Abstract
Background
Surgery used to be the treatment of choice in cases of blunt hepatic injury, but this approach gradually changed over the last two decades as increasing non‐operative management (NOM) of splenic injury led to its use for hepatic injury. The improvement in critical care monitoring and computed tomographic scanning, as well as the more frequent use of interventional radiology techniques, has helped to bring about this change to non‐operative management. Liver trauma ranges from a small capsular tear, without parenchymal laceration, to massive parenchymal injury with major hepatic vein/retrohepatic vena cava lesions. In 1994, the Organ Injury Scaling Committee of the American Association for the Surgery of Trauma (AAST) revised the Hepatic Injury Scale to have a range from grade I to VI. Minor injuries (grade I or II) are the most frequent liver injuries (80% to 90% of all cases); severe injuries are grade III‐V lesions; grade VI lesions are frequently incompatible with survival. In the medical literature, the majority of patients who have undergone NOM have low‐grade liver injuries. The safety of NOM in high‐grade liver lesions, AAST grade IV and V, remains a subject of debate as a high incidence of liver and collateral extra‐abdominal complications are still described.
Objectives
To assess the effects of non‐operative management compared to operative management in high‐grade (grade III‐V) blunt hepatic injury.
Search methods
The search for studies was run on 14 April 2014. We searched the Cochrane Injuries Group's Specialised Register, The Cochrane Library, Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R), Embase Classic+Embase (Ovid), PubMed, ISI WOS (SCI‐EXPANDED, SSCI, CPCI‐S & CPSI‐SSH), clinical trials registries, conference proceedings, and we screened reference lists.
Selection criteria
All randomised trials that compare non‐operative management versus operative management in high‐grade blunt hepatic injury.
Data collection and analysis
Two authors independently applied the selection criteria to relevant study reports. We used standard methodological procedures as defined by the Cochrane Collaboration.
Main results
We were unable to find any randomised controlled trials of non‐operative management versus operative management in high‐grade blunt hepatic injury.
Authors' conclusions
In order to further explore the preliminary findings provided by animal models and observational clinical studies that suggests there may be a beneficial effect of non‐operative management versus operative management in high‐grade blunt hepatic injury, large, high quality randomised trials are needed.
Plain language summary
Is surgery or observation better for people who have a severe blunt injury to the liver?
Background
The liver is the most commonly affected organ when a person is injured in the abdomen. Abdominal injury is usually caused by motor vehicle crashes, falling, being punched in the stomach, or from other causes. When a person is badly injured in the abdomen, they have a 10% to 15% chance of death. According to previous research, the chance of death following a liver injury has not reduced over the past 30 years.
Liver injury is classified on a scale from 1 to 6. A grade 1 injury is least severe, whereas a grade 6 injury is most severe. The majority of people with a grade 6 injury die. Usually, people with grade 1 and 2 liver injuries receive observation as their treatment; so their body can heal naturally. People with higher grade injuries may need surgery. During surgery doctors may stitch the liver together to help it heal.
Review question
We wanted to find out whether surgery or observation is better for people who have a severe blunt liver injury. Studies were included if people had a liver injury of grade 3, 4 or 5. We were interested in finding out if there is a difference in death, illness, or quality of life.
We searched for every randomised controlled trial undertaken worldwide, of surgery or observation for people with grade 3, 4 or 5 liver injury. We searched for trials on 14 April 2014.
Results
We found no randomised controlled trials on this topic. No studies are included in this review.
Conclusion
Trials are needed so that doctors and patients have research to use when making treatment decisions.
Background
The liver is the most commonly injured organ following blunt abdominal trauma (Ahmed 2011). The mechanism of injury often includes motor vehicle and pedestrian crashes, falls or other causes. Hepatic injury is the primary cause of death after severe abdominal trauma, with related mortality of 10% to 15% (Parks 1999). In the last three decades the total number of hepatic injury cases diagnosed has increased due to a rise in urban road traffic crashes together with more accurate diagnosis (Matthes 2003).
Surgery used to be the treatment of choice in cases of blunt hepatic injury, but this approach gradually changed over the last two decades (Christmas 2005; Durham 1992) as increasing non‐operative management (NOM) of splenic injury led to its use for hepatic injury (Richardson 2000). The improvement in critical care monitoring and computed tomographic scanning, as well as the more frequent use of interventional radiology techniques, has helped to bring about this change to non‐operative management (Richardson 2005).
Non‐operative management for blunt hepatic injuries is safe and considered the treatment of choice in haemodynamically stable patients with only low‐grade injuries. In the study described by Christmas 2005, 561 patients with hepatic injury from blunt trauma between 1993 and 2003 underwent NOM: 59% were low‐grade injuries and 41% were high‐grade injuries (grade III‐IV‐V). Of those with grade IV or V injuries, 54.7% underwent NOM, whereas 70% with grade I‐II‐III injuries were managed without surgery.
Failure of NOM is defined as the need for a laparotomy to be performed more than six hours after hospital admission (Norrman 2009). The most common cause of failure of NOM is delayed bleeding and missed associated injuries. Predictive factors of the failure of NOM are: transfusion requirements of more than four units prior to surgery, injury severity score (ISS) of more than 34, injuries of grade IV‐V, large haemoperitoneum, and pooling of contrast (Norrman 2009). In patients with failure of NOM the incidence of postoperative complications is very high and differences have been found between small‐volume and specialised centres (Norrman 2009). In general, in hospitals with low trauma incidence NOM is feasible and safe (Giannopoulos 2009) but surgery may still be required. A study reported by Velmahos 2003 found that 206 patients with blunt injuries to the liver, spleen and kidney underwent NOM. NOM failed in 22%; the rate of failure for spleen injury (34%) was higher than for liver (17%) and kidney (18%) injury.
In the medical literature, the majority of patients who have undergone NOM have low‐grade liver injuries. The safety of NOM in high‐grade liver lesions (American Association for the Surgery of Trauma (AAST) grade IV and V) remains a subject of debate as a high incidence of liver (11%) and collateral extra‐abdominal (17%) complications are still described. Liver‐related and extra‐abdominal complication rates in NOM patients were 11% and 17% respectively in one study (Schnüriger 2009). The length of recovery is a consequence of the grade of liver lesion: mild hepatic injuries heal within seven days whereas high‐grade injuries need up to nine months (Bulas 1993).
Description of the condition
Liver trauma ranges from a small capsular tear, without parenchymal laceration, to massive parenchymal injury with major hepatic vein/retrohepatic vena cava lesions. In 1994, the Organ Injury Scaling Committee of the AAST revised the Hepatic Injury Scale (Table 1). Minor injuries (grade I or II) are the most frequent liver injuries (80% to 90% of all cases); severe injuries are grade III‐V lesions; grade VI lesions are frequently incompatible with survival (Clancy 2001). In this review we analysed only high‐grade blunt hepatic injury (grade III‐V).
1. AAST revised the Hepatic Injury Scale.
| Grade* | Type of injury | Description of injury |
| I | Haematoma | Subcapsular, < 10% surface area |
| Laceration | Capsular tear, < 1 cm parenchymal depth | |
| II | Haematoma | Subcapsular, 10% to 50% surface area or intraparenchymal, < 10 cm in diameter |
| Laceration | 1 to 3 cm parenchymal depth, less than 10 cm in length | |
| III | Haematoma | Subcapsular, > 50% surface area, expanding ruptured subcapsular or parenchymal haematoma with active bleeding, or intraparenchymal haematoma > 10 cm or expanding |
| Laceration | > 3 cm depth | |
| IV | Laceration | Parenchymal disruption involving 25% to 75% of hepatic lobe or 1 to 3 Couinaud segments within a single lobe |
| V | Laceration | Parenchymal disruption involving > 75% of hepatic lobe or more than 3 Couinaud segments within a single lobe |
| Vascular | Juxtahepatic venous injuries, i.e. retrohepatic vena cava/major hepatic veins | |
| VI | Vascular | Hepatic avulsion |
| *Advance 1 grade for multiple injuries *Quoted from Moore (Moore 1995) and others | ||
Description of the intervention
Non‐operative management for blunt hepatic trauma involves monitoring and radiological intervention, including angio‐embolisation (Asensio 2003). In critical care units, patients undergo a protocol for intensive monitoring and an experienced surgical team must follow the patient closely (Buccoliero 2010). With the operative approach, patients immediately undergo hepatic resection (Polanco 2008) and conservative liver techniques (hepatorrhaphy, packing and wrapping) (Cirocchi 1999).
The goal of NOM is to avoid unnecessary laparotomy in selected patients with blunt abdominal injury. For patients with haemodynamic instability (Eastridge 2007), signs of peritonitis or associated extra‐abdominal injuries requiring surgery, it may be impossible to perform emergency surgery (Demetriades 2005). Within an hour of admission to the hospital CT scanning is recommended, because it enables classification of the severity of the abdominal injury and the presence of associated injuries (4.2%) (Schnuriger 2011); missed injuries are more frequently associated with hepatic rather than splenic injuries (Miller 2002). Patients are monitored in the critical care unit by an experienced team. Monitoring of abdominal trauma may include measuring vital signs, abdominal echography, CT scan, CBC (complete blood count), clotting profile, SGOT (aspartate aminotransferase), SGPT (alanine aminotransferase), and urine output. NOM is discontinued in the presence of active bleeding or when there is unstable haemodynamic status (Buccoliero 2010). This approach also applies in high‐grade hepatic injury cases provided that there are no other abdominal injuries that require surgical intervention (Norrman 2009). NOM can also be considered in patients with multiple solid organ injuries, even if it is associated with a higher failure rate (Kozar 2005).
How the intervention might work
The advantage of non‐operative management compared to operative management is a decrease in non‐therapeutic laparotomy with its related mortality, morbility, length of hospitalisation and blood transfusion. The disadvantages of non‐operative management include no immediate control of bleeding, with a risk of delayed bleeding, and missed associated abdominal injuries that require surgical treatment.
Why it is important to do this review
In current clinical practice non‐operative management is best for haemodynamically stable adult and paediatric low‐grade liver injuries. However, uncertainty still exists about its efficacy for high‐grade blunt hepatic injury.
Objectives
To assess the effectiveness of non‐operative management compared to operative management in high‐grade (grade III‐V) blunt hepatic injury.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Cluster‐RCTs are excluded.
Types of participants
Any person who is haemodynamically stable and has high‐grade (grade III‐V) blunt abdominal trauma.
Types of interventions
Non‐operative management compared with operative management.
Types of outcome measures
Primary outcomes
Mortality in the first 30 days following trauma.
Secondary outcomes
30‐day all‐cause morbidity. All‐cause morbidity is defined as having documentation of serious morbidity or at least one of the following American College of Surgeons, National Surgical Quality Improvement Program (ACS‐NSQIP) complications (American College of Surgeons 2008): "superficial surgical site infection, deep surgical site infection, pneumonia, unplanned intubation (without preoperative ventilator dependence), peripheral neurological deficit, urinary tract infection, and deep vein thrombosis" (Ingraham 2011). Morbidity is a binary outcome (presence or absence of complication in each patient).
30‐day serious morbidity. Serious morbidity is defined as having documentation of at least one of the following ACS‐NSQIP complications (American College of Surgeons 2008): "organ space surgical site infection, wound dehiscence, neurological event (cerebrovascular accident or coma lasting 24 hours), cardiac arrest, myocardial infarction, bleeding requiring transfusion, pulmonary embolism, ventilator dependence more than 48 hours, progressive or acute renal insufficiency, and sepsis or septic shock" (Ingraham 2011). We treated morbidity as a binary outcome (presence or absence of complication in each patient).
Quality of life at final follow‐up. Quality of life assessed using the Gastrointestinal Quality of Life Index (GLQI). The GLQI was developed by a board of experts to measure quality of life in patients with gastrointestinal diseases, particularly those undergoing an operation. The questionnaire contains up to 36 items in five main categories: gastrointestinal symptoms (19 questions), physical condition (seven questions), emotions (five questions), social function (four questions) and effect of medical treatment (one question) (Eypasch 1995). Each question is scored on a five‐point Likert scale (range 0 to 144, where a higher score indicates a better quality of life). In the French version of the questionnaire a healthy control population scored 126 points out of a total of 144 (Slim 1999).
Search methods for identification of studies
In order to reduce publication and retrieval bias we did not restrict our search by language, date or publication status.
Electronic searches
The Cochrane Injuries Group's Trials Search Co‐ordinator searched the following:
Cochrane Injuries Group specialised register (14th April 2014);
Cochrane Central Register of Controlled Trials Online (CRSO) (14th April 2014);
Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R) (1946 to 14th April 2014);
Embase Classic + Embase (OvidSP) (1947 to 14th April 2014);
PubMed (15th May 2014);
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to April 2014);
ISI Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (1990 to April 2014);
Clinicaltrials.gov (www.clinicaltrials.gov) (accessed 14th April 2014);
International Clinical Trials Registry Platform http://apps.who.int/trialsearch/ (accessed 14th April 2014).
We adapted the MEDLINE search strategy illustrated in Appendix 1 as necessary for each of the other databases. We used search filters, a modified version of the 'Cochrane Highly Sensitive Search Strategies, for identifying randomized trials in MEDLINE and Embase (Lefebvre 2011).
Searching other resources
We handsearched abstracts presented at the following international scientific society conferences:
American Association for the Surgery of Trauma from 2003 to 2014
Eastern Association for the Surgery of Trauma from 2012 to 2014
Western Trauma Association from 2010 to 2014
We checked the reference lists of all relevant studies retrieved from our search and from relevant, published systematic reviews in order to identify other possible studies for inclusion. On 30 April 2014 we conducted an Internet search for grey literature and other information related to our topic on the following websites:
http://www.trauma.org/
http://traumamon.com/
http://archtrauma.com/
http://www.omicsgroup.org/journals/trauma‐treatment.php
http://tra.sagepub.com/
Data collection and analysis
We conducted the review according to the recommendations of the Cochrane Collaboration (Higgins 2011a; MECIR 2011). We used Review Manager software (RevMan 2012) to conduct the review.
Selection of studies
Two authors (RC, SA) assessed titles or abstracts of all records identified by the initial search and excluded clearly irrelevant studies. We obtained the full text of potentially relevant studies, including any studies with unclear methodologies. Two authors independently assessed the full‐text articles to determine whether they met the inclusion criteria for this review and to evaluate the method of randomisation. There were no disagreements about the inclusion of studies.
Data extraction and management
Two authors independently extracted the following information for each included trial: year of the study, study design, number of participants in each arm, number of participants who received the treatment they were allocated in each arm, number of participants who were allocated to receive one treatment but receive a different treatment, characteristics of non‐operative or operative management, method of generating the randomisation sequence, method of concealing the randomisation sequence, outcomes, drop‐outs, blinding of outcome evaluators and balance of prognostic factors.
Assessment of risk of bias in included studies
In the future if studies are included in the review the risk of bias regarding items of methodological quality in the included studies will be assessed independently by two review authors (RC, ST) using a pilot‐tested data extraction form. Disagreements will be resolved by a consensus meeting with a third review author (EP). In the case of discrepancies in the data extracted, we will contact the authors of the paper for clarification or missing information. We will assess and summarise the potential risk of bias for each trial by using the 'Risk of bias' tool described in theCochrane Handbook (Higgins 2011b).
Measures of treatment effect
In the future if studies are included in the review and the data extracted by the two authors (RC, ST) are different, a third author (EP) will resolve differences. We will express dichotomous outcomes as risk ratios (RR) with 95% confidence intervals (CI). We will express continuous outcomes, if possible, as mean differences (MD) with 95% CI. We will express time‐to‐event outcomes as hazard ratios (HR) with 95% CI. An intention‐to‐treat analysis will be performed in the first instance by extracting the number of patients originally allocated to each treatment group, irrespective of compliance.
Unit of analysis issues
A patient is the unit of analysis. In abdominal surgery, unit of analysis issues are usually not a problem, because cross‐over trials or self controlled trials are not feasible. We do not anticipate unit of analysis issues.
Dealing with missing data
In the future if studies are included in the review we will contact trial investigators to request missing data.
Assessment of heterogeneity
In the future if studies are included in the review RC and ST will assess heterogeneity according to the approach provided in section 9.5 of the Cochrane Handbook (Deeks 2011); 15 studies must be included in the review in order to assess statistical heterogeneity (Thorlund 2012). We will use the Chi2 test with a P value of <0.1 to indicate statistical significance. An I2 value of more than 50% will also be used as an indicator of moderate statistical heterogeneity (Higgins 2003).
Assessment of reporting biases
In the future if studies are included in the review we will use funnel plots to assess small study effects if there are 10 or more studies for a given outcome (Sterne 2011). Due to several explanations for funnel plot asymmetry, RC and ST will carefully interpret results (Egger 1997).
Data synthesis
In the future if studies are included in the review, when calculating risk ratios we will use a random‐effect Mantel‐Haenszel model for meta‐analysis. We will interpret random‐effects meta‐analyses with due consideration of the whole distribution of effects, ideally by presenting a prediction interval (Higgins 2011a). For hazard ratios we will use the generic inverse variance method, and for mean differences we will use the inverse variance method. We will present results on a forest plot. We will perform statistical analyses according to the statistical guidelines referenced in the newest version of theCochrane Handbook (Higgins 2011a).
Subgroup analysis and investigation of heterogeneity
In the future if studies are included in the review we will conduct the following subgroup analysis: hospitals with low‐volume trauma (less than 240 versus 240 or more trauma admissions per year involving patients with an injury severity score greater than 15) (Demetriades 2005).
Sensitivity analysis
In the future if studies are included in the review we will perform sensitivity analyses in order to explore the quality of allocation concealment (low risk of bias versus unclear or high risk of bias).
Results
Description of studies
Results of the search
The search identified 1305 references (Figure 1). After removal of duplicates, 816 references remained. We excluded 733 irrelevant references based on the title or abstract, or both. After reading 83 abstracts, we excluded them all because they were not randomised clinical trials. We retrieved the full‐text of 11 studies reporting on high‐grade blunt hepatic injury, but they did not meet the inclusion criteria. No cluster‐randomised trials were identified by the search for studies.
1.
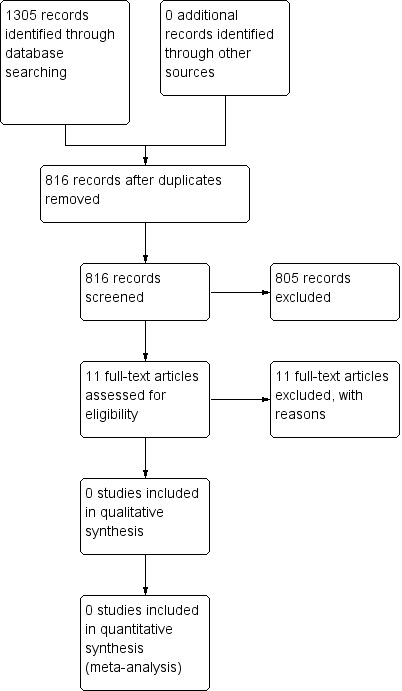
Study flow diagram.
Included studies
We found no randomised controlled trials comparing non‐operative management with operative management for high‐grade blunt hepatic injury.
Excluded studies
We excluded all the trials identified, as they were not randomised, or not relevant to the topic of this review.
Risk of bias in included studies
We included no trials in this review.
Effects of interventions
We included no trials in this review.
Discussion
We were unable to identify any randomised controlled trials of non‐operative management versus operative management in high‐grade blunt hepatic injury.
Summary of main results
We found no published or ongoing randomised controlled trials that compared non‐operative management versus operative management in high‐grade blunt hepatic injury.
Agreements and disagreements with other studies or reviews
We have not identified any other systematic reviews on this topic.
Authors' conclusions
Implications for practice.
There are no completed or ongoing randomised controlled trials of non‐operative management versus operative management in high‐grade blunt hepatic injury.
Implications for research.
Trials comparing non‐operative management with operative management in high‐grade blunt hepatic injury are needed.
Acknowledgements
Emma Sydenham
Appendices
Appendix 1. Search strategies
Cochrane Injuries Group specialised register
#1 ((liver or hepatic)) AND ( INREGISTER) [REFERENCE] [STANDARD] #2 ((nonpenetrating or non‐penetrating) AND (wound* or injur* or trauma*)) AND ( INREGISTER) [REFERENCE] [STANDARD] #3 (traumatology OR (non‐operative or nonoperative or non‐surg* or nonsurg*)) AND ( INREGISTER) [REFERENCE] [STANDARD] #4 #1 OR #2 [REFERENCE] [STANDARD] #5 #3 AND #4 [REFERENCE] [STANDARD]
Cochrane Central Register of Controlled Trials (CRSO)
#1MESH DESCRIPTOR liver EXPLODE ALL TREES #2(hepatic or liver):TI,AB,KY #3#1 OR #2 #4MESH DESCRIPTOR Wounds, Nonpenetrating #5MESH DESCRIPTOR Liver EXPLODE ALL TREES WITH QUALIFIERS IN #6((blunt or non‐penetrat* or nonpenetrat*) AND (injur* or wound* or trauma*)):TI,AB,KY #7#4 OR #6 #8#3 AND #7 #9#5 OR #8 #10MESH DESCRIPTOR Traumatology EXPLODE ALL TREES #11(non?operative or nonoperative or non?surg* or nonsurg*):TI,AB,KY #12#10 OR #11 #13#9 AND #12
Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R)
1. exp Liver/in [Injuries] 2. exp Liver/ 3. (hepatic or liver).ab,ti. 4. 2 or 3 5. wounds, nonpenetrating/ 6. ((blunt or non?penetrat* or nonpenetrat*) adj3 (injur* or wound* or trauma*)).tw. 7. 5 or 6 8. 4 and 7 9. 1 or 8 10. exp Traumatology/ 11. (non?operative or nonoperative or non?surg* or nonsurg*).ab,ti. 12. 10 or 11 13. 9 and 12
Embase Classic + Embase (OvidSP)
1. exp Liver/ 2. (hepatic or liver).ab,ti. 3. 1 or 2 4. Wounds, Nonpenetrating/ 5. ((blunt or non?penetrat* or nonpenetrat*) adj3 (injur* or wound* or trauma*)).tw. 6. 4 or 5 7. 3 and 6 8. exp Traumatology/ 9. (non?operative or nonoperative or non?surg* or nonsurg*).ab,ti. 10. 8 or 9 11. 7 and 10
PubMed
((((("Traumatology"[Mesh]) OR ((((((nonoperative[Title/Abstract]) OR non‐operative[Title/Abstract]) OR non operative[Title/Abstract]) OR non surg*[Title/Abstract]) OR non‐surg*[Title/Abstract]) OR nonsurg*[Title/Abstract]))) AND (((((((blunt[Title/Abstract]) OR non‐penetrat*[Title/Abstract]) OR nonpenetrat*[Title/Abstract])) AND (((trauma*[Title/Abstract]) OR injur*[Title/Abstract]) OR wound*[Title/Abstract]))) AND (((((liver[Title/Abstract]) OR hepatic[Title/Abstract]) OR abdomen[Title/Abstract])) OR (("Liver"[Mesh]) OR "Abdomen"[Mesh:NoExp]))))) NOT (medline[SB])
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) & Conference Proceedings Citation Index‐Science (CPCI‐S)
| #10 | #9 AND #8 AND #7 |
| #9 | TS=(traumatology OR non‐operative OR nonoperative or nonsurg* or non‐surg*) |
| #8 | TS=(blunt or non‐penetrating or nonpenetrating) AND TS=(injur* or wound* or trauma*) |
| #7 | TS=liver or TS=hepatic |
| #6 | #5 AND #4 |
| #5 | TS=(human*) |
| #4 | #3 OR #2 OR #1 |
| #3 | TS=((singl* OR doubl* OR trebl* OR tripl*) SAME (blind* OR mask*)) |
| #2 | TS=(controlled clinical trial OR controlled trial OR clinical trial OR placebo) |
| #1 | TS=(randomised OR randomized OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random OR randomized controlled trial) |
Clinical Trials Registries
Condition: hepatic injury or blunt abdominal trauma
The search was not limited in any way.
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beuran 2010 | Retrospective case series |
| Croce 1995 | Controlled clinical trial |
| Gourgiotis 2007 | Retrospective cohort study |
| Leppäniemi 2011 | Retrospective cohort study |
| Sherman 1994 | Prospective case series |
| Stassen 2012 | Systematic review |
| Thapar 2013 | Case report |
| van der Wilden 2012 | Retrospective case series |
| Velmahos 2003 | Controlled clinical trial |
| Zago 2012 | Retrospective case‐control studies |
| Zago 2012b | Retrospective case‐control studies |
Contributions of authors
Roberto Cirocchi and Stefano Avenia: conception and design of the review, development of all stages of the review, drafting the review, assessing the trials for inclusion.
Eleonora Pressi, Eriberto Farinella, Stefano Trastulli: drafting the review.
Luis M Barrera, Carlos Hernando Morales Uribe, Ana Maria Botero: drafting and revising the review, assessing the trials for inclusion.
Declarations of interest
All authors: None known.
New
References
References to studies excluded from this review
Beuran 2010 {published data only}
- Beuran M, Nego I, Ispas AT, Păun S, Runcanu A, Lupu G, et al. Nonoperative management of high degree hepatic trauma in the patient with risk factors for failure: have we gone too far?. Journal of Medicine and Life 2010;3:289‐96. [PMC free article] [PubMed] [Google Scholar]
Croce 1995 {published data only}
- Croce MA, Fabian TC, Menke PG, Waddle‐Smith L, Minard G, Kudsk KA, et al. Nonoperative management of blunt hepatic trauma is the treatment of choice for hemodynamically stable patients. Results of a prospective trial. Annals of Surgery 1995;221:744‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gourgiotis 2007 {published data only}
- Gourgiotis S, Vougas V, Germanos S, Dimopoulos N, Bolanis I, Drakopoulos S, et al. Operative and nonoperative management of blunt hepatic trauma in adults: a single‐center report. Journal of Hepato‐Biliary‐Pancreatic Surgery 2007;14:387‐91. [DOI] [PubMed] [Google Scholar]
Leppäniemi 2011 {published data only}
- Leppäniemi AK, Mentula PJ, Streng MH, Koivikko MP, Handolin LE. Severe hepatic trauma: nonoperative management, definitive repair, or damage control surgery?. World Journal of Surgery 2011;35:2643‐9. [DOI] [PubMed] [Google Scholar]
Sherman 1994 {published data only}
- Sherman HF, Savage BA, Jones LM, Barrette RR, Latenser BA, Varcelotti JR, et al. Nonoperative management of blunt hepatic injuries: safe at any grade?. Journal of Trauma‐Injury Infection and Critical Care 1994;37:616‐21. [DOI] [PubMed] [Google Scholar]
Stassen 2012 {published data only}
- Stassen NA, Bhullar I, Cheng JD, Crandall M, Friese R, Guillamondegui O, et al. Nonoperative management of blunt hepatic injury: an Eastern Association for the Surgery of Trauma practice management guideline. The Journal of Trauma and Acute Care Surgery 2012;73:S288‐93. [DOI] [PubMed] [Google Scholar]
Thapar 2013 {published data only}
- Thapar PM, Ghawat RM, Dalvi AN, Rokade ML, Philip RM, Warawdekar GM, et al. Massive liver trauma ‐ multidisciplinary approach and minimal invasive surgery can salvage patients. Indian Journal of Surgery 2013;75:S449‐S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Wilden 2012 {published data only}
- Wilden GM, Velmahos GC, Emhoff T, Brancato S, Adams C, Georgakis G, et al. Successful nonoperative management of the most severe blunt liver injuries: a multicenter study of the research consortium of New England centers for trauma. Archives of Surgery 2012;147:423‐8. [DOI] [PubMed] [Google Scholar]
Velmahos 2003 {published data only}
- Velmahos GC, Toutouzas KG, Radin R, Chan L, Demetriades D, Mullins RJ, et al. Nonoperative treatment of blunt injury to solid abdominal organs ‐ A prospective study. Archives of Surgery 2003;138:844‐9. [DOI] [PubMed] [Google Scholar]
Zago 2012 {published data only}
- Zago TM, Tavares Pereira BM, Araujo Calderan TR, Godinho M, Nascimento B, Fraga GP. Nonoperative management for patients with grade IV blunt hepatic trauma. World Journal of Emergency Surgery 2012;27:S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zago 2012b {published data only}
- Zago TM, Pereira BM, Calderan TRA, Hirano ES, Rizoli S, Fraga GP. Blunt hepatic trauma: comparison between surgical and non operative treatment. Revista do Colegio Brasileiro de Cirurgioes 2012;39:307‐13. [DOI] [PubMed] [Google Scholar]
Additional references
Ahmed 2011
- Ahmed N, Vernick JJ. Management of liver trauma in adults. Journal of Emergencies, Trauma and Shock 2011;4:114‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
American College of Surgeons 2008
- American College of Surgeons, National Surgical Quality Improvement Program (ACS‐NSQIP). ACS‐NSQIP Participant Use Data File. http://acsnsqip.org/puf/PufRequestHomepage.aspx. http://acsnsqip.org/puf/PufRequestHomepage.aspx., 2008.
Asensio 2003
- Asensio JA, Roldán G, Petrone P, Rojo E, Tillou A, Kuncir E, et al. Operative management and outcomes in 103 AAST‐OIS grades IV and V complex hepatic injuries: trauma surgeons still need to operate, but angioembolization helps. Journal of Trauma 2003;54:647‐53. [DOI] [PubMed] [Google Scholar]
Buccoliero 2010
- Buccoliero F, Ruscelli P. Current trends in polytrauma management. Diagnostic and therapeutic algorithms operational in the Trauma Center of Cesena, Italy. Annali Italiani di Chirurgia 2010;81:81‐93. [PubMed] [Google Scholar]
Bulas 1993
- Bulas DI, Eichelberger MR, Sivit CJ, Wright CJ, Gotschall CS. Hepatic injury from blunt trauma in children: follow‐up evaluation with CT. American Journal of Roentgenology 1993;160:347‐51. [DOI] [PubMed] [Google Scholar]
Christmas 2005
- Christmas AB, Wilson AK, Manning B, Franklin GA, Miller FB, Richardson JD, et al. Selective management of bunt hepatic injuries including non operative management is a safe and effective strategy. Surgery 2005;138:606‐10. [DOI] [PubMed] [Google Scholar]
Cirocchi 1999
- Cirocchi R, Contine A, Mazieri M, Bisacci R, Fabbri C, Bisacci C, et al. Perihepatic packing combined with wrapping in the treatment of major bi‐lobar hepatic trauma. Chirurgia Italiana 1999;51:259‐64. [PubMed] [Google Scholar]
Clancy 2001
- Clancy TV, Gary Maxwell J, Covington DL, Brinker CC, Blackman D. A statewide analysis of level I and II trauma centers for patients with major injuries. Journal of Trauma 2001;51:346‐51. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Demetriades 2005
- Demetriades D, Martin M, Salim A, Rhee P, Brown C, Chan L. The effect of trauma centre designation and trauma volume on outcome in specific severe injuries. Annals of Surgery 2005;242:512‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Durham 1992
- Durham RM, Buckley J, Keegan M, Fravell S, Shapiro MJ, Mazuski J. Management of blunt hepatic injuries. American Journal of Surgery 1992;164:477‐81. [DOI] [PubMed] [Google Scholar]
Eastridge 2007
- Eastridge BJ, Salinas J, McManus JG, Blackburn L, Bugler EM, Cooke WH, et al. Hypotension begins at 110 mmHg: redefining “hypotension” with data. Journal of Trauma 2007;63:291‐7. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. The British Medical Journal 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eypasch 1995
- Eypasch E, Williams JI, Wood‐Dauphinee S, Ure BM, Schmülling C, Neugebauer E, et al. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. The British Journal of Surgery 1995;82:216‐22. [DOI] [PubMed] [Google Scholar]
Giannopoulos 2009
- Giannopoulos GA, Katsoulis IE, Tzanakis NE, Patsaouras PA, Digalakis MK. Non‐operative management of blunt abdominal trauma. Is it safe and feasible in a district general hospital?. Scandinavian Journal of Trauma, Resuscitation Emergency Medicine 2009;17:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne, JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ingraham 2011
- Ingraham AM, Cohen ME, Raval MV, Ko CY, Nathens AB. Effect of trauma centre status on 30‐day outcomes after emergency general surgery. Journal of the American College of Surgeons 2011;212:277‐86. [DOI] [PubMed] [Google Scholar]
Kozar 2005
- Kozar RA, Moore JB, Niles SE, Holcomb JB, Moore EE, Cothren CC, et al. Complications of nonoperative management of high‐grade blunt hepatic injuries. Journal of Trauma 2005;59:1066‐71. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Matthes 2003
- Matthes G, Stengel D, Seifert J, Rademacher G, Mutze S, Ekkernkamp A. Blunt liver injuries in polytrauma: results from a cohort study with the regular use of whole‐body helical computed tomography. World Journal of Surgery 2003;27:1124‐30. [DOI] [PubMed] [Google Scholar]
MECIR 2011
- The Cochrane Collaboration. Methodological Expectations of Cochrane Intervention Reviews. http://www.editorial‐unit.cochrane.org/mecir 2011.
Miller 2002
- Miller PR, Croce MA, Bee TK, Malhotra AK, Fabian TC. Associated injuries in blunt solid organ trauma: implications for missed injury in nonoperative management. Journal of Trauma 2002;53:238‐44. [DOI] [PubMed] [Google Scholar]
Moore 1995
- Moore EE, Cogbill TH, Jurkovich GJ, Shackford SR, Malangoni MA, Champion HR. Organ injury scaling: spleen and liver (1994 revision). American Association for the Surgery of Trauma. Journal of Trauma 1995;38:323‐4. [DOI] [PubMed] [Google Scholar]
Norrman 2009
- Norrman G, Tingstedt B, Ekelund M, Andersson R. Non‐operative management of blunt liver trauma: feasible and safe also in centres with a low trauma incidence. HPB: the official journal of the International Hepato Pancreato Biliary Association. 2009;11:50‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parks 1999
- Parks RW, Chrysos E, Diamond T. Management of liver trauma. British Journal of Surgery 1999;86:1121‐35. [DOI] [PubMed] [Google Scholar]
Polanco 2008
- Polanco P, Leon S, Pineda J, Puyana JC, Ochoa JB, Alarcon L, et al. Hepatic resection in the management of complex injury to the liver. Journal of Trauma 2008;65:1264‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Richardson 2000
- Richardson JD, Franklin GA, Lukan JK, Carrillo Eh, Spain DA, Miller FB, et al. Evolution in the management of hepatic trauma: 25 year perspective study. Annals of Surgery 2000;232:324‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Richardson 2005
- Richardson JD. Changes in the management of Injuries to the liver and spleen. Journal of American College of Surgeons 2005;200:648‐69. [DOI] [PubMed] [Google Scholar]
Schnuriger 2011
- Schnüriger B, Talving P, Barbarino R, Barmparas G, Inaba K, Demetriades D. Current practice and the role of the CT in the management of penetrating liver injuries at a Level I trauma center. Journal of Emergencies, Trauma and Shock 2011;4:53‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schnüriger 2009
- Schnüriger B, Inderbitzin D, Schafer M, Kickuth R, Exadaktylos A, Candinas D. Concomitant injuries are an important determinant of outcome of high‐grade blunt hepatic trauma. British Journal of Surgery 2009;96:104‐10. [DOI] [PubMed] [Google Scholar]
Slim 1999
- Slim K, Bousquet J, Kwiatkowski F, Lescure G, Pezet D, Chipponi J. First validation of the french version of the gastrointestinal Quality of Life Index (GIQLI). Gastroentérologie Clinique et Biologique 1999;23:25‐31. [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:4002. [DOI] [PubMed] [Google Scholar]
Thorlund 2012
- Thorlund K, Imberger G, Johnston BC, Walsh M, Awad T, Thabane L, et al. Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta‐analyses. PLoS One 2012;7:e39471. [DOI] [PMC free article] [PubMed] [Google Scholar]


