Characterized by episodic airway obstruction, asthma requires spirometry to determine disease severity and therapeutic response.1 Low level of pre-bronchodilator forced expiratory volume in 1 second (FEV1) is associated with difficult-to-treat disease and increased risk of exacerbation rates and mortality.2 It has been suggested that improvement in pre-bronchodilator FEV1 > 230 mL is perceived by patients as an indication of meaningful treatment effect (as in perception of change in their asthma, in questionnaire format at the end of treatment).3
In the Phase 3 LIBERTY ASTHMA QUEST study (NCT02414854) which enrolled patients aged ≥12 years with uncontrolled, moderate-to-severe asthma, add-on dupilumab almost halved the annualized rate of severe asthma exacerbations (AER) over the course of the 52-week treatment period.4 Additionally, patients’ pre-bronchodilator FEV1 increased by 320–340 mL following 12 weeks of treatment. Those patients with evidence of a type 2 inflammatory asthma phenotype (blood eosinophils ≥150 cells/µL or fractional exhaled nitric oxide ≥25 parts per billion at study baseline) demonstrated the best treatment responses.4 A post hoc analysis of the QUEST study demonstrated that dupilumab vs placebo manifested better lung function, such as pre- and post-bronchodilator FEV1, forced vital capacity, and forced expiratory flow at 25–75% of pulmonary volume.5 Spirometric improvements were sustained consistently over 3 years of treatment.6
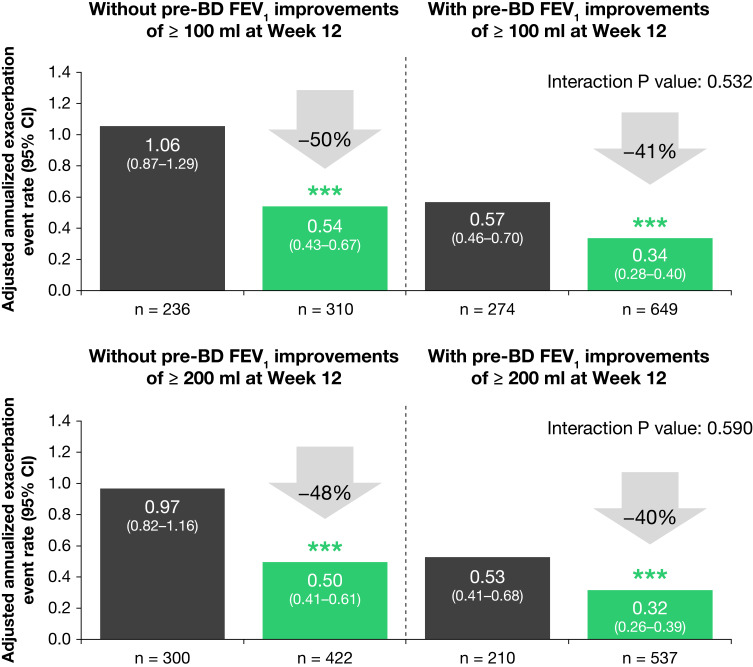
In this post hoc analysis of QUEST, we assessed the effect of dupilumab on AER in those patients who achieved clinically meaningful improvements in pre-bronchodilator FEV1 and in those who did not.
In QUEST, patients were randomized 2:2:1:1 to receive add-on subcutaneous dupilumab 200 mg/300 mg or matched-volume placebo every two weeks for 52 weeks. The study was conducted between April 27, 2015, and November 23, 2017, in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guideline, and applicable regulatory requirements. An independent data and safety monitoring committee conducted blinded monitoring of patient safety data. The local institutional review board or ethics committee at each study center oversaw trial conduct and documentation. All patients provided written informed consent before participating in the trial. The Institutional Review Board (IRB) of the study was the Copernicus Group. For the analyses presented here, the QUEST study population consisted of patients aged ≥12 years old with a type 2 inflammatory asthma phenotype. Analyses were done on the combined 200/300 mg dupilumab vs combined placebo treatment groups. Study populations were stratified into subgroups with and without clinically meaningful improvements of ≥100 or ≥200 mL in pre-bronchodilator FEV1 from study baseline at Week 12 of treatment. The subgroup of patients without improvements included patients with lower levels of improvements (<100 or 200 mL) and patients with no improvements at all. We analyzed the adjusted AER in patients from QUEST using a negative binomial regression model derived with the total number of events onsets from on or after Week 12 up to Week 52 or last contact date as the response variable, and assigned intervention, age, regions, baseline eosinophil strata, baseline inhaled corticosteroid dose level, and the number of exacerbations in the previous year and from baseline to Week 12 as covariates.
Of the 1902 patients randomized in QUEST, 1469 (dupilumab: 959; placebo: 510) with a type 2 inflammatory asthma phenotype were included in these analyses. Among these patients, demographic and disease characteristics at study initiation were comparable between treatment groups (Supplementary Table 1), with the exception of FeNO levels, which were higher in the subgroup of patients who showed improvement in lung function.
Patients on dupilumab vs placebo had improvements in pre-bronchodilator FEV1 after treatment initiation. In the dupilumab group, 649 (68%) patients had FEV1 improvements ≥100 mL, and 537 (57%) had FEV1 improvements ≥200 mL at Week 12, vs corresponding figures of 274 (54%) and 210 (41%) patients in the placebo group. Improvements in lung function (where observed) and reduction in exacerbation events did not follow the same cadence – improved lung function occurred within weeks of treatment with dupilumab whereas the reduction in AER was required to be evaluated over the course of the 52 weeks-treatment period.
Dupilumab vs placebo reduced AER, irrespective of the pre-bronchodilator FEV1 response to treatment at Week 12 (Figure 1). Compared with placebo, dupilumab significantly reduced AER by 41% and 40% in patients achieving ≥100 and ≥200 mL FEV1 improvements, respectively (p < 0.001 for both comparisons). In patients with treatment responses <100 mL and <200 mL, dupilumab vs placebo significantly reduced AER by 50% and 48%, respectively (p < 0.0001 for both comparisons). Subgroup analysis demonstrated that the impact of dupilumab in the reduction of AER was comparable between patients who experienced improvements in lung function >100 and 200 mL, and those that did not (interaction p value >0.05). Of note, very low exacerbation rates were found in patients treated with dupilumab who also showed improvements in lung function.
Figure 1.
Adjusted annualized rate of severe asthma exacerbations in patients with and without clinically meaningful improvements in pre-bronchodilator FEV1 during the QUEST study (patients with uncontrolled, moderate-to-severe asthma and a type 2 phenotype) were analyzed using a negative binomial regression model. ***p < 0.001 vs placebo.
Abbreviations: BD, bronchodilator; CI, confidence interval; FEV1, forced expiratory volume in 1 second.
In conclusion, treatment with dupilumab reduced exacerbation rates independent of clinically meaningful improvements in pre-bronchodilator FEV1 in the patients during the study. Furthermore, the number of patients receiving dupilumab that showed lung function improvement was greater compared to placebo. Nonetheless, our data suggest that exacerbation-reducing effects of dupilumab cannot be predicted by the effect on lung function at 12 weeks, and as such, treatment should be continued regardless of improvements, or lack thereof, in pre-bronchodilator FEV1. More studies are currently undergoing to better characterize the long-term benefits of dupilumab on lung function, and impact on exacerbations and patients’ quality of life.
Funding Statement
Research sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. ClinicalTrials.gov Identifier: NCT02414854. Medical writing/editorial assistance was provided by Natalia Crespo, of Excerpta Medica, and was funded by Sanofi and Regeneron Pharmaceuticals, Inc., according to the Good Publication Practice guideline.
Disclosure
Nicole A Hanania has received research support from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline; and reports consultancy for Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Mylan, Novartis, Regeneron Pharmaceuticals, Inc., Sanofi, Teva. Jorge F Maspero is a consultant for AstraZeneca, Sanofi, Teva; has received speaker fees from GlaxoSmithKline, Inmunotek, Menarini, Novartis, Uriach; and research grants from Novartis. David MG Halpin reports advisory board and speaker fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, GlaxoSmithKline, Novartis, Pfizer, Sandoz, Sanofi. David Jackson has received advisory board fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Sanofi, Teva. Reynold A Panettieri is a consultant for AstraZeneca, Avillion, MedImmune, Bayer, Teva, RIFM, Equillium, Theravance Biopharma; and a speaker for AstraZeneca, Genentech, Regeneron Pharmaceuticals, Inc., Sanofi; has received research grants from ACTIV-1, Origo, NIH, Janssen, Vault Health, AstraZeneca, Equillium, Genentech, MedImmune, OncoArendi Therapeutics, RIFM. Mario Castro reports research support from American Lung Association, AstraZeneca, GlaxoSmithKline, NIH, Novartis, PCORI, Pulmatrix, Sanofi-Aventis, Shionogi; is a consultant for Genentech, Novartis, Sanofi-Aventis, Teva; has received speaker fees from AstraZeneca, Genentech, GlaxoSmithKline, Regeneron Pharmaceuticals, Inc., Sanofi, Teva, Merck and Amgen; and royalties from Elsevier. Christian Domingo has received travel and speaker fees from ALK, Allergy Therapeutics, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Ferrer, GlaxoSmithKline, HAL Allergy, Inmunotek, Menarini, Novartis, Pfizer, Sanofi-Aventis, Stallergenes Greer, Takeda, Teva. Nadia Daizadeh is a former Sanofi employee and may hold stock and/or stock options in the company. Juby A Jacob-Nara, Michel Djandji, Paul J Rowe are Sanofi employees and may hold stock and/or stock options in the company. Rebecca Gall, Benjamin Ortiz, Yamo Deniz are employees and shareholders of Regeneron Pharmaceuticals, Inc. Rebecca Gall also reports non-financial support from Excerpta Medica. The authors report no other conflicts of interest in this work.
References
- 1.Tepper RS, Wise RS, Covar R, et al. Asthma outcomes: pulmonary physiology. J Allergy Clin Immunol. 2012;129(3 Suppl):S65–S87. doi: 10.1016/j.jaci.2011.12.986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2021. Available from: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf. Accessed December 10, 2021.
- 3.Santanello NC, Zhang J, Seidenberg B, Reiss TF, Barber BL. What are minimal important changes for asthma measures in a clinical trial? Eur Respir J. 1999;14(1):23–27. doi: 10.1034/j.1399-3003.1999.14a06.x [DOI] [PubMed] [Google Scholar]
- 4.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi: 10.1056/NEJMoa1804092 [DOI] [PubMed] [Google Scholar]
- 5.Castro M, Rabe KF, Corren J, et al. Dupilumab improves lung function in patients with uncontrolled, moderate-to-severe asthma. ERJ Open Res. 2020;6(1):00204–2019. doi: 10.1183/23120541.00204-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papi A, Castro M, Corren J, et al. Long-term effect of dupilumab on lung function in patients with type 2 asthma: LIBERTY ASTHMA TRAVERSE study. Presented at the 2021 CHEST Annual Meeting; October 17-20; 2021; Orlando, FL, USA. [Google Scholar]