Abstract
Childhood maladaptive aggression is associated with disrupted functional connectivity within amygdala-prefrontal circuitry. In this study, neural correlates of childhood aggression were probed using the intrinsic connectivity distribution, a voxel-wise metric of global resting-state brain connectivity. This sample included 38 children with aggressive behavior (26 boys, 12 girls) ages 8–16 years and 21 healthy controls (14 boys, 6 girls) matched for age and IQ. Functional MRI data were acquired during resting state, and differential patterns of intrinsic functional connectivity were tested in a priori regions of interest implicated in the pathophysiology of aggressive behavior. Next, correlational analyses tested for associations between functional connectivity and severity of aggression measured by the Reactive-Proactive Aggression Questionnaire in children with aggression. Children with aggressive behavior showed increased global connectivity in the bilateral amygdala relative to controls. Greater severity of aggressive behavior was associated with decreasing global connectivity in the dorsal anterior cingulate and ventromedial prefrontal cortex. Follow-up seed analysis revealed that aggression was also positively correlated with left amygdala connectivity with the dorsal anterior cingulate, ventromedial and dorsolateral prefrontal cortical regions. These results highlight the potential role of connectivity of the amygdala and medial prefrontal and anterior cingulate cortices in modulating the severity of aggressive behavior in treatment-seeking children.
Keywords: aggression, amygdala, resting-state functional connectivity, anterior cingulate, ventromedial prefrontal cortex
Introduction
Maladaptive aggression, defined by behaviors performed in response to frustration or provocation that can result in harm to self or others, is one of the most frequent reasons for referral to child mental health services (Connor et al., 2019). In the current diagnostic classification, aggressive behavior is associated with disruptive behavior disorders, including conduct disorder (CD) and oppositional defiant disorder (ODD), and disruptive mood dysregulation disorder (DMDD), of which anger or irritability are core symptoms (American Psychiatric Association, 2013). In clinical samples, however, most childhood psychiatric disorders can confer elevated risk for aggressive behavior (Jensen et al., 2007), underscoring the relevance of using a dimensional approach to understand underlying brain mechanisms of aggression across diagnostic categories. Here, we investigated the neural correlates of aggression in a transdiagnostic sample of treatment-seeking children using resting-state functional connectivity.
On a neural-systems level, aggressive behavior is associated with structural and functional perturbations in frontolimbic circuitry, most notably in the amygdala and regions of the ventral prefrontal cortex (PFC) and the anterior cingulate cortex (ACC) (Blair, 2016; Bertsch et al., 2020). Theoretical models of aggression suggest disruptions in limbic and striatal circuitry in response to emotional stimuli and reinforcement-based decision-making, respectively (Blair et al., 2018), coupled with disruptions in ‘top-down’ prefrontal regulatory circuitry (Alegria et al., 2016), and dysconnectivity between these systems (Blair, 2016). Supporting the notion that frontoparietal abnormalities are linked to aberrant functioning of the cognitive control network, structural MRI studies have shown reduced gray matter volumes in the ventral PFC (Rogers and De Brito, 2016), as well as reduced cortical thickness and/or gray matter volume in the ventral PFC/orbitofrontal cortex and the supramarginal gyrus/inferior parietal cortex (Smaragdi et al., 2017; Ibrahim et al., 2021), in children with disruptive behavior disorders relative to unaffected controls. In turn, reduced cortical thickness of the ventral PFC and orbitofrontal and anterior cingulate cortices was associated with continuous measures of aggressive behavior (Ducharme et al., 2011; Ameis et al., 2014).
In task-based functional magnetic resonance imaging (fMRI) studies that utilized emotion-processing paradigms, maladaptive aggression in children was shown to be related to over-reactivity of the amygdala (Herpertz et al., 2008; Passamonti et al., 2010; Viding et al., 2012) and underactivity in prefrontal regions, particularly the ventromedial and ventrolateral prefrontal cortices (vmPFC and vlPFC, respectively) (Marsh et al., 2008; Decety et al., 2009). In addition, frustration and irritability, two constructs closely linked to aggression, have been associated with reduced dorsal ACC (dACC) activity during emotion processing (Alegria et al., 2016). The dACC plays a central role in performance monitoring and serves as a hub where negative affect is linked with the execution of goal-directed behavior (Etkin et al., 2011; Shenhav et al., 2016). To this end, reduced dACC activation during frustration has been interpreted as failed emotion regulation (Perlman et al., 2015), and increased activation of the dACC has been interpreted as greater effort needed to exercise cognitive control of frustration (Tseng et al., 2019). However, studies utilizing competitive reaction-time tasks that enable manipulation of characteristics of provocation and aggressive responses reported positive relationships between aggression and ventral PFC activity in response to high vs low provocation when prestige or money is at stake (Repple et al., 2017; Buades-Rotger et al., 2021) or between aggression and dorsal anterior cingulate during reactivity to threat (Beyer et al., 2015), indicating that the direction of brain–behavior association may vary by context and phase of aggressive behavior response. On balance, Beyer et al. reported a negative correlation between medial orbitofrontal cortex reactivity to the angry facial expression of the opponent and the ensuing aggressive response during a competitive reactive time task. In the same study, ACC activity was positively associated with aggressive behavior on task trials where the opponents displayed angry facial expressions. These results underscore that the neural circuitry involved in encoding provocation overlaps with the circuitry underlying the decision to retaliate. Greater activation of prefrontal regions may reflect both the cognitive effort required to control impulsive response and the cognitive effort required for the calibration of appropriate retaliatory response to provocation.
While a large body of neuroimaging research has utilized task-based fMRI, including tasks of emotion processing and competitive social interactions, relatively few studies have utilized resting-state fMRI in children with disruptive behavior disorders. While disruptions in cognitive control networks during socioemotional processing have been related to disruptive behavior disorders, it is unknown whether such effects are reflecting transitory (state-like) factors, such as anger in response to an experimental task, or capturing stable (trait-like) variation in the functional brain architecture. Resting-state fMRI may offer advantages to investigate the latter. First, resting-state fMRI provides a measure of spontaneous fluctuations of neural signals across the brain in functionally related regions that are correlated with each other in the absence of external stimuli, revealing the intrinsic functional network organization (a pattern of functional connectivity during resting state) (Beckmann et al., 2005). Second, while activation studies reveal transitory responses to stimulus provocation, resting-state measures of connectivity are thought to reveal the intrinsic functional architecture, reflecting stable aspects of functional brain organization shaped by long-term experiences engaging these large-scale networks (Gratton et al., 2018). Third, while task-based fMRI studies have elucidated the key elements of the neural circuitry involved in aggression, the investigation of intrinsic functional connectivity also holds promise in identifying neural markers of psychopathology in diverse clinical populations without requiring any task performance. Resting-state fMRI can be performed in pediatric populations unable to cooperate during cognitive tasks and can also circumvent concerns that fMRI tasks may vary when conducted across laboratories and research groups.
Prior resting-state studies suggest that disruptive behavior is related to altered resting-state connectivity in the amygdala and lateral and medial prefrontal cortices. In particular, increased resting-state amygdala connectivity (Aghajani et al., 2017; Zhu et al., 2019) and reduced connectivity of the vmPFC regions (Roy et al., 2018) have been implicated in maladaptive aggression. A recent study of children with aggressive behavior reported aberrant resting-state functional connectivity in the amygdala and cingulate cortex (Werhahn et al., 2020). Increased functional connectivity was reported between the amygdala and vmPFC/rostral anterior cingulate in children with attention-deficit hyperactivity disorder (ADHD) complicated by high emotional liability (Hulvershorn et al., 2014). Similarly, externalizing behavior problems in typically developing children without psychiatric disorders were associated with increased amygdala connectivity with the anterior cingulate and lateral orbitofrontal cortex (Thijssen et al., 2021). Thus, the prevailing view is that disruption in frontolimbic circuitry sets the stage for the onset of aggressive behavior, particularly given the shared reciprocal connections between the vmPFC and amygdala (Motzkin et al., 2015) and essential role of this circuitry in emotion regulation (Etkin et al., 2015; Silvers et al., 2017). Collectively, these results suggest that maladaptive aggression could be reflected in the integrity of the amygdala-ventral PFC circuitry. Thus, our resting-state analyses focused on three a priori regions: amygdala, dACC and vmPFC (Alegria et al., 2016; Noordermeer et al., 2016). Measures of global intrinsic connectivity, reflecting the levels of functional connectivity between each region and all other regions, have the potential to advance the understanding of dysfunction in the neural architecture of childhood aggression and help to identify practical biomarkers for pediatric populations. To our knowledge, no resting-state study has investigated global connectivity in children seeking treatment for aggressive behavior using the intrinsic connectivity distribution.
Here, we investigated whether functional connectivity at rest is associated with the presence and severity of aggressive behavior. A popular measure of global connectivity is degree centrality (Buckner and Vincent, 2007; Bullmore and Sporns, 2009). However, this measure requires the selection of an arbitrary threshold, and the choice of threshold can impact results. For this reason, we opted to use an alternate measure of global connectivity known as the intrinsic connectivity distribution, which was designed to avoid the use of an arbitrary threshold (Scheinost et al., 2012). Our study also included a well-characterized, transdiagnostic sample of children with aggressive behavior in the age range from 8 to 16 years. This age range was selected because aggressive behavior tends to be stable during this age period (Nagin and Tremblay, 1999; Brame et al., 2001) and represents the most common reason for referral to mental health services. Although the brain mechanisms of neurocognitive processes underlying aggression may vary over developmental periods (Vijayakumar et al., 2019), we included a sample of children in the age range where children are likely to seek clinical services for aggressive behavior (i.e. 8–16), making our sample more representative of treatment-seeking youth.
The first aim of this study was to examine differential patterns of global connectivity between children with aggression and healthy controls in the amygdala, dorsal anterior cingulate and vmPFC. As described in our paper presenting the protocol for this study that was published prior to data analysis (Sukhodolsky et al., 2016), these three regions were selected as a priori regions of interest (ROIs) based on socio-affective networks implicated in aggression (Blair, 2016) and meta-analyses of fMRI studies of children with aggressive behavior (Alegria et al., 2016; Noordermeer et al., 2016). We also conducted a whole-brain analysis of global connectivity in order to assess regions not included in our initial hypotheses. We hypothesized that relative to typically developing controls, treatment-seeking children with aggression would show aberrant connectivity in the amygdala and prefrontal regions. The second aim of the study was to test the association between intrinsic connectivity and severity of aggression in the parent-rated Reactive-Proactive Aggression Questionnaire (RPQ) (Raine et al., 2006). These correlational analyses were conducted in the group of children with aggressive behavior, first in the a priori regions and then in the whole brain. While these correlational analyses were exploratory, we predicted significant associations between connectivity within ROIs and aggression severity in children with aggressive behavior.
Methods
Participants
The sample included 38 children with aggressive behavior (12 females) and 20 typically developing healthy controls (HC group; 6 females) matched for age and IQ. All participants were 8 to 16 years old. Table 1 shows demographic and clinical characteristics. Children with aggressive behavior participated in a clinical trial of behavior therapy for aggression (Sukhodolsky et al., 2016). Here, we report resting-state fMRI and clinical characterization data that were collected at baseline prior to initiating the treatment. Children with aggressive behavior were recruited from the outpatient child psychiatry clinic at the Yale University Child Study Center and from outreach to local schools, pediatricians and mental health providers.
Table 1.
Demographic and clinical characteristics of participants
| Aggressive behavior | Healthy controls | Test | P-value | |
|---|---|---|---|---|
| Age, mean (s.d.) | 11.87 (2.49) | 13.02 (2.06) | t 56 = 2.42 | 0.08 |
| Sex, number (%) | Chi 2 1 = 0.02 | 0.90 | ||
| Boys | 26 (68.4%) | 14 (70.0%) | ||
| Girls | 12 (31.6%) | 6 (30.0%) | ||
| Race/ethnicity, number (%) | Chi 2 4 = 4.19 | 0.52 | ||
| White | 23 (60.5%) | 15 (75.0%) | ||
| Black | 8 (21.1%) | 5 (25.0%) | ||
| Hispanic | 4 (10.5%) | 0 | ||
| Other | 2 (5.2%) | 0 | ||
| Family characteristics | ||||
| Two-parent family | 21 (55.3%) | 14 (70.0%) | Chi 2 1 = 1.19 | 0.27 |
| Full-scale IQ, mean (s.d.) | 108.39 (12.45) | 111.04 (10.92) | t 56 = 0.89 | 0.37 |
| CBCL-aggression T-score, mean (s.d.) | 76.32 (7.91) | 51.00 (2.96 | t56 = 13.76 | <0.001 |
| RPQ total score, mean (s.d.) | 19.89 (7.40) | 3.30 (3.21) | t56 = 9.57 | <0.001 |
| ICU total score, mean (s.d.) | 32.89 (10.39) | 15.20 (6.62) | t56 = 6.61 | <0.001 |
| fMRI motion (FD), mean (s.d.) | 0.12 (0.07) | 0.11 (0.06) | t56 = 0.83 | 0.41 |
| Diagnoses, number (%) | ||||
| ODD | 28 (73.7%) | |||
| CD | 4 (10.5%) | |||
| ADHD | 30 (78.9%) | |||
| Any anxiety disorder | 7 (18.4%) | |||
| Depression | 4 (10.5%) | |||
| DMDD a | 8 (21.1.4%) | |||
| Other b | 9 (23.72%) | |||
| Medication status, number (%) | ||||
| No medication | 13 (34.2%) | |||
| Taking medication | 25 (65.8%) | |||
| Stimulants | 10 (26.3%) | |||
| α-Agonists | 5 (13.2%) | |||
| Antipsychotics | 3 (7.9%) | |||
| Selective serotonin reuptake inhibitors (SSRIs) | 2 (5.3%) | |||
| Mood stabilizers | 1 (2.6) | |||
Following DSM-5, ODD diagnosis was not assigned to children who met criteria for DMDD.
Other diagnoses category included Enuresis (n = 4), Obsessive Compulsive Disorder (n = 1) and Tic Disorder (n = 4).
Children with aggression were required to have a T-score of 65 or greater on the Aggressive Behavior Scale of the Child Behavior Checklist (CBCL) (Achenbach and Rescorla, 2001), which is 1.5 standard deviation (s.d.) units above the mean of the standardization sample of typically developing children stratified by age and sex. Of note, the current study was developed in response to the National Institute of Mental Health Research Domain Criteria (RDoC) initiative to evaluate the neural underpinnings of symptom profiles across diagnostic boundaries (Insel and Wang, 2010). Thus, co-occurring psychiatric disorders, such as ADHD and anxiety, were allowed if the presence of co-occurring disorders did not require immediate treatment. Untreated PTSD and severe depression were exclusionary because these disorders may present with pressing treatment needs. In addition to a high level of aggression on the CBCL, all children met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria for ODD, CD or DMDD based on a structured diagnostic assessment (Kaufman et al., 2016). All subjects who met the criteria for DMDD diagnosis also met the criteria for ODD, but only DMDD diagnosis was assigned following DSM-5 (American Psychiatric Association, 2013). Participants were excluded if they: (i) had a significant medical condition, such as a seizure disorder based on medical history; (ii) were unable to meet MRI safety requirements, such as the absence of metal medical implants and claustrophobia; or (iii) had a history of head trauma or loss of consciousness. Exclusionary criteria for healthy control subjects were any history of anxiety, ADHD, disruptive behavior disorders or other psychiatric, genetic or neurological disorders. Seventy children were recruited and had fMRI data available for this analysis (48 with aggressive behavior and 22 controls). Twelve participants were removed from the analyses due to excessive head motion (10 children with aggressive behavior and 2 controls). There were no differences in demographic characteristics and measures of aggressive behavior between the 10 children in the aggressive behavior group who were excluded from fMRI data analysis due to head motion and the 38 children with aggressive behavior who were retained in fMRI analysis.
Each participant’s parent provided informed consent according to specifications by the institutional review board at the Yale University School of Medicine. Each child provided verbal and written assent.
Clinical assessment
Children received a comprehensive diagnostic evaluation that included the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL) (Kaufman et al., 2016), a structured interview conducted with the parent and child by an expert clinician to establish DSM-5 diagnoses of disruptive behavior disorders and co-occurring psychopathology. Parents also completed the CBCL (Achenbach and Rescorla, 2001). Full-scale IQ was evaluated with the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1997). Parents completed demographics and medical history forms.
The Aggressive Behavior scale of the parent-rated CBCL, a well-established measure of child psychopathology (Achenbach and Rescorla, 2001), was used as the inclusion criterion of clinically significant aggressive behavior (based on T-score ≥65). The scale consists of 16 items that assess inappropriate anger outbursts and verbal and physical aggression. HC participants were required to have no current or history of psychiatric or neurological disorders, as well as a CBCL-aggression T-score below 55. The Chronbach’s alpha internal consistency of CBCL Aggressive Behavior scale in the present sample was 0.94.
The RPQ (Raine et al., 2006) is a 23-item parent-report scale that measures aggression on a 3-point Likert scale. Twelve items index proactive aggression (e.g. ‘Had fights with others to show who was on top’) and 11 items index reactive aggression (e.g. ‘Reacted angrily when provoked by others’). The RPQ Aggression Total score was used as a continuous measure of aggression in the analyses. The Chronbach’s alpha of RPQ in the present sample was 0.88.
Parents completed the Inventory of Callous-Unemotional Traits (ICU) (Frick, 2003), a 24-item questionnaire with excellent internal consistency and construct validity. The ICU total score was used as a continuous measure of callous-unemotional (CU) traits. The Chronbach’s alpha of ICU in the present sample was 0.94.
MRI acquisition and processing
All participants were scanned on 3 T Siemens Trio scanners with a 32-channel head coil. Each session began with a localizing scan, followed by the collection of a high-resolution anatomical image using an MPRAGE sequence: repetition time (TR) = 2530 ms, echo time (TE) = 3.31 ms, TI = 1100 ms, flip angle = 7°, resolution = 1 × 1 × 1 mm, and the collection of 34 4-mm-thick axial-oblique T1-weighted slices aligned with the 13th slice at the anterior commissure–posterior commissure line. Functional data were collected at the same slice locations as the T1-weighted anatomical data, using a T2*-sensitive gradient-recalled single-shot echo-planar pulse sequence: TR = 2000 ms, TE = 25 ms, flip angle = 60°, field of view (FOV) = 220 mm, matrix size = 642. Participants completed one resting-state run (165 volumes, 5 min 30 s). During the resting run, participants were instructed to rest with their eyes open while viewing a crosshair. The first three volumes (6 s) were discarded to allow the signal to reach a steady state.
Standard preprocessing and individual subject analyses were conducted using the BioImage Suite software package (http://bioimagesuite.yale.edu/). Group-level analyses were conducted in FMRIB Software Library (FSL) (Woolrich et al., 2009; Jenkinson et al., 2012; Winkler et al., 2014). Functional imaging data were also motion corrected using the SPM12 algorithm (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Participants with average framewise displacement greater than 0.3 mm were excluded (which is a commonly used motion criterion for pediatric populations) (Greene et al., 2018; Vanderwal et al., 2021). The time courses of several variables of no interest were removed by regression. These included the six motion parameter time courses, the average signal in the white matter, the average signal in the cerebrospinal fluid and up to third order polynomial drift (polynomial fits were voxel specific and removed frequency fluctuations below those of interest). The data were temporally smoothed using a Gaussian filter with a cutoff frequency of 0.09 Hz and masked to include only voxels in the gray matter. Prior to the actual fMRI session, a mock scanner was used to acclimate participants to the scanning environment (see Supplement for more details). During the mock scan, motion tracker software (Polhemus FASTRAK head motion sensor) was used to provide real-time movement feedback to participants and teach children to minimize head motion.
Computation of global connectivity maps
The intrinsic connectivity distribution (Scheinost et al., 2012) was computed in a voxel-wise manner for each subject. This computation yields a threshold-free measure of global connectivity for each voxel. The time course of each voxel is correlated with the time course of every other voxel. For each voxel, a survival function of the distribution of positive correlations to that voxel is modeled with a stretched exponential with variance α. Alpha represents the spread of the distribution of connections; therefore, a larger alpha is associated with a greater number of strong connections for the seed voxel. Alpha is computed separately for each voxel to yield a map of global connectivity patterns across the brain.
To examine regionally specific differences in connectivity, the map from each subject was normalized to have a mean of 0 and an s.d. of 1. This normalization maintains the spatial pattern of connectivity in a given subject while adjusting for global differences in connectivity. This form of normalization has been shown to reduce head motion-related confounds (Yan et al., 2013).
Transformation to common space
Maps from individual subjects were transformed to the coordinate space of the Colin brain (Holmes et al., 1998) via a concatenation of three registrations: (i) a linear rigid transformation of the functional data to the axial-oblique anatomical data collected in the same scanning session, (ii) a linear rigid transformation of the axial-oblique anatomical data to that subject’s MPRAGE image and (iii) a nonlinear registration of that subject’s MPRAGE image (Papademetris et al., 2004) to the Colin brain. All registrations were inspected visually to ensure accuracy.
Definition of ROIs
As specified in our publication describing the hypotheses for this study (Sukhodolsky et al., 2016), there were three a priori ROIs: the amygdala, dorsal anterior cingulate and vmPFC. The cortical regions of interest ROIs were defined with respect to Brodmann areas from the Talairach atlas in MNI space, included in BioImage Suite (Lacadie et al., 2008). Amygdala ROIs were as defined in Harvard-Oxford subcortical structural atlas included in FSL (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases) (Desikan et al., 2006). The dorsal anterior cingulate region included all gray matter in BA 24, 32 and 33 above z = 10. The vmPFC region included all gray matter on the medial surface (−20 ≤ x ≤ 20) in BA 10, 11, 12, 13, 14, 25 and 32 below z = 10. These three ROIs are shown in Supplementary Figure S1 in the supplement.
Computation of group difference maps
A voxel-wise t-test comparing global connectivity maps of the aggressive behavior and HC groups was computed. Small volume correction was used for the three a priori defined ROIs (amygdala, dorsal anterior cingulate and vmPFC) and whole-brain correction was also performed for exploratory purposes. All corrections for multiple comparisons were performed using nonparametric cluster correction as recommended by Eklund et al. (2016) in the FSL randomise tool (Winkler et al., 2014) with 5000 permutations.
Dimensional analyses examining correlations of global connectivity with aggression
Within the group of children with aggression, voxel-wise correlations between RPQ total aggression score and global connectivity maps were computed. These were corrected for multiple comparisons both at the whole-brain level and at a small volume correction level for each of the three a priori ROIs using the FSL randomise tool with 5000 permutations.
Follow-up amygdala-seed connectivity correlations with aggression
The clusters in the bilateral amygdala that were significantly different between groups were defined as ROIs for follow-up seed connectivity analyses in the group of children with aggression. The two amygdala regions were transformed to individual subject space and seed connectivity maps were computed in BioImage Suite for each subject. The resulting seed maps were transformed to common space and correlations were run across subjects with RPQ aggression measures. Results were corrected for whole-brain multiple comparisons. Because the primary interest of this study was in general maladaptive aggression, we designated RPQ total score as the primary behavioral variable of interest.
Previous research has demonstrated that controlling for CU traits can reveal relationships between amygdala function and aggression severity that are obscured when callous traits are not taken into account (Viding et al., 2012; Lozier et al., 2014). Therefore, we repeated the whole-brain voxel-wise amygdala-seed correlation analysis with the ICU total score as a covariate to explore if the pattern of results changed (see Supplemental Results and Supplementary Figure S2).
Additional analyses evaluating potential confounds
To assess the influence of potentially confounding variables (age, motion and CU traits), connectivity values from significant clusters in each analysis (i.e. group differences in global connectivity and correlations of global connectivity and amygdala-seed connectivity with RPQ scores in the aggressive behavior group) were extracted to conduct follow-up analyses in SPSS v28 (see Supplemental Results and Supplementary Table S2).
Results
Clinical characteristics
Compared to children in the healthy control group, subjects in the aggressive behavior group had significantly higher CBCL aggressive behavior scores (mean ± s.d. = 76.32 ± 7.91 vs 51.0 ± 2.96, t56 = 13.79, P < 0.001) and RPQ scores (mean ± s.d = 19.89 ± 7.40 vs 3.30 ± 3.21, t56 = 9.57, P < 0.001). Twenty-eight children in the aggressive behavior group met the criteria for ODD (73.7%), four met the criteria for CD (10.5%) and eight met the criteria for DMDD (21.1%). There were no differences between the healthy controls and children in the aggressive behavior group on age, sex, race/ethnicity, full-scale IQ and family composition. Demographic and clinical characteristics of the sample are reported in Table 1.
Group differences in global connectivity
A significant group difference was found in the bilateral amygdala between the group of children with aggressive behavior and healthy controls (Figure 1, Supplementary Table S2). Specifically, children with aggressive behavior showed greater global intrinsic connectivity in the bilateral amygdala than children in the healthy controls group [P < 0.05 family-wise error (FWE) corrected]. No significant findings were observed in the other two ROIs or in the exploratory whole-brain analyses.
Fig. 1.
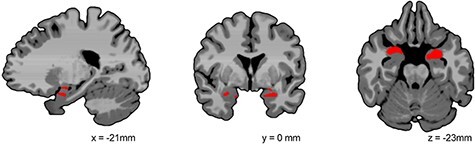
Region of the bilateral amygdala with a significant group difference in global connectivity in P < 0.05 small volume-corrected analysis. Children with aggression showed over- connectivity in the amygdala compared to healthy controls. Images are shown using radiological convention (left is on the right).
Correlations between global connectivity and aggression severity
In the group of children with aggressive behavior, global connectivity was significantly and negatively correlated with the RPQ aggression total score in the small volume-corrected analyses for both the dorsal anterior cingulate and the vmPFC (FWE-corrected P < 0.05, Figure 2A and B, Supplementary Table S2). There was no significant correlation in the amygdala. In the whole-brain analysis, global connectivity was significantly negatively correlated with RPQ aggression total score in the vmPFC and dACC (overlapping with the small volume-corrected finding) and the dorsolateral PFC (FWE-corrected P < 0.05, Figure 2C, Supplementary Table S2).
Fig. 2.
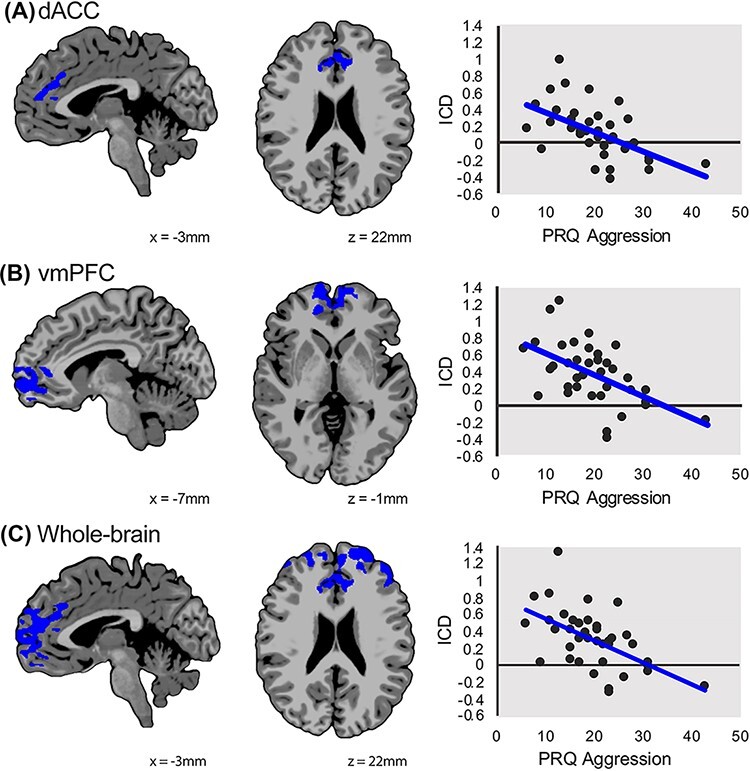
Prefrontal global connectivity is associated with aggression severity. In the aggressive behavior group, dimensional analyses showed that reduced global connectivity in the dACC (A) and vmPFC (B) was negatively associated with severity of aggression. Results are shown at a P < 0.05 threshold with small volume correction analysis for dACC and vmPFC. Results from the whole-brain corrected analysis showed that global connectivity was negatively correlated with aggression severity in the dACC, vmPFC and dorsolateral PFC (C). The x-axis shows the severity of aggression using the RPQ total score, and the y-axis shows the intrinsic connectivity distribution or strength of global connectivity. Images are shown using radiological convention (left is on the right).
Follow-up correlations between amygdala-seed connectivity and aggression severity
In children with aggressive behavior, connectivity to the left and right amygdala regions identified in the group contrasts of global connectivity were then related to aggression severity using the RPQ aggression total score. The severity of aggression was significantly and positively correlated with left amygdala connectivity with a cluster of the PFC, including vmPFC, dACC and the dorsolateral PFC at a whole-brain corrected level (FWE-corrected P < 0.05; Figure 3, Supplementary Table S2). There were no significant correlations with right amygdala connectivity. We repeated this analysis including ICU total score as a covariate, and the pattern of results was largely unchanged (see Supplemental Results and Supplementary Figure S2).
Fig. 3.
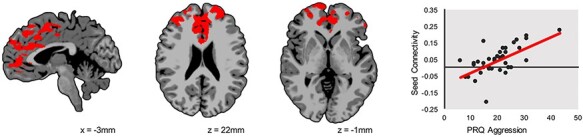
Follow-up analyses examining the correlation between left amygdala seed-based connectivity and aggression severity. In the aggressive behavior group, severity of aggression is positively associated with left amygdala connectivity with a cluster in the PFC, including vmPFC, dorsolateral PFC and dACC. Results are whole-brain corrected at a P < 0.05 level. The x-axis shows the severity of aggression using the RPQ total score, and the y-axis shows the strength of left amygdala connectivity. Images are shown using radiological convention (left is on the right).
Additional analyses of potential confounds
The SPSS post hoc analyses revealed that after covarying for age, CU traits and motion, main results for global connectivity remained significant for between-group (Figure 1), small volume-corrected (Figure 2A and B) and whole-brain (Figure 2C) analyses. Amygdala-seed connectivity (Figure 3) was also unchanged by the inclusion of these covariates (see Supplemental Results and Supplementary Table S2 for additional details).
Discussion
Abnormal patterns of connectivity within amygdala-PFC circuitry in children with aggressive behavior have been well documented using task-based fMRI. As a novel approach, this study utilized a metric of global connectivity—reflecting the overall inter-connectedness of a region with all other regions of the brain—to investigate disruptions in frontolimbic circuitry associated with aggressive behavior. Three important findings were observed in this study that advance the understanding of associations between disruptions in intrinsic functional connectivity and aggression in children. First, children with aggressive behavior showed increased global connectivity in the bilateral amygdala relative to unaffected controls. Second, in children with aggressive behavior, decreased global connectivity in the dorsal anterior cingulate and vmPFC was associated with greater severity of aggressive behavior. Third, the severity of aggression was positively correlated with left amygdala-PFC connectivity in children with aggressive behavior. Additionally, the amygdala connectivity findings remained significant after covarying for CU traits.
The amygdala has been a primary area of interest in neuroimaging studies of aggressive behavior due to its key role in emotion reactivity (Phelps and LeDoux, 2005) and frustration processing (Yu et al., 2014). Given the bidirectional flow of information between the amygdala and PFC structures, our finding may indicate an intrinsic functional architecture reflecting excessive bottom-up signaling at the expense of the top-down regulation of negative affect. The positive association of increased amygdala-dACC and vmPFC connectivity with behavioral problems was reported in healthy adolescents (Saxbe et al., 2018; Thijssen et al., 2021) and in juvenile offenders (Aghajani et al., 2017). Resting-state studies of adults have also reported positive associations between increased amygdala intrinsic connectivity and anger (Gilam et al., 2017), a related construct of aggression, as well as between amygdala connectivity with frontotemporal regions and aggression (Buades-Rotger et al., 2019). Our study expands upon this earlier work by demonstrating increased intrinsic connectivity of amygdala in a transdiagnostic sample of children seeking treatment for aggressive behavior. Increased resting-state connectivity of the amygdala with the vmPFC and ACC structures suggests a more vigilant state of neural networks involved in emotional processing (Thijssen et al., 2021). We speculate that the greater state of vigilance at rest and in the absence of emotional stimuli may deplete cognitive resources required for the performance of the emotion-processing tasks, resulting in reduced amygdala-PFC connectivity observed in task-based fMRI studies of emotion processing in children with aggressive behavior.
In dimensional analyses conducted in the group of children with aggressive behavior, global connectivity of the dACC and vmPFC was negatively associated with the severity of aggression. That is, children with aggressive behavior showed decreasing levels of dACC and vmPFC connectivity with increasing degree of aggression. The vmPFC is a central node in the circuitry supporting the cognitive control of emotion (Etkin et al., 2015), which modulates limbic reactivity through reciprocal cortico-cortical connections with other regions of the lateral and ventral PFC (Hartley and Phelps, 2010; Ochsner et al., 2012; Silvers et al., 2017). The anterior cingulate is involved in the awareness of affective states (Craig, 2009), the processing of empathic concern for others (Decety et al., 2008; Mutschler et al., 2013) and cognitive processes, including monitoring of response conflicts and decision-making (Bush et al., 2000). Interactions between the anterior cingulate, vmPFC and amygdala are also involved in the top-down regulation of emotion (Buhle et al., 2014; Silvers et al., 2017). Aberrant function of anterior cingulate and vmPFC are implicated in childhood aggressive behavior and may impede adaptive emotion processing and regulation (Alegria et al., 2016). Additionally, findings from resting-state fMRI studies suggest a similar pattern of aberrant intrinsic functional connectivity in ventral PFC regions associated with aggressive behavior (Broulidakis et al., 2016; Roy et al., 2018; Werhahn et al., 2020; Zhu et al., 2020). Similarly, in a resting-state study of adults, Varkesvisser and colleagues reported reduced connectivity between the dorsolateral PFC and the basolateral subregion of the amygdala in the aggression group relative to controls (Varkevisser et al., 2017). Our finding that reduced dACC and vmPFC intrinsic connectivity is related to increased aggressive behavior severity is consistent with prior task-based and resting-state fMRI studies and is particularly important in the context of the dimensional RDoC approach as prefrontal circuitry subserving multiple functions of cognitive control are impaired in various neuropsychiatric disorders (McTeague et al., 2018). In support of this, in a recent longitudinal study of the developmental trajectories of aggression from 7 to 14 years of age, cognitive control emerged as a key buffering factor of desisting aggression (Hawes et al., 2016). Additionally, frontolimbic connectivity has also been shown to modulate the relationship between amygdala over-reactivity and increased levels negative affect in childhood (Gaffrey et al., 2021). Thus, atypical PFC connectivity may represent a consistent finding across fMRI studies in children with disruptive behavior problems.
While different regions emerged as significant for group differences between children with and without aggression and dimensional associations of connectivity with severity of aggressive behavior, these regions were located within the prefrontal and limbic regions and thus aligned with our a priori hypotheses of frontolimbic dysfunction in childhood aggression. Other resting-state fMRI studies have also reported differences in regions emerging as significant between dimensional and categorical approaches in youths with aggression (Zhou et al., 2015; Aghajani et al., 2017; Roy et al., 2018; Werhahn et al., 2020). Dimensional vs categorical approaches reveal related but distinct features of brain organization as related to resting-state fMRI (Parkes et al., 2020). For instance, while categorical or case-control designs have the potential to assist in identifying biomarkers that are disorder specific, dimensional approaches may capture a wider range of variation in symptomatology, which has clinical implications for understanding the link between brain dysfunction and psychiatric disorders. Here, group differences in connectivity between children with aggression vs healthy controls could indicate a biomarker of risk of being in a group characterized by clinically significant levels of aggressive behavior, while within-group dimensional analysis reveal intrinsic connectivity biomarkers of aggression severity.
Follow-up seed connectivity tests were also conducted for the amygdala ROI, which emerged as significant in the group comparisons. Here, seed-based connectivity analyses in the aggressive behavior group revealed that increasing left amygdala connectivity with PFC and ACC was correlated with increasing severity of aggression. These findings are consistent with a study of adolescents with CD showing over-connectivity between the right basolateral amygdala with ventromedial PFC regions (Aghajani et al., 2017). Similarly, greater amygdala-ACC connectivity was associated with greater levels of emotional liability in 5- to 13 year-old children with ADHD (Hulvershorn et al., 2014). Thus, correlation of severity of aggression with increased intrinsic functional connectivity between the amygdala and prefrontal and anterior cingulate cortices could suggest excessive or inefficient top-down control of amygdala, possibly reflecting the failure to suppress the heightened emotional salience of frustration. This interpretation is consistent with the other two findings in our study: increased amygdala global connectivity in children with aggression relative to controls and reduced global connectivity of dorsal anterior cingulate and medial PFC in children with aggressive behavior.
Given that frontolimbic projections are critical to dampening the acquisition and expression of negatively valenced emotions (Ochsner et al., 2012), amygdala-prefrontal over-connectivity, as reported here, could indicate a compensatory mechanism to modulate emotions in children with high levels of aggressive behavior. Alternatively, given the bidirectional flow of information between the amygdala and PFC structures (Hartley and Phelps, 2010), our finding may also indicate an intrinsic functional architecture reflecting excessive bottom-up signaling or recruitment of cognitive resources toward positive or motivationally salient stimuli at the expense of the top-down regulation of negative affect (Sadeh and Verona, 2008). It is also important to note that, in the current study, decreased global connectivity was observed in the vmPFC and dACC ROIs, but increased seed connectivity to the amygdala was observed in these same regions. It is possible that these regions may be functionally hyper-coupled to the amygdala in children with aggressive behavior and less modulated by other areas of the brain. For instance, increased amygdala-prefrontal connectivity may come at the expense of diminished functional connections between the PFC and other cortical regions.
Study limitations
Some limitations should be considered. First, the sample size was modest and replication of these findings will be needed in larger samples. Replication of findings in large-scale open-access datasets, such as the Adolescent Brain Cognitive Development study (Casey et al., 2018), offers opportunities to test the generalization of neural markers identified in this clinical sample of treatment-seeking youth with aggression to other populations. Second, our sample consisted of predominantly males and showed a male to female ratio that is comparable to reported estimates of male to female ratios for children with disruptive behavior disorders (2–3:1) (Demmer et al., 2017). Given that sex differences in adolescence have been shown to influence the development of the intrinsic functional architecture (Alarcón et al., 2015), particularly amygdala connectivity, an understanding of sex differences may inform future research of the neural features associated with increased risk for aggression. While the current study was not statistically powered or designed to test sex differences, future work is necessary to examine whether boys and girls with aggressive behavior demonstrate unique patterns of intrinsic functional connectivity. Third, the cross-sectional nature of this study is a fundamental limitation. Future longitudinal resting-state fMRI studies will be essential for understanding the effects of intrinsic functional brain networks on the trajectory of childhood maladaptive aggression. Finally, the lack of assessment of pubertal development should be noted as a limitation in the current study. Future resting-state fMRI studies are needed with matched groups based on pubertal development in order to reduce the possibility of group differences in brain developmental stages. Our post hoc analysis with age as a covariate also indicated that age did not significantly affect the association of intrinsic connectivity with aggression, suggesting that these results are robust to and/or stable across changes in neural developmental. Future longitudinal studies will be essential for understanding the developmental effects of puberty on associations between intrinsic functional connectivity and aggressive behavior.
Conclusions
This study is the first to apply a metric of global connectivity to identify underlying disruptions in the intrinsic functional architecture in children seeking treatment for aggressive behavior. Children with aggressive behavior showed over-connectivity in the bilateral amygdala compared to healthy controls. Dimensional analyses revealed associations between increased aggression severity and decreased global connectivity in prefrontal regions, including the dACC and vmPFC. Further, we found that greater aggression severity was associated with decreased left amygdala-prefrontal connectivity. These findings suggest disruptions in frontolimbic networks involved in the cognitive control of emotion, which may increase the risk of aggressive behavior in children. These findings may also inform the development of neural biomarkers of maladaptive aggression.
Supplementary Material
Acknowledgements
We thank Dr. Megan Tudor for subject characterization assessments and Mrs. Emilie Bertschinger, Ms. Tess Gladstone and Ms. Carolyn Marsh for study coordination. This work was supported by the High Performance Computing (HPC) facilities operated by, and the staffs of, the Yale Center for Research Computing and Yale’s W.M. Keck Biotechnology Laboratory.
Contributor Information
Denis G Sukhodolsky, Child Study Center, Yale School of Medicine, New Haven, CT 06520, USA.
Karim Ibrahim, Child Study Center, Yale School of Medicine, New Haven, CT 06520, USA.
Carla B Kalvin, Child Study Center, Yale School of Medicine, New Haven, CT 06520, USA.
Rebecca P Jordan, Child Study Center, Yale School of Medicine, New Haven, CT 06520, USA.
Jeffrey Eilbott, Child Study Center, Yale School of Medicine, New Haven, CT 06520, USA; SurveyBott Consulting, Guilford, CT 06437, USA.
Michelle Hampson, Child Study Center, Yale School of Medicine, New Haven, CT 06520, USA; Department of Radiology and Biomedical Imaging, Yale School of Medicine, New Haven, CT 06520, USA; Department of Psychiatry, Yale School of Medicine, New Haven, CT 06520, USA; Department of Biomedical Engineering, Yale University, New Haven, CT 06520, USA.
Funding
This study was supported by National Institute of Mental Health (NIMH) grant R01MH101514 (D.G.S.), National Center for Advancing Translational Science grant TL1 TR001864 and KL2 TR001862 (K.I.), and NIMH grant T32 MH18268 fellowship (K.I. and C.B.K.). National Institutes of Health grants RR19895 and RR029676 helped fund the HPC cluster that was used for data analysis.
Conflict of interest
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary data is available at SCAN online.
References
- Achenbach T.M., Rescorla L.A. (2001). Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families. [Google Scholar]
- Aghajani M., Klapwijk E.T., van der Wee N.J., et al. (2017). Disorganized amygdala networks in conduct-disordered juvenile offenders with callous-unemotional traits. Biological Psychiatry, 82(4), 283–93. [DOI] [PubMed] [Google Scholar]
- Alarcón G., Cservenka A., Rudolph M.D., Fair D.A., Nagel B.J. (2015). Developmental sex differences in resting state functional connectivity of amygdala sub-regions. NeuroImage, 115, 235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegria A.A., Radua J., Rubia K. (2016). Meta-analysis of fMRI studies of disruptive behavior disorders. American Journal of Psychiatry, 173(11), 1119–30. [DOI] [PubMed] [Google Scholar]
- Ameis S.H., Ducharme S., Albaugh M.D., et al. (2014). Cortical thickness, cortico-amygdalar networks, and externalizing behaviors in healthy children. Biological Psychiatry, 75(1), 65–72. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th edn (DSM-5). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. (2005). Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society B: Biological Sciences, 360(1457), 1001–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch K., Florange J., Herpertz S.C. (2020). Understanding brain mechanisms of reactive aggression. Current Psychiatry Reports, 22(12), Article 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer F., Münte T.F., Göttlich M., Krämer U.M. (2015). Orbitofrontal cortex reactivity to angry facial expression in a social interaction correlates with aggressive behavior. Cerebral Cortex, 25(9), 3057–63. [DOI] [PubMed] [Google Scholar]
- Blair R.J.R. (2016). The neurobiology of impulsive aggression. Journal of Child and Adolescent Psychopharmacology, 26(1), 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J.R., Veroude K., Buitelaar J.K. (2018). Neuro-cognitive system dysfunction and symptom sets: a review of fMRI studies in youth with conduct problems. Neuroscience and Biobehavioral Reviews, 91, 69–90. [DOI] [PubMed] [Google Scholar]
- Brame B., Nagin D.S., Tremblay R.E. (2001). Developmental trajectories of physical aggression from school entry to late adolescence. Journal of Child Psychology and Psychiatry, 42(4), 503–12. [PubMed] [Google Scholar]
- Broulidakis M.J., Fairchild G., Sully K., Blumensath T., Darekar A., Sonuga-Barke E.J.S. (2016). Reduced default mode connectivity in adolescents with conduct disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 55(9), 800–8.e801. [DOI] [PubMed] [Google Scholar]
- Buades-Rotger M., Engelke C., Krämer U.M. (2019). Trait and state patterns of basolateral amygdala connectivity at rest are related to endogenous testosterone and aggression in healthy young women. Brain Imaging and Behavior, 13(2), 564–76. [DOI] [PubMed] [Google Scholar]
- Buades-Rotger M., Göttlich M., Weiblen R., et al. (2021). Low competitive status elicits aggression in healthy young men: behavioral and neural evidence. Social Cognitive and Affective Neuroscience, 16(11), 1123–37.doi: 10.1093/scan/nsab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Vincent J.L. (2007). Unrest at rest: default activity and spontaneous network correlations. NeuroImage, 37(4), 1091–6. [DOI] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wage T.D., et al. (2014). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience, 10(3), 186–98. [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 222. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Cannonier T., Conley M.I., et al. (2018). The Adolescent Brain Cognitive Development (ABCD) study: imaging acquisition across 21 sites. Developmental Cognitive Neuroscience, 32, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor D.F., Newcorn J.H., Saylor K.E., et al. (2019). Maladaptive aggression: with a focus on impulsive aggression in children and adolescents. Journal of Child and Adolescent Psychopharmacology, 29(8), 576–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. (2009). How do you feel - now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10(1), 59–70. [DOI] [PubMed] [Google Scholar]
- Decety J., Michalska K.J., Akitsuki Y. (2008). Who caused the pain? An fMRI investigation of empathy and intentionality in children. Neuropsychologia, 46(11), 2607–14. [DOI] [PubMed] [Google Scholar]
- Decety J., Michalska K.J., Akitsuki Y., Lahey B.B. (2009). Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biological Psychology, 80(2), 203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer D.H., Hooley M., Sheen J., McGillivray J.A., Lum J.A.G. (2017). Sex differences in the prevalence of oppositional defiant disorder during middle childhood: a meta-analysis. Journal of Abnormal Child Psychology, 45(2), 313–25. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–80. [DOI] [PubMed] [Google Scholar]
- Ducharme S., Hudziak J.J., Botteron K.N., et al. (2011). Right anterior cingulate cortical thickness and bilateral striatal volume correlate with child behavior checklist aggressive behavior scores in healthy children. Biological Psychiatry, 70(3), 283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. (2016). Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113(28), 7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Büchel C., Gross J.J. (2015). The neural bases of emotion regulation. Nature Reviews Neuroscience, 16(11), 693–700. [DOI] [PubMed] [Google Scholar]
- Frick P.J. (2003). The Inventory of Callous-Unemotional Traits. New Orleans, LA: University of New Orleans. [Google Scholar]
- Gaffrey M.S., Barch D.M., Luby J.L., Petersen S.E. (2021). Amygdala functional connectivity is associated with emotion regulation and amygdala reactivity in 4- to 6-year-olds. Journal of the American Academy of Child and Adolescent Psychiatry, 60(1), 176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilam G., Lin T., Fruchter E., Hendler T. (2017). Neural indicators of interpersonal anger as cause and consequence of combat training stress symptoms. Psychological Medicine, 47(9), 1561–72. [DOI] [PubMed] [Google Scholar]
- Gratton C., Laumann T.O., Nielsen A.N., et al. (2018). Functional brain networks are dominated by stable group and individual factors, not cognitive or daily variation. Neuron, 98(2), 439–52.e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D.J., Koller J.M., Hampton J.M., et al. (2018). Behavioral interventions for reducing head motion during MRI scans in children. NeuroImage, 171, 234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley C.A., Phelps E.A. (2010). Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology, 35(1), 136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes S.W., Perlman S.B., Byrd A.L., Raine A., Loeber R., Pardini D.A. (2016). Chronic anger as a precursor to adult antisocial personality features: the moderating influence of cognitive control. Journal of Abnormal Psychology, 125(1), 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpertz S.C., Huebner T., Marx I., et al. (2008). Emotional processing in male adolescents with childhood-onset conduct disorder. Journal of Child Psychology and Psychiatry, 49(7), 781–91. [DOI] [PubMed] [Google Scholar]
- Holmes C.J., Hoge R., Collins L., Woods R., Toga A.W., Evans A.C. (1998). Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography, 22(2), 324–33. [DOI] [PubMed] [Google Scholar]
- Hulvershorn L.A., Mennes M., Castellanos F.X., et al. (2014). Abnormal amygdala functional connectivity associated with emotional lability in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 53(3), 351–61.e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim K., Kalvin C., Li F., et al. (2021). Sex differences in medial prefrontal and parietal cortex structure in children with disruptive behavior. Developmental Cognitive Neuroscience, 47, Article 100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T.R., Wang P.S. (2010). Rethinking mental illness. JAMA, 303(19), 1970–1. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. (2012). Fsl. NeuroImage, 62, 782–90. [DOI] [PubMed] [Google Scholar]
- Jensen P.S., Youngstrom E.A., Steiner H., et al. (2007). Consensus report on impulsive aggression as a symptom across diagnostic categories in child psychiatry: implications for medication studies. Journal of the American Academy of Child and Adolescent Psychiatry, 46(3), 309–22. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Axelson D., Perepletchikova F., Brent D., Ryan N. (2016). Schedule for affective disorders and schizophrenia for school aged children: present and lifetime version for DSM-5 (K-SADS-PL). https://www.pediatricbipolar.pitt.edu/resources/instruments [October 8, 2021].
- Lacadie C.M., Fulbright R.K., Rajeevan N., Constable R.T., Papademetris X. (2008). More accurate Talairach coordinates for neuroimaging using non-linear registration. NeuroImage, 42(2), 717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier L.M., Cardinale E.M., Van Meter J.W., Marsh A.A. (2014). Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry, 71(6), 627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh A.A., Finger E.C., Mitchell D.G.V., et al. (2008). Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry, 165(6), 712–20. [DOI] [PubMed] [Google Scholar]
- McTeague L.M., Laplante M.-C., Bulls H.W., Shumen J.R., Lang P.J., Keil A. (2018). Face perception in social anxiety: visuocortical dynamics reveal propensities for hypervigilance or avoidance. Biological Psychiatry, 83(7), 618–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin J.C., Philippi C.L., Wolf R.C., Baskaya M.K., Koenigs M. (2015). Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biological Psychiatry, 77(3), 276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler I., Reinbold C., Wankerl J., Seifritz E., Ball T. (2013). Structural basis of empathy and the domain general region in the anterior insular cortex. Frontiers in Human Neuroscience, 7, 177.doi: 10.3389/fnhum.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagin D., Tremblay R.E. (1999). Trajectories of boys’ physical aggression, opposition, and hyperactivity on the path to physically violent and nonviolent juvenile delinquency. Child Development, 70(5), 1181–96. [DOI] [PubMed] [Google Scholar]
- Noordermeer S.D.S., Luman M., Oosterlaan J. (2016). A systematic review and meta-analysis of neuroimaging in Oppositional Defiant Disorder (ODD) and Conduct Disorder (CD) taking Attention-Deficit Hyperactivity Disorder (ADHD) into account. Neuropsychology Review, 26(1), 44–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papademetris X., Jackowski A.P., Schultz R.T., Staib L.H., Duncan J.S. (2004). Integrated intensity and point-feature nonrigid registration. Lecture Notes in Computer Science, 3216, 763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes L., Satterthwaite T.D., Bassett D.S. (2020). Towards precise resting-state fMRI biomarkers in psychiatry: synthesizing developments in transdiagnostic research, dimensional models of psychopathology, and normative neurodevelopment. Current Opinion in Neurobiology, 65, 120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L., Fairchild G., Goodyer I.M., et al. (2010). Neural abnormalities in early-onset and adolescence-onset conduct disorder. Archives of General Psychiatry, 67(7), 729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S.B., Jones B.M., Wakschlag L.S., Axelson D., Birmaher B., Phillips M.L. (2015). Neural substrates of child irritability in typically developing and psychiatric populations. Developmental Cognitive Neuroscience, 14, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron, 48(2), 175–87. [DOI] [PubMed] [Google Scholar]
- Raine A., Dodge K., Loeber R., et al. (2006). The reactive–proactive aggression questionnaire: differential correlates of reactive and proactive aggression in adolescent boys. Aggressive Behavior, 32(2), 159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repple J., Pawliczek C.M., Voss B., et al. (2017). From provocation to aggression: the neural network. BMC Neuroscience, 18(1), Article 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J.C., De Brito S.A. (2016). Cortical and subcortical gray matter volume in youths with conduct problems ameta-analysis. JAMA Psychiatry, 73(1), 64–72. [DOI] [PubMed] [Google Scholar]
- Roy A.K., Bennett R., Posner J., Hulvershorn L., Castellanos F.X., Klein R.G. (2018). Altered intrinsic functional connectivity of the cingulate cortex in children with severe temper outbursts. Development and Psychopathology, 30(2), 571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh N., Verona E. (2008). Psychopathic personality traits associated with abnormal selective attention and impaired cognitive control. Neuropsychology, 22(5), 669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe D., Lyden H., Gimbel S.I., et al. (2018). Longitudinal associations between family aggression, externalizing behavior, and the structure and function of the amygdala. Journal of Research on Adolescence, 28(1), 134–49. [DOI] [PubMed] [Google Scholar]
- Scheinost D., Benjamin J., Lacadie C.M., et al. (2012). The intrinsic connectivity distribution: a novel contrast measure reflecting voxel level functional connectivity. NeuroImage, 62(3), 1510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A., Cohen J.D., Botvinick M.M. (2016). Dorsal anterior cingulate cortex and the value of control. Nature Neuroscience, 19(10), 1286–91. [DOI] [PubMed] [Google Scholar]
- Silvers J.A., Insel C., Powers A., et al. (2017). vlPFC-vmPFC-amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cerebral Cortex (New York, N.Y.: 1991), 27(7), 3502–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaragdi A., Cornwell H., Toschi N., et al. (2017). Sex differences in the relationship between conduct disorder and cortical structure in adolescents. Journal of the American Academy of Child and Adolescent Psychiatry, 56(8), 703–12. [DOI] [PubMed] [Google Scholar]
- Sukhodolsky D.G., Wyk B.C.V., Eilbott J.A., et al. (2016). Neural mechanisms of cognitive-behavioral therapy for aggression in children and adolescents: design of a randomized controlled trial within the national institute for mental health research domain criteria construct of frustrative non-reward. Journal of Child and Adolescent Psychopharmacology, 26(1), 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen S., Collins P.F., Weiss H., Luciana M. (2021). The longitudinal association between externalizing behavior and frontoamygdalar resting-state functional connectivity in late adolescence and young adulthood. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 62(7), 857–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng W.L., Deveney C.M., Stoddard J., et al. (2019). Brain mechanisms of attention orienting following frustration: associations with irritability and age in youths. American Journal of Psychiatry, 176(1), 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwal T., Eilbott J., Kelly C., et al. (2021). Stability and similarity of the pediatric connectome as developmental measures. NeuroImage, 226, Article 117537. [DOI] [PubMed] [Google Scholar]
- Varkevisser T., Gladwin T.E., Heesink L., van Honk J., Geuze E. (2017). Resting-state functional connectivity in combat veterans suffering from impulsive aggression. Social Cognitive and Affective Neuroscience, 12(12), 1881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E., Sebastian C.L., Dadds M.R., et al. (2012). Amygdala response to preattentive masked fear in children with conduct problems: the role of callous-unemotional traits. American Journal of Psychiatry, 169(10), 1109–16. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N., Pfeifer J.H., Flournoy J.C., Hernandez L.M., Dapretto M. (2019). Affective reactivity during adolescence: associations with age, puberty and testosterone. Cortex, 117, 336–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (1997). WAIS-III Administration and Scoring Manual. San Antonio, TX: The Psychological Association. [Google Scholar]
- Werhahn J.E., Mohl S., Willinger D., et al. (2020). Aggression subtypes relate to distinct resting state functional connectivity in children and adolescents with disruptive behavior. European Child & Adolescent Psychiatry, 30(4), 1237–49.doi: 10.1007/s00787-020-01601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. (2014). Permutation inference for the general linear model. NeuroImage, 92, 381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M.W., Jbabdi S., Patenaude B., et al. (2009). Bayesian analysis of neuroimaging data in FSL. NeuroImage, 45(1 Suppl), S173–86. [DOI] [PubMed] [Google Scholar]
- Yan C.G., Cheung B., Kelly C., et al. (2013). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage, 76, 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R., Mobbs D., Seymour B., Rowe J.B., Calder A.J. (2014). The neural signature of escalating frustration in humans. Cortex, 54(1), 165–78. [DOI] [PubMed] [Google Scholar]
- Zhou J., Yao N., Fairchild G., et al. (2015). Disrupted default mode network connectivity in male adolescents with conduct disorder. Brain Imaging and Behavior, 10(4), 995–1003.doi: 10.1007/s11682-015-9465-6. [DOI] [PubMed] [Google Scholar]
- Zhu W., Zhou X., Xia L.X. (2019). Brain structures and functional connectivity associated with individual differences in trait proactive aggression. Scientific Reports, 9(1), Article 7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Wang K., Cao A., Zhang Y., Qiu J. (2020). Personality traits and negative affect mediate the relationship between cortical thickness of superior frontal cortex and aggressive behavior. Neuroscience Letters, 718, Article 134728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


