Abstract
To complete cytokinesis, abscission of the proteinaceous and microtubule-rich intercellular bridge needs to occur. The midbody (MB), a structure that forms on the intercellular bridge, is a key regulator of cytokinesis and appears to play a role in downstream signaling after abscission. Initially, it was thought that after abscission was completed, the MB was degraded. However, a new body of evidence has emerged suggesting that one daughter cell or a surrounding non–daughter cell can inherit or internalize the MB, thus leading to changes in cell proliferation and differentiation. In this review, we highlight the role that the MB has after mitosis. We will focus on the rising evidence that the MB plays an important role in establishment of cell polarity, such as apical lumen formation, neurite extension, and ciliation. Additionally, we will discuss the evidence suggesting that MBs can also serve the role of signaling organelles (MBsomes) that lead to cell proliferation, differentiation, and even tumorigenicity.
INTRODUCTION
Mitotic cell division is a key event during the development, growth, and function of all organisms. Owing to the importance of mitotic cell division, the entire process is highly regulated from DNA synthesis, nuclear envelope breakdown, separation of chromosomes, and cytokinesis to the final stage of mitotic cell division, abscission. Abscission is the physical separation of the plasma membranes of two daughter cells. During abscission, a microtubule-rich, proteinaceous structure, known as the midbody (MB), forms between the dividing cells. The MB was initially described by Walther Flemming more than 100 years ago and is well-known for its role in regulating the timing and location of abscission. Intriguingly, numerous studies over the past few years have suggested that the MB function extends beyond mitotic cell division and that postmitotic MBs may regulate cell fate, polarization, proliferation, and even tumorigenicity through internalization or inheritance. Because numerous reviews have been written about the formation of the MB and its function during abscission (Steigemann and Gerlich, 2009; D’Avino and Capalbo, 2016; Antanaviciute et al., 2018; Peterman and Prekeris, 2019), this review will mainly focus on the postmitotic MB roles as a polarity cue and postmitotic signaling organelle.
ROLE OF THE MB DURING ABSCISSION
To complete cytokinesis, cells need to cleave the intercellular bridge that links the two daughter cells. One of the best-described roles for the MB is to recruit abscission-regulating proteins during cytokinesis (Dambournet et al., 2011; Elia et al., 2011; Schiel et al., 2012; Mierzwa and Gerlich, 2014). A complex of proteins that controls abscission at the MB is the endosomal sorting complex required for transport (ESCRT) (Henne et al., 2011), originally described as a complex that mediates budding and scission of intracellular vesicles during formation of multivesicular bodies, eventually leading to lysosomal degradation. The ESCRT complex is primarily composed of four protein complexes: ESCRT-0, -I, -II, and -III, along with the AAA-ATPase and VPS4 (Fededa and Gerlich, 2012). Briefly, it was suggested that the ESCRT-I component TSG101 and/or ALIX interact directly with the MB protein CEP55 (Yang et al., 2008; Elia et al., 2011; Christ et al., 2016). However, recent studies show that the loss of CEP55 still results in complete abscission, suggesting that other CEP55-independent mechanisms may also contribute to ESCRT-III recruitment (Tedeschi et al., 2020). Indeed, in Drosophila, which lacks CEP55, TSG101 and ALIX are recruited by centralspindlin component Pavarotti (MKLP1 in vertebrates) (Lie-Jensen et al., 2019). Following the recruitment of TSG101/ALIX to the MB, the ESCRT-III complex is then targeted to the MB and subsequently to the abscission site. Previous studies working on the ESCRT complexes have shown that the ESCRT-III complex drives membrane scission during late-stage cytokinesis (Lafaurie-Janvore et al., 2013; Christ et al., 2016, 2017) by forming complex spiral structures with progressively smaller diameters that are capable of associating with the membrane, ultimately driving membrane scission (Elia et al., 2011; Guizetti et al., 2011; Cashikar et al., 2014; Mierzwa et al., 2017; Goliand et al., 2018). All these studies implicate MB as a staging site for initial recruitment of ESCRT components, as well as for regulating activation and spatiotemporal dynamics of ESCRT-III polymerization and abscission.
MBs AS A POLARITY CUE
In the past several years it has become apparent that MBs also have postmitotic functions, especially in serving as a polarity cue in many different cellular contexts. The idea of mitotic division serving as a polarity cue is not new, because it has been well-established that during yeast budding, the bud formation site leaves a “bud scar” that then directly regulates the activation and recruitment of a small monomeric GTPase, Cdc42, which in turn determines the site of the new bud during the next cycle of yeast budding (Nelson, 2003; Etienne-Manneville, 2004; Harris and Tepass, 2010; Miller et al., 2020). Similarly, recent work suggests that MB formation may regulate the formation of several polarized cellular structures in vertebrates, such as the apical lumen, neurites, and apical cilia (Schluter et al., 2009; Pollarolo et al., 2011; Buckley et al., 2013; Li et al., 2014; Wang et al., 2014; Bernabe-Rubio et al., 2016; Klinkert et al., 2016; Mangan et al., 2016; Lujan et al., 2017). The MB is perfectly suited to play the role of a polarity cue, because during cell division only one MB is formed and the location of this MB in many cases is tightly controlled by the cell. Below we summarize what is currently known about potential functions of the MB during cell polarization.
Role of MBs in apical lumen formation
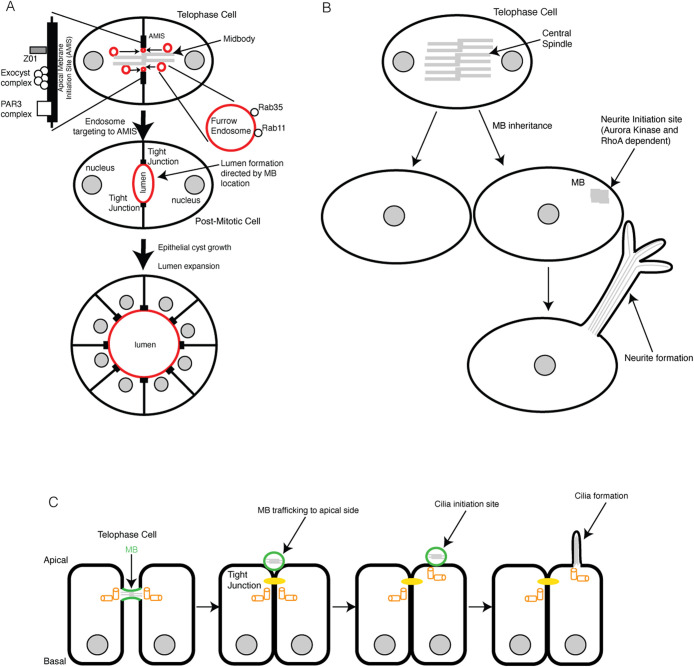
Epithelial tissues are polarized with an apical surface facing the lumen and basolateral surfaces connecting to neighboring cells or the extracellular matrix. During polarization, epithelial cells need to coordinate with each other to ensure that all their apical surfaces are pointed in the same direction, thus forming an apical lumen that eventually develops into the apical duct system (Figure 1A). How epithelial cells polarize to form the apical lumen has been a topic of study for many years. It has been shown that, at least in vitro, renal and hepatic epithelial cells initiate apical lumen formation after initial division of nonpolarized daughter cells. The MB appears to play a key role in this de novo lumen formation by acting as a symmetry-breaking structure and polarity cue. Studies using Madin–Darby canine kidney (MDCK) three-dimensional tissue culture models have shown that the formation of the MB during cell division marks the site of nascent apical lumen formation (Figure 1A) (Schluter et al., 2009; Li et al., 2014). To further support the role of the MB in apical lumen formation, it has been shown that the apical membrane initiation site (AMIS) interacts with the MB by forming ring-like structures around it, which then can act as a protein scaffolding complex that recruits apical cargo that is trafficked via Rab11 and Rab35 endosomes (Figure 1A) (Klinkert et al., 2016; Mangan et al., 2016). The targeting of these endosomes, along with proteins needed for abscission at the MB, is at least partially controlled by the exocyst complex, suggesting that abscission and cell polarity determination are linked cellular events (Figure 1A) (Klinkert et al., 2016; Mangan et al., 2016). Additional studies have shown that protein phosphatase PRL-3, commonly up-regulated in cancer, regulates apical lumen formation by controlling the MB localization, thus further suggesting the importance of the MB during cell and tissue polarization (Lujan et al., 2017). It is important to note that the MB plays a role in lumen formation not only in MDCK cells but hepatocytes as well. The polarity proteins in hepatocytes, Par3 and Mdr, along with the tight junction protein, ZO1, have all been shown to localize with the MB just before abscission occurs (Figure 1A) (Wang et al., 2014).
FIGURE 1:
Role of the MB as a polarity cue. (A) The formation of the MB marks the site of nascent apical lumen. (B) The daughter cell that inherits the MB, along with Aurora kinase and RhoA, marks the site for the formation of the first neurite. (C) Localization of the MB to the apical side of the cell after asymmetric abscission marks the site and leads to the formation of a primary cilium.
The idea that MBs play an important role in apical lumen formation is a relatively new concept that has several questions left to be answered. While most studies looking at the role of the MB in apical lumen formation have been done using cell culture, the question remains whether MBs play a role in cell polarity in vivo. A few studies have started to answer this question. A study using Caenorhabditis elegans showed that MBs translocate and align at the intestinal lumen formation site (Bai et al., 2018). Additionally, work in Drosophila melanogaster showed that disruption of the MB causes cell polarity defects, furthering supporting the idea that the MB plays a crucial role in acting as a hub for polarity proteins during lumen formation (Le Bras and Le Borgne, 2014; Daniel et al., 2018). Using zebrafish, studies have shown that the abscission site marks the apical lumen site during neural tube formation in zebrafish, and the MB provides a location for directed membrane transport of apical polarity proteins needed for lumen formation (Distel et al., 2011; Girdler et al., 2013; Rathbun et al., 2020). Finally, a study using frog epithelium showed that MBs migrate and associate with tight junctions and apical poles (Higashi et al., 2016). While these in vivo studies have intriguing observations that suggest a role for the MB in maintaining and establishing cell polarity, many studies will have to be done to elucidate the exact mechanism of how the MB fulfills this role.
Role of MBs in neurite formation
In addition to playing a role in epithelial cells and hepatocytes, the MB has been shown to regulate neuronal polarity by marking the site for the first neurite during Drosophila development (Figure 1B) (Pollarolo et al., 2011). Pollarolo et al. (2011) showed that furrow molecules, RhoA and Aurora kinase, were targeted to the MB and are the earliest landmarks of neuronal polarity, suggesting that the MB must be present for the first neurite to form (Figure 1B). Additionally, in zebrafish, the MBs regulate development of the neural rod, because oriented cell divisions (C-divisions) have a dominant influence in establishing the site of the nascent neuronal lumen (Buckley et al., 2013). While there are not many studies on the role of the MB in regulating neurite formation, the studies that are present suggest a strong role for the MB in the proper formation of the first neurite during development. Further studies are needed to determine which proteins or protein complexes interact with the MB, or are part of the MB, in order to fulfill this function.
Role of MBs in cilia formation
In the 1960s, researchers reported a negative correlation between mitotic cell division and cilia formation (Dingemans, 1969), with subsequent studies elucidating the mechanisms of the pathways that result in the resorption of the cilia in early mitosis (Pugacheva et al., 2007). The reason for the inhibition of ciliation by mitotic cell division is primarily that the same centrosomes are used to assemble the mitotic spindle and cilia; thus, at any given time cells can either divide or ciliate, but not both. Until recently, there had been no evidence to support the idea that MBs can stimulate ciliogenesis. However, a recent study suggested that the MB may have a role in stimulating cilia formation (Bernabe-Rubio et al., 2016), although this idea remains quite controversial. These studies suggested that after the final stages of mitosis, the MB remnant remains associated with one of the daughter cells and that over time the MB remnant moves to the apical surface (Figure 1C) (Bernabe-Rubio et al., 2016). The model from this study suggests that after the remnant moves along the apical surface and becomes proximal to the centrosome at the center of the apical surface, ciliogenesis occurs (Bernabe-Rubio et al., 2016), and removal of MBs inhibits formation of the cilium. Thus, it was proposed that the MB may somehow induce cilia formation (Bernabe-Rubio et al., 2016). This is an intriguing and novel connection between MBs and ciliogenesis; however, several questions need to be answered before this becomes an accepted model. Specifically, how do MBs stimulate cilia formation? Do MBs mediate transfer-specific proteins to the basal body, licensing it to form cilia? If yes, what are they? Finally, how do nondividing cells form cilia if they need MBs to license them?
MBs AS REGULATORS OF CELL FATE AND DIFFERENTIATION
Numerous studies have clearly defined that in certain contexts MBs can serve as polarity cues. In all these cases, cell polarization is tightly connected to cell division and MBs appear to predominately function as a physical landmark rather than a signaling structure. Recent work, however, suggested that MBs can also function as a postmitotic signaling organelle that affects cell fate, proliferation, and differentiation long after completion of mitotic cell division.
Role of the MB and the central spindle in regulating differential segregation of signaling endosomes
Asymmetric division has emerged as a key contributor to the process that allows the generation of two daughter cells that assume different fates during development. At least in principle, asymmetric cell division leads to differential inheritance of various signaling molecules that ultimately either keep a daughter cell in a stem cell–like state or lead to its differentiation (Neumuller and Knoblich, 2009). Many known mechanisms lead to asymmetric inheritance of signaling molecules, and recently the MB and central spindle have emerged as important regulators of this process. It was shown that in some cases, partitioning of signaling molecules is driven by differential distribution of signaling endosomes at the MB due to asymmetry in the central spindle microtubule (Coumailleau et al., 2009; Derivery et al., 2015; Kressmann et al., 2015). For example, Smad anchor for receptor activation (Sara) signaling endosomes have been found to accumulate on one side of the MB due to the asymmetric distribution of the microtubules, where the side with fewer microtubules will obtain more of the Sara endosomes after abscission (Derivery et al., 2015). Studies on Sara endosomes have resulted in the discovery of the importance of asymmetric division in downstream signaling activation. When these Sara endosomes, containing the molecules for downstream Notch signaling, are distributed unequally due to the microtubule content, one daughter cell inherits more Sara endosomes, resulting in that cell having higher Notch signaling activity than the other daughter cell (Coumailleau et al., 2009; Derivery et al., 2015). Another study was able to show this phenomenon in neurons, suggesting that the daughter cell that inherits the Sara endosomes divides again before differentiating into a mature neuron, as compared with the other daughter cell, which differentiates into a neuron before dividing again (Kressmann et al., 2015). Overall, differential distribution of signaling molecules, driven by the central spindle and MB asymmetry during cell division, has emerged as one of the contributors to establishing daughter cell fate and differentiation.
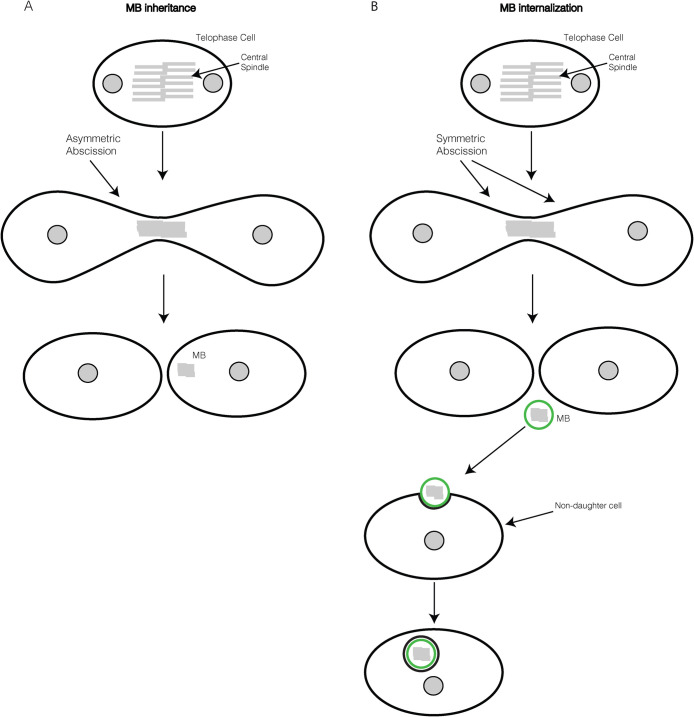
MB inheritance versus internalization
Traditionally, it was thought that after abscission, the MB was either discarded into the extracellular space or degraded via autophagosomal pathways (Barr and Gruneberg, 2007; Pohl and Jentsch, 2009). However, recent studies suggested that the postmitotic MB can be inherited during cell division, which may pass on MB-associated signaling cues to one of the daughter cells (Figure 2A) (Wilcock et al., 2007; Ettinger et al., 2011; Kuo et al., 2011; Pollarolo et al., 2011; Singh and Pohl, 2014). MB inheritance is thought to be caused by the asymmetric abscission on only one side of the MB, resulting in one of the daughter cells retracting the intracellular bridge and therefore inheriting the MB (Figure 2A). Asymmetric MB inheritance is an intriguing concept because it may contribute to unequal segregation of MB-associated cell fate determinants between the two daughter cells, similar to what has been mentioned in the preceding section. Conversely, other studies suggest that extracellular MBs can be internalized by other cells and function as a signaling platform that affects cell differentiation and proliferation (Figure 2B) (Marzesco et al., 2005; Dubreuil et al., 2007; Ettinger et al., 2011; Crowell et al., 2014). This model suggests that abscission occurs symmetrically, releasing the MB into the extracellular matrix, where it can be taken in by one of the daughter cells or surrounding non–daughter cells (Figure 2B). This internalization of the MB results in a double membrane–bound organelle, also known as MBsome, that has been shown to remain in the cytosol for a prolonged time while presumably signaling to regulate cell fate and proliferation (Figure 2B) (Peterman et al., 2019; Rai et al., 2021). While studies have proposed two different mechanisms of intracellular MB accumulation, recent data suggest that in tissue culture, postmitotic MBs accumulate within cells predominantly via internalization (Crowell et al., 2014). It is important to note that most of the studies looking at inheritance versus internalization have used HeLa or MDA-MB-231 cells. Thus, it is possible that certain cell types may prefer to use the MB-inheritance pathway. Indeed, a recent study looking at polarized MDCK cells suggests that the MB remains in contact with one of the daughter cells by a thin intercellular bridge instead of being internalized (Bernabe-Rubio et al., 2016).
FIGURE 2:
MB inheritance or internalization. (A) The MB is inherited by one of the daughter cells due to the asymmetric abscission of the intercellular bridge. (B) The MB is released into the extracellular space due to the symmetric abscission of the intercellular bridge, allowing for the internalization of the MB by a non–daughter cell.
MB accumulation and its role in regulating stemness and cancer progression
A persistent question in the field is whether intracellular MB accumulation plays a role in maintaining stemness. Indeed, while some work has shown the role of the postmitotic MB in these processes, the data remain somewhat confusing and controversial. For example, a recent study has shown that upon differentiation of stem cells, MBs are released at a greater rate (Ettinger et al., 2011), suggesting that MBs may contribute to signaling that maintains cell stemness. Consistent with this hypothesis, several studies have shown that stem cells and cancer stem cells can accumulate MBs (Ettinger et al., 2011; Kuo et al., 2011). However, the correlation between MB accumulation and stemness is not always clear. Indeed, recent studies looking at D. melanogaster suggested that the gender of the fly will determine the fate of the MB in germ stem cells (Salzmann et al., 2014). It was shown in male flies that germ stem cells inherit the mother centrosome but release the MB, while the female germ stem cells retain the mother centrosome and MB, suggesting that it is a context-dependent mechanism as to whether the MB is required for maintaining stemness (Salzmann et al., 2014). Additionally, MB accumulation in cancer progression has become a recent topic, as studies have shown that the intracellular MB accumulation might play an important role in stimulating cancer progression. It was suggested that intracellular MB accumulation is induced by inhibiting MB degradation by autophagy, therefore resulting in increased tumorigenicity (Kuo et al., 2011). Similarly, work in our lab has identified a protein responsible for MB degradation, named FYCO1, and if FYCO1 is lost, an increase in MB accumulation and invasive capacity was found in squamous cell carcinoma (Dionne et al., 2017). Finally, some studies have shown that MB accumulation leads to increases in proliferation and anchorage-independent growth in HeLa and MDA-MB-231 cells (Kuo et al., 2011; Peterman et al., 2019; Rai et al., 2021). Thus, while there are intriguing correlations between postmitotic MB and stemness, it is clear that postmitotic MBs play different roles in different contexts, and it is unlikely that postmitotic MBs will emerge as a universal stemness maker.
Role of the MB as an intracellular signaling organelle
Until recently, the mechanism of how MBs were internalized into cells was poorly understood. The first characterization of mechanisms mediating MB internalization came from research using C. elegans embryos (Chai et al., 2012). It was shown that during early development, C. elegans uses a Rac1 GTPase and actin-dependent mechanisms to internalize MBs that are left over after the first and second embryonic divisions (Chai et al., 2012). Since then, additional experiments from several laboratories have shown that in mammalian cells, Rac1 and actin-dependent polymerization are required for MB internalization (Crowell et al., 2014; Peterman et al., 2019; Rai et al., 2021). Based on these studies, it has been suggested that MBs are internalized by using a specialized MB receptor, similar to the receptors used by macrophages to detect apoptotic bodies. Importantly, it has been shown that C. elegans uses apoptotic corpse receptors that bind to phosphatidylserine (PS) to internalize MBs (Crowell et al., 2014). Apoptotic bodies flip their PS to the outer membrane leaflet in order to be recognized by macrophages; thus, PS is often considered to be an “eat me” signal (Dupuy and Caron, 2008; Flannagan et al., 2012). In a recent study using mammalian cells, MFG-E8 was identified as an adaptor protein that bridges PS on the outer leaflet of MBs and αVβ3 integrins on the cell surface (Peterman et al., 2019). Consistent with these findings, a recent lipidomic screen shows that PS is highly enriched in MBs and that the PS accumulates at the outer leaflet of the MB, providing strong evidence for a PS-dependent internalization mechanism (Atilla-Gokcumen et al., 2014; Peterman et al., 2019). Another interesting point of study is trying to elucidate the mechanism of how the MB tethers to the internalizing cell. A recent study suggests that the BST2/tetherin, originally described as a protein that blocks the release of enveloped viruses from the cell surface of infected cells (Neil et al., 2008; Van Damme et al., 2008; Neil, 2013; Sauter, 2014), plays a similar role in the retention of MBs (Presle et al., 2021). The study was able to show that BST2 is enriched at the MB and that upon depletion of BST2, the MB was detached from the cell surface and accumulated in the extracellular medium or taken in by other cells (Presle et al., 2021). Overall, these studies have shown that internalization of MBs is a Rac1- and actin-dependent mechanism, with recognition of the MB appearing to be dependent on the presence of PS on the outer leaflet of the MB.
It is now well-established that the MB plays various roles in more than just cell division. However, the mechanisms of how MB-dependent signaling occurs remain poorly understood. When MBs are internalized, they are encapsulated in a double membrane (Figure 2B), which leads to the hypothesis that the signaling must occur through some type of transmembrane protein or receptor that is found in the membrane. To prove this hypothesis, there are studies showing that internalized MBs, which have an enrichment of PS on the outer leaflet, along with MFG-E8, can bind and activate αVβ3 integrins, leading to the activation of the FAK-dependent signaling pathway (Peterman et al., 2019). Intriguingly, cells that have internalized MBs become anchorage-independent, presumably due to the integrin-dependent inside-in signal coming from the internalized postmitotic MB (Peterman et al., 2019; Rai et al., 2021). A similar type of inside-in signaling coming from signaling endosomes has been previously reported in cancer cells; thus, internalized MBs can be considered as a type of MB-associated signaling endosome or MBsome (Hamidi and Ivaska, 2018; Peterman et al., 2019). While the MBsome has been shown to increase the ability of a cell to become anchorage-dependent, presumably via integrin signaling, little is known about the specific mechanisms that drive this process. A recent study has shown that ligated epidermal growth factor receptors (EGFRs) are present on the MBsome and may contribute to MBsome-dependent proliferation (Peterman et al., 2019) because the clustering and cosignaling from integrins and receptor tyrosine kinases (RTKs), like EGFR, are required for obtaining the full complement of RTK signaling (Guo et al., 2006; Barrow-McGee et al., 2016). The fact that both integrins and EGFR are found on MBsomes leads to the intriguing possibility that MBsomes can be a hub for RTK signaling. However, further studies need to be completed to fully validate this hypothesis. The search for other RTKs that may be recruited to the MBsome is the next step in elucidating the mechanism behind which the MBsome can act as a signaling hub.
SUMMARY AND FUTURE DIRECTIONS
The fate of the postmitotic MB is much less well-understood than other MB functions. Thus, there are many questions that remain to be answered in order to define the molecular mechanism mediating postmitotic MB functions. While recent work has been able to describe multiple scenarios where cells use postmitotic MBs to regulate stemness, tumorigenicity, and proliferation, the problem with these studies is that they seem to be cell dependent or organism dependent, making it hard to define a more generalized theme for what signals the MB is passing on. One possibility is that MBs derived from different cell types may contain different proteins (or RNAs), thus leading to different signaling. Alternatively, cells may respond to MB inheritance/internalization differentially depending on the context. Many of these cell- and tissue-specific MB roles remain to be defined and certainly will be a focus of many studies in the future.
The other big mystery in the field of postmitotic MB function is how internalized MBs can signal when they are surrounded by two membranes. Recent work proposed that MBsomes can mediate inside-in signaling (Peterman et al., 2019), which does not require the contents of the MB to enter the cytosol. While this is the case, at least initially, it is likely that eventually these MB membranes fuse, allowing the release of MB-associated proteins and RNAs into cytosol. Several proteomic studies have identified numerous MB-associated proteins (Skop et al., 2004; Gnazzo et al., 2016; Capalbo et al., 2019; Peterman et al., 2019); thus, it is plausible that the release of these proteins (or MB-associated RNAs) is another way of conveying MB-associated signal. However, whether these MBsome membranes actually fuse, what mechanisms are mediating this fusion, and what MB-associated signals are released in cytosol to mediate MB function remain to be determined.
Acknowledgments
We appreciate Migle Prekeryte for critical reading and editing of the manuscript. The work in the R. P. laboratory is funded by National Institutes of Health grants DK064380, GM122768, and GM143774.
Abbreviations used:
- ALIX
ALG-2-interacting protein X
- AMIS
apical membrane initiation site
- BST2
bone marrow stromal cell antigen 2
- Cdc42
cell division cycle 42
- CEP55
centrosomal protein 55
- EGFR
epidermal growth factor receptor
- ESCRT
endosomal sorting complex required for transport
- FYCO1
FYVE and coiled-coil domain autophagy adaptor 1
- MB
midbody
- Mbsomes
midbody signaling organelle
- MDCK
Madine-Darby Canine Kidney
- Mdr
multidrug resistant
- MFG-E8
milk fat globule-egf factor 8
- MKLP1
mitotic kinesin-like protein 1
- Par3
partitioning defective 3
- PRL-3
phosphatase of regenerating liver 3
- PS
phosphatidylserine
- Rab11
ras-related protein 11
- Rab35
ras-related protein 35
- Rac1
Ras-related C3 botulinum toxin substrate 1
- RhoA
Ras homolog family member A
- RTK
receptor tyrosine kinases
- Sara
smad anchor for receptor activation
- TSG101
tumor susceptibility gene 101
- VPS4
vacuolar protein sorting-associated protein 4
- ZO1
zonula occludens protein 1.
Footnotes
REFERENCES
- Antanaviciute I, Gibieza P, Prekeris R, Skeberdis VA (2018). Midbody: from the regulator of cytokinesis to postmitotic signaling organelle. Medicina (Kaunas) 54, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atilla-Gokcumen GE, Muro E, Relat-Goberna J, Sasse S, Bedigian A, Coughlin ML, Garcia-Manyes S, Eggert US (2014). Dividing cells regulate their lipid composition and localization. Cell 156, 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Lee P-Y, Chen C-Y, Simmons JR, Nebenfuehr B, Mitchell D, Klebanow LR, Mattson N, Sorensen Turpin CG, Chen B-C, et al. (2018). Aurora B is required for programmed variations of cytokinesis during morphogenesis in the C. elegans embryo. bioRxiv, 319657. [Google Scholar]
- Barr FA, Gruneberg U (2007). Cytokinesis: placing and making the final cut. Cell 131, 847–860. [DOI] [PubMed] [Google Scholar]
- Barrow-McGee R, Kishi N, Joffre C, Menard L, Hervieu A, Bakhouche BA, Noval AJ, Mai A, Guzman C, Robbez-Masson L, et al. (2016). Beta 1-integrin-c-Met cooperation reveals an inside-in survival signalling on autophagy-related endomembranes. Nat Commun 7, 11942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabe-Rubio M, Andrés G, Casares-Arias J, Fernandez-Barrera J, Rangel L, Reglero-Real N, Gershlick DC, Fernandez JJ, Millan J, Correas I, et al. (2016). Novel role for the midbody in primary ciliogenesis by polarized epithelial cells. J Cell Biol 214, 259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CE, Ren X, Ward LC, Girdler GC, Araya C, Green MJ, Clark BS, Link BA, Clarke JD (2013). Mirror-symmetric microtubule assembly and cell interactions drive lumen formation in the zebrafish neural rod. EMBO J 32, 30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capalbo L, Bassi ZI, Geymonat M, Todesca S, Copoiu L, Enright AJ, Callaini G, Riparbelli MG, Yu L, Choudhary JS, et al. (2019). The midbody interactome reveals unexpected roles for PP1 phosphatases in cytokinesis. Nat Commun 10, 4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashikar AG, Shim S, Roth R, Maldazys MR, Heuser JE, Hanson PI (2014). Structure of cellular ESCRT-III spirals and their relationship to HIV budding. eLife 3, e02184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Tian D, Yang Y, Feng G, Cheng Z, Li W, Ou G (2012). Apoptotic regulators promote cytokinetic midbody degradation in C. elegans. J Cell Biol 199, 1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ L, Raiborg C, Wenzel EM, Campsteijn C, Stenmark H (2017). Cellular functions and molecular mechanisms of the ESCRT membrane-scission machinery. Trends Biochem Sci 42, 42–56. [DOI] [PubMed] [Google Scholar]
- Christ L, Wenzel EM, Liestol K, Raiborg C, Campsteijn C, Stenmark H (2016). ALIX and ESCRT-I/II function as parallel ESCRT-III recruiters in cytokinetic abscission. J Cell Biol 212, 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coumailleau F, Furthauer M, Knoblich JA, Gonzalez-Gaitan M (2009). Directional Delta and Notch trafficking in Sara endosomes during asymmetric cell division. Nature 458, 1051–1055. [DOI] [PubMed] [Google Scholar]
- Crowell EF, Gaffuri AL, Gayraud-Morel B, Tajbakhsh S, Echard A (2014). Engulfment of the midbody remnant after cytokinesis in mammalian cells. J Cell Sci 127, 3840–3851. [DOI] [PubMed] [Google Scholar]
- Dambournet D, Machicoane M, Chesneau L, Sachse M, Rocancourt M, El Marjou A, Formstecher E, Salomon R, Goud B, Echard A (2011). Rab35 GTPase and OCRL phosphatase remodel lipids and F-actin for successful cytokinesis. Nat Cell Biol 13, 981–988. [DOI] [PubMed] [Google Scholar]
- Daniel E, Daude M, Kolotuev I, Charish K, Auld V, Le Borgne R (2018). Coordination of septate junctions assembly and completion of cytokinesis in proliferative epithelial tissues. Curr Biol 28, 1380–1391.e1384. [DOI] [PubMed] [Google Scholar]
- D’Avino PP, Capalbo L (2016). Regulation of midbody formation and function by mitotic kinases. Semin Cell Dev Biol 53, 57–63. [DOI] [PubMed] [Google Scholar]
- Derivery E, Seum C, Daeden A, Loubery S, Holtzer L, Julicher F, Gonzalez-Gaitan M (2015). Polarized endosome dynamics by spindle asymmetry during asymmetric cell division. Nature 528, 280–285. [DOI] [PubMed] [Google Scholar]
- Dingemans KP (1969). The relation between cilia and mitoses in the mouse adenohypophysis. J Cell Biol 43, 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne LK, Peterman E, Schiel J, Gibieza P, Skeberdis VA, Jimeno A, Wang XJ, Prekeris R (2017). FYCO1 regulates accumulation of post-mitotic midbodies by mediating LC3-dependent midbody degradation. J Cell Sci 130, 4051–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel M, Jennifer CH, Koster RW (2011). In vivo cell biology using Gal4-mediated multicolor subcellular labeling in zebrafish. Commun Integr Biol 4, 336–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Marzesco AM, Corbeil D, Huttner WB, Wilsch-Brauninger M (2007). Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1. J Cell Biol 176, 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy AG, Caron E (2008). Integrin-dependent phagocytosis: spreading from microadhesion to new concepts. J Cell Sci 121, 1773–1783. [DOI] [PubMed] [Google Scholar]
- Elia N, Sougrat R, Spurlin TA, Hurley JH, Lippincott-Schwartz J (2011). Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission. Proc Natl Acad Sci USA 108, 4846–4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S (2004). Cdc42—the centre of polarity. J Cell Sci 117, 1291–1300. [DOI] [PubMed] [Google Scholar]
- Ettinger AW, Wilsch-Brauninger M, Marzesco AM, Bickle M, Lohmann A, Maliga Z, Karbanova J, Corbeil D, Hyman AA, Huttner WB (2011). Proliferating versus differentiating stem and cancer cells exhibit distinct midbody-release behaviour. Nat Commun 2, 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fededa JP, Gerlich DW (2012). Molecular control of animal cell cytokinesis. Nat Cell Biol 14, 440–447. [DOI] [PubMed] [Google Scholar]
- Flannagan RS, Jaumouille V, Grinstein S (2012). The cell biology of phagocytosis. Annu Rev Pathol 7, 61–98. [DOI] [PubMed] [Google Scholar]
- Girdler GC, Araya C, Ren X, Clarke JD (2013). Developmental time rather than local environment regulates the schedule of epithelial polarization in the zebrafish neural rod. Neural Dev 8, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnazzo MM, Uhlemann EE, Villarreal AR, Shirayama M, Dominguez EG, Skop AR (2016). The RNA-binding protein ATX-2 regulates cytokinesis through PAR-5 and ZEN-4. Mol Biol Cell 27, 3052–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goliand I, Adar-Levor S, Segal I, Nachmias D, Dadosh T, Kozlov MM, Elia N (2018) Resolving ESCRT-III spirals at the intercellular bridge of dividing cells using 3D STORM. Cell Rep 24, 1756–1764. [DOI] [PubMed] [Google Scholar]
- Guizetti J, Schermelleh L, Mantler J, Maar S, Poser I, Leonhardt H, Muller-Reichert T, Gerlich DW (2011). Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science 331, 1616–1620. [DOI] [PubMed] [Google Scholar]
- Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, Giancotti FG (2006). Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 126, 489–502. [DOI] [PubMed] [Google Scholar]
- Hamidi H, Ivaska J (2018). Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer 18, 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KP, Tepass U (2010). Cdc42 and vesicle trafficking in polarized cells. Traffic 11, 1272–1279. [DOI] [PubMed] [Google Scholar]
- Henne WM, Buchkovich NJ, Emr SD (2011). The ESCRT pathway. Dev Cell 21, 77–91. [DOI] [PubMed] [Google Scholar]
- Higashi T, Arnold TR, Stephenson RE, Dinshaw KM, Miller AL (2016). Maintenance of the epithelial barrier and remodeling of cell-cell junctions during cytokinesis. Curr Biol 26, 1829–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkert K, Rocancourt M, Houdusse A, Echard A (2016). Rab35 GTPase couples cell division with initiation of epithelial apico-basal polarity and lumen opening. Nat Commun 7, 11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressmann S, Campos C, Castanon I, Furthauer M, Gonzalez-Gaitan M (2015). Directional Notch trafficking in Sara endosomes during asymmetric cell division in the spinal cord. Nat Cell Biol 17, 333–339. [DOI] [PubMed] [Google Scholar]
- Kuo TC, Chen CT, Baron D, Onder TT, Loewer S, Almeida S, Weismann CM, Xu P, Houghton JM, Gao FB, et al. (2011). Midbody accumulation through evasion of autophagy contributes to cellular reprogramming and tumorigenicity. Nat Cell Biol 13, 1214–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaurie-Janvore J, Maiuri P, Wang I, Pinot M, Manneville JB, Betz T, Balland M, Piel M (2013). ESCRT-III assembly and cytokinetic abscission are induced by tension release in the intercellular bridge. Science 339, 1625–1629. [DOI] [PubMed] [Google Scholar]
- Le Bras S, Le Borgne R (2014). Epithelial cell division—multiplying without losing touch. J Cell Sci 127, 5127–5137. [DOI] [PubMed] [Google Scholar]
- Li D, Mangan A, Cicchini L, Margolis B, Prekeris R (2014). FIP5 phosphorylation during mitosis regulates apical trafficking and lumenogenesis. EMBO Rep 15, 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie-Jensen A, Ivanauskiene K, Malerod L, Jain A, Tan KW, Laerdahl JK, Liestol K, Stenmark H, Haglund K (2019). Centralspindlin recruits ALIX to the midbody during cytokinetic abscission in Drosophila via a mechanism analogous to virus budding. Curr Biol 29, 3538–3548.e3537. [DOI] [PubMed] [Google Scholar]
- Lujan P, Rubio T, Varsano G, Kohn M (2017). Keep it on the edge: the post-mitotic midbody as a polarity signal unit. Commun Integr Biol 10, e1338990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan AJ, Sietsema DV, Li D, Moore JK, Citi S, Prekeris R (2016). Cingulin and actin mediate midbody-dependent apical lumen formation during., polarization of epithelial cells. Nat Commun 7, 12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzesco AM, Janich P, Wilsch-Brauninger M, Dubreuil V, Langenfeld K, Corbeil D, Huttner WB (2005). Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci 118, 2849–2858. [DOI] [PubMed] [Google Scholar]
- Mierzwa B, Gerlich DW (2014). Cytokinetic abscission: molecular mechanisms and temporal control. Dev Cell 31, 525–538. [DOI] [PubMed] [Google Scholar]
- Mierzwa BE, Chiaruttini N, Redondo-Morata L, von Filseck JM, Konig J, Larios J, Poser I, Muller-Reichert T, Scheuring S, Roux A, Gerlich DW (2017). Dynamic subunit turnover in ESCRT-III assemblies is regulated by Vps4 to mediate membrane remodelling during cytokinesis. Nat Cell Biol 19, 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Kang PJ, Park HO (2020). Regulation of Cdc42 for polarized growth in budding yeast. Microb Cell 7, 175–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil SJD (2013). The antiviral activities of tetherin. In: Intrinsic Immunity. Current Topics in Microbiology and Immunology, ed. Cullen B, vol. 371, Berlin: Springer, 67–104. [DOI] [PubMed] [Google Scholar]
- Neil SJ, Zang T, Bieniasz PD (2008). Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451, 425–430. [DOI] [PubMed] [Google Scholar]
- Nelson WJ (2003). Mum, this bud’s for you: where do you want it? Roles for Cdc42 in controlling bud site selection in Saccharomyces cerevisiae. Bioessays 25, 833–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumuller RA, Knoblich JA (2009). Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev 23, 2675–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterman E, Gibieza P, Schafer J, Skeberdis VA, Kaupinis A, Valius M, Heiligenstein X, Hurbain I, Raposo G, Prekeris R (2019). The post-abscission midbody is an intracellular signaling organelle that regulates cell proliferation. Nat Commun 10, 3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterman E, Prekeris R (2019). The postmitotic midbody: regulating polarity, stemness, and proliferation. J Cell Biol 218, 3903–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl C, Jentsch S (2009). Midbody ring disposal by autophagy is a post-abscission event of cytokinesis. Nat Cell Biol 11, 65–70. [DOI] [PubMed] [Google Scholar]
- Pollarolo G, Schulz JG, Munck S, Dotti CG (2011). Cytokinesis remnants define first neuronal asymmetry in vivo. Nat Neurosci 14, 1525–1533. [DOI] [PubMed] [Google Scholar]
- Presle A, Fremont S, Salles A, Commere PH, Sassoon N, Berlioz-Torrent C, Gupta-Rossi N, Echard A (2021). The viral restriction factor tetherin/BST2 tethers cytokinetic midbody remnants to the cell surface. Curr Biol 31, 2203–2213.e2205. [DOI] [PubMed] [Google Scholar]
- Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA (2007). HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129, 1351–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai A, Greening DW, Xu R, Chen M, Suwakulsiri W, Simpson RJ (2021). Secreted midbody remnants are a class of extracellular vesicles molecularly distinct from exosomes and microparticles. Commun Biol 4, 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbun LI, Colicino EG, Manikas J, O’Connell J, Krishnan N, Reilly NS, Coyne S, Erdemci-Tandogan G, Garrastegui A, Freshour J, et al. (2020). Cytokinetic bridge triggers de novo lumen formation in vivo. Nat Commun 11, 1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzmann V, Chen C, Chiang CY, Tiyaboonchai A, Mayer M, Yamashita YM (2014). Centrosome-dependent asymmetric inheritance of the midbody ring in Drosophila germline stem cell division. Mol Biol Cell 25, 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter D (2014). Counteraction of the multifunctional restriction factor tetherin. Front Microbiol 5, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiel JA, Simon GC, Zaharris C, Weisz J, Castle D, Wu CC, Prekeris R (2012). FIP3-endosome-dependent formation of the secondary ingression mediates ESCRT-III recruitment during cytokinesis. Nat Cell Biol 14, 1068–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter MA, Pfarr CS, Pieczynski J, Whiteman EL, Hurd TW, Fan S, Liu CJ, Margolis B (2009). Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol Biol Cell 20, 4652–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Pohl C (2014). Coupling of rotational cortical flow, asymmetric midbody positioning, and spindle rotation mediates dorsoventral axis formation in C. elegans. Dev Cell 28, 253–267. [DOI] [PubMed] [Google Scholar]
- Skop AR, Liu H, Yates J 3rd, Meyer BJ, Heald R (2004). Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science 305, 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigemann P, Gerlich DW (2009). Cytokinetic abscission: cellular dynamics at the midbody. Trends Cell Biol 19, 606–616. [DOI] [PubMed] [Google Scholar]
- Tedeschi A, Almagro J, Renshaw MJ, Messal HA, Behrens A, Petronczki M (2020). Cep55 promotes cytokinesis of neural progenitors but is dispensable for most mammalian cell divisions. Nat Commun 11, 1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J (2008). The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3, 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Yanger K, Stanger BZ, Cassio D, Bi E (2014). Cytokinesis defines a spatial landmark for hepatocyte polarization and apical lumen formation. J Cell Sci 127, 2483–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock AC, Swedlow JR, Storey KG (2007). Mitotic spindle orientation distinguishes stem cell and terminal modes of neuron production in the early spinal cord. Development 134, 1943–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Rismanchi N, Renvoise B, Lippincott-Schwartz J, Blackstone C, Hurley JH (2008). Structural basis for midbody targeting of spastin by the ESCRT-III protein CHMP1B. Nat Struct Mol Biol 15, 1278–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]