Abstract
Objectives
To determine the relative effectiveness of medications for preventing hypertensive disorders in high-risk pregnant women and to provide a ranking of medications using network meta-analysis.
Methods
All randomized controlled trials comparing the most commonly used medications to prevent hypertensive disorders in high-risk pregnant women that are nulliparity and pregnant women having family history of preeclampsia, history of pregnancy-induced hypertension in previous pregnancy, obstetric risks, or underlying medical diseases. We received the search results from the Cochrane Pregnancy and Childbirth’s Specialised Register of Controlled Trials, searched on 31st July 2020. At least two review authors independently selected the included studies and extracted the data and the methodological quality. The comparative risk ratios (RR) and 95% confidence intervals (CI) were analyzed using pairwise and network meta-analyses, and treatment rankings were estimated by the surface under the cumulative ranking curve for preventing preeclampsia (PE), gestational hypertension (GHT), and superimposed preeclampsia (SPE). Safety of the medications is also important for decision-making along with effectiveness which will be reported in a separate review.
Results
This network meta-analysis included 83 randomized studies, involving 93,864 women across global regions. Three medications, either alone or in combination, probably prevented PE in high-risk pregnant women when compared with a placebo or no treatment from network analysis: antiplatelet agents with calcium (RR 0.19, 95% CI 0.04 to 0.86; 1 study; low-quality evidence), calcium (RR 0.61, 95% CI 0.47 to 0.80; 13 studies; moderate-quality evidence), antiplatelet agents (RR 0.69, 95% CI 0.57 to 0.82; 31 studies; moderate-quality evidence), and antioxidants (RR 0.77, 95% CI 0.63 to 0.93; 25 studies; moderate-quality evidence). Calcium probably prevented PE (RR 0.63, 95% CI 0.46 to 0.86; 11 studies; moderate-quality evidence) and GHT (RR 0.89, 95% CI 0.84 to 0.95; 8 studies; high-quality evidence) in nulliparous/primigravida women. Few included studies for the outcome of superimposed preeclampsia were found.
Conclusion
Antiplatelet agents, calcium, and their combinations were most effective medications for preventing hypertensive disorders in high-risk pregnant women when compared with a placebo or no treatment. Any high-risk characteristics for women are important in deciding the best medications. The qualities of evidence were mostly rated to be moderate.
Systematic review registration
PROSPERO CRD42018096276
Supplementary Information
The online version contains supplementary material available at 10.1186/s13643-022-01978-5.
Keywords: Medications, Hypertension prevention, Hypertensive disorders in pregnancy, High-risk pregnant women, Network meta-analysis
Background
Hypertensive disorders in pregnancy (HDP) are one of the five common complications during pregnancy, causing maternal and fetal deaths globally. The incidence of HDP ranges from 1 to 35% worldwide, with a wide variation across regions [1–3]. Due to the lack of a clear understanding of the underlying etiology of HDP, the antiplatelet agents, anticoagulants, antioxidants, nitric oxide, and calcium, which have been widely studied for their possible use in reducing or preventing HDP, were systematically reviewed [4–9]. To date, there have been two network meta-analyses. One was a conference abstract, which reported that calcium supplements reduced the risk of preeclampsia (PE) compared to aspirin, fish oil, and vitamin C or E [10]. Additionally, another network meta-analysis found that either vitamin D or calcium supplements may be effective [11].
A recent study demonstrated the superiority of Doppler and serum markers over conventional risk factor-based screening [12], and a new screening algorithm has recently demonstrated the effectiveness of aspirin prophylaxis in high-risk women [13, 14]. However, aspirin has been shown to be effective for high-risk women not only based on new screening algorithms but also on more traditional ways of defining high-risk, as shown in a Cochrane review [4]. In addition, these screening methods require experienced technicians and are not routinely available in health facilities in low-or middle-income countries where HDP are common.
Pregnant women with a history of hypertensive disorders in a previous pregnancy, those having chronic kidney disease, autoimmune disease, diabetes mellitus, or chronic hypertension, as well as nulliparous women, advanced age, or obese women, and those having multiple pregnancies, or family history of PE, were considered as risk factors of being advised to take aspirin for prevention of PE by the National Institute for Health and Care Excellence (NICE) 2019 and the American College of Obstetricians and Gynecologists’ Committee [15, 16]. To date, only aspirin has been recommended for PE prophylaxis in women with risk factors in the NICE guideline and the US Preventive Services Task Force recommendation [15, 17], not the other medications reported in previous systematic reviews [4–9]. The objectives of this analysis were to determine the relative effectiveness and provide a ranking of the available medications for preventing hypertensive disorders in high-risk pregnant women classified by the NICE 2019 using a network meta-analysis.
Methods
Eligibility criteria
We included all randomized controlled trials or cluster-randomized trials comparing the most commonly used medications by any route or doses in high-risk women during pregnancy for preventing hypertensive disorders. Only one main publication/report of the studies was selected to be reviewed and analyzed. Eligibility criteria were the studies that included pregnant women, at any gestational age, and at high risk of developing hypertensive disorders based on one of these following risk factors: nulliparity, family history of PE, history of pregnancy-induced hypertension in a previous pregnancy, obstetric risks (advanced maternal age, obesity, or multiple pregnancies), and underlying medical diseases (polycystic ovarian syndrome, autoimmune diseases, chronic renal diseases, diabetes, or chronic hypertension) in which medications were commenced only during pregnancy.
The studies were eligible if they used any of these groups of medications (antiplatelet agents, anticoagulants, antioxidants, nitric oxide, or calcium supplements) for preventing HDP and compared them against each other, placebo, or no treatment/conventional management. We considered medications routinely prescribed during pregnancy, such as ferrous, folic, or multivitamin supplementation, as conventional standard treatments. The medications prescribed before conception and continued during pregnancy were excluded. Two-arm or multi-arm trials that compared drug(s) in different dosages or regimens in the same medication group were included, if the comparison of medication groups could be made after the drug(s) in the same medication group were combined. Both primary outcomes (PE, gestational hypertension (GHT), and chronic hypertension with superimposed preeclampsia (SPE)) and secondary outcomes (placental abruption, postpartum hemorrhage, neonatal intraventricular hemorrhage, and neonate with small gestational age or growth restriction) were included in the protocol registered in PROSPERO [18]. However, in this network meta-analysis, the primary outcomes on relative effectiveness were focused, and the secondary outcomes on safety will be reported in other separate review with network meta-analysis.
Information sources and search strategy
We received the search results from the database of the Cochrane Pregnancy and Childbirth’s Specialised Register of Controlled Trials, on 31st July 2020, using the topic area of “hypertension, prevention,” as the assigned search. This is a database, containing the results of over 30 years of searching for trials related to pregnancy and childbirth as a whole.
The full search methods, including individual strategies for each database search, can be found within the Trials Register section of the group’s webpage (https://pregnancy.cochrane.org/pregnancy-and-childbirth-groups-trials-register). The register is stored in the Cochrane Register of Studies. Each review receives its own specific search results, and no language was restricted.
Selection and data collection process
Two review authors (TL, YY) screened the titles and abstracts of all search results independently, considering the criteria for included studies using the RAYYAN web-based application. Any discrepancies were solved by discussion. Two pairs of review authors (TL-YY, TL-CK) assessed the full texts independently to decide which of all the potential studies would be included using an electronic checklist form. We resolved any disagreements through discussion or in consultation with an independent reviewer (EO, RM), if required. A study flow diagram of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) was used to present the number of records identified, excluded, or included.
We reviewed all included reports; however, if more than one reports come from the same study, we chose one main primary report as the main cited reference which the data were extracted for this review to avoid the data duplication. At least two of the review authors (TL, YY, CK, RM, EO) independently assessed the risk of bias for each study, using the criteria in the Cochrane Handbook for Systematic Reviews of Interventions [19]. TL and CK independently extracted the data.
Study risk-of-bias assessment and certainty assessment
The criteria for assessing risk of bias included random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, other bias, and overall risk of bias. TL and CK assessed the quality of the evidence, using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methods [20]. Any disagreements were resolved by discussion, and the information was entered into Review Manager 5 software, for risk of bias [21]. The summary of findings of each outcome was presented using the template of GRADE network meta-analysis–summary of findings (NMA-SoF) tables for multiple treatment comparisons compared with placebo [22].
Effect measures
We evaluated the assumption of transitivity epidemiologically, by comparing the clinical and methodological characteristics of sets of studies grouped by treatment comparisons. The drugs in the same two-arm or multiple-arm trials that are in the same groups of medication of interest in this review were grouped to be the same treatment node, regardless of regimens or doses. When the trials had more than one drugs in different treatment nodes in one arm, we defined them as the combinations group of medications. A network plot was drawn with the nodes representing interventions, the size of the nodes representing sample sizes, and the thickness of the lines connecting between nodes indicating the number of direct comparisons between pairs of interventions. A separate network plot was presented for primary outcomes on PE, GHT, and SPE.
We evaluated the inconsistency of the evidence on the network using the global inconsistency test [23] and the Dias’s side-splitting approach [24]. The heterogeneity of pairwise studies in the meta-analysis was assessed using the I2 statistic. If substantial heterogeneity, I2 > 50%, was identified, subgroup analysis considering different high-risk characteristics was explored [23, 25]. The comparative risk ratios (RR) and 95% confidence interval (CI) were estimated for pooled direct evidence, using a random-effects model and network meta-analysis using multivariate random-effects models. We estimated the surface under the cumulative ranking curve (SUCRA) to provide a hierarchy of the medications in numerical presentations. The SUCRA values ranged from 0 to 100%, with values close to 0 indicating a higher likelihood that a medication is in one of the bottom ranks, while values close to 100% indicate a higher likelihood that a medication is in one of the top ranks [26]. We also assessed the publication bias using a comparison-adjusted funnel plot and the Egger’s test, if at least 10 studies with the same comparisons and outcome were found because the power of the test is usually low to differentiate the chance of real asymmetry in fewer than 10 studies [27, 28].
The data were analyzed using STATA 15, with the “network” commands (The StataCorp, Texas, USA). Multivariate random-effects models were used to analyze both direct and indirect pairwise comparisons and network meta-analysis. The visualizations of RR and 95% CI of effect size of pairwise and network meta-analyses as well as ranking treatments among medications were operated in R software (R version 3.6.1, R Core Team 2019, Vienna, Austria), with “tidyverse,” “ggplot2,” “gridExtra,” and “RColorBrewer” packages. We reported this systematic review in accordance with the recommendations in PRISMA 2020 [29].
Results
Study selection
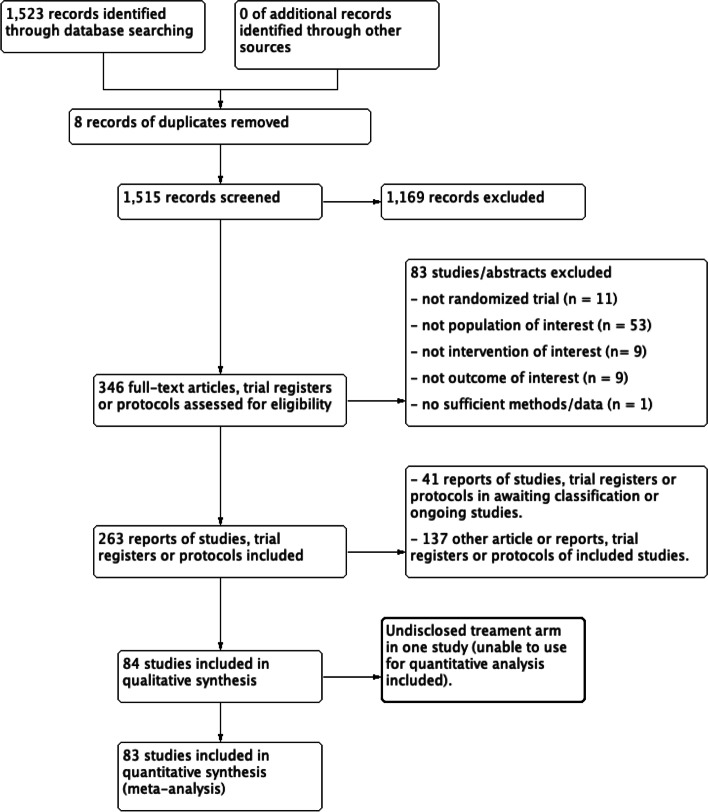
From 84 studies, there were 6998 women with outcomes of interest among 93,971 included women (7.4%). One included study, conducted in Columbia, comparing 100 mg aspirin with a placebo did not disclose the drug groups (drug 1, n = 54) and drug 2, n = 43) in the results of the study; hence, we could not use the data from this study for the analysis [30]. The results of search and selection process are presented in the PRISMA flow diagram as shown in Fig. 1.
Fig. 1.
PRISMA study flow diagram
Study characteristics and risk of bias
Among 83 studies with the outcomes of interest in this network meta-analysis, 77 studies reported PE [31–107], 39 studies reported gestational hypertension [31, 34, 36, 37, 39, 40, 42, 50, 51, 57, 58, 60, 61, 63, 66, 68, 69, 71, 72, 77, 81–83, 86, 88, 90, 91, 94, 96, 97, 102, 103, 106–112], and four studies reported SPE [56, 58, 112, 113]. The incidences of PE, GHT, and SPE in control groups using a placebo or no treatment were 7.8% (3559/45,449), 14.9% (4463/30,002), and 1.4% (45/3174), respectively. Risks-of-bias domain is summarized across all studies and presented in Fig. 2; 38 studies were judged to have a low risk of bias [34, 38, 42–45, 50, 51, 53, 58–64, 67, 68, 70, 72–74, 78, 80, 82, 86–89, 91, 94, 95, 101–103, 105, 106, 108]. There was no evidence of global inconsistency in the network analysis for all primary outcomes on PE, GHT, and SPE.
Fig. 2.
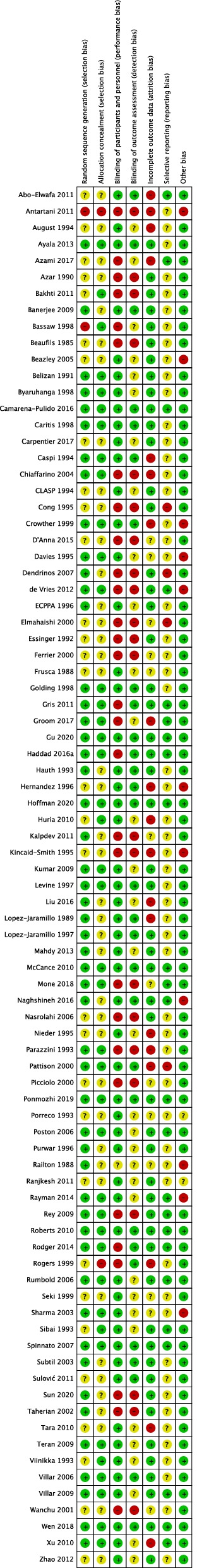
Risk-of-bias graph presented as percentages across all included studies
Results of synthesis and certainty of evidence
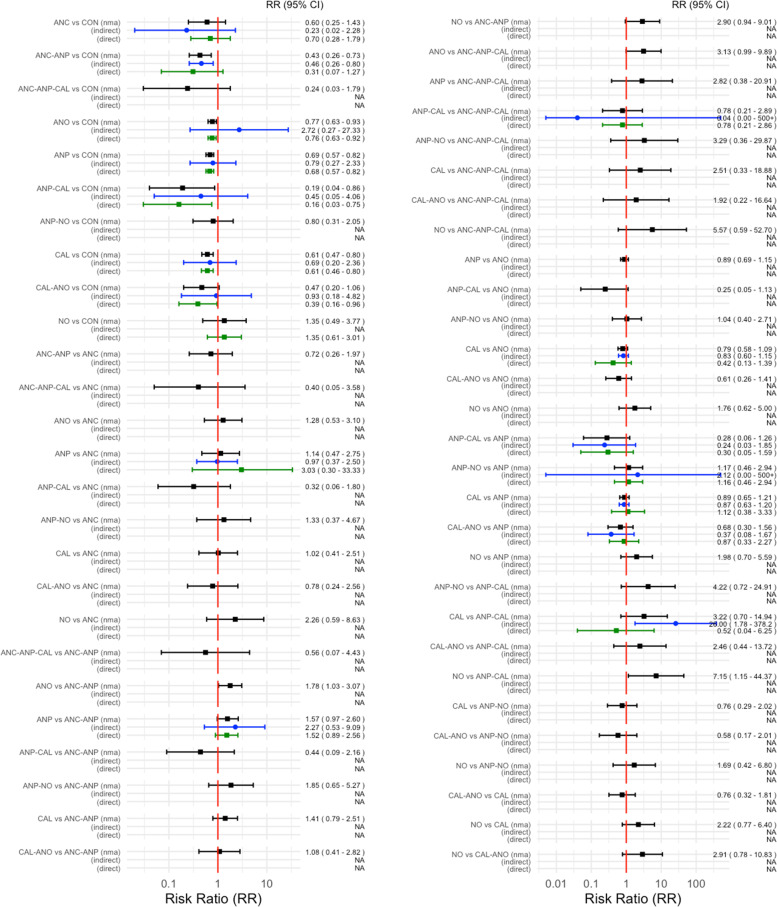
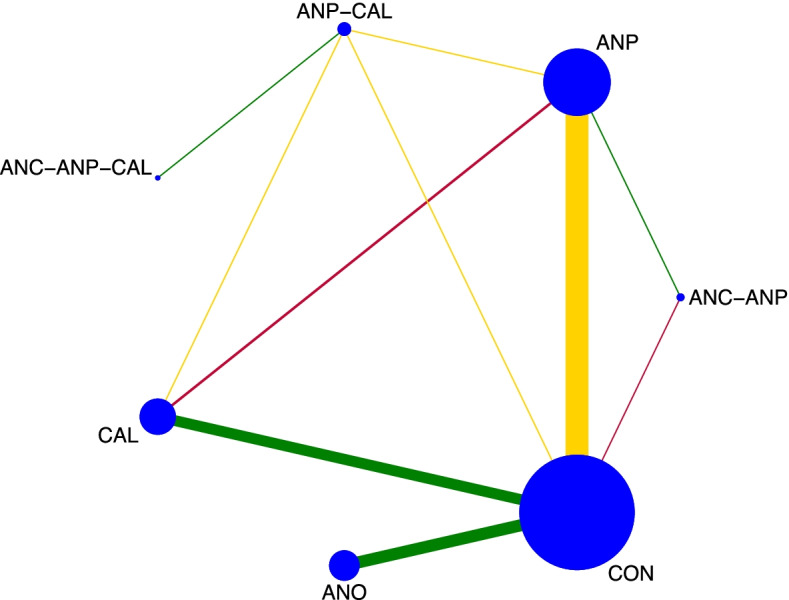
The network diagram of 77 studies for preventing PE in all high-risk women is presented in Fig. 3. Antiplatelet agents were the most frequently investigated medications, in 38 of 77 studies (49.4%), followed by antioxidants in 25 studies (32.4%), calcium in 14 studies (18.2%), and various combinations in nine studies (11.7%). Pooled effect sizes, from direct estimates as well as network meta-analysis, are presented in Fig. 4. Calcium, antiplatelet agents, and combinations of antiplatelet agents with calcium probably had a moderately preventive effect for PE when compared with a placebo or no treatment as the evidence from network analysis accounted for antiplatelet agents with calcium (RR 0.19, 95% CI 0.04 to 0.86; 1 study; 334 participants; low-quality evidence); calcium (RR 0.61, 95% CI 0.47 to 0.80; 13 studies; 26,021 participants; moderate-quality evidence); antiplatelet agents (RR 0.69, 95% CI 0.57 to 0.82; 31 studies; 41,953 participants; moderate-quality evidence); and antioxidants (RR 0.77, 95% CI 0.63 to 0.93; 25 studies; 24,768 participants; moderate-quality evidence).
Fig. 3.

Network plot of medications for preventing preeclampsia. CON, control; ANC, anticoagulants; ANO, antioxidants; ANP, antiplatelet agents; CAL, calcium; N, nitric oxide; CAL-ANO, calcium plus antioxidants; ANC-ANP, anticoagulants plus antiplatelet agents; ANP-NO, antiplatelet agents plus nitric oxide; ANP-CAL, antiplatelet agents plus calcium; ANC-ANP-CAL, anticoagulants plus antiplatelet plus calcium
Fig. 4.
Direct, indirect, and network meta-analysis estimates of medications for preventing preeclampsia. CON, control; ANC, anticoagulants; ANO, antioxidants; ANP, antiplatelet agents; CA, calcium; NO, nitric oxide; CAL-ANO, calcium plus antioxidants; ANC-ANP, anticoagulants plus antiplatelet agents; ANP-NO, antiplatelet agents plus nitric oxide; ANP-CAL, antiplatelet agents plus calcium; ANC-ANP-CAL, anticoagulants plus antiplatelet plus calcium
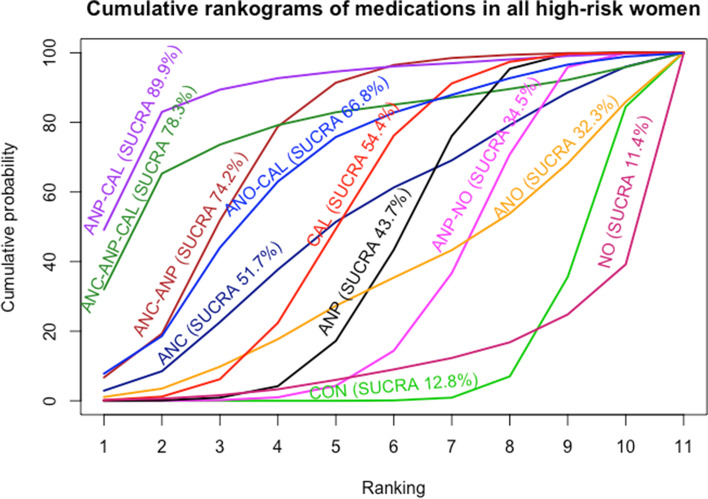
Antiplatelet agents with calcium in all high-risk women reported in one study showed highest SUCRA (89.9%) (Fig. 5). For the consistency of evidence on the network, the global inconsistency test was not significant (P = 0.459). The direct and indirect comparison estimates of each treatment pair by the Dias’s side splitting are presented in Fig. 4, and no significant treatment pairs were detected by the Dias’s inconsistency tests. The summary of findings for medication to prevent PE in all high-risk women is presented in Table 1. Certainty of evidence of the medications compared with a placebo or no treatment to prevent PE ranged from very low to moderate. Due to substantial heterogeneity (I2 59.0%), subgroup analyses based on the high-risk subgroup population were performed, and the findings are shown in the summary of findings for subgroups on prevention of PE (Additional file 1: Appendices 1–3).
Fig. 5.
Cumulative rankograms of medications for preventing preeclampsia. CON, control; ANC, anticoagulants; ANO, antioxidants; ANP, antiplatelet agents; CAL, calcium; NO, nitric oxide; CAL-ANO, calcium plus antioxidants; ANC-ANP, anticoagulants plus antiplatelet agents; ANP-NO, antiplatelet agents plus nitric oxide; ANP-CAL, antiplatelet agents plus calcium; ANC-ANP-CAL, anticoagulants plus antiplatelet plus calcium
Table 1.
Summary of findings for medications to prevent preeclampsia
| Patient or population: Pregnant women at any gestational age at high risk of developing hypertensive disorders in pregnancy Settings: Hospital setting Intervention: Antiplatelet agents, anticoagulants, antioxidants, calcium, nitric oxide, and their combinations Comparator: Placebo or no treatment Outcome: Preeclampsia | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total studies: 77 RCTs Total participants: 93,234 |
Direct estimates RR (95% CI) |
Certainty of evidence | Indirect estimates RR (95% CI) |
Certainty of evidence | Network estimates RR (95% CI) [95% PrI] |
Certainty of evidence | SUCRA | Comments |
| Antiplatelets + calcium (1 RCT; 334 participants) | 0.16 (0.03 to 0.75) |
⊕⊕⊝⊝ Lowa,b |
0.45 (0.05 to 4.06) |
⊕⊕⊕⊝ Moderateb |
0.19 (0.04 to 0.86) [0.04 to 1.01] |
⊕⊕⊝⊝ Lowa,b |
89.9% | There was no evidence of inconsistency for global inconsistency test (P = 0.459) and Dias’s inconsistency tests of the node splitting |
| Anticoagulants + antiplatelets + calcium (2 RCTs; 156 participants) | Not estimable | 0.24 (0.03 to 1.79) |
⊕⊝⊝⊝ Very lowc,d |
0.24 (0.03 to 1.79) [0.03 to 2.07] |
⊕⊝⊝⊝ Very lowc,d |
78.3% | ||
| Anticoagulants + antiplatelets (1 RCT; 20 participants) | 0.31 (0.07 to 1.27) |
⊕⊝⊝⊝ Very lowd,e |
0.46 (0.26 to 0.80) |
⊕⊕⊝⊝ Lowd |
0.43 (0.26 to 0.73) [0.19 to 1.01] |
⊕⊕⊝⊝ Lowd |
74.2% | |
| Calcium + antioxidants (1 RCT; 660 participants) | 0.39 (0.16 to 0.96) |
⊕⊕⊝⊝ Lowe |
0.93 (0.18 to 4.82) |
⊕⊕⊕⊝ Moderateb |
0.47 (0.20 to 1.06) [0.16 to 1.35] |
⊕⊕⊕⊝ Moderateb |
66.8% | |
| Calcium (13 RCTs; 26,021 participants) | 0.61 (0.46 to 0.80) |
⊕⊕⊕⊝ Moderatef |
0.69 (0.20 to 2.36) |
⊕⊕⊕⊝ Moderateb |
0.61 (0.47 to 0.80) [0.30 to 1.24] |
⊕⊕⊕⊝ Moderatef |
54.4% | |
| Anticoagulants (2 RCTs; 399 participants) | 0.70 (0.28 to 1.79) |
⊕⊕⊕⊝ Moderateb |
0.23 (0.02 to 2.28) |
⊕⊕⊕⊝ Moderateb |
0.60 (0.25 to 1.43) [0.20 to 1.80] |
⊕⊕⊕⊝ Moderateb |
51.7% | |
| Antiplatelets (31 RCTs; 41,953 participants) | 0.68 (0.57 to 0.82) |
⊕⊕⊝⊝ Lowa,f |
0.79 (0.27 to 2.33) |
⊕⊕⊕⊝ Moderateb |
0.69 (0.57 to 0.82) [0.35 to 1.35] |
⊕⊕⊕⊝ moderatef |
43.7% | |
| Antiplatelets + nitric oxide (No direct comparison) | Not estimable | Not estimable |
0.80 (0.31 to 2.05) [0.25 to 2.55] |
⊕⊕⊝⊝ lowd |
34.5% | |||
| Antioxidants (25 RCTs; 24,768 participants) | 0.76 (0.63 to 0.92) |
⊕⊕⊕⊝ Moderatef |
2.72 (0.27 to 27.33) |
⊕⊕⊕⊝ Moderateb |
0.77 (0.63 to 0.93) [0.39 to 1.52] |
⊕⊕⊕⊝ Moderatef |
32.3% | |
| Nitric oxide (1 RCT; 68 participants) | 1.35 (0.61 to 3.01) |
⊕⊝⊝⊝ Very lowd,e |
Not estimable |
1.35 (0.49 to 3.77) [0.39 to 4.65] |
⊕⊝⊝⊝ Very lowd,e |
11.4% | ||
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate
Very low quality: We are very uncertain about the estimate
The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
CI Confidence interval, PrI Prediction interval, RR Relative risk
aWe downgraded (1) level for serious limitations in study design due to most of the studies being at unclear risk of bias
bWe downgraded (1) level for serious imprecision due to wide confidence interval
cWe downgraded (1) level for serious intransitivity due to without closed loop of intervention
dWe downgraded (2) level for very serious imprecision due to wide confidence interval and small number of events and sample size
eWe downgraded (2) level for very serious limitations in study design due to most of the studies being at high risk of bias
fWe downgraded (1) level for serious publication bias due to asymmetry funnel plot and P-value of Egger’s test < 0.05
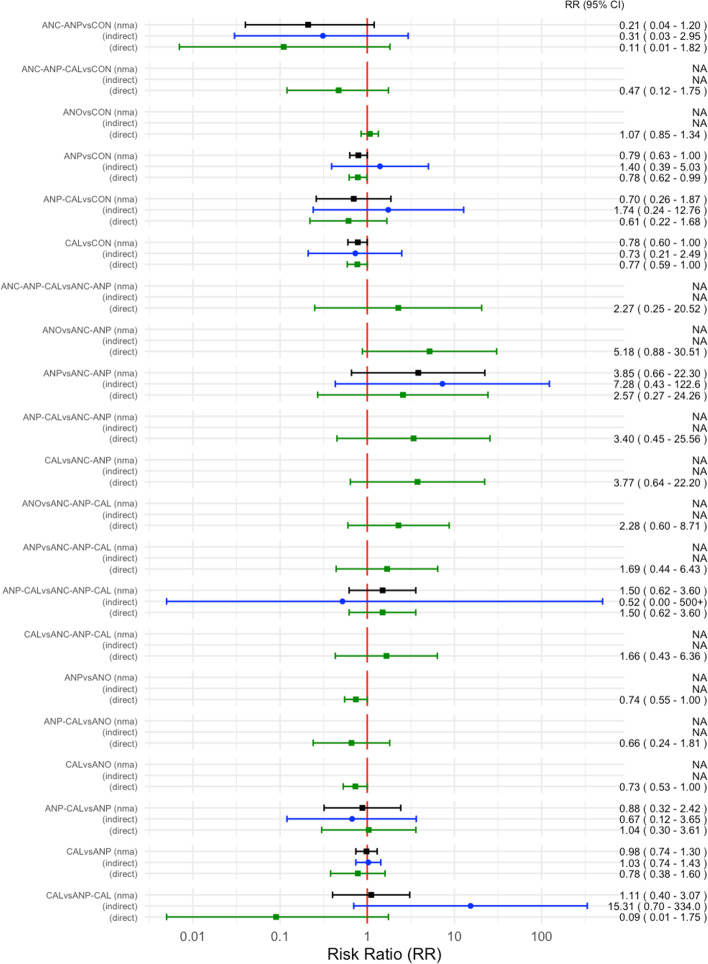
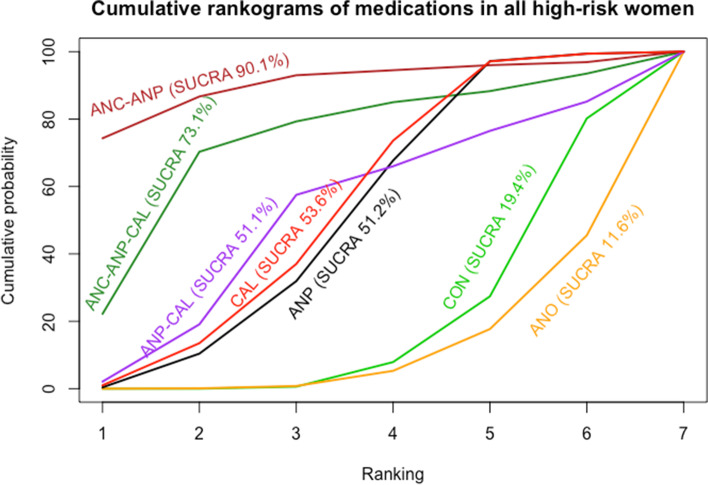
The network diagram of 39 studies for preventing gestational hypertension is presented in Fig. 6. Antiplatelet agents were the most frequently investigated medications in 19 out of 39 studies (48.7%), followed by antioxidants in 10 studies (25.6%) and calcium in nine studies (23.1%). Pooled effect sizes from direct estimates as well as network meta-analysis (Fig. 7) suggested antiplatelet agents (RR 0.78, 95% CI 0.62 to 0.99 from direct estimates and RR 0.80, 95% CI 0.64 to 1.00 from network meta-analysis; 19 studies; 16,813 participants; moderate-quality evidence) or calcium (RR 0.77, 95% CI 0.59 to 1.00 from direct estimates and RR 0.78, 95% CI 0.61 to 1.00 from network meta-analysis; 9 studies; 24,534 participants; moderate-quality evidence) may prevent GHT. It is the uncertain effect of a combination of antiplatelet agents with anticoagulants in network meta-analysis estimate (RR 0.21, 95% CI 0.04 to 1.20; 1 study; 20 participants; very low-quality evidence) with highest SUCRA (90.1%) to prevent GHT in all high-risk women, as shown in Fig. 8.
Fig. 6.
Network plot of medications for preventing gestational hypertension. CON, control; ANO, antioxidants; ANP, antiplatelet agents; CAL, calcium; ANC-ANP, anticoagulants plus antiplatelet agents; ANP-CAL, antiplatelet agents plus calcium; ANC-ANP-CA, anticoagulants plus antiplatelet plus calcium
Fig. 7.
Direct, indirect, and network meta-analysis estimates of medications for preventing gestational hypertension. CON, control; ANO, antioxidants; ANP, antiplatelet agents; CAL, calcium; ANC-ANP, anticoagulants plus antiplatelet agents; ANP-CAL, antiplatelet agents plus calcium; ANC-ANP-CAL, anticoagulants plus antiplatelet plus calcium
Fig. 8.
Cumulative rankograms of medications for preventing gestational hypertension. CON, control; ANO, antioxidants; ANP, antiplatelet agents; CAL, calcium; ANC-ANP, anticoagulants plus antiplatelet agents; ANP-CAL, antiplatelet agents plus calcium; ANC-ANP-CAL, anticoagulants plus antiplatelet plus calcium
For the consistency of evidence on the network, the global inconsistency test was not significant (P = 0.512). The direct and indirect comparison estimates of each treatment pair by the Dias’s side splitting are presented in Fig. 7, and no significant treatment pairs were detected by the Dias’s inconsistency tests. Summary of findings for medication to prevent gestational hypertension in all high-risk women is presented in Table 2. Certainty of evidence of the medications compared with a placebo or no treatment to prevent GHT ranged from very low to moderate. Due to substantial heterogeneity (I2 63.2%), the subgroup analyses based on the high-risk subgroup population were performed, and the findings are shown in the table of summary of findings for subgroups on prevention of gestational hypertension (Additional file 1: Appendices 4–6).
Table 2.
Summary of findings for medications to prevent gestational hypertension
| Patient or population: Pregnant women at any gestational age at high risk of developing hypertensive disorders in pregnancy Settings: Hospital setting Intervention: Antiplatelet agents, anticoagulants, antioxidants, calcium, nitric oxide, and their combinations Comparator: Placebo or no treatment Outcome: Gestational hypertension | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total studies: 39 RCTs Total participants: 60,953 |
Direct estimates RR (95% CI) |
Certainty of evidence | Indirect estimates RR (95% CI) |
Certainty of evidence | Network estimates RR (95% CI) [95% PrI] |
Certainty of evidence | SUCRA | Comments |
| Anticoagulants + antiplatelets (1 RCT; 20 participants) | 0.11 (0.007 to 0.50) |
⊕⊝⊝⊝ Very lowa,b |
0.31 (0.03 to 2.98) |
⊕⊕⊝⊝ Lowc |
0.21 (0.04 to 1.20) [0.03 to 1.39] |
⊕⊝⊝⊝ Very lowa,c |
90.1% | There was no evidence of inconsistency for global inconsistency test (P = 0.512) and Dias’s inconsistency tests of the node splitting |
| Anticoagulants + antiplatelets + calcium (1 RCT; 149 participants) | Not estimable | Not estimable |
0.47 (0.13 to 1.74) [0.11 to 2.05] |
⊕⊕⊕⊝ Lowc |
73.1% | |||
| Calcium (9 RCTs; 24,534 participants) | 0.77 (0.59 to 1.00) |
⊕⊕⊕⊝ Moderateb |
0.75 (0.22 to 2.53) |
⊕⊕⊕⊝ Moderateb |
0.78 (0.61 to 1.00) [0.43 to 1.39] |
⊕⊕⊕⊝ Moderateb |
53.6% | |
| Antiplatelets (19 RCTs; 16,813 participants) | 0.78 (0.62 to 0.99) |
⊕⊕⊕⊝ Moderated |
1.41 (0.40 to 5.00) | ⊕⊕⊕⊝Moderateb |
0.80 (0.64 to 1.00) [0.44 to 1.41] |
⊕⊕⊕⊝ Moderateb |
51.2% | |
| Antiplatelets + calcium (1 RCT; 334 participants) | 0.61 (0.22 to 1.68) |
⊕⊕⊝⊝ Lowb,d |
1.78 (0.24 to 12.92) |
⊕⊕⊕⊝ Moderateb |
0.70 (0.26 to 1.87) [0.22 to 2.22] |
⊕⊕⊝⊝ Lowb,d |
51.1% | |
| Antioxidants (10 RCTs; 53,057 participants) | 1.06 (0.85 to 1.34) |
⊕⊕⊕⊝ Moderateb |
Not estimable |
1.07 (0.85 to 1.34) [0.60 to 1.90] |
⊕⊕⊕⊝ Moderateb |
11.6% | ||
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate
Very low quality: We are very uncertain about the estimate
The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
CI Confidence interval, PrI Prediction interval, RR Relative risk
aWe downgraded (2) level for very serious limitations in study design due to most of the studies being at high risk of bias
bWe downgraded (1) level for serious imprecision due to wide confidence interval
cWe downgraded (2) level for very serious imprecision due to wide confidence interval and small number of events and sample size
dWe downgraded (1) level for serious limitations in study design due to most of the studies being at unclear risk of bias
The network diagram of four studies for preventing SPE in all high-risk women is presented in Fig. 9. Pooled effect sizes from the network meta-analysis of four studies suggested the uncertainty of the evidence on antiplatelet agents when compared with a placebo or no treatment in network meta-analysis (RR 0.72, 95% CI 0.46 to 1.14; 3 study; 6298 participants; low-quality evidence). The summary of findings for medications in the prevention of SPE is presented in Table 3. Certainty of evidence of the medications compared with a placebo or no treatment to prevent SPE was very low or low. The inconsistency test using side-splitting approach was significant for SPE.
Fig. 9.

Network plot of medications for preventing superimposed preeclampsia. CON, control; ANO, antioxidants; ANP, antiplatelet agents
Table 3.
Summary of findings for medications to prevent superimposed preeclampsia
| Patient or population: Pregnant women at any gestational age at high risk of developing hypertensive disorders in pregnancy Settings: Hospital setting Intervention: Antiplatelet agents, anticoagulants, antioxidants, calcium, nitric oxide, and their combinations Comparator: Placebo or no treatment Outcome: Superimposed preeclampsia | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total studies: 4 RCTs Total participants: 6,342 |
Direct estimates RR (95% CI) |
Certainty of evidence | Indirect estimates RR (95% CI) |
Certainty of evidence | Network estimates RR (95% CI) [95% PrI] |
Certainty of evidence | SUCRA | Commentsa |
| Antiplatelets (3 RCTs; 6,298 participants) | 0.72 (0.46 to 1.14) |
⊕⊕⊝⊝ Lowb,c |
Not estimable |
0.72 (0.46 to 1.14) [0.04 to 14.21] |
⊕⊕⊝⊝ Lowb,c |
69.0% | There was no evidence of inconsistency for global inconsistency test (P = 0.165) | |
| Antioxidants (1 RCT; 44 participants) | 0.67 (0.12 to 3.61) |
⊕⊝⊝⊝ Very lowd,e |
Not estimable |
0.67 (0.12 to 3.61) [< 0.001 to 37900] |
⊕⊝⊝⊝ Very lowd,e |
60.6% | ||
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate
The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
CI Confidence interval, PrI Prediction interval, RR Relative risk
aDias’s inconsistency tests of the node splitting not estimable
bWe downgraded (1) level for serious limitations in study design due to most of the studies being at unclear risk of bias
cWe downgraded (1) level for serious imprecision due to wide confidence interval
dWe downgraded (2) level for very serious limitations in study design due to most of the studies being at high risk of bias
eWe downgraded (2) level for very serious imprecision due to wide confidence interval and small number of events and sample size
Reporting biases
The summary on the tests of heterogeneity, effect of intervention, and tests of publication bias for direct comparisons in a network meta-analysis are presented for all primary outcomes (Additional file 1: Appendix 7). Publication biases, using comparison-adjusted funnel plot for preventing PE and GHT, were found with a P-value of Egger’s test < 0.001 (Additional files 2 and 3).
Discussion
This network meta-analysis found that antiplatelet agents, calcium, antioxidants, and their combinations were more effective medications for preventing hypertensive disorders in pregnancy than a placebo or no treatment in different women’s contexts. It was uncertain that one medication was superior to the others. The qualities of evidence were rated to be moderate, due to the limitation of risk of bias, publication bias, or imprecision. There is the potential for medication combinations, such as antiplatelet agents with calcium, anticoagulants with antiplatelet agents, or calcium with antioxidants, to be slightly better, but evidence was limited with only few current studies and large confidence intervals. More studies investigating these combination treatments are needed.
The effectiveness of antiplatelet agents and calcium on prevention of PE was similar to the findings of two previous systematic reviews and a meta-analysis [4, 7]. Doses of antiplatelet agents used in the included studies in this network meta-analysis ranged from 50 to 150 mg daily aspirin or 300 mg dipyridamole. For calcium, the daily doses ranged from 1000 to 2000 mg elemental calcium. Our findings support the WHO guidelines of 2011, which strongly recommends 1.5–2.0 g elemental calcium/day in areas where dietary calcium intake is low, or 75 mg of aspirin for the prevention of PE in women at high risk of developing the condition with moderate quality of evidence [114], and the NICE recommendation for the use of 75–150 mg aspirin [15]. The majority of antioxidants used were a combination of daily 1000 mg vitamin C plus 200–400 mg vitamin E. Our network meta-analysis found a preponderance of evidence that antioxidants could reduce PE and gestational hypertension, although this finding was opposite to the finding of a previous systematic review [115]. The combinations of antiplatelet agents with calcium or antioxidants with calcium, and antiplatelet agents with anticoagulants, had high cumulative probabilities for being the highest rank for preventing PE and/or GHT with low- to moderate-quality evidence, even though the studies were small. More research on combining antiplatelet agents with calcium may be needed.
The findings of our network meta-analysis were consistent with the results of two previous network meta-analyses, which found that calcium supplementation could reduce the risk of PE; however, these systematic reviews did not rate the quality of evidence using GRADE [10, 11]. In addition, the first review did not clearly describe risk characteristics of women in the results [10], and the latter defined nulliparous women as low-risk women [11]. The probability of being the most effective treatment for calcium in our review was higher than that in the study of Sanchez-Ramos (2017) [10]. The effectiveness of antiplatelet agents in our review supports the suggestion of using aspirin prophylaxis for PE from a previous systematic review and meta-analysis [116]. However, the qualities of evidence for the outcomes in our review were rated as ranging from very low to moderate. These were then downgraded, due to the risk of bias, imprecision, and publication bias regarding a GRADE approach.
There were limitations of this network meta-analysis. First, a wide range of high-risk pregnant women were included, resulting in the heterogeneous findings of included studies. This may be explained by different responses to the medications in various risk characteristics. Second, we focused on the studies conducted in hospital settings using high-risk factors suggested by NICE 2019, not Doppler, laboratory tests, or serum markers for screening risk of hypertensive disorders in pregnancy. Third, the subgroup analysis on intervention (different drugs in the same group of medication in the intervention arm, different doses of the same drug, or gestational age at the time the medication was given) and gestational age at the time the outcome occurred was not performed in this network meta-analysis. Fourth, this review presented parts of the results on relative effectiveness of medications, and safety outcomes will be reported in a separate review. Both aspects of effectiveness and safety are essential to consider the benefits outweigh the risks of medications to pregnant women. Lastly, PE with preterm birth was not included as the outcome in this network analysis.
Conclusions
Antiplatelet agents, calcium, antioxidants, and their combinations were more effective medications than a placebo or no treatment for preventing hypertensive disorders in different risks of pregnant women’s context. It was uncertain that one medication was superior to the others. The combinations of antiplatelet agents with calcium or anticoagulants were in one of the top ranks to prevent PE; however, the evidence was limited due to imprecision and heterogeneity leading to different clinical decisions in a future study. Calcium was in one of the top ranks to prevent GHT in nulliparous or primigravida women. Further network meta-analyses considering different drugs in the same groups of medications, different doses of the same drug, gestational age at the time the medications are given, and gestational age at the time the outcome occurred are required, so as to identify the most effective regimen of drugs for preventing hypertensive disorders in pregnancy.
Supplementary Information
Additional file 1: Appendix 1. Summary of findings for medications to prevent pre-eclampsia in subgroup: the studies including high-risk women with underlying diseases. Appendix 2. Summary of findings for medications to prevent pre-eclampsia in subgroup: the studies including high-risk women with no underlying diseases or mixed nulliparous women and women with no underlying diseases. Appendix 3. Summary of findings for medications to prevent pre-eclampsia in subgroup: the studies including nulliparous or primigravida women. Appendix 4. Summary of findings for medications to prevent gestational hypertension in subgroup: studies including high-risk women with underlying diseases or mixed other high-risk women. Appendix 5. Summary of findings for medications to prevent gestational hypertension in subgroup: the studies including high-risk women with no underlying diseases or mixed nulliparous women and women with no underlying diseases. Appendix 6. Summary of findings for medications to prevent gestational hypertension in subgroup: the studies including nulliparous or primigravida women. Appendix 7. Findings on the tests of heterogeneity, effect of intervention and tests of publication bias for direct comparisons in a network meta-analysis.
Additional file 2. Publication biases using comparison-adjusted funnel plot for preventing preeclampsia. 01: anticoagulants; 02: anticoagulants plus antiplatelet agents; 03: anticoagulants plus antiplatelet plus calcium; 04: antioxidants; 05: antiplatelet agents; 06: antiplatelet agents plus calcium; 07: antiplatelet agents plus nitric oxide; 08: calcium; 09: calcium plus antioxidants; 10: control; 11: nitric oxide.
Additional file 3. Publication biases using comparison-adjusted funnel plot for preventing gestational hypertension. 01: anticoagulants plus antiplatelet agents; 02: anticoagulants plus antiplatelet plus calcium; 03: antioxidants; 04: antiplatelet agents; 05: antiplatelet agents plus calcium; 06: calcium; 07: control.
Acknowledgements
This study was made possible through a grant offered by the Training Scholarship Program from the Faculty of Medicine, Prince of Songkla University, Thailand, the training programs from the KKU-WHO Long-Term Institutional Development HUBs, Thailand, and the Department of Health Policy, National Centre for Child Health and Development, Japan. We would like to thank the Editorial Team of Cochrane Pregnancy and Childbirth for their comments. We gratefully thank Assistant Professor Edward McNeil, Prince of Songkla University, for his assistance on graphic presentation of ranking treatments with SUCRA and forest plots in R software and the International Affairs Office, Faculty of Medicine, Prince of Songkla University for the English editing.
Abbreviations
- CI
Confidence interval
- GHT
Gestational hypertension
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- HDP
Hypertensive disorders in pregnancy
- NICE
National Institute for Health and Care Excellence
- NMA-SoF
Network meta-analysis–summary of findings
- PE
Preeclampsia
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- RR
Risk ratios
- SUCRA
Surface under the cumulative ranking curve
- SPE
Superimposed preeclampsia
Authors’ contributions
All authors participated in the concept of the study and approved the protocol. TL prepared the protocol, conducted the screening and selection of studies, extracted the data, assessed the risks of bias and GRADE, entered the data, analyzed and interpreted the results, and prepared the draft for review. YY participated in screening and selecting the studies, extracting the data, assessing the risks of bias, and approved the draft of the review. CK participated in selection of studies, extracted as before the data, assessed risk of bias and GRADE, entered the data, and approved the draft for review. RM and EO participated in the data extraction, assessing risk of bias, and approved the draft for review. HN was involved in data analysis and interpretation. All authors have approved the final version of the review.
Funding
Educational grants were provided to the principal author to attend the training programs and workshops on network meta-analysis. The funding body has not involved in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The protocol of this systematic review and network meta-analysis was approved by Institute Ethics Committee, Faculty of Medicine, Prince of Songkla University in consideration of exempt determination REC.61-139-18-1.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170:1–7. doi: 10.1016/j.ejogrb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PFA. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 3.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–e333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 4.Duley L, Meher S, Hunter KE, Seidler AL, Askie LM. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2019;2019(10):CD004659. doi: 10.1002/14651858.CD004659.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mastrolia SA, Novack L, Thachil J, Rabinovich A, Pikovsky O, Klaitman V, et al. LMWH in the prevention of preeclampsia and fetal growth restriction in women without thrombophilia. a systematic review and meta-analysis. Thromb Haemost. 2016;116:868–878. doi: 10.1160/TH16-02-0169. [DOI] [PubMed] [Google Scholar]
- 6.ACOG Hypertension in pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 7.Hofmeyr GJ, Lawrie TA, Atallah ÁN, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2018;10(10):CD001059. doi: 10.1002/14651858.CD001059.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rumbold A, Duley L, Crowther CA, Haslam RR. Antioxidants for preventing pre-eclampsia. Cochrane Database Syst Rev. 2008;2008(1):CD004227. doi: 10.1002/14651858.CD004227.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zullino S, Buzzella F, Simoncini T. Nitric oxide and the biology of pregnancy. Vascul Pharmacol. 2018;110:71–74. doi: 10.1016/j.vph.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Ramos L, Roeckner JT, Kaunitz AM. Which agent most effectively prevents preeclampsia? a systematic review with multitreatment comparison (network meta-analysis) of large multicenter randomized controlled trials. Am J Obstet Gynecol. 2017;216:S504. doi: 10.1016/j.ajog.2016.11.792. [DOI] [Google Scholar]
- 11.Khaing W, Vallibhakara SA-O, Tantrakul V, Vallibhakara O, Rattanasiri S, McEvoy M, et al. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. 2017;9:1141. doi: 10.3390/nu9101141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan MY, Wright D, Syngelaki A, Akolekar R, Cicero S, Janga D, et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstet Gynecol. 2018;51:743–750. doi: 10.1002/uog.19039. [DOI] [PubMed] [Google Scholar]
- 13.Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–622. doi: 10.1056/NEJMoa1704559. [DOI] [PubMed] [Google Scholar]
- 14.Guy GP, Leslie K, Diaz Gomez D, Forenc K, Buck E, Khalil A, et al. Implementation of routine first trimester combined screening for pre-eclampsia: a clinical effectiveness study. Br J Obstet Gynecol. 2020;1:1–8. doi: 10.1111/1471-0528.16361. [DOI] [PubMed] [Google Scholar]
- 15.NICE . Hypertension in pregnancy: diagnosis and management. London: NICE; 2019. p. 55. [Google Scholar]
- 16.ACOG No202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1–25. [DOI] [PubMed]
- 17.US Preventive Services Task Force. Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, et al. Aspirin use to prevent preeclampsia and related morbidity and mortality: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:1186–1191. doi: 10.1001/jama.2021.14781. [DOI] [PubMed] [Google Scholar]
- 18.PROSPERO . Guidance notes for registering a systematic review protocol with PROSPERO. York: University of York; 2016. [Google Scholar]
- 19.Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Cochrane handbook for systematic reviews of interventions version 620/0/00 0:00:00 AM (updated February 2021) Cochrane: Cochrane; 2021. Chapter 8: assessing risk of bias in a randomized trial; pp. 1–25. [Google Scholar]
- 20.Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. Br Med J. 2014;349:g5630. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 21.Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
- 22.Yepes-Nuñez JJ, Li SA, Guyatt G, Jack SM, Brozek JL, Beyene J, et al. Development of the summary of findings table for network meta-analysis. J Clin Epidemiol. 2019;115:1–13. doi: 10.1016/j.jclinepi.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3:98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932–944. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 25.Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking metaanalyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Available from: www.training.cochrane.org/handbook.
- 26.Mbuagbaw L, Rochwerg B, Jaeschke R, Heels-Andsell D, Alhazzani W, Thabane L, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. 2017;6:79. doi: 10.1186/s13643-017-0473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Br Med J. 2011;342:1–8. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 28.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8:e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez F, Martinez MF, Camero A, Pinzon JA. Low dose aspirin as prophylactic therapy of pregnancy induced hypertension. Rev Colomb Obstet Ginecol. 1996;47:197–201. doi: 10.18597/rcog.1446. [DOI] [Google Scholar]
- 31.Abo-Elwafa HA, Ahmed NS, Ameen M. The possible effect of gestational antioxidant on coagulopathy associated preeclampsia in women at risk of preeclampsia. 2011. A preprint published in university website 2011 at https://staffsites.sohag-univ.edu.eg/uploads/448/1538011756%20-%20BLOOD3_article_2.pdf.
- 32.Antartani R, Ashok K. Effect of lycopene in prevention of preeclampsia in high risk pregnant women. J Turk Ger Gynecol Assoc Artemis. 2011;12:35–38. doi: 10.5152/jtgga.2011.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.August P, Helseth G, Edersheim TG, Hutson JM, Druzin M. Proceedings of the 9th International Congress, International Society for the Study of Hypertension. 1994. Sustained release, low-dose aspirin ameliorates but does not prevent preeclampsia (PE) in a high risk population; p. 280. [Google Scholar]
- 34.Ayala DE, Ucieda R, Hermida RC. Chronotherapy with low-dose aspirin for prevention of complications in pregnancy. Chronobiol Int. 2013;30:260–279. doi: 10.3109/07420528.2012.717455. [DOI] [PubMed] [Google Scholar]
- 35.Azami M, Azadi T, Farhang S, Rahmati S, Pourtaghi K. The effects of multi mineral-vitamin D and vitamins (C) supplementation in the prevention of preeclampsia: an RCT. Int J Reprod Biomed Yazd Iran. 2017;15:273–278. [PMC free article] [PubMed] [Google Scholar]
- 36.Azar R, Turpin D. Effect of antiplatelet therapy in women at high risk for pregnancy-induced hypertension [abstract]. In: Cosmi EV, Di Renzo GC, editors. Perugia: Proceedings of 7th World Congress of Hypertension in Pregnancy; 1990. p. 257.
- 37.Bakhti A, Vaiman D. Prevention of gravidic endothelial hypertension by aspirin treatment administered from the 8th week of gestation. Hypertens Res - Clin Exp. 2011;34:1116–1120. doi: 10.1038/hr.2011.111. [DOI] [PubMed] [Google Scholar]
- 38.Banerjee S, Jeyaseelan S, Guleria R. Trial of lycopene to prevent pre-eclampsia in healthy primigravidas: results show some adverse effects. J Obstet Gynaecol Res. 2009;35:477–482. doi: 10.1111/j.1447-0756.2008.00983.x. [DOI] [PubMed] [Google Scholar]
- 39.Bassaw B, Roopnarinesingh S, Roopnarinesingh A, Homer H. Prevention of hypertensive disorders of pregnancy. J Obstet Gynaecol. 1998;18:123–126. doi: 10.1080/01443619867830. [DOI] [PubMed] [Google Scholar]
- 40.Beaufils M, Uzan S, Donsimoni R, Colau JC. Prevention of pre-eclampsia by early antiplatelet therapy. Lancet. 1985;1:840–842. doi: 10.1016/S0140-6736(85)92207-X. [DOI] [PubMed] [Google Scholar]
- 41.Beazley D, Ahokas R, Livingston J, Griggs M, Sibai BM. Vitamin C and E supplementation in women at high risk for preeclampsia: a double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2005;192:520–521. doi: 10.1016/j.ajog.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Belizan JM, Villar J, Gonzalez L, Campodonico L, Bergel E. Calcium supplementation to prevent hypertensive disorders of pregnancy. N Engl J Med. 1991;325:1399–1405. doi: 10.1056/NEJM199111143252002. [DOI] [PubMed] [Google Scholar]
- 43.Byaruhanga RN, Chipato T, Rusakaniko S. A randomized controlled trial of low-dose aspirin in women at risk from pre-eclampsia. Int J Gynecol Obstet. 1998;60:129–135. doi: 10.1016/S0020-7292(97)00257-9. [DOI] [PubMed] [Google Scholar]
- 44.Camarena-Pulido EE, Benavides LG, Baron JGP, Gonzalez SP, Saray AJM, Padilla FEG, et al. Efficacy of l-arginine for preventing preeclampsia in high-risk pregnancies: a double-blind, randomized, clinical trial. Hypertens Pregnancy. 2016;35:217–225. doi: 10.3109/10641955.2015.1137586. [DOI] [PubMed] [Google Scholar]
- 45.Caritis S, Sibai B, Hauth J, Lindheimer MD, Klebanoff M, Thom E, et al. Low-dose aspirin to prevent preeclampsia in women at high risk National Institute of Child Health and human development network of maternal-fetal medicine units [see comments] N Engl J Med. 1998;338:701–705. doi: 10.1056/NEJM199803123381101. [DOI] [PubMed] [Google Scholar]
- 46.Carpentier C, Bujold E, Camire B, Tapp S, Boutin A, Demers S. P08.03: low-dose aspirin for prevention of fetal growth restriction and pre-eclampsia in twins: the GAP pilot randomised trial. Ultrasound Obstet Gynecol. 2017;50:178. doi: 10.1002/uog.18072. [DOI] [Google Scholar]
- 47.Chiaffarino F, Parazzini F, Paladini D, Acaia B, Ossola W, Marozio L, et al. A small randomised trial of low-dose aspirin in women at high risk of pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2004;112:142–144. doi: 10.1016/S0301-2115(03)00269-0. [DOI] [PubMed] [Google Scholar]
- 48.CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative G r. o. u. p CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. Lancet. 1994;343:619–629. doi: 10.1016/S0140-6736(94)92633-6. [DOI] [PubMed] [Google Scholar]
- 49.Cong KJ, Chi SL, Liu GR. Calcium supplementation during pregnancy to reduce pregnancy induced hypertension. Beijing Med J. 1992;5:268. [Google Scholar]
- 50.Crowther CA, Hiller JE, Pridmore B, Bryce R, Duggan P, Hague WM, et al. Calcium supplementation in nulliparous women for the prevention of pregnancy-induced hypertension, preeclampsia and preterm birth: an Australian randomized trial Fracog and the act study group. Aust N Z J Obstet Gynaecol. 1999;39:12–18. doi: 10.1111/j.1479-828X.1999.tb03434.x. [DOI] [PubMed] [Google Scholar]
- 51.Davies NJ, Gazvani R, Farquharson RG, Walkinshaw SA. Low-dose aspirin in the prevntion of hypertensive disorders or pregnancy in relatively low-risk nulliparous women. Hypertens Pregnancy. 1995;14:49–55. doi: 10.3109/10641959509058050. [DOI] [Google Scholar]
- 52.Dendrinos S, Kalogirou I, Makrakis E, Theodoridis T, Mahmound EA, Christopoulou-Cokkinou V, et al. Safety and effectiveness of tinzaparin sodium in the management of recurrent pregnancy loss. Clin Exp Obstet Gynecol. 2007;34:143–145. [PubMed] [Google Scholar]
- 53.de Vries JI, van Pampus MG, Hague WM, Bezemer PD, Joosten JH, Investigators Fruit Low-molecular-weight heparin added to aspirin in the prevention of recurrent early-onset pre-eclampsia in women with inheritable thrombophilia: the FRUIT-RCT. J Thromb Haemost. 2012;10:64–72. doi: 10.1111/j.1538-7836.2011.04553.x. [DOI] [PubMed] [Google Scholar]
- 54.ECPPA ECPPA: randomised trial of low dose aspirin for the prevention of maternal and fetal complications in high risk pregnant women. Br J Obstet Gynaecol. 1996;103:39–47. doi: 10.1111/j.1471-0528.1996.tb09513.x. [DOI] [PubMed] [Google Scholar]
- 55.Elmahaishi. The uses of low dose aspirin (150 mg/day) in primigravida reduces the severity and complications of pregnancy induced hypertension [abstract]. In: Washington DC: XVI FIGO World Congress of Obstetrics & Gynecology; 2000. p. 98.
- 56.Essinger S. The use of low-dose acetylsalicylic acid in prevention of pregnancy-induced hypertension. Rev Col Bras Cir. 1992;19:58–62. [Google Scholar]
- 57.Ferrier C, Koferl U, Durig P, Schneider H. LMW-heparin and low-dose aspirin for prevention of preeclampsia: preliminary data of a randomized prospective study [abstract] Hypertens Pregnancy. 2000;19:82. [Google Scholar]
- 58.Golding J. A randomised trial of low dose aspirin for primiparae in pregnancy. The jamaica low dose aspirin study group. Br J Obstet Gynaecol. 1998;105:293–299. doi: 10.1111/j.1471-0528.1998.tb10089.x. [DOI] [PubMed] [Google Scholar]
- 59.Gris JC, Chauleur C, Molinari N, Mares P, Fabbro-Peray P, Quere I, et al. Addition of enoxaparin to aspirin for the secondary prevention of placental vascular complications in women with severe pre-eclampsia: the pilot randomised controlled NOH-PE trial. Thromb Haemost. 2011;106:1053–1061. doi: 10.1160/TH11-05-0340. [DOI] [PubMed] [Google Scholar]
- 60.Groom KM, McCowan LM, Mackay LK, Chamley LW, Stone PR, Lee AC, et al. Enoxaparin for the prevention of preeclampsia and intrauterine growth restriction in women with a history: a randomized trial. Am J Obstet Gynecol. 2017;216:296.e1–296.14. doi: 10.1016/j.ajog.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 61.Gu W, Lin J, Hou YY, Lin N, Song MF, Zeng WJ, et al. Effects of low-dose aspirin on the prevention of preeclampsia and pregnancy outcomes: a randomized controlled trial from Shanghai, China. Eur J Obstet Gynecol Reprod Biol. 2020;248:156–163. doi: 10.1016/j.ejogrb.2020.03.038. [DOI] [PubMed] [Google Scholar]
- 62.Haddad B, Winer N, Chitrit Y, Houfflin-Debarge V, Chauleur C, Bages K, et al. Enoxaparin and aspirin compared with aspirin alone to prevent placenta-mediated pregnancy complications: a randomized controlled trial. Obstet Gynecol. 2016;128:1053–1063. doi: 10.1097/AOG.0000000000001673. [DOI] [PubMed] [Google Scholar]
- 63.Hauth JC, Goldenberg RL, Parker CR, Philips JB, III, Copper RL, DuBard MB, et al. Low-dose aspirin therapy to prevent preeclampsia. Am J Obstet Gynecol. 1993;168:1083–1093. doi: 10.1016/0002-9378(93)90351-I. [DOI] [PubMed] [Google Scholar]
- 64.Hoffman MK, Goudar SS, Kodkany BS, Metgud M, Somannavar M, Okitawutshu J, et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;395:285–293. doi: 10.1016/S0140-6736(19)32973-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huria A, Gupta P, Kumar D, Sharma MK. Vitamin C and vitamin E supplementation in pregnant women at risk for pre-eclampsia: a randomized controlled trial. Internet J Health. 2010;10:1–5. [Google Scholar]
- 66.Kincaid-Smith P, North RA, Fairley KF, Kloss M, Ihle BU. Prevention of pre-eclampsia in high risk women with renal disease: a prospective randomized trial of heparin and dipyridamole. Nephrology. 1995;1:297–300. doi: 10.1111/j.1440-1797.1995.tb00043.x. [DOI] [Google Scholar]
- 67.Kumar A, Devi SG, Batra S, Singh C, Shukla DK. Calcium supplementation for the prevention of pre-eclampsia. Int J Gynecol Obstet. 2009;104:32–36. doi: 10.1016/j.ijgo.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 68.Levine RJ, Hauth JC, Curet LB, Sibai BM, Catalano PM, Morris CD, et al. Trial of calcium to prevent preeclampsia. N Engl J Med. 1997;337:69–76. doi: 10.1056/NEJM199707103370201. [DOI] [PubMed] [Google Scholar]
- 69.Liu FM, Zhao M, Wang M, Yang HL, Li L. Effect of regular oral intake of aspirin during pregnancy on pregnancy outcome of high-risk pregnancy-induced hypertension syndrome patients. Eur Rev Med Pharmacol Sci. 2016;20:5013–5016. [PubMed] [Google Scholar]
- 70.Lopez-Jaramillo P, Delgado F, Jacome P, Teran E, Ruano C, Rivera J. Calcium supplementation and the risk of preeclampsia in Ecuadorian pregnant teenagers. Obstet Gynecol. 1997;90:162–167. doi: 10.1016/S0029-7844(97)00254-8. [DOI] [PubMed] [Google Scholar]
- 71.Mahdy ZA, Siraj HH, Khaza’ai H, Mutalib MS, Azwar MH, Wahab MA, et al. Does palm oil vitamin E reduce the risk of pregnancy induced hypertension? Acta Medica (Hradec Kralove) 2013;56:104–109. doi: 10.14712/18059694.2014.17. [DOI] [PubMed] [Google Scholar]
- 72.McCance DR, Holmes VA, Maresh MJ, Patterson CC, Walker JD, Pearson DW, et al. Vitamins C and E for prevention of pre-eclampsia in women with type 1 diabetes (DAPIT): a randomised placebo-controlled trial. Lancet. 2010;376:259–266. doi: 10.1016/S0140-6736(10)60630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mone F, Mulcahy C, McParland P, Breathnach F, Downey P, McCormack D, et al. Trial of feasibility and acceptability of routine low-dose aspirin versus early screening test indicated aspirin for pre-eclampsia prevention (test study): a multicentre randomised controlled trial. BMJ Open. 2018;8:e022056. doi: 10.1136/bmjopen-2018-022056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Naghshineh E, Sheikhaliyan S. Effect of vitamin D supplementation in the reduce risk of preeclampsia in nulliparous women. Adv Biomed Res. 2016;5:7. doi: 10.4103/2277-9175.175239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nasrolahi SH, Alimohammady SH, Zamani M. The effect of antioxidants (vitamin e and c) on preeclampsia in primiparous women. J Gorgan Univ Med Sci. 2006;8:17–21. [Google Scholar]
- 76.Nieder J, Claus P, Augustin W. Prevention of pre-eclampsia and fetal growth retardation by trapidil. Zentralbl Gynakol. 1995;117:23–28. [PubMed] [Google Scholar]
- 77.Parazzini F, Benedetto C, Frusca T, Gregorini G, Bocciolone L, Marozio L, et al. Low-dose aspirin in prevention and treatment of intrauterine growth retardation and pregnancy-induced hypertension. Italian study of aspirin in pregnancy. Lancet. 1993;341:396–400. doi: 10.1016/0140-6736(93)93108-D. [DOI] [PubMed] [Google Scholar]
- 78.Pattison NS, Chamley LW, Birdsall M, Zanderigo AM, Liddell HS, McDougall J. Does aspirin have a role in improving pregnancy outcome for women with the antiphospholipid syndrome? A randomized controlled trial. Am J Obstet Gynecol. 2000;183:1008–1012. doi: 10.1067/mob.2000.106754. [DOI] [PubMed] [Google Scholar]
- 79.Picciolo C, Roncaglia N, Neri I, Pasta F, Arreghini A, Facchinetti F. Nitric oxide in the prevention of pre-eclampsia. Prenat Neonatal Med. 2000;5:212–215. [Google Scholar]
- 80.Ponmozhi G, Keepanasseril A, Mathaiyan J, Manikandan K. Nitric oxide in the prevention of pre-eclampsia (NOPE): a double-blind randomized placebo-controlled trial assessing the efficacy of isosorbide mononitrate in the prevention of pre-eclampsia in high-risk women. J Obstet Gynecol India. 2019;69:S103–S110. doi: 10.1007/s13224-018-1100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Porreco RP, Hickok DE, Williams MA, Krenning C. Low-dose aspirin and hypertension in pregnancy. Lancet. 1993;341:312. doi: 10.1016/0140-6736(93)92672-G. [DOI] [PubMed] [Google Scholar]
- 82.Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH, for the Vitamins in Pre-eclampsia trial consortium Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367:1145–1154. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [PubMed] [Google Scholar]
- 83.Purwar M, Kulkarni H, Motghare V, Dhole S. Calcium supplementation and prevention of pregnancy induced hypertension. J Obstet Gynaecol Res. 1996;22:425–430. doi: 10.1111/j.1447-0756.1996.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 84.Railton A, Davey DA. Aspirin and dipyridamole in the prevention of pre-eclampsia: effect on plasma 6 keto PGF1alpha and TxB2 and clinical outcome of pregnancy. In: Vienna: Proceedings of 1st European Congress on Prostaglandins in Reproduction; 1988. p. 48.
- 85.Ranjkesh F, Laluha F, Pakniat H, Kazemi H, Golshahi T, Esmaeili S. Effect of omeg-3 supplementation on preeclampsia in high risk pregnant women. J Qazvin Univ Med Sci. 2011;15:28–33. [Google Scholar]
- 86.Rayman MP, Searle E, Kelly L, Johnsen S, Bodman-Smith K, Bath SC, et al. Effect of selenium on markers of risk of pre-eclampsia in UK pregnant women: a randomised, controlled pilot trial. Br J Nutr. 2014;112:99–111. doi: 10.1017/S0007114514000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rey E, Garneau P, David M, Gauthier R, Leduc L, Michon N, et al. Dalteparin for the prevention of recurrence of placental-mediated complications of pregnancy in women without thrombophilia: a pilot randomized controlled trial. J Thromb Haemost. 2009;7:58–64. doi: 10.1111/j.1538-7836.2008.03230.x. [DOI] [PubMed] [Google Scholar]
- 88.Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362:1282–1291. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodger MA, Hague WM, Kingdom J, Kahn SR, Karovitch A, Sermer M, et al. Antepartum dalteparin versus no antepartum dalteparin for the prevention of pregnancy complications in pregnant women with thrombophilia (TIPPS): a multinational open-label randomised trial. Lancet. 2014;384:1673–1683. doi: 10.1016/S0140-6736(14)60793-5. [DOI] [PubMed] [Google Scholar]
- 90.Rogers MS, Fung HYM, Hung CY. Calcium and low-dose aspirin prophylaxis in women at high risk of pregnancy-induced hypertension. Hypertens Pregnancy. 1999;18:165–172. doi: 10.3109/10641959909023076. [DOI] [PubMed] [Google Scholar]
- 91.Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS, for the Acts Study G r. o. u. p Vitamins C and E and the risks of preeclampsia and perinatal complications. N Engl J Med. 2006;354:1796–1806. doi: 10.1056/NEJMoa054186. [DOI] [PubMed] [Google Scholar]
- 92.Seki H, Kuromaki K, Takeda S, Kinoshita K, Satoh K. Trial of prophylactic administration of TXA2 synthetase inhibitor, ozagrel hydrochloride for preeclampsia. Hypertens Pregnancy. 1999;18:157–164. doi: 10.3109/10641959909023075. [DOI] [PubMed] [Google Scholar]
- 93.Sharma JB, Kumar A, Kumar A, Malhotra M, Arora R, Prasad S, et al. Effect of lycopene on pre-eclampsia and intra-uterine growth retardation in primigravidas. Int J Gynecol Obstet. 2003;81:257–262. doi: 10.1016/S0020-7292(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 94.Sibai BM, Caritis SN, Thom E, Klebanoff M, McNellis D, Rocco L, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. N Engl J Med. 1993;329:1213–1218. doi: 10.1056/NEJM199310213291701. [DOI] [PubMed] [Google Scholar]
- 95.Spinnato JA, Freire S, Silva JL, Cunha Rudge MV, Martins-Costa S, Koch MA, et al. Antioxidant therapy to prevent preeclampsia: a randomized controlled trial. Obstet Gynecol. 2007;110:1311–1318. doi: 10.1097/01.AOG.0000289576.43441.1f. [DOI] [PubMed] [Google Scholar]
- 96.Subtil D, Goeusse P, Puech F, Lequien P, Biausque S, Breart G, et al. Aspirin (100 mg) used for prevention of pre-eclampsia in nulliparous women: the essai regional aspirine mere-enfant study (part 1) BJOG Int J Obstet Gynaecol. 2003;110:475–484. doi: 10.1046/j.1471-0528.2003.02096.x. [DOI] [PubMed] [Google Scholar]
- 97.Šulović N, Kontić-Vučinić, Relic G, Šulović L. Did calcium management prevent preeclampsia? Abstract no: P33. Pregnancy Hypertens. 2011;1:287. doi: 10.1016/j.preghy.2011.08.093. [DOI] [PubMed] [Google Scholar]
- 98.Sun H, Cai Y, Ma Z, Yuan J, Zhang L, Yang H, et al. Preventive effect of low-dose aspirin on preeclampsia occured in preeclampsia high-risk pregnant women and its mechanism. J Jilin Univ Med Ed. 2020;46:138–143. [Google Scholar]
- 99.Taherian AA, Taherian A, Shirvani A. Prevention of preeclampsia with low-dose aspirin or calcium supplementation. Arch Iran Med. 2002;5:151–156. [Google Scholar]
- 100.Tara F, Maamouri G, Rayman MP, Ghayour-Mobarhan M, Sahebkar A, Yazarlu O, et al. Selenium supplementation and the incidence of preeclampsia in pregnant Iranian women: a randomized, double-blind, placebo-controlled pilot trial. Taiwan J Obstet Gynecol. 2010;49:181–187. doi: 10.1016/S1028-4559(10)60038-1. [DOI] [PubMed] [Google Scholar]
- 101.Teran E, Hernandez I, Nieto B, Tavara R, Ocampo JE, Calle A. Coenzyme Q10 supplementation during pregnancy reduces the risk of pre-eclampsia. Int J Gynecol Obstet. 2009;105:43–45. doi: 10.1016/j.ijgo.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 102.Villar J, Abdel-Aleem H, Merialdi M, Mathai M, Ali MM, Zavaleta N, et al. World Health Organization randomized trial of calcium supplementation among low calcium intake pregnant women. Am J Obstet Gynecol. 2006;194:639–649. doi: 10.1016/j.ajog.2006.01.068. [DOI] [PubMed] [Google Scholar]
- 103.Villar J, Purwar M, Merialdi M, Zavaleta N, Thi Nhu Ngoc N, Anthony J, et al. World Health Organisation multicentre randomised trial of supplementation with vitamins C and E among pregnant women at high risk for pre-eclampsia in populations of low nutritional status from developing countries. BJOG Int J Obstet Gynaecol. 2009;116:780–788. doi: 10.1111/j.1471-0528.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 104.Wanchu M, Malhotra S, Khullar M. Calcium supplementation in pre-eclampsia. J Assoc Physicians India. 2001;49:795–798. [PubMed] [Google Scholar]
- 105.Wen SW, White RR, Rybak N, Gaudet LM, Robson S, Hague W, et al. Effect of high dose folic acid supplementation in pregnancy on pre-eclampsia (FACT): double blind, phase III, randomised controlled, international, multicentre trial. BMJ. 2018;362:k3478. doi: 10.1136/bmj.k3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu H, Perez-Cuevas R, Xiong X, Reyes H, Roy C, Julien P, et al. An international trial of antioxidants in the prevention of preeclampsia (INTAPP) Am J Obstet Gynecol. 2010;202:239.e1–239.10. doi: 10.1016/j.ajog.2010.01.050. [DOI] [PubMed] [Google Scholar]
- 107.Zhao YM, Xiao LP, Hu H, Yang XN, Xu YQ, Guo LM. Low-dose aspirin prescribed at bed time for the prevention of pre-eclampsia in high-risk pregnant women. Reprod Contracept. 2012;32:355–359. [Google Scholar]
- 108.Caspi E, Raziel A, Sherman D, Arieli S, Bukovski I, Weinraub Z. Prevention of pregnancy-induced hypertension in twins by early administration of low-dose aspirin: a preliminary report. Am J Reprod Immunol. 1994;31:19–24. doi: 10.1111/j.1600-0897.1994.tb00842.x. [DOI] [PubMed] [Google Scholar]
- 109.D’Anna R, Santamaria A, Corrado F, Benedetto AD, Petrella E, Facchinetti F. Myo-inositol in the prevention of gestational diabetes and its complications. Pregnancy Hypertens. 2015;5:6. doi: 10.1016/j.preghy.2014.10.015. [DOI] [Google Scholar]
- 110.Frusca T, Gregorini G, Ballerini S, Marchesi D, Bruni M. Low dose aspirin in preventing preeclampsia and IUGR. In: Proceedings of 6th World Congress of Hypertension in Pregnancy. Montreal; 1988. p. 232.
- 111.Lopez-Jaramillo P, Narvaez M, Weigel RM, Yepez R. Calcium supplementation reduces the risk of pregnancy-induced hypertension in an Andes population. Br J Obstet Gynaecol. 1989;96:648–655. doi: 10.1111/j.1471-0528.1989.tb03278.x. [DOI] [PubMed] [Google Scholar]
- 112.Viinikka L, Hartikainen-Sorri AL, Lumme R, Hiilesmaa V, Ylikorkala O. Low dose aspirin in hypertensive pregnant women: effect on pregnancy outcome and prostacyclin-thromboxane balance in mother and newborn. Br J Obstet Gynaecol. 1993;100:809–815. doi: 10.1111/j.1471-0528.1993.tb14304.x. [DOI] [PubMed] [Google Scholar]
- 113.Kalpdev A, Saha SC, Dhawan V. Vitamin C and e supplementation does not reduce the risk of superimposed PE in pregnancy. Hypertens Pregnancy. 2011;30:447–456. doi: 10.3109/10641955.2010.507840. [DOI] [PubMed] [Google Scholar]
- 114.World Health Organization . WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 115.Tenório MB, Ferreira RC, Moura FA, Bueno NB, Goulart MOF, Oliveira ACM. Oral antioxidant therapy for prevention and treatment of preeclampsia: meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2018;28:865–876. doi: 10.1016/j.numecd.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 116.Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2018;218:287–93.e1. doi: 10.1016/j.ajog.2017.11.561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Appendix 1. Summary of findings for medications to prevent pre-eclampsia in subgroup: the studies including high-risk women with underlying diseases. Appendix 2. Summary of findings for medications to prevent pre-eclampsia in subgroup: the studies including high-risk women with no underlying diseases or mixed nulliparous women and women with no underlying diseases. Appendix 3. Summary of findings for medications to prevent pre-eclampsia in subgroup: the studies including nulliparous or primigravida women. Appendix 4. Summary of findings for medications to prevent gestational hypertension in subgroup: studies including high-risk women with underlying diseases or mixed other high-risk women. Appendix 5. Summary of findings for medications to prevent gestational hypertension in subgroup: the studies including high-risk women with no underlying diseases or mixed nulliparous women and women with no underlying diseases. Appendix 6. Summary of findings for medications to prevent gestational hypertension in subgroup: the studies including nulliparous or primigravida women. Appendix 7. Findings on the tests of heterogeneity, effect of intervention and tests of publication bias for direct comparisons in a network meta-analysis.
Additional file 2. Publication biases using comparison-adjusted funnel plot for preventing preeclampsia. 01: anticoagulants; 02: anticoagulants plus antiplatelet agents; 03: anticoagulants plus antiplatelet plus calcium; 04: antioxidants; 05: antiplatelet agents; 06: antiplatelet agents plus calcium; 07: antiplatelet agents plus nitric oxide; 08: calcium; 09: calcium plus antioxidants; 10: control; 11: nitric oxide.
Additional file 3. Publication biases using comparison-adjusted funnel plot for preventing gestational hypertension. 01: anticoagulants plus antiplatelet agents; 02: anticoagulants plus antiplatelet plus calcium; 03: antioxidants; 04: antiplatelet agents; 05: antiplatelet agents plus calcium; 06: calcium; 07: control.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.