Abstract
Background
Lung cancer is the leading cause of cancer-related death globally and in South Africa. Historically, the majority of patients diagnosed with lung cancer are incurable at presentation.
Objectives
To assess the tumour, nodes, metastasis (TNM) staging of lung cancer in a centre with access to both positron emission tomography-computed tomography (PET-CT) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) using a structured diagnostic approach and to compare results with a historical cohort from 2009 from the same hospital.
Methods
A retrospective descriptive observational study was performed using the registry of a high-volume tertiary hospital’s weekly multidisciplinary thoracic oncology meeting (MDT). A structured diagnostic approach was used for staging purposes. All patients with a tissue diagnosis of primary lung cancer and adequate imaging (chest CT and/or PET-CT) who presented at the MDT during the period from 1 January - 31 December 2019 were included. Final staging and tissue diagnoses were documented and compared with a historical cohort from 2009 from the same institution.
Results
Adenocarcinoma was the most common subtype (38.8%; n=116). Less than a tenth of patients (6.3%; n=16/254) with non-small cell lung cancer had potentially curable lung cancer (stage IA to IIIA) at presentation, significantly less than the 2009 cohort (14.5%; n=25/173; p=0.007). The most common procedure administered on patients was transthoracic needle aspiration (37.54%; n=112), followed by conventional bronchoscopic needle aspiration or biopsy (20.4%; n=61), and EBUS-TBNA (17.1%; n=51/299). After PET-CT, 19/30 cases were upstaged including 9/18 from potentially resectable to unresectable. Two of these cases were down-staged to potentially resectable following EBUS-TBNA.
Conclusion
There was a significant decline in resectable and potentially curable lung cancer at presentation over a 10-year period. PET-CT and EBUS-TBNA improved the accuracy of non-small cell lung cancer staging among patients with resectable and potentially curable lung cancer but have exposed a higher stage profile.
Keywords: lung cancer, cancer staging, endobronchial ultrasound, positron emission tomography
Background
Lung cancer is the leading cause of cancer-related death in South Africa (SA) and globally.[1,2] Western Cape Province has the highest lung cancer mortality rate in the country at 27 per 100 000 population.[3] High rates of smoking, a large HIV burden and an array of occupational exposures contribute to the problem.[4,5]
A previous study at Tygerberg Hospital in Cape Town, SA, found that 14.5% of patients with non-small cell lung cancer were potentially curable at presentation (stage I to IIIA), with a much lower proportion of patients potentially resectable at presentation (6.2%).[6] The relatively low proportion was attributed to the long subclinical course and late presentation with clinically advanced tumours and diagnostic delay in a high tuberculosis (TB) burden setting.[6]
Lung cancer is currently staged according to the eighth edition of the International Union Against Cancer, Tumour, Node, Metastasis Classification staging system (TNM).[7] Patients with non-small cell lung cancer (NSCLC) who present with stage I and II disease are generally offered surgical resection and chemotherapy. Stage IIIA is managed with chemotherapy and radiotherapy with curative intent. Higher stage disease (stage IIIB to IVB) is typically offered palliation.[7] Positron emission tomography-computed tomography (PET-CT) scanning and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) are now routinely employed in the staging of potentially curable lung cancer.[8] Malignant tumours are often highly metabolically active, with increased glucose metabolism and consequently, 18F-fluorodeoxyglucose (FDG) uptake can be detected as avidity on the PET-CT. Likewise, nodal spread and distant metastases that may not be apparent on routine CT may be detected as ‘hot spots’. As a staging modality, PET-CT has been shown to up-stage a proportion of patients with presumed curable lung cancer at presentation.[9–12] The modalities of PET-CT and EBUS-TBNA became readily available locally in 2012.
Lymph nodes are usually staged radiologically unless operability depends on it, in which case, EBUS-TBNA is performed. This plays a pivotal role in sampling lymph nodes that have been identified by means of routine contrasted chest CT or on PET-CT as potentially involved.[13,14] The use of PET-CT and EBUS-TBNA in addition to CT scan improves the accuracy of lung cancer staging, particularly in early stage disease.[15]
The objectives of the present study were to assess the TNM staging (eighth edition) of lung cancer in a centre with access to both PET-CT and EBUS-TBNA using a structured diagnostic approach and comparing results with a historical cohort from 2009 from the same institution. The secondary objective was to describe the effect of PET-CT and EBUS-TBNA on the staging of individual patients who underwent these investigations.
Methods
A retrospective descriptive study was performed using the registry of Tygerberg Hospital’s weekly multidisciplinary team (MDT) thoracic oncology meeting. All patients with a tissue diagnosis of primary lung cancer and adequate imaging (chest CT and/or PET-CT) who presented during the period from 1 January 2019 - 31 December 2019 were included. Tygerberg Hospital, a 1 380-bed facility, is one of two referral centres in Cape Town rendering a tertiary service to a population of ~1.5 million people. The study was approved by the Stellenbosch University Health Research Ethics Committee (ref. no. S19/12/286).
The inclusion criteria were age ≥18 years old, tissue confirmation of primary lung cancer, and adequate imaging to perform TNM staging. The exclusion criteria were age <18 years old, tissue diagnoses other than primary lung cancer, no tissue diagnosis available, and incorrect or missing data.
Patients were identified from the existing Division of Pulmonology’s MDT registry. Data were extracted from the division’s records, the Picture Archiving and Communication Systems (PACS) of the various hospitals and the National Health Laboratory Service (NHLS) TrakCare web results viewer. These data included demographics, HIV status, method of tissue diagnosis, final diagnosis, staging according to CT scan and PET-CT where available, results of follow-up lymph node sampling where available and outcome at MDT meeting (including final TNM staging). The results of patients with non-small cell lung cancer were compared with a historical cohort from the same institution from 2009.[6]
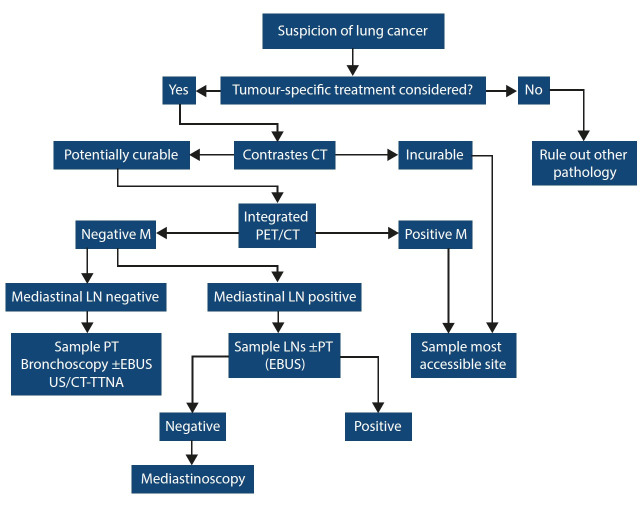
The hospital used a structured algorithm for the diagnosis and staging of lung cancer at the time (Fig. 1). Patients with potentially curable disease underwent PET-CT. If PET-CT identified metastasis, the most accessible site was sampled and patients were referred to oncology for palliative care. If no mediastinal lymph nodes were identified on PET-CT, the most accessible site was sampled for tissue diagnosis and patients were referred to surgery with curative intent. If mediastinal lymph nodes were deemed positive on PET-CT, patients underwent conventional TBNA or EBUS-TBNA depending on accessibility of lymph nodes. If patients remained potentially curable after sampling, then they were referred for mediastinoscopy to sample PET avid but EBUS-TBNA negative lymph nodes. In patients with incurable disease, EBUS-TBNA was only used to access the most accessible site as a last resort.
Fig. 1.
Staging algorithm for lung cancer at Tygerberg Hospital.
CT = computed tomography
PET = positron emission tomography
M = metastasis
LN = lymph node
EBUS = endobronchial ultrasound
TTNA = transthoracic needle aspirate
The average waiting time at our institution was two weeks for contrasted CT in inpatients in 2019. The waiting time for PET-CT was one week. Our institution has an intervention list every day of the week. The waiting time for intervention in potentially curable cases was a day during the week. Adequacy of the samples for a laboratory diagnosis of malignancy was assessed routinely at the time of intervention as our institution has rapid on-site cytology (ROSE) available.
All descriptive numerical data with a normal distribution were described using means and standard deviation (SD), whereas non-normal data were described using median and interquartile ranges (IQR). Chi-squared or Fisher’s exact test were used to identify statistical significance for all categorical outcomes. Statistical significance was set at p<0.05 and a 95% confidence interval (CI) was used. Data analysis was performed using EpiCalc 2000, version 1.02 (Brixton Books, England).
Results
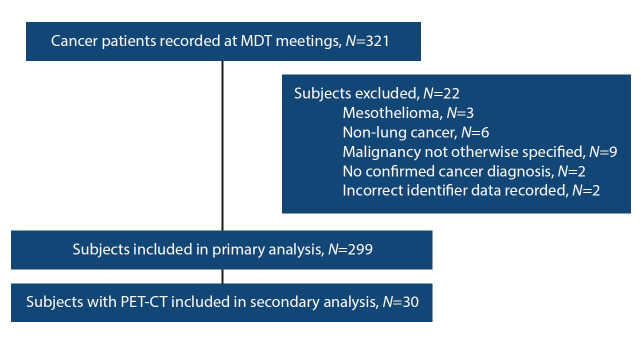
Records of the 321 cancer patients discussed at the MDT meeting during the present study period were reviewed (Fig. 2). Twenty-two subjects were excluded, including cases where the type of malignancy (primary v. secondary) could not be specified (n=9), cancers other than primary lung cancer (n=6), mesothelioma (n=3), cases with no tissue-confirmed cancer diagnosis (n=2), and cases with incorrect or missing identifying data (n=2).
Fig. 2.
Consort diagram of patients included in the study and analyses.
A total of 299 patients was included in the study, with a mean (SD) of 60.2 (9.9) years. The majority of patients (62.9%; n=188) were male (Table 1). Less than a tenth of patients (6.0%) were HIV positive and 34.1% (n=102) had an unknown HIV status. Thirty subjects who had undergone PET-CT were included in the subgroup analysis.
Table 1. Baseline characteristics of study population and method of diagnosis (N=299).
| Characteristic | n (%) |
| Baseline characteristics | |
| Age, mean (SD) | 60.2 (9.9) |
| Male | 188 (62.9) |
| Female | 111 (37.1) |
| HIV status | |
| Positive | 18 (6.0) |
| CD4 cell count, mean (SD) | 468 (262) |
| Negative | 179 (60.0) |
| Unknown | 102 (34.1) |
| Diagnostic investigation | |
| Bronchoscopy | 112 (37.5) |
| Conventional bronchoscopy | 60 (20.1) |
| EBUS-TBNA | 51 (17.1) |
| Bronchial washings | 3 (1.0) |
| Bronchial brushing | 3 (1.0) |
| Transthoracic procedures | 136 (45.5) |
| TTNA | 109 (36.5) |
| Thoracentesis | 19 (6.4) |
| Pleural biopsy | 6 (2.0) |
| Tru-cut biopsy | 2 (0.7) |
| Interventional radiology | 9 (3.0) |
| CT-guided biopsy | 9 (3.0) |
| Other FNA | 30 (10.0) |
| Lymph node FNA | 24 (8.0) |
| FNA of metastasis | 3 (1.0) |
| Liver FNA | 3 (1.0) |
| Surgical | 9 (3.0) |
| Bone Biopsy | 5 (1.7) |
| Craniotomy | 2 (0.7) |
| VATS | 1 (0.3) |
| Lung Resection | 1 (0.3) |
| Other | 3 (1.0) |
| Sputum | 3 (1.0) |
CT = computed tomography
EBUS = endobronchial ultrasound
FNA = fine needle aspiration
SD = standard deviation
TBNA = transbronchial nodal aspiration
TTNA = transthoracic nodal aspiration
VATS = video-assisted thoracoscopic surgery
* Unless otherwise specified
Transthoracic procedures (n=136) were the most common techniques employed for histological diagnosis of lung cancer, followed by bronchoscopy (n=112). Of the latter, EBUS-TBNA was used in 17.1% (n=51) of the cases, of which 46 were used primarily for diagnostic purposes, one for staging and diagnostic purposes, and five for staging purposes. Other techniques (Table 1) included fine needle aspiration (FNA) of lymph nodes, metastases to other sites including the liver (n=30), CT-guided biopsy (n=9), surgical techniques such as bone biopsy, craniotomy, video-assisted thorascopic surgery and lung resection (n=9) and sputum cytology (n=3). The most common histological subtype of lung cancer was adenocarcinoma (n=116), followed by squamous cell carcinoma (n=92) (Table 2).
Table 2. Subtypes of lung cancer in the study population (N=299).
| Histological subtype | n (%) |
| Non-small cell lung cancer | 254 (84.9) |
| Adenocarcinoma | 116 (38.8) |
| Squamous carcinoma | 92 (30.8) |
| Large cell/poorly differentiated | 46 (15.4) |
| Small cell/neuroendocrine lung cancer | 44 (14.7) |
| Small cell lung cancer | 39 (13.0) |
| Large cell neuroendocrine | 5 (1.7) |
| Other | 1 (0.3) |
| Carcinoid tumour | 1 (0.3) |
The final TNM (8th edition) stage of all patients as well as those ultimately diagnosed with NSCLC are summarised in Table 3. Most patients had stage IV lung cancer (n=229). Less than a tenth of patients (1.2%; n=3) with NSCLC had potentially operable lung cancer according to final staging (stage I or II). In comparison, 7.5% (n=13/75) of patients in the 2009 study at our institution had potentially operable lung cancer (odds ratio (OR) 0.15; 95% confidence interval (CI) 0.04 - 0.52); p<0.001). Patients were also less likely to have potentially curable lung cancer (up to and including stage IIIa) in our cohort at presentation (6.3%; n=16/254) compared with the 2009 cohort where the TNM 6th edition was used for staging (14.5%; n=25/173; OR 0.40; 95% CI 0.21 - 0.77; p=0.007).
Table 3. Tumour, node, metastasis (TNM) staging of the study population overall and by histological subtype.
| Staging |
Overall
(N=299), n(%) |
Non-small cell (N=254), n(%) | Small cell (N=44), n(%) |
| IA | 3 (1.0) | 3 (1.2) | 0 |
| IB | 0 | 0 | 0 |
| IIA | 0 | 0 | 0 |
| IIB | 1 (0.3) | 0 | 0 |
| IIIA | 13 (4.3) | 13 (5.1) | 0 |
| IIIB | 18 (6.0) | 14 (5.5) | 4 (7.4) |
| IIIC | 35 (11.7) | 29 (11.4) | 6 (11.1) |
| IVA | 149 (49.8) | 128 (50.4) | 21 (38.9) |
| IVB | 80 (26.8) | 67 (26.4) | 13 (24.1) |
The staging of 30 patients who underwent PET-CT was further assessed. PET-CT was found to have increased the staging in 19 of 30 cases. Nine of 18 cases were up-staged from potentially curable to incurable. Further investigation after PET-CT down-staged 6 of 30 cases. Two of these cases went from not resectable to potentially resectable in both cases this was based on EBUS-TBNA sampling.
Discussion
We found a clinically meaningful and statistically significant decline in the number of resectable and potentially curable lung cancer patients managed at our institution between 2009 and 2019. The introduction of the two staging modalities of PET-CT and EBUS-TBNA expectedly played a major role in this decline among patients with resectable and potentially curable lung cancer through improved accuracy of staging and detection of advanced disease (both nodal and distant) that would have gone undetected prior to their introduction into routine service.
PET-CT improved staging accuracy. Half of patients with potentially surgically curable lung cancer on initial staging were up-staged after PET-CT and found to be unresectable. This is important as it avoided unnecessary and potentially harmful surgery and expedited referral to oncology services. However, up-staging by PET-CT may be overly sensitive as demonstrated in 2 of the 9 patients who were subsequently down-staged back to potentially operable after further investigation. This is of particular concern in our setting where we have a high burden of TB and other respiratory diseases, which can account for these false positives.[9,10] A study performed at our institution showed that the diagnostic accuracy of PET-CT in the evaluation of pulmonary mass lesions using the conventional maximum standardised uptake value (SUVmax) cut-off of 2.5 was reduced in a TB-endemic area.[16] An SUVmax cut-off of 5.0 has a higher specificity and diagnostic accuracy for malignancy, with a comparable sensitivity.[16] This study also confirmed the ongoing trend towards adenocarcinoma over squamous cell carcinoma as the predominant subtype in lung cancer.[6,17,18]
The prognosis for lung cancer is directly related to stage at the time of diagnosis and ranges from 92% (stage IA) to 0% (stage IVB), illustrating the importance of early diagnosis.[7] Our data unfortunately once again highlight the very low proportion of patients who present with curable lung cancer in SA compared with developed countries such as the USA, where 15 - 25% of patients are treated with curative intent.[19]
There are many contributing factors to the late presentation, most notably the high incidence of TB and subsequent post-TB lung disease, often resulting in empiric treatment for TB in patients who present with symptomatic lung cancer due to apparent overlap in symptoms and imaging. Common presenting symptoms include weight loss, fever, cough, haemoptysis, breathlessness and increased sputum production. Radiologically, both disease entities include parenchymal disease, lymphadenopathy, miliary disease, pleural effusion, cavitation and pulmonary nodules. Granulomas from previous TB infection and those due to active disease frequently manifest as solitary pulmonary nodules in the lung.[20] Primary care physicians may be inexperienced and the majority of chest radiographs performed at that level are not reported by specialist radiologists. The high burden of HIV may also be an important contributor, as HIV has been shown to be associated with incurable lung cancer at presentation.[5] The HIV status was unknown in a third of our patients, which limited any meaningful conclusions in this regard. In our present study population, there were no delays from initial CT diagnosis to final diagnosis in potentially curable lung cancer as the average waiting time was 3 weeks from the initial CT to diagnosis in patients admitted to hospital. Limited access and long waiting times to imaging modalities such as CT scans in a poorly resourced and overburdened healthcare system undoubtedly add to the problem.[4] Despite a protracted preclinical course, even regular chest radiographs have been shown to be ineffective in screening for early lung cancer.[19] It merely introduces so-called lead time bias without any real impact on mortality.[21]
The recently released recommendations for lung cancer screening in southern Africa may be an important step in improving cure rates for lung cancer through early detection.[22] These guidelines recommend annual screening by low-dose CT for patients between 55 and 74 years of age who are current or former smokers (having quit within the preceding 15 years), with a 30 pack-year history of smoking and no history of lung cancer, who are willing and able to undergo further investigation and screening. However, such a programme is resource intensive and may not be practical in an overburdened public sector with already poor access to CT services. We postulate that a major reason the USA has a higher percentage of potentially curable lung cancer at presentation is because post the National Lung Screening Trial (NLST), low-dose CT (LDCT) screening was used, which also showed an all-cause mortality benefit.[23]
Preventative measures, most importantly smoking cessation, must continue to be promoted while healthcare workers must maintain a high index of suspicion with early referral of suspected cases.
Study strength and limitations
Our study has certain strengths, most notably the fact that it was performed in a high-volume centre that served the same drainage area and hence comparable patient demographics in 2009 and 2019. The limitations of the present study included the facts that it was conducted retrospectively, smoking status of patients was not recorded, HIV testing was incomplete and the surgical pathology was not available to see how many patients were actually under-staged in 2009. The present study included patients referred to our tertiary service and it is likely that some inoperable patients, especially those with poor functional status, were not referred. It should be noted that there was a change in staging edition of the TNM staging between 2009 and 2019. In 2009, the cohort was staged according to the 6th edition TNM staging to allow for uniformity in staging as the 7th edition was used at the health institution in the latter part of 2009. We can only postulate potential other reasons for higher numbers of resectable and potential curable lung cancer in 2009, but the difference remains clinically significant, as the nodal staging did not change significantly from the 6th or 7th to the 8th edition of the TNM staging system.[7] It is unlikely that radiologically lymph node interpretation was misinterpreted as the study specifically looked at radiological findings in lung cancer as well as extensively.
Conclusion
We found a clinical meaningful and statistically significant decline in the number of resectable and potentially curable lung cancer patients managed at our institution between 2009 and 2019. PET-CT and EBUS-TBNA have improved the accuracy of lung cancer staging for NSCLC at presentation among patients with potentially curable disease, but exposed a higher TNM stage profile and thus a significantly lower chance of having operable or potentially curable lung cancer. Preventative measures, most importantly smoking cessation, must continue to be promoted aggressively while healthcare workers must maintain a high index of suspicion with early referral of suspected cases.
Acknowledgments
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Bello B, Fadahun O, Kielkowski D, Nelson G. Trends in lung cancer mortality in South Africa: 1995-2006. BMC Public Health. 2011;11(1):209. doi: 10.1186/1471-2458-11-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Made F, Wilson K, Jina R, et al. Distribution of cancer mortality rates by province in South Africa. Cancer Epidemiol. 2017;51:56–61. doi: 10.1016/j.canep.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Sheridan J, Collins AM. Adult lung cancer in Southern Africa: Epidemiology and aetiology. Am J Respir Med. 2013;8(2):10–12. [Google Scholar]
- 5.Koegelenberg CFN, van der Made T, Taljaard JJ, Irusen EM. The impact of HIV infection on the presentation of lung cancer in South Africa. S Afr Med J. 2016;106(7):666–668. doi: 10.7196/SAMJ.2016v106i7.10737. [DOI] [PubMed] [Google Scholar]
- 6.Nanguzgambo AB, Aubeelack K, von Groote-Bidlingmaier F, et al. Radiologic features, staging, and operability of primary lung cancer in the Western Cape, South Africa. J Thorac Oncol. 2011;6(2):343–350. doi: 10.1097/JTO.0b013e3181fd40ec. [DOI] [PubMed] [Google Scholar]
- 7.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151(1):193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Madsen PH, Holdgaard PC, Christensen JB, Høilund-Carlsen PF. Clinical utility of F-18 FDG PET-CT in the initial evaluation of lung cancer. Eur J Nucl Med Mol Imaging. 2016;43(11):2084–2097. doi: 10.1007/s00259-016-3407-4. [DOI] [PubMed] [Google Scholar]
- 9.Hochhegger B, Rafael G, Alves T, Irion KL, Fritscher CC, Fritscher LG. PET/CT imaging in lung cancer: Indications and findings. J Bras Pneumol. 2015;41(3):264–274. doi: 10.1590/S1806-37132015000004479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpi S, Ali JM, Tasker A, Peryt A, Aresu G, Coonar AS. The role of positron emission tomography in the diagnosis, staging and response assessment of non-small cell lung cancer. Ann Transl Med. 2018;6(5):95. doi: 10.21037/atm.2018.01.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritz E, Vanholder R, Uk M, Sever U, Erek E, Lameire N. Disease of the month. J Am Soc Nephrol. 2000;8(11):1553–1561. doi: 10.1681/ASN.V1181553. http://jasn.asnjournals.org/content/11/8/1553.full [DOI] [PubMed] [Google Scholar]
- 12.van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: The PLUS multicentre randomised trial. Lancet. 2002;359(9315):1388–1393. doi: 10.1016/s0140-6736(02)08352-6. [DOI] [PubMed] [Google Scholar]
- 13.Navani N, Nankivell M, Lawrence DR, et al. Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: An open-label, pragmatic, randomised controlled trial. Lancet Respir. 2015;3(4):282–289. doi: 10.1016/S2213-2600(15)00029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Liao W, Wu B, Chen C. Endobronchial ultrasound changed the world of lung cancer patients: A 11-year institutional experience. PLoS ONE. 2015;10(11):e0142336. doi: 10.1371/journal.pone.0142336Jones. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones GS, Baldwin DR. Recent advances in the management of lung cancer. R Coll physicians. 2018;18(2):s41–s46. doi: 10.7861/clinmedicine.18-2s-s41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du Toit R, Shaw JA, Irusen EM, von Groote-Bidlingmaier F, Warwick JM, Koegelenberg CFN. The diagnostic accuracy of integrated positron emission tomography/computed tomography in the evaluation of pulmonary mass lesions in a tuberculosis-endemic area. S Afr Med J. 2015;105(12):1049. doi: 10.7196/SAMJ.2015.v105i12.10300. [DOI] [PubMed] [Google Scholar]
- 17.Koegelenberg CFN, Aubeelack K, Nanguzgambo AB, et al. Adenocarcinoma the most common cell type in patients presenting with primary lung cancer in the Western Cape. S Afr Med J. 2011;101(5):321. doi: 10.7196/samj.4554. [DOI] [PubMed] [Google Scholar]
- 18.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality. JAMA. 2011;306(17):1865. doi: 10.1001/jama.2011.1591. [DOI] [PubMed] [Google Scholar]
- 20.Hahm CR, Park HY, Jeon K, et al. Solitary pulmonary nodules caused by Mycobacterium tuberculosis and Mycobacterium avium complex. Lung. 2010;188(1):25–31. doi: 10.1007/s00408-009-9203-1. [DOI] [PubMed] [Google Scholar]
- 21.Brenner DJ. Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology. 2004;231(2):440–445. doi: 10.1148/radiol.2312030880. [DOI] [PubMed] [Google Scholar]
- 22.Koegelenberg CFN, Dorfman S, Schewitz I, et al. Recommendations for lung cancer screening in Southern Africa. J Thorac Dis. 2019;11(9):3696–3703. doi: 10.21037/jtd.2019.08.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The National Lung Screening Trial Research Team. 21. Vol. 368. N Engl J Med; 2013. Results of initial low-dose computed tomographic screening for lung cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]