Abstract
Background
COVID-19 vaccination and infection are speculated to increase the activity of immune-mediated diseases, including multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD). The aim of this study was to evaluate a short-term risk of relapse after COVID-19 vaccination and COVID-19 infection in patients with these demyelinating disorders of the central nervous system and to determine disease exacerbation risk factors.
Methods
Data in this retrospective, observational cohort study was collected via the Czech nationwide registry ReMuS from March 1, 2020, to October 30, 2021. We compared the proportion of patients with at least one clinical relapse in the 90 days following vaccination or infection to the 90-day intervals during the year before. For the evaluation of the risk factors of relapse, a comparison between groups with and without relapses after COVID-19 vaccination or infection was made.
Results
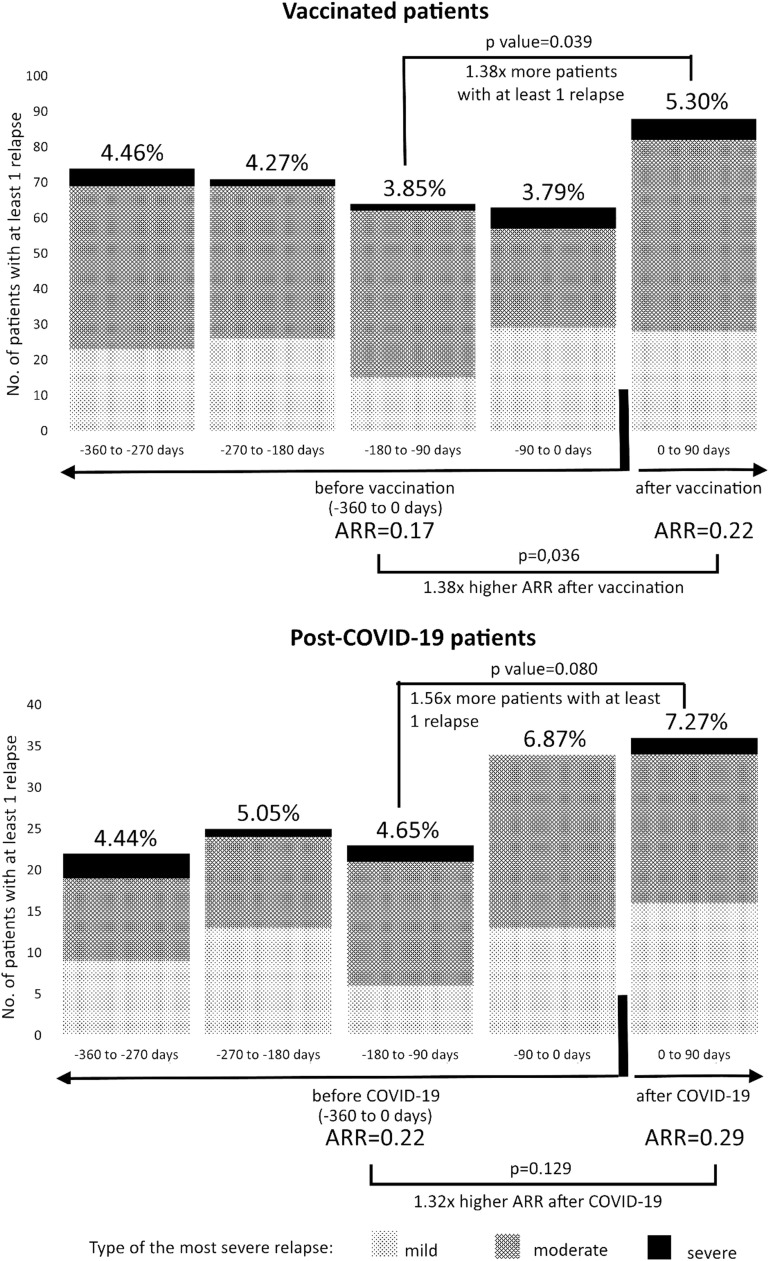
We identified 1661 vaccinated (90.11% BNT162b2) patients with MS without a history of COVID-19 and 495 unvaccinated patients with MS who experienced COVID-19. A mild increase in the proportion of patients with at least one clinical relapse (-360 to -270 days: 4.46%; -270 to -180: 4.27%; -180 to -90: 3.85%; -90 to 0: 3.79% vs. 0 to +90 days: 5.30%) after vaccination in patients with MS was observed, as well as a rise in the proportion of patients with at least one clinical relapse after COVID-19. Lower age was associated with MS relapse after vaccination or infection. Although there were only 17 vaccinated and eight post-COVID-19 patients with NMOSD, the results were broadly consistent with those of patients with MS.
Conclusion
There is a mild increase in the relapse incidence after the COVID-19 vaccination. The risks, however, need to be balanced against the risks of COVID-19 itself, also leading to the rise in relapse rate and particularly to morbidity and mortality.
Keywords: Multiple sclerosis, Neuromyelitis optica, COVID-19, Vaccination, Acute relapse
1. Introduction
Globally, there have been almost 300 million confirmed cases of coronavirus disease 19 (COVID-19), including nearly 3.5 million deaths, at the beginning of 2022 (World Health Organisation, 2022). The key element of strategies to reduce the severity of the disease and to suppress the spread of infection is vaccination. COVID-19 vaccines authorised by the European Medicines Agency (EMA) (European Medicines Agency, 2022) have been tested in randomised clinical trials (Baden et al., 2021; Polack et al., 2020; Sadoff et al., 2021; Voysey et al., 2021) which were designed to establish efficacy and safety in the general population. However, these clinical trials were not designed to evaluate safety profile in patients with multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD).
MS and NMOSD are chronic autoimmune inflammatory diseases of the central nervous system. Generally, vaccines work by stimulating the immune system. Their immunogenicity is key to achieving protection against specific pathogens. Therefore, there is one more potential risk of vaccination in patients with MS and NMOSD in comparison with the healthy population: the risk of induction of disease exacerbation. The risks, however, need to be balanced against the risks of COVID-19 itself, leading to morbidity and death even in people without disabilities. Moreover, numerous data is demonstrating a higher risk of more severe COVID-19 course in patients with MS and NMOSD treated with high-dose glucocorticoids or anti-CD20 monoclonal antibodies (Barzegar et al., 2021a; Prosperini et al., 2022; Stastna et al., 2021) and there are also data showing an association between systemic infection and risk of MS or NMOSD relapse (Correale et al., 2006; Ma et al., 2020).
To our knowledge, data assessing the effect of COVID-19 infection and vaccination on the risks of relapse in MS and NMOSD are scarce, inconsistent, and mostly evaluated on relatively small cohorts (Achiron et al., 2021; Barzegar et al., 2021b; Cai et al., 2021; di Filippo et al., 2021; Dinoto et al., 2021; Etemadifar et al., 2021; Fragoso et al., 2021; Jovicevic et al., 2021; Lotan et al., 2021b, 2021a). Thus, in the light of the ongoing global COVID-19 pandemic situation and the continuing need for vaccination, we aimed to evaluate the short-term risks of clinical relapse in the 90-day period after the first dose COVID-19 vaccine administration and COVID-19 infection as well as the safety profile of COVID-19 vaccines in patients with MS and NMOSD. Our goal was also to determine factors related to the potential increase of immediate disease activity.
2. Methods
2.1. Data collection
Two of the largest Czech MS centres representing almost one-third of the Czech MS population participated in this retrospective, observational cohort study. Data from the largest one (General University Hospital in Prague) were used for the main analysis, data from the second MS centre (Hospital Teplice) for the independent validation of results. The data was collected via the Czech nationwide registry ReMuS from March 1, 2020, to October 30, 2021. The guarantor of expertise of this registry is the Section for Neuroimmunology and Liquorology of the Czech Neurological Society. Data in register ReMuS are collected using standardized software iMed. Before the release of the coded patient-level data to investigators, there is a multiple-level quality control process (Horakova et al., 2019).
2.2. Standard protocol approvals, registrations, and patient consents
ReMuS was established by the Endowment Fund IMPULS (NF IMPULS, 2022) and approved by local ethics committees in each MS centre (The Ethics Committee of the General University Hospital in Prague and The Ethics Committee of The KZ a.s. - Hospital Teplice). ReMuS is based on informed consent, thus it is possible to use retrospective data for scientific and research purposes without requiring new approvals. An informed consent was obtained from all patients enroled in ReMuS.
2.3. Population of interest
The inclusion criteria for participants of this study were (1) MS or NMOSD diagnosis, (2) administration of at least one dose of COVID-19 vaccine or a COVID-19 diagnosis based on a positive result of a SARS-CoV-2 polymerase chain reaction test (PCR) or positive antigen test or positive serological test, (3) together with a healthcare professional completed vaccine adverse events (AEs) questionnaire (containing questions about most common AEs (Baden et al., 2021; Meo et al., 2021; Polack et al., 2020; Sadoff et al., 2021; European Medicines Agency, 2022; Voysey et al., 2021), their severity and free text option) or known outcome of acute SARS-CoV-2 infection (return to normal activities or end of self-isolation in asymptomatic cases; or death), (4) minimum of 90-day follow up taking as baseline the day of the first vaccine dose administration or COVID-19 symptoms appearance or positive COVID-19 laboratory test (what earlier). Patients who experienced both COVID-19 vaccination and infection in the 90-day period after baseline were excluded.
2.4. Variables assessed and definitions
Data on demographics, MS or NMOSD, COVID-19 course, vaccination (together with AEs and their severity) were collected (Table 1 , 2 and 3 ). MS exacerbation/relapse was defined as the development of a new neurological abnormality, or worsening of a pre-existing one, for more than 24 h. It must have been preceded by a stable or improving neurological state for at least 30 days, in the absence of concurrent fever, infection or steroid withdrawal. Severe relapse was defined by the increase of Expanded Disability Status Scale (EDSS) by 2.5 steps in total or by two steps in three and more functional systems (FS) or by hospitalisation due to MS or NMOSD relapse. Moderate relapse was defined by an increase of EDSS by one or two steps in total or increase by two steps in one or two FS or by one step in four or more FS. Mild relapse was defined by lower severity than a moderate one.
Table 1.
Clinical and demographic characteristics of MS patients at the date of the 1st dose of COVID-19 vaccine or COVID-9 onset.
| Vaccinated | Post-COVID-19 | |
|---|---|---|
| Study population | 1661 | 495 |
| Age, mean (SD), y | 48.49 (11.43) | 45.84 (11.00) |
| <55 | 1188 (71.52%) | 382 (77.17%) |
| >=55 | 473 (28.48%) | 113 (22.83%) |
| Female | 1202 (72.37%) | 365 (73.74%) |
| MS duration (SD), y | 17.33 (10.22) | 15.44 (9.46) |
| EDSS1 | ||
| EDSS < 4 | 1029 (63.44%) | 342 (70.95%) |
| EDSS >= 4 | 593 (36.56%) | 140 (29.05%) |
| DMT | 1259 (75.80%) | 396 (80.00%) |
| Interferons | 343 (20.53%) | 96 (19.39%) |
| Glatiramer acetate | 129 (7.77%) | 47 (9.49%) |
| Teriflunomide | 92 (5.54%) | 30 (6.06%) |
| Dimethyl fumarate | 92 (5.54%) | 22 (4.44%) |
| Natalizumab | 103 (6.20%) | 42 (8.48%) |
| Fingolimod | 273 (16.44%) | 79 (15.96%) |
| Ocrelizumab | 122 (7.34%) | 46 (9.29%) |
| Rituximab | 26 (1.57%) | 8 (1.62%) |
| Alemtuzumab | 19 (1.14%) | 6 (1.21%) |
| Cladribine | 60 (3.61%) | 20 (4.04%) |
| No DMT | 402 (24.20%) | 99 (20.00%) |
| COVID-19 vaccine | ||
| BNT162b2 (Comirnaty) – mRNA | 1495 (90.01%)2 | |
| Time between doses (SD), d | 38.47 (13.13) | |
| mRNA-1273 (Spikevax) – mRNA | 96 (5.78%)3 | |
| Time between doses (SD), d | 36.45 (8.22) | |
| AZD1222 (Vaxzevria) – vector | 57 (3,43%)4 | |
| Time between doses (SD), d | 76.86 (16.83) | |
| Gam-COVID-Vac (Sputnik V) – vector | 1 (0.06%)5 | |
| Time between doses, d | 21 | |
| Ad26.COV2.S (Janssen) – vector | 12 (0.72%) | |
| COVID-19 severity | ||
| Death | 1 (0.20%) | |
| Need of invasive ventilation or extracorporeal membrane oxygenation | 2 (0.40%) | |
| Need of non-invasive ventilation or high-flow oxygen therapy | 9 (1.82%) | |
| Need of supplemental oxygen | 11 (2.22%) | |
| Radiologically confirmed pneumonia | 25 (5.05%) | |
| Suspected pneumonia (dry cough, fever and shortness of breath) | 45 (9.09%) | |
| Symptomatic | 389 (78.59%) | |
| Asymptomatic | 13 (2.63%) | |
MS = multiple sclerosis; EDSS = expanded disability status scale; DMT = disease-modifying treatment; 1. EDSS is not available for all individuals, the percentage corresponds to the proportion of patients with a known value; 2. 1477 (98,80%) patients got 2 doses of the vaccine; 3. all patients got 2 doses of the vaccine; 4. 56 (98.25%) patients got 2 doses of the vaccine; 5. 2 (66.67%) patients got 2 doses of the vaccine.
Table 2.
Adverse events following vaccination.1
| Type of adverse event | Total | 1st Comirnaty | 2nd Comirnaty | 1st Spikevax | 2nd Spikevax | 1st Vaxzevria | 2nd Vaxzevria | Janssen |
|---|---|---|---|---|---|---|---|---|
| Fatigue | 693 | 288 | 326 | 24 | 36 | 13 | 4 | 2 |
| Pain in the injected extremity | 626 | 342 | 234 | 22 | 20 | 2 | 4 | 2 |
| Injection site pain | 569 | 295 | 216 | 26 | 18 | 8 | 6 | 0 |
| Headache | 226 | 90 | 108 | 9 | 10 | 5 | 3 | 1 |
| Worsening of neurological symptoms | 207 | 94 | 93 | 6 | 9 | 2 | 2 | 1 |
| Subfebrile temperature | 181 | 64 | 89 | 8 | 12 | 6 | 2 | 0 |
| Chills | 164 | 54 | 85 | 5 | 10 | 5 | 2 | 3 |
| Arthralgia | 110 | 37 | 50 | 5 | 11 | 5 | 1 | 1 |
| Fever | 97 | 21 | 48 | 5 | 14 | 6 | 1 | 2 |
| Muscle ache | 91 | 37 | 45 | 3 | 3 | 2 | 0 | 1 |
| Injection site redness | 28 | 8 | 13 | 3 | 2 | 1 | 1 | 0 |
| Lymphadenopathy | 26 | 10 | 13 | 3 | 0 | 0 | 0 | 0 |
| Nausea | 25 | 7 | 16 | 1 | 1 | 0 | 0 | 0 |
| Infection | 23 | 12 | 9 | 0 | 0 | 1 | 1 | 0 |
| Faintness | 22 | 9 | 9 | 1 | 3 | 0 | 0 | 0 |
| Injection site oedema | 21 | 3 | 14 | 3 | 1 | 0 | 0 | 0 |
| Anxiety | 18 | 7 | 9 | 0 | 1 | 1 | 0 | 0 |
| Local allergic reaction | 16 | 4 | 5 | 2 | 4 | 1 | 0 | 0 |
| Hot flash | 13 | 2 | 8 | 0 | 2 | 1 | 0 | 0 |
| insomnia | 9 | 2 | 6 | 1 | 0 | 0 | 0 | 0 |
| diarrhoea | 8 | 4 | 4 | 0 | 0 | 0 | 0 | 0 |
| Sweating | 6 | 1 | 4 | 1 | 0 | 0 | 0 | 0 |
| Injection site haematoma | 6 | 2 | 3 | 0 | 0 | 1 | 0 | 0 |
| Loss of appetite | 5 | 1 | 2 | 0 | 2 | 0 | 0 | 0 |
| Vomiting | 5 | 1 | 3 | 0 | 1 | 0 | 0 | 0 |
| Menstrual changes | 4 | 0 | 4 | 0 | 0 | 0 | 0 | 0 |
| Dizziness | 3 | 1 | 2 | 0 | 0 | 0 | 0 | 0 |
| Other | 15 | 6 | 9 | 0 | 0 | 0 | 0 | 0 |
Only 1 patient received Sputnik V, he did not refer any adverse events.
Table 3.
Clinical relapses in patients from General University Hospital in Prague.1
| NO. OF PATIENTS WITH AT LEAST 1 CLINICAL RELAPSE (ALL) | p value | |
|---|---|---|
| In 90 days after vaccination | 88 (5.30%)2 | |
| Time between 1st dose of vaccine and relapse (SD), d | 41.08 (26.80) | |
| Between 0–90 days before vaccination | 63 (3.79%)3 | 0.043 |
| Between 90–180 days before vaccination | 64 (3.85%)4 | 0.039 |
| Between 180–270 days before vaccination | 71 (4.27%)5 | 0.181 |
| Between 360–270 days before vaccination | 74 (4.46%)6 | 0.279 |
| In 90 days after COVID-19 | 36 (7.27%)7 | |
| Time between COVID-19 onset and relapse (SD), d | 38.17 (25.10) | |
| Between 0–90 days before COVID-19 | 34 (6.87%)8 | 0.896 |
| Between 90–180 days before COVID-19 | 23 (4.65%)9 | 0.080 |
| Between 180–270 days before COVID-19 | 25 (5.05%)10 | 0.185 |
| Between 270–360 days before COVID-19 | 22 (4.44%)11 | 0.077 |
| Annualised relapse rate after vaccination | 0.22 | 0.036 |
| Annualised relapse rate before vaccination | 0.17 | |
| Annualised relapse rate after COVID-19 | 0.29 | 0.129 |
| Annualised relapse rate before COVID-19 | 0.22 | |
| NO. OF PATIENTS WITH AT LEAST 1 CLINICAL RELAPSE (PATIENTS WITH DMT CHANGES EXCLUDED) | ||
| In 90 days after vaccination | 79 (4.90%) | 0.020 |
| Between 0 - 90 days before vaccination | 52 (3.22%) | |
| In 90 days after vaccination | 75 (4.80%) | <0.001 |
| Between 90 - 180 days before vaccination | 38 (2.43%) | |
| In 90 days after vaccination | 71 (4.67%) | 0.003 |
| Between 180 - 270 days before vaccination | 39 (2.57%) | |
| In 90 days after vaccination | 64 (4.36%) | <0.001 |
| Between 270 - 360 days before vaccination | 23 (1.57%) | |
| In 90 days after COVID-19 | 32 (6.88%) | 0.665 |
| Between 0 - 90 days before COVID-19 | 28 (6.02%) | |
| In 90 days after COVID-19 | 30 (6.67%) | 0.006 |
| Between 90 - 180 days before COVID-19 | 12 (2.67%) | |
| In 90 days after COVID-19 | 28 (6.39%) | 0.025 |
| Between 180 - 270 days before COVID-19 | 13 (2.97%) | |
| In 90 days after COVID-19 | 27 (6.46%) | 0.003 |
| Between 270 - 360 days before COVID-19 | 9 (2.15%) | |
| Annualised relapse rate after vaccination | 0.18 | <0.001 |
| Annualised relapse rate before vaccination | 0.09 | |
| Annualised relapse rate after COVID-19 | 0.26 | 0.009 |
| Annualised relapse rate before COVID-19 | 0.13 | |
DMT = disease modifying therapy; 1. 1661 patients with MS from General University Hospital in Prague were vaccinated, 495 experienced COVID-19; 2. 3 patients had 2 relapses, 29 (31.87%) relapses were mild, 56 (61.54%) moderate and 6 (6.59%) severe; 3. 1 patient had 2 relapses, 30 (46.88%) relapses were mild, 28 (43.75%) moderate and 6 (9.38%) severe; 4. 1 patient had 2 relapses, 16 (24.62%) relapses were mild, 47 (72.31%) moderate and 2 (3.08%) severe; 5. 3 patients had 2 relapses, 28 (37.84%) relapses were mild, 44 (59.46%) moderate and 2 (2.70%) severe; 6. 2 patients had 2 relapses, 25 (32.89%) relapses were mild, 46 (60.53%) moderate and 5 (6.58%) severe; 7. relapse was mild in 16 (44.44%), moderate in 18 (50.00%) and severe in 2 (5.56%) patients; 8. 1 patient had 2 relapses, 14 (40.00%) relapses were mild, 21 (60.00%) moderate and 0 severe; 9. relapse was mild in 6 (26.09%), moderate in 15 (65.22%) and severe in 2 (8.70%) patients; 10. 1 patient had 2 relapses, 13 (50.00%) relapses were mild, 12 (46.15%) moderate and 1 (3.85%) severe; 11. 1 patient had 2 relapses, 9 (39.13%) relapses were mild, 11 (47.83%) moderate and 3 (13.04%) severe.
AEs were defined as medical occurrences temporally associated with the vaccine administration, but not necessarily causally related. They were classified as life-threatening, severe (requiring hospitalization or invasive intervention), moderate (lasting at least seven days or requiring interventional treatment) and mild (lasting less than seven days, not requiring intervention, hospitalisation, or treatment). All information was collected by healthcare professionals.
2.5. Statistical analyses
Vaccinated patients and patients who experienced COVID-19 were analysed separately. Also, patients with MS and NMOSD were analysed independently. For the assessment of the short-term risk of an acute exacerbation, we compared the proportion of patients with at least one clinical relapse (PPR) in the 90 days following the baseline to the 90-day intervals during the year before via McNemar paired test. As supportive evidence, we also compared annualised relapse rates (ARR) before and after baseline using paired t-test. The ARR after baseline was estimated by extrapolation of the first 90 days after baseline. To avoid bias caused by disease-modifying treatment (DMT) changes, we repeated the whole analysis excluding patients with DMT changes in the observation periods. For the evaluation of the risk factors of MS relapse, a comparison between groups with and without relapses after COVID-19 vaccination or infection was made using t-test and chi-squared test with continuity correction. We did not use any data imputations. Alpha was set to 0.05. Data analyses were performed in R version 4.0.4. For independent validation of results based on data from General University hospital in Prague, repeating all analyses were used on data from the Hospital Teplice.
2.6. Data availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
3. Results
3.1. Effect of COVID-19 vaccination on relapsing activity in patients with MS
By October 30, 2021, we identified 1661 vaccinated patients with MS without a history of COVID-19 infection (details in Table 1). The majority (95.79%) of patients were vaccinated with mRNA vaccine (98.87% of them got two doses). All patients got the same type of the first and second vaccine dose.
A mild increase in ARR and PPR after vaccination was observed. Not all differences were statistically significant. However, after the exclusion of patients with DMT change in each 90-day period, the ARR and PPR increases were accentuated and became significant in all comparisons (Table 3). Results in the validation group of 394 vaccinated MS patients from Hospital Teplice were similar (Table 4 ).
Table 4.
Clinical relapses in patients from Hospital Teplice.1
| NO. OF PATIENTS WITH AT LEAST 1 CLINICAL RELAPSE (ALL) | p value | |
|---|---|---|
| In 90 days after vaccination | 15 (3.81%) | |
| Between 0–90 days before vaccination | 9 (2.28%) | 0.307 |
| Between 90–180 days before vaccination | 11 (2.79%) | 0.540 |
| Between 180–270 days before vaccination | 7 (1.78%) | 0.135 |
| Between 360–270 days before vaccination | 17 (4.31%) | 0.850 |
| In 90 days after COVID-19 | 12 (4.48%) | |
| Between 0–90 days before COVID-19 | 14 (5.22%) | 0.844 |
| Between 90–180 days before COVID-19 | 15 (5.60%) | 0.663 |
| Between 180–270 days before COVID-19 | 11 (4.10%) | 1.000 |
| Between 270–360 days before COVID-19 | 10 (3.73%) | 0.831 |
| NO. OF PATIENTS WITH AT LEAST 1 CLINICAL RELAPSE (PATIENTS WITH DMT CHANGES EXCLUDED) | ||
| In 90 days after vaccination | 13 (3.38%) |
0.264 |
| Between 0 - 90 days before vaccination | 7 (1.82%) | |
| In 90 days after vaccination | 13 (3.42%) |
0.522 |
| Between 90 - 180 days before vaccination | 9 (2.36%) | |
| In 90 days after vaccination | 13 (3.47%) |
0.010 |
| Between 180 - 270 days before vaccination | 2 (0.53%) | |
| In 90 days after vaccination | 13 (3.51%) | 0.502 |
| Between 270 - 360 days before vaccination | 9 (2.43%) | |
| In 90 days after COVID-19 | 11 (4.42%) | 1.000 |
| Between 0 - 90 days before COVID-19 | 11(4.42%) | |
| In 90 days after COVID-19 | 11 (4.70%) | 0.267 |
| Between 90 - 180 days before COVID-19 | 6 (2.56%) | |
| In 90 days after COVID-19 | 11 (4.89%) | 0.332 |
| Between 180 - 270 days before COVID-19 | 6 (2.67%) | |
| In 90 days after COVID-19 | 11 (5.16%) | 0.027 |
| Between 270 - 360 days before COVID-19 | 2 (0.94%) |
DMT = disease modifying therapy; 1. 394 MS patients from Hospital Teplice were vaccinated, 268 experienced COVID-19.
Most relapses before as well as after vaccination were mild or moderate (Table 3, Fig. 1 ). The mean time from the first dose of vaccine to the first relapse was 41.08 days (SD 26.80). Relapses were reported more often in patients with younger age and shorter MS duration. DMT type was not shown as a significant risk factor of relapse after COVID-19 vaccination. For a detailed comparison of patients with and without relapses after vaccination see Table 5 .
Fig. 1.
No. of patients with at least 1 clinical relapse before and after COVID-19 vaccination or infection
1661 MS patients from General University Hospital in Prague were vaccinated; 495 experienced COVID-19.
Table 5.
Risk factors of an acute MS relapse after vaccination or COVID-19.1
| Without relapse after vaccination | With relapse after vaccination | p value2 | Without relapse after COVID-19 | With relapse after COVID-19 | p value2 | |
|---|---|---|---|---|---|---|
| Study population | 1573 | 88 | 459 | 36 | ||
| Age, mean (SD), y | 48.70 (11.39) | 44.79 (11.57) | 0.003 | 46.14 (11.00) | 41.93 (10.36) | 0.024 |
| <55 | 1114 (70.82%) | 74 (84.09%) | 0.007 | 350 (76.25%) | 32 (88.89%) | 0.082 |
| >=55 | 459 (29.18%) | 14 (15.91%) | 0.367 | 109 (23.75%) | 4 (11.11%) | 0.830 |
| Female | 1142 (72.60) | 60 (68.18) | 339 (73.86) | 26 (72.22%) | ||
| MS duration (SD), y | 17.55 (10.16) | 13.37 (10.49) | <0.001 | 15.57 (9.44) | 13.71 (9.63) | 0.271 |
| EDSS | ||||||
| EDSS < 4 | 978 (63.42%) | 51 (63.75)3 | 0.953 | 317 (70.92%) | 25 (71.43%) | 0.949 |
| EDSS >= 4 | 564 (36.58%) | 29 (36.25%)3 | 130 (28.32%) | 10 (28.57%) | ||
| DMT | 1195 (75.97%) | 64 (72.73%) | 0.511 | 364 (79.30%) | 32 (88.89%) | 0.011 |
| Interferons | 328 (20.85%) | 15 (17.05%) | 88 (19.17%) | 8 (22.22%) | ||
| Teriflunomide | 86 (5.47%) | 6 (6.82%) | 30 (6.54%) | 0 (0%) | ||
| Glatiramer acetate | 123 (7.82%) | 6 (6.82%) | 42 (9.15%) | 5 (13.89%) | ||
| Dimethyl fumarate | 89 (5.66%) | 3 (3.41%) | 16 (3.49%) | 6 (16.67%) | ||
| Anti-CD20 | 138 (8.77%) | 10 (11.36%) | 51 (11.11%) | 3 (8.33%) | ||
| Fingolimod | 263 (16.72%) | 10 (11.36%) | 73 (15.90%) | 6 (16.67%) | ||
| Other 2nd line DMT | 168 (10.68%) | 14 (15.91%) | 64 (13.94%) | 4 (11.11%) | ||
| No DMT | 378 (24.03%) | 24 (27.27%) | 95 (20.70%) | 4 (11.11%) | ||
| COVID-19 vaccine | 0.645 | |||||
| BNT162b2 (Comirnaty) | 1414 (88.89%)4 | 81 (92.05%)7 | ||||
| Time between doses (SD), d | 36.80 (10.06) | 43.70 (19.16) | ||||
| mRNA-1273 (Spikevax) | 91 (5.79%)5 | 5 (5.68%)5 | ||||
| Time between doses (SD), d | 36.13 (7.63) | 42.20 (15.75) | ||||
| Vector vaccines | 68 (4.32%)6 | 2 (2.27%) | ||||
| Time between doses (SD), d | 77.75 (17.85) | 109.00 | ||||
| COVID-19 severity | ||||||
| Mild COVID-19 | 375 (81.70%) | 27 (75%) | 0.322 | |||
| More severe COVID-198 | 84 (18.30%) | 9 (25%) | ||||
MS = multiple sclerosis; EDSS = expanded disability status scale; DMT = disease modifying drug, Anti-CD20 = ocrelizumab + rituximab; Other 2nd line DMT = alemtuzumab + natalizumab + cladribine; 1. All data not available for all individuals, the percentage corresponds to the proportion of patients with known value; 2. a comparison between groups with and without relapses after COVID-19 vaccination or infection; 3. Data were missing for 9.09% of patients; 4. A total of 1400 (99.01%) of patients got 2 doses of vaccine; 5. All patients got 2 doses of vaccine; 6. A total of 56 (82.35%) patients got 2 doses of vector vaccine; 7. A total of 77 (95.06%) patients got 2 doses of vaccine; 8. Patient with confirmed or suspected (dry cough, fever and shortness of breath) pneumonia.
3.2. The safety profile of COVID-19 vaccination in patients with MS
Early AEs following at least one dose of vaccine were reported by 1221 (73.51%) patients. A total of 3002 (93.32%) AEs were mild, 214 (6.65%) moderate and one (0.03%) severe (anaphylaxis after the second dose of BNT162b2 vaccine in a 47-year-old female treated with fingolimod). There was also a report of death in a 57-year-old female, who died 73 days after the first dose of BNT162b2, and a 66-year-old female, who died 62 days after the first dose of BNT162b2. According to medical reports, the cause of death was not related to the vaccination in either patient. The number and seriousness of AEs after the first and second dose was quite similar. In general, AEs were reported less frequently in the BNT162b2 vaccine compared to the mRNA-1273 vaccine and vector vaccines. The spectrum and proportion of AEs are shown in Table 2.
3.3. Effect of COVID-19 infection on relapsing activity in patients with MS
A total of 495 patients with MS who experienced COVID-19 infection without previous vaccination were included in this analysis. COVID-19 diagnosis was based on a positive PCR in 435 (87.88%) patients, an antigen test in 38 (7.68%) patients and a serological test in 22 (4.44%) patients. Altogether, 99 (20.00%) patients had clinically suspected (dry cough, fever and shortness of breath) or radiologically confirmed pneumonia. One 69-year-old female treated with ocrelizumab died due to COVID-19 pneumonia. For more characteristics see Table 1.
Statistically insignificant increases in ARR and PPR after COVID-19 infection were noticed. Nevertheless, after the exclusion of patients with DMT change in each followed period, the ARR and PPR increases were accentuated and became significant in all comparisons except for the comparison between the 90-day period before and after the COVID-19 onset (Table 3, Fig. 1). We found similar results in the validation group of 268 post-COVID-19 patients with MS from the Hospital Teplice (Table 4).
Most relapses before as well as after COVID-19 infection were mild or moderate. The mean time from the COVID-19 onset to the first relapse was 38.17 days (SD 25.10) (Table 3). Patients with relapses were younger and were treated more often with the first-line DMT than non-relapsing post-COVID-19 patients (Table 5).
3.4. Patients with NMOSD
Characteristics of 17 vaccinated patients with NMOSD without COVID-19 in their medical history and 8 patients with NMOSD who experienced COVID-19 infection without previous vaccination are presented in Table 6 . Although there were only a few patients with NMOSD and the differences did not reach statistical significance, the results were broadly consistent with those of patients with MS.
Table 6.
Clinical and demographic characteristics of NMOSD patients at the date of the 1st dose of COVID-19 vaccine or COVID-9 onset.
| Vaccinated | Post-COVID-19 | |
|---|---|---|
| Study population | 17 | 8 |
| Age, mean (SD), y | 49.09 (14.50) | 53.33 (10.27) |
| <55 | 11 (64.71%) | 6 (75.00%) |
| >=55 | 6 (35.29%) | 2 (25.00%) |
| Female | 16 (94.12%) | 5 (62.50%) |
| NMOSD duration (SD), y | 17.17 (11.26) | 10.66 (5.04) |
| EDSS | ||
| EDSS < 4 | 7 (41.18%) | 6 (75.00%) |
| EDSS >= 4 | 10 (58.82%) | 2 (25.00%) |
| DMT | 15 (88.24%) | 5 (62.50%) |
| Rituximab | 13 (76.47%) | 5 (62.50%) |
| Inebilizumab | 2 (11.76%) | 0 |
| No DMT | 2 (11.76%) | 3 (37.50%) |
| COVID-19 vaccine | ||
| BNT162b2 (Comirnaty) – mRNA | 17 (100%) | |
| Time between doses (SD), d | 28.82 (7.84) | |
| COVID-19 severity | ||
| Death | 2 (25%) | |
| Need of invasive ventilation or extracorporeal membrane oxygenation | 0 | |
| Need of non-invasive ventilation or high-flow oxygen therapy | 0 | |
| Need of supplemental oxygen | 0 | |
| Radiologically confirmed pneumonia | 2 (25%) | |
| Suspected pneumonia (dry cough, fever and shortness of breath) | 0 | |
| Symptomatic | 4 (50%) | |
| Asymptomatic | 0 | |
| Patients with at least 1 clinical relapse | ||
| In 90 days after vaccination | 1 (5.88%) | |
| Time between 1st dose of vaccine and relapse (SD), d | 41 | |
| Between 0–90 days before vaccination | 0 | |
| Between 90–180 days before vaccination | 0 | |
| Between 180–270 days before vaccination | 0 | |
| Between 360–270 days before vaccination | 2 (11.76%) | |
| In 90 days after COVID-19 | 2 (25%) | |
| Time between COVID-19 onset and acute relapse (SD), d | 30 (7.07) | |
| Between 0–90 days before COVID-19 | 0 | |
| Between 90–180 days before COVID-19 | 0 | |
| Between 180–270 days before COVID-19 | 1 (12.5%) | |
| Between 270–360 days before COVID-19 | 1 (12.5%) |
NMOSD = neuromyelitis optica spectrum disorder; EDSS = expanded disability status scale; DMT = disease modifying treatment.
4. Discussion
Vaccines work in principle by stimulating the immune system. Therefore, over and above classical AEs, there is an additional potential risk in patients with autoimmune diseases such as MS and NMOSD, the risk of disease exacerbation. This was confirmed in our analysis of 1661 vaccinated patients with MS, where a slight increase in the proportion of patients with at least one clinical relapse (PPR) and ARR in 90 days after vaccination against COVID-19 was found. After the exclusion of patients with DMT change in followed periods (less stable patients), the increase was even more pronounced.
Conversely, two previous cohort studies addressing this issue did not show an increase in relapse frequency after COVID-19 vaccination (Achiron et al., 2021; di Filippo et al., 2021). The Israeli observational study (Achiron et al., 2021) on 555 patients with MS compared the incidence of relapses after BNT162b2 vaccine administration with the incidence of relapses in non-vaccinated patients in the same period during the prepandemic era. This study, however, suffers from the limitation of a heterogenous follow-up period (about 20% of patients with relapses were followed for less than 14 days after vaccination) which might have lowered the number of recorded relapses. In addition, vaccinated and unvaccinated cohorts from this study (Achiron et al., 2021) might include patients with different MS activities. An Italian study (di Filippo et al., 2021) with 324 patients with MS compared the incidence of relapses 60 days before and after BNT162b2 vaccination. The main limits of this study were a relatively small size associated with the small number of clinical relapses, heterogeneity of the sample (8.6% of patients had experienced COVID-19 infection before vaccination) and a short reference period before vaccination. In addition, there was an international recommendation not to vaccinate patients shortly after relapse treated with high-dose glucocorticoids (Horakova, 2021; Multiple sclerosis international federation , 2022). Therefore, the comparison of PPR after with the period directly before the vaccine administration is at least problematic. Especially for this reason, we consider the main outcome of our study to be a PPR difference when comparing 90-day period after with the period between 90 and 180 days before vaccination (that mean 1,38 times increase, respectively two-fold increase when excluding patients with DMT change).
Another goal of our study was to identify patients at higher risk of relapse after vaccination. As we assumed, clinical relapses occurred more frequently in patients with younger age (<55 years) and shorter MS durations. This is probably due to a more vigorous immune response mounted by younger individuals, as the immune system tends to gradually deteriorate with age (Müller et al., 2019; Sadighi Akha, 2018; Uher et al., 2021). Regarding the vaccination-related AEs, corresponding to previous publications (Achiron et al., 2021; Lotan et al., 2021b), their spectrum was comparable to that reported in the general population (Baden et al., 2021; Meo et al., 2021; Polack et al., 2020; Sadoff et al., 2021; “Safety of COVID-19 vaccines | European Medicines Agency”; Voysey et al., 2021). Also, as well as in the general population, AEs were reported to be less frequent in the BNT162b2 vaccine (Meo et al., 2021). However, the overall rate of adverse events amongst patients with MS in our and previous studies is lower than reported in the general population (Achiron et al., 2021; Baden et al., 2021; Lotan et al., 2021b; Meo et al., 2021; Polack et al., 2020; Sadoff et al., 2021; “Safety of COVID-19 vaccines | European Medicines Agency”; Voysey et al., 2021). This could be potentially explained by the high proportion of patients treated with DMT, most of which have an immunosuppressive effect.
For a comprehensive view of this issue, it is necessary to look at the risks associated with the COVID-19 infection itself, leading to morbidity or even death and a higher risk of relapse, according to our findings. After the exclusion of patients with DMT change during each followed period, the ARR and PPR increases became statistically significant, except for the comparison between the 90-day periods before and after COVID-19. We suggest the increase of PPR before COVID-19 could be caused by a higher risk of COVID-19 infection due to high-dose glucocorticoids indicated for the treatment of relapses.
Our findings are supported by the recent small sample size study (Barzegar et al., 2021b) comparing the incidence of relapses after COVID-19 infection in 41 patients with MS with an incidence in the previous two years. The second small sample size study (Etemadifar et al., 2021) (54 patients with MS) dealing with this issue did not show a higher incidence of relapses after COVID-19. However, in this study, they compared the period after infection to only six months preceding the vaccination. Thus, the bias caused by the relapse rate increase shortly before infection plays a bigger role here.
As for NMOSD, a few post-vaccination disease relapses have been reported via smaller studies (Dinoto et al., 2021; Fragoso et al., 2021; Jovicevic et al., 2021; Lotan et al., 2021a). The frequency of relapses within one month of vaccination in the largest one was 4% (one out of 26 patients) (Dinoto et al., 2021), which corresponds with our findings. COVID-19 infection itself has already been demonstrated as a risk factor for NMOSD relapses (Cai et al., 2021) and above all, the largest systematic review suggests that one-third of COVID-19 infected NMOSD patients were hospitalised, approximately 15% admitted to ICU and more than 3% died (Barzegar et al., 2021a). Based on this unfortunately scarce data, the potential benefits of vaccination seem to clearly overcome the risk of relapses in NMOSD patients.
We present an extensive study dealing with the risk of relapses after COVID-19 infection or vaccination. Additionally, we added the independent validation of results by evaluating data from the Teplice Hospital (394 vaccinated and 268 post-COVID-19 patients with MS). Results in vaccinated patients and patients who experienced COVID-19 were similar in this control cohort. On the other hand, our work has several limitations. First, there is a relatively low number of patients with NMOSD insufficient to generalize conclusions. Second, patients vaccinated with vector vaccines are also relatively underrepresented. For this reason, we cannot evaluate the effect of individual types of vaccines. Third, there is also the possibility that patients may have focused more on their health problems after vaccination or infection compared to previous times. This may have increased the number of recorded relapses. Fourth, in vaccine safety studies, the 28 to even 60-day risk intervals in the search for autoimmune neurological adverse events are often used because of the dynamics of immune response (Aly et al., 2022; Confavreux et al., 2001; Kim et al., 2022; Lee et al., 2011; Li et al., 2022; Watad et al., 2021). For the most widely used vaccine in Czechia (BNT162b2), the recommended interval between vaccine doses was 38–42 days, later replaced by 21 days. Therefore, based on the predicted risk interval and the interval between the two vaccine doses, a cut-off of at least 90 days seems to be reasonable. However, the ARR evaluation after vaccination could be more representative of the longer follow-up period. Fifth, another important limitation of our work is the lack of an objective biomarker, such as magnetic resonance imaging. And finally, given that characteristics of patients after COVID-19 vaccination and infection were different, it is impossible to directly compare these two cohorts. The adjustment or matching could be performed only on known and collected factors such as age or EDSS. However, the selection bias caused, for example, by the vaccination strategy in patients with MS is not removable by a statistical method - the risk of confounding would remain too great and could lead to misinterpretation.
5. Conclusion
To sum up, there was a mild increase in relapse risk after the COVID-19 vaccination in comparison to the previous year in MS patients, mainly in those younger than 55 years. The risks, however, need to be balanced against the risks of COVID-19 itself, also leading to the rise in relapse rate and particularly morbidity and mortality, especially in elderly patients (Stastna et al., 2021). Thus, based on our findings, patients who have the lowest risk of relapse after vaccination are at the greatest risk for a more severe COVID-19 course. However, further studies are needed to confirm our conclusion. Results in patients with NMOSD are broadly consistent, but the risks from COVID-19 infection are more pronounced. Unfortunately, the relatively low number of patients with NMOSD is insufficient to generalize conclusions. We believe these results can improve clinical practice by facilitating clinical decisions about vaccination and preventive measures during COVID-19 pandemic.
Funding
This work was supported by the Czech Ministry of Health, the institutional support of hospital research [grant number RVO-VFN 64,165], the Czech Ministry of Health grant [grant number NU22-A-121] and by the National Institute for Neurological Research project funded by the European Union - Next Generation EU (Programme EXCELES, ID Project No. LX22NPO5107).
CRediT authorship contribution statement
Dominika Stastna: Conceptualization, Data curation, Investigation, Methodology, Formal analysis, Project administration, Validation, Visualization, Writing – original draft. Ingrid Menkyova: Conceptualization, Data curation, Investigation, Methodology, Project administration, Validation, Writing – review & editing. Jiri Drahota: Conceptualization, Data curation, Methodology, Formal analysis, Validation, Writing – review & editing. Tereza Hrnciarova: Conceptualization, Methodology, Formal analysis, Validation, Writing – review & editing. Eva Kubala Havrdova: Conceptualization, Investigation, Writing – review & editing. Marta Vachova: Conceptualization, Investigation, Writing – review & editing. Michaela Andelova: Conceptualization, Investigation, Writing – review & editing. Pavlina Kleinova: Conceptualization, Investigation, Writing – review & editing. Ivana Kovarova: Conceptualization, Investigation, Writing – review & editing. Eva Krasulova: Conceptualization, Investigation, Writing – review & editing. Jana Lizrova Preiningerova: Conceptualization, Investigation, Writing – review & editing. Iveta Novakova: Conceptualization, Investigation, Writing – review & editing. Klara Novotna: Conceptualization, Investigation, Writing – review & editing. Martina Novotna: Conceptualization, Investigation, Writing – review & editing. Petra Nytrova: Conceptualization, Investigation, Writing – review & editing. Jana Pavlickova: Conceptualization, Investigation, Writing – review & editing. Barbora Srpova: Conceptualization, Investigation, Writing – review & editing. Katerina Storey: Conceptualization, Investigation, Writing – review & editing. Veronika Ticha: Conceptualization, Investigation, Writing – review & editing. Michaela Tyblova: Conceptualization, Investigation, Writing – review & editing. Tomas Uher: Conceptualization, Investigation, Writing – review & editing. Karolina Vodehnalova: Conceptualization, Investigation, Writing – review & editing. Dana Horakova: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: D. Stastna received financial support for conference travel from Novartis, Biogen, Merck, Bayer and Janssen-Cilag; E. Kubala Havrdova received speaker honoraria and consultant fees from Biogen, Merck, Novartis, Sanofi and Teva, as well as support for research activities from Biogen and Merck; M. Vachova received compensation for travelling, conference fees, consulting fees and speaker honoraria from Biogen, Merck, Novartis, Roche, Sanofi and Teva; M. Andelova received financial support for conference travel from Novartis, Sanofi, Merck, Biogen and Roche; J. Preiningerova Lizrova received financial support for research activities from Biogen and compensation for travelling, conference fees and consulting fees from Biogen, Novartis, Sanofi, Roche and Merck; K. Novotna received compensation for travelling, conference fees and speaker honoraria from Biogen, Merck, Novartis, Roche, Sanofi and Teva; B. Srpova received compensation for travelling and conference fees from Novartis, Sanofi, Biogen, Roche and Merck as well as support for research activities from Biogen; M. Tyblova received financial support for conference travel from Novartis, Sanofi, Merck, Teva and Biogen; T. Uher received financial support for conference travel from Biogen, Novartis, Sanofi, Roche and Merck and speaker honoraria from Biogen, Novartis and Roche as well as support for research activities from Biogen and Sanofi; K. Vodehnalova received compensation for travelling, conference fees and consulting fees from Merck, Teva, Sanofi Genzyme, Biogen, Novartis and Roche; D. Horakova received compensation for travel, speaker honoraria, and consultant fees from Biogen, Novartis, Merck, Bayer, Sanofi, Roche and Teva, as well as support for research activities from Biogen. All authors were supported by the Charles University: Cooperatio Program in Neuroscience.
Acknowledgments
The authors are very grateful to all employees of MS centres (especially Tereza Cernanska) participating in the data collection. Without their hard work and dedication, this study would never have been possible. Special acknowledgements are also due to Christine Fogl for language editing.
References
- Achiron A., Dolev M., Menascu S., Zohar D.N., Dreyer-Alster S., Miron S., Shirbint E., Magalashvili D., Flechter S., Givon U., Guber D., Stern Y., Polliack M., Falb R., Gurevich M. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult. Scler. 2021;27:864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly A.S., Alkolfat F., Mansour E.R., Salama S. Guillain-Barre syndrome following COVID-19 vaccination: a case report and an updated review. Neuroimmunol. Reports. 2022;2 doi: 10.1016/J.NEREP.2022.100083. [DOI] [Google Scholar]
- Baden L.R., el Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMOA2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzegar M., Mirmosayyeb O., Ebrahimi N., Bagherieh S., Afshari-Safavi A., Hosseinabadi A.M., Shaygannejad V., Asgari N. COVID-19 susceptibility and outcomes among patients with neuromyelitis optica spectrum disorder (NMOSD): a systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2021 doi: 10.1016/j.msard.2021.103359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzegar M., Vaheb S., Mirmosayyeb O., Afshari-Safavi A., Nehzat N., Shaygannejad V. Can coronavirus disease 2019 (COVID-19) trigger exacerbation of multiple sclerosis? A retrospective study. Mult. Scler. Relat. Disord. 2021;52 doi: 10.1016/J.MSARD.2021.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H., Zhou R., Jiang F., Zeng Q., Yang H. Vaccination in neuromyelitis optica spectrum disorders: friend or enemy? Mult. Scler. Relat. Disord. 2021 doi: 10.1016/J.MSARD.2021.103394. [DOI] [PubMed] [Google Scholar]
- Confavreux C., Suissa S., Saddier P., Bourdès V., Vukusic S. Vaccinations and the risk of relapse in multiple sclerosis. Vaccines in Multiple Sclerosis Study Group. N. Engl. J. Med. 2001;344:319–326. doi: 10.1056/NEJM200102013440501. [DOI] [PubMed] [Google Scholar]
- Correale J., Fiol M., Gilmore W. The risk of relapses in multiple sclerosis during systemic infections. Neurology. 2006;67:652–659. doi: 10.1212/01.WNL.0000233834.09743.3B. [DOI] [PubMed] [Google Scholar]
- di Filippo M., Cordioli C., Malucchi S., Annovazzi P., Cavalla P., Torri Clerici V., Ragonese P., Nociti V., Radaelli M., Laroni A., Buttari F., Lorefice L., Ferraro D., Gajofatto A., Prosperini L., Fantozzi R., Boffa L., Lanzillo R., Moccia M., Clerico M., de Luca G., Tomassini V., Calabrese M., Borrelli A., Paolicelli D., Maniscalco G.T., Gazzola P., Gallo A., Solaro C., Cocco E., Gasperini C., Tortorella C. 2021. mRNA COVID-19 vaccines do not increase the short-term risk of clinical relapses in multiple sclerosis. J Neurology, Neurosurg Psychiatry jnnp. 2021 doi: 10.1136/jnnp-2021-327200. [DOI] [PubMed] [Google Scholar]
- Dinoto A., Sechi E., Ferrari S., Gajofatto A., Orlandi R., Solla P., Maccabeo A., Maniscalco G.T., Andreone V., Sartori A., Manganotti P., Rasia S., Capra R., Mancinelli C.R., Mariotto S. Risk of disease relapse following COVID-19 vaccination in patients with AQP4-IgG-positive NMOSD and MOGAD. Mult. Scler. Relat. Disord. 2021 doi: 10.1016/J.MSARD.2021.103424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemadifar M., Sedaghat N., Aghababaee A., Kargaran P.K., Maracy M.R., Ganjalikhani-Hakemi M., Rayani M., Abhari A.P., Khorvash R., Salari M., Nouri H. COVID-19 and the risk of relapse in multiple sclerosis patients: a fight with no bystander effect? Mult. Scler. Relat. Disord. 2021;51 doi: 10.1016/J.MSARD.2021.102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso Y.D., Gomes S., Gonçalves M.V.M., Mendes Junior E., Oliveira B.E.S.de, Rocha C.F., Santos G.A.C.dos, Tauil C.B., Araujo R.V., Peron J.P.S. New relapse of multiple sclerosis and neuromyelitis optica as a potential adverse event of AstraZeneca AZD1222 vaccination for COVID-19. Mult. Scler. Relat. Disord. 2021 doi: 10.1016/J.MSARD.2021.103321. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Horakova, D., 2021. Doporučení Výboru Sekce klinické neuroimunologie a likvorologie ČNS ČLS JEP [WWW Document]. URL https://www.czech-neuro.cz/content/uploads/2021/03/2021_3_6-dh-doporuceni-update.pdf (accessed 1.22.22).
- Horakova D., Rockova P., Jircikova J., Dolezal T., Vachova M., Hradilek P., Valis M., Sucha J., Martinkova A., Ampapa R., Grunermelova M., Stetkarova I., Stourac P., Mares J., Dufek M., Kmetova E., Adamkova J., Hrnciarova T. Initiation of first disease-modifying treatment for multiple sclerosis patients in the Czech republic from 2013 to 2016: data from the national registry ReMuS. Mult. Scler. Relat. Disord. 2019;35:196–202. doi: 10.1016/J.MSARD.2019.08.003. [DOI] [PubMed] [Google Scholar]
- Jovicevic V., Ivanovic J., Andabaka M., Tamas O., Veselinovic N., Momcilovic N., Mesaros S., Pekmezovic T., Drulovic J. COVID-19 and vaccination against SARS-CoV-2 in patients with neuromyelitis optica spectrum disorders. Mult. Scler. Relat. Disord. 2021 doi: 10.1016/J.MSARD.2021.103320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E., Min Y.G., Shin J.Y., Kwon Y.N., Bae J.S., Sung J.J., Hong Y.H. Guillain–Barré syndrome and variants following COVID-19 vaccination: report of 13 Cases. Front Neurol. 2022;12:2636. doi: 10.3389/FNEUR.2021.820723/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G.M., Greene S.K., Weintraub E.S., Baggs J., Kulldorff M., Fireman B.H., Baxter R., Jacobsen S.J., Irving S., Daley M.F., Yin R., Naleway A., Nordin J.D., Li L., McCarthy N., Vellozzi C., Destefano F., Lieu T.A. H1N1 and seasonal influenza vaccine safety in the Vaccine Safety datalink Project. Am. J. Prev. Med. 2011;41:121–128. doi: 10.1016/J.AMEPRE.2011.04.004/ATTACHMENT/29AFE14A-359C-4B25-9DEE-B1B986E9A127/MMC1.PDF. [DOI] [PubMed] [Google Scholar]
- Li X., Gao L., Tong X., Chan V.K.Y., Chui C.S.L., Lai F.T.T., Wong C.K.H., Wan E.Y.F., Chan E.W.Y., Lau K.K., Lau C.S., Wong I.C.K. Autoimmune conditions following mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccination: a descriptive cohort study among 1.1 million vaccinated people in Hong Kong. J. Autoimmun. 2022;130 doi: 10.1016/J.JAUT.2022.102830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan I., Romanow G., Levy M. Patient-reported safety and tolerability of the COVID-19 vaccines in persons with rare neuroimmunological diseases. Mult. Scler. Relat. Disord. 2021;55 doi: 10.1016/J.MSARD.2021.103189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan I., Wilf-Yarkoni A., Friedman Y., Stiebel-Kalish H., Steiner I., Hellmann M.A. Safety of the BNT162b2 COVID-19 vaccine in multiple sclerosis (MS): early experience from a tertiary MS center in Israel. Eur. J. Neurol. 2021;28:3742–3748. doi: 10.1111/ENE.15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Kermode A.G., Hu X., Qiu W. Risk of relapse in patients with neuromyelitis optica spectrum disorder: recognition and preventive strategy. Mult. Scler. Relat. Disord. 2020;46 doi: 10.1016/J.MSARD.2020.102522. [DOI] [PubMed] [Google Scholar]
- Meo S.A., Bukhari I.A., Akram J., Meo A.S., Klonoff D.C. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021;25:1663–1679. doi: 10.26355/EURREV_202102_24877. [DOI] [PubMed] [Google Scholar]
- Müller L., di Benedetto S., Pawelec G. The immune system and its dysregulation with aging. Subcell. Biochem. 2019;91:21–43. doi: 10.1007/978-981-13-3681-2_2. [DOI] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.v., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMOA2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosperini L., Tortorella C., Haggiag S., Ruggieri S., Galgani S., Gasperini C. Determinants of COVID-19-related lethality in multiple sclerosis: a meta-regression of observational studies. J. Neurol. 2022;(1):1–11. doi: 10.1007/S00415-021-10951-6. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadighi Akha A.A. Aging and the immune system: an overview. J. Immunol. Methods. 2018;463:21–26. doi: 10.1016/J.JIM.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., Offergeld K., Scheper G., Taylor K.L., Robb M.L., Treanor J., Barouch D.H., Stoddard J., Ryser M.F., Marovich M.A., Neuzil K.M., Corey L., Cauwenberghs N., Tanner T., Hardt K., Ruiz-Guiñazú J., le Gars M., Schuitemaker H., van Hoof J., Struyf F., Douoguih M. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/NEJMOA2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stastna D., Menkyova I., Drahota J., Mazouchova A., Adamkova J., Ampapa R., Grunermelova M., Peterka M., Recmanova E., Rockova P., Rous M., Stetkarova I., Valis M., Vachova M., Woznicova I., Horakova D. Multiple sclerosis, neuromyelitis optica spectrum disorder and COVID-19: a pandemic year in Czechia. Mult. Scler. Relat. Disord. 2021;54 doi: 10.1016/J.MSARD.2021.103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher T., Krasensky J., Malpas C., Bergsland N., Dwyer M.G., Kubala Havrdova E., Vaneckova M., Horakova D., Zivadinov R., Kalincik T. Evolution of brain volume loss rates in early stages of multiple sclerosis. Neurol. - Neuroimmunol. Neuroinflammation. 2021;8 doi: 10.1212/NXI.0000000000000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet North Am. Ed. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1/ATTACHMENT/BD910DFE-2C8A-4512-A277-6B1DBA6322EE/MMC2.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watad A., de Marco G., Mahajna H., Druyan A., Eltity M., Hijazi N., Haddad A., Elias M., Zisman D., Naffaa M.E., Brodavka M., Cohen Y., Abu-Much A., Elhija M.A., Bridgewood C., Langevitz P., McLorinan J., Bragazzi N.L., Marzo-Ortega H., Lidar M., Calabrese C., Calabrese L., Vital E., Shoenfeld Y., Amital H., McGonagle D. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines (Basel) 2021;9 doi: 10.3390/VACCINES9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.