Abstract
Background
Olaparib, the world’s first poly ADP-ribose polymerase (PARP) inhibitor (PARPi), has been approved for treatment of ovarian cancer, breast cancer, pancreatic cancer and prostate cancer by FDA. The current study was to assess olaparib-related adverse events (AEs) of real-world through data mining of the US Food and Drug Administration Adverse Event Reporting System (FAERS).
Methods
Disproportionality analyses, including the reporting odds ratio (ROR), the proportional reporting ratio (PRR), the Bayesian confidence propagation neural network (BCPNN) and the multi-item gamma Poisson shrinker (MGPS) algorithms were employed to quantify the signals of olaparib-associated AEs.
Results
Out of 8,450,009 reports collected from the FAERS database, 6402 reports of olaparib-associated AEs were identified. A total of 118 significant disproportionality preferred terms (PTs) conforming to the four algorithms simultaneously were retained. The most common AEs included anemia, thrombocytopenia, nausea, decreased appetite, blood creatinine increased and dermatomyositis, which were corresponding to those reported in the specification and clinical trials. Unexpected significant AEs as interstitial lung disease, Pneumocystis jirovecii pneumonia, folate deficiency, renal impairment and intestinal obstruction might also occur. The median onset time of olaparib-related AEs was 61 days (interquartile range [IQR] 14–182 days), and most of the cases occurred within the first 1 month after olaparib initiation.
Conclusion
Results of our study were consistent with clinical observations, and we also found potential new and unexpected AEs signals for olaparib, suggesting prospective clinical studies were needed to confirm these results and illustrate their relationship. Our results could provide valuable evidence for further safety studies of olaparib.
Keywords: olaparib, PARP inhibitor, pharmacovigilance, data mining, FAERS
Introduction
Ovarian cancer is the most lethal of the gynecologic malignancies with a 5-year survival rate of about 47.4%, and approximately 70% have a relapse within the subsequent 3 years.1–3 Because poly ADP-ribose polymerase (PARP) inhibitors (PARPis) significantly prolonged the progression-free survival (PFS) in patients with ovarian cancer and had a relatively low incidence of serious side effects, they had become an effective treatment option for patients with both primary and recurrent ovarian cancer, which had transformed the oncology landscape. BRCA1/2 gene is a tumor suppressor gene, which plays an important role in DNA damage repair and cell growth.4,5 Mutations in BRCA genes can inhibit normal DNA repair and cause homologous recombination deficiency (HRD), so that the double strand broken DNA cannot carry out homologous recombination repair, thus resulting in carcinogenesis. Studies have reported that carriers of a pathogenic BRCA1 variant have an estimated 48.3% (95% CI, 38.8–57.9%) cumulative risk of ovarian cancer by age 70, whereas the cumulative risk by age 70 is 20.0% (95% CI, 13.3–29.0%) for carriers of a pathogenic BRCA2 variant.6 In homologous recombination-deficient tumors, PARPi eliminates an alternative DNA repair pathway essential for maintaining viability, thus forming a “synthetic lethal” effect and finally leading to tumor cells’ death.7 As the first PARPi, olaparib has been widely used in clinic and updated continuously by oncology societies and guidelines to treat ovarian, prostate, breast and pancreatic cancers.3,8–11
According to the product description and early evaluation of post-marketing safety, the most common adverse drug reactions (ADRs) of olaparib were anemia, thrombocytopenia, neutropenia, leucopenia, fatigue and nausea. Because of the relatively recent introduction of olaparib, currently, most of the safety studies on olaparib are reported in clinical trials and several meta-analyses.12–14 The spectrum of infrequent adverse events (AEs) continues to occur with the increasing use of olaparib, such as respiratory, renal, metabolic and nutritional side-effects. Moreover, systematic research on olaparib-related AEs signals based on real world and large international databases is still lacking.
Spontaneous reporting system (SRS) has become the main information source for exploring the post-marketing drug safety with the characteristics of wide monitoring range and earlier detection of suspected ADR signals. The Food and Drug Administration Adverse Event Reporting System (FAERS) is a public and accessible database designed to support the FDA’s post-marketing safety monitoring of drugs and therapeutic biologic products. Reports were quantitatively assessed by signal detections, where a signal meant a drug-related AE. In the present study, we retrospectively analyzed the AEs reported from the first quarter of 2015 to the first quarter of 2021 with olaparib through data mining of FAERS.
Methods
Study Design and Data Sources
FAERS is a well-known publicly available post-marketing safety surveillance database for collecting AEs reports by health professionals, pharmaceutical manufacturers, individual patients and others. FAERS data include the following seven datasets: demographic and administrative information, drug information, adverse events, patient outcomes, report sources, start and end dates for reported drugs, and indications for use. This real-world, retrospective pharmacovigilance study is a disproportionality analysis based on FAERS database, extracting data from the first quarter of 2015 to the first quarter of 2021. This study was approved (No. 20220185) by the institutional ethics board of the Union Hospital of Tongji Medical College of Huazhong University of Science and Technology.
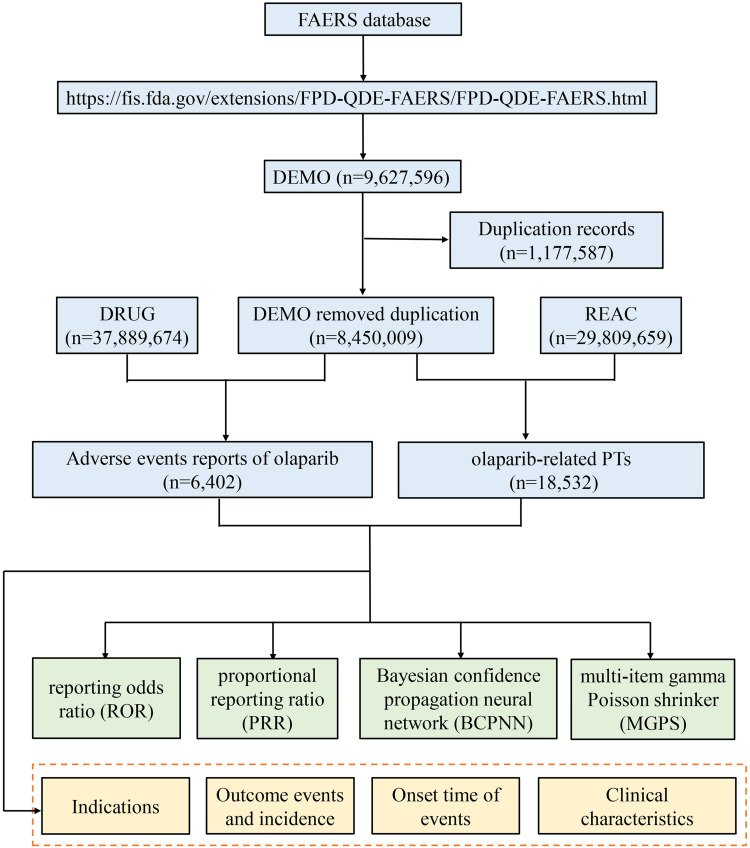
The system organ class (SOC) is the top level of Medical Dictionary for Regulatory Activities (MedDRA). We extracted all preferred terms (PTs) based on MedDRA and had more than three records in FAERS. According to FDA’s recommendations, we selected the latest FDA_DT when the PRIMARYIDs were the same, and chose the higher PRIMARYID when the FDA_DT and the CASEID were the same, to remove duplicate reports submitted by various individuals and institutions. Both brand names and generic names were used to identify olaparib associated records because FAERS had two variables, DRUGNAME and PROD_AI. In this study, olaparib/Lynparza was used to search. The reported drugs in FAERS were categorized into four patterns: PS (primary suspect), SS (secondary suspect), C (concomitant), and I (interacting). Serious patient outcomes were defined as death (DE), life-threatening (LT), hospitalization-initial or prolonged (HO), disability (DS), congenital anomaly (CA) or other important medical event (OT). Clinical characteristics including gender, age, reporting area, reporter, reporting time and outcomes of patients with olaparib-related AEs were collected. Additionally, we assessed the time-to-onset of AEs caused by olaparib with the following formula: (Time-to-onset = Adverse event onset date–start date of olaparib use) by excluding the incorrect records. The flow diagram of our study is shown in Figure 1.
Figure 1.
The flow diagram of selecting olaparib-related AEs from FAERS database.
Statistical Analysis
Descriptive analysis was used to show the characteristics of all AE reports regarding to olaparib. Disproportionality analysis, which is widely used in pharmacovigilance study, was performed to identify potential signals between olaparib and all AEs in our investigation. Reporting odds ratio (ROR), the proportional reporting ratio (PRR), the Bayesian confidence propagation neural network (BCPNN), and the multi-item gamma Poisson shrinker (MGPS) are four major specific indices that were calculated using standard formulas to assess potential associations between olaparib and AEs as presented in Table 1.15–17 Only those signals with at least three target AE records to target drugs were calculated in our study. At least one of the four algorithm meets the criteria should be considered as a positive signal of drug-associated AEs. In this study, we selected AE signals that simultaneously met all of the above four algorithm standards for research. All data processing and statistical analyses were performed using MYSQL 8.0, Navicat Premium 15, Microsoft EXCEL 2019 and the GraphPad Prism 8 (GraphPad Software, CA, USA).
Table 1.
Four Major Algorithms Used to Assess Potential Associations Between Olaparib and AEs.
| Algorithms | Equation | Criteria |
|---|---|---|
| ROR | ROR=ad/b/c | lower limit of 95% CI>1, N≥3 |
| 95%CI=eln(ROR)±1.96(1/a+1/b+1/c+1/d)^0.5 | ||
| PRR | PRR=a(c+d)/c/(a+b) | PRR≥2, χ2≥4, N≥3 |
| χ2=[(ad-bc)^2](a+b+c+d)/[(a+b)(c+d)(a+c)(b+d)] | ||
| BCPNN | IC=log2a(a+b+c+d)/((a+c)(a+b)) | IC025>0 |
| 95%CI= E(IC) ± 2V(IC)^0.5 | ||
| MGPS | EBGM=a(a+b+c+d)/(a+c)/(a+b) | EBGM05>2 |
| 95%CI=eln(EBGM)±1.96(1/a+1/b+1/c+1/d)^0.5 |
Notes: Equation: a, number of reports containing both the target drug and target adverse drug reaction; b, number of reports containing other adverse drug reaction of the target drug; c, number of reports containing the target adverse drug reaction of other drugs; d, number of reports containing other drugs and other adverse drug reactions.
Abbreviations: 95% CI, 95% confidence interval; N, the number of reports; χ2, chi-squared; IC, information component; IC025, the lower limit of 95% CI of the IC; E(IC), the IC expectations; V(IC), the variance of IC; EBGM, empirical Bayesian geometric mean; EBGM05, the lower limit of 95% CI of EBGM.
Results
General Characteristics
From January 2015 to March 2021, a total of 8,450,009 AE reports submitted to FAERS database, among which 6402 reports on olaparib were reported. The characteristics of AE reports submitted for olaparib are presented in Table 2. The number of reported AEs had gradually increased from 2015 to 2020. With the exception of 9.76% reported in the first quarter of 2021, the most reported year was 2020 (31.16%), followed by 2019 (20.82%). More female than male patients (78.40% vs 13.26%) were reported due to the specific indications for ovarian and breast cancer. The largest percentages of reports (31.27%) were in patients aged 18–65 years and elderly individuals (aged >65 years) also accounted for a high proportion with 22.21% (n=1422). Ovarian cancer was the most reported indication (48.61%), followed by breast cancer (8.06%), prostate cancer (5.03%), pancreatic carcinoma (2.37%) and fallopian tube cancer (1.00%). Almost half of the reports were submitted by health professionals, including physicians (35.11%), pharmacists (3.64%), and other health professionals (11.19%). The country that reported the most was America (52.37%), followed by Japan (13.01%), France (7.58%), Germany (2.94%) and China (2.69%). Olaparib was the primary suspect (86.14%) in most reports. Other medical events (48.16%) was the most frequently reported severe outcome, followed by death and hospitalization occurring in 2025 (31.63%) and 1206 (18.84%) cases, respectively. The high percentage of death events might be more related to disease progression of cancer.
Table 2.
Clinical Characteristics of Reports with Olaparib from the FAERS Database (January 2015–March 2021)
| Characteristics | Case Number, n | Case Proportion, % |
|---|---|---|
| Number of events | 6402 | |
| Gender | ||
| Female | 5019 | 78.40 |
| Male | 849 | 13.26 |
| Unknown | 534 | 8.34 |
| Age (years) | ||
| <18 | 7 | 0.11 |
| 18≤ and ≤65 | 2002 | 31.27 |
| >65 | 1422 | 22.21 |
| Unknown | 2971 | 46.41 |
| Indications (top five) | ||
| Ovarian cancer | 3112 | 48.61 |
| Breast cancer | 516 | 8.06 |
| Prostate cancer | 322 | 5.03 |
| Pancreatic carcinoma | 152 | 2.37 |
| Fallopian tube cancer | 64 | 1.00 |
| Serious outcome | ||
| Death (DE) | 2025 | 31.63 |
| Life-threatening (LT) | 242 | 3.78 |
| Hospitalization – initial or prolonged (HO) | 1206 | 18.84 |
| Disability (DS) | 77 | 1.20 |
| Congenital anomaly (CA) | 6 | 0.09 |
| Other serious (important medical event) (OT) | 3083 | 48.16 |
| Reported countries (top five) | ||
| America (US) | 3353 | 52.37 |
| Japan (JP) | 833 | 13.01 |
| France (FR) | 485 | 7.58 |
| Germany (DE) | 188 | 2.94 |
| China (CN) | 172 | 2.69 |
| Reported person | ||
| Health profession | ||
| Physician (MD) | 2248 | 35.11 |
| Pharmacist (PH) | 233 | 3.64 |
| Other health-professional (OT) | 716 | 11.19 |
| Non-healthcare professional | ||
| Consumer (CN) | 1216 | 18.99 |
| Unknown | 1989 | 31.07 |
| Reporting year | ||
| 2021Q1 | 625 | 9.76 |
| 2020 | 2187 | 31.16 |
| 2019 | 1333 | 20.82 |
| 2018 | 824 | 12.87 |
| 2017 | 616 | 9.62 |
| 2016 | 519 | 8.11 |
| 2015 | 298 | 4.66 |
| Role code | ||
| Primary suspect (PS) | 5515 | 86.14 |
| Secondary suspect (SS) | 1992 | 31.12 |
| Concomitant (C) | 168 | 2.62 |
| Interacting (I) | 48 | 0.75 |
Note: 2021Q1, the first quarter of 2021.
Signal Detection
Signal reports of olaparib at the SOC level are shown in Table 3. Obviously, olaparib-associated AEs occurrence targeted 27 organ systems. The significant SOCs were “neoplasms benign, malignant and unspecified (incl cysts and polyps) (SOC: 10029104)” and “blood and lymphatic system disorders (SOC: 10005329)”. The signal detections of “hepatobiliary disorders (SOC: 10019805)”, “investigations (SOC: 10022891)” and “gastrointestinal disorders (SOC: 10017947)” were positive with ROR and IC methods while not with PRR and EBGM, suggesting these signals might also be important and frequent.
Table 3.
Signal Strength of AEs of Olaparib at the System Organ Class (SOC) Level in FAERS Database
| System Organ Class (SOC) | Olaparib Cases Reporting SOC | ROR (95% Two-Sided CI) | PRR (95% Two-Sided CI) | χ2 | IC (IC025) | EBGM (EBGM05) |
|---|---|---|---|---|---|---|
| General disorders and administration site conditions | 3095 | 1.45 (1.38–1.53) | 1.23 (1.20–1.27) | 226.57 | 0.30 (0.24) | 1.23 (1.18) |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 1862 | 6.84 (6.48–7.22) | 5.14 (4.95–5.34) | 6559.87 | 2.36 (2.28) | 5.13 (4.86) |
| Gastrointestinal disorders | 1567 | 1.47 (1.38–1.55) | 1.35 (1.29–1.41) | 174.76 | 0.43 (0.35) | 1.35 (1.28) |
| Blood and lymphatic system disorders | 1485 | 6.08 (5.74–0.6.45) | 4.91 (4.69–5.13) | 4828.85 | 2.29 (2.20) | 4.89 (4.61) |
| Investigations | 1101 | 1.56 (1.46–1.67) | 1.46 (1.39–1.55) | 183.67 | 0.55 (0.45) | 1.46 (1.37) |
| Injury, poisoning and procedural complications | 1001 | 0.52 (0.49–0.56) | 0.60 (0.56–0.63) | 372.34 | −0.75 (−0.84) | 0.60 (0.56) |
| Nervous system disorders | 830 | 0.54 (0.49–0.58) | 0.60 (0.56–0.63) | 290.93 | −0.75 (−0.85) | 0.60 (0.55) |
| Respiratory, thoracic and mediastinal disorders | 814 | 0.86 (0.80–0.92) | 0.88 (0.82–0.93) | 16.75 | −0.19 (−.30) | 0.88 (0.81) |
| Vascular disorders | 562 | 0.50 (0.45–0.54) | 0.54 (0.50–0.58) | 262.21 | −0.89 (−1.01) | 0.54 (0.50) |
| Musculoskeletal and connective tissue disorders | 530 | 0.62 (0.57–0.68) | 0.65 (0.60–0.70) | 113.47 | −0.62 (−0.75) | 0.65 (0.60) |
| Cardiac disorders | 519 | 0.65 (0.59–0.71) | 0.67 (0.62–0.73) | 92.76 | −0.57 (−0.70) | 0.67 (0.62) |
| Skin and subcutaneous tissue disorders | 514 | 0.44 (0.41–0.49) | 0.49 (0.45–0.53) | 327.09 | −1.03 (−1.16) | 0.49 (0.45) |
| Infections and infestations | 485 | 0.65 (0.59–0.72) | 0.68 (0.63–0.74) | 83.199 | −0.56 (−0.70) | 0.68 (0.62) |
| Metabolism and nutrition disorders | 476 | 1.01 (0.92–1.11) | 1.01 (0.93–1.10) | 0.08 | 0.02 (−0.12) | 1.01 (0.92) |
| Psychiatric disorders | 361 | 0.41 (0.36–0.45) | 0.44 (0.40–0.49) | 297.12 | −1.19 (−1.34) | 0.44 (0.39) |
| Renal and urinary disorders | 344 | 0.83 (0.74–0.93) | 0.84 (0.76–0.93) | 11.36 | −0.25 (−0.42) | 0.84 (0.75) |
| Immune system disorders | 326 | 0.52 (0.46–0.58) | 0.54 (0.49–0.60) | 138.32 | −0.88 (−1.05) | 0.54 (0.49) |
| Reproductive system and breast disorders | 310 | 1.37 (1.22–1.53) | 1.35 (1.21–1.50) | 29.01 | 0.43 (0.26) | 1.35 (1.20) |
| Hepatobiliary disorders | 275 | 1.75 (1.55–1.97) | 1.72 (1.53–1.93) | 84.36 | 0.78 (0.59) | 1.72 (1.52) |
| Eye disorders | 93 | 0.33 (0.26–0.40) | 0.34 (0.28–0.41) | 125.98 | −1.56 (−1.88) | 0.34 (0.28) |
| Surgical and medical procedures | 58 | 0.26 (0.20–0.34) | 0.27 (0.21–0.35) | 118.68 | −1.89 (−2.28) | 0.27 (0.21) |
| Endocrine disorders | 47 | 0.27 (0.20–0.36) | 0.28 (0.21–0.37) | 90.25 | −1.84 (−2.27) | 0.28 (0.21) |
| Congenital, familial and genetic disorders | 45 | 1.21 (0.90–1.62) | 1.20 (0.90–1.61) | 1.57 | 0.27 (−0.20) | 1.20 (0.90) |
| Ear and labyrinth disorders | 32 | 0.38 (0.27–0.54) | 0.38 (0.27–0.54) | 32.02 | −1.38 (−1.91) | 0.38 (0.27) |
| Product issues | 20 | 0.07 (0.05–0.11) | 0.08 (0.05–0.12) | 236.92 | −3.73 (−4.38) | 0.08 (0.05) |
| Social circumstances | 13 | 0.19 (0.11–0.32) | 0.19 (0.11–0.32) | 45.89 | −2.41 (−3.23) | 0.19 (0.11) |
| Pregnancy, puerperium and perinatal conditions | 4 | 0.03 (0.01–0.09) | 0.04 (0.01–0.09) | 108.09 | −4.83 (−6.29) | 0.04 (0.01) |
Notes: Bold indicates statistically significant signals in four algorithms.
Abbreviations: ROR, reporting odds ratio; CI, confidence interval; PRR, proportional reporting ratio; χ2, chi-squared; IC, information component; EBGM, empirical Bayesian geometric mean.
A total of 118 significant PTs of interest conforming to all of the four algorithms simultaneously are described in Table 4. In this study, anaemia (PT: 10002034), thrombocytopenia (PT: 10043554), acute leukaemia (PT: 10000830), acute myeloid leukaemia (PT: 10000880), myelodysplastic syndrome (PT: 10028533), blood magnesium decreased (PT: 10005654), musculoskeletal toxicity (PT: 10082578), nausea (PT: 10028813), blood creatinine increased (PT: 10005483), neuropathy peripheral (PT: 10029331), pneumonitis (PT: 10035742) dermatomyositis (PT: 10012503) and erythema nodosum (PT: 10015226) were present, which consistent with the instructions and medication warnings. Of note, unexpected significant AEs, including folate deficiency (PT: 10016880), glomerular filtration rate decreased (PT: 10018358), renal impairment (PT: 10062237) and hydronephrosis (PT: 10020524), acquired gene mutation (PT: 10069754), intestinal obstruction (PT: 10022687), ascites (PT: 10003445), hepatic cyst (PT: 10019646), pleural effusion (PT: 10035598), interstitial lung disease (PT: 10022611), Pneumocystis jirovecii pneumonia (PT: 10073755), tumor marker increased (PT: 10048621), pelvic mass (PT: 10034260) and so on, were uncovered in the label. Cardiovascular events have been reported in patients treated with olaparib, such as venous thrombosis, heart failure and cardiopulmonary failure, which are indicated in the drug label. However, they did not meet the criteria for at least one of the four algorithms in our analysis.
Table 4.
Signal Strength of Reports of Olaparib at the Preferred Terms Level in FAERS Database
| SOC | Preferred Terms (PTs) | PT/N | ROR (95% Two-Sided CI) | PRR (95% Two-Sided CI) | χ2 | IC (IC025) | EBGM (EBGM05) |
|---|---|---|---|---|---|---|---|
| Blood and lymphatic system disorders | Anaemia | 637 | 12.72 (11.72–13.81) | 11.55 (10.73–12.44) | 6140.34 | 3.52 (3.37) | 11.46 (10.56) |
| Thrombocytopenia | 137 | 4.55 (3.84–5.39) | 4.47 (3.79–5.28) | 369.56 | 2.16 (1.86) | 4.46 (3.76) | |
| Pancytopenia | 106 | 7.23 (5.97–8.77) | 7.13 (5.90–8.62) | 556.90 | 2.83 (2.45) | 7.10 (5.85) | |
| Bone marrow failure | 79 | 9.17 (7.34–11.46) | 9.07 (7.28–11.30) | 564.30 | 3.17 (2.69) | 9.02 (7.21) | |
| Haematotoxicity | 47 | 19.30 (14.45–25.76) | 19.16 (14.38–25.53) | 797.78 | 4.24 (3.33) | 18.90 (14.16) | |
| Myelosuppression | 36 | 20.26 (14.56–21.18) | 20.15 (14.51–27.98) | 645.61 | 4.31 (3.19) | 19.86 (14.28) | |
| Haemolytic anaemia | 17 | 6.90 (4.28–11.13) | 6.89 (4.28–11.09) | 85.16 | 2.78 (1.59) | 6.86 (4.26) | |
| Cytopenia | 15 | 4.47 (2.69–7.43) | 4.46 (2.69–7.40) | 40.17 | 2.15 (1.03) | 4.45 (2.68) | |
| Blood disorder | 11 | 4.87 (2.69–8.81) | 4.87 (2.69–8.79) | 33.67 | 2.28 (0.88) | 4.85 (2.68) | |
| Anaemia macrocytic | 7 | 14.07 (6.68–29.63) | 14.05 (6.68–29.58) | 83.98 | 3.80 (1.12) | 13.9 (6.60) | |
| Macrocytosis | 5 | 15.07 (6.23–36.39) | 15.06 (6.24–36.34) | 64.88 | 3.90 (0.61) | 14.90 (6.17) | |
| Bone marrow disorder | 5 | 6.55 (2.72–15.78) | 6.55 (2.72–15.76) | 23.39 | 2.71 (0.21) | 6.52 (2.71) | |
| Aplasia pure red cell | 5 | 6.64 (2.75–15.99) | 6.63 (2.76–15.97) | 23.81 | 2.72 (0.21) | 6.61 (2.74) | |
| Congenital, familial and genetic disorders | Acquired gene mutation* | 17 | 33.50 (20.69–54.25) | 33.41 (20.66–54.04) | 521.39 | 5.03 (2.77) | 32.61 (20.14) |
| BRCA2 gene mutation* | 13 | 592.4 (307.84–1140.13) | 591.23 (307.50–1136.77) | 5289.10 | 8.67 (2.74) | 408.5 (212.3) | |
| Porokeratosis* | 6 | 95.43 (41.66–218.62) | 95.34 (41.65–218.26) | 522.36 | 6.48 (1.27) | 88.98 (38.84) | |
| BRCA1 gene mutation* | 4 | 229.52 (79.35–663.85) | 229.37 (79.35–663.07) | 774.77 | 7.61 (0.42) | 195.51 (67.61) | |
| Gastrointestinal disorders | Nausea | 707 | 3.18 (2.94–3.44) | 2.94 (2.74–3.15) | 936.70 | 1.55 (1.43) | 2.93 (2.71) |
| Ileus* | 64 | 19.91 (15.54–25.52) | 19.72 (15.43–25.21) | 1121.26 | 4.28 (3.53) | 19.45 (15.17) | |
| Ascites* | 64 | 7.51 (5.87–9.62) | 7.45 (5.83–9.51) | 355.671 | 2.89 (2.37) | 7.41 (5.79) | |
| Intestinal obstruction* | 53 | 4.86 (3.71–6.38) | 4.83 (3.69–6.32) | 160.71 | 2.27 (1.74) | 4.82 (3.67) | |
| Small intestinal obstruction* | 22 | 6.10 (4.01–9.28) | 6.09 (4.01–9.24) | 93.12 | 2.60 (1.63) | 6.06 (3.98) | |
| Subileus* | 9 | 14.7 (7.66–28.53) | 14.76 (7.66–28.47) | 114.22 | 3.87 (1.51) | 14.61 (7.57) | |
| Large intestinal obstruction* | 7 | 9.23 (4.39–19.42) | 9.22 (4.39–19.39) | 50.97 | 3.20 (0.89) | 9.17 (4.36) | |
| Intra-abdominal fluid collection* | 7 | 10.21 (4.85–21.49) | 10.20 (4.85–21.45) | 57.66 | 3.34 (0.95) | 10.13 (4.81) | |
| Abdominal mass* | 6 | 8.28 (3.71–18.47) | 8.27 (3.71–18.45) | 38.10 | 3.04 (0.61) | 8.22 (3.68) | |
| General disorders and administration site conditions | Death | 1464 | 6.76 (6.37–7.16) | 5.44 (5.20–5.69) | 5515.66 | 2.44 (2.35) | 5.42 (5.11) |
| Disease progression | 157 | 5.46 (4.66–6.40) | 5.35 (4.59–6.25) | 556.20 | 2.42 (2.13) | 5.34 (4.55) | |
| Drug resistance | 32 | 3.41 (2.41–4.83) | 3.40 (2.41–4.81) | 54.17 | 1.76 (1.11) | 3.40 (2.40) | |
| Disease recurrence | 29 | 4.00 (2.77–5.76) | 3.98 (2.77–5.73) | 64.64 | 1.99 (1.27) | 3.97 (2.76) | |
| Therapy partial responder | 12 | 5.61 (3.18–9.90) | 5.60 (3.18–9.87) | 45.19 | 2.48 (1.09) | 5.58 (3.17) | |
| Implant site reaction | 6 | 43.05 (19.08–97.10) | 43.01 (19.08–96.94) | 238.42 | 5.38 (1.19) | 41.68 (18.48) | |
| Pelvic mass* | 5 | 23.40 (9.66–56.68) | 23.38 (9.66–56.60) | 105.27 | 4.52 (0.74) | 22.99 (9.49) | |
| Hepatobiliary disorders | Hepatic cyst* | 5 | 5.54 (2.30–13.34) | 5.54 (2.30–13.32) | 18.51 | 2.46 (0.10) | 5.52 (2.29) |
| Infections and infestations | Pneumocystis jirovecii pneumonia* | 14 | 4.02 (2.38–6.80) | 4.01 (2.38–6.78) | 31.59 | 2.00 (0.87) | 4.00 (2.37) |
| Injury, poisoning and procedural complications | Product use in unapproved indication | 198 | 2.52 (2.19–2.90) | 2.47 (2.15–2.83) | 175.21 | 1.30 (1.08) | 2.47 (2.14) |
| Investigations | Carbohydrate antigen 125 increased* | 161 | 123.95 (105.27–145.93) | 120.86 (103.03–141.76) | 17534.7 | 6.79 (5.80) | 110.81 (94.13) |
| Platelet count decreased | 157 | 5.28 (4.51–6.19) | 5.18 (4.43–6.04) | 529.54 | 2.37 (2.09) | 5.16 (4.40) | |
| Haemoglobin decreased | 140 | 4.87 (4.11–5.75) | 4.78 (4.06–5.63) | 419.06 | 2.25 (1.96) | 4.77 (4.03) | |
| White blood cell count decreased | 106 | 3.35 (2.76–4.06) | 3.31 (2.74–4.00) | 171.32 | 1.72 (1.40) | 3.30 (2.73) | |
| Neutrophil count decreased | 96 | 9.25 (7.55–11.32) | 9.12 (7.47–11.13) | 690.62 | 3.18 (2.75) | 9.07 (7.41) | |
| Blood creatinine increased | 83 | 4.72 (3.80–5.87) | 4.67 (3.77–5.79) | 239.47 | 2.22 (1.82) | 4.66 (3.75) | |
| Tumour marker increased* | 61 | 42.65 (33.01–55.10) | 42.25 (32.78–54.46) | 2381.26 | 5.36 (4.24) | 40.97 (31.72) | |
| Red blood cell count decreased | 50 | 6.15 (4.65–8.12) | 6.11 (4.63–8.05) | 212.78 | 2.60 (2.03) | 6.08 (4.60) | |
| Prostatic specific antigen increased* | 39 | 7.97 (5.81–10.93) | 7.93 (5.79–10.85) | 234.93 | 2.98 (2.25) | 7.89 (5.75) | |
| Blood count abnormal | 36 | 4.09 (2.94–5.67) | 4.07 (2.94–5.64) | 83.16 | 2.02 (1.38) | 4.06 (2.92) | |
| Full blood count decreased | 26 | 3.83 (2.60–5.63) | 3.82 (2.60–5.61) | 54.02 | 1.93 (1.17) | 3.81 (2.59) | |
| Haematocrit decreased | 18 | 3.57 (2.25–5.67) | 3.56 (2.24–5.65) | 33.10 | 1.83 (0.89) | 3.56 (2.24) | |
| Glomerular filtration rate decreased* | 17 | 4.96 (3.08–7.99) | 4.95 (3.07–7.96) | 53.35 | 2.30 (1.23) | 4.93 (3.06) | |
| Blood magnesium decreased | 13 | 5.07 (2.94–8.75) | 5.07 (2.94–8.73) | 42.29 | 2.34 (1.06) | 5.05 (2.93) | |
| White blood cell count abnormal | 9 | 4.25 (2.21–8.17) | 4.24 (2.21–8.16) | 22.23 | 2.08 (0.56) | 4.23 (2.20) | |
| Platelet count abnormal | 9 | 4.60 (2.39–8.86) | 4.60 (2.39–8.84) | 25.25 | 2.20 (0.64) | 4.58 (2.38) | |
| Creatinine renal clearance decreased | 9 | 8.71 (4.52–16.78) | 8.70 (4.52–16.74) | 60.91 | 3.11 (1.18) | 8.65 (4.49) | |
| Tumour marker abnormal | 7 | 68.97 (32.25–147.53) | 68.90 (32.24–147.25) | 445.14 | 6.03 (1.54) | 65.53 (30.64) | |
| Blood creatine increased | 7 | 6.79 (3.23–14.28) | 6.78 (3.23–14.25) | 34.35 | 2.76 (0.69) | 6.75 (3.21) | |
| Mean cell volume increased | 6 | 8.38 (3.75–18.71) | 8.37 (3.75–18.68) | 38.72 | 3.06 (0.62) | 8.33 (3.73) | |
| Carbohydrate antigen 19–9 increased* | 6 | 49.20 (21.77–111.18) | 49.15 (21.77–110.99) | 272.87 | 5.57 (1.21) | 47.42 (20.98) | |
| Carbohydrate antigen 15–3 increased* | 6 | 31.81 (14.15–71.51) | 31.78 (14.15–71.40) | 174.67 | 4.96 (1.14) | 31.06 (13.81) | |
| SARS-CoV-2 test negative* | 4 | 23.05 (8.76–61.96) | 23.04 (8.58–61.89) | 82.88 | 4.50 (0.31) | 22.66 (8.43) | |
| Red blood cell count abnormal | 4 | 8.70 (3.25–23.25) | 8.69 (3.25–23.23) | 27.05 | 3.11 (0.00) | 8.64 (3.23) | |
| Eastern Cooperative Oncology Group performance status worsened* | 4 | 13.07 (4.88–34.99) | 13.06 (4.88–34.95) | 44.11 | 3.69 (0.16) | 12.94 (4.83) | |
| Blood electrolytes decreased | 4 | 10.29 (3.84–27.53) | 10.28 (3.85–27.50) | 33.27 | 3.35 (0.07) | 10.21 (3.82) | |
| Metabolism and nutrition disorders | Decreased appetite | 196 | 2.76 (2.39–3.18) | 2.71 (2.36–3.11) | 212.93 | 1.43 (1.21) | 2.70 (2.34) |
| Hypophagia | 25 | 3.20 (2.16–4.74) | 3.19 (2.16–4.72) | 37.58 | 1.67 (0.92) | 3.19 (2.15) | |
| Folate deficiency* | 6 | 18.99 (8.48–42.54) | 18.98 (8.48–42.47) | 100.73 | 4.23 (1.00) | 18.72 (8.36) | |
| Musculoskeletal and connective tissue disorders | Musculoskeletal toxicity | 8 | 229.66 (108.35–486.78) | 229.37 (108.30–485.78) | 1549.55 | 7.61 (1.85) | 195.51 (92.31) |
| Nervous system disorders | Neuropathy peripheral | 90 | 3.12 (2.53–3.84) | 3.09 (2.51–3.79) | 127.35 | 1.62 (1.27) | 3.083 (2.50) |
| Dysgeusia | 66 | 3.16 (2.48–4.03) | 3.14 (2.47–3.99) | 96.11 | 1.65 (1.22) | 3.13 (2.46) | |
| Taste disorder | 30 | 7.77 (5.42–11.13) | 7.73 (5.41–11.07) | 175.02 | 2.94 (2.09) | 7.70 (5.37) | |
| Renal impairment* | 84 | 3.38 (2.73–4.20) | 3.35 (2.71–4.15) | 138.89 | 1.74 (1.37) | 3.35 (2.70) | |
| Hydronephrosis* | 9 | 4.51 (2.34–8.68) | 4.51 (2.34–8.67) | 24.48 | 2.17 (0.62) | 4.49 (2.33) | |
| Respiratory, thoracic and mediastinal disorders | Interstitial lung disease* | 107 | 7.92 (6.54–9.59) | 7.80 (6.46–9.42) | 632.38 | 2.96 (2.57) | 7.76 (6.41) |
| Pneumonitis | 67 | 8.41 (6.61–10.71) | 8.34 (6.56–10.59) | 430.41 | 3.05 (2.53) | 8.29 (6.51) | |
| Pleural effusion* | 48 | 2.77 (2.08–3.68) | 2.75 (2.08–3.65) | 53.66 | 1.46 (0.96) | 2.75 (2.07) | |
| Pulmonary mass* | 20 | 4.04 (2.60–6.27) | 4.03 (2.60–6.24) | 45.44 | 2.01 (1.10) | 4.02 (2.59) | |
| Skin and subcutaneous tissue disorders | Erythema nodosum | 8 | 9.34 (4.66–18.73) | 9.33 (4.66–18.69) | 59.08 | 3.21 (1.08) | 9.27 (4.62) |
| Dermatomyositis | 5 | 8.19 (3.40–19.73) | 8.18 (3.40–19.70) | 31.33 | 3.02 (0.34) | 8.14 (3.38) |
Note: *New and significant signals of olaparib-associated AEs from FAERS database.
Abbreviations: SOC, System Organ Class; ROR, reporting odds ratio; CI, confidence interval; PRR, proportional reporting ratio; χ2, chi-squared; IC, information component; EBGM, empirical Bayesian geometric mean. The PTs in neoplasms benign, malignant and unspecified (incl cysts and polyps) are not included because the majority of indications are cancers.
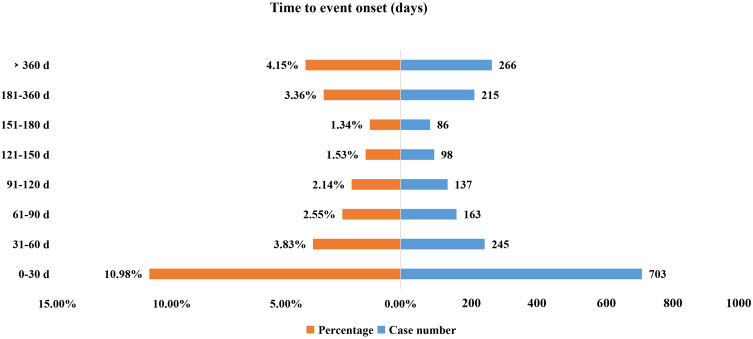
Onset Time Events
The onset times of olaparib-associated AEs were extracted from the database. A total of 1913 olaparib-associated AEs reported onset time and the median onset time was 61 days (interquartile range [IQR] 14–182 days). As shown in Figure 2, results indicated that most of the AE cases occurred within the first 1 (n=703, 10.98%), 2 (n=245, 3.83%) and 3 months (n=163, 2.55%) after olaparib initiation. It was noteworthy that AEs might still occur after 1 year of olaparib treatment with the proportion of 4.15% as shown in our data.
Figure 2.
Time to onset of olaparib-related AEs.
Discussion
It was reported that approximately 10–20% advanced cancer patients experienced serious AEs considered causally related to olaparib.18 To our knowledge, this study was the first analysis to investigate the potential link between olaparib and its AEs using a pharmacovigilance approach to evaluate the post-marketing safety of olaparib. The AEs of olaparib occurred more commonly in females (78.40%) than in males (13.26%), which was consistent with the indications of olaparib mainly for ovarian cancer and breast cancer. Almost half of the reports (49.94%) were submitted by health professionals, which might be considered a more reliable source of reporting.
According to the disproportionality analysis, the most common and significant AEs at SOC levels were “blood and lymphatic system disorders” and “neoplasms benign, malignant and unspecified (incl cysts and polyps)”. The AEs of neoplasms benign and malignant were most likely due to disease progression of cancer patients rather than olaparib. Hematologic toxicity is one of the most common ADRs of olaparib, including anemia, thrombocytopenia and neutropenia, which is included in the label and our results precisely supported this. Anemia, as one of the most common serious hematologic toxicity, can lead to discontinuation and interruption of olaparib and affect the treatment efficacy. Matulonis’s study indicated that 8% patients with advanced relapsed ovarian cancer and a germline BRCA1/2 mutation had AEs leading to olaparib interruptions, with the most common causes being vomiting (7%) and anemia (4%).19 Several studies had also shown that the incidences of anemia, thrombocytopenia, neutropenia were about 50%, 30% and 25%, respectively.3,20,21 In addition, significant AEs signal strengths of myelodysplastic syndrome (MDS) and acute myeloid leukaemia (AML) were presented in our analysis, which were corresponding to that in the instruction and clinical safety data.22
Folate deficiency was reported as a new and significant AE of olaparib with signal strength being ROR 18.99 (8.48–42.54), PRR 18.98 (8.48–42.47), IC 4.23 (1.00) and EBGM 18.72 (8.36), respectively, in our results. A study has reported a severe folate deficiency case associated with olaparib therapy, which may explain anemia and other hematologic toxicities associated with the agent.21 An advanced ovarian cancer patient was found to have an exceedingly low serum folate level (<1.6 ng/mL; normal range, 7–31.4 ng/mL) after taking olaparib, while the level of which was 14.0 ng/mL before initiation of olaparib.21 It demonstrated that olaparib might cause folate deficiency with anemia. Folic acid supplementation could reduce the need for blood transfusions and dose modification. Therefore, identifying and treat folate deficiency is necessary and may improve the safety of olaparib therapy.
Another mechanism of hematologic toxicity of olaparib might be that olaparib would inhibit or kill the normal proliferating hematopoietic cells while killing tumor cells, leading to abnormal hematopoiesis.23 Therefore, regular blood routine monitoring and effective management in olaparib therapy are important interventions to reduce the risk of olaparib-associated hematotoxicity. Besides, drug–drug interactions (DDI) of olaparib should be considered especially when used in combination with other anticancer drugs that have a myelosuppressive effect, because olaparib is metabolized mainly by CYP3A. The metabolic clearance and plasma concentration of olaparib may be affected by co-administration with CYP3A inhibitors or inducers. DDI simulation has provided dose recommendations for olaparib co-administration with clinically relevant CYP3A4 inhibitors and inducers to eliminate potential risk. When olaparib is administered with strong/moderate CYP3A inhibitor, the dose should be reduced to 100/150 mg bid (tablet), and 150/200 mg bid (capsule).24 Moreover, olaparib administration is not recommended with strong/moderate CYP3A inducers.24
Creatinine is usually considered as a functional parameter to evaluate renal function of patients in clinical practice. Recently, increased serum creatinine levels have been observed in majority of patients taking olaparib, but the underlying mechanism is unclear. Studies have shown that olaparib likely causes inhibition of renal transporters leading to a reversible, dose-dependent increase in creatinine.25 Consistently, strong signal strength of blood creatinine increased (PT: 10005483) has also been reported in our analysis. However, some unexpected and significant safety signals related to olaparib on renal function such as glomerular filtration rate decreased (PT: 10018358), renal impairment (PT: 10062237) and hydronephrosis (PT: 10020524) were shown simultaneously based on our results of FAERS data. Therefore, renal function should be monitored before and after olaparib treatment, especially in patients with renal impairment and the dose of olaparib should be adjusted to reduce the risk. The FDA had approved the revised instructions for olaparib in October 2016. Dose adjustment was not required for patients with mild renal impairment [creatinine clearance (CCR) 51–80 mL/min], and reduction with administration of 300 mg once, twice a day was recommended for patients with moderate renal impairment [creatinine clearance (CCR) 31–50 mL/min].
Notably, long-term use of olaparib is associated with a risk of skin AEs, and the most common is dermatitis. A clinical study has shown that the incidence of dermatitis was up to 38% with olaparib treatment.26 In addition, there have been reports of post-marketing erythema nodosum.27 Both AEs presented significant signal strengths in our data analysis, which were also consistent with the instructions. Conformably, the FDA added ADRs of dermatitis and erythema nodosum to the instructions of olaparib in August 2017 and March 2021, respectively, most likely based on an analysis of the FAERS database.
In our analysis, in addition to nausea, intestinal obstruction, ascites and abdominal mass were AEs with significant signal strength in the gastrointestinal disorders of olaparib, which were not included in the label but intestinal obstruction was reported in a clinical trial with an incidence of 2%.20 In the SOC of respiratory, thoracic and mediastinal systems and infections, only pneumonitis was mentioned in the instructions. Treatment-related pneumonitis was first reported in the Phase I clinical trial of olaparib in 2012.28 A meta-analysis has also reported that PARPis showed a significant increase in the risk of pneumonitis events (Peto OR 2.68 [95% CI1.31–5.47], p=0.007) compared with control arms, and the fatality rate of pneumonitis was up to 16%.29 Interstitial lung disease, pleural effusion, pulmonary mass and Pneumocystis jirovecii pneumonia were new and significant serious AEs of olaparib validated in our analysis. Interstitial lung disease, in particular, has the highest number of reported cases (107) and showed a high correlation with significant signal strength being ROR 7.92 (6.54–9.59), PRR 7.80 (6.46–9.42), IC 2.96 (2.57) and EBGM 7.76 (6.41), respectively. However, these AEs were not in package insert. To date, the mechanisms of PARPis-induced pneumonitis are still unclear, but studies have shown that PARP-1 and PARP-2, which are the main members of the PARP family, are highly expressed in the lung, and PARPis can alter the sensitivity of the two members to oxidative stress, thus resulting in chronic lung inflammation alleviation.30–32 Clinicians should be aware of these new and unexpected complications, and FDA could revise and give warnings in the label if necessary, especially as PARPis are now being more widely used in cancer patients.
Results of this study indicated that the median onset time was 61 days, and most of the cases occurred within the first 1 (n=703, 10.98%), 2 (n=245, 3.83%) and 3 months (n=163, 2.55%) after olaparib initiation, which was consistent with 1 month onset time of olaparib-related AEs by a previous Phase III study with a great number of 391 patients.3 Therefore, a longer follow-up period is needed to observe the ADRs of olaparib in future clinical studies.
There are some limitations to our study. First, database reporting is voluntary and thus the quality might be variable. Second, multiple unmeasured confounders such as potential drug-drug interactions, comorbidities and drug combinations, which might affect AEs, were not included in the data analysis. Third, despite having access to thousands of case reports, the safety reports do not provide detailed information of patients exposed to the drug without AEs. Therefore, the true incidence of AEs cannot be determined from FAERS data. Fourth, it was unable to infer an exact causal relationship. The disproportionality analysis neither quantified risk nor existed causality, but only provided an estimation of the signal strength, which was only statistically significant. Prospective clinical studies are still needed to confirm the causal relationship between them.
Conclusion
In conclusion, the present study using pharmacovigilance analysis of FAERS database scientifically and systematically quantified the potential risks, time to AE onsets and the safety signal spectrum with olaparib treatment. Unexpected and new significant AEs as interstitial lung disease, Pneumocystis jirovecii pneumonia, folate deficiency, renal impairment and intestinal obstruction might also occur. Common hematologic toxicity, gastrointestinal, respiratory and skin subcutaneous AEs are frequent AEs for which patients should be monitored. Our study could provide valuable evidence for further studies and clinical practice of olaparib.
Funding Statement
This study was supported by grants from the National Natural Science Foundation of China (No. 82104476).
Data Sharing Statement
The data presented in this study are available on request from the corresponding author.
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.Kuroki L, Guntupalli SR. Treatment of epithelial ovarian cancer. BMJ. 2020;371:m3773. doi: 10.1136/bmj.m3773 [DOI] [PubMed] [Google Scholar]
- 2.Stewart C, Ralyea C, Lockwood S. Ovarian cancer: an integrated review. Semin Oncol Nurs. 2019;35(2):151–156. doi: 10.1016/j.soncn.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 3.Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495–2505. doi: 10.1056/NEJMoa1810858 [DOI] [PubMed] [Google Scholar]
- 4.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–917. doi: 10.1038/nature03443 [DOI] [PubMed] [Google Scholar]
- 5.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–921. doi: 10.1038/nature03445 [DOI] [PubMed] [Google Scholar]
- 6.Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(1):77–102. doi: 10.6004/jnccn.2021.0001 [DOI] [PubMed] [Google Scholar]
- 7.Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355(6330):1152–1158. doi: 10.1126/science.aam7344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussain M, Mateo J, Fizazi K, et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med. 2020;383(24):2345–2357. doi: 10.1056/NEJMoa2022485 [DOI] [PubMed] [Google Scholar]
- 9.Robson ME, Tung N, Conte P, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30(4):558–566. doi: 10.1093/annonc/mdz012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–533. doi: 10.1056/NEJMoa1706450 [DOI] [PubMed] [Google Scholar]
- 11.Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317–327. doi: 10.1056/NEJMoa1903387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz-Schutz VC, Gomes LM, Mariano RC, et al. Risk of fatigue and anemia in patients with advanced cancer treated with olaparib: a meta-analysis of randomized controlled trials. Crit Rev Oncol Hematol. 2019;141:163–173. doi: 10.1016/j.critrevonc.2019.06.012 [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Meng J, Wang G. Risk of selected gastrointestinal toxicities associated with poly (ADP-ribose) polymerase (PARP) inhibitors in the treatment of ovarian cancer: a meta-analysis of published trials. Drug Des Devel Ther. 2018;12:3013–3019. doi: 10.2147/DDDT.S164553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou JX, Feng LJ, Zhang X. Risk of severe hematologic toxicities in cancer patients treated with PARP inhibitors: a meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2017;11:3009–3017. doi: 10.2147/DDDT.S147726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11(1):3–10. doi: 10.1002/pds.668 [DOI] [PubMed] [Google Scholar]
- 16.Ang PS, Chen Z, Chan CL, Tai BC. Data mining spontaneous adverse drug event reports for safety signals in Singapore - a comparison of three different disproportionality measures. Expert Opin Drug Saf. 2016;15(5):583–590. doi: 10.1517/14740338.2016.1167184 [DOI] [PubMed] [Google Scholar]
- 17.Shu Y, Ding Y, Dai B, Zhang Q. A real-world pharmacovigilance study of axitinib: data mining of the public version of FDA adverse event reporting system. Expert Opin Drug Saf. 2022;21(4):563–572. doi: 10.1080/14740338.2022.2016696 [DOI] [PubMed] [Google Scholar]
- 18.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–250. doi: 10.1200/JCO.2014.56.2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matulonis UA, Penson RT, Domchek SM, et al. Olaparib monotherapy in patients with advanced relapsed ovarian cancer and a germline BRCA1/2 mutation: a multistudy analysis of response rates and safety. Ann Oncol. 2016;27(6):1013–1019. doi: 10.1093/annonc/mdw133 [DOI] [PubMed] [Google Scholar]
- 20.Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, Phase 3 trial. Lancet Oncol. 2017;18(9):1274–1284. doi: 10.1016/S1470-2045(17)30469-2 [DOI] [PubMed] [Google Scholar]
- 21.Shammo JM, Usha L, Richardson KJ, et al. Olaparib-induced severe folate deficiency in a patient with advanced ovarian cancer. J Oncol Pract. 2019;15(7):405–407. doi: 10.1200/JOP.18.00705 [DOI] [PubMed] [Google Scholar]
- 22.Shenolikar R, Durden E, Meyer N, Lenhart G, Moore K. Incidence of secondary myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) in patients with ovarian or breast cancer in a real-world setting in the United States. Gynecol Oncol. 2018;151(2):190–195. doi: 10.1016/j.ygyno.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 23.Hopkins TA, Ainsworth WB, Ellis PA, et al. PARP1 trapping by parp inhibitors drives cytotoxicity in both cancer cells and healthy bone marrow. Mol Cancer Res. 2019;17(2):409–419. doi: 10.1158/1541-7786.MCR-18-0138 [DOI] [PubMed] [Google Scholar]
- 24.Pilla Reddy V, Bui K, Scarfe G, Zhou D, Learoyd M. Physiologically Based Pharmacokinetic Modeling for Olaparib Dosing Recommendations: bridging Formulations, Drug Interactions, and Patient Populations. Clin Pharmacol Ther. 2019;105(1):229–241. doi: 10.1002/cpt.1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruin MAC, Korse CM, van Wijnen B, et al. A real or apparent decrease in glomerular filtration rate in patients using olaparib? Eur J Clin Pharmacol. 2021;77(2):179–188. doi: 10.1007/s00228-020-03070-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karam SD, Reddy K, Blatchford PJ, et al. Final report of a phase I trial of olaparib with cetuximab and radiation for heavy smoker patients with locally advanced head and neck cancer. Clin Cancer Res. 2018;24(20):4949–4959. doi: 10.1158/1078-0432.CCR-18-0467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngu SF, Tse KY, Chu MMY, Ngan HYS, Chan KKL. Olaparib dose re-escalation in ovarian cancer patients who experienced severe and/or uncommon adverse events: a case series. Asia Pac J Clin Oncol. 2021;17(Suppl 3):3–11. doi: 10.1111/ajco.13584 [DOI] [PubMed] [Google Scholar]
- 28.Del Conte G, Sessa C, von Moos R, et al. Phase I study of olaparib in combination with liposomal doxorubicin in patients with advanced solid tumours. Br J Cancer. 2014;111(4):651–659. doi: 10.1038/bjc.2014.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma Z, Sun X, Zhao Z, et al. Risk of pneumonitis in cancer patients treated with PARP inhibitors: a meta-analysis of randomized controlled trials and a pharmacovigilance study of the FAERS database. Gynecol Oncol. 2021;162(2):496–505. doi: 10.1016/j.ygyno.2021.05.012 [DOI] [PubMed] [Google Scholar]
- 30.Bai P. Biology of poly(ADP-ribose) polymerases: the factotums of cell maintenance. Mol Cell. 2015;58(6):947–958. doi: 10.1016/j.molcel.2015.01.034 [DOI] [PubMed] [Google Scholar]
- 31.Dharwal V, Naura AS. PARP-1 inhibition ameliorates elastase induced lung inflammation and emphysema in mice. Biochem Pharmacol. 2018;150:24–34. doi: 10.1016/j.bcp.2018.01.027 [DOI] [PubMed] [Google Scholar]
- 32.Sethi GS, Sharma S, Naura AS. PARP inhibition by olaparib alleviates chronic asthma-associated remodeling features via modulating inflammasome signaling in mice. IUBMB Life. 2019;71(7):1003–1013. doi: 10.1002/iub.2048 [DOI] [PubMed] [Google Scholar]