Abstract
A direct viable count (DVC) procedure was developed which clearly and easily discriminates the viability of bacterial cells. In this quantitative DVC (qDVC) procedure, viable cells are selectively lysed by spheroplast formation caused by incubation with antibiotics and glycine. This glycine effect leads to swollen cells with a very loose cell wall. The viable cells then are lysed easily by a single freeze-thaw treatment. The number of viable cells was obtained by subtracting the number of remaining cells after the qDVC procedure from the total cell number before the qDVC incubation. This improved procedure should provide useful information about the metabolic potential of natural bacterial communities.
The introduction of the concept of viable but nonculturable cells by Byrd and Colwell's group in the 1980s has led to important research work concerning the existence and significance of these kinds of cells within natural bacterial communities (5, 7). The enumeration of living bacteria in the natural environment has been carried out by using various methods (2, 4, 27, 30), including the direct viable count (DVC) method. The original DVC method was first reported by Kogure's group in 1979 for enumeration of viable bacteria in a natural marine environment (15). This method is based on the incubation of samples with a single antimicrobial agent (nalidixic acid) and nutrients (yeast extract). Nalidixic acid acts as a specific inhibitor of DNA synthesis and prevents cell division without affecting other cellular metabolic activities (8). The resulting cells can continue to metabolize nutrients and become elongated and/or fattened after incubation.
The original DVC method, however, poses two difficulties for application to complex communities. First, in a natural aquatic environment, there are bacteria which are resistant to the antimicrobial agent used, and they are able to grow without the formation of elongated and/or fattened cells. Joux and LeBaron used an antibiotic cocktail to overcome this difficulty and then demonstrated the effectiveness of their improved DVC procedure (12). Second, it is often difficult to discriminate between elongated (fattened) cells and cells which are not elongated or fattened (6). Elongated cells may be smaller than the population average and may be missed during a count. This difficulty can be resolved somewhat by combination with image analysis (1, 25). Even this combined technique, however, still includes some difficulties. For example, the diversity of bacterial size in certain samples (e.g., surface river water) renders the determination of further enlargement of the bacteria quite difficult (28). Furthermore, an image analyzing system is required and can be troublesome to operate.
The objective of this study was to overcome the second difficulty of cell discrimination by taking advantage of the fact that viable cells are selectively lysed by spheroplast formation. Glycine was used to induce spheroplast formation by viable cells. Glycine interferes with several steps in peptidoglycan synthesis for bacterial cell wall formation (9), and this effect leads to swollen cells with a very loose cell wall. The viable cells are lysed easily by physical or chemical treatment following this spheroplast formation.
This quantitative DVC (qDVC) method is a modification of Kogure's original method (15) and the improved method reported by Joux and LeBaron (12). Escherichia coli K-12 strain W3110 in logarithmic phase was incubated in twofold-diluted Luria-Bertani (LB) broth containing nalidixic acid (40 μg/ml; Sigma) and glycine (2% [wt/vol]; Wako Pure Chemical, Osaka, Japan) (10) for 140 min at 37°C in the dark (qDVC incubation) to determine the effectiveness of the qDVC procedure (see Fig. 1). Selective lysis of viable cells in samples after qDVC incubation was carried out by freeze-thaw treatment as follows: samples were frozen for 1 min in liquid nitrogen and then thawed at room temperature (approximately 25°C). Bacterial cells remaining after the freeze-thaw cycles were stained with a 1/10,000 dilution of a stock solution of SYBR green I (Molecular Probes) for 5 min at room temperature (approximately 25°C) and filtered through a black polycarbonate filter (pore size, 0.2 μm; Advantec). Then the filter was air dried and mounted on glass microscope slides with nonfluorescence immersion oil (Olympus). Cells on the filter were counted by using an epifluorescence microscope (AX-70; Olympus) equipped with a filter block for blue excitation (U-MWIBA). The bacterial number was adjusted to approximately 50 cells per field of view, and a minimum of 1,000 cells per sample were counted. The following formula for calculating the number of viable cells was used: number of viable cells = number of lysed cells = total cell number before qDVC incubation − number of cells after freeze-thaw treatment following qDVC incubation.
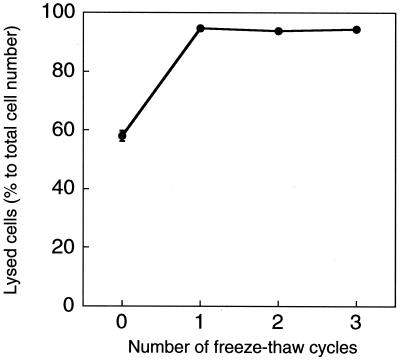
FIG. 1.
Change in ratio of number of lysed cells to total cell number during freeze-thaw cycles. E. coli cells were incubated with medium, nalidixic acid (40 μg/ml), and glycine (2% [wt/vol]). The experiments were replicated twice.
In addition, samples before qDVC incubation were freeze-thawed and observed by epifluorescence microscopy to determine whether bacterial cells not subjected to the qDVC procedure were not lysed.
One-half of all viable cells were already lysed during qDVC incubation, before the freeze-thaw treatment (Fig. 1). This result indicates that many viable cells were lysed during incubation alone, while the remaining half were not. Four physical or chemical treatments were used to lyse the remaining cells: (i) shaking (shuffling) at 2,500 rpm for 1 h by vortex machine, (ii) sonication for 6 min at 125 W and 400 kHz in a bath-type sonicator (JUS-S01; JEOL), (iii) enzymatic treatment in 0.5 mg of lysozyme/ml at 4°C for 15 min, and (iv) freeze-thaw treatment (described above). Shaking, sonication, or enzymatic treatments were found to be unsuitable due to nonselective lysis (data not shown). More than 95% of all viable cells were lysed by a single freeze-thaw treatment (Fig. 1); however, the number of lysed cells was not altered substantially with repeat freeze-thaw cycles. These results showed that a single freeze-thaw treatment is enough for cell lysis.
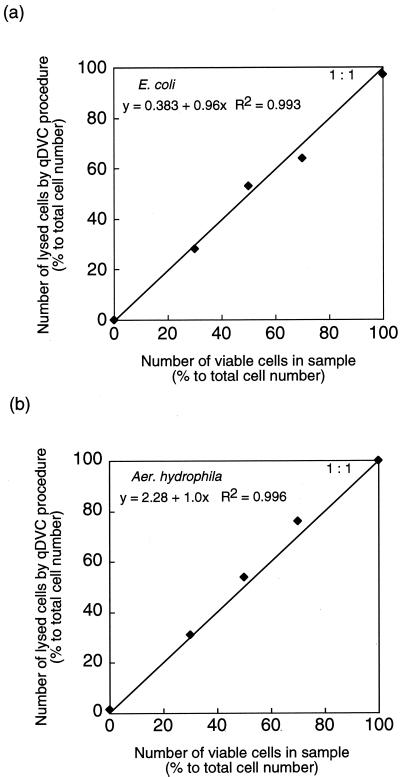
The effectiveness of the qDVC procedure was evaluated with four bacterial strains: E. coli K-12 strain W3110, Aeromonas hydrophila ATCC 7966, Staphylococcus epidermidis IFO 3762, and strain S1 isolated from tap water. A. hydrophila is found in freshwater and is widely distributed in aquatic environments (3, 14, 16, 26, 31). S. epidermidis is a gram-positive bacterium whose viability is difficult to determine by the original DVC method (24). Strain S1 was isolated from tap water using R2A medium (21). Cultured bacterial cells of each strain were mixed with dead cells (heat-treated cells) of the same strain in various ratios (10:0, 7:3, 5:5, 3:7, and 0:10). Cells were freeze-thawed following the qDVC incubation, and the number of lysed cells was determined (Fig. 2). The conditions of qDVC incubation for E. coli were previously described. A. hydrophila was incubated in LB broth containing nalidixic acid (1 μg/ml) and glycine (2% [wt/vol]) for 140 min at 30°C in the dark. S. epidermidis was incubated in LB broth containing ciprofloxacin (25 μg/ml; Bayer) for 4 h at 37°C in the dark. Strain S1 was incubated in R2A broth containing nalidixic acid (40 μg/ml) and glycine (2% [wt/vol]) for 12 h at 25°C in the dark. There was a good correlation between the number of viable cells in the sample and the number of lysed cells revealed by the qDVC procedure (E. coli, y = 0.383 + 0.96x, R2 = 0.993; A. hydrophila, y = 2.28 + 1.0x, R2 = 0.996; S. epidermidis, y = 8.05 + 0.91x, R2 = 0.998; strain S1, y = 1.52 + 0.98x, R2 = 0.994). That is, the qDVC procedure was effectively applied to various types of bacteria, both gram negative and gram positive, for viable counting.
FIG. 2.
Relationship between number of viable cells in sample and number of lysed cells determined by the qDVC procedure with E. coli (a) and A. hydrophila (b). The straight line corresponds to a 1:1 relationship.
DVC and qDVC procedures were applied to several environmental samples to determine the effectiveness of the qDVC procedure for viable bacterial cells in natural environments (Table 1). Surface river water samples were collected from two points on the Minoh and Neya Rivers in Osaka, Japan. The Kitahashi sampling point is located in a commercial area, Osaka Business Park, and is highly polluted (29, 32, 33); domestic water flows into this river upstream. The Takiue site is located in a forested area, Minoh National Park, and is unpolluted (29, 32, 33); at this point the river is narrow, shallow, and fast flowing. The streambed is rocky and the water is not exposed to either domestic or industrial effluents. Springwater samples were collected at Tarumi Shrine in Osaka, Japan. Tarumi Shrine is located in a residential area. The sampling period was from 1 February to 8 March 2000. All samples were collected in 1-liter autoclaved glass bottles and tested within 2 h of sampling.
TABLE 1.
Bacterial counts and activity measurements at different sampling points by various methods
| Sampling point (type of source) | Date (mo/day/yr) | Water temp (°C) | Total cell no. (105)/mla | % Respiring cellsb | % Esterase-active cellsc | % of cells with membrane integrityd | % CFU on R2A medium | DVC method
|
qDVC method
|
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of DVC+ cells (105)/mle | % DVC+ cellsa | No. of remaining cells (105)/mlaf | % qDVC+ cellsg | ||||||||
| Kitahashi (river water) | 02/24/2000 | 7.8 | 24 (2.0)** | 15 | 25 | 58 | 16 | NDh | ND | 14 (1.0)** | 42 |
| 02/25/2000 | 6.8 | 21 (1.5)** | 9.4 | 28 | 62 | 13 | ND | ND | 10 (0.73)** | 52 | |
| 02/28/2000 | 11 | 83 (18)** | 61 | 38 | 66 | ND | 20 (2.5)** | 24 | 17 (1.0)** | 80 | |
| 02/29/2000 | 10 | 76 (0.58)** | 29 | 66 | 54 | 34 | 20 (6.1)** | 26 | 27 (2.5)** | 78 | |
| 03/01/2000 | 10 | 69 (7.8)** | 16 | 41 | 49 | 22 | 35 (5.1)** | 51 | 23 (0.58)** | 67 | |
| Takiue (river water) | 03/06/2000 | 7.3 | 16 (0.00)** | 6.4 | 20 | 69 | 3.1 | 0.35 (0.061)** | 2.2 | 12 (1.0)** | 25 |
| 03/07/2000 | 6.1 | 16 (1.2)** | 2.7 | 40 | 70 | 1.5 | 0.25 (0.045)** | 1.6 | 12 (1.0)** | 25 | |
| 03/08/2000 | 5.0 | 17 (1.2)** | 3.5 | 44 | 67 | 0.59 | 0.25 (0.080)** | 1.5 | 10 (1.5)** | 41 | |
| Tarumi Shrine (springwater) | 02/01/2000 | ND | 0.74* | 27 | 38 | —i | 9.1 | 0.031* | 4.1 | 0.52* | 35 |
| 02/03/2000 | ND | 1.0* | 18 | 14 | — | 6.0 | 0.047* | 4.8 | 0.80* | 23 | |
| 02/04/2000 | ND | 0.64* | 22 | 38 | — | 14 | 0.065 | 9.3 | 0.43* | 32 | |
| 02/07/2000 | ND | 1.2* | 43 | 25 | — | 25 | 0.50* | 45 | 0.62* | 49 | |
| 02/16/2000 | 11 | 1.0 (0.058)** | 21 | 32 | — | 9.1 | 0.045 (0.016)** | 45 | 0.61 (0.046)** | 39 | |
| 02/17/2000 | 12 | 0.94 (0.14)** | 13 | 39 | — | 7.3 | 0.028 (0.0023)** | 3.0 | 0.57 (0.025)** | 40 | |
| 02/18/2000 | 11 | 0.96 (0.040)** | 8.8 | 27 | — | 10 | 0.023 (0.0066)** | 2.4 | 0.58 (0.046)** | 40 | |
Mean cell counts, with standard deviations in parentheses. *, results from single replicate; **, results from three replicates.
Cells that are able to reduce CTC.
Cells that are able to hydrolyze ester bonds of 6CFDA.
Cells that are not able to be stained by PI.
DVC+, substrate responsive, i.e., cells are elongated and/or fattened during DVC incubation.
Cell number after freeze-thaw treatment following qDVC incubation.
qDVC+, cells lysed by qDVC procedure.
ND, not done.
—, undetectable because of low fluorescence intensity.
The DVC method was carried out as described by Joux and LeBaron (12). All environmental samples were enriched with yeast extract (50 μg/ml), nalidixic acid (20 μg/ml; Sigma), piromidic acid (10 μg/ml; Sigma), pipemidic acid (10 μg/ml; Sigma), cephalexin (10 μg/ml; Sigma), and ciprofloxacin (0.5 μg/ml; Bayer) and incubated at 25°C for 24 h in the dark. Nalidixic, piromidic, and pipemidic acids were dissolved in 0.05 M NaOH. Cephalexin and ciprofloxacin were dissolved in sterile distilled water. All antimicrobial solutions were filter sterilized through 0.2-μm-pore-size membrane filters (Advantec).
For qDVC incubation, river and springwater samples were incubated in yeast extract solution (50 μg/ml) containing nalidixic acid (20 μg/ml), piromidic acid (10 μg/ml), pipemidic acid (10 μg/ml), cephalexin (10 μg/ml), ciprofloxacin (0.5 μg/ml), and glycine (2% [wt/vol]) for 24 h at 25°C. After the DVC or qDVC procedure, bacterial cells were stained with SYBR green I and enumerated by epifluorescence microscopy as described above.
Without qDVC incubation, 4.4% ± 1.0% of bacterial cells were lysed by one freeze-thaw treatment, and bacteria in the freshwater environments where we obtained the water samples were hardly lysed by a single freeze-thaw treatment.
The qDVC procedure resulted in higher viable counts than previous DVC procedures for all water samples (Table 1). The percentage of viable cells with the qDVC procedure was 1.1 to 27 times higher than that with the DVC procedure. Surprisingly, the previous DVC procedure sometimes resulted in viable counts that were lower than culturable counts. These results are different from those reported by Joux and LeBaron for different aquatic environments, for which the percentages of substrate-responsive cells were higher than those of culturable counts (12). The low percentages of substrate-responsive cells reported in this study can be explained by our difficulty in discriminating between elongated cells and others, since the water samples used in this study contained bacterial cells in a wide range of sizes. The previous DVC procedure is quite dependent on bacterial morphology, while the qDVC procedure is independent of bacterial morphology and is suited to use in populations with bacterial cells of many different sizes. The previous DVC procedure is better suited for use with bacterial populations with cells of similar size. Peele and Colwell reported that cell elongation, by the DVC procedure, reflects the habitat and species composition of the bacterial population (19). Moreover, Tabor and Neihof reported that a relatively large amount of energy and numerous metabolic steps are necessary for sufficient synthesis of cellular components (27) to allow recognition of cells as elongated and/or fattened by the DVC procedure. Cell lysis in the qDVC procedure indicates an ability to form the cell wall, an earlier step in the cell cycle. This means that the qDVC procedure may be more sensitive in the determination of reproducing or growing viable cells.
The numbers of total bacteria, CFU, and respiring, esterase-active, and dead cells in natural samples were also determined, and the results were compared with the ratio of viable cells determined by the qDVC method (Table 1). Bacterial cells in the samples were stained with SYBR green I and counted using an epifluorescence microscope, as described above, for the enumeration of total bacteria (both viable and dead cells) in all samples. The numbers of colony-forming bacteria were determined by the enumeration of colonies formed on R2A agar plates (21) after 1 week of incubation at 25°C. The percentage of colony-forming bacteria was determined as the ratio of the number of colony-forming bacteria to the total cell number.
Respiring bacteria were defined as cells able to reduce 5-cyano-2,3-ditolyl tetrazolium chloride (CTC; Polysciences) in red fluorescent formazan (22, 23, 32). Counts were determined by a modification of the original method (22). Briefly, samples were incubated with 2 mM CTC (fresh stock solution, 50 mM) for 1 h at 37°C in the dark. Respiring bacterial cells with red fluorescence were enumerated under blue-light excitation by epifluorescence microscopy.
Esterase-active bacteria were defined as cells able to hydrolyze ester binding of 6-carboxyfluorescein diacetate (6CFDA; Sigma) (13, 20). Samples were incubated in 6CFDA buffer (0.1 mM phosphate buffer [pH 8.5], 5% [wt/vol] NaCl, 0.5 mM EDTA) containing 150 μg of 6CFDA/ml (concentration of stock solution in acetone is 10 mg/ml) for 5 min at room temperature (32). Esterase-active bacterial cells with green fluorescence were enumerated under blue-light excitation.
There are many fluorescent dyes which can indicate loss of membrane integrity, which, in turn, can often act as an indicator of cell viability (11, 17, 18). For instance, propidium iodide (PI) has a polarity and penetrates only cells with compromised plasma membranes (18). In this study, dead bacterial cells were detected by staining with PI (2 μg/ml; Sigma) for 5 min at room temperature (approximately 25°C) (32). The number of cells with membrane integrity was obtained by subtracting the number of PI-stained cells from the total cell number.
Respiring, esterase-active, or PI-stained cells were enumerated using an epifluorescence microscope (BH-2; Olympus). The filter combination consisted of two excitation filters (BP490 and EY455), a dichroic mirror (DM500), and an absorption filter (O515).
The differences between culturable and lysed cells detected by the qDVC procedure revealed a large fraction of viable but nonculturable cells (Table 1). The large qDVC-positive fraction can be explained by the detection of some microbial cells that may have undergone a few cell wall formations but in insufficient numbers to produce bacterial colonies. The detection of these cells can be achieved by other methods, such as dilution culture (4) or enumeration of microcolonies (30).
The qDVC procedure resulted in high percentages of viable cells (23 to 80%), underlining the high metabolic potentials of bacterial communities in natural freshwater environments. The differences between respiring cells and cells lysed by the qDVC procedure may represent difficulties in optimizing CTC staining conditions. The detection of CTC reduction by bacterial respiration is sensitive to changes in experimental conditions, especially the incubation time and CTC concentration. Rodriguez et al. (22) and Coallier et al. (6) reported that the lower number of bacteria obtained with a high concentration of CTC is perhaps related to a toxic effect of this dye or to the presence of impurities or deposits that make counting difficult. However, CTC reduction does not seem to be influenced by the presence of a substrate such as the yeast extract used in this study. This method can easily be adapted for in situ conditions. Actually, CTC was suitable for enumeration of living bacteria in the springwater samples from Tarumi Shrine. Determination of esterase-active cell counts is also an effective method for enumeration of living bacteria in heterogeneous populations (13, 20, 32). In this study, the esterase-active cell counts were similar to the viable counts determined by the qDVC procedure. PI staining can be used to evaluate cell viability based on membrane integrity (18, 32). However, in this study, staining with PI did not correlate with other indicators. This result might show that membrane integrity is insufficient to ensure viability of a cell in some aquatic environments, a theory also suggested by LeBaron et al. (17).
It is concluded that the qDVC procedure can easily discriminate between viable cells and others. Lysed cells in the qDVC procedure are a result of metabolism and growth of viable cells. This procedure provides very useful information about the metabolic potentials of natural communities. This qDVC procedure is simple to use and may be applicable for various freshwater environmental samples. However, the qDVC procedure should be modified for each ecosystem since glycine cannot interfere with the synthesis of peptidoglycan for cell wall formation in some environmental bacteria. Addition of other inhibitors of bacterial cell wall synthesis, such as penicillin or ampicillin, should be effective in accelerating selective lysis of viable bacterial cells. The qDVC procedure could complement methods which permit the quantitative estimation of bacterial activity in freshwater environments.
ACKNOWLEDGMENT
This study was supported partially by the Science and Technology Agency (STA), Japan (Promotion System for Intellectual Infrastructure of Research and Development, under Special Coordination Funds for Promoting Science and Technology).
REFERENCES
- 1.Barcina I, Arana I, Santorum P, Iriberri J, Egea L. Direct viable count of gram-positive and gram-negative bacteria using ciprofloxacin as inhibitor of cellular division. J Microbiol Methods. 1995;22:139–150. [Google Scholar]
- 2.Bianchi A, Giuliano L. Enumeration of viable bacteria in the marine pelagic environment. Appl Environ Microbiol. 1996;62:174–177. doi: 10.1128/aem.62.1.174-177.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandi G, Sisti M, Giardini F, Schiavano G F, Albano A. Survival ability of cytotoxic strains of motile Aeromonas spp. in different types of water. Lett Appl Microbiol. 1999;29:211–215. doi: 10.1046/j.1365-2672.1999.00584.x. [DOI] [PubMed] [Google Scholar]
- 4.Button D K, Schut F, Quang P, Martin R, Robertson B R. Viability and isolation of marine bacteria by dilution culture: theory, procedures, and initial results. Appl Environ Microbiol. 1993;59:881–891. doi: 10.1128/aem.59.3.881-891.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrd J J, Xu H-S, Colwell R R. Viable but nonculturable bacteria in drinking water. Appl Environ Microbiol. 1991;57:875–878. doi: 10.1128/aem.57.3.875-878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coallier J, Prévost M, Rompré A. The optimization and application of two direct viable count methods for bacteria in distributed drinking water. Can J Microbiol. 1994;40:830–836. doi: 10.1139/m94-132. [DOI] [PubMed] [Google Scholar]
- 7.Colwell R R, Brayton P R, Grimes D J, Roszak D B, Huq S A, Palmer L M. Viable but non-culturable Vibrio cholerae and related pathogens in the environment: implications for release of genetically engineered microorganisms. Bio/Technology. 1985;3:817–820. [Google Scholar]
- 8.Goss W A, Deitz W H, Cook T M. Mechanism of action of nalidixic acid on Escherichia coli. J Bacteriol. 1964;88:1112–1118. doi: 10.1128/jb.88.4.1112-1118.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammes W, Schleifer K H, Kandler O. Mode of action of glycine on the biosynthesis of peptidoglycan. J Bacteriol. 1973;116:1029–1053. doi: 10.1128/jb.116.2.1029-1053.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hines W D, Freeman B A, Pearson G R. Production and characterization of Brucella spheroplasts. J Bacteriol. 1964;87:438–445. doi: 10.1128/jb.87.2.438-445.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jepras R I, Carter J, Pearson S C, Paul F E, Wilkinson M J. Development of a robust flow cytometric assay for determining numbers of viable bacteria. Appl Environ Microbiol. 1995;61:2696–2701. doi: 10.1128/aem.61.7.2696-2701.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joux F, LeBaron P. Ecological implications of an improved direct viable count method for aquatic bacteria. Appl Environ Microbiol. 1997;63:3643–3647. doi: 10.1128/aem.63.9.3643-3647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawai M, Yamaguchi N, Nasu M. Rapid enumeration of physiologically active bacteria in purified water used in the pharmaceutical manufacturing process. J Appl Microbiol. 1999;86:496–504. doi: 10.1046/j.1365-2672.1999.00689.x. [DOI] [PubMed] [Google Scholar]
- 14.Kersters I, Huys G, Duffel H V, Vancanneyt M, Kersters K, Verstraete W. Survival potential of Aeromonas hydrophila in freshwaters and nutrient-poor waters in comparison with other bacteria. J Appl Bacteriol. 1996;80:266–276. doi: 10.1111/j.1365-2672.1996.tb03219.x. [DOI] [PubMed] [Google Scholar]
- 15.Kogure K, Simidu U, Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979;25:415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- 16.Kühn I, Allestam G, Huys G, Janssen P, Kersters K, Krovacek K, Stenström T-A. Diversity, persistence, and virulence of Aeromonas strains isolated from drinking water distribution systems in Sweden. Appl Environ Microbiol. 1997;63:2708–2715. doi: 10.1128/aem.63.7.2708-2715.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeBaron P, Catala P, Parthuisot N. Effectiveness of SYTOX Green stain for bacterial viability assessment. Appl Environ Microbiol. 1998;64:2697–2700. doi: 10.1128/aem.64.7.2697-2700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López-Amorós R, Castel S, Comas-Riu J, Vives-Rego J. Assessment of E. coli and Salmonella viability and starvation by confocal laser microscopy and flow cytometry using rhodamine 123, DiBAC4(3), propidium iodide and CTC. Cytometry. 1997;29:298–305. doi: 10.1002/(sici)1097-0320(19971201)29:4<298::aid-cyto6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Peele E R, Colwell R R. Application of a direct microscopic method for enumeration of substrate-responsive marine bacteria. Can J Microbiol. 1981;27:1071–1075. [Google Scholar]
- 20.Porter J, Diaper J, Edwards C, Pickup R. Direct measurement of natural planktonic bacterial community viability by flow cytometry. Appl Environ Microbiol. 1995;61:2783–2786. doi: 10.1128/aem.61.7.2783-2786.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reasoner D J, Geldreich E E. A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol. 1985;49:1–7. doi: 10.1128/aem.49.1.1-7.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez G G, Phipps D, Ishiguro K, Ridgway H F. Use of fluorescent redox probe for direct visualization of actively respiring bacteria. Appl Environ Microbiol. 1992;58:1801–1808. doi: 10.1128/aem.58.6.1801-1808.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaule G, Flemming H-C, Ridgway H F. Use of 5-cyano-2,3-ditolyl tetrazolium chloride for quantifying planktonic and sessile respiring bacteria in drinking water. Appl Environ Microbiol. 1993;59:3850–3857. doi: 10.1128/aem.59.11.3850-3857.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Servis N A, Nichols S, Adams J C. Development of a direct viable count procedure for some gram-positive bacteria. Lett Appl Microbiol. 1995;20:237–239. doi: 10.1111/j.1472-765x.1995.tb00436.x. [DOI] [PubMed] [Google Scholar]
- 25.Singh A, Pyle B H, McFeters G A. Rapid enumeration of viable bacteria by image analysis. J Microbiol Methods. 1989;10:91–101. doi: 10.1016/0167-7012(89)90005-5. [DOI] [PubMed] [Google Scholar]
- 26.Sisti M, Albano A, Brandi G. Bactericidal effect of chlorine on motile Aeromonas spp. in drinking water supplies and influence of temperature on disinfection efficacy. Lett Appl Microbiol. 1998;26:347–351. doi: 10.1046/j.1472-765x.1998.00346.x. [DOI] [PubMed] [Google Scholar]
- 27.Tabor P S, Neihof R A. Improved microautoradiographic method to determine individual microorganisms active in substrate uptake in natural waters. Appl Environ Microbiol. 1982;44:945–953. doi: 10.1128/aem.44.4.945-953.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabor P S, Neihof R A. Direct determination of activities for microorganisms of Chesapeake Bay populations. Appl Environ Microbiol. 1984;48:1012–1019. doi: 10.1128/aem.48.5.1012-1019.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tani K, Chen J M, Yamaguchi N, Nasu M. Estimation of bacterial biovolume and biomass by scanning electron microscopic image analysis. Microb Environ. 1996;11:11–17. [Google Scholar]
- 30.Torrella F, Morita R Y. Microcultural study of bacterial size changes and microcolony and ultramicrocolony formation by heterotrophic bacteria in seawater. Appl Environ Microbiol. 1981;41:518–527. doi: 10.1128/aem.41.2.518-527.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warburton D W, McCormick J K, Bowen B. Survival and recovery of Aeromonas hydrophila in water: development of methodology for testing bottled water in Canada. Can J Microbiol. 1994;40:145–148. doi: 10.1139/m94-023. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi N, Nasu M. Flow cytometric analysis of bacterial respiratory and enzymatic activity in the natural aquatic environment. J Appl Microbiol. 1997;83:43–52. [Google Scholar]
- 33.Yamaguchi N, Ohmori H, Welikala N, Nasu M. Biodegradation of chemical compounds in a newly developed modified river die-away test. Jpn J Toxicol Environ Health. 1997;43:209–214. [Google Scholar]