Introduction
We present a case of a 22-year-old patient who presented with acute vulvar ulcers that developed 2 days after they got the COVID-19 Pfizer booster. While these ulcers have historically been identified after a variety of triggers, more recently, reports have been made following the COVID-19 vaccination and infection. Here, we have identified a case following a Pfizer booster dose.
Case report
A 22-year-old genderqueer adult presented to the dermatology clinic with an 11-day history of vulvar ulcers. The patient reported tenderness and swelling of the labia initially and subsequently noticed discrete, deeply erythematous lesions. These gradually coalesced into gray, tan exudates and pseudomembranes with continued extensive vulvar swelling. The patient also noted severe pain, particularly with urination, a fever, and axillary and supraclavicular lymphadenopathy with ulcer onset. Two days prior to symptom onset, the patient had received the Pfizer COVID-19 booster vaccine and had a negative COVID polymerase chain reaction test after the booster.
The patient reported a history of 2 similar episodes, at age 13 years and approximately 6 months prior to presentation. The first episode was presumed to be trauma secondary to shaving, although they denied any history of shaving. The second episode was preceded by a viral upper respiratory illness. The patient noted a history of oral aphthous ulcer outbreaks 3 times per year. They denied any vaginal intercourse for over 2 years, new medications, personal care products, or inciting trauma. Testing for human immunodeficiency virus (HIV), gonorrhea, and chlamydia was all negative in the past and on repeat testing. Their recent pap smear was unremarkable.
Prior to presenting to dermatology, the patient saw infectious disease and was prescribed amoxicillin/clavulanate, doxycycline, and fluconazole as empiric therapy. A punch biopsy of the right labia minora showed epidermal ulceration with dense dermal mixed inflammation and favored an infectious etiology. Bacterial vulvar tissue culture and smear showed neutrophils and moderate mixed gram-positive organisms. Serologic testing revealed herpes simplex virus (HSV)-1 IgG. HSV-2, HIV, acid-fast bacilli, varicella zoster, hepatitis B, hepatitis C, cytomegalovirus, and syphilis testing were all negative.
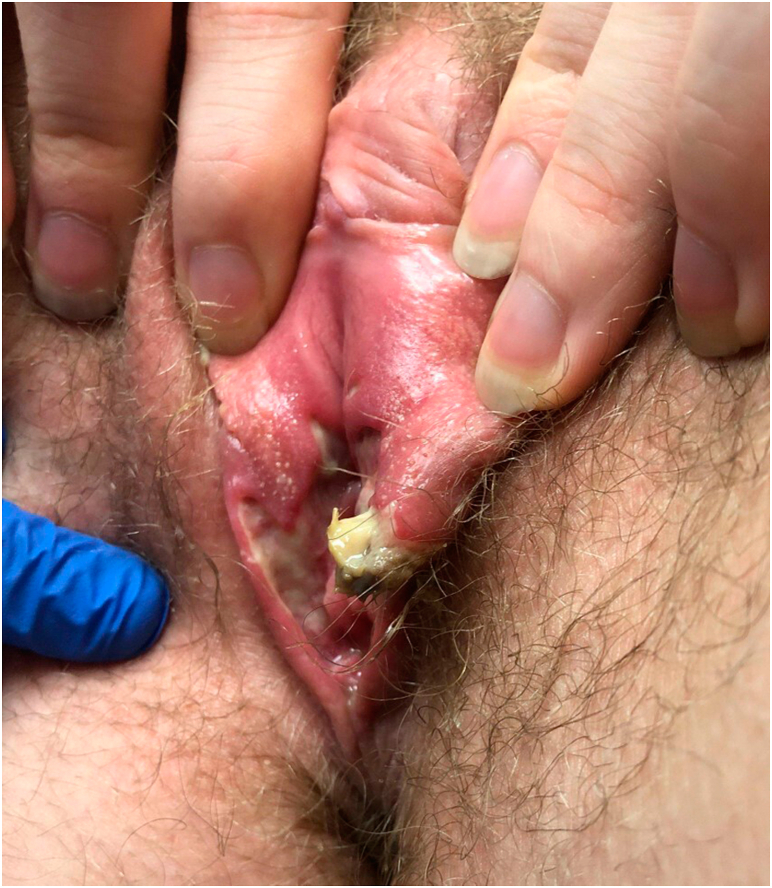
On examination, no oral ulcers were present, but vulvar edema and extensive ulceration were present bilaterally (left worse than right), with yellow, gray purulent drainage and pseudomembrane (Fig 1). A second punch biopsy of nonulcerated skin and tissue culture of the left vulva was completed. The patient was advised to continue oral antibiotics and to empirically start mupirocin and clobetasol ointment twice daily. The second punch biopsy of the left vulva showed dermal edema with vascular ectasia and sparse chronic inflammation and no signs of bacterial or fungal organisms. Tissue culture results showed Escherichia coli and rare candida, which were interpreted as asymptomatic colonization from GI/GU flora. Antinuclear antibody, Epstein-Barr virus panel, Lyme, and thyroid cascade were negative. C3 and C4 complement were mildly elevated at 181 and 50, respectively, though noted to be insignificant.
Fig 1.
Acute vulvar ulcers. Extensive vulvar ulceration and edema is apparent with yellow, gray purulent drainage and pseudomembrane.
Approximately 1 week later, they noted improvement with topical clobetasol, but persistent pain. The patient started 60-mg oral prednisone with a 15-day taper. Two weeks after their first appointment, they noted improvement in discomfort and treatment was continued with prednisone taper, clobetasol ointment, and the mupirocin ointment. The patient was counseled that this may recur in the future.
Discussion
Reactive nonsexually related acute genital ulcers (RNSRAGU) or acute vulvar ulcers (formerly known as Lipschutz ulcers) present as painful ulcers of the lower vagina or vulva and are often associated with a viral prodrome.1 These ulcers are known to form in response to many infectious triggers, but most recently, reports have been made of RNSRAGU from both COVID-19 infection and vaccination.1, 2, 3, 4, 5
There have been many dermatological reactions to both COVID infection and vaccine in the literature thus far, including acro-ischemia, pityriasis rosea, chilblain-like lesions, vesicular eruptions, and diffuse erythematous rash.1,2,6 Mucosal ulcerations are a known side effect of vaccines historically, but a growing number of cases to the COVID-19 vaccine have been reported.1,2 This case of acute vulvar ulcers in a 22-year-old adult 2 days after receiving the COVID-19 Pfizer booster vaccine adds to the evolving compendium of COVID-19 vaccine–related adverse reactions.
Acute vulvar ulcers have been historically known to form reactively to a wide range of infectious triggers, most commonly Epstein-Barr virus.7 Other reported triggers include cytomegalovirus, Mycoplasma, influenza, viral upper resipiratory infections, and gastroenteritis, Lyme disease, and most recently COVID-19 infection and vaccine.4,7,8 The etiology behind these ulcerations is not well understood, but it may be due to a type III hypersensitivity reaction causing microthromboses and necrosis of the genital mucosa. Another theory is that a virus instigates cytolysis from hematological spread or autoinoculation.8
The classic presentation is typically a 0.3- to 5-cm painful, purulent well-demarcated ulceration with a necrotic center of the lower vagina or vulva, though the labia minora is a common location.1,3 RNSRAGU is a clinical diagnosis of exclusion; thus, many other conditions are considered: infectious etiologies (HSV, syphilis, HIV), autoimmune (pemphigus and Behcet’s disease), local reactions to systemic inflammatory conditions (Crohns), drug reactions, and trauma.9
The treatment for acute vulvar ulcers secondary to COVID-19 or the vaccine has been focused on symptomatic pain relief, but antibiotics can be considered in settings where bacterial superinfection seem likely.10 For outpatient treatment, patients with moderate to severe infection may benefit from topical or oral steroid treatments, respectively. In the case of RNSRAGU and the COVID-19 infection and vaccine, multiple case reports note successful treatment with both oral and topical steroid use.1,4
Our hope is that this case will add to the growing literature on RNSRAGU following COVID-19 vaccination but also highlight the Pfizer booster vaccine specifically. Our patient had multiple other risk factors in addition to their booster, including a history of oral aphthous ulcers and 2 previous episodes of acute vulvar ulcers. Their infectious, autoimmune, and inflammatory workup was overall unremarkable, and thus, RNSRAGU was the diagnosis of exclusion. While the patient did not report gastrointestinal symptoms, we still recommended the patient follow-up with gastroenterology to rule out underlying disease. In the absence of reported common triggers for RNSRAGU, including recent infection (including COVID-19) and stress, their Pfizer booster was likely the trigger.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
Prior presentation: No prior presentations have been done on this case.
Consent: The patient gave consent for their medical information to be published in print and online and with the understanding that this information may be publicly available.
References
- 1.Cymerman R.M., Hoffmann R.K., Schaffer P.R., Pomeranz M.K. Vulvar infections: beyond sexually transmitted infections. Int J Dermatol. 2017;56(4):361–369. doi: 10.1111/ijd.13464. [DOI] [PubMed] [Google Scholar]
- 2.Krapf J.M., Casey R.K., Goldstein A.T. Reactive non-sexually related acute genital ulcers associated with COVID-19. BMJ Case Rep. 2021;14(5):E242653. doi: 10.1136/bcr-2021-242653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ueda Y., Kamiya K., Ohtsuki M. Reactive nonsexually related acute genital ulcers. J Gen Fam Med. 2017;19(1):30–31. doi: 10.1002/jgf2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosman I.S., David R.B., Bayliss S.J., White A.J., Merritt D.F. Acute genital ulcers in nonsexually active young girls: case series, review of the literature, and evaluation and management recommendations. Pediatr Dermatol. 2012;29(2):147–153. doi: 10.1111/j.1525-1470.2011.01589.x. [DOI] [PubMed] [Google Scholar]
- 5.Popatia S., Chiu Y.E. Vulvar aphthous ulcer after COVID-19 vaccination. Pediatr Dermatol. 2022;39(1):153–154. doi: 10.1111/pde.14881. [DOI] [PubMed] [Google Scholar]
- 6.Falkenhain-López D., Agud-Dios M., Ortiz-Romero P.L., Sánchez-Velázquez A. COVID-19-related acute genital ulcers. J Eur Acad Dermatol Venereol. 2020;34(11):e655–e656. doi: 10.1111/jdv.16740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drucker A., Corrao K., Gandy M. Vulvar aphthous ulcer following Pfizer-BioNTech COVID-19 vaccine - a case report. J Pediatr Adolesc Gynecol. 2021;35(2):165–166. doi: 10.1016/j.jpag.2021.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wojcicki A.V., O'Flynn O'Brien K.L. Vulvar aphthous ulcer in an adolescent after Pfizer-BioNTech (BNT162b2) COVID-19 vaccination. J Pediatr Adolesc Gynecol. 2021;35(2):167–170. doi: 10.1016/j.jpag.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falkenhain-López D., Agud-Dios M., Ortiz-Romero P.L., Sánchez-Velázquez A. COVID-19-related acute genital ulcers. J Eur Acad Dermatol Venereol. 2020;34(11):e655–e656. doi: 10.1111/jdv.16740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacyntho C.M.A., Lacerda M.I., Carvalho M.S.R., Ramos M.R.M.S., Vieira-Baptista P., Bandeira S.H.A.D. COVID-19 related acute genital ulcer: a case report. Einstein (Sao Paulo) 2022;20 doi: 10.31744/einstein_journal/2022RC6541. [DOI] [PMC free article] [PubMed] [Google Scholar]