Abstract
Background and aims
Interferon-induced transmembrane protein 3 (IFITM3) plays a critical role in the adaptive and innate immune response by preventing membrane hemifusion between the host and viral cell cytoplasm. This study aimed to evaluate whether IFITM3 rs12252 polymorphism is related to an increased mortality rate of coronavirus disease 2019 (COVID-19).
Methods
The IFITM3 rs12252 polymorphism was genotyped using the amplification refractory mutation system-polymerase chain reaction (ARMS-PCR) in 548 dead and 630 improved patients positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Results
In the present study, the minor allele frequency of IFITM3 rs12252 (C) was significantly more frequent in dead patients than in improved cases. The results of the multivariate logistic regression analysis indicated that the lower lipid profiles, PCR Ct value, 25-hydroxyvitamin D, and uric acid and higher levels of erythrocyte sedimentation rate (ESR), liver enzymes, and creatinine, and IFITM3 rs12252 CC genotypes were related to the COVID-19 infection mortality.
Conclusions
In summary, our findings suggested a possible link between the mortality of COVID-19 infection, the CC genotypes of IFITM3 rs12252, and clinical parameters. Further investigations are required worldwide to prove the link relationship of COVID-19 mortality with host genetic factors.
Keywords: Interferon-induced transmembrane protein 3, Coronavirus disease 2019, Severe acute respiratory syndrome coronavirus 2
1. Introduction
Despite the global emergence of various new prevention and control approaches, severe acute respiratory syndrome-Coronavirus-2 (SARS-CoV-2) continues to spread at an alarming rate throughout the world [1]. There have been approximately 495 million confirmed cases of coronavirus disease 2019 (COVID-19) globally, including 6,170,283 deaths [2]. People with COVID-19 display a wide range of clinical symptoms, from asymptomatic to severe pneumonia with multiple organ failure [3].
COVID-19 mortality, as well as its clinical manifestations and consequences, are correlated to the internalization process of a virus into the host cell, host genetic factors, comorbidities, advanced age, and gender [4]. In this regard, in both adaptive and innate immune responses, the interferon-induced transmembrane (IFITM) proteins play an important role in antiviral defense. The human IFITM locus, which includes IFITM3, is found on chromosome 11p15.5 and consists of five genes. The IFITM3 gene is an IFN-stimulated gene (ISG), and the protein of this gene is mainly expressed in endosomes and lysosomes. It is effective against a wide range of enveloped viruses by preventing the hemifusion of the viral membrane and host cell membrane, such as Ebola, Marburg, influenza A, and SARS-CoV [5].
Previous research has suggested that single-nucleotide polymorphisms (SNPs) in the gene IFITM3 may impair the antiviral activity of the gene, resulting in increased susceptibility to infection and higher disease mortality in susceptible individuals [6]. In Asians and Caucasians, the C allele of the SNP rs12252 was strongly linked with the mortality of H1N1 and H7N9 influenza A virus infection [7], [8]. After receiving trivalent vaccination with inactivated H1N1, and B viruses, CC homozygotes decreased seroconversion compared to rs12252 T carrier [9]. The C allele of the rs12252 gene has been related to the progression of human immunodeficiency virus-1 (HIV-1) infection, with the C allele being considerably higher in patients with a low CD4 + T-cell count [10]. The functional impact of this polymorphism is still up for debate. The predicted alternative splicing of the transcript, which results in IFITM3 protein truncation and altered localization, has yet to be indicated [6], [11].
In a functional in vitro study of SARS-CoV, the limitation of spike (S) protein-mediated entrance by IFITM1 and IFITM3 was observed [12]. SARS-CoV-2 also enters cells via the S protein, which binds to angiotensin-converting enzyme 2 (ACE2). Consequently, it is speculated that IFITM3 plays a role in SARS-CoV-2 infection as well [13]. With this background in mind, we evaluated whether the previously mentioned connections between the variations of rs12252 in the IFITM3 gene might also be found in an Iranian cohort with SARS-CoV-2 infection.
2. Material and methods
2.1. Study population
During July 2021-January 2022, 1178 patients with COVID-19 were studied at Pasteur Institute of Iran. The study was approved by the Ethics Committee of Ilam University of Medical Sciences, Iran (IR.MEDILAM.REC.I400.237), and was carried out following the 1975 Declaration of Helsinki and applicable local rules. Written informed consent was obtained from all subjects.
SARS-CoV-2 was detected in this investigation using reverse transcriptase real-time polymerase chain reaction (rtReal Time-PCR) from nasopharyngeal or oropharyngeal swab samples.
The exclusion criteria were patients with underlying diseases such as cystic fibrosis, asthma, chronic obstructive pulmonary disease, cancer, HIV, pregnancy, obesity, diabetes, liver disease, heart disease, chronic kidney disease, and others.
The laboratory parameters, including real-time PCR cycle threshold (Ct) values, cholesterol, triglyceride (TG), high density lipoprotein (HDL), low-density lipoprotein (LDL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), fasting blood glucose (FBS), serum creatinine, uric acid, triiodothyronine (T3), 25-hydroxyvitamin D, C-reactive protein (CRP), white blood cells (WBC), platelets, hemoglobin, erythrocyte sedimentation rate (ESR), thyroxine (T4), and thyroid-stimulating hormone (TSH), were retrieved from the medical records of the patients.
2.2. DNA extraction and IFITM3 rs12252 genotyping
Approximately 10 ml of blood samples were taken from patients. Peripheral blood mononuclear cells (PBMCs) were isolated from the samples by Ficoll (Ficoll-Paque PLUS, GE Healthcare, USA) density gradient centrifugation and were stored at −70 °C.
The genomic DNA of patients with COVID-19 was isolated from PBMCs according to the manufacturer's instructions using the High Pure PCR Template Preparation Kit (Roche Diagnostics Deutschland GmbH, Mannheim, Germany).
The IFITM3 rs12252 was genotyped by using the amplification refractory mutation system-polymerase chain reaction (ARMS-PCR) method. The 5′ ARMS primers for the IFITM3 rs12252 gene were designed with one base mismatch at the 3′ end. The “T” and “C” allele forward primers were 5́-CTTCTTCTCTCCTGTCAACCGT-3́ and 5́-CTTCTTCTCTCCTGTCAACCGC-3́, respectively, and the reverse primer was 5́-GCACCCTCTGAGCATTCCCT-3́. The product size was 263 bp. Furthermore, we used a 441 bp product as internal control with the primers set of 5́-CCCCTGCCAAATCCCGAT-3́ and 5́-GCACCCTCTGAGCATTCCCT-3́ (Supplementary Fig. 1 ). The PCR was performed by initial denaturation at 95 °C for 20 min, followed by 40 cycles of 95 °C for 35 sec, 59 °C for 40 sec, 72 °C for 40 sec, and final extension at 72 °C for 10 min.
The 441 bp product was sequenced using the Sanger technique on an ABI 3500 DX Genetic Analyzer (ABI, Thermo Fisher Scientific, Waltham, MA, USA) to confirm the ARMS-PCR results. The raw sequencing data were evaluated using MEGA Version 11.0 (https://www.megasoftware.net/) (Supplementary Figure 2).
2.3. Statistical analysis
The statistical software IBM SPSS for Windows version 22.0 was used to analyze all the data (SPSS. Inc, Chicago, IL, USA). The Shapiro-Wilk test was applied to determine whether continuous variables had a normal distribution. The Pearson's chi-square and Mann-Whitney U tests were utilized for quantitative and continuous variables. In order to investigate the relationships between COVID-19 resistance and some risk factors for susceptibility, a multivariate logistic regression analysis was carried out using the Hosmer-Lemeshow test. Statistical significance was defined as a two-tailed P-value < 0.05. The influence of IFITM3 rs12252 on the mortality of COVID-19 cases was evaluated using the area under the curve-receiver operating characteristic curve (AUC-ROC) analysis. The correlation between COVID-19 mortality and IFITM3 rs12252 was investigated using the SNPStats software inheritance mode analysis. The best fit model for each SNP was determined using the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) [14].
3. Results
3.1. Baseline characteristics of COVID-19 patients
A total of 1178 COVID-19 patients were enrolled in this study. They were divided into two groups: dead (n = 548) and improved (n = 630) individuals. The mean ages of dead and improved patients were 57.7 ± 11.3 and 49.6 ± 13.1 years, respectively. Low concentrations of cholesterol (P = 0.001), TG (P = 0.025), LDL (P < 0.001), HDL (P < 0.001), uric acid (P < 0.001), creatinine (P < 0.001), 25-hydroxyvitamin D (P < 0.001), and real-time PCR Ct value (P = 0.001) and high ALT (P < 0.001), AST (P < 0.001), ALP (P < 0.001), and ESR (P < 0.001) were shown to be significantly linked with higher mortality rates following COVID-19 infection. The laboratory and clinical characteristics of the patient are shown in Table 1 .
Table 1.
Comparison laboratory parameters between dead and improved patients infected with COVID-19.
| Variables | Dead patients (n = 548) | Improved patients (n = 630) | P-value |
|---|---|---|---|
| Mean age ± SD | 57.7 ± 11.3 | 49.6 ± 13.1 | <0.001* |
| Distribution (%) | |||
| 20–50 (years) | 119 (21.7%) | 295 (46.8%) | <0.001* |
| 51–80 (years) | 429 (78.3%) | 335 (53.2%) | |
| Gender (male/female) | 294/254 (53.6/46.4%) | 323/307 (51.3/48.7%) | 0.415 |
| ALT, IU/L (mean ± SD) (Reference range: 5–40) | 44.1 ± 23.3 | 32.9 ± 25.0 | <0.001* |
| AST, IU/L (mean ± SD) (Reference range: 5–40) | 37.2 ± 13.1 | 30.8 ± 15.9 | <0.001* |
| ALP, IU/L (mean ± SD) (Reference range: up to 306) | 202.8 ± 67.7 | 169.3 ± 89.8 | <0.001* |
| Cholesterol, mg/dL (mean ± SD) (Reference range: 50–200) | 115.9 ± 38.0 | 122.0 ± 36.4 | <0.001* |
| TG, mg/dL (mean ± SD) (Reference range: 60–165) | 116.6 ± 42.1 | 133.5 ± 62.5 | 0.001* |
| LDL, mg/dL (mean ± SD) (Reference range: up to 150) | 70.7 ± 36.3 | 104.9 ± 49.3 | <0.001* |
| HDL, mg/dL (mean ± SD) (Reference range: >40) | 30.1 ± 11.1 | 34.5 ± 11.5 | <0.001* |
| WBC, 109/L (mean ± SD) (Reference range: 4000–10000) | 7582.7 ± 2689.6 | 7738.9 ± 2950.3 | 0.572 |
| CRP, mg/L (mean ± SD) (Reference range: <10 mg/L Negative) | 66.0 ± 21.6 | 56.6 ± 20.1 | <0.001* |
| ESR, mm/1st h (mean ± SD) (Reference range: 0–15) | 54.8 ± 15.9 | 46.0 ± 15.2 | <0.001* |
| FBS, mg/dL (mean ± SD) (Reference range: 70–100) | 110.4 ± 43.3 | 105.6 ± 40.6 | 0.001* |
| Platelets × 1000/cumm (mean ± SD) (Reference range: 140000–400000) | 185 ± 77 | 183 ± 67 | 0.612 |
| Hemoglobin, g/dL (mean ± SD) (Reference range: 12–18) | 15.4 ± 3.7 | 13.8 ± 2.6 | 0.378 |
| Uric acid, mg/dL (mean ± SD) (Reference range: 3.6–6.8) | 3.2 ± 1.2 | 5.9 ± 1.5 | <0.001* |
| Creatinine, mg/dL (mean ± SD) (Reference range: 0.6–1.4) | 1.1 ± 0.3 | 0.7 ± 0.3 | <0.001* |
| T3, ng/dL (mean ± SD) (Reference range: 2.3–4.2) | 3.0 ± 1.2 | 2.5 ± 1.1 | 0.054 |
| T4, mcg/dL (mean ± SD) (Reference range: 5.6–13.7) | 8.9 ± 6.2 | 8.5 ± 6.0 | 0.310 |
| TSH, mu/L (mean ± SD) (Reference range: 0.4–4.5) | 3.4 ± 1.9 | 3.3 ± 1.8 | 0.991 |
| 25-hydroxy vitamin D, ng/mL (mean ± SD) (Sufficiency: 21–150) | 26.8 ± 10.2 | 37.3 ± 13.1 | <0.001* |
| Real-time PCR Ct values | 13.1 ± 7.2 | 28.6 ± 9.5 | 0.001* |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; TG, triglyceride; LDL, low density lipoprotein; HDL, high density lipoprotein; WBC, white blood cells; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FBS, fasting blood glucose; T3, triiodothyronine; T4, thyroxine; TSH, Thyroid-stimulating hormone; Ct, cycle threshold; SD, standard deviation. *Statistically significant (<0.05).
3.2. Association of IFITM3 rs12252 and COVID-19 mortality
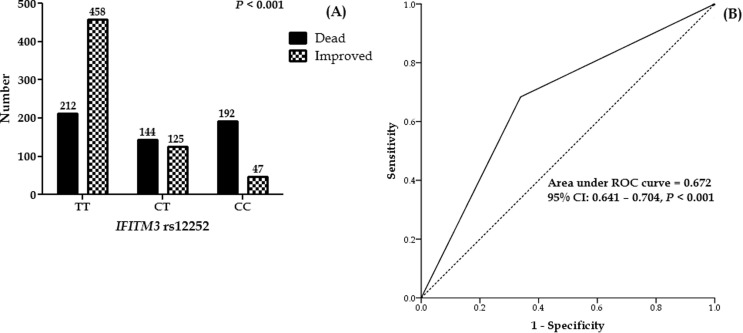
Fig. 1 depicts the effect of IFITM3 rs12252 on COVID-19 mortality. The mortality of COVID-19 was significantly higher in patients with IFITM3 rs12252 CC genotypes than in other genotypes, whereas TT genotypes were observed in improved patients (Fig. 1A).
Fig. 1.
(A) Frequency of IFITM3 rs12252 in COVID-19 patients. (B) ROC curve with the IFITM3 rs12252 for prediction the mortality rate in COVID-19 patients.
SNPStats was used to construct the IFITM3 rs12252 inheritance model (codominant, dominant, recessive, and overdominant). The best fit inheritance model for IFITM3 rs12252 was codominant with the lowest AIC and BIC. The C/C genotype was associated with a higher mortality rate (OR 8.83, 95% CI 6.17–12.63, P < 0.0001). Minor allele frequency (C) in improved, dead, and all patients was 0.17, 0.48, and 0.32, respectively (Table 2 ).
Table 2.
IFITM3 rs12252 association with COVID-19 mortality.
| Groups |
|||||||
|---|---|---|---|---|---|---|---|
| Model | Genotype | Improved patients | Dead patients | OR (95% CI) | P-value | AIC | BIC |
| Allele | T | 1041 (83.0%) | 568 (52.0%) | – | – | – | – |
| C | 219 (17.0%) | 528 (48.0%) | – | – | – | – | |
| Codominant | T/T | 458 (72.7%) | 212 (38.7%) | 1.00 | <0.0001 |
1450.9 |
1466.1 |
| C/T | 125 (19.8%) | 144 (26.3%) | 2.49 (1.86–3.33) | ||||
| C/C | 47 (7.5%) | 192 (35.0%) | 8.83 (6.17–12.63) | ||||
| Dominant | T/T | 458 (72.7%) | 212 (38.7%) | 1.00 | <0.0001 |
1490.7 |
1500.8 |
| C/T-C/C | 172 (27.3%) | 336 (61.3%) | 4.22 (3.30–5.39) | ||||
| Recessive | T/T-C/T | 583 (92.5%) | 356 (65.0%) | 1.00 | <0.0001 |
1487.3 |
1497.4 |
| C/C | 47 (7.5%) | 192 (35.0%) | 6.69 (4.74–9.45) | ||||
| Overdominant | T/T-C/C | 505 (80.2%) | 404 (73.7%) | 1.00 | 0.0087 | 1624.5 | 1634.6 |
| C/T | 125 (19.8%) | 144 (26.3%) | 1.44 (1.10–1.89) | ||||
| Minor allele frequency (C) | 0.17 | 0.48 | – | – | – | – | |
OR: Odds ratios; CI: confidence intervals; AIC: Akaike information criterion; BIC: Bayesian information criterion.
Moreover, the AUC-ROC value for IFITM3 rs12252 was 0.672, implying that host genetic variables are essential in viral infection mortality (Fig. 1B).
3.3. Factors linked with the mortality of COVID-19 infection
We evaluated some of the parameters linked to COVID-19 infection mortality using multivariate logistic regression analysis. COVID-19 mortality was found to be related to mean age (P < 0.001), ALT (P = 0.002), AST (P = 0.047), ALP (P < 0.001), TG (P = 0.035), HDL (P < 0.001), LDL (P < 0.001), creatinine (P < 0.001), uric acid (P < 0.001), 25-hydroxyvitamin D (P < 0.001), ESR (P < 0.001), Real-time PCR Ct values (P = 0.044), and IFITM3 rs12252 CC (P < 0.001) (Table 3 ).
Table 3.
Factors associated with dead patients infected with COVID-19.
| Factors |
||
|---|---|---|
| Baseline Predictors | OR (95 % CI) | P-value |
| Mean age ± SD | 0.937 (0.917–0.957) | <0.001* |
| ALT, IU/L | 0.981 (0.962–1.000) | 0.002* |
| AST, IU/L | 0.981 (0.962–1.000) | 0.047* |
| ALP, IU/L | 0.995 (0.992–0.998) | <0.001* |
| TG, mg/dL | 1.006 (1.000–1.011) | 0.035* |
| HDL, mg/dL | 1.051 (1.028–1.074) | <0.001* |
| LDL, mg/dL | 1.018 (1.013–1.024) | <0.001* |
| Creatinine, mg/dL | 0.009 (0.004–0.022) | <0.001* |
| Uric acid, mg/dL | 2.915 (2.424–3.504) | <0.001* |
| 25-hydroxyvitamin D, (ng/Ml) | 1.106 (1.080–1.133) | <0.001* |
| ESR, (mm/1st h) | 0.968 (0.951–0.984) | <0.001* |
| Real-time PCR Ct values | 0989 (0.977–1.000) | 0.044* |
| IFITM3 rs12252 (CC) | 0.382 (0.276–0.528) | <0.001* |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; TG, triglyceride; LDL, low density lipoprotein; HDL, high density lipoprotein; ESR, erythrocyte sedimentation rate; Ct, cycle threshold; IFITM3, Interferon-induced transmembrane protein-3; SD, standard deviation; OR: Odds ratios; CI: confidence intervals; *Statistically significant (<0.05).
4. Discussion
This comprehensive study evaluated the relationship of IFITM3 rs12252 in COVID-19 patients with severity and mortality in Iranian patients infected with SARS-CoV-2. Our findings suggested that IFITM3 rs12252 is linked to COVID-19 mortality.
The IFITM3 is critical in the first line of defense against SARS-COV-2, and alleles, such as IFITM3 rs12252, may change its role from an inhibitor to the promoter of SARS-COV-2 entrance into cells. The altered viral endocytosis in patients with the IFITM3 rs12252 allele may generate a high viral load and cytokine storm accompanied by severe COVID-19 in vulnerable patients.
In our study, the minor allele frequency was 32.0% (C allele). Following an examination of a Chinese cohort, it has been proposed that the minor allele IFITM3 rs12252 (C) is connected to greater COVID-19 severity [15]. The minor allele IFITM3 rs12252 (C) was associated with worse outcomes in almost every related report; for example, increased influenza severity [16] or much more rapid progression of HIV [10], although it is usually found in Chinese patients but not in or European cohorts. According to the results of the 1000 Genomes Project, this is significant because the minor allele IFITM3 rs12252 (C) is frequently found in Chinese populations (nearly 50%). On the other hand, it is uncommon among European populations (1–8%), South Asian populations (10–18%), and African people (21–33%) [17], [18]. Interestingly, the frequency of the IFITM3 rs12252 mutant allele was much greater in East Asia than in other populations. Allelic variations in IFITM3 SNPs are directly associated with SARS-CoV-2 clinical symptoms. In European population research, our data indicate that the mutant allele frequency of IFITM3 rs12252 was higher in the COVID-19 group than in the healthy group [18].
Our findings showed that the CC and TT genotypes of IFITM3 rs12252 might be a risk factor for mortality and a protective factor for COVID-19 recovery in Iranian patients, respectively. These results were consistent with previous research in different regions of the world that found a link between IFITM3 polymorphisms and severe COVID-19. In preliminary research, Zhang et al. found a considerably greater prevalence of IFITM3 rs12252 C-allele carriers in patients with severe COVID-19 than in patients with moderate COVID-19 [15]. In a recent study, C-allele carriers of IFITM3 rs12252 were found to have a 2-fold greater risk of SARS-CoV-2 infection than the control group collected before the pandemic [19]. An investigation in Saudi Arabia revealed that the presence of the C allele substantially doubled the risk of COVID-19 mortality. The prognostic usefulness of the C allele was independent of well-established clinical risk variables, such as age, gender and even significant underlying comorbidities. They also showed that IFITM3 rs12252 with the T/C genotype significantly augmented the risk of mortality in younger people infected with SARS-CoV-2, raising a unique theory on the pathogenic pathways that may lead to death in this group of people [20]. Cuesta-Llavona et al. indicated that the controls had a considerably greater frequency of rs12252 (AA) genotype carriers, implying a protective effect [21]. Only one study found no association between IFITM3 rs12252 polymorphisms and infection severity [13].
Therefore, IFITM3 rs12252 is thought to be a COVID-19 risk-related genetic factor, and individuals who carry the rs12252 (C) allele may be at a higher risk of developing severe COVID-19 disease.
Excess mortality in patients aged 51–80 years was generally higher than in the younger age groups in our study. The IFITM3 rs12252 CC genotype dramatically increases the risk of death in older people infected with SARS-CoV-2. These findings were in accordance with a report in Saudi Arabia who found that hospital admission and mortality in patients with 60 years rate were correlated with the “C” allele [20].
In the present study, liver enzyme levels were significantly higher in patients who died than in improved patients. COVID-19 individuals have elevated liver enzymes in a median of 15% and up to 58% of cases [22]. Several mechanisms have been proposed to explain COVID-19 liver damage. ACE-2 receptors, the primary portal through which SARS-CoV-2 enters cells, are present in greater abundance on bile duct cholangiocytes than on hepatocytes [23]. Consequently, SARS-CoV-2 infection of cells should result in biliary inflammation and an obstructive pattern of liver injury rather than a hepatocellular pattern [24]. In addition, drug-induced or cytokine-driven damage are two more possible causes of injury. Even in the absence of viral replication in the liver, the generation of inflammatory cytokines resulted in hepatic oxidative stress, which led to the hepatocellular injury in the animal models of influenza [25].
Our findings showed that low uric acid levels were linked to a higher likelihood of hospital death. The uric acid concentration in the dead cases was substantially lower than in the recovered individuals. This result was in agreement with several reports [26], [27]. Uric acid is a natural byproduct of purine catabolism that plays a variety of intricate and changeable roles in the body and is more than just a metabolic byproduct. Nucleic acid metabolites have been proven to impact the natural immune system substantially [28]. The uric acid crystals can stimulate dendritic cells to boost the release of cytokines associated with T-helper (Th)-17 polarization in the presence of nuclear factor-kappa B signal, whereas uric acid can accelerate Th-17 cell differentiation [29]. Proinflammatory cytokines have been demonstrated to affect uric acid excretion and serum uric acid levels. In SARS-CoV patients, serum IL-8 level was positively related to uric acid fraction excretion while negatively correlated with serum uric acid level. Moreover, IL-6 levels during gouty attach were related to changes in blood uric acid [30], [31].
In the current investigation, the low levels of lipids and 25-hydroxyvitamin D were significantly correlated with COVID-19 mortality. These findings were consistent with prior research, which found that disease mortality was associated with lower total lipid levels. Lowering cellular lipids can enhance circulation cholesterol uptake, resulting in lower serum lipid concentration. This may result in the augmented expression of lipoprotein receptors, particularly scavenger receptor class B type 1, increasing cholesterol uptake into the plasma membrane and SARS-CoV-2 infection rates [32], [33].
T-regulatory lymphocytes are the body's first line of defense against excessive inflammation and frequent viral infections. Treg levels are low in many SARS-CoV-2 patients and can be boosted with 25-hydroxyvitamin D treatment. Low 25-hydroxyvitamin D levels are associated with a rise in inflammatory cytokines and a dramatically higher risk of pneumonia and viral upper respiratory tract infections. Furthermore, the lack of 25-hydroxyvitamin D is linked to increased thrombotic events, which are prevalent in COVID-19 [34], [35].
Because of evidence of ethnic heterogeneity is very important, lack of the impact of this polymorphism among Iranian ethnic groups was main limitation of this study.
5. Conclusion
The results of this study indicated a strong correlation between the clinical parameters and the mortality rate of COVID-19. Moreover, we indicated that patients with the IFITM3 rs12252 (C) allele were prone to higher COVID-19 mortality than the (T) allele. Early detection of patients with the C-allele may assist in preventive and therapeutic efforts in patients at a higher risk of death. It is suggested that additional studies be conducted to corroborate the findings.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like to thank all of the patients who participated in the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cyto.2022.155957.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Li J., Du P., Yang L., Zhang J., Song C., Chen D., Song Y., Ding N., Hua M., Han K., Song R., Xie W., Chen Z., Wang X., Liu J., Xu Y., Gao G., Wang Q., Pu L., Di L., Li J., Yue J., Han J., Zhao X., Yan Y., Yu F., Wu A.R., Zhang F., Gao Y.Q., Huang Y., Wang J., Zeng H., Chen C. Two-step fitness selection for intra-host variations in SARS-CoV-2. Cell reports. 2022;38(2) doi: 10.1016/j.celrep.2021.110205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus (COVID-19) Dashboard. . 2022.
- 3.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S.C., Du B., Li L.-J., Zeng G., Yuen K.-Y., Chen R.-C., Tang C.-l., Wang T., Chen P.-Y., Xiang J., Li S.-Y., Wang J.-L., Liang Z.-J., Peng Y.-X., Wei L.I., Liu Y., Hu Y.-H., Peng P., Wang J.-M., Liu J.-Y., Chen Z., Li G., Zheng Z.-J., Qiu S.-Q., Luo J., Ye C.-J., Zhu S.-Y., Zhong N.-S. in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monticelli M., Mele B.H., Andreotti G., Cubellis M.V., Riccio G. Why does SARS-CoV-2 hit in different ways? Host genetic factors can influence the acquisition or the course of COVID-19. European journal of medical genetics. 2021;64(6) doi: 10.1016/j.ejmg.2021.104227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diamond M.S., Farzan M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat. Rev. Immunol. 2013;13(1):46–57. doi: 10.1038/nri3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everitt A.R., Clare S., Pertel T., John S.P., Wash R.S., Smith S.E., Chin C.R., Feeley E.M., Sims J.S., Adams D.J., Wise H.M., Kane L., Goulding D., Digard P., Anttila V., Baillie J.K., Walsh T.S., Hume D.A., Palotie A., Xue Y., Colonna V., Tyler-Smith C., Dunning J., Gordon S.B., Smyth R.L., Openshaw P.J., Dougan G., Brass A.L., Kellam P. IFITM3 restricts the morbidity and mortality associated with influenza. Nature. 2012;484(7395):519–523. doi: 10.1038/nature10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen T., Xiao M., Yang J., Chen Y.K., Bai T., Tang X.J., Shu Y.L. Association between rs12252 and influenza susceptibility and severity: an updated meta-analysis. Epidemiol. Infect. 2019;147 doi: 10.1017/S0950268818002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim Y.-C., Jeong B.-H. Ethnic variation in risk genotypes based on single nucleotide polymorphisms (SNPs) of the interferon-inducible transmembrane 3 (IFITM3) gene, a susceptibility factor for pandemic 2009 H1N1 influenza A virus. Immunogenetics. 2020;72(9-10):447–453. doi: 10.1007/s00251-020-01188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei N., Li Y., Sun Q., Lu J., Zhou J., Li Z., Liu L., Guo J., Qin K., Wang H., Zhao J., Li C., Sun L., Wang D., Zhao Z., Shu Y. IFITM3 affects the level of antibody response after influenza vaccination. Emerging Microbes Infect. 2020;9(1):976–987. doi: 10.1080/22221751.2020.1756696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Makvandi-Nejad S., Qin L., Zhao Y., Zhang T., Wang L., Repapi E., Taylor S., McMichael A., Li N., Dong T., Wu H. Interferon-induced transmembrane protein-3 rs12252-C is associated with rapid progression of acute HIV-1 infection in Chinese MSM cohort. AIDS (London, England). 2015;29(8):889–894. doi: 10.1097/QAD.0000000000000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenney A.D., Dowdle J.A., Bozzacco L., McMichael T.M., St. Gelais C., Panfil A.R., Sun Y., Schlesinger L.S., Anderson M.Z., Green P.L., López C.B., Rosenberg B.R., Wu L., Yount J.S. Human genetic determinants of viral diseases. Annu. Rev. Genet. 2017;51(1):241–263. doi: 10.1146/annurev-genet-120116-023425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang I.-C., Bailey C.C., Weyer J.L., Radoshitzky S.R., Becker M.M., Chiang J.J., Brass A.L., Ahmed A.A., Chi X., Dong L., Longobardi L.E., Boltz D., Kuhn J.H., Elledge S.J., Bavari S., Denison M.R., Choe H., Farzan M., Baric R.S. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus. PLoS Pathog. 2011;7(1) doi: 10.1371/journal.ppat.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schönfelder K., Breuckmann K., Elsner C., Dittmer U., Fistera D., Herbstreit F., Risse J., Schmidt K., Sutharsan S., Taube C., Jöckel K.-H., Siffert W., Kribben A., Möhlendick B. The influence of IFITM3 polymorphisms on susceptibility to SARS-CoV-2 infection and severity of COVID-19. Cytokine. 2021;142 doi: 10.1016/j.cyto.2021.155492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sole X., Guino E., Valls J., Iniesta R., Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics. 2006;22(15):1928–1929. doi: 10.1093/bioinformatics/btl268. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Qin L, Zhao Y, Zhang P, Xu B, Li K, et al. Interferon-induced transmembrane protein 3 genetic variant rs12252-C associated with disease severity in coronavirus disease 2019. The Journal of infectious diseases. 2020;222:34-7. [DOI] [PMC free article] [PubMed]
- 16.Zhang Y.-H., Zhao Y., Li N., Peng Y.-C., Giannoulatou E., Jin R.-H., et al. Interferon-induced transmembrane protein-3 genetic variant rs12252-C is associated with severe influenza in Chinese individuals. Nat. Commun. 2013;4:1–6. doi: 10.1038/ncomms2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikoloudis D., Kountouras D., Hiona A. The frequency of combined IFITM3 haplotype involving the reference alleles of both rs12252 and rs34481144 is in line with COVID-19 standardized mortality ratio of ethnic groups in England. PeerJ. 2020;8 doi: 10.7717/peerj.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y., Wei L., He L., Sun J., Liu N. Interferon-induced transmembrane protein 3 gene polymorphisms are associated with COVID-19 susceptibility and severity: A meta-analysis. J. Infect. 2022;84(6):825–833. doi: 10.1016/j.jinf.2022.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gómez J., Albaiceta G.M., Cuesta-Llavona E., García-Clemente M., López-Larrea C., Amado-Rodríguez L., López-Alonso I., Melón S., Alvarez-Argüelles M.E., Gil-Peña H., Vidal-Castiñeira J.R., Corte-Iglesias V., Saiz M.L., Alvarez V., Coto E. The Interferon-induced transmembrane protein 3 gene (IFITM3) rs12252 C variant is associated with COVID-19. Cytokine. 2021;137 doi: 10.1016/j.cyto.2020.155354. [DOI] [PubMed] [Google Scholar]
- 20.Alghamdi J., Alaamery M., Barhoumi T., Rashid M., Alajmi H., Aljasser N., Alhendi Y., Alkhalaf H., Alqahtani H., Algablan O., Alshaya A.I., Tashkandi N., Massadeh S., Almuzzaini B., Ehaideb S.N., Bosaeed M., Ayoub K., Yezli S., Khan A., Alaskar A., Bouchama A. Interferon-induced transmembrane protein-3 genetic variant rs12252 is associated with COVID-19 mortality. Genomics. 2021;113(4):1733–1741. doi: 10.1016/j.ygeno.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuesta-Llavona E., Albaiceta G.M., García-Clemente M., Duarte-Herrera I.D., Amado-Rodríguez L., Hermida-Valverde T., Enríquez-Rodriguez A.I., Hernández-González C., Melón S., Alvarez-Argüelles M.E., Boga J.A., Rojo-Alba S., Vázquez-Coto D., Gómez J., Coto E. Association between the interferon-induced transmembrane protein 3 gene (IFITM3) rs34481144/rs12252 haplotypes and COVID-19. Current Research Virological Sci. 2021;2 doi: 10.1016/j.crviro.2021.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon A.M., Barritt A.S. Elevated liver enzymes in patients with COVID-19: look, but not too hard. Springer. 2021;66(6):1767–1769. doi: 10.1007/s10620-020-06585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kukla M., Skonieczna-Żydecka K., Kotfis K., Maciejewska D., Łoniewski I., Lara L.F., Pazgan-Simon M., Stachowska E., Kaczmarczyk M., Koulaouzidis A., Marlicz W. COVID-19, MERS and SARS with concomitant liver injury—systematic review of the existing literature. J. Clinical Medicine. 2020;9(5):1420. doi: 10.3390/jcm9051420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. lancet Gastroenterology Hepatology. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papic N., Pangercic A., Vargovic M., Barsic B., Vince A., Kuzman I. Liver involvement during influenza infection: perspective on the 2009 influenza pandemic. Influenza Other Respir. Viruses. 2012;6:e2–e5. doi: 10.1111/j.1750-2659.2011.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen B., Lu C., Gu H.-Q., Li Y., Zhang G., Lio J., Luo X., Zhang L., Hu Y., Lan X., Chen Z., Xie Q., Pan H. Serum uric acid concentrations and risk of adverse outcomes in patients with COVID-19. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.633767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G., Wu X., Zhou C.-L., Wang Y.-M., Song B., Cheng X.-B., Dong Q.-F., Wang L.-L., You S.-S., Ba Y.-M. Uric acid as a prognostic factor and critical marker of COVID-19. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-96983-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishii K.J., Akira S. Potential link between the immune system and metabolism of nucleic acids. Curr. Opin. Immunol. 2008;20(5):524–529. doi: 10.1016/j.coi.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Conforti-Andreoni C., Spreafico R., Qian H.L., Riteau N., Ryffel B., Ricciardi-Castagnoli P., Mortellaro A. Uric acid-driven Th17 differentiation requires inflammasome-derived IL-1 and IL-18. J. Immunol. 2011;187(11):5842–5850. doi: 10.4049/jimmunol.1101408. [DOI] [PubMed] [Google Scholar]
- 30.Wu V.-C., Huang J.-W., Hsueh P.-R., Yang Y.-F., Tsai H.-B., Kan W.-C., Chang H.-W., Wu K.-D. Renal hypouricemia is an ominous sign in patients with severe acute respiratory syndrome. Am. J. Kidney Dis. 2005;45(1):88–95. doi: 10.1053/j.ajkd.2004.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urano W., Yamanaka H., Tsutani H., Nakajima H., Matsuda Y., Taniguchi A., et al. The inflammatory process in the mechanism of decreased serum uric acid concentrations during acute gouty arthritis. The Journal of Rheumatology. 2002;29:1950–1953. [PubMed] [Google Scholar]
- 32.Kočar E., Režen T., Rozman D. Cholesterol, lipoproteins, and COVID-19: Basic concepts and clinical applications. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of. Lipids. 2021;1866(2):158849. doi: 10.1016/j.bbalip.2020.158849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiner Ž., Hatamipour M., Banach M., Pirro M., Al-Rasadi K., Jamialahmadi T., Radenkovic D., Montecucco F., Sahebkar A. Statins and the COVID-19 main protease: in silico evidence on direct interaction. Archives Medical Sci.: AMS. 2020;16(3):490–496. doi: 10.5114/aoms.2020.94655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weir E.K., Thenappan T., Bhargava M., Chen Y. Does vitamin D deficiency increase the severity of COVID-19? Clinical Medicine. 2020;20(4) doi: 10.7861/clinmed.2020-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahimi P., Tarharoudi R., Rahimpour A., Mosayebi Amroabadi J., Ahmadi I., Anvari E., Siadat S.D., Aghasadeghi M., Fateh A. The association between interferon lambda 3 and 4 gene single-nucleotide polymorphisms and the recovery of COVID-19 patients. Virology J. 2021;18(1) doi: 10.1186/s12985-021-01692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.