. J Physiol. 2008;586:3313–3324. doi: 10.1113/jphysiol.2008.155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizuta M., Kurose T., Miki T., Shoji-Kasai Y., Takahashi M., Seino S., et al. Localization and functional role of synaptotagmin III in insulin secretory vesicles in pancreatic beta-cells. Diabetes. 1997;46:2002–2006. doi: 10.2337/diab.46.12.2002. [DOI] [PubMed] [Google Scholar]
- 35.Gao Z., Reavey-Cantwell J., Young R.A., Jegier P., Wolf B.A. Synaptotagmin III/VII isoforms mediate Ca2+-induced insulin secretion in pancreatic islet beta -cells. J Biol Chem. 2000;275:36079–36085. doi: 10.1074/jbc.M004284200. [DOI] [PubMed] [Google Scholar]
- 36.Ohara-Imaizumi M., Fujiwara T., Nakamichi Y., Okamura T., Akimoto Y., Kawai J., et al. Imaging analysis reveals mechanistic differences between first- and second-phase insulin exocytosis. J Cell Biol. 2007;177:695–705. doi: 10.1083/jcb.200608132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang T., Qin T., Xie L., Dolai S., Zhu D., Prentice K.J., et al. New roles of syntaxin-1A in insulin granule exocytosis and replenishment. J Biol Chem. 2017;292:2203–2216. doi: 10.1074/jbc.M116.769885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lam P.P., Ohno M., Dolai S., He Y., Qin T., Liang T., et al. Munc18b is a major mediator of insulin exocytosis in rat pancreatic β-cells. Diabetes. 2013;62:2416–2428. doi: 10.2337/db12-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiseman D.A., Kalwat M.A., Thurmond D.C. Stimulus-induced S-nitrosylation of Syntaxin 4 impacts insulin granule exocytosis. J Biol Chem. 2011;286:16344–16354. doi: 10.1074/jbc.M110.214031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gheibi S., Ghasemi A. Insulin secretion: the nitric oxide controversy. EXCLI J. 2020;19:1227–1245. doi: 10.17179/excli2020-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurohane Kaneko Y.K., Ishikawa T. Dual role of nitric oxide in pancreatic β-cells. J Pharmacol Sci. 2013;123:295–300. doi: 10.1254/jphs.13r10cp. [DOI] [PubMed] [Google Scholar]
- 42.Matschinsky F.M. Regulation of pancreatic beta-cell glucokinase: from basics to therapeutics. Diabetes. 2002;51:S394–S404. doi: 10.2337/diabetes.51.2007.s394. [DOI] [PubMed] [Google Scholar]
- 43.Meffert M.K., Calakos N.C., Scheller R.H., Schulman H. Nitric oxide modulates synaptic vesicle docking fusion reactions. Neuron. 1996;16:1229–1236. doi: 10.1016/s0896-6273(00)80149-x. [DOI] [PubMed] [Google Scholar]
- 44.Palmer Z.J., Duncan R.R., Johnson J.R., Lian L.Y., Mello L.V., Booth D., et al. S-nitrosylation of syntaxin 1 at Cys(145) is a regulatory switch controlling Munc18-1 binding. Biochem J. 2008;413:479–491. doi: 10.1042/BJ20080069. [DOI] [PubMed] [Google Scholar]
- 45.Clayton J.A., Collins F.S. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray H.E., Paget M.B., Downing R. Preservation of glucose responsiveness in human islets maintained in a rotational cell culture system. Mol Cell Endocrinol. 2005;238:39–49. doi: 10.1016/j.mce.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 47.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. Faseb J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 48.Bryan N.S., Ivy J.L. Inorganic nitrite and nitrate: evidence to support consideration as dietary nutrients. Nutr Res. 2015;35:643–654. doi: 10.1016/j.nutres.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Wong J.C., Vo V., Gorjala P., Fiscus R.R. Pancreatic-β-cell survival and proliferation are promoted by protein kinase G type Iα and downstream regulation of AKT/FOXO1. Diabetes Vasc Dis Res. 2017;14:434–449. doi: 10.1177/1479164117713947. [DOI] [PubMed] [Google Scholar]
- 50.Tejedo J.R., Cahuana G.M., Ramírez R., Esbert M., Jiménez J., Sobrino F., et al. Nitric oxide triggers the phosphatidylinositol 3-kinase/akt survival pathway in insulin-producing RINm5F cells by arousing src to activate insulin receptor substrate-1. Endocrinology. 2004;145:2319–2327. doi: 10.1210/en.2003-1489. [DOI] [PubMed] [Google Scholar]
- 51.Tejedo J.R., Ramírez R., Cahuana G.M., Rincón P., Sobrino F., Bedoya F.J. Evidence for involvement of c-Src in the anti-apoptotic action of nitric oxide in serum-deprived RINm5F cells. Cell Signal. 2001;13:809–817. doi: 10.1016/s0898-6568(01)00206-6. [DOI] [PubMed] [Google Scholar]