Abstract
Background
Social participation (SP) may be an effective measure for decreasing frailty risks. This study investigated whether frequency and type of SP is associated with decreased frailty risk among Chinese middle-aged and older populations.
Methods
Data were derived from the China Health and Retirement Longitudinal Study (CHARLS). Frailty was assessed using the Rockwood’s Cumulative Deficit Frailty Index. SP was measured according to frequency (none, occasional, weekly and daily) and type (interacting with friends [IWF]; playing mah-jong, chess, and cards or visiting community clubs [MCCC], going to community-organized dancing, fitness, qigong and so on [DFQ]; participating in community-related organizations [CRO]; voluntary or charitable work [VOC]; using the Internet [INT]). Smooth curves were used to describe the trend for frailty scores across survey waves. The fixed-effect model (N = 9,422) was applied to explore the association between the frequency/type of SP and frailty level. For baseline non-frail respondents (N = 6,073), the time-varying Cox regression model was used to calculate relative risk of frailty in different SP groups.
Results
Weekly (β = − 0.006; 95%CI: [− 0.009, − 0.003]) and daily (β = − 0.009; 95% CI: [− 0.012, − 0.007]) SP is associated with lower frailty scores using the fixed-effect models. Time-varying Cox regressions present lower risks of frailty in daily SP group (HR = 0.76; 95% CI: [0.69, 0.84]). SP types that can significantly decrease frailty risk include IWF, MCCC and DFQ. Daily IWF and daily DFQ decreases frailty risk in those aged < 65 years, female and urban respondents, but not in those aged ≥ 65 years, male and rural respondents. The impact of daily MCCC is significant in all subgroups, whereas that of lower-frequent MCCC is not significant in those aged ≥ 65 years, male and rural respondents.
Conclusion
This study demonstrated that enhancing participation in social activities could decrease frailty risk among middle-aged and older populations, especially communicative activities, intellectually demanding/engaging activities and community-organized physical activities. The results suggested very accurate, operable, and valuable intervening measures for promoting healthy ageing.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-022-03219-9.
Keywords: Frailty, Social participation, Frequency, Communicative activities, Intellectually demanding/engaging activities, Community-organized physical activities
Background
Frailty describes the vulnerability of an individual to the poor resolution of homeostasis in response to endogenous and exogenous stressors after a sudden change in health status. It denotes a transition phase between healthy ageing and disability, which is undoubtedly an emerging global health burden [1, 2]. Frailty is associated with the independence, physical function, and cognition of an individual, which consequently exposes the individual to a high risk of negative health-related outcomes [3, 4]. Population ageing is rapidly accelerating worldwide, which leads to great challenges associated with the economy, medicine, and society [5]. Thus, ameliorating frailty to promote healthy ageing is a critical and urgent initiative around the world.
Social participation (SP) may be an effective measure for decreasing frailty risks. The continuity theory holds that older people have health barriers due to aging, but alternative activities such as volunteer service and physical training can continue to help them reduce the health damage caused by aging [6, 7]. Previous studies provided evidence that social frailty or social isolation is associated with physical functioning, cognition, and depression, and predicts mortality [8–10]. Social frailty can be defined as the absence of social resources, social activities, and self-management abilities considered important for fulfilling the basic social needs [11, 12]. Moreover, social isolation is typically defined as having less or infrequent social contacts [13]. SP has been demonstrated effective in preventing social frailty/isolation [14], in increasing happiness [15], physical, and cognitive functioning [16, 17], and in reducing the risk of disability in activities or instrumental activities of daily living [18] among older adults. In addition, some studies indicated that individuals with more SP can expand their social network, improve their perception of social cohesion, gain a stronger sense of trust and reciprocity, therefore leading to healthier outcomes [19, 20]. At the same time, they can perceive the strength of social support and improve their own level of social support [21]. To sum up, SP is effective in improving the older population’s physical and psychological health status, and has a potential to prevent frailty, or reduce existing frailty. However, research that directly proves the impact of SP on frailty is scarce.
With the improvement in living standards, SP has become a component of the daily life of many residents, whereas an increasing number of types of SP have emerged. SP with different frequencies and types may generate varying levels of effectiveness. For example, Wang [22] argued that transitioning from no SP to one or more types of SP or to a once a week or higher frequency was associated with a decline in depressive symptoms. An observational study conducted in Japan demonstrates that exercise-based social participation has associations with reversing frailty progression [23]. SP is one of the most effective interventions with relatively low costs of resources, and can be promoted through advocacy, education, and community activities. Therefore, exploring the association between SP and frailty, and identifying the most effective type and frequency of SP can be a promising initiative for promoting healthy ageing.
This study aims to explore the effect of SP on frailty among older populations by comparing its impacts according to the frequency and type of SP. Moreover, this study hypothesizes that high-frequency SP is associated with less risk of frailty, whereas the impact differs among the types of SP.
Methods
Data sources
Data were derived from the China Health and Retirement Longitudinal Study (CHARLS) for 2011, 2013, 2015, and 2018. CHARLS was conducted by the National School of Development of Peking University, which collected high-quality microdata from middle-aged and older individuals associated with health status, demographics, and economic information in China. Data are nationally representative of China due to the use of multistage stratified probability-proportionate-to-size sampling. A detailed description of the objectives and methods of CHARLS has been reported previously [24].
The current study included data from participants who were interviewed at baseline (2011), aged 45 years and older as of 2011, and remained for the following waves. Participants with missing values for the dependent or independent variables were excluded. Figure 1 presents the details of sample selection.
Fig. 1.
Flow chart of sample selection
Variables
Frailty
The outcome of this study is frailty, which is defined following Rockwood's Cumulative Deficit Frailty Index (FI) [25]. As suggested by Moorhouse & Rockwood, the FI allows inclusion of any health deficit providing that a minimum of 30 deficits in total are included and that each deficit is associated with adverse health outcomes; increases in prevalence with age at least into the tenth decade; has a prevalence of at least 1% in the population; and does not saturate [26]. A total of 54 items were selected to calculate FI in this study, including physical limitations, psychological symptoms, comorbidities, history of trauma, cognitive deficits [27, 28]. All variables were coded as 0 or 1, with details shown in Supplementary Table S1. The frailty index was calculated by summing the number of deficits reported by the respondents and dividing it by the total number of answered possible deficits. For the chronic disease variables (e.g., hypertension), a score of 1 in one wave was allocated to all subsequent waves, because these conditions are irreversible. A frailty index with potential ranging from 0 to 1 was generated. High scores indicate high levels of frailty. Given the accuracy of the frailty index, individuals with a denominator of less than 43 (80% out of the total 54 items) were excluded from the study [7, 29]. When analyzing the risk of frailty by hazard ratio, frailty was categorized using a defined cutoff point of 0.25 according to previous studies (i.e., non-frail or prefrail: < 0.25; frail: ≥ 0.25–1.00) [30, 31].
Social participation
SP is the exposure variable in this study. SP in CHARLS included (1) interacting with friends (IWF), (2) playing mah-jong, chess, and cards or visiting community clubs (MCCC), (3) going to (community-organized) dancing, fitness, Chinese Qigong (a system of deep breathing exercises) and so on (DFQ), (4) participating in community-related organizations (CRO), (5) undertaking voluntary or charitable work, (6) caring for a distant sick or disabled adult, (7) providing help to relatives, friends, or neighbors, and (8) using the Internet (INT). Types 5–7 were categorized into one group as voluntary or charitable work (VOC) [22, 32]. Frequency of each type of SP was categorized into four groups, namely, (1) none, (2) occasional, (3) weekly, and (4) nearly daily. The frequency of the comprehensive SP in each respondent was defined as his/her highest frequency of the above SP types. Supplementary Table S2 indicates the distribution of SP types. The frequencies of IWF and MCCC are higher than those of other SP types.
Covariates
The covariates were first identified within the literature. Then we explored their association with the independent variable and their impact on the change of the association between the independent variable and dependent variable. Covariates were included in the main analysis as potential confounders if they changed the estimates of the effect of SP on frailty by more than 10% or were significantly associated with the frailty score [33]. Confounders were selected based on a generalized estimating equation, as the data were repeated measurements. The final covariates included: age, gender, marital status, hukou status (which is a special identifier in China and affects many aspects of life in China such as buying a house, buying a car, children’s school enrollment and other welfare) [34], level of education, rural/urban residence, public health insurance coverage, current work status [35], alcohol intake, smoking status, and household per capita consumption (which is a a proxy for socioeconomic status) [36]. Supplementary Table S3 presents the definitions and grouping details for the covariates.
Statistical analysis
In description analysis of the respondents’ baseline characteristics within different SP groups, "number (percentage)" and "mean ± standard deviation (SD) " were used for the description of binary or categorical variables and continuous variables, respectively.
To observe the trajectory of frailty level along with time, we adopted the general additive models (GAM) to fit the regression for frailty on survey wave with identity used as link functions for the outcomes and all the covariates adjusted. GAM extends the generalized linear model, in which the predictor function may contain one or more user-specified sums of smooth functions of the covariates plus a conventional parametric component of the linear predictor [37]. That means, with the cubic spline smoothing function to control for the confounding factors, an additional smoothing function of survey wave could be constructed to filter out the trajectory of frailty level [38, 39]. In addition, the curve could reveal the trajectory variance between different groups. Therefore, we also compared the trajectory variance between those frail at baseline and non-frail at baseline.
Moreover, this study employed a longitudinal linear fixed-effect regression model to estimate the association between SP frequency, SP type, and frailty scores across the four waves. F-test and Hausman test were employed for model selection among ordinary least squares (OLS), random-effects model, and fixed-effects model. The F-test between the pooled ordinary least squares (OLS) and fixed-effect models yielded statistical significance (P < 0.001), which indicated that the the fixed-effect model was prior to the OLS model. A Hausman specification test was then conducted between the fixed- and the random-effect models, which also yielded statistical significance (P < 0.001), and indicated that the fixed-effect model was prior to the random-effect model [40]. The fixed effects model treats each individual as their own control and has the advantage of reducing biases brought about by between-individual and hard-to-observe factors [22].
The Cox proportional hazard models were further performed to calculate relative risk with survey waves as the timescale. However, SP conditions may vary across waves; thus, we were unable to roughly define exposure, which may lead to immortal time bias [41]. Therefore, we performed the Cox regression with time-varying exposure and covariates to avoid this bias [42]. HRs with 95% CIs were calculated. The respondents classified as frail at baseline were excluded from analysis, whereas those who remained non-frail as of 2018 were considered censored data. When analyzing SP types, fixed-effects models and time-varying Cox regression models were also performed to explore the associations between frequency of each SP type and frailty risk, with all the selected covariates controlled.
To avoid statistical test performance reduction and bias due to the direct exclusion of missing values, multiple imputations (MIs) were conducted to impute the missing covariate values at baseline based on five replications and a chained equation approach. Supplementary Table S4 provides the number of missing values and MI evaluation. The missing values in the subsequent waves were then imputed using baseline data, because the majority of the conditions were stable or changed little.
To validate the results, two sensitivity analyses were conducted. First, we used the data without imputation to repeat the fixed-effect and Cox analyses. Second, some studies suggested the deficit to calculate frailty index should have a missingness of 5.0% or less [43, 44]. Therefore, we identified participants who responded to 52 or more items on frailty-related deficits and repeated the analysis. Furthermore, as individuals may experience worsened or improved frailty state over time, we identified respondents who were categorized under the frailty group at baseline and set frailty improvement as the outcome variable to explore the association between SP and improvement in frailty.
In addition, we performed several subgroup analyses to identify the associations among specific respondents, including (1) those aged ≥ 65 years versus those aged < 65 years; (2) male versus female participants; and (3) rural versus urban participants.
We did not account for sampling and non-response weight in the analysis, because many studies that employed the CHARLS data suggested that the results of regression analyses with and without weighting were similar [36]. P values were two-tailed, where statistical significance was set at an alpha level of 0.05. Data were analyzed using Stata (version 15) and R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
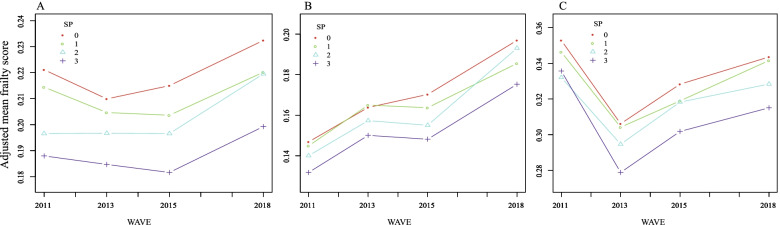
Out of the 17,708 participants in the baseline, we included 9,422 respondents in the analysis (Fig. 1). Among them, 4,667, 1,411, 1,101, and 2,243 respondents were grouped under non-SP, occasionally, weekly, and daily, respectively (Table 1). Participants under the non-SP and daily groups are older than the other two groups and are composed of more women. The mean frailty score increased from 0.22 in 2011 to 0.28 in 2018, whereas the prevalence of frailty increased from 35.54% to 53.50% correspondingly (Supplementary Table S5). Figure 2 reveals the dynamic changes in frailty scores across the survey waves. After adjusting for all covariates, we observed a remarkable variance in the trends within groups, where the adjusted mean scores for the daily group were lower than those for three other groups were (Fig. 2A). For the baseline non-frail respondents (Fig. 2B), the frailty score for the daily SP group was also the lowest. Moreover, we observed a remarkable intersection between occasional and weekly SP from 2015 to 2018, after which the frailty score for weekly SP exceeded that of occasional SP. As for respondents who were frail at baseline (Fig. 2C), the daily SP group continued to score lowest infrailty.
Table 1.
Baseline description of the sample within different SP frequency groups*
| All | Non-SP | Occasional | Weekly | Daily | |
|---|---|---|---|---|---|
| Number of participants | 9422 | 4667 | 1411 | 1101 | 2243 |
| Age | 57.68 ± 8.24 | 58.06 ± 8.05 | 56.44 ± 7.94 | 56.72 ± 8.34 | 58.17 ± 8.64 |
| Gender | |||||
| Male | 4362 (46.30%) | 2130 (45.64%) | 714 (50.60%) | 586 (53.22%) | 932 (41.55%) |
| Female | 5060 (53.70%) | 2537 (54.36%) | 697 (49.40%) | 515 (46.78%) | 1311 (58.45%) |
| Education levels | |||||
| Less than lower secondary | 8362 (88.75%) | 4298 (92.09%) | 1239 (87.81%) | 934 (84.83%) | 1891 (84.31%) |
| Upper secondary & vocational training | 933 (9.90%) | 344 (7.37%) | 154 (10.91%) | 146 (13.26%) | 289 (12.88%) |
| Tertiary | 127 (1.35%) | 25 (0.54%) | 18 (1.28%) | 21 (1.91%) | 63 (2.81%) |
| Marital status | |||||
| Divorced or widowed | 951 (10.09%) | 476 (10.20%) | 120 (8.50%) | 89 (8.08%) | 266 (11.86%) |
| Married | 8471 (89.91%) | 4191 (89.80%) | 1291 (91.50%) | 1012 (91.92%) | 1977 (88.14%) |
| Hukou status | |||||
| Agricultural | 7711 (81.85%) | 4009 (85.92%) | 1182 (83.77%) | 880 (79.93%) | 1640 (73.12%) |
| Non-agricultural | 1647 (17.48%) | 625 (13.39%) | 223 (15.80%) | 213 (19.35%) | 586 (26.13%) |
| Other | 63 (0.67%) | 32 (0.69%) | 6 (0.43%) | 8 (0.73%) | 17 (0.76%) |
| Rural/urban residence | |||||
| Rural | 6153 (65.30%) | 3160 (67.71%) | 974 (69.03%) | 717 (65.12%) | 1302 (58.05%) |
| Urban | 3269 (34.70%) | 1507 (32.29%) | 437 (30.97%) | 384 (34.88%) | 941 (41.95%) |
| Morbidity | |||||
| None | 2843 (30.17%) | 1397 (29.93%) | 431 (30.55%) | 337 (30.61%) | 678 (30.23%) |
| Single | 2805 (29.77%) | 1419 (30.40%) | 442 (31.33%) | 309 (28.07%) | 635 (28.31%) |
| Morbidity | 3774 (40.06%) | 1851 (39.66%) | 538 (38.13%) | 455 (41.33%) | 930 (41.46%) |
| Public health insurance coverage | |||||
| Not covered | 566 (6.02%) | 289 (6.21%) | 69 (4.90%) | 57 (5.18%) | 151 (6.76%) |
| Covered | 8834 (93.98%) | 4367 (93.79%) | 1340 (95.10%) | 1043 (94.82%) | 2084 (93.24%) |
| Current work status | |||||
| Not working | 2319 (24.68%) | 1036 (22.27%) | 252 (17.89%) | 242 (22.04%) | 789 (35.25%) |
| Working | 7078 (75.32%) | 3616 (77.73%) | 1157 (82.11%) | 856 (77.96%) | 1449 (64.75%) |
| Alcohol intake | |||||
| Do not drink | 6308 (66.95%) | 3246 (69.55%) | 858 (60.81%) | 668 (60.67%) | 1536 (68.48%) |
| Drink | 3114 (33.05%) | 1421 (30.45%) | 553 (39.19%) | 433 (39.33%) | 707 (31.52%) |
| Smoking status | |||||
| Never | 5810 (61.67%) | 2939 (62.99%) | 794 (56.27%) | 620 (56.31%) | 1457 (64.96%) |
| Quit now | 752 (7.98%) | 362 (7.76%) | 124 (8.79%) | 104 (9.45%) | 162 (7.22%) |
| Smoke | 2859 (30.35%) | 1365 (29.25%) | 493 (34.94%) | 377 (34.24%) | 624 (27.82%) |
| Household per capita consumption | |||||
| Low | 3178 (39.28%) | 1699 (42.71%) | 489 (39.79%) | 344 (36.13%) | 646 (33.45%) |
| Low to middle | 2369 (29.28%) | 1192 (29.96%) | 363 (29.54%) | 259 (27.21%) | 555 (28.74%) |
| Middle | 1706 (21.09%) | 767 (19.28%) | 260 (21.16%) | 230 (24.16%) | 449 (23.25%) |
| High | 837 (10.35%) | 320 (8.04%) | 117 (9.52%) | 119 (12.50%) | 281 (14.55%) |
SP Social participation
*Mean ± standard deviation was used to describe continuous variables, and number (constituent ratio [%]) was used to describe categorical variables
Fig. 2.
Smoothing curves fitting for the dynamic changes in frailty scores across the 4 survey waves. A. Smoothing curves fitting for the change of mean frailty scores within different SP groups. B. Smoothing curves fitting for the change of mean frailty scores within different SP groups among respondents not frail at baseline. C. Smoothing curves fitting for the change of mean frailty scores within different SP groups among respondents frail at baseline. (0 = non-SP; 1 = occasional SP; 2 = weekly SP; 3 = daily SP. Smoothing curves were constructed based on general additive models, with all the covariates adjusted.)
The fixed-effect model indicates the positive effect of weekly (β = − 0.006; 95% CI: [− 0.009, − 0.003]; P < 0.001) and daily (β = − 0.009; 95% CI: [− 0.012, − 0.007]; P < 0.001) SP on frailty score compared with those of the non-SP group (Table 2). Data from 6,073 respondents underwent time-varying Cox analysis. Supplementary Table S6 presents the baseline characteristics of these respondents. The Cox regression presents a lower risk of frailty in the daily SP group compared with the non-SP group (HR = 0.76; 95% CI: [0.69, 0.84]; P < 0.001). The two models also consistently indicate that age, educational level, marital status, Hukou status, comorbidity and smoking status are risk factors of frailty.
Table 2.
The association between SP frequency and frailty based on fixed-effects model and time-varying Cox regression
| Fixed-effects model (n = 9422) | Time-varying Cox regression (n = 6073) | |||
|---|---|---|---|---|
| Coefficient (95%CI) | P value | HR(95%CI) | P value | |
| Frequency of SP (ref. Non SP) | ||||
| Occasional | 0.000 (-0.003, 0.002) | 0.776 | 0.93 (0.83, 1.04) | 0.187 |
| Weekly | -0.006 (-0.009, -0.003) | < 0.001 | 0.97 (0.86, 1.09) | 0.577 |
| Daily | -0.009 (-0.012, -0.007) | < 0.001 | 0.76 (0.69, 0.84) | < 0.001 |
| Age | 0.002 (0.001, 0.002) | < 0.001 | 1.01 (1.00, 1.01) | < 0.001 |
| Gender (ref. Male) | ||||
| Female | 0.019 (-0.043, 0.080) | 0.554 | 1.87 (1.67, 2.10) | < 0.001 |
| Rural/urban residence (ref. Rural) | ||||
| Urbana | – | – | 0.75 (0.68, 0.82) | < 0.001 |
| Education level (ref. Less than lower secondary) | ||||
| Upper secondary & vocational training | -0.014 (-0.025, -0.003) | 0.015 | 0.73 (0.64, 0.84) | < 0.001 |
| Tertiary | -0.025 (-0.046, -0.004) | 0.020 | 0.62 (0.42, 0.90) | 0.013 |
| Marital status (ref. Divorced or widowed) | ||||
| Married | -0.027 (-0.032, -0.021) | < 0.001 | 0.77 (0.68, 0.86) | < 0.0001 |
| Hukou status (ref. Agricultural) | ||||
| Non-agricultural | -0.010 (-0.016, -0.004) | 0.002 | 0.74 (0.66, 0.84) | < 0.001 |
| Other | -0.010 (-0.019, 0.000) | 0.044 | 0.81 (0.56, 1.18) | 0.274 |
| Public health insurance coverage (ref. Not covered) | ||||
| Covered | 0.002 (-0.002, 0.006) | 0.441 | 0.83 (0.69, 1.00) | 0.045 |
| Current work status (ref. Not working) | ||||
| Working | -0.018 (-0.020, -0.015) | < 0.001 | 0.94 (0.86, 1.03) | 0.194 |
| Comorbidity (ref. None) | ||||
| Single | 0.030 (0.026, 0.034) | < 0.001 | 2.10 (1.83, 2.41) | < 0.001 |
| Morbidity | 0.072 (0.067, 0.076) | < 0.001 | 5.03 (4.43, 5.70) | < 0.001 |
| Household per capita consumption group (ref. low) | ||||
| Low to middle | 0.000 (-0.003, 0.002) | 0.890 | 0.91 (0.81, 1.02) | 0.089 |
| Middle | 0.008 (0.005, 0.010) | < 0.001 | 0.93 (0.84, 1.04) | 0.232 |
| High | 0.014 (0.011, 0.016) | < 0.001 | 0.97 (0.87, 1.09) | 0.612 |
| Alcohol intake (ref. Do not drink) | ||||
| Drink | -0.001 (-0.004, 0.002) | 0.407 | 0.96 (0.88, 1.06) | 0.434 |
| Smoking status (ref. Never) | ||||
| Quit now | 0.011 (0.006, 0.016) | < 0.001 | 1.20 (1.05, 1.38) | 0.008 |
| Smoke now | 0.000 (-0.005, 0.005) | 0.973 | 1.25 (1.11, 1.40) | < 0.001 |
HR Hazard ratio, CI Confidence Interval
aThe variable “Rural/urban residence” was omitted in the fixed-effects model because of collinearity
According to SP type (Table 3), daily IWF, MCCC and DFQ can significantly decrease the risk of frailty in the fixed-effect model (IWF: β = − 0.008; 95% CI: [− 0.010, − 0.005], P < 0.001; MCCC: β = − 0.014; 95% CI: [− 0.019, − 0.010], P < 0.001; DFQ: β = − 0.015; 95% CI: [− 0.020 to − 0.010], P < 0.001) and the time-varying Cox regression (IWF: HR = 0.89; 95% CI: [0.80, 0.99], P = 0.031; MCCC: HR = 0.68; 95% CI: [0.57, 0.80]; P < 0.001; DFQ: HR = 0.72; 95% CI: [0.60, 0.86]; P < 0.001). For MCCC, occasional frequency was associated with lower frailty risk through the fixed-effect model (β = − 0.007; 95% CI: [− 0.011, − 0.003], P < 0.001) and the Cox model (HR = 0.86; 95% CI: [0.74, 0.98], P = 0.028). Weekly MCCC is significantly associated with lower frailty risk through the fixed-effect model (β = − 0.010; 95% CI: [− 0.014, − 0.006], P < 0.001), while this association is almost significant through the Cox model (HR = 0.86; 95% CI: [0.74, 1.00], P = 0.056). The weekly DFQ was also associated with decreased frailty risk through the two models (fixed-effect model: β = − 0.011; 95% CI: [− 0.019, − 0.002], P = 0.015; Cox model: HR = 0.59; 95% CI: [0.37, 0.92], P = 0.020). The impacts of CRO, VOC and INT on frailty are not significant, except that daily INT is associated with lower frailty risk through fixed-effects model.
Table 3.
The association of frailty with different SP types based on fixed-effects model and time-varying Cox regression
| Fixed-effects model (n = 9422)a | Time-varying Cox model (n = 6073)b | |||
|---|---|---|---|---|
| Coefficient (95%CI) | P value | HR (95%CI) | P value | |
| IWF | ||||
| Occasional | 0.002 (-0.001, 0.004) | 0.270 | 0.96 (0.86, 1.08) | 0.496 |
| Weekly | -0.004 (-0.008, -0.001) | 0.009 | 0.99 (0.87, 1.13) | 0.902 |
| Daily | -0.008 (-0.010, -0.005) | < 0.001 | 0.89 (0.80, 0.99) | 0.031 |
| MCCC | ||||
| Occasional | -0.007 (-0.011, -0.003) | < 0.001 | 0.86 (0.74, 0.98) | 0.028 |
| Weekly | -0.010 (-0.014, -0.006) | < 0.001 | 0.86 (0.74, 1.00) | 0.056 |
| Daily | -0.014 (-0.019, -0.010) | < 0.001 | 0.68 (0.57, 0.80) | < 0.001 |
| DFQ | ||||
| Occasional | -0.008 (-0.016, 0.000) | 0.049 | 0.80 (0.56, 1.13) | 0.200 |
| Weekly | -0.011 (-0.019, -0.002) | 0.015 | 0.59 (0.37, 0.92) | 0.020 |
| Daily | -0.015 (-0.020, -0.010) | < 0.001 | 0.72 (0.60, 0.86) | < 0.001 |
| CRO | ||||
| Occasional | -0.003 (-0.012, 0.005) | 0.423 | 1.04 (0.76, 1.42) | 0.823 |
| Weekly | 0.000 (-0.012, 0.012) | 0.992 | 0.74 (0.48, 1.16) | 0.192 |
| Daily | 0.002 (-0.016, 0.020) | 0.811 | 0.55 (0.22, 1.36) | 0.193 |
| VOC | ||||
| Occasional | 0.002 (-0.001, 0.005) | 0.197 | 0.97 (0.86, 1.09) | 0.641 |
| Weekly | -0.001 (-0.006, 0.005) | 0.854 | 0.97 (0.77, 1.23) | 0.824 |
| Daily | 0.001 (-0.006, 0.008) | 0.823 | 0.78 (0.57, 1.09) | 0.143 |
| INT | ||||
| Occasional | -0.001 (-0.012, 0.011) | 0.910 | 0.70 (0.42, 1.17) | 0.173 |
| Weekly | 0.003 (-0.010, 0.016) | 0.699 | 0.57 (0.30, 1.12) | 0.102 |
| Daily | 0.011 (0.005, 0.017) | < 0.001 | 0.90 (0.75, 1.08) | 0.245 |
IWF Interacting with friends, MCCC Playing mah-jong, chess, cards or visiting community clubs, DFQ Going to community-organized dancing, fitness, qigong and so on; CRO, participating in community-related organizations, VOC Voluntary or charitable work, INT Using the Internet, HR Hazard ratio, CI Confidence Interval
a“None” group was set as the reference in each type analysis. Controlled covariates included age, gender, marital status, hukou status, education levels, rural/urban residence, public health insurance coverage, current work status, alcohol intake, smoking status and household per capita consumption
bThe intensity of each SP type was set as time-variant exposure. “None” group was set as the reference. Age, marital status, hukou status, public health insurance coverage, current work status, alcohol intake, smoking status and household per capita consumption were controlled as time-variant covariates, and gender, education level and rural/urban residence were controlled as fixed covariates
For those categorized as frail at baseline (baseline characteristics are presented in Supplementary Table S7), the time-varying Cox regression (outcome: frailty improvement) also demonstrated a significant association between daily SP and lower frailty risk (HR = 1.39; 95% CI: [1.23, 1.57], P < 0.001). For different SP types, weekly and daily IWF, daily MCCC, daily DFQ, daily CRO and occasional INT are effective in improving frailty status. (Table 4).
Table 4.
The association between frequency and type of SP and frailty based on time-varying Cox regression among respondents frail at baseline (n = 3349)*
| Respondents frail at the baseline | ||
|---|---|---|
| HR (95%CI) | P value | |
| SP | ||
| Occasional | 1.02 (0.88, 1.19) | 0.763 |
| Weekly | 1.13 (0.95, 1.33) | 0.157 |
| Daily | 1.39 (1.23, 1.57) | < 0.001 |
| IWF | ||
| Occasional | 1.01 (0.85, 1.19) | 0.941 |
| Weekly | 1.27 (1.05, 1.53) | 0.012 |
| Daily | 1.34 (1.18, 1.53) | < 0.001 |
| MCCC | ||
| Occasional | 1.08 (0.87, 1.35) | 0.476 |
| Weekly | 1.20 (0.96, 1.50) | 0.116 |
| Daily | 1.60 (1.29, 1.98) | < 0.001 |
| DFQ | ||
| Occasional | 1.39 (0.84, 2.29) | 0.198 |
| Weekly | 0.90 (0.50, 1.64) | 0.733 |
| Daily | 1.63 (1.27, 2.08) | < 0.001 |
| CRO | ||
| Occasional | 1.10 (0.62, 1.94) | 0.753 |
| Weekly | 1.72 (0.92, 3.22) | 0.088 |
| Daily | 3.06 (1.27, 7.37) | 0.013 |
| VOC | ||
| Occasional | 0.96 (0.8, 1.15) | 0.662 |
| Weekly | 0.90 (0.65, 1.23) | 0.499 |
| Daily | 1.09 (0.72, 1.64) | 0.689 |
| INT | ||
| Occasional | 2.39 (1.11, 5.16) | 0.027 |
| Weekly | 1.60 (0.56, 4.54) | 0.380 |
| Daily | 0.87 (0.42, 1.80) | 0.713 |
SP Social participation, IWF Interacting with friends, MCCC Playing mah-jong, chess, cards or visiting community clubs, DFQ Going to community-organized dancing, fitness, qigong and so on, CRO Participating in community-related organizations, HR Hazard ratio, CI Confidence Interval
*The intensity of each SP type of was set as time-variant exposure. “None” group was set as the reference. Age, marital status, hukou status, public health insurance coverage, current work status, alcohol intake, smoking status and household per capita consumption were controlled as time-variant covariates, and gender, education level and rural/urban residence were controlled as fixed covariates
Additionally, we conducted two sensitivity analyses to validate the results (Table 5). First, we used data without imputation to repeat the fixed-effects and Cox analysis. Second, we included respondents with frailty related items having a missingness of 5.0% or less (at least 52 items). In conclusion, the findings were consistent with those of the previous analysis. In other words, the direction and magnitude of the effects remained consistent, which validated our main analysis.
Table 5.
Sensitivity analysis*
| Fixed-effects model | Time-varying Cox model | |||
|---|---|---|---|---|
| Estimate (95%CI) | P value | HR (95%CI) | P value | |
| Without imputation | ||||
| Occasional | 0.001 (-0.002, 0.005) | 0.409 | 0.93 (0.83, 1.04) | 0.186 |
| Weekly | -0.004 (-0.008, -0.001) | 0.022 | 0.97 (0.86, 1.09) | 0.593 |
| Daily | -0.009 (-0.012, -0.006) | < 0.001 | 0.76 (0.70, 0.84) | < 0.001 |
| Deficit have a missingness of 5.0% or less | ||||
| Occasional | 0.000 (-0.004, 0.003) | 0.949 | 0.99 (0.86, 1.13) | 0.873 |
| Weekly | -0.007 (-0.011, -0.003) | 0.001 | 0.97 (0.83, 1.14) | 0.747 |
| Daily | -0.009 (-0.012, -0.005) | < 0.001 | 0.83 (0.74, 0.94) | 0.002 |
HR Hazard ratio, CI Confidence Interval
*“None” group was set as the reference. Age, marital status, hukou status, public health insurance coverage, current work status, alcohol intake, smoking status and household per capita consumption were controlled as time-variant covariates, and gender, education level and rural/urban residence were controlled as fixed covariates
In the subgroup analysis, we used the time-varying Cox regression to predict risk. Daily SP was associated with decreased risk of frailty in all subgroups. Weekly SP could decrease frailty risk only in urban respondents. We further identified the impacts of IWF, MCCC and DFQ on frailty in the subgroups, and found that daily MCCC decreases frailty risk in all subgroups. Daily IWF decreases frailty risk in those aged < 65 years, female and rural respondents, but not in those aged ≥ 65 years, male and urban respondents. The effects of occasional and daily MCCC were significant in those aged < 65 years and female participants, whereas only daily MCCC was significant for those aged ≥ 65 years and male participants. The impact of daily DFQ was observed in those aged < 65 years, female and urban respondents, but not in those aged ≥ 65 years, male and rural respondents. Table 6 presents the results in detail.
Table 6.
Subgroup analysis based on time-varying Cox regression
| Aged ≥ 65 in 2011 (n = 1013)a | Aged < 65 in 2011 (n = 5060)a | Male (n = 3233)b | Female (n = 2840)b | Rural (n = 3653)c | Urban (n = 2420)c | |
|---|---|---|---|---|---|---|
| SP | ||||||
| Occasional | 0.92 (0.75, 1.11) | 0.93 (0.82, 1.06) | 0.89 (0.76, 1.04) | 0.97 (0.84, 1.13) | 0.98 (0.86, 1.11) | 0.83 (0.68, 1.02) |
| Weekly | 0.96 (0.77, 1.19) | 0.97 (0.84, 1.12) | 1.02 (0.86, 1.21) | 0.91 (0.77, 1.08) | 1.06 (0.91, 1.22) | 0.80 (0.64, 1.00)* |
| Daily | 0.76 (0.65, 0.89)** | 0.76 (0.68, 0.86)*** | 0.75 (0.65, 0.87)*** | 0.77 (0.68, 0.87)*** | 0.77 (0.68, 0.87)*** | 0.74 (0.63, 0.86)*** |
| IWF | ||||||
| Occasional | 1.10 (0.89, 1.35) | 0.90 (0.78, 1.04) | 0.90 (0.76, 1.08) | 1.01 (0.86, 1.17) | 1.05 (0.91, 1.22) | 0.81 (0.66, 0.99)* |
| Weekly | 1.08 (0.85, 1.38) | 0.95 (0.81, 1.12) | 1.13 (0.94, 1.37) | 0.86 (0.71, 1.04) | 1.10 (0.94, 1.30) | 0.80 (0.63, 1.03) |
| Daily | 0.93 (0.78, 1.11) | 0.87 (0.76, 1.00)* | 0.90 (0.76, 1.07) | 0.87 (0.76, 1.00) | 0.86 (0.75, 0.98)* | 0.94 (0.79, 1.12) |
| MCCC | ||||||
| Occasional | 0.86 (0.66, 1.13) | 0.85 (0.72, 1.00)* | 0.92 (0.76, 1.11) | 0.80 (0.65, 0.98)* | 0.90 (0.76, 1.07) | 0.78 (0.61, 1.00) |
| Weekly | 0.90 (0.68, 1.17) | 0.84 (0.70, 1.02) | 0.86 (0.70, 1.06) | 0.87 (0.70, 1.09) | 0.98 (0.81, 1.18) | 0.70 (0.54, 0.91)** |
| Daily | 0.71 (0.55, 0.92)* | 0.65 (0.53, 0.81)*** | 0.68 (0.54, 0.87)** | 0.68 (0.54, 0.85)** | 0.66 (0.53, 0.82)*** | 0.68 (0.53, 0.87)** |
| DFQ | ||||||
| Occasional | 0.40 (0.12, 1.27) | 0.89 (0.63, 1.28) | 0.85 (0.44, 1.67) | 0.78 (0.52, 1.16) | 0.58 (0.33, 1.00) | 1.05 (0.67, 1.63) |
| Weekly | 0.56 (0.21, 1.48) | 0.58 (0.35, 0.97)* | 0.52 (0.21, 1.24) | 0.62 (0.37, 1.05) | 0.49 (0.23, 1.03) | 0.65 (0.37, 1.16) |
| Daily | 0.89 (0.66, 1.19) | 0.63 (0.49, 0.79)*** | 0.74 (0.54, 1.02) | 0.72 (0.57, 0.90)*** | 0.75 (0.55, 1.01) | 0.71 (0.56, 0.90)*** |
*P < 0.05; **P < 0.01; ***P < 0.001. SP Social participation, IWF Interacting with friends, MCCC Playing mah-jong, chess, cards or visiting community clubs, DFQ Going to community-organized dancing, fitness, qigong and so on, HR Hazard ratio, CI Confidence Interval
a The frequency of each type of SP was set as time-variant exposure. “None” group was set as the reference. Age, marital status, hukou status, public health insurance coverage, current work status, alcohol intake, smoking status and household per capita consumption were controlled as time-variant covariates, and gender, education level and residence were controlled as fixed covariates
b All the covariates were controlled except for gender
c All the covariates were controlled except for rural/urban residence
Discussion
To the best of our knowledge, this study is the first to assess the impact of SP on frailty from the perspectives of frequency and type. The result indicates that, SP, especially a high-frequency one, can significantly decrease the risk of frailty among the middle-aged and older populations. For different types of SP, high-frequency of IWF, MCCC and DFQ are effective exhibits a potential in preventing frailty among non-frail populations and improving the frailty status of already-frail populations. In addition, moderate-frequency of MCCC and DFQ are also associated with decreased risk of frailty.
The prevalence of frailty ranges from 35.5% to 53.5%, which increase with the increase in age. A recent meta-analysis reveals a pooled prevalence of frailty as 10% in China, which is much lower than our study [45]. This could be explained by the measurement of frailty. For example, a study taken into their calculation of frailty prevalence, which also used the CHARLS data, found 7.0% of adults aged 60 years or older were frail [46]. Frailty in that study was measured by the physical frailty phenotype (PFP) scale in which five elements are included: weakness, slowness, exhaustion, inactivity, and shrinking. Some studies indicated that the accumulation of deficits model, as in this study, usually come up with higher percentages of frail than the phenotype measure, as the accumulation of deficits model includes other dimensions of frailty [31]. Chronic diseases and depression were measured as risk factors in Wu’s study [46], nevertheless, which were defined as deficits to calculate frailty score in our analysis. In this study, the prevalences of depressive symptoms are high (almost half of the respondents), and prevalences of chronic diseases, such as hypertension, arthritis and stomach/digestive disease (25.3%, 36.6% and 24.1% at baseline, respectively) are also common. These could further explain the high prevalence of frailty in our study.
The previous literature has suggested that participating in social activities may incentivize mutual support, provide one with a sense of belonging, and largely reduce social isolation, which, therefore, may improve mental health or prevent depression [47]. Furthermore, studies have demonstrated that SP is associated with better cognitive function and lower risk of dementia [48, 49]. Other studies also pointed to the positive effect of SP on physical function [50]. These results indicate the potential association between SP and frailty, where the current study provides a clear evidence for this association.
In this study, SP refers to communicative activities (i.e., interaction with friends), intellectually demanding/engaging activities (i.e., playing mah-jong, chess, and cards or visiting other community clubs), public welfare activities (e.g., participating in community-related organizations and voluntary or charitable work), several community-organized physical activities (going to dancing, fitness, qigong and so on), and online activities (using the Internet). Our analysis indicated that communicative activities, intellectually demanding/engaging activities and community-organized physical activities are more effective in preventing frailty of older population than other SP types. High-frequency communicative activities, such as interacting with friends, can help the older adults to build social networks and increase the opportunities to form intimate relationships, thus helping them to improve social connection and reduce loneliness [51]. In addition, communicative activities are proved to be significantly associated with social network support and effective in decreasing the risk of social isolation and depression [52], consequently decreasing frailty risk. The effectiveness of intellectually demanding/engaging activities, which is observed in all the subgroups, may due to their functions on mental-exercise. As mental-exercise hypothesis indicates, continuous mental cognitive training can prevent individual cognitive decline [53]. Therefore, intellectually demanding/engaging activities can stimulate intellect, and, consequently, maintain cognitive function and prevent frailty among older adults [54]. Daily physical activities, such as exercising or dancing, are also significantly associated with lowered risk of frailty. This result, consistent with those of previous studies [23, 55, 56], indicates that moderate or high-frequency physical activities may be associated with the reduced incidence of disabilities, reduced progression of disabilities, and improved physical functioning, which are associated with lower frailty risk. In addition, we found high frequency of public welfare activities are associated with reversing frailty progression. This can be explained that volunteer activities emphasize individual performance and team cohesion, which are also proved to be more likely to weaken the mortality of older population [57].
Interestingly, the impacts of SP frequency, as well as type, vary among subgroups. For example, the impact of daily IWF, occasional MCCC, and weekly DFQ is significant for those aged < 65 years but not for those aged ≥ 65 years. This difference may be due to the intrinsic property or the irreversible trend of high levels of frailty among older adults aged ≥ 65 years and above, which will be increasingly difficult to control. In addition, the differences in the results for adults in rural and urban areas may be due to the variance in social resources and the stability of social networks. For example, daily IWF is significant in rural but not urban residents, whereas occasional IWF is significant in urban but not rural residents. The potential underlying reasons may be that the social network among rural residents is limited but strong and stable. Nevertheless, the social network among urban residents is relatively weaken. Due to sensitivity from a perception of non-existence to existence for urban respondents, their frailty status is more sensitive to occasional IWF than the rural respondents [22, 58]. Furthermore, variances also existed between male and female respondents. Specifically, female respondents are more sensitive to daily IWF, occasional MCCC, and daily DFQ, which may be due to gender differences in terms of personality, preferences for social interaction, and internal frailty risks [59, 60].
This study provided evidence of the negative association between SP and frailty, which has several implications for healthy ageing strategies. This study employed a nationwide representative database and employed high-quality microdata among middle-aged and older populations. Furthermore, it utilized cohort analysis and different statistical models and comprehensive subgroup analysis. The results suggested other accurate intervention strategies and directions for further prospective mechanism research. Encourageing SP, building esthetic, walkable, and cohesive neighborhood, and providing additional materials and organizational bases for diversified social activities are urgent initiatives for decreasing the risk of frailty for the older population [61].
This study has several limitations. First, the inherent limitations of a retrospective study indicate that the relationship between SP and frailty requires a definitive validation in prospective studies. Second, the study acknowledges the existence of recall bias, because information was self-reported. For example, the indexes associated with physical function and mental health were the main constituent elements of frailty. However, a self-rated level may differ from that of reality. Third, respondents who became frail but died before 2018 have not been included our analysis. Given frailty is associated with mortality, excluding them may introduce a survival bias. Finally, the frailty index covers numerous indicators. During the interview process, many indicators are observed missing among the participants, which led to sample loss. Nevertheless, we applied the cohort design and conducted several sensitivity analyses to overcome this bias.
Conclusions
In summary, this study used the longitudinal and cohort designs with the fixed-effect and time-varying Cox models to validate the results. The findings clearly demonstrated that enhancing participation in social activities, especially high-frequency SP, could significantly decrease the risk of frailty, as well as reverse frailty progression, among middle-aged and older populations. Furthermore, we identified that communicative activities, intellectually demanding/engaging activities and community-organized physical activities are significantly associated with decreased frailty risk. The impact of the frequency and type of SP vary across age, gender, and residence groups. Thus, these findings present important implications for research and public health policy. As the trend of population ageing has become a worldwide challenge, the current study provided extremely operable, easy, and valuable intervening measures to promote healthy ageing among for middle-aged and older populations around the world.
Supplementary Information
Acknowledgements
We gratefully acknowledge the China Health and Retirement Longitudinal Study team for providing data and training in using the datasets.
Abbreviations
- SP
Social participation
- CHARLS
China Health and Retirement Longitudinal Study
- OLS
Ordinary least squares
- MI
Multiple imputation
- FI
Frailty index
- IWF
Interacting with friends
- MCCC
Playing mah-jong, chess, cards or visiting other community clubs
- DFQ
Going to (community-organized) dancing, fitness, qigong and so on
- CRO
Participating in community-related organizations
- VOC
Voluntary or charitable work
- INT
Using the Internet
- HR
Hazard ratio
- CI
Confidence Interval
- PFP
Physical frailty phenotype
Authors’ contributions
CJY, KXY and LHX made substantial contributions to study design and data cleaning. CJY ZF and HH analysed the data in duplicate. SJ, KXY and LHM interpreted the results and drafted the article. All authors revised it critically for important intellectual content. All authors read and approved the final manuscript.
Funding
This work was supported by National Social Science Foundation (16BGL150). The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
Availability of data and materials
All the original data could be obtained from the official website of CHARLS (http://charls.pku.edu.cn/). The analysis dataset is available to other researchers and others upon request by emailing the corresponding author (lihaomiao@whu.edu.cn).
Declarations
Ethical approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations. The Biomedical Ethics Review Committee of Peking University approved CHARLS, and all participants were required to provide written informed consent. The ethical approval number was IRB00001052-11015.
Consent for publication
Not applicable.
Competing interests
The authors report no potential conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rockwood K, Howlett SE. Fifteen years of progress in understanding frailty and health in aging. BMC Med. 2018;16(1):220. doi: 10.1186/s12916-018-1223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 3.Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394(10206):1376–1386. doi: 10.1016/S0140-6736(19)31785-4. [DOI] [PubMed] [Google Scholar]
- 4.Tabue-Teguo M, Simo N, Gonzalez-Colaco HM, Cesari M, Avila-Funes JA, Feart C, Amieva H, Dartigues JF. Frailty in elderly: a brief review. Geriatr Psychol Neuropsychiatr Vieil. 2017;15(2):127–137. doi: 10.1684/pnv.2017.0670. [DOI] [PubMed] [Google Scholar]
- 5.Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel JP, Lloyd-Sherlock P, Epping-Jordan JE, Peeters G, Mahanani WR, et al. The World report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387(10033):2145–2154. doi: 10.1016/S0140-6736(15)00516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atchley RC. A continuity theory of normal aging. Gerontologist. 1989;29(2):183–190. doi: 10.1093/geront/29.2.183. [DOI] [PubMed] [Google Scholar]
- 7.Spini D, Bernardi L, Oris M. Toward a Life Course Framework for Studying Vulnerability. Res Hum Dev. 2017;14(1):5–25. doi: 10.1080/15427609.2016.1268892. [DOI] [Google Scholar]
- 8.Ma L, Sun F, Tang Z. Social Frailty Is Associated with Physical Functioning, Cognition, and Depression, and Predicts Mortality. J Nutr Health Aging. 2018;22(8):989–995. doi: 10.1007/s12603-018-1054-0. [DOI] [PubMed] [Google Scholar]
- 9.Makizako H, Shimada H, Tsutsumimoto K, Lee S, Doi T, Nakakubo S, Hotta R, Suzuki T. Social Frailty in Community-Dwelling Older Adults as a Risk Factor for Disability. J am med dir assoc . 2015;16(11):1003–1007. doi: 10.1016/j.jamda.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Mehrabi F, Beland F. Effects of social isolation, loneliness and frailty on health outcomes and their possible mediators and moderators in community-dwelling older adults: A scoping review. Arch Gerontol Geriatr. 2020;90:104119. doi: 10.1016/j.archger.2020.104119. [DOI] [PubMed] [Google Scholar]
- 11.Bunt S, Steverink N, Olthof J, van der Schans CP, Hobbelen J. Social frailty in older adults: a scoping review. Eur J Ageing. 2017;14(3):323–334. doi: 10.1007/s10433-017-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding YY, Kuha J, Murphy M. Pathways from physical frailty to activity limitation in older people: Identifying moderators and mediators in the English Longitudinal Study of Ageing. Exp Gerontol. 2017;98:169–176. doi: 10.1016/j.exger.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 13.Gale CR, Westbury L, Cooper C. Social isolation and loneliness as risk factors for the progression of frailty: the English Longitudinal Study of Ageing. Age Ageing. 2018;47(3):392–397. doi: 10.1093/ageing/afx188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takashima R, Onishi R, Saeki K, Hirano M. The values and meanings of social activities for older urban men after retirement. PLoS One. 2020;15(11):e242859. doi: 10.1371/journal.pone.0242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinbach U. Social networks, institutionalization, and mortality among elderly people in the United States. J Gerontol. 1992;47(4):S183–S190. doi: 10.1093/geronj/47.4.S183. [DOI] [PubMed] [Google Scholar]
- 16.Saito T, Murata C, Saito M, Takeda T, Kondo K. Influence of social relationship domains and their combinations on incident dementia: a prospective cohort study. J Epidemiol Community Health. 2018;72(1):7–12. doi: 10.1136/jech-2017-209811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SH, Kim YB. Which type of social activities decrease depression in the elderly? An analysis of a population-based study in South Korea. Iran J Public Health. 2014;43(7):903–912. [PMC free article] [PubMed] [Google Scholar]
- 18.Kanamori S, Kai Y, Aida J, Kondo K, Kawachi I, Hirai H, Shirai K, Ishikawa Y, Suzuki K. Social participation and the prevention of functional disability in older Japanese: the JAGES cohort study. PLoS One. 2014;9(6):e99638. doi: 10.1371/journal.pone.0099638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berry HL, Welsh JA. Social capital and health in Australia: An overview from the household, income and labour dynamics in Australia survey. Soc Sci Med. 2010;70(4):588–596. doi: 10.1016/j.socscimed.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Douglas H, Georgiou A, Westbrook J. Social participation as an indicator of successful aging: an overview of concepts and their associations with health. Aust Health Rev. 2017;41(4):455–462. doi: 10.1071/AH16038. [DOI] [PubMed] [Google Scholar]
- 21.Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. PERSPECT PSYCHOL SCI. 2015;10(2):227–237. doi: 10.1177/1745691614568352. [DOI] [PubMed] [Google Scholar]
- 22.Wang R, Chen Z, Zhou Y, Shen L, Zhang Z, Wu X. Melancholy or mahjong? Diversity, frequency, type, and rural-urban divide of social participation and depression in middle- and old-aged Chinese: A fixed-effects analysis. SOC SCI MED. 2019;238:112518. doi: 10.1016/j.socscimed.2019.112518. [DOI] [PubMed] [Google Scholar]
- 23.Takatori K, Matsumoto D. Social factors associated with reversing frailty progression in community-dwelling late-stage elderly people: An observational study. PLoS ONE. 2021;16(3):e247296. doi: 10.1371/journal.pone.0247296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS) INT J EPIDEMIOL. 2014;43(1):61–68. doi: 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moorhouse P, Rockwood K. Frailty and its quantitative clinical evaluation. J R Coll Physicians Edinb. 2012;42(4):333–340. doi: 10.4997/JRCPE.2012.412. [DOI] [PubMed] [Google Scholar]
- 27.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC GERIATR. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. CLIN GERIATR MED. 2011;27(1):17–26. doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Pena FG, Theou O, Wallace L, Brothers TD, Gill TM, Gahbauer EA, Kirkland S, Mitnitski A, Rockwood K. Comparison of alternate scoring of variables on the performance of the frailty index. BMC GERIATR. 2014;14:25. doi: 10.1186/1471-2318-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero-Ortuno R. An alternative method for Frailty Index cut-off points to define frailty categories. Eur Geriatr Med. 2013;4(5):10. [DOI] [PMC free article] [PubMed]
- 31.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62(7):738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 32.Lin W. A study on the factors influencing the community participation of older adults in China: based on the CHARLS2011 data set. Health Soc Care Community. 2017;25(3):1160–1168. doi: 10.1111/hsc.12415. [DOI] [PubMed] [Google Scholar]
- 33.Jaddoe VW, de Jonge LL, Hofman A, Franco OH, Steegers EA, Gaillard R. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ. 2014;348:g14. doi: 10.1136/bmj.g14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen M, Wu Y, Xiang X. Hukou-based rural-urban disparities in maternal health service utilization and delivery modes in two Chinese cities in Guangdong Province. INT J EQUITY HEALTH. 2021;20(1):145. doi: 10.1186/s12939-021-01485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norheim KL, Bøggild H, Andersen JH, Omland Ø, Bønløkke JH, Madeleine P. Retirement status and frailty: a cross-sectional study of the phenotype of manual workers aged 50-70 years. Eur J Public Health. 2021;31(1):116-21. [DOI] [PubMed]
- 36.Zhao Y, Atun R, Oldenburg B, McPake B, Tang S, Mercer SW, Cowling TE, Sum G, Qin VM, Lee JT. Physical multimorbidity, health service use, and catastrophic health expenditure by socioeconomic groups in China: an analysis of population-based panel data. LANCET GLOB HEALTH. 2020;8(6):e840–e849. doi: 10.1016/S2214-109X(20)30127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carvalho FJ, de Santana DG, Sampaio MV. Modeling Overdispersion, Autocorrelation, and Zero-Inflated Count Data Via Generalized Additive Models and Bayesian Statistics in an Aphid Population Study. NEOTROP ENTOMOL. 2020;49(1):40–51. doi: 10.1007/s13744-019-00729-x. [DOI] [PubMed] [Google Scholar]
- 38.Gescheit IM, Dayan A, Ben-David M, Gannot I. Minimal-invasive thermal imaging of a malignant tumor: a simple model and algorithm. MED PHYS. 2010;37(1):211–216. doi: 10.1118/1.3253992. [DOI] [PubMed] [Google Scholar]
- 39.Guo RN, Zheng HZ, Ou CQ, Huang LQ, Zhou Y, Zhang X, Liang CK, Lin JY, Zhong HJ, Song T, et al. Impact of Influenza on Outpatient Visits, Hospitalizations, and Deaths by Using a Time Series Poisson Generalized Additive Model. PLoS ONE. 2016;11(2):e149468. doi: 10.1371/journal.pone.0149468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Y, Chen Z, Shaw I, Wu X, Liao S, Qi L, Huo L, Liu Y, Wang R. Association between social participation and cognitive function among middle- and old-aged Chinese: A fixed-effects analysis. J GLOB HEALTH. 2020;10(2):20801. doi: 10.7189/jogh.10.020801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suissa S. Immortal time bias in pharmaco-epidemiology. AM J EPIDEMIOL. 2008;167(4):492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis-Oudshoorn C. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med. 2018;6(7):121. doi: 10.21037/atm.2018.02.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan J, Yu C, Guo Y, Bian Z, Sun Z, Yang L, Chen Y, Du H, Li Z, Lei Y, et al. Frailty index and all-cause and cause-specific mortality in Chinese adults: a prospective cohort study. Lancet Public Health. 2020;5(12):e650–e660. doi: 10.1016/S2468-2667(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ. 2011;183(8):E487–E494. doi: 10.1503/cmaj.101271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He B, Ma Y, Wang C, Jiang M, Geng C, Chang X, Ma B, Han L. Prevalence and Risk Factors for Frailty among Community-Dwelling Older People in China: A Systematic Review and Meta-Analysis. J NUTR HEALTH AGING. 2019;23(5):442–450. doi: 10.1007/s12603-019-1179-9. [DOI] [PubMed] [Google Scholar]
- 46.Wu C, Smit E, Xue QL, Odden MC. Prevalence and Correlates of Frailty Among Community-Dwelling Chinese Older Adults: The China Health and Retirement Longitudinal Study. J Gerontol A Biol Sci Med Sci. 2017;73(1):102–108. doi: 10.1093/gerona/glx098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hikichi H, Aida J, Matsuyama Y, Tsuboya T, Kondo K, Kawachi I. Community-level social capital and cognitive decline after a natural disaster: A natural experiment from the 2011 Great East Japan Earthquake and Tsunami. SOC SCI MED. 2020;257:111981. doi: 10.1016/j.socscimed.2018.09.057. [DOI] [PubMed] [Google Scholar]
- 48.Kelly ME, Duff H, Kelly S, McHugh PJ, Brennan S, Lawlor BA, Loughrey DG. The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: a systematic review. Syst Rev. 2017;6(1):259. doi: 10.1186/s13643-017-0632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuiper JS, Zuidersma M, Oude VR, Zuidema SU, van den Heuvel ER, Stolk RP, Smidt N. Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. AGEING RES REV. 2015;22:39–57. doi: 10.1016/j.arr.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Ukawa S, Tamakoshi A, Okada Y, Ito YM, Taniguchi R, Tani Y, Sasaki Y, Saito J, Haseda M, Kondo N, et al. Social participation patterns and the incidence of functional disability: The Japan Gerontological Evaluation Study. GERIATR GERONTOL INT. 2020;20(8):765–772. doi: 10.1111/ggi.13966. [DOI] [PubMed] [Google Scholar]
- 51.Park NS, Jang Y, Lee BS, Haley WE, Chiriboga DA. The mediating role of loneliness in the relation between social engagement and depressive symptoms among older Korean Americans: do men and women differ? J Gerontol B Psychol Sci Soc Sci. 2013;68(2):193–201. doi: 10.1093/geronb/gbs062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu F, Sheng Y. Social support network, social support, self-efficacy, health-promoting behavior and healthy aging among older adults: A pathway analysis. Arch Gerontol Geriatr. 2019;85:103934. doi: 10.1016/j.archger.2019.103934. [DOI] [PubMed] [Google Scholar]
- 53.Salthouse TA. Mental Exercise and Mental Aging: Evaluating the Validity of the "Use It or Lose It" Hypothesis. PERSPECT PSYCHOL SCI. 2006;1(1):68–87. doi: 10.1111/j.1745-6916.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- 54.Iizuka A, Suzuki H, Ogawa S, Kobayashi-Cuya KE, Kobayashi M, Takebayashi T, Fujiwara Y. Can cognitive leisure activity prevent cognitive decline in older adults? A systematic review of intervention studies. GERIATR GERONTOL INT. 2019;19(6):469–482. doi: 10.1111/ggi.13671. [DOI] [PubMed] [Google Scholar]
- 55.Tak E, Kuiper R, Chorus A, Hopman-Rock M. Prevention of onset and progression of basic ADL disability by physical activity in community dwelling older adults: a meta-analysis. AGEING RES REV. 2013;12(1):329–338. doi: 10.1016/j.arr.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 56.de Vries NM, van Ravensberg CD, Hobbelen JS, Olde RM, Staal JB, Nijhuis-van DSM. Effects of physical exercise therapy on mobility, physical functioning, physical activity and quality of life in community-dwelling older adults with impaired mobility, physical disability and/or multi-morbidity: a meta-analysis. AGEING RES REV. 2012;11(1):136–149. doi: 10.1016/j.arr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Haak M, Lofqvist C, Ullen S, Horstmann V, Iwarsson S. The influence of participation on mortality in very old age among community-living people in Sweden. AGING CLIN EXP RES. 2019;31(2):265–271. doi: 10.1007/s40520-018-0947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu J, Rozelle S, Xu Q, Yu N, Zhou T: Social Engagement and Elderly Health in China: Evidence from the China Health and Retirement Longitudinal Survey (CHARLS). Int J Environ Res Public Health 2019, 16(2). [DOI] [PMC free article] [PubMed]
- 59.Lee S, Lee S, Lee E, Youm Y, Cho HS, Kim WJ. Gender differences in social network of cognitive function among community-dwelling older adults. GERIATR GERONTOL INT. 2020;20(5):467–473. doi: 10.1111/ggi.13906. [DOI] [PubMed] [Google Scholar]
- 60.Naud D, Genereux M, Alauzet A, Bruneau JF, Cohen A, Levasseur M. Social participation and barriers to community activities among middle-aged and older Canadians: Differences and similarities according to gender and age. GERIATR GERONTOL INT. 2021;21(1):77–84. doi: 10.1111/ggi.14087. [DOI] [PubMed] [Google Scholar]
- 61.Ye B, Gao J, Fu H. Associations between lifestyle, physical and social environments and frailty among Chinese older people: a multilevel analysis. BMC GERIATR. 2018;18(1):314. doi: 10.1186/s12877-018-0982-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the original data could be obtained from the official website of CHARLS (http://charls.pku.edu.cn/). The analysis dataset is available to other researchers and others upon request by emailing the corresponding author (lihaomiao@whu.edu.cn).