Abstract
Background
Aerosol-borne diseases such as COVID-19 may outbreak occasionally in various regions of the world, inevitably resulting in short-term shortage and corresponding reuse of disposable respirators.
Aim
To investigate the effective disinfection methods, reusable duration and frequency of N95 respirators.
Methods
Based on the self-built respirator simulation test system, and under combinations of experimental conditions of three N95 respirators × 0–200 nm NaCl aerosols × three simulated breathing flow rates (15, 50 and 85 L/min) × two disinfection methods (dry heating and ultraviolet (UV) radiation), this study continuously measured the changes in filtration efficiency of all respirators during multi-cycles of ‘8-h simulated donning + disinfection’ until the penetration reached ≥5%.
Findings
Multi-cycles of dry heating and UV radiation treatments on the reused (i.e., multiple 8-h donning) N95 respirators had a minimal effect (<0.5%) on the respirator filtration efficiency, and even at 85 L/min, all tested N95 respirators were able to maintain filtration efficiencies ≥95% for at least 30 h or four reuse cycles of ‘8-h donning + disinfection’, while a lower breathing flow rate (15 L/min) plus the exhalation valve could further extend the N95 respirator's usability duration up to 140 h or 18 reuse cycles of ‘8-h donning + disinfection’. As the respirator wearing time extended, aerosol penetration slowly increased in a quadratic function with a negative second-order coefficient, and the penetration increment during each cycle of 8-h donning was less than 0.9%.
Conclusion
Multi-cycles of N95 respirator reuse in combination with dry heating or UV irradiation disinfection are feasible.
Keywords: N95 respirator, Disinfection, Reuse, Filtration efficiency
Introduction
Since January 2020, the COVID-19 epidemic remains at the global pandemic level. Aerial droplets and aerosols are the main transmission routes of SARS-CoV-2 and its variants [[1], [2], [3], [4], [5]]. Therefore, respirators are a necessity and consumable item during the epidemic. In the past two and a half years, occasional but frequent outbreaks of COVID-19 around the world have induced multiple unpredictable surges in demand for respirators in various regions, inevitably resulting in initial short-term severe shortages of respirators. In view of this fact, the World Health Organization and the national Centres for Disease Control of many countries have recommended appropriately extending the use time and frequency of single-use respirators [[6], [7], [8], [9]], but have not clearly stipulated the specific disinfection methods, reusable duration and frequency of respirators.
Previous studies revealed some disinfection methods that can effectively inactivate micro-organisms on respirators, including soap or hot water immersion, alcohol spraying, high-pressure steam, dry heating, ultraviolet (UV) radiation and vaporized hydrogen peroxide [10,11]. However, because most current respirators use electret filters as the filtration materials, disinfection treatments in the form of aqueous solutions or steam, such as soap or hot water immersion, alcohol spraying and high-pressure steam, will neutralize the static charge on the filters, resulting in a significant decrease in the filtration efficiency of respirators [[12], [13], [14], [15], [16], [17], [18]]. Dry heating, UV radiation and vaporized hydrogen peroxide are disinfection methods that have been proven to be effective in inactivating micro-organisms without compromising the respirator filtration efficiency [19]. Among them, vaporized hydrogen peroxide, which requires specialized equipment [[20], [21], [22]], is not as convenient as microwave ovens for dry heating and UV lamps for UV radiation. Therefore, more studies have focused on the effect of multi-cycles of dry heating and UV radiation on the filtration efficiency of respirators in order to provide guidance for the disinfectable frequency of respirators. At temperatures of 75 °C, 100 °C, 77 °C, 60 & 70 °C, and 85 °C, Côrtes et al. [23], Nguyen et al. [24], Ou et al. [14], Song et al. [25], and Liao et al. [13] performed dry heating on N95 respirators for 45, 50, 30, 60 and 5 min, respectively. They observed no significant changes in the filtration efficiency and structure of the respirators after 5, 20, 10, 3 and 50 cycles of disinfection, respectively. Viscusi et al. [26], Bergman et al. [27], Lindsley et al. [28], Jiang et al. [29], Ou et al. [14] and Liao et al. [13] conducted UV radiation on N95 respirators with 180 mJ/cm2 (40 W, 15 min), 1800 mJ/cm2 (40 W, 15 min), 0–950 J/cm2, 330 mJ/cm2 (5 min), >1000 mJ/cm2 and >3.6 J/cm2 (8 W, 30 min), respectively. Their results suggest that the protective performance of the respirators barely decreased after continuous 1, 3, 1, 5, 10 and 10 cycles of disinfection, respectively.
Although current studies have confirmed that dry heating and UV radiation can be repeatedly applied to disinfecting respirators without damaging their protective performance, these disinfection cycles were continuously performed one after another. That is, the reported filtration efficiencies were actually for the ‘unused + multiple disinfected respirators’. In summary, there is a lack of investigation on the change of filtration efficiency before and after each cycle of disinfection for reused respirators. In addition, existing research mostly adopts a constant flow to test respirators, which neither reflects the breathing flow pattern nor simulates the exhaled hot and humid air [30]. In view of the above research gaps, this study quantitatively explored the changes in filtration efficiency of N95 respirators during multi-cycles of ‘8-h simulated donning + disinfection’. The research results were expected to provide reference for the reusable duration and frequency of multi-cycle disinfected respirators.
Methods
Tested respirators
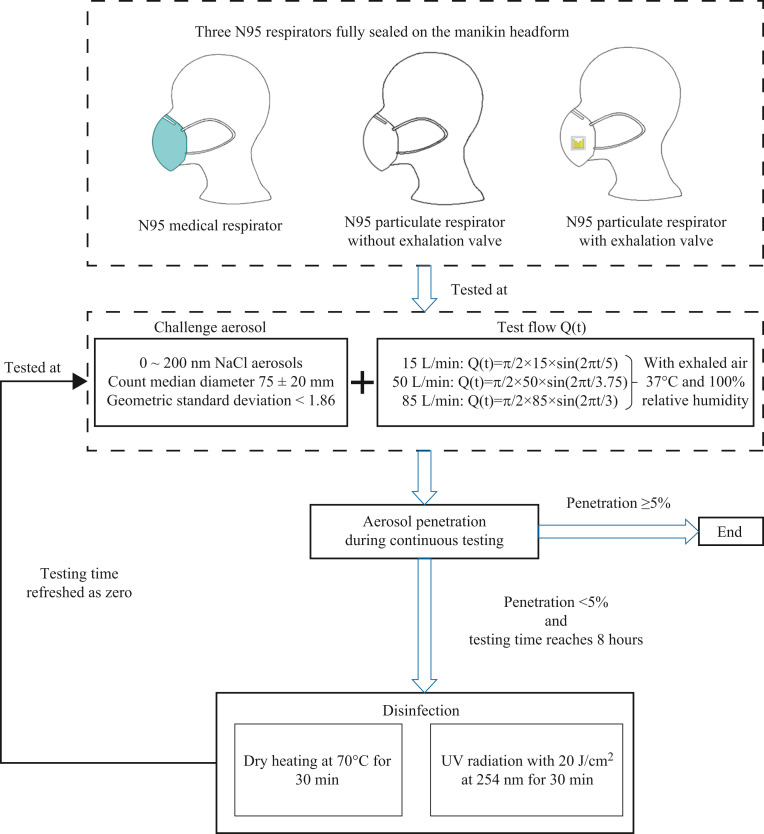
Three models of N95 respirators commonly used during the COVID-19 epidemic were tested: (1) N95 medical respirator (certified by China GB19083-2010 [31], GB2626-2019 [32] and US ASTM F2100-2019 [33], NIOSH 42 CFR Part 84–2019 [34]); (2) N95 particulate respirator without exhalation valve (certified by China GB2626-2019 [32] and US NIOSH 42 CFR Part 84–2019 [34]); and (3) N95 particulate respirator with exhalation valve (certified by China GB2626-2019 [32] and US NIOSH 42 CFR Part 84–2019 [34]). All the three N95 respirators are made with electret filters and have an arch structure.
Challenge aerosols
Traditional respiratory infectious diseases mainly rely on the transmission of micron-sized droplets produced by coughing and sneezing [35]. In contrast, with the increase in asymptomatic infections caused by the variants of SARS-CoV-2, aerosol transmission during normal breathing and speaking processes have attracted more and more attention [36,37]. With aerosols as carriers, 60- to 140-nm SARS-CoV-2 can be stably suspended in the air for a long time [38]. As SARS-CoV-2 can survive for 3 h in outdoor air and up to 16 h in confined spaces [39], it can be easily inhaled into the human respiratory system to cause infection [[40], [41], [42], [43]]. NaCl aerosols are able to substitute viral aerosols with similar particle sizes to test the respirator filtration efficiency [44,45], and are currently adopted by respirator testing standards in the USA, the EU and China. In this study, NaCl aerosols with the particle size 0–200 nm were regarded as the challenge aerosols. This particle size covers the most penetrating particle size of electret filters (40–70 nm) [46,47], and thus can represent the most stringent testing conditions for respirators.
Test flow rates
Human breathing flow approximates the sinusoidal time-varying cyclic flow [[48], [49], [50]], and at the same mean inhalation flow, the aerosol penetration of a respirator measured under the sinusoidal cyclic flow may be higher than that of the constant flow adopted in the current respirator testing standards [[50], [51], [52]]. In view of this, our research team developed a breathing simulator, which can generate sinusoidal cyclic flow and has the function of heating and humidifying the exhaled air (for more details, see Zhu et al. [47]). In this study, three sinusoidal time-varying cyclic flow rates with mean inhalation flows of 15, 50 and 85 L/min and corresponding breathing frequencies of 12, 16 and 20 breaths/min were set as the test flow rates, respectively, and the exhaled air was set to 37 °C and 100% relative humidity.
Test system
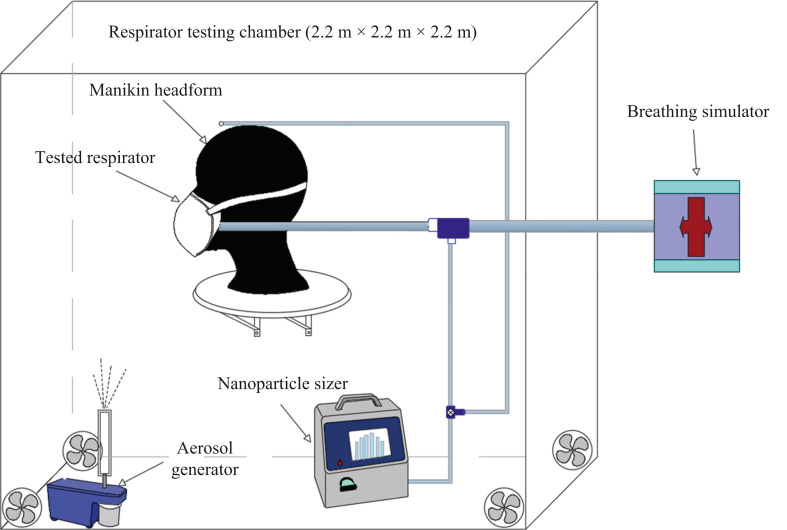
Figure 1 shows the respirator simulation test system developed in this study. As can be seen, the synthetic resin made test chamber is 2.2 m × 2.2 m × 2.2 m in size. In the chamber, an aerosol generator (Model 8026, TSI Inc, Shoreview, MN, USA) was used to atomize and generate NaCl aerosol at a steady flow rate. The respirator to be tested was fully sealed on the manikin headform with adhesive tape and placed in the centre of the test chamber. The mouth and nose area of the headform was connected to the self-developed breathing simulator. A nanoparticle sizer (Model 3910, TSI Inc, Shoreview, MN, USA) was adopted to measure the count concentration of NaCl aerosol inside and outside the tested respirator, respectively. For more details about the experimental set-up, see Zhu et al. [47].
Figure 1.
Respirator simulation test system.
Disinfection methods
Effective disinfection methods for respirators should simultaneously meet the following four requirements [53]: (1) effectively inactivate micro-organisms on the respirator; (2) not damage the respirator filtration efficiency; (3) not affect the respirator face seal fit; and (4) be safe for the respirator wearer. Based on the existing research findings, this study selected dry heating and UV radiation to disinfect the three N95 respirators after each cycle of ‘8-h simulated donning’. On the premise of ensuring the complete inactivation of micro-organisms on the respirator, specific disinfection parameters were set as follows: (1) dry heating: drying the respirator at 70 °C for 30 min, according to Xiang et al. [25] and China National Health Commission [54]. (2) UV radiation: irradiating both sides of the respirator for 30 min by using 20 J/cm2 UV rays at 254 nm wavelength, based on Liao et al. [13] and Lindblad et al. [55].
Testing flow chart
The experimental conditions of this study are summarized as follows: three N95 respirators × one challenge aerosol × three breathing flow rates × two disinfection methods. For each combination of experimental conditions, one brand new respirator was tested for multi-cycles of ‘8-h simulated donning + disinfection’ until the penetration reached ≥5%. The specific testing flow chart is illustrated in Figure 2 .
Figure 2.
Testing flow chart of multi-cycles of ‘8-h simulated donning + disinfection’ for three N95 respirators at three breathing flow rates.
Results
Change of penetration during the first 8-h simulated donning process
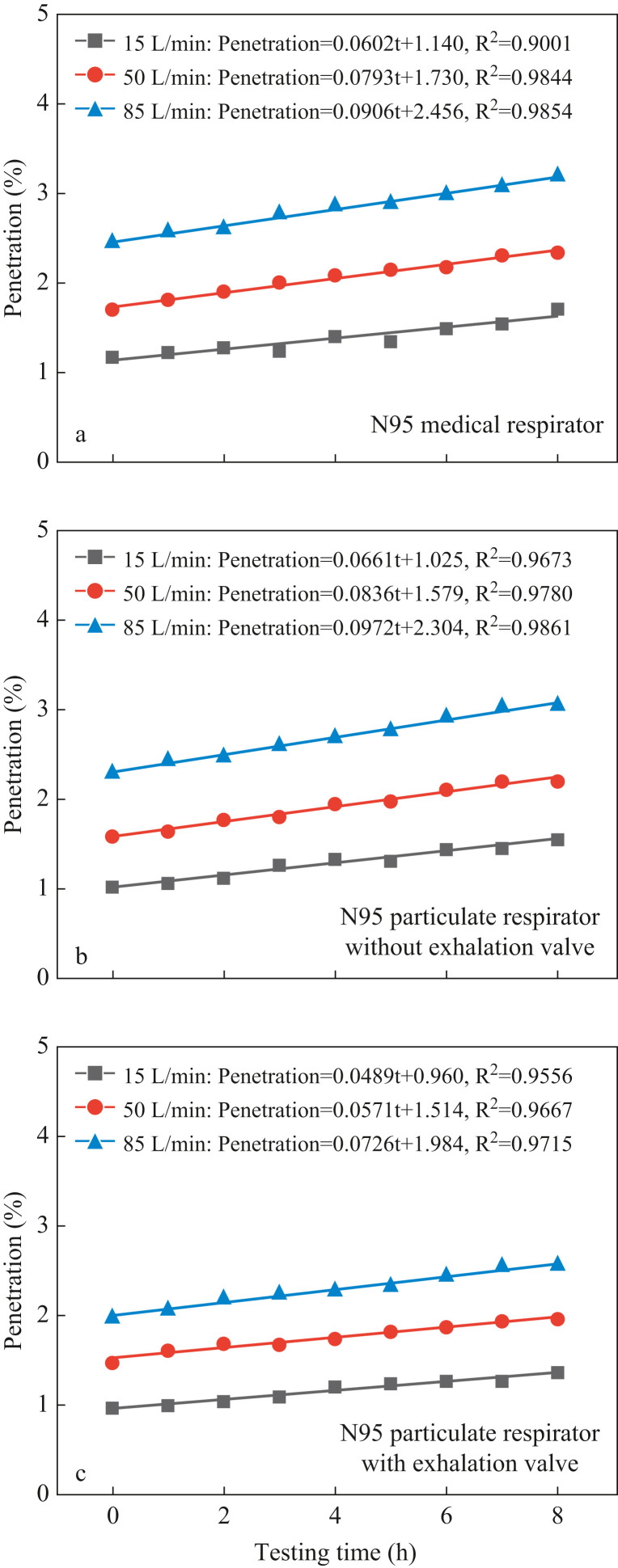
The aerosol penetration of three N95 respirators at three breathing flow rates during the first 8-h simulated donning process is shown in Figure 3 . As can be observed, under all three simulated breathing flow rates, the aerosol penetration of all three tested N95 respirators increases linearly with the passage of testing time. This finding, which is consistent with several previous studies [[56], [57], [58], [59], [60]], can be explained by the following: First, during the simulated donning process, aerosols in the inhaled airflow will continuously deposit on the electret fibre. Second, the 37 °C and 100% relative humidity exhaled airflow will also cause continuous condensation of water vapour on the electret fibre. These two factors jointly induce the gradual weakening of electrostatic attraction of the electret filter, resulting in the gradual increase in penetration with the passage of testing time [61]. Furthermore, it was found that the linear regression slope was greater for a higher breathing flow rate, which could be attributed to the higher weakening speed of the electrostatic attraction caused by the higher aerosol deposition and water vapour condensation rate under a higher flow rate. To be specific, for all three tested N95 respirators, the linear slopes under flow rates of 15, 50 and 85 L/min were generally smaller than 0.07, 0.085 and 0.1, respectively. Correspondingly, the penetration increments during the first 8-h simulated donning process are less than 0.55%, 0.65% and 0.8%, respectively. With the initial penetration values smaller than 2.5% and the 8-h increment less than 0.8%, all three N95 respirators after 8-h simulated donning continued to guarantee filtration efficiencies >96.7%, indicating that the extended use of N95 respirators over 8 h is feasible.
Figure 3.
Aerosol penetration during the first 8-h simulated donning process at three breathing flow rates for three N95 respirators.
It was also found that as the breathing flow rate rose, the penetration of all three N95 respirators was significantly promoted. Zhu et al. [46], Mahdavi et al. [48], Qian et al. [49] and Kunda et al. [62] also reported similar findings. This is because the electret filter mainly relies on filtration mechanisms of electrostatic attraction and diffusion to capture nanoscale aerosols. Specifically, at a higher flow rate, aerosols can pass through the respirator filter within a shorter period of time; resultantly, the function of electrostatic attraction and diffusion is weakened, such that more aerosols penetrate into the respirator cavity [63]. In this study, the penetration values for N95 respirators under 15, 50 and 85 L/min during the first 8-h simulated donning process are smaller than 1.5%, 2.5% and 3.3%, respectively, indicating that the protection level and usable time of respirators are closely related to the activity intensity.
Influence of each cycle of disinfection on the penetration
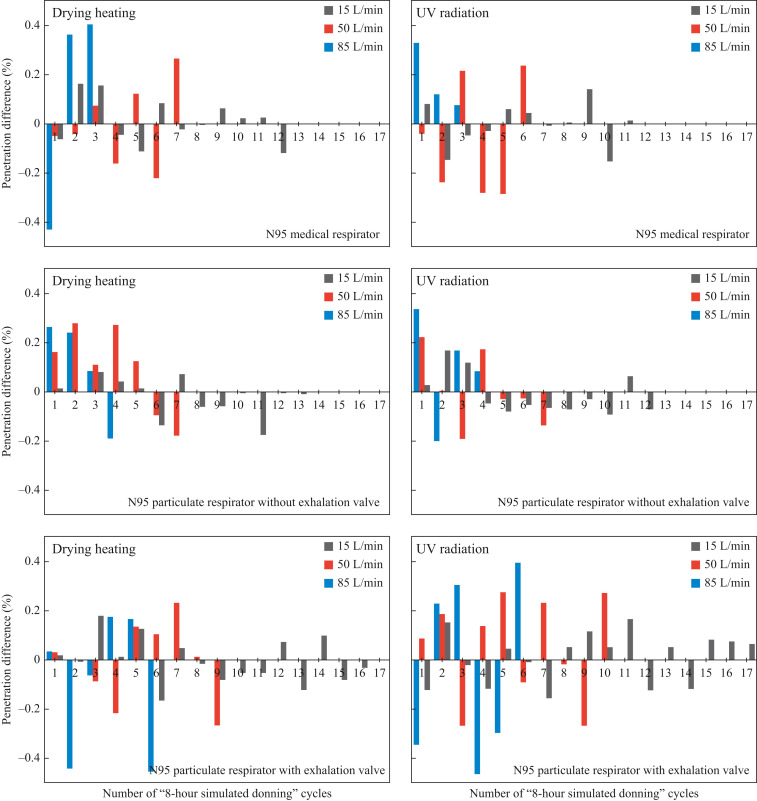
During multi-cycles of ‘8-h simulated donning + disinfection’, the penetration value after each cycle of disinfection minus the corresponding value before that cycle of disinfection, referred to as ‘penetration difference’ in this study, was obtained and is presented in Figure 4 . The penetration differences for three N95 respirators donned with three simulated breathing flow rates were all within ± 0.5%, indicating that dry heating and UV radiation treatments rarely affect the filtration efficiency of ‘reused respirators subject to multi-cycles of 8-h disinfection’. Under each combination of experimental conditions, no clear changing trend was observed for the penetration differences among different cycles. The above findings agree well with previous studies related to multi-cycles of dry heating or UV radiation on N95 respirators [[10], [11], [12], [13], [14], [15]], which confirms that both dry heating and UV radiation have little influence on the charging state and structure of the electret filter. Moreover, it was disclosed that the penetration differences at 15, 50 and 85 L/min were within ± 0.2%, ± 0.3% and ± 0.5%, respectively. The reason is that for the same respirator, a higher testing flow measures a higher penetration value (see Figure 3); in other words, a higher breathing flow rate will enlarge the ‘measured penetration differences’ and report a greater penetration difference.
Figure 4.
Penetration differences before and after each cycle of disinfection for three N95 respirators donned at three simulated breathing flow rates. UV, ultraviolet.
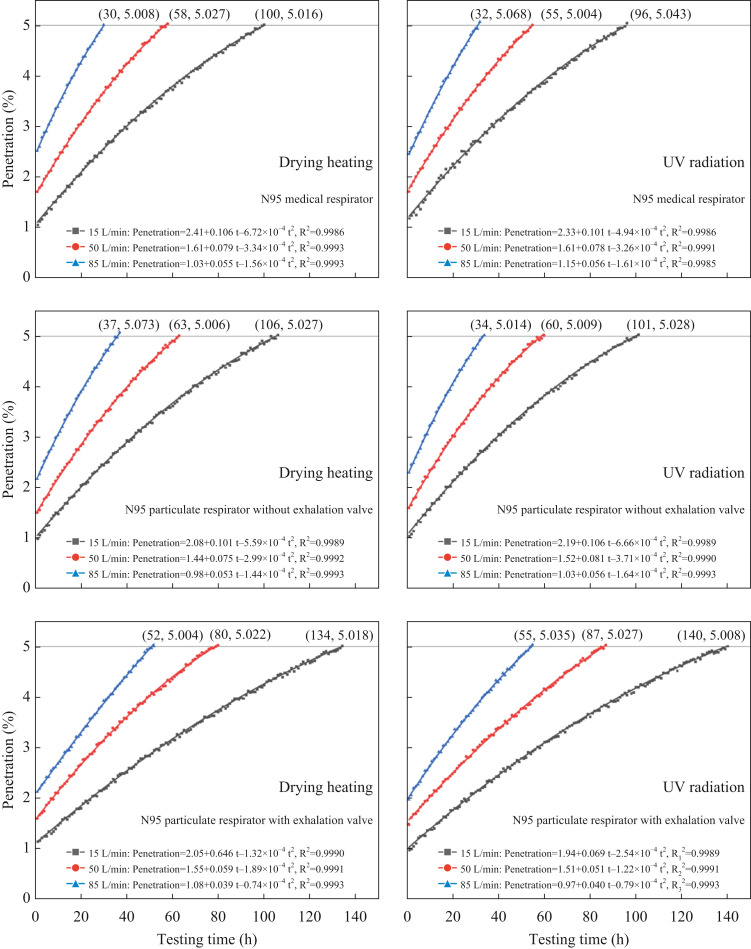
Change of penetration during multi-cycles of ‘8-h simulated donning + disinfection’
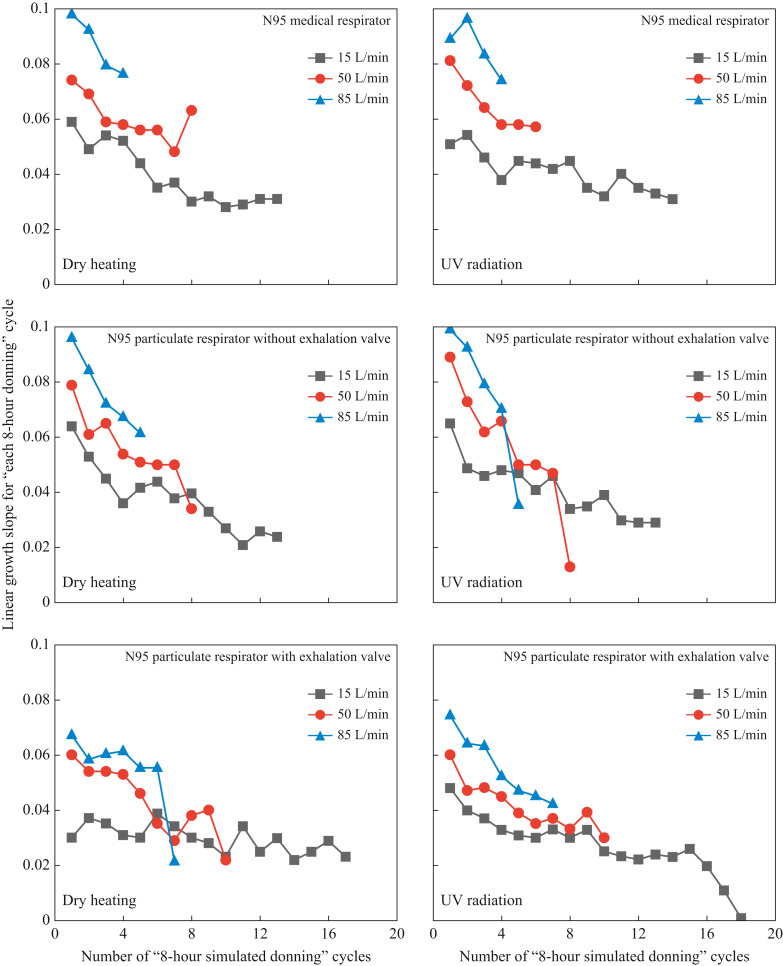
As presented in Figure 5 , all the change curves of aerosol penetration during multi-cycles of ‘8-h simulated donning + disinfection’ until the penetration value ≥5% could be well fitted by quadratic functions of testing time, with correlation coefficients R2 ≈ 0.999. As the testing time went by, the penetration value gradually increased at a decelerating rate. This finding can also be confirmed by Figure 6 where the slopes of linear regression of penetration on testing time during each cycle of 8-h donning are illustrated (data processing method similar to that given above and Figure 3). That is, as the number of test cycles grew, the linear growth slopes for each 8-h donning cycle showed an obvious downward trend. This phenomenon can be explained as follows: during the respirator donning process, the deposition of inhaled aerosols on the filter and the wetting of filter by exhaled hot and humid air occured simultaneously. The former weakened the electrostatic attraction of the filter on the one hand and promoted the mechanical filtration efficiency (including diffusion, interception, and inertial collision) on the other hand [56,57], while the latter continuously neutralized the charge on the electret filters [[58], [59], [60]]. It is well known that electrostatic attraction and diffusion dominate the filtration of nanoscale aerosols by electret filters [63]. Besides, during the simulated donning process, the decay of electrostatic attraction was stronger than the enhancement of diffusion caused by aerosol deposition. Hence, the aerosol penetration was gradually promoted with the passage of testing time. However, as the charge on the filter reduced and the aerosols continuously accumulated on the filter, the electrostatic attraction was constantly weakening, while the mechanical filtration mechanism, which included diffusion, was becoming dominant [59]. Consequently, the increase in aerosol penetration decelerated.
Figure 5.
Aerosol penetration during multi-cycles of ‘8-h simulated donning + disinfection’ for three N95 respirators donned at three breathing flow rates. UV, ultraviolet.
Figure 6.
Slopes of linear regression on penetration during each cycle of 8-h donning process for three N95 respirators tested at three breathing flow rates. UV, ultraviolet.
Figure 5, Figure 6 show that the higher the breathing flow rate, the higher the penetration values at different moments, and the earlier the penetration values exceed 5%. Such a result was attributed to the following two aspects: First, under a higher breathing flow rate, aerosols will penetrate through the electret filter more quickly, leaving less time for the electrostatic attraction and diffusion to function well to capture aerosols [46,48,49,62]. Second, from the perspective of both aerosol deposition in the inspiratory process and water vapour condensation in the expiratory stage, a higher breathing flow rate will speed up the charge removal rate of the electret filter, resulting in an accelerated increase in aerosol penetration (see the penetration increasing trend in Figure 5 and the linear growth slopes in Figure 6). To be specific, under 15, 50 and 85 L/min, the tested N95 respirators can keep filtration efficiencies ≥95% for at least about 100, 60 and 30 h, corresponding to 12, 7 and 4 reuse cycles of ‘8-h simulated donning + disinfection’, respectively.
In addition, it was noted that under different breathing flow rates, the increasing speed of penetration for N95 respirators with exhalation valves was much lower than those for N95 medical respirators and N95 respirators without exhalation valves (see Figure 4, Figure 5, Figure 6). To be specific, with filtration efficiency ≥95%, under 15, 50 and 85 L/min, the N95 respirator with exhalation valve can be used for about 36, 24 and 20 h longer than the N95 respirator without an exhalation valve, corresponding to 4.5, 3 and 2.5 more reuse cycles of ‘8-h simulated donning + disinfection’. This finding confirms the explanation that the 37 °C and 100% relative humidity exhaled airflow will weaken the electrostatic attraction of the electret filter. Because the exhaled air of the N95 particulate respirator with exhalation valve mainly pass through the exhalation valve [64], the corresponding weakening speed of electrostatic attraction should be lower, resulting in a relatively slower increase in penetration.
Guidelines for healthcare workers on disinfection and reuse of N95 respirators
Firstly, both dry heating and UV irradiation are applicable to disinfection of reused N95 respirators. Secondly, the respirator needs to be disinfected before each time of reuse, even if it is used less than 8 h after its last disinfection. Thirdly, the face seal fitness and breakage condition of the respirator should be carefully checked before reuse. Lastly and most importantly, N95 respirators with exhalation valves cannot used by healthcare workers in sterile rooms.
The main conclusions in this study are summarized as follows: (1) For N95 respirators after multi-cycles of 8-h donning, dry heating and UV radiation treatments have a minimal effect on their filtration efficiency (within ± 0.5%). That is, both methods are applicable to disinfection of reused respirators. (2) As the respirator wearing time extends, aerosol penetration slowly increases in quadratic function with negative second-order coefficient, and the penetration increment during each cycle of 8-h donning is less than 0.8%. (3) N95 respirators are able to maintain filtration efficiencies of ≥95% for at least 30 h or four reuse cycles of ‘8-h donning + disinfection’, while lower breathing flow rate plus exhalation valve can further extend this time duration up to 140 h or 18 reuse cycles of ‘8-h donning + disinfection’.
Conflict of interest statement
None declared.
Funding sources
This work was supported by the National Natural Science Foundation of China (Nos. 51904291, 52174222, 51674252 and 51974300), the Basic Research Program of Jiangsu Province (No. BK20190638), the Project funded by China Postdoctoral Science Foundation (No. 2020M681781), and the Jiangsu Planned Projects for Postdoctoral Research Funds (No. 2020Z076).
References
- 1.Dhand R., Li J. Coughs and sneezes: their role in transmission of respiratory viral infections, including SARS-CoV-2. Am J Respir Crit Care Med. 2020;202:651–659. doi: 10.1164/rccm.202004-1263PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong S.C., Chen H., Lung D.C., Ho P.L., Yuen K.Y., Cheng V.C. To prevent SARS-CoV-2 transmission in designated quarantine hotel for travelers: is the ventilation system a concern? Indoor Air. 2021;31:1295–1297. doi: 10.1111/ina.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Y., Huang J., Zhang L., Chen S., Gao J., Jiao H. The global transmission of new coronavirus variants. Environ Res. 2022;206 doi: 10.1016/j.envres.2021.112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong S.C., Au A.K., Chen H., Yuen L.L., Li X., Lung D.C., et al. Transmission of Omicron (B.1.1.529) – SARS-CoV-2 Variant of Concern in a designated quarantine hotel for travelers: a challenge of elimination strategy of COVID-19. Lancet Reg Health West Pac. 2022;18 doi: 10.1016/j.lanwpc.2021.100360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyerowitz E.A., Richterman A., Gandhi R.T., Sax P.E. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med. 2021;174:69–79. doi: 10.7326/M20-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . WHO; Geneva: 2020. Advice on the use of masks in the context of COVID-19: interim guidance, 5 June 2020.https://apps.who.int/iris/handle/10665/332293 Available at: [last accessed May 2022] [Google Scholar]
- 7.Centers for Disease Control and Prevention . GDC; Atlanta, GA: 2020. Implementing filtering facepiece respirator (FFR) reuse, including reuse after decontamination, when there are known shortages of N95 respirators.https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html Available at: [last accessed May 2022] [Google Scholar]
- 8.Food and Drug Administration . U.S FDA; 2020. Investigating decontamination and reuse of respirators in public Health emergencies.https://www.fda.gov/emergency-preparedness-and-response/mcm-regulatory-science/investigating-decontamination-and-reuse-respirators-public-health-emergencies Available at: [last accessed May 2022] [Google Scholar]
- 9.Pereira-Ávila F.M.V., Lam S.C., Góes F.G.B., Gir E., Pereira-Caldeira N.M.V., Teles S.A., et al. Factors associated with the use and reuse of face masks among Brazilian individuals during the COVID-19 pandemic. Rev Lat Am Enfermagem. 2020;28 doi: 10.1590/1518-8345.4604.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Martinez C.E., Sossa-Briceño M.P., Cortés J.A. Decontamination and reuse of N95 filtering facemask respirators: a systematic review of the literature. Am J Infect Control. 2020;48:1520–1532. doi: 10.1016/j.ajic.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnatta J.R., Souza R.Q., Lemos C.S., Oliveira R.A., Martins L.R., Moriya G.A.A., et al. Safety in the practice of decontaminating filtering facepiece respirators: a systematic review. Am J Infect Control. 2021;49:825–835. doi: 10.1016/j.ajic.2020.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubio-Romero J.C., Pardo-Ferreira M.D.C., Torrecilla-García J.A., Calero-Castro S. Disposable masks: Disinfection and sterilization for reuse, and non-certified manufacturing, in the face of shortages during the COVID-19 pandemic. Saf Sci. 2020;129 doi: 10.1016/j.ssci.2020.104830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao L., Xiao W., Zhao M., Yu X., Wang H., Wang Q., et al. Can N95 respirators be reused after disinfection? How many times? ACS Nano. 2020;14(5):6348–6356. doi: 10.1021/acsnano.0c03597. [DOI] [PubMed] [Google Scholar]
- 14.Ou Q., Pei C., Chan Kim S., Abell E., Pui D.Y.H. Evaluation of decontamination methods for commercial and alternative respirator and mask materials – view from filtration aspect. J Aerosol Sci. 2020;150 doi: 10.1016/j.jaerosci.2020.105609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juang P.S.C., Tsai P. N95 Respirator cleaning and reuse methods proposed by the inventor of the N95 mask material. J Emerg Med. 2020;58:817–820. doi: 10.1016/j.jemermed.2020.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackenzie D. Reuse of N95 Masks. Engineering. 2020;6:593–596. doi: 10.1016/j.eng.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grinshpun S.A., Yermakov M., Khodoun M. Autoclave sterilization and ethanol treatment of re-used surgical masks and N95 respirators during COVID-19: impact on their performance and integrity. J Hosp Infect. 2020;105:608–614. doi: 10.1016/j.jhin.2020.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Vossen J.M.B.M., Heerikhuisen M., Traversari R.A.A.L., van Wuijckhuijse A.L., Montijn R.C. Heat sterilization dramatically reduces filter efficiency of the majority of FFP2 and KN95 respirators. J Hosp Infect. 2021;107:87–90. doi: 10.1016/j.jhin.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludwig-Begall L.F., Wielick C., Dams L., Nauwynck H., Demeuldre P.F., Napp A., et al. The use of germicidal ultraviolet light, vaporized hydrogen peroxide and dry heat to decontaminate face masks and filtering respirators contaminated with a SARS-CoV-2 surrogate virus. J Hosp Infect. 2020;106:577–584. doi: 10.1016/j.jhin.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumkrong P., Scoles L., Brunet Y., Baker S., Mercier P.H.J., Poirier D. Evaluation of hydrogen peroxide and ozone residue levels on N95 masks following chemical decontamination. J Hosp Infect. 2021;111:117–124. doi: 10.1016/j.jhin.2021.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger D., Gundermann G., Sinha A., Moroi M., Goyal N., Tsai A. Review of aerosolized hydrogen peroxide, vaporized hydrogen peroxide, and hydrogen peroxide gas plasma in the decontamination of filtering facepiece respirators. Am J Infect Control. 2022;50:203–213. doi: 10.1016/j.ajic.2021.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grossman J., Pierce A., Mody J., Gagne J., Sykora C., Sayood S., et al. Institution of a novel process for N95 respirator disinfection with vaporized hydrogen peroxide in the setting of the COVID-19 pandemic at a large academic medical center. J Am Coll Surg. 2020;231:275–280. doi: 10.1016/j.jamcollsurg.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Côrtes M.F., Espinoza E.P.S., Noguera S.L.V., Silva A.A., de Medeiros M.E.S.A., Villas Boas L.S., et al. Decontamination and re-use of surgical masks and respirators during the COVID-19 pandemic. Int J Infect Dis. 2021;104:320–328. doi: 10.1016/j.ijid.2020.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh C., Araud E., Puthussery J.V., Bai H., Clark G.G., Wang L., et al. Dry heat as a decontamination method for N95 respirator reuse. Environ Sci Technol Lett. 2020;7:677–682. doi: 10.1021/acs.estlett.0c00534. [DOI] [PubMed] [Google Scholar]
- 25.Xiang Y., Song Q., Gu W. Decontamination of surgical face masks and N95 respirators by dry heat pasteurization for one hour at 70°C. Am J Infect Control. 2020;48:880–882. doi: 10.1016/j.ajic.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viscusi D.J., Bergman M.S., Eimer B.C., Shaffer R.E. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg. 2009;53:815–827. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergman M.S., Viscusi D.J., Heimbuch B.K., Wander J.D., Shaffer R.E. Evaluation of multiple (3-cycle) decontamination processing for filtering facepiece respirators. J Eng Fibers Fabr. 2010;5:33–41. [Google Scholar]
- 28.Lindsley W.G., Martin S.B., Thewlis R.E., Sarkisian K., Nwoko J.O., Mead K.R., et al. Effects of ultraviolet germicidal irradiation (UVGI) on N95 respirators filtration performance and structural integrity. j Occup Environ Hyg. 2015;12:509–517. doi: 10.1080/15459624.2015.1018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang Z.Y., Huang Z., Schmale I., Brown E.L., Lorenz M.C., Patlovich S.J., et al. N95 respirator reuse, decontamination methods, and microbial burden: a randomized controlled trial. Am J Otolaryngol. 2021;42 doi: 10.1016/j.amjoto.2021.103017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coooper E.A. A comparison of the respiratory work done against an external resistance by man and by a sine-wave pump. Q J Exp Physiol Cogn Med Sci. 1960;45:179–191. doi: 10.1113/expphysiol.1960.sp001456. [DOI] [PubMed] [Google Scholar]
- 31.State Administration for Market Regulation of China (SAMR) 2010. Standardization Administration of China (SA) GB19083-2010: Technical requirements for protective face mask for medical use (in Chinese) [Google Scholar]
- 32.State Administration for Market Regulation of China (SAMR) 2019. Standardization Administration of China (SA) [GB2626-2019: Respiratory protection—non-powered air-purifying particle respirator] (in Chinese) [Google Scholar]
- 33.ASTM International . West Conshohocken; PA, USA: 2021. Astm F2100-21: standard specification for performance of materials used in medical face masks. [Google Scholar]
- 34.National Institute for Occupational Safety and Health (NIOSH) 2019. 42 CFR Part 84-2019: respiratory protective devices. [Google Scholar]
- 35.Ehsanifar M. Airborne aerosols particles and COVID-19 transition. Environ Res. 2021;200 doi: 10.1016/j.envres.2021.111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bourouiba L., Dehandschoewercker E., Bush John W.M. Violent expiratory events: on coughing and sneezing. J Fluid Mech. 2014;745:537–563. [Google Scholar]
- 37.Lindsley W.G., Reynolds J.S., Szalajda J.V., Noti J.D., Beezhold D.H. A cough aerosol simulator for the study of disease transmission by human cough-generated aerosols. Aerosol Sci Tech. 2013;47:937–944. doi: 10.1080/02786826.2013.803019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fears A.C., Klimstra W.B., Duprex P., Hartman A., Weaver S.C., Plante K.S., et al. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerg Infect Dis. 2020;26:2168–2171. doi: 10.3201/eid2609.201806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheuch G. Breathing is enough: for the spread of influenza virus and SARS-CoV-2 by breathing only. J Aerosol Med Pulm Drug Deliv. 2020;33:230–234. doi: 10.1089/jamp.2020.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asadi S., Bouvier N., Wexler A.S., Ristenpart W.D. The coronavirus pandemic and aerosols: does COVID-19 transmit via expiratory particles? Aerosol Sci Tech. 2020:1–4. doi: 10.1080/02786826.2020.1749229. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y., Xie J., Huang F., Cao L. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang S., Mao Y., Jones R.M., Tan Q., Ji J.S., Li N., et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ Int. 2020;144 doi: 10.1016/j.envint.2020.106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bałazy A., Toivola M., Adhikari A., Sivasubramani S.K., Reponen T., Grinshpun S.A. Do N95 masks provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am J Infect Control. 2006;34:51–57. doi: 10.1016/j.ajic.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 45.Eninger R.M., Honda T., Adhikari A., Heinonen-Tanski H., Reponen T., Grinshpun S.A. Filter performance of n99 and n95 facepiece respirators against viruses and ultrafine particles. Ann Occup Hyg. 2008;52:385–396. doi: 10.1093/annhyg/men019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu J., He X., Guffey S., Wang L., Wang H., Cheng J. Performance comparison of N95 and P100 filtering facepiece respirators with presence of artificial leakage. Ann Work Expo Health. 2020;64:202–216. doi: 10.1093/annweh/wxz086. [DOI] [PubMed] [Google Scholar]
- 47.Zhu J., He X., Wang L., Liao X., Teng G., Jing P. Performance of N95 elastomeric respirators in high humidity and high coal dust concentration environment. Int J Min Sci Techno. 2022;32:215–224. [Google Scholar]
- 48.Bahloul A., Mahdavi A., Haghighat F., Ostiguy C. Evaluation of N95 filtering facepiece respirator efficiency with cyclic and constant flows. J Occup Environ Hyg. 2014;11:499–508. doi: 10.1080/15459624.2013.877590. [DOI] [PubMed] [Google Scholar]
- 49.Qian Y., Willeke K., Grinshpun S.A., Donnelly J., Coffey C.C. Performance of N95 respirators: filtration efficiency for airborne microbial and inert particles. Am Ind Hyg Assoc J. 1998;59:128–132. doi: 10.1080/15428119891010389. [DOI] [PubMed] [Google Scholar]
- 50.Eshbaugh J.P., Gardner P.D., Richardson A.W., Hofacre K.C. N95 and p100 respirator filter efficiency under high constant and cyclic flow. J Occup Environ Hyg. 2009;6:52–61. doi: 10.1080/15459620802558196. [DOI] [PubMed] [Google Scholar]
- 51.Gardner P.D., Eshbaugh J.P., Harpest S.D., Richardson A.W., Hofacre K.C. Viable viral efficiency of N95 and P100 respirator filters at constant and cyclic flow. J Occup Environ Hyg. 2013;10:564–572. doi: 10.1080/15459624.2013.818228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCullough N.V., Brosseau L.M., Vesley D. Collection of three bacterial aerosols by respirator and surgical mask filters under varying conditions of flow and relative humidity. Ann Occup Hyg. 1997;41:677–690. doi: 10.1016/S0003-4878(97)00022-7. [DOI] [PubMed] [Google Scholar]
- 53.Mackenzie D. Reuse of N95 Masks. Engineering (Beijing) 2020;6:593–596. doi: 10.1016/j.eng.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.National Health and Health Commission of the People’s Republic of China Diagnosis and treatment guidelines for 2019 novel coronavirus pneumonia (Draft version 9) http://www.nhc.gov.cn/yzygj/s7653p/202203/b74ade1ba4494583805a3d2e40093d88.shtml Available at: [last accessed May 2022]
- 55.Lindblad M., Tano E., Lindahl C., Huss F. Ultraviolet-C decontamination of a hospital room: amount of UV light needed. Burns. 2020;46:842–849. doi: 10.1016/j.burns.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Kerner M., Schmidt K., Schumacher S., Asbach C., Antonyuk S. Ageing of electret filter media due to deposition of submicron particles – experimental and numerical investigations. Sep Purif Technol. 2020;251 [Google Scholar]
- 57.Cai R.-R., Lu H., Zhang L.-Z. Mechanisms of performance degradation and efficiency improvement of electret filters during neutral particle loading. Powder Technol. 2021;382:133–143. [Google Scholar]
- 58.Li Y., Wong T., Chung J., Guo Y.P., Hu J.Y., Guan Y.T., et al. In vivo protective performance of N95 respirator and surgical facemask. Am J Ind Med. 2010;49:1056–1065. doi: 10.1002/ajim.20395. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y., Lin Z., Zhang W. Comparison of effects of particle charging, media characteristics, humidity and aerosols on loading performance of electret media. Build Environ. 2020;179 [Google Scholar]
- 60.Wu S., Cai R., Zhang L. Modeling and prediction of loading characteristics of electret filter media for PM2.5. Build Environ. 2022;207 [Google Scholar]
- 61.Mahdavi A., Haghighat F., Bahloul A., Brochot C., Ostiguy C. Particle loading time and humidity effects on the efficiency of an N95 filtering facepiece respirator model under constant and inhalation cyclic flows. Ann Occup Hyg. 2015;59:629–640. doi: 10.1093/annhyg/mev005. [DOI] [PubMed] [Google Scholar]
- 62.Konda A., Prakash A., Moss G., Schmoldt M., Grant G., Guha S. Correction to aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano. 2020;14:10742–10743. doi: 10.1021/acsnano.0c04676. [DOI] [PubMed] [Google Scholar]
- 63.Tcharkhtchi A., Abbasnezhad N., Zarbini Seydani M., Zirak N., Farzaneh S., Shirinbayan M. An overview of filtration efficiency through the masks: mechanisms of the aerosols penetration. Bioact Mater. 2020;6:106–122. doi: 10.1016/j.bioactmat.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang J.C., Johnson J.S., Olmsted R.N. Demystifying theoretical concerns involving respirators with exhalation valves during COVID-19 pandemic. Am J Infect Control. 2020;48:1564–1565. doi: 10.1016/j.ajic.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]