Abstract
Background
Third vaccination against SARS-CoV-2 is recommended for patients with multiple sclerosis (pwMS), usually six months after the last vaccination.
Methods
In this prospective multicenter study on 292 pwMS and 46 healthy controls (HC), who had all received two vaccinations prior to study enrollment, SARS-CoV-2 IgG response was measured in the month before and 2–4 months after third vaccination. PwMS were categorized as follows: untreated (N-DMT, n = 32), receiving disease-modifying therapy (DMT) with expected humoral response (er-DMT: interferon-beta preparations, glatiramer acetate, dimethyl fumarate, teriflunomide, natalizumab, cladribine, alemtuzumab; n = 120) or no expected humoral response (nr-DMT: S1PMs, CD20mAb; n = 140).
Results
PwMS on nr-DMT had significantly lower median antibody levels before (12.1 U/ml [0.4–2500]) and after third vaccination (305 U/ml [0.4–2500]) in comparison to other groups (p<0.001). We did not find differences in antibody levels after homologous (n = 281; 2500 [0.4–2500]) and heterologous (n = 57; 2500 [0.4–2500]) vaccination regime regardless of the DMT group. The DMT group (= –0.60; 95% CI –1195.73, –799.10; p<0.001) was associated with antibody levels after third vaccination, while time to revaccination (6 months [1–13]) was not. After third vaccination, seropositivity was reached in 75.8% and 82.2% of pwMS on anti-CD20 mAbs and S1PMs, respectively. Complete B-cell depletion significantly decreased the probability of seroconversion even after the third vaccination (OR 0.14; p = 0.021), whereas time interval to last DMT intake and time to revaccination did not. Twenty-two patients reported a SARS-CoV-2 infection (3 N-DMT, 9 er-DMT, 10 nr-DMT), one being asymptomatic and the rest having a mild course.
Conclusion
Humoral response to SARS-CoV-2 third vaccination in pwMS is excellent. While reduced by S1PMs and CD20mAb, protective response is still expected in the majority of patients
Key words: Multiple sclerosis, Disease-modifying therapy, SARS-CoV-2, Vaccination, Third
Abbreviations: DMT, disease-modifying treatment; EDSS, Expanded Disability Status Scale; er-DMT, disease-modifying treatment with expected humoral response; HC, healthy control; N-DMT, no disease-modifying treatment; nr-DMT, disease-modifying treatment with no expected humoral response; PPMS, primary progressive multiple sclerosis; pwMS, patients with multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis; SPMS, secondary progressive multiple sclerosis; S1PM, sphingosine-1-phosphate receptor modulator
1. Introduction
Patients with multiple sclerosis (pwMS) are most commonly treated with disease-modifying therapies (DMT) that interfere with the immune system, potentially limiting immune response to vaccination and the extent of protection achieved but not altering the adverse effect profile. Several studies have shown that the humoral response to SARS-CoV-2 vaccines is not reduced in pwMS when treated with DMT other than sphingosine-1-phosphate receptor modulators (S1PMs) and anti-CD20 monoclonal antibodies (mAbs) (Bsteh et al., 2022c; Kornek et al., 2022; Sormani et al., 2021; Achiron et al., 2021). Despite poor antibody response in those patients, current guidelines do advise against discontinuation of DMT for the high risk of disease reactivation. Rather, the timing of the vaccination should be carefully planned as treatment with anti-CD20 mAbs is associated with increased COVID-19 severity (Simpson-Yap et al., 2021; Bsteh et al., 2022a; Sormani et al., 2022). Until now, the third vaccination has already shown an adequate safety profile with a comparable adverse event rate as compared to the first vaccination cycle (Dreyer-Alster et al., 2022).
We investigated humoral response and the adverse event profile of SARS-CoV-2 third vaccination in pwMS compared to healthy controls (HC), as well as the role of the third vaccination in the primarily seronegative and therefore more vulnerable group of treated pwMS (S1PMs, anti-CD20 mAbs).
2. Methods
We conducted a multicenter (Departments of Neurology of Medical Universities Vienna, Innsbruck and Linz) prospective observational study including 292 pwMS and 45 HC, who had all received two vaccinations during the original study (first immunization), which was originally described elsewhere (Bsteh et al., 2022c). PwMS were first subgrouped according to DMT status at the time of first immunization. Further on, pwMS were subgrouped based on the expected probability of humoral response to first immunization as follows: untreated (N-DMT), receiving DMT with expected humoral response (er-DMT: interferon-beta preparations, glatiramer acetate, dimethyl fumarate, teriflunomide, natalizumab, cladribine, alemtuzumab) or receiving DMT with no expected humoral response (nr-DMT: S1PMs, CD20mAb) at the time of third vaccination. Inclusion criteria for pwMS were age 18 years and a diagnosis of MS according to the 2017 version of the McDonald criteria (Thompson et al., 2018).
The primary endpoint was the humoral immunogenicity 2–4 months after the third dose, measured by the level of SARS-CoV-2 serum antibodies. Secondary endpoints included the proportion of patients developing antibodies against SARS-CoV-2 (seroconversion) and safety variables (local or systemic adverse events, severe adverse events). Patients were vaccinated by mRNA (Pfizer-BioNTech, Moderna) or vector vaccines (AstraZeneca, Johnson & Johnson). Choice of vaccine type was not part of the study protocol – as it was designed as a non-interventional trial. Vaccination regimes were post-hoc defined as homologous (only mRNA or only vector vaccine) or heterologous (both mRNA and vector vaccine); however, due to country regulations, only two patients received the vector vaccine at the third vaccination, the majority of patients receiving heterologous vaccination regime being vaccinated with the vector vaccine first, followed by the mRNA vaccine.
Venous blood samples were drawn within 1 month before and 2–4 months after the third vaccination. The quantification of antibodies to the receptor-binding domain of the viral spike protein was performed centrally by the commercially available anti-SARS-CoV-2 immunoassay (IgG; Elecsys) (Higgins et al., 2021), with results shown in standardized units per milliliter (U/ml). Antibody levels ranged from 0.4 to 2500 U/ml, and 0.8 U/ml was used as the cut-off for positive samples. Seroconversion was defined as an initially negative sample that reached the cut-off of 0.8 U/ml at the next timepoint.
Statistical analyses were performed using SPSS 26.0 (SPSS Inc.). Categorical variables were expressed in frequencies and percentages, continuous variables as mean and standard deviation or median and range as appropriate. Continuous variables were tested for normal distribution by the Kolmogorov–Smirnov test. Univariate comparisons were done by Fisher exact test, McNemar test, Kruskal-Wallis test or one-way ANOVA test as appropriate. To test for potential bias due to different timepoints of blood samples for antibody levels, we compared median values of two groups (2–3 months vs. 3–4 months after third vaccination). Linear stepwise regression models were calculated with antibody level after third vaccination as a dependent variable, and DMT group as independent variable, adjusted for sex, age, disease duration, treatment duration, time interval to last DMT intake, time to revaccination and vaccination regime (homologous/heterologous). Predictors of seroconversion were investigated in the nr-DMT group by multivariable logistic regression analyses, with seroconversion as the dependent variable and DMT as independent variable, and age, sex, disease duration, time interval to last DMT intake, time to revaccination, vaccination regime, absolute lymphocyte count and complete B-cell depletion (<1 cell/µl) as covariates. A value of p<0.05 was considered statistically significant. All multiple analyses were corrected for using Bonferroni method.
The study was approved by the ethics committees of the Medical Universities of Vienna, Innsbruck and Linz (EK 1029/2021). Written informed consent was obtained from all study participants. Data supporting the findings of this study are available from the corresponding author upon reasonable request and upon approval by the ethics committee of the Medical University of Vienna.
3. Results
In the study, 292 pwMS and 46 HC were enrolled. The characteristics of the study cohort are given in Table 1 .
Table 1.
Characteristics of the study cohort.
| HC (n = 46) | pwMS (n = 292) | |||
| No DMT (n = 32) | er-DMT (n = 120) | nr-DMT (n = 140) | ||
| Femalea | 33 (71.7) | 27 (84.4) | 80 (66.7) | 96 (68.6) |
| Age (years)b | 42.2 (10.9) | 55.0 (10.5) | 37.8 (9.9) | 41.0 (12.1) |
| Disease duration (years)c | NA | 14 (0–47) | 6 (0–41) | 8 (0–35) |
| Treatment duration (years)c | NA | NA | 2.5 (0.5–15.0) | 3.3 (0.3–17.9) |
| EDSSc | NA | 3.0 (0–8.5) | 1.3 (0–6.5) | 2.5 (0–7.0) |
| Disease course | ||||
| RRMSa | NA | 17 (53.1) | 118 (98.1) | 94 (67.1) |
| SPMSa | NA | 13 (40.6) | 2 (1.9) | 28 (20.0) |
| PPMSa | NA | 2 (6.3) | 0 (0.0) | 18 (12.9) |
| er-DMT | ||||
| Alemtuzumaba | NA | NA | 4 (3.3) | NA |
| Cladribinea | NA | NA | 26 (21.7) | NA |
| Dimethyl fumaratea | NA | NA | 44 (36.7) | NA |
| Glatiramer acetatea | NA | NA | 12 (10.0) | NA |
| Interferon betaa | NA | NA | 13 (10.9) | NA |
| Natalizumaba | NA | NA | 14 (11.7) | NA |
| Teriflunomidea | NA | NA | 7 (5.8) | NA |
| nr-DMT | ||||
| Anti-CD20 mAbsa | NA | NA | NA | 95 (67.9) |
| Complete B-cell depletiona | NA | NA | NA | 63 (66.3) |
| S1PMsa | NA | NA | NA | 45 (32.1) |
| Absolute lymphocyte countc | NA | NA | NA | 0.5 (0.2–1.5) |
| Lymphopenia before vaccinationa | NA | NA | NA | 40 (88.9) |
| Grade 3 or highera | NA | NA | NA | 21 (46.7) |
| Vaccination regime | ||||
| Homologousa | 23 (50.0) | 27 (84.4) | 104 (86.7) | 127 (90.7) |
| Heterologousa | 23 (50.0) | 5 (15.6) | 16 (13.3) | 13 (9.3) |
| SARS-CoV-2 infection | ||||
| Before third vaccinationa | 1 (2.2) | 1 (3.1) | 10 (8.3) | 9 (6.4) |
| After third vaccinationa | 0 (0.0) | 3 (9.4) | 9 (7.5) | 10 (7.1) |
Absolute number and percentage.
Mean and standard deviation.
Median and range
Anti-CD20 mAbs: monoclonal antibodies against cluster of differentiation 20 (ocrelizumab, ofatumumab, rituximab), DMT: disease-modifying therapy, EDSS: Expanded Disability Status Scale, er-DMT: expected response DMT, nr-DMT: no expected response DMT, S1PMs: spingosin-1-phosphate receptor modulator (fingolimod, ozanimod, ponesimod, siponimod).
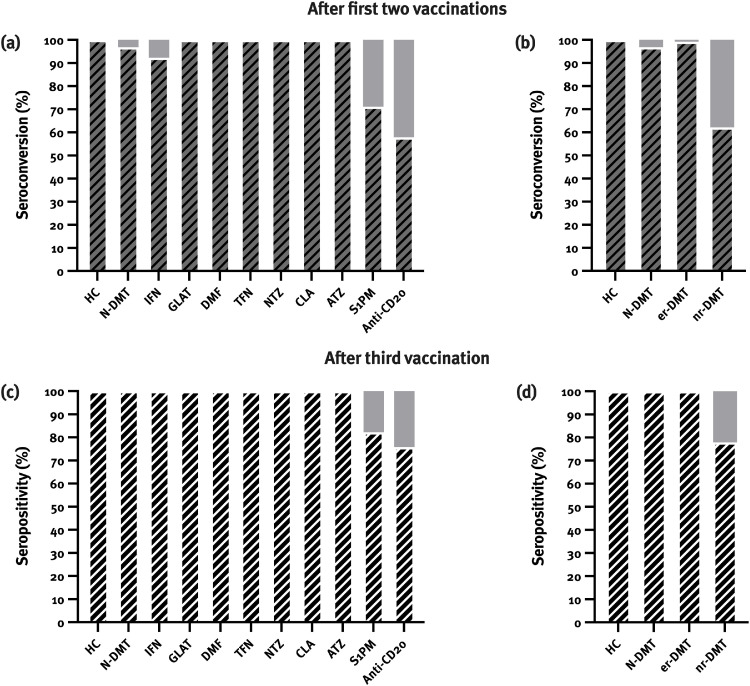
Differentiating according to DMTs, we found seroconversion in 100% of patients on glatiramer acetate, dimethyl fumarate, teriflunomide, natalizumab, cladribine and alemtuzumab (12/12, 44/44, 7/7, 14/14, 26/26, and 4/4, respectively), and 92.3% (12/13) of patients on interferon beta which did not significantly differ compared to HC and patients in the N-DMT group (Fig. 1 a). Patients on S1PMs (71.1%; 32/45) and anti-CD20 monoclonal antibodies (57.9%; 55/95) showed significantly lower rates of seroconversion (p<0.001) (Fig. 1b).
Fig. 1.
Seroconversion rates after first two vaccinations were lower in patients on sphingosine-1-phosphate receptor modulators (S1PMs) and anti-CD20 monoclonal antibodies (a, b). After third vaccination, all patients were positive for anti-SARS-CoV-2 antibodies apart from patients on S1PM and anti-CD20 monoclonal antibodies (c, d).
ATZ: alemtuzumab, CLA: cladribine, DMT: dimethyl fumarate, GLAT: glatiramer acetate, HC: healthy controls, IFN: interferon, NTZ: natalizumab, TFN: teriflunomide.
After third vaccination, 42.5% (17/40) and 38.5% (5/13) of patients on anti-CD20 mAbs and S1PMs seroconverted, respectively, reaching seropositivity in 75.8% and 82.2% of patients on anti-CD20 mAbs and S1PMs. In HC, N-DMT and er-DMT groups, all patients were positive for SARS-CoV-2 antibodies after third vaccination (Fig. 1c,d). No differences in seroconversion rates between homologous and heterologous vaccination regime were seen.
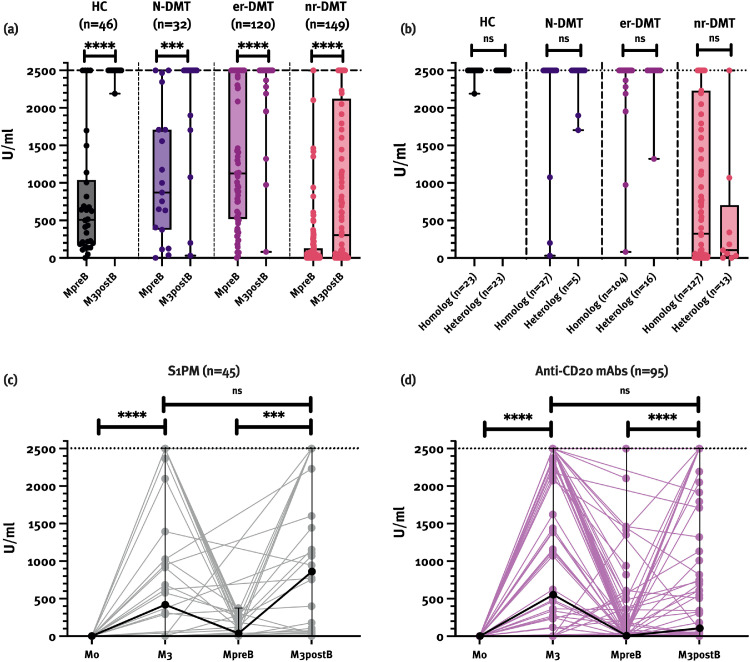
Patients on nr-DMT had significantly lower median antibody levels before third vaccination (12.1 [0.4–2500]) compared to N-DMT (871.8 [0.4–2500]), er-DMT (1126.0 [0.4–2500]) and HC (508.6 [0.4–2500]; p<0.001). After third vaccination, median absolute antibody levels were as follows: HC (2500 [2190–2500]), N-DMT (2500 [32.2–2500]), er-DMT (2500 [80.2–2500]), and nr-DMT (305 [0.4–2500]; p<0.001) (Fig. 2 a). The highest measurable antibody levels were reached in 45/46 (97.8%) HC, and in 26/32 (81.8%), 109/120 (90.8%) and 33/140 (23.6%) patients on N-DMT, er-DMT and nr-DMT, respectively (p<0.001). Patients on anti-CD20 mAbs had a median antibody level of 104 U/ml (0.4–2500) and patients on S1PMs had a median antibody level of 859.5 U/ml (0.4–2500) (p = 0.056) after third vaccination (Fig. 2c,d). We did not find differences in antibody levels after homologous (n = 281; 2500 [0.4–2500]) and heterologous (n = 57; 2500 [0.4–2500]) vaccination regime regardless of the DMT group (Fig. 2b).
Fig. 2.
Patients on nr-DMT had significantly lower SARS-CoV-2 antibody levels before and after third vaccination compared to other pwMS and HC (a). No differences in antibody levels between homologous and heterologous vaccination regime were found (b). Antibody levels in patients on S1PMs and anti-CD20 mAbs increased significantly after third vaccination. However, median antibody levels after first immunization and third vaccination did not differ in either of the groups (c, d). Data are presented as median values with range (minimum, maximum).
Anti-CD20 mAbs: monoclonal antibodies against cluster of differentiation 20, DMT: disease-modifying therapy, HC: healthy controls, M0: one week before first immunization, M3: three months after first immunization, MpreB: one month before third vaccination, M3postB: 2–4 months after third vaccination, ns: not significant, pwMS: patients with multiple sclerosis, S1PMs: sphingosine-1-phosphate receptor modulator
** p<0.01, *** p<0.001, **** p<0.0001
Median time to third vaccination was 6 months (1–13). In multivariate analyses, only the DMT group (= –0.60; 95% CI –1195.73, –799.10; p<0.001) was associated with antibody levels after the third vaccination but not age, sex, disease duration, treatment duration, time interval to last DMT intake, time to revaccination and vaccination regime.
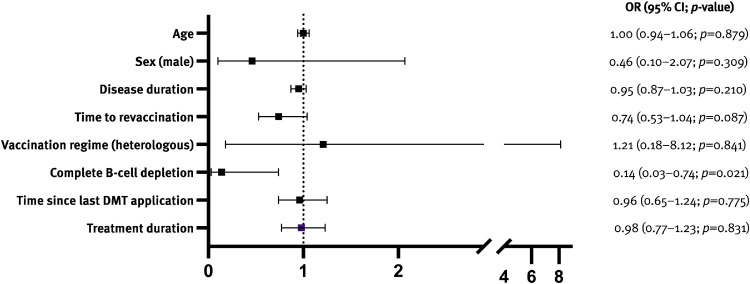
In patients on anti-CD20 mAbs, complete B-cell depletion significantly decreased the probability of seroconversion even after third vaccination (OR 0.14; p = 0.021), whereas time interval to last DMT intake, time to revaccination and vaccination regime did not (Fig. 3 ). The S1PMs subgroup was too small to conduct any subanalysis.
Fig. 3.
Complete B-cell depletion was the only predictor of no seroconversion in patients on anti-CD20 mAbs.
Adverse events (AE) were reported in 41.8% of all study participants, most of them complaining about local AE (39.0%), less commonly about systemic AE (18.1%). No differences between the groups in the rates of AE were noted.
Twenty-two pwMS reported SARS-CoV-2 infection after third vaccination, 3 pwMS in the N-DMT, 9 pwMS in the er-DMT, and 10 pwMS in the nr-DMT. One patient was asymptomatic and 21 patients had a mild course, no one requiring hospitalization or intensive care. Among those, only one patient on anti-CD20 mAbs was seronegative before the infection and also remained negative thereafter.
4. Discussion
In this multicenter prospective observational study on 292 pwMS and 46 HC, we aimed to evaluate the SARS-CoV-2 antibody levels after third vaccination in pwMS. As our previous study showed lower seroconversion rates in patients treated with S1PMs and anti-CD20 mAbs (Bsteh et al., 2022c), our primary focus was to evaluate this group of patients.
With regard to absolute antibody levels, the N-DMT group and the er-DMT group did not differ from the HC group, while the nr-DMT group had significantly lower levels before and after third vaccination. Although patients with S1PMs seem to develop higher levels of antibodies compared to patients with anti-CD20 mAbs, the difference was not statistically significant. Besides, even after third vaccination, seropositivity (75.8–82.2%) still did not reach rate of seropositivity of other pwMS or HC. Our results are in line with the literature, in which seroconversion rates after third vaccination in those patients are reported to be 44–46% (Milo et al., 2022), reaching seropositivity in 75.0% and 58.3% of patients on S1PMs anti-CD20 mAbs (Maglione et al., 2022; Tallantyre et al., 2022).
We also analyzed whether antibody levels and the seroconversion rate are associated with the time to revaccination or treatment duration, but that seems to play little if any role. The majority of our patients received third vaccination after a median of 6 months which seems to be reasonable as antibody levels were already reduced before third vaccination (Fig. 2).
In our recent study, the degree of lymphopenia was a factor influencing humoral response in patients on S1PMs (Bsteh et al., 2022c). While this was not statistically significant in the present study, this may be primarily due to the lower sample size. However, complete B-cell depletion plays – as expected – a pivotal role in the seroconversion after third vaccination. Therefore, monitoring of B-cell reconstitution might aid in estimating chances of humoral response to third vaccination. However, extending dosing intervals in order to facilitate vaccination response is currently not recommended due to the risk of disease reactivation (Otero-Romero, 2022). In this context, we should keep in mind that even seronegative patients still generate a robust T-cell response which may help to protect against severe COVID-19 (Kornek et al., 2022). Thus, it is not warranted to withhold the opportunity for potential protection from COVID-19 by vaccination from any pwMS, independent of DMT or lymphocyte status.
Although several studies suggested that heterologous vaccination regime could provide a higher immune response (He et al., 2021; Atmar et al., 2021; Liu et al., 2021), we did not find any differences between homologous and heterologous vaccination regime. These findings are also in accordance with a lately published study, in which a third vaccination enhanced humoral and cellular immune response in seronegative patients with different immunological diseases on anti-CD20 mAbs irrespective of the vaccination regime (Bonelli et al., 2022).
Twenty-two patients reported a SARS-CoV-2 infection, with the majority of patients having a mild course and no one suffering from a severe course or dying from the infection. It is reassuring that vaccination seems to provide protection from a severe course even in patients on nr-DMT with lower antibody levels or even no antibodies at all (Bsteh et al., 2022b). However, this data should be interpreted cautiously as there have been several variants of SARS-CoV-2, causing not only different disease severity, but also being less susceptible to the neutralizing activity of SARS-CoV-2 vaccine-elicited antibodies (Wang et al., 2021).
Some limitations to this study should be acknowledged. Despite relatively low seroconversion rates after the first vaccination in those patients, only 13 seronegative patients on S1PMs and 40 seronegative patients on anti-CD20 mAbs were included in this study, limiting subanalysis of probability for seroconversion. Besides, the majority has undergone the homologous vaccination regime, which is why our results could be biased in favor of the latter. Moreover, patients were tested for antibody levels in a relatively broad timeframe (2–4 months after third vaccination) which could lead to misinterpretation of our results. However, we performed sensitivity analyses which revealed no differences in antibody levels between these two timepoints, bringing the bias in this regard to a bare minimum. Also, our study was limited by the ceiling effect of the antibody testing, with the majority of pwMS (except for nr-DMT) and HC reaching the highest measurable antibody levels.
In conclusion, SARS-CoV-2 third vaccination is safe in pwMS with an excellent humoral response. Though not statistically significant, more patients seroconverted after third vaccination, and reached higher SARS-CoV-2 antibody levels after third vaccination, speaking in favor of recommending it to every pwMS. Because time to revaccination did not play a role in our multivariable model, it seems reasonable to plan it approximately 6 months after the second vaccination when antibody levels are decreasing. As the most important factor for seroconversion in patients on anti-CD20 mAbs is the B-cell repletion, third vaccination should be planned accordingly.
Author contributions
Nik Krajnc: Conceptualization, Investigation, Visualization, Data curation, Formal analysis, Writing – original draft.
Harald Hegen: Investigation, Writing – review and editing.
Gerhard Traxler: Investigation, Data curation, Writing – review and editing.
Fritz Leutmezer: Conceptualization, Investigation, Visualization, Writing – review and editing.
Franziska Di Pauli: Investigation, Writing – review and editing.
Barbara Kornek: Investigation, Writing – review and editing.
Paulus Rommer: Investigation, Writing – review and editing.
Gudrun Zulehner: Investigation, Writing – review and editing.
Katharina Riedl: Investigation, Writing – review and editing.
Sophie Dürauer: Investigation, Data curation, Writing – review and editing.
Angelika Bauer: Investigation, Data curation, Writing – review and editing.
Sarah Kratzwald: Investigation, Data curation, Writing – review and editing.
Sigrid Klotz: Investigation, Writing – review and editing.
Michael Winklehner: Investigation, Writing – review and editing.
Florian Deisenhammer: Investigation, Writing – review and editing, Supervision.
Michael Guger: Investigation, Writing – review and editing, Supervision.
Romana Höftberger: Investigation, Writing – review and editing, Supervision.
Thomas Berger: Conceptualization, Investigation, Visualization, Writing – review and editing.
Gabriel Bsteh: Conceptualization, Visualization, Investigation, Funding acquisition, Data curation, Writing – review and editing, Supervision.
Disclosures
Nik Krajnc: has participated in meetings sponsored by, received speaker honoraria or travel funding from BMC/Celgene, Merck, Novartis, Roche and Sanofi-Genzyme and held a grant for a Multiple Sclerosis Clinical Training Fellowship Programme from the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Harald Hegen: has participated in meetings sponsored by, received speaker honoraria or travel funding from Bayer, Biogen, Celgene, Merck, Novartis, Sanofi-Genzyme, Siemens and Teva, and received honoraria for consulting Biogen, Celgene, Novartis and Teva.
Gerhard Traxler: has participated in meetings sponsored by, received honoraria (lectures, advisory boards, consultations) or travel funding from Biogen, Celgene/BMS, Janssen-Cilag, Merck, Novartis, Roche, Sanofi-Genzyme and Teva.
Fritz Leutmezer: has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Bayer, Biogen, Celgene/BMS, Janssen, MedDay, Merck, Novartis, Roche, Sanofi-Genzyme and Teva.
Franziska Di Pauli: has participated in meetings sponsored by, received honoraria (lectures, advisory boards, consultations) or travel funding from Bayer, Biogen, Celgene/BMS, Merck, Novartis, Sanofi-Genzyme, Roche and Teva.
Barbara Kornek: has received honoraria for speaking and for consulting from Biogen, BMS-Celgene, Johnson&Johnson, Merck, Novartis, Roche, Teva and Sanofi-Genzyme outside of the submitted work. No conflict of interest with respect to the present study.
Paulus Rommer: has received honoraria for consultancy/speaking from AbbVie, Allmiral, Alexion, Biogen, Merck, Novartis, Roche, Sandoz, Sanofi Genzyme, has received research grants from Amicus, Biogen, Merck, Roche.
Gudrun Zulehner: has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Merck, Novartis, Roche, Sanofi-Genzyme and Teva.
Katharina Riedl: has participated in meetings sponsored by or received travel funding from Almirall.
Sophie Dürauer: has nothing to disclose.
Angelika Bauer: has participated in meetings sponsored by or received travel funding from Novartis, Sanofi Genenzyme, Merck, Almirall and Biogen.
Sarah Kratzwald: has nothing to disclose.
Sigrid Klotz: has nothing to disclose.
Michael Winklehner: has nothing to disclose.
Florian Deisenhammer: has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Alexion, Almirall, Biogen, Celgene/BMS, Janssen, Merck, Novartis, Roche and Sanofi-Genzyme. His-institution received scientific grants from Biogen and Sanofi-Genzyme.
Michael Guger: has received support and honoraria for research, consultation, lectures and education from Almirall, Bayer, Biogen, Celgene/BMS, Genzyme, Janssen-Cilag, MedDay, Merck, Novartis, Octapharma, Roche, Sanofi-Genzyme, Shire and Teva.
Romana Höftberger: has received honoraria for lectures from Novartis and Biogen.
Thomas Berger: has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS: Allergan, Bayer, Biogen, Bionorica, BMS/Celgene, GSK, GW/Jazz Pharma, Horizon, Janssen-Cilag, MedDay, Merck, Novartis, Octapharma, Roche, Sandoz, Sanofi-Genzyme, Teva and UCB. His-institution has received financial support in the past 12 months by unrestricted research grants (Biogen, Bayer, BMS/Celgene, Merck, Novartis, Roche, Sanofi-Genzyme, Teva and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi-Genzyme, Teva.
Gabriel Bsteh: has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Celgene/BMS, Lilly, Merck, Novartis, Roche, Sanofi-Genzyme and Teva, and received honoraria for consulting Biogen, Celgene/BMS, Novartis, Roche, Sanofi-Genzyme and Teva. He has received unrestricted research grants from Celgene/BMS and Novartis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Acknowledgments
The authors explicitly thank Sonja Wieszmüllner and Christiane Göls for their diligent and tireless work in organizing and administrating this study. This work was partly supported by a grant from the Austrian Science Fund FWF: project DOC 33-B27 (Michael Winklehner, Romana Höftberger).
References
- Achiron A., Mandel M., Dreyer-Alster S., Harari G., Magalashvili D., Sonis P., Dolev M., Menascu S., Flechter S., Falb R., Gurevich M. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021;14 doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmar, R.L., Lyke, K.E., Deming, M.E., Jackson, L.A., Branche, A.R., El Sahly, H.M., Rostad, C.A., Martin, J.M., Johnston, C., Rupp, R.E., Mulligan, M.J., Brady, R.C., Frenck, R.W., Backer, M., Kottkamp, A.C., Babu, T.M., Rajakumar, K., Edupuganti, S., Dobryzynski, D., Posavad, C.M., Archer, J.I., Crandon, S., Nayak, S.U., Szydlo, D., Zemanek, J., Islas, C.P.D., Brown, E.R., Suthar, M.S., McElrath, M.J., McDermott, A.B., O'Connell, S.E., Montefiori, D.C., Eaton, A., Neuzil, K.M., Stephens, D.S., Roberts, P.C., Beigel, J.H. Group, D.S., 2021. Heterologous SARS-CoV-2 Booster Vaccinations - Preliminary Report. medRxiv.
- Bonelli M., Mrak D., Tobudic S., Sieghart D., Koblischke M., Mandl P., Kornek B., Simader E., Radner H., Perkmann T., Haslacher H., Mayer M., Hofer P., Redlich K., Husar-Memmer E., Fritsch-Stork R., Thalhammer R., Stiasny K., Winkler S., Smolen J.S., Aberle J.H., Zeitlinger M., Heinz L.X., Aletaha D. Additional heterologous versus homologous booster vaccination in immunosuppressed patients without SARS-CoV-2 antibody seroconversion after primary mRNA vaccination: a randomised controlled trial. Ann. Rheum. Dis. 2022;81:687–694. doi: 10.1136/annrheumdis-2021-221558. [DOI] [PubMed] [Google Scholar]
- Bsteh G., Gradl C., Assar H., Heschl B., Hegen H., Di Pauli F., Leutmezer F., Traxler G., Zulehner G., Rommer P., Hiller M.S., Krajnc N., Wipfler P., Guger M., Enzinger C., Berger T. ÖGN Jahrestagung; Graz: 2022. COVID-19 in Multiple sclerosis: Update from the Nation-Wide Austrian registry. [Google Scholar]
- Bsteh G., Gradl C., Heschl B., Hegen H., Di Pauli F., Assar H., Leutmezer F., Traxler G., Krajnc N., Zulehner G., Hiller M.S., Rommer P., Wipfler P., Guger M., Enzinger C., Berger T. Impact of vaccination on COVID-19 outcome in multiple sclerosis. Eur. J. Neurol. 2022 doi: 10.1111/ene.15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsteh G., Hegen H., Traxler G., Krajnc N., Leutmezer F., Di Pauli F., Kornek B., Rommer P., Zulehner G., Durauer S., Bauer A., Kratzwald S., Klotz S., Winklehner M., Deisenhammer F., Guger M., Hoftberger R., Berger T. Comparing humoral immune response to SARS-CoV2 vaccines in people with multiple sclerosis and healthy controls: an Austrian prospective multicenter cohort study. Eur. J. Neurol. 2022 doi: 10.1111/ene.15265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer-Alster S., Menascu S., Mandel M., Shirbint E., Magalashvili D., Dolev M., Flechter S., Givon U., Guber D., Stern Y., Miron S., Polliack M., Falb R., Sonis P., Gurevich M., Achiron A. COVID-19 vaccination in patients with multiple sclerosis: safety and humoral efficacy of the third booster dose. J. Neurol. Sci. 2022;434 doi: 10.1016/j.jns.2022.120155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q., Mao Q., An C., Zhang J., Gao F., Bian L., Li C., Liang Z., Xu M., Wang J. Heterologous prime-boost: breaking the protective immune response bottleneck of COVID-19 vaccine candidates. Emerg. Microbes Infect. 2021;10:629–637. doi: 10.1080/22221751.2021.1902245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins V., Fabros A., Kulasingam V. Quantitative Measurement of Anti-SARS-CoV-2 Antibodies: analytical and Clinical Evaluation. J. Clin. Microbiol. 2021:59. doi: 10.1128/JCM.03149-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornek B., Leutmezer F., Rommer P.S., Koblischke M., Schneider L., Haslacher H., Thalhammer R., Zimprich F., Zulehner G., Bsteh G., Dal-Bianco A., Rinner W., Zebenholzer K., Wimmer I., Steinmaurer A., Graninger M., Mayer M., Roedl K., Berger T., Winkler S., Aberle J.H., Tobudic S. B Cell Depletion and SARS-CoV-2 Vaccine Responses in Neuroimmunologic Patients. Ann. Neurol. 2022 doi: 10.1002/ana.26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Shaw R.H., Stuart A.S.V., Greenland M., Aley P.K., Andrews N.J., Cameron J.C., Charlton S., Clutterbuck E.A., Collins A.M., Dinesh T., England A., Faust S.N., Ferreira D.M., Finn A., Green C.A., Hallis B., Heath P.T., Hill H., Lambe T., Lazarus R., Libri V., Long F., Mujadidi Y.F., Plested E.L., Provstgaard-Morys S., Ramasamy M.N., Ramsay M., Read R.C., Robinson H., Singh N., Turner D.P.J., Turner P.J., Walker L.L., White R., Nguyen-Van-Tam J.S., Snape M.D., Com C.O.V.S.G. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglione A., Morra M., Meroni R., Matta M., Clerico M., Rolla S. Humoral response after the booster dose of anti-SARS-CoV-2 vaccine in multiple sclerosis patients treated with high-efficacy therapies. Mult. Scler. Relat. Disord. 2022;61 doi: 10.1016/j.msard.2022.103776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milo R., Staun-Ram E., Karussis D., Karni A., Hellmann M.A., Bar-Haim E., Miller A. Humoral and Cellular Immune Responses to SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis: an israeli multi-center experience following 3 vaccine doses. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.868915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Romero, S., 2022. Title. Journal Volume, [in press].
- Simpson-Yap S., De Brouwer E., Kalincik T., Rijke N., Hillert J.A., Walton C., Edan G., Moreau Y., Spelman T., Geys L., Parciak T., Gautrais C., Lazovski N., Pirmani A., Ardeshirdavanai A., Forsberg L., Glaser A., McBurney R., Schmidt H., Bergmann A.B., Braune S., Stahmann A., Middleton R., Salter A., Fox R.J., van der Walt A., Butzkueven H., Alroughani R., Ozakbas S., Rojas J.I., van der Mei I., Nag N., Ivanov R., Sciascia do Olival G., Dias A.E., Magyari M., Brum D., Mendes M.F., Alonso R.N., Nicholas R.S., Bauer J., Chertcoff A.S., Zabalza A., Arrambide G., Fidao A., Comi G., Peeters L. Associations of Disease-Modifying Therapies With COVID-19 Severity in Multiple Sclerosis. Neurology. 2021;97:e1870–e1885. doi: 10.1212/WNL.0000000000012753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani, M.P., Inglese, M., Schiavetti, I., Carmisciano, L., Laroni, A., Lapucci, C., Da Rin, G., Serrati, C., Gandoglia, I., Tassinari, T., Perego, G., Brichetto, G., Gazzola, P., Mannironi, A., Stromillo, M.L., Cordioli, C., Landi, D., Clerico, M., Signoriello, E., Frau, J., Ferro, M.T., Di Sapio, A., Pasquali, L., Ulivelli, M., Marinelli, F., Callari, G., Iodice, R., Liberatore, G., Caleri, F., Repice, A.M., Cordera, S., Battaglia, M.A., Salvetti, M., Franciotta, D., Uccelli, A. CovaXi, M.S.s.g.o.b.o.t.I.C.-A.i.M.S., 2021. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine 72, 103581. [DOI] [PMC free article] [PubMed]
- Sormani M.P., Schiavetti I., Inglese M., Carmisciano L., Laroni A., Lapucci C., Visconti V., Serrati C., Gandoglia I., Tassinari T., Perego G., Brichetto G., Gazzola P., Mannironi A., Stromillo M.L., Cordioli C., Landi D., Clerico M., Signoriello E., Cocco E., Frau J., Ferro M.T. Breakthrough SARS-CoV-2 infections after COVID-19 mRNA vaccination in MS patients on disease modifying therapies during the Delta and the Omicron waves in Italy. EBioMedicine. 2022;80 doi: 10.1016/j.ebiom.2022.104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallantyre E.C., Scurr M.J., Vickaryous N., Richards A., Anderson V., Baker D., Chance R., Evangelou N., George K., Giovannoni G., Harding K.E., Hibbert A., Ingram G., Jolles S., Jones M., Kang A.S., Loveless S., Moat S.J., Robertson N.P., Rios F., Schmierer K., Willis M., Godkin A., Dobson R. Response to COVID-19 booster vaccinations in seronegative people with multiple sclerosis. Mult. Scler. Relat. Disord. 2022;64 doi: 10.1016/j.msard.2022.103937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A.J., Banwell B.L., Barkhof F., Carroll W.M., Coetzee T., Comi G., Correale J., Fazekas F., Filippi M., Freedman M.S., Fujihara K., Galetta S.L., Hartung H.P., Kappos L., Lublin F.D., Marrie R.A., Miller A.E., Miller D.H., Montalban X., Mowry E.M., Sorensen P.S., Tintore M., Traboulsee A.L., Trojano M., Uitdehaag B.M.J., Vukusic S., Waubant E., Weinshenker B.G., Reingold S.C., Cohen J.A. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C.O., Finkin S., Schaefer-Babajew D., Cipolla M., Gaebler C., Lieberman J.A., Oliveira T.Y., Yang Z., Abernathy M.E., Huey-Tubman K.E., Hurley A., Turroja M., West K.A., Gordon K., Millard K.G., Ramos V., Da Silva J., Xu J., Colbert R.A., Patel R., Dizon J., Unson-O'Brien C., Shimeliovich I., Gazumyan A., Caskey M., Bjorkman P.J., Casellas R., Hatziioannou T., Bieniasz P.D., Nussenzweig M.C. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]