Abstract
The germinal center serves as a site of B cell selection and affinity maturation, critical processes for productive adaptive immunity. In autoimmune disease tolerance is broken in the germinal center reaction, leading to production of autoreactive B cells that may propagate disease. Follicular T cells are crucial regulators of this process, providing signals necessary for B cell survival in the germinal center. Here we review the emerging roles of follicular T cells in the autoreactive germinal center. Recent advances in immunological techniques have allowed study of the gene expression profiles and repertoire of follicular T cells at unprecedented resolution. These studies provide insight into the potential role follicular T cells play in preventing or facilitating germinal center loss of tolerance. Improved understanding of the mechanisms of T cell help in autoreactive germinal centers provides novel therapeutic targets for diseases of germinal center dysfunction.
Introduction
T cell dependent B cell activation is a key step in the formation of humoral immune responses. Following antigen priming, T and B cells meet at the T/B border in the spleen or interfollicular region in lymph nodes, forming extrafollicular foci (EF) or migrating to developing germinal centers (GC)1. Follicular helper T (TFH) cells differentiate from naïve CD4 T cells that have received antigen priming and co-stimulation from dendritic cells, leading to downregulation of CCR7 and PSGL-1 and upregulation of CXCR5 which enables follicular entry2. In the GC, B cells receive help from TFH cells via CD40L, IL-4, and IL-21, which leads to somatic hypermutation (SHM), class switch recombination (CSR), affinity maturation, and memory B cell formation2,3. This well-orchestrated yet intricate dance between T and B cells in the GC reaction underlies the remarkable ability of our immune system to produce robust, functional, and long-lasting responses to both infection and vaccination.
While GC responses help fight disease, they might also contribute to autoimmunity4. Pathogenic autoantibodies have been implicated in autoimmune disease5 and are commonly used for diagnostic6 and prognostic7 clinical evaluation. These autoantibodies may be oligoclonal, class-switched, and hypermutated8–11, suggesting a GC origin. However, both EF and GC-derived autoantibodies have been described in mouse models of autoimmunity4. As B cells can develop extrafollicularly without T cell help12, these observations suggest that not all autoantibody responses are T cell dependent. Peripheral tolerance mechanisms prevent activation of autoreactive B cells, mediated by availability of T cell help, antigen abundance, intrinsic B cell signaling, and stromal regulation in the GC13. The breakdown of peripheral tolerance in autoreactive GCs suggests that TFH cells are dysfunctional in autoantibody disease.
Despite recognition of the importance of follicular T cells in autoreactive GC reactions, the molecular features of dysfunction in TFH and follicular regulatory T (TFR) cells have only recently been characterized. Developments in single cell sequencing and mass cytometry have facilitated interrogation of numerous cell types, including TFH and TFR cells, with unparalleled detail. These studies have both provided mechanistic insight and generated new hypotheses for the multifaceted roles follicular T cells may play in loss of B cell tolerance. Here we review the historical evidence that T cell help is a necessary and sufficient driver of autoantibody formation, describe emerging studies of follicular T cell dysfunction in autoimmune disease, and pose open questions to be addressed by future studies.
Main Text
T cell dependence of autoantibody responses
Autoantibodies have been identified in over 80 different autoimmune diseases5. These autoantibodies may have specificity for either tissue-specific antigens, such as thyroid peroxidase in autoimmune thyroid disease14, or for ubiquitous antigens, such as dsDNA in systemic lupus erythematosus (SLE)15. Autoantibodies may also be pathogenic16, such as anti-acetylcholine receptor in myasthenia gravis (MG)17, or may be associated with disease, such as anti-cyclic citrullinated peptides in rheumatoid arthritis (RA)18. Autoantibody specificity may also evolve over time in a single patient, leading to reactivity against different autoantigens, termed epitope spreading19. The degree of epitope spreading correlates with disease severity, allowing autoantibody specificity to serve as a biomarker for both clinical diagnosis and prognosis20–25. Somatic mutations have also been identified in autoantibody sequences11,26–32. The evolving specificity, affinity maturation, oligoclonality, class switching, and chronic production of autoantibodies suggest that some may have T cell dependent GC origins5.
GC-derived autoantibodies represent a breakdown of tolerance mechanisms normally in place to maintain GC homeostasis. Autoreactive B cells that escape central tolerance either die following failure to receive T cell help33–36 or adopt an IgM low state with reduced antibody secretion in the periphery, termed clonal anergy37,38. Accumulating evidence has suggested that these anergic B cells are recruited into germinal centers where they undergo clonal redemption and lose autoreactivity39,40, a process that if dysregulated could lead to autoantibody development and epitope spreading (Figure 1). Clonal redemption in HEL3X bone marrow chimeras requires foreign antigen elicited T cell help41 or in MRL/lpr mice requires CD40L but not B7-CD28 mediated TFH help42. Current models of clonal redemption state that only once B cells have mutated away from self and toward foreign antigen binding are they able to receive sufficient T cell help39,40,43–45, suggesting that the failures of clonal redemption observed in autoantibody-mediated disease are due to provision of T cell help despite maintenance of B cell autoreactivity. Alternatively, autoreactive B cells may overcome T cell dependence due to TLR adjuvanticity, driven by large amounts of self-DNA and RNA from dying cells in the GC46,47. The stochastic nature of somatic hypermutation also leads to the generation of autoreactive BCRs in both healthy and autoimmune patients11,48–50, but only results in high affinity pathogenic autoantibody production in disease51, likely representing a fundamental breakdown of GC tolerance.
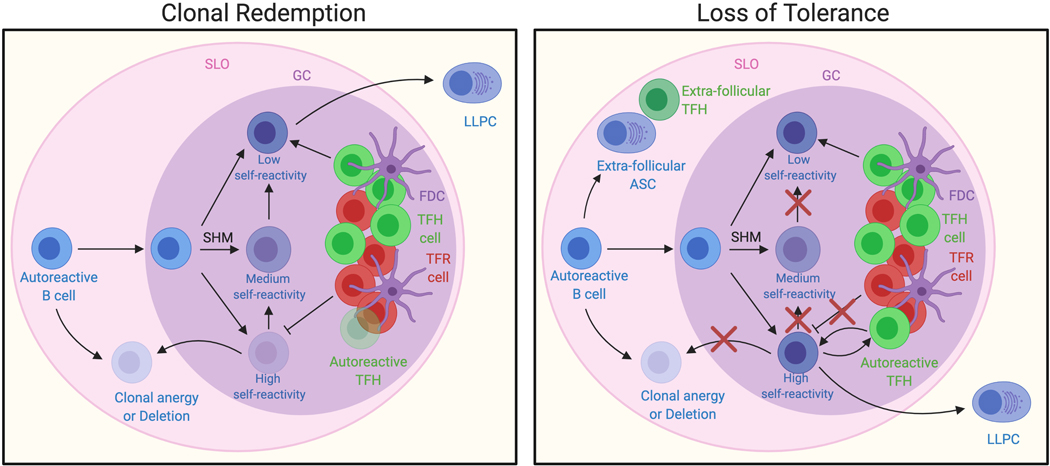
Figure 1. Failures in clonal redemption lead to loss of tolerance.
Autoreactive B cells normally undergo SHM to lose self-reactivity in a process termed clonal redemption. Anergic B cells that have escaped central tolerance are recruited into the GC of SLOs and mutate away from self to receive T cell help, which facilitates CSR and LLPC development. This process is regulated by checkpoints such as limited antigen-specific TFH cells, limited antigen availability on FDCs, co-stimulation provided by TFH cells, and TFR cells. We propose that in autoantibody disease an autoreactive B cell clone escapes clonal redemption due to failures of these checkpoints, triggering TFH dysfunction and epitope spreading towards autoantigens. SHM, somatic hypermutation; CSR, class switch recombination; GC, germinal center; TFH, follicular helper T cell; TFR, follicular regulatory T cell; FDC, follicular dendritic cell; LLPC, long lived plasma cell; SLO, secondary lymphoid organ; ASC, antibody secreting cell.
Given the importance of follicular T cells for maintaining GC tolerance, dysregulated TFH and TFR cells have long been thought to contribute to the development of autoreactive B cells. Increased TFH frequency has been observed in SLE, MG, Sjögren’s syndrome (SS), multiple sclerosis (MS), autoimmune thyroid disease, and RA52–62, and altered TFR frequency has been observed in SLE, RA, ankylosing spondylitis, SS, MG, and MS63–67. Mouse models of systemic autoimmunity such as OX40L overexpression, TLR7 overexpression, or Roquinsan/san have increased TFH cells43,68–70, which can also confer disease when adoptively transferred. Loss of GC TFH cells either through SAP deficiency71 or Bcl6 haploinsufficiency72,73 decreases GC cells, autoantibody development, and glomerulonephritis in Roquinsan/san mice. Spontaneous GC formation and ensuing epitope spreading in a mixed 564Igi bone marrow chimera model of autoimmunity is CD40L dependent74. CTLA4Ig and anti-CD40L alone or in combination abrogates autoreactive GCs by inducing tolerance, prevents autoantibody development, and ameliorates disease75–79. These observations in human and murine autoantibody disease suggest that T cell help is both necessary and sufficient to propagate disease.
Mechanisms of T cell mediated peripheral tolerance in the GC likely involve crosstalk between autoreactive T and B cells. CD40LG and MHC class II are genetic risk loci for elevated levels of autoreactive B cells80, suggesting that CD40 and TCR signals from follicular T cells to autoreactive B cells help maintain B cell tolerance. MRL/lpr mice with MHC class II-deficient B cells have decreased TFH frequency and autoantibody production81. IPEX patients bearing mutations in FOXP3 have defective peripheral B cell tolerance and increased B cell homeostatic proliferation82, suggesting that regulatory T (TREG) or TFR cells maintain peripheral B cell tolerance. These observations suggest that TCR and co-stimulation mediated help from follicular T cells likely serves as a checkpoint against autoreactive GC development. Despite recognition that some autoantibody responses are T cell dependent, more detailed characterization of the follicular T cell dysfunction underlying loss of tolerance have only recently been characterized through advances in immunological techniques.
Follicular T cell dysfunction
Comparisons of follicular T cell phenotypes between foreign and autoimmune responses have provided insight into the characteristics of follicular T cells that permit autoantibody development. Flow cytometric profiling of follicular T cells in combination with more recent advances in single cell sequencing and mass cytometry have allowed for transcriptome and repertoire wide characterization of autoimmune follicular T cells. These approaches have led to appreciation for the heterogeneity amongst the follicular T cell population, identification of novel subsets of follicular T cells, gene expression to clonality correlational analyses, and developmental trajectory inference. Collectively, these studies have revealed changes in follicular T cell transcription factor expression, chemokine and cytokine profiles, co-stimulation, metabolism, exhaustion, trafficking, and differentiation in autoantibody disease (Figure 2).
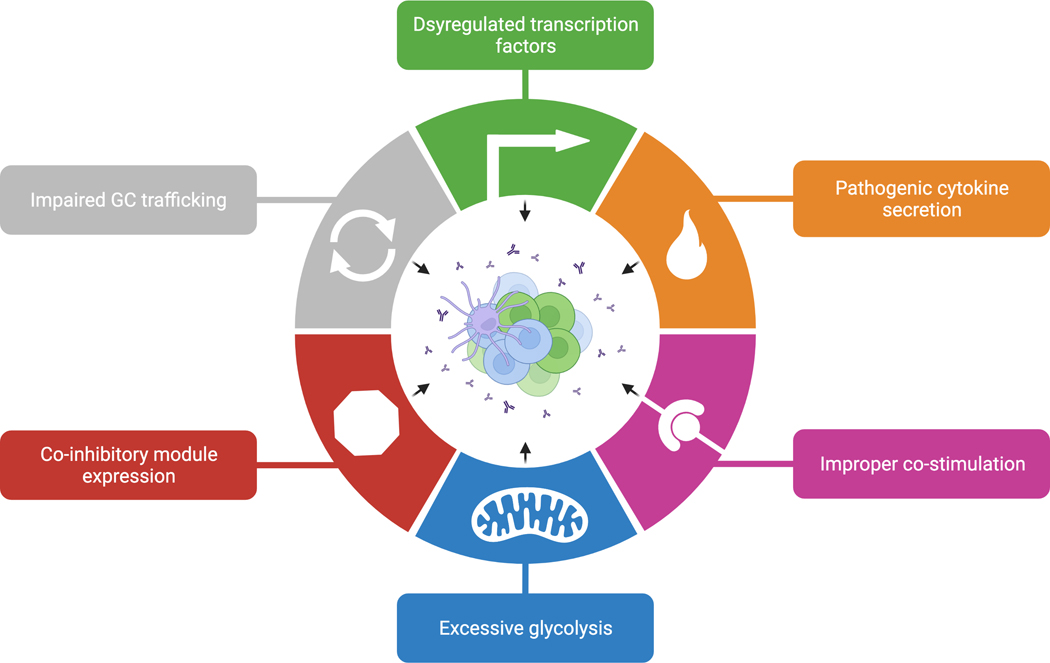
Figure 2. Hallmarks of TFH dysregulation in autoimmune disease.
TFH cells isolated from mice and humans with autoimmune disease exhibit transcriptional and functional changes. We propose that loss of GC tolerance is due to TFH dysregulation, albeit through various mechanisms of dysfunction. TFH cells might permit or promote autoreactive B cell development due to dysregulated transcription factor expression, cytokine and chemokine profiles, co-stimulation, metabolism, exhaustion, and trafficking. GC, germinal center; TFH, follicular helper T cell.
Dysregulated transcription factors
Transcription factor expression not only governs TFH differentiation but can also influence TFH function83. TFH cells may express lineage-specific transcription factors in response to pathogen identity, ultimately influencing cytokine production and ensuing CSR84–88. TFH Bcl6 expression normally suppresses T-bet and RORγt expression, thereby reducing IL-17 production89,90, and dysregulated IL-17 signaling contributes to SLE91–95. Gata3 and RORγt expressing circulating TFH cells are more prevalent and correlate with disease severity in juvenile dermatomyositis, SLE, SS, RA, and MS96–98. ETS1 is an SLE risk locus and deletion of the transcription factor Ets1 in T cells leads to Gata-3 TFH2 cell-driven SLE-like autoimmunity in mice99. Id3 expression was decreased on TFH and TFR cells in 564Igi bone marrow chimeras100 and peripheral blood CD4 T cells from SLE patients101. ID3 is a transcription factor necessary for TFR maturation102,103, suggesting that impaired TFR development might contribute to autoreactive GCs. Tcf7 was negatively associated with clonal expansion in autoimmune TFH and TFR cells, but not foreign antigen elicited TFH and TFR cells100. TCF-1 (protein name for Tcf7) promotes TFH fate determination over TH1 cells by repressing Blimp1104,105. Maf and Ikzf2 are upregulated in CD4 T cells from B6.Sle1yaa mice106 and human SLE kidney biopsies107. c-Maf (protein name for Maf) is an AP-1 transcription factor necessary for TFH development and IL-21 production108. The transcription factor Helios (protein name for Ikzf2), although commonly used to identify thymic-derived TREG cells109, has also been observed on activated TFH cells110. TFH upregulation of FoxP3 facilitates GC contraction111, failure of which could lead to chronic GCs observed in autoantibody disease. We hypothesize that these transcription factor changes represent instability in TFH identity following autoantigen-driven expansion.
Pathogenic cytokine secretion and improper co-stimulation
Follicular T cells canonically provide help to GC B cells through cytokine release and co-stimulation, which if dysregulated might permit autoreactivity. CD4 T cells from B6.Sle1yaa mice had dysregulated cytokine and chemokine profiles including increased expression of Il12rb1 Il15ra, Il21, Il2rb, Ccl5, Ccl4, Il10ra, Il3ra, Il4, and Ifng70. Human SLE risk loci include variants in IL2, IL6, IL10, IL12A, TYK2, TNFSF4, STAT4, IRF5, IRF8, IL21, and IL21R112–125. Administration of low dose IL-2 to SLE patients led to TFH contraction and reduced disease severity126, supporting TREG independent IL-2 mediated immunosuppression via Blimp-1 and T-bet suppression of Bcl6127–129, suggesting that IL-2 deficiency might contribute to autoreactive GC development. In contrast, IL-6 deficiency in Was−/− murine lupus prevents TFH expansion and autoimmune pathology130, suggesting that excessive IL-6 signaling might promote loss of GC tolerance. Il7r expression was decreased on TFH cells from 564Igi bone marrow chimeras100 and human SLE kidney biopsies107, as well as peripheral blood CD4 T cells from SLE patients101. IL-7R is repressed by Bcl-6 and can negatively regulate TFH identity131–133, possibly reflecting transcription factor influence on TFH fate in autoimmunity. TFH cell-derived IL-17 can increase RGS13 and RGS16 expression on B cells, contributing to autoantibody development in BXD2 and NZB/W F1 mice91,92,134–137. IL-17 in combination with TFH cell-derived BAFF can prevent BCR mediated B cell apoptosis, retain B cells in the GC, and traffic TFH to the light zone (LZ) of GCs91,92,138, processes that if dysregulated in the setting of excess IL-17 might lead to autoreactive GC formation. Autoantibody production decreased in B6.Sle1yaa and NZB/W F1 mice following IL-21 blockade139–142 and BXSB/Yaa mice have elevated serum IL-21143, a TFH defining cytokine that promotes CSR and long-lived plasma cell (LLPC) formation but might also contribute to loss of GC tolerance by increasing the TFH to TFR ratio144–146. IL-23 correlates with disease severity in SLE147 and IL-23 deficiency in B6/lpr or MRL/lpr mice leads to decreased TFH cells, autoantibodies, and glomerulonephritis93,148,149, suggesting that IL-23 signaling could also promote loss of GC tolerance. A milieu of cytokines with disparate functions are dysregulated in autoimmune TFH cells, representing potential pathogenic drivers and therapeutic targets.
In addition to dysregulated cytokines, surface molecule expression on TFH cells is altered in autoimmune disease with potential functional consequences. Overexpression of ICOS in TFH cells is necessary and sufficient to induce autoreactive GC responses in the Roquinsan/san mouse model of SLE43,71,150,151 and is associated with autoantibodies and end organ damage in human disease152–154. CD95-deficient B cells can lead to fatal systemic immunity155–159 whereas CD95L overexpression on T cells can suppress autoreactive B cells160,161, suggesting that TFH cells can mediate negative selection of autoreactive B cells via CD95/CD95L. Cd74 was more strongly associated with clonal expansion in TFR cells from 564Igi bone marrow chimeras100 and FaslprCd74−/− mice have decreased autoantibodies and kidney pathology162. CD74 serves multiple functions in facilitating MHC class II peptide loading163, regulating CD95/CD95L signaling164, and in complex with CXCR4 serving as a receptor for the cytokine MIF165–167. Serum MIF levels correlate with SLE severity168,169 and MIF deficiency reduces kidney pathology in MRL/lpr and NZB/W F1 mice170,171, suggesting that MIF mediated CD74 signaling in follicular T cells might contribute to autoantibody production. CD153 was increased on TFH cells from 564Igi bone marrow chimeras100 and on CD4 T cells from B6.Sle1yaa mice106. CD153+ follicular T cells are senescent yet pathogenic due to their ability to initiate spontaneous GCs and glomerulonephritis via osteopontin secretion172. Given the functional importance of these surface molecules, their dysregulated expression in autoimmune follicular T cells likely contributes to loss of GC tolerance.
Altered metabolic states
Autoimmune disease might also be influenced by altered metabolic states of lymphocyte populations, including follicular T cells. TFH differentiation and function require metabolic tuning, as HDL components can inhibit TFH formation173,174 and 7α,25-dihydroxycholesterol can guide TFH positioning via EBI2 signaling175. ApoE-deficient bone marrow chimeras reconstituted with BXD2 bone marrow develop increased TFH cells, GCs, and autoantibodies176, suggesting that dyslipidemia may contribute to TFH associated loss of GC tolerance. Bcl6177 and PD-1178 suppress glycolysis, but mTOR signaling is necessary for TFH activation179–181. CD4 T cells from SLE patients have increased mTORC1 signaling and glycolysis182–190 and mTORC1 promotes Bcl6 expression in autoreactive TFH cells191,192. Inhibition of glycolysis in B6.Sle1.Sle2.Sle3, NZB/W F1, BXSB.yaa, or B6.lpr mice with 2-deoxyglucose and metformin reduced autoantibody and TFH levels but preserved the ability to mount humoral responses to foreign antigen184,185,191,193. Follicular T cells have increased glycolysis and hypoxia related gene signatures in 564Igi bone marrow chimeras100 and computational metabolic modeling of CD4 T cells from B6.Sle1yaa mice revealed widespread increase in metabolic activity, including glycolysis106. These results suggest that glucose inhibition is an appealing therapeutic strategy to selectively target autoreactive TFH cells while preserving immunity against foreign pathogens.
Co-inhibitory module induction
Although best characterized in CD8 T cells, exhaustion and co-inhibitory receptor expression on follicular T cells might have functional consequences in autoimmunity. Lag3 expression was increased on TFH cells in 564Igi bone marrow chimeras100 and Tigit, Lag3 and Pdcd1 are upregulated in CD4 T cells from B6.Sle1yaa mice106 and human SLE kidney biopsies107. Co-inhibitory module upregulation most commonly follows continuous TCR engagement and T cell activation to facilitate homeostatic contraction194,195, and therefore their upregulation on TFH cells in autoimmune disease likely signals antigen experience in the GC. Alternatively, type I interferon signaling can also increase co-inhibitory module expression196, and both 564Igi and B6.Sle1yaa have elevated interferon signaling197–199. As in CD8 T cells200, PD-1 signaling can attenuate TFH expansion and activation201. PD-1 might also have GC specific functions, including promotion of IL-21 and IL-4 release and ensuing LLPC production202,203. PD-1 also influences TFH positioning within the follicle as PD-L1 expression on bystander B cells can limit follicular entry of ICOS negative CD4 T cells while facilitating GC entry of TFH cells204. Lag-3 inhibition in mice infected with Plasmodium resulted in increased TFH cells and malarial clearance205,206. Tim-3 expressing TFH cells identified in cancer patients were less responsive to stimulation and less capable to promote CSR207–209. We hypothesize that increased co-inhibitory receptor expression on autoimmune TFH cells is reflective of the chronic nature of autoreactive GCs and that targeting these pathways in autoantibody disease warrants caution given their multifaceted roles in TFH function.
Impaired GC trafficking
Follicular T cell differentiation and positioning within the GC are tightly regulated processes that may be dysregulated in the autoreactive GC. Following extrafollicular engagement with B cells, TFH fate determination and guidance towards the follicle is necessary for GC formation and is dictated by chemokine gradients and molecular cues such as downregulation of CCR7210, miR-17–9289, and PSGL-1211 and expression of CXCR5210, S1PR2212, EPHB6213, miR-155214, EBI2215, Sema4C216, LFA-1217,218, VLA-4219, integrin αV220 and SAP221. TFH cell extrinsic signals also influence TFH migration towards and maintenance within the GC, such as B cell expression of PD-L1204,222, SLAM receptors221, PlxnB2216, and EFNB1213, and FDC expression of CXCL13223. Itgb1 and Ahnak are upregulated in CD4 T cells from B6.Sle1yaa mice106 and human SLE kidney biopsies107. Integrin β1 is a component of VLA-4 (α4β1), whose expression on TFH cells may influence T-B conjugate formation219 or interactions with FDCs via VCAM-1224,225. AHNAK is a scaffold protein necessary for Ca++ signaling in CD4 T cells226,227 that also promotes pseudopod protrusion and migration on metastatic cancer cells228. Itgb7 and Selplg are upregulated in TFH cells belonging to TCR specificity groups expanded in 564Igi bone marrow chimeras100. Integrin β7 is a component of α4β7, the receptor for the gut homing molecule MAdCAM-1229, while PSGL-1 (protein name for Selplg) limits TFH entry to the GC211 and may doubly serve as a checkpoint molecule230, suggesting that TCR autoreactivity might influence T cell trafficking out of the GC. This hypothesis is supported by the observation that Selplg was negatively associated with clonal expansion in foreign antigen elicited TFH cells but not TFH cells from 564Igi bone marrow chimeras100. PSGL-1 mediated autoreactive TFH suppression or GC exit might have functional consequences, as a subset of central memory-like CD44+CD62L+ TFH cells are decreased in 564Igi bone marrow chimeras with a corresponding increase in PSGL-1loCD62Llo extrafollicular CD4 T cells100. CD4 T cells from B6.Sle1yaa mice also increased expression of Cxcr3 and Cxcr4106, chemokine receptors that regulate migration towards the interferon-inducible ligands CXCL9, CXCL10, and CXCL11231 or between the LZ and dark zone (DZ)232, respectively. TFH cells in autoreactive GCs modulate expression of key trafficking molecules, which we hypothesize represent a homeostatic response to autoantigen recognition that limits T cell help via GC exit.
TFR dysfunction
TFR cells are central regulators of the GC reaction that curtail autoreactive B cells233. TFR cells may suppress GC B cells directly by downregulating B cell expression of MHC class II and B7–1 and B7–2 via TFR expression of CTLA-4234, suppressing GC B cell activation via release of neuritin, IL-10, and TGB-β235–237, or killing GC B cells via granzyme mediated cytolysis238. TFR cells may also limit TFH cell help directly by granzyme mediated killing of TFH cells239 or mechanically disrupting their interaction with GC B cells240, a process potentially governed by TCR affinity241,242. FoxP3CreBcl6fl/fl mice have increased autoantibodies after influenza infection, pristane injection, experimental SS, and spontaneously after 30 weeks239,243–247. Conditional deletion of TFR cells in FoxP3CreCxcr5LSL-DTR mice after immunization led to the production of autoreactive IgG248. Single cell sequencing of TFR cells in 564Igi bone marrow chimeras revealed similar transcriptional changes as in TFH cells, including upregulation of Ly6a and Gm42031 and downregulation of Id3 and Lag3100. ID3 is necessary for maintenance of the regulatory T cell pool and ID3 depletion impairs TFR localization103. We propose that just as TFH cells aberrantly provide help to autoreactive B cells in autoantibody disease, dysfunctional TFR cells fail to suppress autoreactive B cells, representing an orthogonal breakdown of peripheral tolerance in the autoreactive GC.
Autoreactive follicular T cell repertoire
Maintenance of peripheral B cell tolerance is at least partially governed by limiting of T cell help dictated by cognate TCR interactions between TFH and GC B cells5,249–251. Inefficiency of central B cell tolerance252 in combination with the stochastic nature of SHM therefore rely on the relatively greater efficiency of central T cell tolerance to prevent autoantibody development250,252. Clonal redemption of autoreactive B cells is T cell dependent42, with TCR specificity42,242,253 and antigen availability41,51 likely serving as selective pressures for BCR mutation away from self-reactivity. Despite long held models of the importance of follicular T cell specificity, only recently have advances in single cell sequencing allowed for unbiased study of the follicular T cell repertoire. These studies have provided insight into not only TFH and TFR clonality in normal immune responses, but also hypotheses for how these regulatory processes might fail in the autoreactive GC.
TFH and TFR cells have distinct repertoires254, likely reflecting disparate ontogenies and functions. TCR transgenic experiments demonstrated that TFH recruitment into the GC is governed by specificity for the immunizing antigen, whereas TFR entry is not influenced by antigen identity254. Foreign immunization of TCR transgenic mice also results in oligoclonal expansion of TFH but not TFR cells, whose repertoire instead more closely resembles thymic-derived TREG cells254. A low frequency of antigen-specific FoxP3- T cell-derived TFR cells emerged following immunization with either self or foreign antigen in IFA255, suggesting that adjuvant identity may influence TFR development and clonality. TFR cells expressed greater Ki-67 than TFH cells in B6.Sle1yaa mice106, possibly reflecting selective expansion in response to encounter with autoantigen-driven by differences in TFH and TFR specificities. These observations lead to the model that antigen-specific TFH cells focus B cell affinity maturation towards foreign antigens, while autoreactive TFR cells limit survival of B cells that gain autoreactivity.
TCR transgenic mice or reliance on tetramers likely fail to capture the true diversity of wild type TFH and TFR repertoires. Although less diverse than non-follicular T cell repertoires, TFH and TFR repertoires from non TCR transgenic mice were also polyclonal but non-overlapping256. Surprisingly, immunization of non TCR transgenic mice expands TFH and TFR pools but does not significantly alter their clonality256,257, although greater clonotype overlap was observed amongst TFH cells following foreign immunization and amongst TFR cells following autoantigen immunization256. Instead, immunization resulted in non-specific TFH and TFR bystander activation256 possibly due to TCR cross-reactivity258–261. Bystander activation may also be TCR independent and be driven by TLR signaling and cytokines rather than antigen262–265. TFH and TFR repertoires were also polyclonal and non-overlapping in 564Igi bone marrow chimera mice100, suggesting that non-specific TFH and TFR bystander activation occurs in autoimmune disease as well. Notably, the polyclonal nature of follicular T cell responses may simply represent a generalizable T cell phenomenon that is only now being appreciated due to the unbiased nature of repertoire wide sequencing in a field that has previously relied on TCR transgenics and tetramer enrichment.
While repertoire wide analyses of TFH and TFR TCRs have provided insight into clonality, antigen specificity has remained elusive. Autoreactive CD4+CD25- T cells regularly escape central tolerance and are found in the periphery of healthy mice and humans261,266,267 but are curtailed by peripheral tolerance mechanisms including TREG cells, anergy, and suppressive CD8 T cells268–270. Both conventional CD4 T (TCON) and TREG cells exhibit increased oligoclonality in B6.Sle1yaa mice compared to foreign antigen immunized mice106. Autoreactive CD4 T cells correlate with autoantibody mediated disease severity271–276. In transgenic mouse models these autoreactive T cells can receive autoantigenic stimulation from autoreactive B cells, leading to TLR independent loss of tolerance and autoantibody production 46,47. The representation of these autoreactive CD4 T cells in the homeostatic repertoire, their spatiotemporal location, and mechanism of driving loss of tolerance remain uknown277–279. Given the inherent differences between follicular and non-follicular T cell repertoires256, conclusions regarding CD4 T cell specificity in autoimmune disease are difficult to extrapolate to TFH and TFR cells.
Although autoreactive T cells that escape central tolerance are incapable of SHM and clonal redemption like autoreactive B cells39,40,43–45, they may still be subject to activation due to the highly cross-reactive nature of the TCR258–261. Just as TCR affinity influences thymic education280, low TCR signal strength can promote TFH clonality281 and polarization over TH1 commitment241,282, possibly permitting lower affinity yet autoreactive TCRs283–285 in the TFH repertoire. Computational prediction of antigen specificity revealed that follicular T cell repertoires from both foreign antigen immunized and 564Igi bone marrow chimeras had highly similar predicted specificities100. Similarly, TCR repertoires from foreign antigen immunized and B6.Sle1yaa mice shared the majority of predicted specificities106. This overlap likely represents bystander activation of non-antigen-specific follicular T cells with polyreactive TCRs256,261. A minority of predicted specificities were enriched in 564Igi bone marrow chimeras100, possibly representing an autoantigen-specific response slightly detectable in a sea of non-specific proliferation, akin to the tetramer-specific responses observed after foreign antigen immunization254,255. Indeed, TCR database annotation could predict antigen specificities for follicular T cell clonotypes commonly expanded in both foreign antigen immunized mice and 564Igi bone marrow chimeras but not clonotypes enriched in 564Igi bone marrow chimeras100, potentially reflecting the lack of annotated autoantigens in TCR databases. Further studies are needed to determine whether the follicular T cell clonotypes expanded in both foreign and autoimmune disease are specific for foreign antigens, autoantigens, or both.
Given the consistent bystander activation of TFH and TFR cells and convergence of follicular T cell predicted specificities in foreign antigen immunized and autoimmune mice, we hypothesize that autoreactive B cells co-opt cross-reactive TCRs present within the follicular T cell repertoire to license T cell help in developing autoreactive GCs. A follicular T cell repertoire that is cross-reactive with self might also explain how epitope spreading and molecular mimicry propagate. Given the importance of MHC class II expression to maintain peripheral B cell tolerance46,47,80,81, we propose that autoreactive B cells overcome clonal anergy by internalizing and presenting self-antigens to cross-reactive follicular T cells which then provide help to B cells with specificities against unrelated antigens (Figure 3). Marginal zone (MZ) B cells have polyreactive BCRs and relatively decreased activation thresholds286–288, and therefore might represent a B cell population capable of polarizing cross-reactive T cells against self. Notably, this model assumes both the presence of TCRs cross-reactive with autoantigens in the follicular T cell repertoire and the ability of autoreactive B cells to overcome mechanisms of peripheral tolerance.
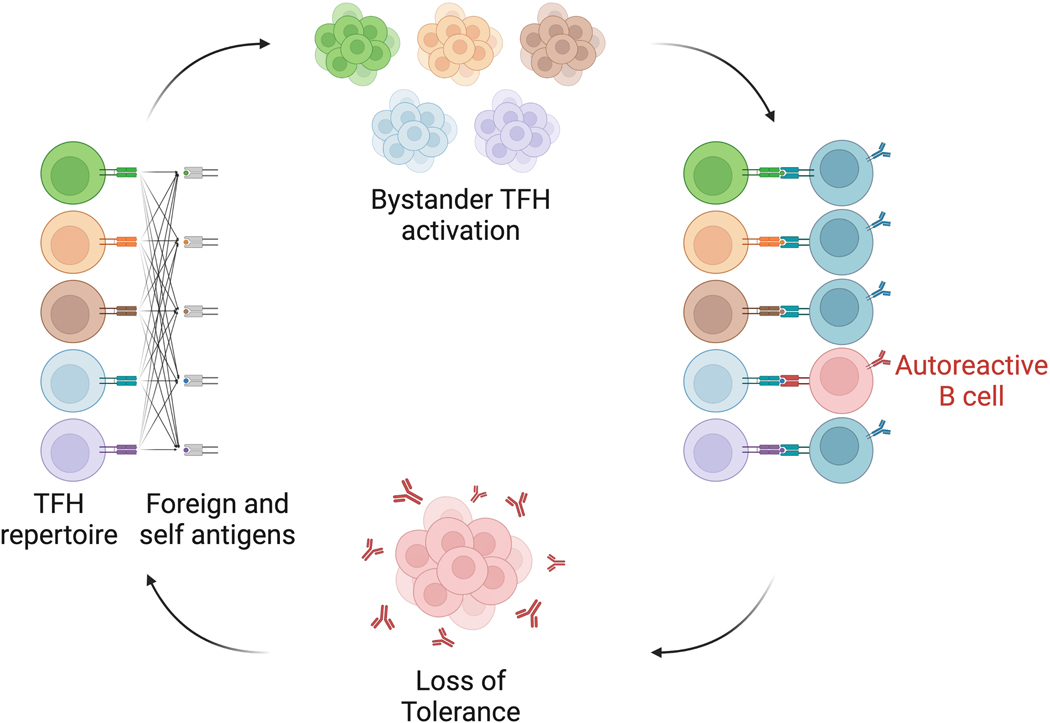
Figure 3. Bystander activation and cross-reactivity of the TFH repertoire.
Repertoire wide analyses have revealed that both immunization and autoimmune disease result in polyclonal responses of TFH and TFR cells. Computational prediction of antigen specificities also suggests overlap between autoimmune and foreign antigen elicited follicular T cell repertoire specificities. We propose that the cross reactivity of the TCR is responsible for non-specific bystander activation, which allows for provision of T cell help to autoreactive B cells, particularly in situations of chronic GC or extrafollicular B cell activation. GC, germinal center; TFH, follicular helper T cell; TFR, follicular regulatory T cell.
Extrafollicular autoantibody development
In addition to TFH mediated help in autoreactive GCs, T cell help mediated by other cell types and in other immunologic niches have been identified in autoantibody disease (Figure 4). T cell help might be provided to autoreactive B cells not only by TFH and TFR cells, but also by peripheral helper T (TPH) cells, circulating TFH (cTFH) cells, and CD8 T cells. Autoreactive B cells might also develop in EF and tertiary lymphoid structures (TLS) or ectopic GCs. Indeed, mounting evidence suggests that extrafollicular origins might serve as the dominant source of autoantibodies in disease.
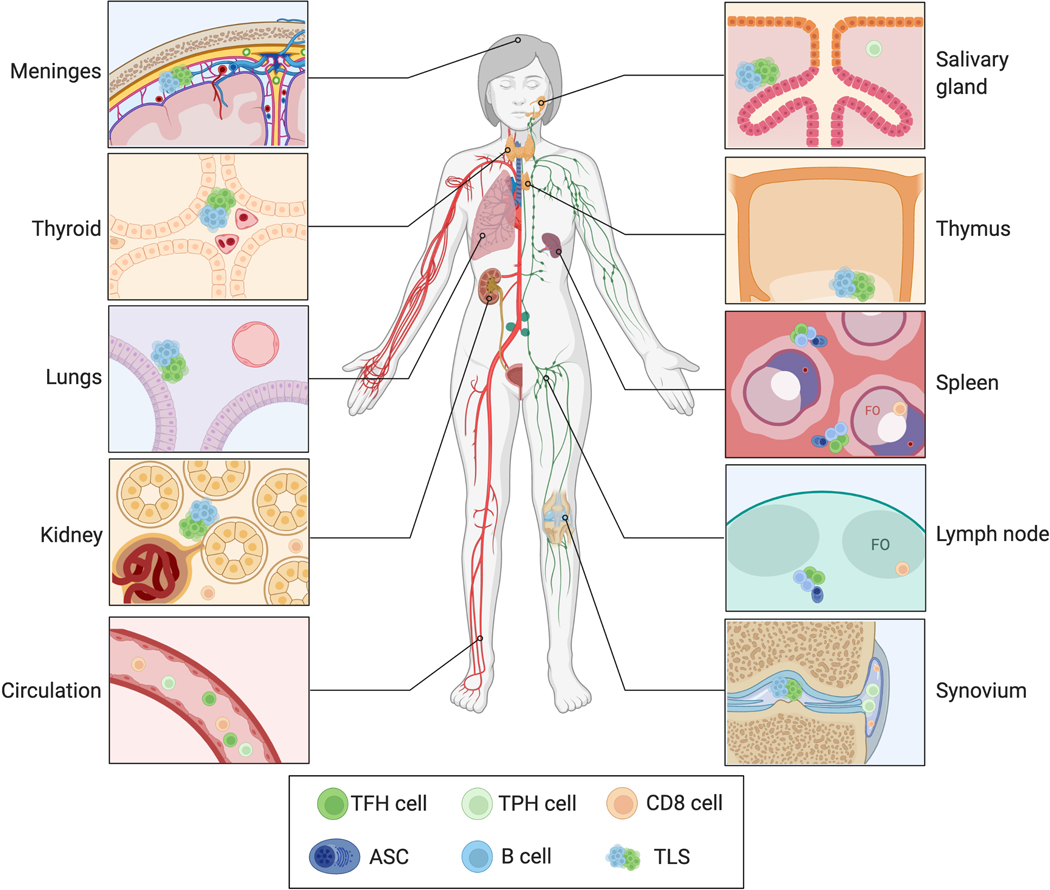
Figure 4. Extrafollicular sites of autoantibody production.
T cell help to autoreactive B cells may be provided by cells other than follicular T cells and at locations other than GCs. TPH, cTFH, TRH, TH17, and CD8 T cells exhibit dysregulation in autoantibody disease and might also contribute to loss of GC tolerance. Autoantibodies might also originate from SLO EF and TLS, which might serve as sites of loss of tolerance or epitope spreading, respectively. GC, germinal center; TPH, peripheral helper T cell; cTFH, circulating follicular helper T cell; TRH, resident helper T cell; EF, extrafollicular foci; TLS, tertiary lymphoid structure; FO, follicle; ASC, antibody secreting cell; SLO, secondary lymphoid organ.
High resolution analyses of patient biopsies have led to the characterization of alternative T cell populations capable of providing T cell help107,289. Circulating, pre-follicular, and extrafollicular TFH cells all express CXCR5 and PD-1290. Indeed, the canonical markers of GC TFH cells are likely imprecise290, as CXCR5+PD-1+ cells encompass both GC and non-GC TFH cells, two populations with distinct antigen requirements, gene expression, and repertoires291. TFH cells can inefficiently enter circulation292, with varying levels of retained Bcl6, CXCR5, and PD-1 expression293. SLE patients have increased cTFH that correlate with disease severity, plasmablasts (PBs), and autoantibody levels52,294–297. These cTFH cells might simply be a marker of active GC reactions292 or might represent a memory population capable of secondary lymphoid organ homing and GC re-entry298,299.
TPH cells were identified in the synovium of seropositive RA patients and are distinct from cTFH cells due to their PD-1hiCXCR5-Bcl6- profile and ability to migrate to peripheral sites of inflammation107,300–303. TPH cells produce IL-21 and CXCL13304, express TFH signature genes289, exhibit hallmarks of exhaustion289, can stimulate PB formation in vitro289,304,305, and correlate with age-associated CD11c+CD21- B cell (ABC) levels304,306. Antigen-specific TPH cells have been identified in celiac disease and autoimmune hepatitis307,308, suggesting that TCR specificity influences TPH fate and possibly function. Other CD4 T cell populations such as resident helper T (TRH) cells309,310, CXCR3+PD1hi CD4 T cells301, or TH17 cells147,311 might also provide tissue-specific help to autoreactive B cells in autoimmune disease. Non-TFH sources of T cell help must be considered when designing therapy for autoantibody disease, as Bcl6-deficient mice are still capable of neutralizing antibody responses312,313.
CD8 T cells exhibit dysfunctional phenotypes in autoantibody disease, including impaired cytotoxicity of circulating CD8 T cells314–318, impaired suppressive ability of regulatory CD8 T cells269,319–322, and effector memory phenotypes of tissue resident CD8 T cells323. Regulatory CD8 T cells represent a CD8 T cell subset that exert Qa-1 restricted suppression of TFH and GC B cells, preventing autoantibody development and glomerulonephritis268. KIR+ CD8 T cells can eliminate autoreactive CD4 T cells and are increased in the circulation of celiac disease, MS, SLE, and COVID-19 patients324. CXCR5+ follicular CD8 T cells represent a distinct subset capable of GC entry where they facilitate autoreactive B cell CSR and autoantibody development325–327. CD40L+ CD8 T cells are necessary for ectopic GC formation in RA328,329. These results suggest that CD8 T cells can provide B cell help, potentially in collaboration with TFH cells. Single cell sequencing of SLE kidneys revealed increased cytotoxicity of infiltrating CD8 T cells107,317 whereas circulating CD8 T cells has decreased cytolytic capacity in SLE316. B6.Sle1yaa mice had decreased frequency of splenic CD8 T cells, which also exhibited metabolic rewiring and clonal expansion of an effector subset106. Given the differences in circulating and tissue resident CD8 T cells, we hypothesize that tissue residence drives altered CD8 T cell metabolic profiles that facilitate cytotoxicity and contribute to autoantibody pathogenesis. Alternatively, these cytotoxic CD8 T cell phenotypes might be driven by TFH cell-derived IL-21, which can license CD8 T cell responses in both cancer330 and chronic viral infection331.
SHM, CSR, and memory B cell formation may occur outside GCs in EF in both T cell dependent and T cell independent manners4,72,332–334. Immunization results in early CD4 T and B cell proliferation in EF, ultimately leading to emergence of short-lived plasma cells in an ICOS and CD40L dependent manner277,335–339. Extrafollicular Bcl6+CXCR5loICOSlo CD4 T cells secrete IL-21 and IL-10 and can more efficiently convert naïve B cells into antibody secreting cells (ASC) than GC TFH cells340. Autoantibody levels decrease quickly following B cell depletion with anti-CD20341–345, suggesting production by extrafollicular short lived plasma cells. SLE flares correlate with circulating PC frequency346 and ASC development from activated naïve B cells followed by production of lowly mutated autoantibody347, reminiscent of a non-GC origin. BAFF transgenic mice produce class switched autoantibody outside GCs in a T cell independent manner348,349 and B6.56R mice loss B cell tolerance in a T cell and TLR9 independent manner350. TLR7 dependent extrafollicular ABCs expressing T-bet and CD11c, particularly CXCR5-CD21- DN2 cells351, are pathogenic in lupus347,352. T-bet+CD11c+ B cells arise outside the GC in the marginal zone, are TFH dependent, and can rapidly become ASC353. Indeed, RNA velocity analysis of B6.Sle1yaa splenic B cells suggested that autoreactive plasma cells (PCs) originate at least partially from MZ B cells106, consistent with T cell independent activation of extrafollicular autoreactive MZ B cells observed in autoantibody disease286–288,354–356. These observations provide multiple distinct potential ontogenies of autoantibody secreting cells.
CD4 T cells with a TFH signature are also present in EF in lupus prone mice. B6.Sle1yaa and NZW/BXSB mice eventually lose splenic GC organization accompanied by extrafollicular localization of TFH cells106,357. 564Igi bone marrow chimeras have increased extrafollicular cells that also expressed increased PD-1 and Selplg expression on follicular T cells correlated with predicted autoreactivity100, suggesting that TCR autoreactivity promotes EF responses. MRL/lpr mice develop autoantibodies from T cell dependent extrafollicular SHM and ASC production72,358, likely due to increased extrafollicular PSGL-1lo T cells that express CXCR4 and facilitate IgG production via IL-21 and CD40L277,335. Although SHM and ASC production is T cell dependent in MRL/lpr mice, autoantibody production is T cell independent and instead requires TLR9 or TLR7 mediated MYD88 signaling358,359. In contrast, autoantibody production in Dnase1l3−/− mice by extrafollicular short lived ASCs is dependent on CD40L mediated T cell help, although is also promoted by TLR9 and TLR7 signaling360. Both GC TFH and extrafollicular T cells in BXSB/yaa mice express increased IL-21143, suggesting simultaneous EF and GC responses. Autoantibody responses might originate in EF due to lack of tolerogenic pressures that normally facilitate negative selection or clonal redemption in the GC. We hypothesize that ultimately both EF and GC serve as sites of loss of tolerance with potential differences in threshold, kinetics, and T cell dependence in autoantibody disease.
Beyond EF in secondary lymphoid organs, TLS or ectopic GCs may also promote autoantibody development. TLS develop following chronic inflammation locally at sites of infection, autoimmune processes, or solid tumors361. Pathogenic TLS have been identified in the lungs of Wegner granulomatosis362,363 patients, thyroid in Hashimoto thyroiditis364,365, meninges in MS366–370, thymus in myasthenia gravis371, synovium in RA372,373, salivary gland in Sjogren syndrome374,375, and kidney in SLE376,377. Peripheral tolerance mechanisms are dysregulated in autoimmune TLS, as autoreactive B cells are capable of TLS entry and ensuing autoantibody production372,378. Gut associated lymphoid tissue is also capable of shaping the immature transitional B cell repertoire away from autoreactivity, a process that fails in SLE379. Autoimmune TLS harbor latent Epstein-Barr virus (EBV)380,381 and EBV transformed B cells can produce autoantibodies382,383. Abnormal T cell help might also influence TLS autoreactivity, as CD8 and TH17 cells are necessary for TLS formation in RA synovium329 and experimental autoimmune encephalomyelitis CNS384–386, respectively. Although TFH like cells have also been identified in autoimmune TLS385,387, their ontogeny and function remain unclear. We propose that TLS are established following secondary lymphoid organ loss of tolerance due to local antigen availability and their persistence allows for continual evolution of the autoantibody repertoire.
Other forms of germinal center dysfunction
In addition to contributing to autoantibody development, dysfunctional T cell help likely contributes to other abnormal GC responses. Insufficient, overactive, or dysfunctional GCs have been described in autoimmune disease, infection, and cancer. Molecular mechanisms of T cell help dysfunction in both autoreactive GCs and other diseases might reciprocally provide biological insight and therapeutic targets for each other. Recent discoveries have revolutionized our understanding of GC dysfunction in molecular mimicry, cancer, and COVID-19 (Figure 5).
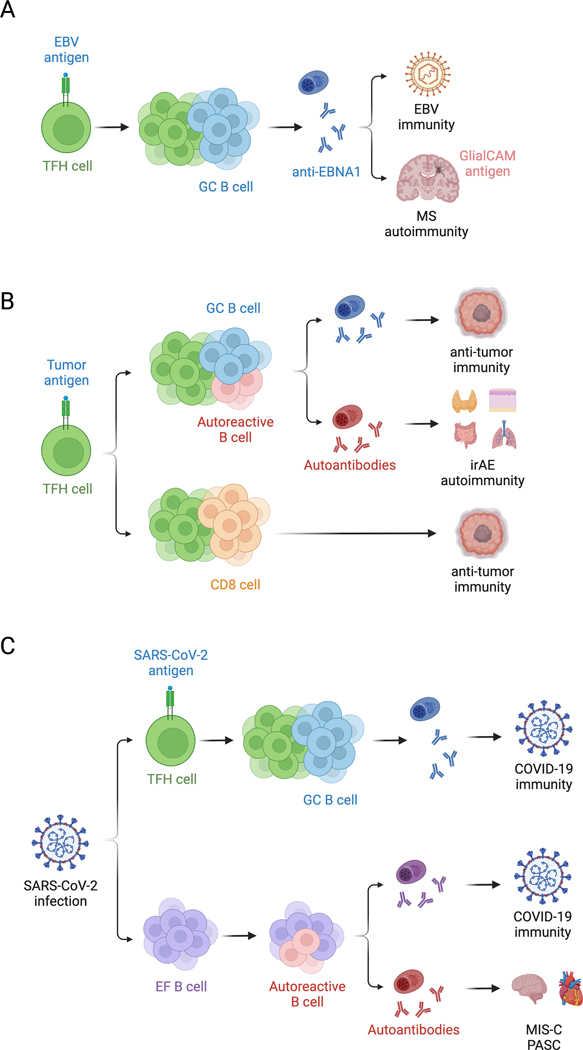
Figure 5. Dysregulated GCs in autoimmune disease, cancer, and COVID-19.
Mechanisms of dysfunctional T cell help in autoantibody disease are applicable to other forms of GC dysfunction. (A) Molecular mimicry is an etiology of MS and might be driven by the cross reactivity of the TFH repertoire. (B) In contrast, epitope spreading might enhance anti-tumor immune responses, although might also exacerbate irAE following immune checkpoint blockade in cancer. (C) T cell independent neutralizing antibodies against SARS-CoV-2 are made in COVID-19, although EF and pathogenic autoantibodies are also observed in severe COVID-19. GC, germinal center; TFH, follicular helper T cell; EBV, Epstein-Barr virus; MS, multiple sclerosis; irAE, immune related adverse event; EF, extrafollicular foci; MIS-C, multisystem inflammatory syndrome in children; PASC, post-acute sequelae of COVID-19.
Molecular mimicry is the process by which immune responses against foreign antigens lead to autoimmune responses against homologous antigens likely through epitope spreading or cross-reactivity388,389. Documented triggers include Streptococcus pyogenes infection leading to rheumatic heart disease390, Campylobacter jejuni infection leading to Guillain-Barre syndrome391, and EBV infection leading to MS392. BCR repertoire analysis from MS CSF revealed that SHM of an EBNA1 antibody led to cross-reactivity with the CNS restricted protein GlialCAM26. CSF oligoclonal PBs increased expression of HLA-DR26 and latent EBV persists in meningeal TLS380, suggesting that EBV reactive B cells may break T cell tolerance in meningeal TLS, allowing them to receive T cell help and mutate towards autoreactivity in MS. Molecular mimicry was exacerbated by phosphorylation of GlialCAM26 and citrullinated peptides or phosphorylated Ro/La are targets in RA393 and SLE394, respectively, suggesting that tissue-specific post translational modification might explain escape from central tolerance mechanisms. Even after loss of tolerance, epitope spreading to an evolving set of autoantigens may occur with both diagnostic and prognostic utility in autoimmune disease20–25,395,396. We hypothesize that molecular mimicry and epitope spreading is facilitated by the cross-reactivity of the T cell repertoire285, which allows for non-specific bystander TFH activation and provision of T cell help leading to B cell SHM and autoreactivity, particularly in situations of chronic GCs.
Although cancer immunotherapies have historically focused on anti-tumor CD8 T cells, there is growing appreciation for the importance of CD4 T and B cells in anti-tumor immunity. Aberrant epitope spreading might also contribute to both beneficial anti-tumor immune responses397–405 and adverse autoimmune events406–410 following checkpoint blockade cancer immunotherapy. Paradoxically, epitope spreading and worse immune related adverse events (irAE) correlate with better anti-tumor immunity411–415 and anti-tumor antibodies can evolve from pre-existing autoantibodies through SHM416, suggesting that overcoming GC tolerance mechanisms might benefit cancer immunotherapy. TPH, TFH, and B cells have been identified in tumors and tumor associated TLS, and their presence correlates with improved survival303,417–433. Mice genetically engineered to express B cell neoantigens in lung cancer develop tumor-specific TFH and B cell responses with greater anti-tumor immunity330. IL-21 production by tumor-specific TFH cells is necessary for anti-tumor CD8 T cell cytotoxicity330, suggesting cross talk from B cells to TFH cells to CD8 T cells. T cell help via IL-21 promotes formation of cytolytic CX3CR1+ CD8 T cells that are capable of controlling both chronic LCMV and tumor growth434. Similarly, TFH cell-derived IL-21 promotes antigen-specific CD8 T cell responses in chronic LCMV infection331. Given the increased CD8 T cell cytotoxicity observed in autoantibody disease106,107,317, these studies highlight convergent mechanisms of T cell help in cancer, infection, and autoimmunity. While epitope spreading is pathogenic in autoantibody disease, it might provide therapeutic benefit in cancer.
GC dysfunction has also been described in COVID-19, as patients with severe disease lack TFH cells and GC structures435. Despite lack of GCs, severe COVID-19 patients still form extrafollicular T-B cell conjugates and class switched antibodies435,436. SARS-CoV-2 infection and vaccination results in generation of both TFH-dependent and TFH-independent antibodies312,437,438. Impaired TFH help in immunocompromised kidney transplant recipients likely accounts for decreased responsiveness to mRNA vaccination439. However, Cd4CreBclf6fl/fl mice demonstrated that although TFH-independent antibodies were less somatically mutated, they were still neutralizing and broadly reactive against SARS-CoV-2 and heterologous viruses312. Although these antibodies appear protective, ongoing extrafollicular responses correlate with worse outcomes of COVID-19436. Autoantibodies targeting type I interferons correlate with COVID-19 severity440, likely due to the protection conferred by type I interferons against SARS-CoV-2441. Pathologic autoantibodies against other immunomodulatory proteins such as cytokines, chemokines, and complement also emerge in COVID-19 and multisystem inflammatory syndrome in children (MIS-C), similarly affecting clinical outcomes442–446. Given the association between autoantibodies and atherogenesis and arrhythmias447–450, aberrant autoantibody production might explain the cardiovascular complications of COVID-19. Autoantibody levels in COVID-19 patients also correlate with anti-SARS-CoV-2 antibody levels and is an independent risk factor for development of post-acute sequelae of COVID-19 (PASC) or long COVID-19451. Informed by the role of extrafollicular responses in autoantibody disease, we hypothesize that although capable of producing neutralizing antibodies against SARS-CoV-2, ongoing extrafollicular TFH-independent B cell responses also lead to autoantibody development which directly exacerbates COVID-19 severity.
Conclusion
Technological advances have allowed for the study of the autoreactive GC at single cell resolution, transforming our understanding of the role of T cell help in loss of tolerance. These developments have led to new models of TFH and B cell crosstalk in not only autoantibody disease, but also other autoimmune diseases, cancer, and infection. Gene expression profiling has identified metabolism and adhesion molecule expression as therapeutic targets specific to autoreactive TFH cells. Repertoire analyses have revealed a surprising degree of bystander activation of follicular T cells, leading to the hypothesis that cross-reactive T cells might license B cell loss of tolerance and epitope spreading. Mass cytometry of patient biopsies identified novel T helper subsets such as TPH cells and highlighted the contribution of extrafollicular responses to autoantibody development. Lessons learned from the autoreactive GC can also provide mechanistic insight into the autoantibody development observed in MS and COVID-19. These hypotheses highlight the therapeutic potential of targeting alternative pathways such as TFH metabolism, bystander T cells, or extrafollicular B cells in autoantibody disease.
In addition to generating new hypotheses, recent discoveries have opened the door to many remaining questions of autoreactive GC biology. Although TFH cells share predicted specificities in foreign antigen immunized and autoimmune mice, the antigenic targets of these TCRs remains unknown. Indeed, reactivities against overlapping antigens between TFH cells and epitope spread B cells has not been established. Identification of target antigens might clarify whether bystander follicular T cell activation is a necessary component of autoreactive GC responses or is simply a consequence of GC development. The degree of T cell dependence of extrafollicular responses and the tolerance mechanisms that normally restrain MZ or DN2 B cell conversion to autoantibody secreting cells also remain unclear. The nature of T cell help provided by other populations such as TPH and CD8 T cells and in other locations such as TLS, as well as their ability to propagate autoantibody responses, is poorly characterized. Pressing questions such as why SARS-CoV-2 infection generates extrafollicular responses and whether these EF are the source of pathogenic autoantibody must be answered. Answers to these questions have the potential to not only improve our understanding of GC biology, but also lead to the development of more efficacious therapies for diseases ranging from autoimmunity to cancer.
Acknowledgments
We thank members of the laboratory for helpful discussions. E.A.G. was supported by NIH T32GM007753, T32AI007529, and F30AI160909. M.C.C. is supported by NIH R01AR074105 and R01AI130307. Schematics were created with BioRender.com.
Abbreviations:
- EF
extrafollicular foci
- GC
germinal center
- TFH
follicular helper T cell
- SHM
somatic hypermutation
- CSR
class switch recombination
- TFR
follicular regulatory T cell
- SLE
systemic lupus erythematosus
- MG
myasthenia gravis
- RA
rheumatoid arthritis
- SS
Sjögren’s syndrome
- MS
multiple sclerosis
- LZ
light zone
- LLPC
long-lived plasma cell
- DZ
dark zone
- MZ
marginal zone
- TPH
peripheral helper T cell
- cTFH
circulating follicular helper T cell
- TLS
tertiary lymphoid structures
- PB
plasmablast
- ABC
age-associated B cell
- TRH
resident helper T cell
- ASC
antibody secreting cell
- PC
plasma cell
- EBV
Epstein-Barr virus
- irAE
immune-related adverse event
- MIS-C
multisystem inflammatory syndrome in children
- PASC
post-acute sequelae of COVID-19
Footnotes
Conflict of Interest Statement
The authors declare no competing interests.
References
- 1.Allen CDC, Okada T. & Cyster JG Germinal-Center Organization and Cellular Dynamics. Immunity 27, 190–202 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crotty S. Follicular Helper CD4 T Cells (TFH). Annual Review of Immunology 29, 621–663 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Weinstein JS et al. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol 17, 1197–1205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elsner RA & Shlomchik MJ Germinal Center and Extrafollicular B Cell Responses in Vaccination, Immunity, and Autoimmunity. Immunity 53, 1136–1150 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinuesa CG, Sanz I. & Cook MC Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol 9, 845–857 (2009). [DOI] [PubMed] [Google Scholar]
- 6.von Mühlen CA & Tan EM Autoantibodies in the diagnosis of systemicrheumatic diseases. Seminars in Arthritis and Rheumatism 24, 323–358 (1995). [DOI] [PubMed] [Google Scholar]
- 7.Scofield RH Autoantibodies as predictors of disease. The Lancet 363, 1544–1546 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Shlomchik M. et al. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. Journal of Experimental Medicine 171, 265–292 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marion TN, Bothwell AL, Briles DE & Janeway CA IgG anti-DNA autoantibodies within an individual autoimmune mouse are the products of clonal selection. The Journal of Immunology 142, 4269–4274 (1989). [PubMed] [Google Scholar]
- 10.Behar SM, Lustgarten DL, Corbet S. & Scharff MD Characterization of somatically mutated S107 VH11-encoded anti-DNA autoantibodies derived from autoimmune (NZB x NZW)F1 mice. Journal of Experimental Medicine 173, 731–741 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamond B. & Scharff MD Somatic mutation of the T15 heavy chain gives rise to an antibody with autoantibody specificity. Proceedings of the National Academy of Sciences 81, 5841–5844 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allman D, Wilmore JR & Gaudette BT The Continuing Story of T-cell Independent Antibodies. Immunol Rev 288, 128–135 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brink R. & Phan TG Self-Reactive B Cells in the Germinal Center Reaction. Annual Review of Immunology 36, 339–357 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Saravanan P. & Dayan CM THYROID AUTOANTIBODIES. Endocrinology and Metabolism Clinics of North America 30, 315–337 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Sherer Y, Gorstein A, Fritzler MJ & Shoenfeld Y. Autoantibody explosion in systemic lupus erythematosus: More than 100 different antibodies found in SLE patients. Seminars in Arthritis and Rheumatism 34, 501–537 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Elkon K. & Casali P. Nature and functions of autoantibodies. Nat Clin Pract Rheumatol 4, 491–498 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincent A. Unravelling the pathogenesis of myasthenia gravis. Nat. Rev. Immunol 2, 797–804 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Szodoray P. et al. Anti-citrullinated protein/peptide autoantibodies in association with genetic and environmental factors as indicators of disease outcome in rheumatoid arthritis. Autoimmunity Reviews 9, 140–143 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Vanderlugt CL & Miller SD Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nature Reviews Immunology 2, 85–95 (2002). [DOI] [PubMed] [Google Scholar]
- 20.McRae BL, Vanderlugt CL, Dal Canto MC & Miller SD Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med 182, 75–85 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasad S, Kohm AP, McMahon JS, Luo X. & Miller SD Pathogenesis of NOD diabetes is initiated by reactivity to the insulin B chain 9–23 epitope and involves functional epitope spreading. Journal of Autoimmunity 39, 347–353 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Didona D. & Di Zenzo G. Humoral Epitope Spreading in Autoimmune Bullous Diseases. Front. Immunol 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escolà-Vergé L. et al. Mixed Connective Tissue Disease and Epitope Spreading: An Historical Cohort Study. J Clin Rheumatol 23, 155–159 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L. et al. A nephritogenic peptide induces intermolecular epitope spreading on collagen IV in experimental autoimmune glomerulonephritis. J. Am. Soc. Nephrol 17, 3076–3081 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Seitz-Polski B. et al. Epitope Spreading of Autoantibody Response to PLA2R Associates with Poor Prognosis in Membranous Nephropathy. J. Am. Soc. Nephrol 27, 1517–1533 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanz TV et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 603, 321–327 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Es JH et al. Somatic mutations in the variable regions of a human IgG anti-double-stranded DNA autoantibody suggest a role for antigen in the induction of systemic lupus erythematosus. Journal of Experimental Medicine 173, 461–470 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roark JH, Bussel JB, Cines DB & Siegel DL Genetic analysis of autoantibodies in idiopathic thrombocytopenic purpura reveals evidence of clonal expansion and somatic mutation. Blood 100, 1388–1398 (2002). [PubMed] [Google Scholar]
- 29.Zenzo GD et al. Pemphigus autoantibodies generated through somatic mutations target the desmoglein-3 cis-interface. J Clin Invest 122, 3781–3790 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL & Weigert MG The role of clonal selection and somatic mutation in autoimmunity. Nature 328, 805–811 (1987). [DOI] [PubMed] [Google Scholar]
- 31.Olee T. et al. Genetic analysis of self-associating immunoglobulin G rheumatoid factors from two rheumatoid synovia implicates an antigen-driven response. Journal of Experimental Medicine 175, 831–842 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McIntosh RS, Asghar MS, Watson PF, Kemp EH & Weetman AP Cloning and analysis of IgG kappa and IgG lambda anti-thyroglobulin autoantibodies from a patient with Hashimoto’s thyroiditis: evidence for in vivo antigen-driven repertoire selection. The Journal of Immunology 157, 927–935 (1996). [PubMed] [Google Scholar]
- 33.Goodnow CC, Adelstein S. & Basten A. The Need for Central and Peripheral Tolerance in the B Cell Repertoire. Science 248, 1373–1379 (1990). [DOI] [PubMed] [Google Scholar]
- 34.Mueller DL Mechanisms maintaining peripheral tolerance. Nat Immunol 11, 21–27 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Brooks JF, Murphy PR, Barber JEM, Wells JW & Steptoe RJ Peripheral Tolerance Checkpoints Imposed by Ubiquitous Antigen Expression Limit Antigen-Specific B Cell Responses under Strongly Immunogenic Conditions. The Journal of Immunology 205, 1239–1247 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Mayer CT et al. An apoptosis-dependent checkpoint for autoimmunity in memory B and plasma cells. Proceedings of the National Academy of Sciences 117, 24957–24963 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pike BL, Boyd AW & Nossal GJ Clonal anergy: the universally anergic B lymphocyte. Proceedings of the National Academy of Sciences 79, 2013–2017 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cornall RJ, Goodnow CC & Cyster JG The regulation of self-reactive B cells. Current Opinion in Immunology 7, 804–811 (1995). [DOI] [PubMed] [Google Scholar]
- 39.Burnett DL, Reed JH, Christ D. & Goodnow CC Clonal redemption and clonal anergy as mechanisms to balance B cell tolerance and immunity. Immunological Reviews 292, 61–75 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Reed JH, Jackson J, Christ D. & Goodnow CC Clonal redemption of autoantibodies by somatic hypermutation away from self-reactivity during human immunization. Journal of Experimental Medicine 213, 1255–1265 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burnett DL et al. Germinal center antibody mutation trajectories are determined by rapid self/foreign discrimination. Science 360, 223–226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seo S. et al. The Impact of T Helper and T Regulatory Cells on the Regulation of Anti-Double-Stranded DNA B Cells. Immunity 16, 535–546 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Vinuesa CG et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 435, 452–458 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Mietzner B. et al. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. PNAS 105, 9727–9732 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabouri Z. et al. Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proceedings of the National Academy of Sciences 111, E2567–E2575 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giles JR, Neves AT, Marshak-Rothstein A. & Shlomchik MJ Autoreactive helper T cells alleviate the need for intrinsic TLR signaling in autoreactive B cell activation. JCI Insight 2,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan J, Harvey BP, Gee RJ, Shlomchik MJ & Mamula MJ B cells drive early T cell autoimmunity in vivo prior to dendritic cell-mediated autoantigen presentation. J. Immunol 177, 4481–4487 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Wellmann U. et al. The evolution of human anti-double-stranded DNA autoantibodies. Proceedings of the National Academy of Sciences 102, 9258–9263 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schroeder K, Herrmann M. & Winkler TH The role of somatic hypermutation in the generation of pathogenic antibodies in SLE. Autoimmunity 46, 121–127 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Guo W. et al. Somatic hypermutation as a generator of antinuclear antibodies in a murine model of systemic autoimmunity. Journal of Experimental Medicine 207, 2225–2237 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan TD et al. Elimination of germinal-center-derived self-reactive B cells is governed by the location and concentration of self-antigen. Immunity 37, 893–904 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Simpson N. et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis & Rheumatism 62, 234–244 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Yang J-H et al. Expression and function of inducible costimulator on peripheral blood T cells in patients with systemic lupus erythematosus. Rheumatology (Oxford) 44, 1245–1254 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Tzartos JS et al. IL-21 and IL-21 Receptor Expression in Lymphocytes and Neurons in Multiple Sclerosis Brain. The American Journal of Pathology 178, 794–802 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saito R. et al. Altered expression of chemokine receptor CXCR5 on T cells of myasthenia gravis patients. Journal of Neuroimmunology 170, 172–178 (2005). [DOI] [PubMed] [Google Scholar]
- 56.Li X. et al. Role of the frequency of blood CD4+ CXCR5+ CCR6+ T cells in autoimmunity in patients with Sjögren’s syndrome. Biochemical and Biophysical Research Communications 422, 238–244 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Christensen JR et al. Systemic Inflammation in Progressive Multiple Sclerosis Involves Follicular T-Helper, Th17- and Activated B-Cells and Correlates with Progression. PLOS ONE 8, e57820 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma J. et al. Increased Frequency of Circulating Follicular Helper T Cells in Patients with Rheumatoid Arthritis. Clinical and Developmental Immunology vol. 2012 e827480 https://www.hindawi.com/journals/jir/2012/827480/ (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu R. et al. A regulatory effect of IL-21 on T follicular helper-like cell and B cell in rheumatoid arthritis. Arthritis Research & Therapy 14, R255 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szabo K. et al. Follicular helper T cells may play an important role in the severity of primary Sjögren’s syndrome. Clinical Immunology 147, 95–104 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Kawamoto M. et al. Expression and function of inducible co-stimulator in patients with systemic lupus erythematosus: possible involvement in excessive interferon-γ and anti-double-stranded DNA antibody production. Arthritis Research & Therapy 8, R62 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu C. et al. Increased Frequency of Follicular Helper T Cells in Patients with Autoimmune Thyroid Disease. J Clin Endocrinol Metab 97, 943–950 (2012). [DOI] [PubMed] [Google Scholar]
- 63.Shan Y. et al. Higher frequency of peripheral blood follicular regulatory T cells in patients with new onset ankylosing spondylitis. Clin. Exp. Pharmacol. Physiol 42, 154–161 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Kim YU, Lim H, Jung HE, Wetsel RA & Chung Y. Regulation of Autoimmune Germinal Center Reactions in Lupus-Prone BXD2 Mice by Follicular Helper T Cells. PLoS One 10, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wen Y. et al. Imbalance of circulating CD4+CXCR5+FOXP3+ Tfr-like cells and CD4+CXCR5+FOXP3− Tfh-like cells in myasthenia gravis. Neuroscience Letters 630, 176–182 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Dhaeze T. et al. Circulating Follicular Regulatory T Cells Are Defective in Multiple Sclerosis. The Journal of Immunology 195, 832–840 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Fonseca VR et al. Human blood Tfr cells are indicators of ongoing humoral activity not fully licensed with suppressive function. Science Immunology 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brocker T. et al. CD4 T cell traffic control: in vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B follicles. European Journal of Immunology 29, 1610–1616 (1999). [DOI] [PubMed] [Google Scholar]
- 69.Murata K. et al. Constitutive OX40/OX40 Ligand Interaction Induces Autoimmune-Like Diseases. The Journal of Immunology 169, 4628–4636 (2002). [DOI] [PubMed] [Google Scholar]
- 70.Subramanian S. et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. PNAS 103, 9970–9975 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Linterman MA et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med 206, 561–576 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.William J, Euler C, Christensen S. & Shlomchik MJ Evolution of Autoantibody Responses via Somatic Hypermutation Outside of Germinal Centers. Science 297, 2066–2070 (2002). [DOI] [PubMed] [Google Scholar]
- 73.Liu D. et al. BCL6 controls contact-dependent help delivery during follicular T-B cell interactions. Immunity 54, 2245–2255.e4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Degn SE et al. Clonal Evolution of Autoreactive Germinal Centers. Cell 170, 913–926.e19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mihara M. et al. CTLA4Ig inhibits T cell–dependent B-cell maturation in murine systemic lupus erythematosus. J Clin Invest 106, 91–101 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akkerman A. et al. CTLA4Ig Prevents Initiation but not Evolution of Anti-phospholipid Syndrome in NZW/BXSB Mice. Autoimmunity 37, 445–451 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X, Huang W, Mihara M, Sinha J. & Davidson A. Mechanism of Action of Combined Short-Term CTLA4Ig and Anti-CD40 Ligand in Murine Systemic Lupus Erythematosus. The Journal of Immunology 168, 2046–2053 (2002). [DOI] [PubMed] [Google Scholar]
- 78.Mohan C, Shi Y, Laman JD & Datta SK Interaction between CD40 and its ligand gp39 in the development of murine lupus nephritis. The Journal of Immunology 154, 1470–1480 (1995). [PubMed] [Google Scholar]
- 79.Daikh DI, Finck BK, Linsley PS, Hollenbaugh D. & Wofsy D. Long-term inhibition of murine lupus by brief simultaneous blockade of the B7/CD28 and CD40/gp39 costimulation pathways. The Journal of Immunology 159, 3104–3108 (1997). [PubMed] [Google Scholar]
- 80.Kinnunen T. et al. Specific peripheral B cell tolerance defects in patients with multiple sclerosis. J Clin Invest 123, 2737–2741 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giles JR, Kashgarian M, Koni PA & Shlomchik MJ B Cell–Specific MHC Class II Deletion Reveals Multiple Nonredundant Roles for B Cell Antigen Presentation in Murine Lupus. The Journal of Immunology 195, 2571–2579 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kinnunen T. et al. Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood 121, 1595–1603 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu X, Nurieva RI & Dong C. Transcriptional regulation of follicular T-helper (Tfh) cells. Immunological Reviews 252, 139–145 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang P. et al. The Transcription Factor T-Bet Is Required for Optimal Type I Follicular Helper T Cell Maintenance During Acute Viral Infection. Frontiers in Immunology 10, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hercor M. et al. Antigen-presenting cell-derived IL-6 restricts the expression of GATA3 and IL-4 by follicular helper T cells. Journal of Leukocyte Biology 101, 5–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fang D. et al. Transient T-bet expression functionally specifies a distinct T follicular helper subset. Journal of Experimental Medicine 215, 2705–2714 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grados A. et al. T Cell Polarization toward TH2/TFH2 and TH17/TFH17 in Patients with IgG4-Related Disease. Frontiers in Immunology 8, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gowthaman U. et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science 365, eaaw6433 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu D. et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31, 457–468 (2009). [DOI] [PubMed] [Google Scholar]
- 90.Nurieva RI et al. Bcl6 Mediates the Development of T Follicular Helper Cells. Science 325, 1001–1005 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hsu H-C et al. Interleukin 17–producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol 9, 166–175 (2008). [DOI] [PubMed] [Google Scholar]
- 92.Ding Y. et al. IL-17RA Is Essential for Optimal Localization of Follicular Th Cells in the Germinal Center Light Zone To Promote Autoantibody-Producing B Cells. The Journal of Immunology 191, 1614–1624 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Z, Kyttaris VC & Tsokos GC The Role of IL-23/IL-17 Axis in Lupus Nephritis. The Journal of Immunology 183, 3160–3169 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Crispín JC et al. Expanded Double Negative T Cells in Patients with Systemic Lupus Erythematosus Produce IL-17 and Infiltrate the Kidneys. The Journal of Immunology 181, 8761–8766 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wen Z, Xu L, Xu W. & Xiong S. Retinoic Acid Receptor–Related Orphan Nuclear Receptor γt Licenses the Differentiation and Function of a Unique Subset of Follicular Helper T Cells in Response to Immunogenic Self-DNA in Systemic Lupus Erythematosus. Arthritis & Rheumatology 73, 1489–1500 (2021). [DOI] [PubMed] [Google Scholar]
- 96.Le Coz C. et al. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PLoS ONE 8, e75319 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.He J. et al. Circulating Precursor CCR7loPD-1hi CXCR5+ CD4+ T Cells Indicate Tfh Cell Activity and Promote Antibody Responses upon Antigen Reexposure. Immunity 39, 770–781 (2013). [DOI] [PubMed] [Google Scholar]
- 98.Wang J. et al. High frequencies of activated B cells and T follicular helper cells are correlated with disease activity in patients with new-onset rheumatoid arthritis. Clin. Exp. Immunol 174, 212–220 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim CJ et al. The Transcription Factor Ets1 Suppresses T Follicular Helper Type 2 Cell Differentiation to Halt the Onset of Systemic Lupus Erythematosus. Immunity 49, 1034–1048.e8 (2018). [DOI] [PubMed] [Google Scholar]
- 100.Akama-Garren EH et al. Follicular T cells are clonally and transcriptionally distinct in B cell-driven mouse autoimmune disease. Nat Commun 12, 6687 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bradley SJ, Suarez-Fueyo A, Moss DR, Kyttaris VC & Tsokos GC T Cell Transcriptomes Describe Patient Subtypes in Systemic Lupus Erythematosus. PLOS ONE 10, e0141171 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sullivan JM, Höllbacher B. & Campbell DJ Cutting Edge: Dynamic Expression of Id3 Defines the Stepwise Differentiation of Tissue-Resident Regulatory T Cells. The Journal of Immunology (2018) doi: 10.4049/jimmunol.1800917. [DOI] [PMC free article] [PubMed]
- 103.Miyazaki M. et al. Id2 and Id3 maintain the regulatory T cell pool to suppress inflammatory disease. Nature Immunology 15, 767–776 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Choi YS et al. LEF-1 and TCF-1 orchestrate T FH differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nature Immunology 16, 980–990 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu L. et al. The transcription factor TCF-1 initiates the differentiation of T(FH) cells during acute viral infection. Nat Immunol 16, 991–999 (2015). [DOI] [PubMed] [Google Scholar]
- 106.Akama-Garren E. & Carroll M. Lupus susceptibility loci predispose mice to clonal lymphocytic responses and myeloid expansion. The Journal of Immunology. [DOI] [PMC free article] [PubMed]
- 107.Arazi A. et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nature Immunology 20, 902–914 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kroenke MA et al. Bcl6 and Maf Cooperate To Instruct Human Follicular Helper CD4 T Cell Differentiation. The Journal of Immunology 188, 3734–3744 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thornton AM et al. Expression of Helios, an Ikaros Transcription Factor Family Member, Differentiates Thymic-Derived from Peripherally Induced Foxp3+ T Regulatory Cells. The Journal of Immunology 184, 3433–3441 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Serre K. et al. Helios is associated with CD4 T cells differentiating to T helper 2 and follicular helper T cells in vivo independently of Foxp3 expression. PLoS One 6, e20731 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jacobsen JT et al. Expression of Foxp3 by T follicular helper cells in end-stage germinal centers. Science 373, eabe5146 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sawalha AH et al. Genetic association of interleukin-21 polymorphisms with systemic lupus erythematosus. Annals of the Rheumatic Diseases 67, 458–461 (2008). [DOI] [PubMed] [Google Scholar]
- 113.Lan Y, Luo B, Wang J-L, Jiang Y-W & Wei Y-S The association of interleukin-21 polymorphisms with interleukin-21 serum levels and risk of systemic lupus erythematosus. Gene 538, 94–98 (2014). [DOI] [PubMed] [Google Scholar]
- 114.Webb R. et al. A polymorphism within IL21R confers risk for systemic lupus erythematosus. Arthritis Rheum 60, 2402–2407 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bentham J. et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet 47, 1457–1464 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Remmers EF et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N Engl J Med 357, 977–986 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sigurdsson S. et al. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am J Hum Genet 76, 528–537 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cunninghame Graham DS et al. Polymorphism at the TNF superfamily gene TNFSF4 confers susceptibility to systemic lupus erythematosus. Nat Genet 40, 83–89 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stahl EA et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet 42, 508–514 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Graham RR et al. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat Genet 38, 550–555 (2006). [DOI] [PubMed] [Google Scholar]
- 121.Lessard CJ et al. Identification of IRF8, TMEM39A, and IKZF3-ZPBP2 as susceptibility loci for systemic lupus erythematosus in a large-scale multiracial replication study. Am J Hum Genet 90, 648–660 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gateva V. et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet 41, 1228–1233 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sanchez E. et al. Phenotypic associations of genetic susceptibility loci in systemic lupus erythematosus. Annals of the Rheumatic Diseases 70, 1752–1757 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cui YX, Fu CW, Jiang F, Ye LX & Meng W. Association of the interleukin-6 polymorphisms with systemic lupus erythematosus: a meta-analysis. Lupus 24, 1308–1317 (2015). [DOI] [PubMed] [Google Scholar]
- 125.Solus JF et al. Genetics of serum concentration of IL-6 and TNFα in systemic lupus erythematosus and rheumatoid arthritis: a candidate gene analysis. Clin Rheumatol 34, 1375–1382 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Spee-Mayer C. von et al. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Annals of the Rheumatic Diseases 75, 1407–1415 (2016). [DOI] [PubMed] [Google Scholar]
- 127.Ballesteros-Tato A. et al. Interleukin-2 Inhibits Germinal Center Formation by Limiting T Follicular Helper Cell Differentiation. Immunity 36, 847–856 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Johnston RJ, Choi YS, Diamond JA, Yang JA & Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. Journal of Experimental Medicine 209, 243–250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nurieva RI et al. STAT5 Protein Negatively Regulates T Follicular Helper (Tfh) Cell Generation and Function *. Journal of Biological Chemistry 287, 11234–11239 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Arkatkar T. et al. B cell–derived IL-6 initiates spontaneous germinal center formation during systemic autoimmunity. Journal of Experimental Medicine 214, 3207–3217 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McDonald PW et al. IL-7 signalling represses Bcl-6 and the TFH gene program. Nat Commun 7, 10285 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hatzi K. et al. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. Journal of Experimental Medicine 212, 539–553 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu X. et al. Genome-wide Analysis Identifies Bcl6-Controlled Regulatory Networks during T Follicular Helper Cell Differentiation. Cell Reports 14, 1735–1747 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xie S. et al. IL-17 activates the canonical NF-kappaB signaling pathway in autoimmune B cells of BXD2 mice to upregulate the expression of regulators of G-protein signaling 16. J Immunol 184, 2289–2296 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Estes JD et al. Follicular dendritic cell regulation of CXCR4-mediated germinal center CD4 T cell migration. J Immunol 173, 6169–6178 (2004). [DOI] [PubMed] [Google Scholar]
- 136.Mitsdoerffer M. et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A 107, 14292–14297 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu HY, Quintana FJ & Weiner HL Nasal Anti-CD3 Antibody Ameliorates Lupus by Inducing an IL-10-Secreting CD4+CD25−LAP+ Regulatory T Cell and Is Associated with Down-Regulation of IL-17+CD4+ICOS+CXCR5+ Follicular Helper T Cells. The Journal of Immunology 181, 6038–6050 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lesley R. et al. Reduced Competitiveness of Autoantigen-Engaged B Cells due to Increased Dependence on BAFF. Immunity 20, 441–453 (2004). [DOI] [PubMed] [Google Scholar]
- 139.Zhang M. et al. Interleukin-21 receptor blockade inhibits secondary humoral responses and halts the progression of preestablished disease in the (NZB × NZW)F1 systemic lupus erythematosus model. Arthritis Rheumatol 67, 2723–2731 (2015). [DOI] [PubMed] [Google Scholar]
- 140.Choi J-Y et al. Disruption of Pathogenic Cellular Networks by IL-21 Blockade Leads to Disease Amelioration in Murine Lupus. The Journal of Immunology 198, 2578–2588 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Young DA et al. Blockade of the interleukin-21/interleukin-21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis Rheum 56, 1152–1163 (2007). [DOI] [PubMed] [Google Scholar]
- 142.Herber D. et al. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol 178, 3822–3830 (2007). [DOI] [PubMed] [Google Scholar]
- 143.Bubier JA et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proceedings of the National Academy of Sciences 106, 1518–1523 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ding Y. et al. Interleukin-21 promotes germinal center reaction by skewing the follicular regulatory T cell to follicular helper T cell balance in autoimmune BXD2 mice. Arthritis Rheumatol 66, 2601–2612 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jandl C. et al. IL-21 restricts T follicular regulatory T cell proliferation through Bcl-6 mediated inhibition of responsiveness to IL-2. Nat Commun 8, 14647 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nian Y. et al. IL-21 Receptor Blockade Shifts the Follicular T Cell Balance and Reduces De Novo Donor-Specific Antibody Generation. Frontiers in Immunology 12, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wong CK et al. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: Implications for Th17-mediated inflammation in auto-immunity. Clinical Immunology 127, 385–393 (2008). [DOI] [PubMed] [Google Scholar]
- 148.Kyttaris VC, Zhang Z, Kuchroo VK, Oukka M. & Tsokos GC Cutting Edge: IL-23 Receptor Deficiency Prevents the Development of Lupus Nephritis in C57BL/6–lpr/lpr Mice. The Journal of Immunology 184, 4605–4609 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dai H, He F, Tsokos GC & Kyttaris VC IL-23 Limits the Production of IL-2 and Promotes Autoimmunity in Lupus. J Immunol 199, 903–910 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pratama A. et al. Roquin-2 Shares Functions with Its Paralog Roquin-1 in the Repression of mRNAs Controlling T Follicular Helper Cells and Systemic Inflammation. Immunity 38, 669–680 (2013). [DOI] [PubMed] [Google Scholar]
- 151.Yu Di et al. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature 450, 299–303 (2007). [DOI] [PubMed] [Google Scholar]
- 152.Okamoto T. et al. Expression and function of the co-stimulator H4/ICOS on activated T cells of patients with rheumatoid arthritis. The Journal of Rheumatology 30, 1157–1163 (2003). [PubMed] [Google Scholar]
- 153.Hutloff A. et al. Involvement of inducible costimulator in the exaggerated memory B cell and plasma cell generation in systemic lupus erythematosus. Arthritis & Rheumatism 50, 3211–3220 (2004). [DOI] [PubMed] [Google Scholar]
- 154.Shilling RA et al. Cutting Edge: Polymorphisms in the ICOS Promoter Region Are Associated with Allergic Sensitization and Th2 Cytokine Production. The Journal of Immunology 175, 2061–2065 (2005). [DOI] [PubMed] [Google Scholar]
- 155.Hao Z. et al. Fas Receptor Expression in Germinal-Center B Cells Is Essential for T and B Lymphocyte Homeostasis. Immunity 29, 615–627 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shlomchik MJ, Madaio MP, Ni D, Trounstein M. & Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med 180, 1295–1306 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA & Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356, 314–317 (1992). [DOI] [PubMed] [Google Scholar]
- 158.Takahashi T. et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the fas ligand. Cell 76, 969–976 (1994). [DOI] [PubMed] [Google Scholar]
- 159.Butt D. et al. FAS Inactivation Releases Unconventional Germinal Center B Cells that Escape Antigen Control and Drive IgE and Autoantibody Production. Immunity 42, 890–902 (2015). [DOI] [PubMed] [Google Scholar]
- 160.Alabyev B, Vuyyuru R. & Manser T. Influence of Fas on the regulation of the response of an anti-nuclear antigen B cell clonotype to foreign antigen. International Immunology 20, 1279–1287 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Razzaghi R. et al. Compromised counterselection by FAS creates an aggressive subtype of germinal center lymphoma. J Exp Med 218, e20201173 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhou Y. et al. CD74 deficiency mitigates systemic lupus erythematosus-like autoimmunity and pathological findings in mice. J Immunol 198, 2568–2577 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stumptner-Cuvelette P. & Benaroch P. Multiple roles of the invariant chain in MHC class II function. Biochim Biophys Acta 1542, 1–13 (2002). [DOI] [PubMed] [Google Scholar]
- 164.Berkova Z. et al. CD74 interferes with the expression of fas receptor on the surface of lymphoma cells. Journal of Experimental & Clinical Cancer Research 33, 80 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schwartz V. et al. A functional heteromeric MIF receptor formed by CD74 and CXCR4. FEBS Letters 583, 2749–2757 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Starlets D. et al. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood 107, 4807–4816 (2006). [DOI] [PubMed] [Google Scholar]
- 167.Leng L. et al. MIF Signal Transduction Initiated by Binding to CD74. Journal of Experimental Medicine 197, 1467–1476 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Foote A. et al. Macrophage migration inhibitory factor in systemic lupus erythematosus. The Journal of Rheumatology 31, 268–273 (2004). [PubMed] [Google Scholar]
- 169.Lan HY et al. Expression of macrophage migration inhibitory factor in human glomerulonephritis. Kidney International 57, 499–509 (2000). [DOI] [PubMed] [Google Scholar]
- 170.Leng L. et al. A Small-Molecule Macrophage Migration Inhibitory Factor Antagonist Protects against Glomerulonephritis in Lupus-Prone NZB/NZW F1 and MRL/lpr Mice. The Journal of Immunology 186, 527–538 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hoi AY et al. Macrophage Migration Inhibitory Factor Deficiency Attenuates Macrophage Recruitment, Glomerulonephritis, and Lethality in MRL/lpr Mice. The Journal of Immunology 177, 5687–5696 (2006). [DOI] [PubMed] [Google Scholar]
- 172.Tahir S. et al. A CD153+CD4+ T Follicular Cell Population with Cell-Senescence Features Plays a Crucial Role in Lupus Pathogenesis via Osteopontin Production. The Journal of Immunology 194, 5725–5735 (2015). [DOI] [PubMed] [Google Scholar]
- 173.Nus M. et al. Marginal zone B cells control the response of follicular helper T cells to a high-cholesterol diet. Nat Med 23, 601–610 (2017). [DOI] [PubMed] [Google Scholar]
- 174.Gaddis DE et al. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat Commun 9, 1095 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li J, Lu E, Yi T. & Cyster JG EBI2 augments Tfh cell fate by promoting interaction with IL-2-quenching dendritic cells. Nature 533, 110–114 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ryu H. et al. Atherogenic dyslipidemia promotes autoimmune follicular helper T cell responses via IL-27. Nat Immunol 19, 583–593 (2018). [DOI] [PubMed] [Google Scholar]
- 177.Oestreich KJ et al. Bcl-6 directly represses the gene program of the glycolysis pathway. Nat Immunol 15, 957–964 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Patsoukis N. et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun 6, 6692 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Huang B. et al. In vivo CRISPR screens reveal a HIF-1α-mTOR-network regulates T follicular helper versus Th1 cells. Nat Commun 13, 805 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang J. et al. Critical roles of mTOR Complex 1 and 2 for T follicular helper cell differentiation and germinal center responses. eLife 5, e17936 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zeng H. et al. mTORC1 and mTORC2 Kinase Signaling and Glucose Metabolism Drive Follicular Helper T Cell Differentiation. Immunity 45, 540–554 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Perl A. et al. Comprehensive metabolome analyses reveal N-acetylcysteine-responsive accumulation of kynurenine in systemic lupus erythematosus: implications for activation of the mechanistic target of rapamycin. Metabolomics 11, 1157–1174 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Morel L. Immunometabolism in systemic lupus erythematosus. Nat Rev Rheumatol 13, 280–290 (2017). [DOI] [PubMed] [Google Scholar]
- 184.Yin Y. et al. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med 7, 274ra18 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yin Y. et al. Glucose Oxidation Is Critical for CD4+ T Cell Activation in a Mouse Model of Systemic Lupus Erythematosus. The Journal of Immunology 196, 80–90 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lui SL et al. Rapamycin attenuates the severity of established nephritis in lupus-prone NZB/W F 1 mice. Nephrology Dialysis Transplantation 23, 2768–2776 (2008). [DOI] [PubMed] [Google Scholar]
- 187.Fernandez D. & Perl A. mTOR signaling: a central pathway to pathogenesis in systemic lupus erythematosus? Discov Med 9, 173–178 (2010). [PMC free article] [PubMed] [Google Scholar]
- 188.Zheng X, Tsou P-S & Sawalha AH Increased Expression of EZH2 Is Mediated by Higher Glycolysis and mTORC1 Activation in Lupus CD4+ T Cells. Immunometabolism 2, e200013 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fernandez DR et al. Activation of mammalian target of rapamycin controls the loss of TCRzeta in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J Immunol 182, 2063–2073 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kato H. & Perl A. Mechanistic Target of Rapamycin Complex 1 Expands Th17 and IL-4+ CD4−CD8− Double-Negative T Cells and Contracts Regulatory T Cells in Systemic Lupus Erythematosus. The Journal of Immunology 192, 4134–4144 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Choi S-C et al. Inhibition of glucose metabolism selectively targets autoreactive follicular helper T cells. Nature Communications 9, 4369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yi W. et al. The mTORC1–4E-BP-eIF4E axis controls de novo Bcl6 protein synthesis in T cells and systemic autoimmunity. Nat Commun 8, 254 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li W. et al. Targeting T Cell Activation and Lupus Autoimmune Phenotypes by Inhibiting Glucose Transporters. Front. Immunol 10, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chen L. Co-inhibitory molecules of the B7–CD28 family in the control of T-cell immunity. Nat Rev Immunol 4, 336–347 (2004). [DOI] [PubMed] [Google Scholar]
- 195.Schnell A, Bod L, Madi A. & Kuchroo VK The yin and yang of co-inhibitory receptors: toward anti-tumor immunity without autoimmunity. Cell Res 30, 285–299 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sumida TS et al. Type I interferon transcriptional network regulates expression of coinhibitory receptors in human T cells. Nat Immunol 1–11 (2022) doi: 10.1038/s41590-022-01152-y. [DOI] [PMC free article] [PubMed]
- 197.Banchereau J. & Pascual V. Type I Interferon in Systemic Lupus Erythematosus and Other Autoimmune Diseases. Immunity 25, 383–392 (2006). [DOI] [PubMed] [Google Scholar]
- 198.Das A. et al. Follicular Dendritic Cell Activation by TLR Ligands Promotes Autoreactive B Cell Responses. Immunity 46, 106–119 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Han J-H et al. Expression of an anti-RNA autoantibody in a mouse model of SLE increases neutrophil and monocyte numbers as well as IFN-I expression. Eur J Immunol 44, 215–226 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Barber DL et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006). [DOI] [PubMed] [Google Scholar]
- 201.Hams E. et al. Blockade of B7-H1 (programmed death ligand 1) enhances humoral immunity by positively regulating the generation of T follicular helper cells. J Immunol 186, 5648–5655 (2011). [DOI] [PubMed] [Google Scholar]
- 202.Good-Jacobson KL et al. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol 11, 535–542 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kawamoto S. et al. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science 336, 485–489 (2012). [DOI] [PubMed] [Google Scholar]
- 204.Shi J. et al. PD-1 Controls Follicular T Helper Cell Positioning and Function. Immunity 49, 264–274.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Butler NS et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol 13, 188–195 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Furtado R. et al. Blockade of LAG-3 in PD-L1-Deficient Mice Enhances Clearance of Blood Stage Malaria Independent of Humoral Responses. Frontiers in Immunology 11, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Murga-Zamalloa CA, Brown NA & Wilcox RA Expression of the checkpoint receptors LAG-3, TIM-3 and VISTA in peripheral T cell lymphomas. Journal of Clinical Pathology 73, 197–203 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li L, Ma Y, Xu Y. & Maerkeya K. TIM-3 expression identifies a distinctive PD-1+ follicular helper T cell subset, with reduced interleukin 21 production and B cell help function in ovarian cancer patients. International Immunopharmacology 57, 139–146 (2018). [DOI] [PubMed] [Google Scholar]
- 209.Zhu S, Lin J, Qiao G, Wang X. & Xu Y. Tim-3 identifies exhausted follicular helper T cells in breast cancer patients. Immunobiology 221, 986–993 (2016). [DOI] [PubMed] [Google Scholar]
- 210.Hardtke S, Ohl L. & Förster R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood 106, 1924–1931 (2005). [DOI] [PubMed] [Google Scholar]
- 211.Weber JP et al. ICOS maintains the T follicular helper cell phenotype by downregulating Krüppel-like factor 2. Journal of Experimental Medicine 212, 217–233 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Moriyama S. et al. Sphingosine-1-phosphate receptor 2 is critical for follicular helper T cell retention in germinal centers. Journal of Experimental Medicine 211, 1297–1305 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lu P, Shih C. & Qi H. Ephrin B1–mediated repulsion and signaling control germinal center T cell territoriality and function. Science 356, eaai9264 (2017). [DOI] [PubMed] [Google Scholar]
- 214.Hu R. et al. miR-155 Promotes T Follicular Helper Cell Accumulation during Chronic, Low-Grade Inflammation. Immunity 41, 605–619 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Suan D. et al. T follicular helper cells have distinct modes of migration and molecular signatures in naive and memory immune responses. Immunity 42, 704–718 (2015). [DOI] [PubMed] [Google Scholar]
- 216.Yan H. et al. Plexin B2 and Semaphorin 4C Guide T Cell Recruitment and Function in the Germinal Center. Cell Rep 19, 995–1007 (2017). [DOI] [PubMed] [Google Scholar]
- 217.Janssen E. et al. DOCK8 is essential for LFA-1–dependent positioning of T follicular helper cells in germinal centers. JCI Insight 5, e134508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Meli AP et al. The Integrin LFA-1 Controls T Follicular Helper Cell Generation and Maintenance. Immunity 45, 831–846 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Cannons JL et al. Optimal Germinal Center Responses Require a Multistage T Cell:B Cell Adhesion Process Involving Integrins, SLAM-Associated Protein, and CD84. Immunity 32, 253–265 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Schrock DC et al. Pivotal role for αV integrins in sustained Tfh support of the germinal center response for long-lived plasma cell generation. Proceedings of the National Academy of Sciences 116, 4462–4470 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Qi H, Cannons JL, Klauschen F, Schwartzberg PL & Germain RN SAP-controlled T-B cell interactions underlie germinal centre formation. Nature 455, 764–769 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Peng C. et al. BCL6-Mediated Silencing of PD-1 Ligands in Germinal Center B Cells Maintains Follicular T Cell Population. The Journal of Immunology 202, 704–713 (2019). [DOI] [PubMed] [Google Scholar]
- 223.Wang X. et al. Follicular dendritic cells help establish follicle identity and promote B cell retention in germinal centers. J Exp Med 208, 2497–2510 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Koopman G. et al. Adhesion through the LFA-1 (CD11a/CD18)-ICAM-1 (CD54) and the VLA-4 (CD49d)-VCAM-1 (CD106) pathways prevents apoptosis of germinal center B cells. The Journal of Immunology 152, 3760–3767 (1994). [PubMed] [Google Scholar]
- 225.Balogh P, Aydar Y, Tew JG & Szakal AK Appearance and phenotype of murine follicular dendritic cells expressing VCAM-1. The Anatomical Record 268, 160–168 (2002). [DOI] [PubMed] [Google Scholar]
- 226.Matza D. et al. A Scaffold Protein, AHNAK1, Is Required for Calcium Signaling during T Cell Activation. Immunity 28, 64–74 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Matza D. et al. Requirement for AHNAK1-mediated calcium signaling during T lymphocyte cytolysis. Proceedings of the National Academy of Sciences 106, 9785–9790 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Shankar J. et al. Pseudopodial Actin Dynamics Control Epithelial-Mesenchymal Transition in Metastatic Cancer Cells. Cancer Research 70, 3780–3790 (2010). [DOI] [PubMed] [Google Scholar]
- 229.Berlin C. et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195 (1993). [DOI] [PubMed] [Google Scholar]
- 230.Tinoco R, Otero DC, Takahashi AA & Bradley LM PSGL-1: A New Player in the Immune Checkpoint Landscape. Trends in Immunology 38, 323–335 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Groom JR & Luster AD CXCR3 in T cell function. Experimental Cell Research 317, 620–631 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Allen CDC et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol 5, 943–952 (2004). [DOI] [PubMed] [Google Scholar]
- 233.Lu Y. & Craft J. T Follicular Regulatory Cells: Choreographers of Productive Germinal Center Responses. Frontiers in Immunology 12, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sage PT, Paterson AM, Lovitch SB & Sharpe AH The Coinhibitory Receptor CTLA-4 Controls B Cell Responses by Modulating T Follicular Helper, T Follicular Regulatory, and T Regulatory Cells. Immunity 41, 1026–1039 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cai G. et al. A Regulatory Role for IL-10 Receptor Signaling in Development and B Cell Help of T Follicular Helper Cells in Mice. The Journal of Immunology 189, 1294–1302 (2012). [DOI] [PubMed] [Google Scholar]
- 236.McCarron MJ & Marie JC TGF-β prevents T follicular helper cell accumulation and B cell autoreactivity. J Clin Invest 124, 4375–4386 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Gonzalez-Figueroa P. et al. Follicular regulatory T cells produce neuritin to regulate B cells. Cell 184, 1775–1789.e19 (2021). [DOI] [PubMed] [Google Scholar]
- 238.Zhao D-M, Thornton AM, DiPaolo RJ & Shevach EM Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood 107, 3925–3932 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Xie MM et al. Follicular regulatory T cells inhibit the development of granzyme B–expressing follicular helper T cells. JCI Insight 4, e128076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sage PT & Sharpe AH T follicular regulatory cells in the regulation of B cell responses. Trends in Immunology 36, 410–418 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tubo NJ et al. Single Naive CD4+ T Cells from a Diverse Repertoire Produce Different Effector Cell Types during Infection. Cell 153, 785–796 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fazilleau N, McHeyzer-Williams LJ, Rosen H. & McHeyzer-Williams MG The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol 10, 375–384 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Botta D. et al. Dynamic regulation of T follicular regulatory cell responses by interleukin 2 during influenza infection. Nat Immunol 18, 1249–1260 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Xie MM et al. Roles of T Follicular Helper Cells and T Follicular Regulatory Cells in Autoantibody Production in IL-2–Deficient Mice. ImmunoHorizons 3, 306–316 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wu H. et al. Follicular regulatory T cells repress cytokine production by follicular helper T cells and optimize IgG responses in mice. European Journal of Immunology 46, 1152–1161 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Fu W. et al. Deficiency in T follicular regulatory cells promotes autoimmunity. Journal of Experimental Medicine 215, 815–825 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Laidlaw BJ et al. Interleukin-10 from CD4+ follicular regulatory T cells promotes the germinal center response. Science Immunology 2, eaan4767 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Clement RL et al. Follicular regulatory T cells control humoral and allergic immunity by restraining early B cell responses. Nat Immunol 20, 1360–1371 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Linterman MA & Vinuesa CG T follicular helper cells during immunity and tolerance. Prog Mol Biol Transl Sci 92, 207–248 (2010). [DOI] [PubMed] [Google Scholar]
- 250.Fulcher DA et al. The fate of self-reactive B cells depends primarily on the degree of antigen receptor engagement and availability of T cell help. Journal of Experimental Medicine 183, 2313–2328 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Cook MC, Basten A. & de St Groth BF. Outer Periarteriolar Lymphoid Sheath Arrest and Subsequent Differentiation of Both Naive and Tolerant Immunoglobulin Transgenic B Cells Is Determined by B Cell Receptor Occupancy. Journal of Experimental Medicine 186, 631–643 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Adelstein S. et al. Induction of Self-Rolerance in T Cells But Not B Cells of Transgenic Mice Expressing Little Self Antigen. Science 251, 1223–1225 (1991). [DOI] [PubMed] [Google Scholar]
- 253.Cooke MP et al. Immunoglobulin signal transduction guides the specificity of B cell-T cell interactions and is blocked in tolerant self-reactive B cells. Journal of Experimental Medicine 179, 425–438 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Maceiras AR et al. T follicular helper and T follicular regulatory cells have different TCR specificity. Nat Commun 8, 15067 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Aloulou M. et al. Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells. Nat Commun 7, 10579 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ritvo P-G et al. High-resolution repertoire analysis reveals a major bystander activation of Tfh and Tfr cells. PNAS 115, 9604–9609 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ritvo P-GG et al. Tfr cells lack IL-2Rα but express decoy IL-1R2 and IL-1Ra and suppress the IL-1–dependent activation of Tfh cells. Science Immunology 2, eaan0368 (2017). [DOI] [PubMed] [Google Scholar]
- 258.Birnbaum ME et al. Deconstructing the Peptide-MHC Specificity of T Cell Recognition. Cell 157, 1073–1087 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lang HLE et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nature Immunology 3, 940–943 (2002). [DOI] [PubMed] [Google Scholar]
- 260.Mason D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunology Today 19, 395–404 (1998). [DOI] [PubMed] [Google Scholar]
- 261.Nelson RW et al. T Cell Receptor Cross-Reactivity between Similar Foreign and Self Peptides Influences Naive Cell Population Size and Autoimmunity. Immunity 42, 95–107 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Eberl G, Brawand P. & MacDonald HR Selective bystander proliferation of memory CD4+ and CD8+ T cells upon NK T or T cell activation. J Immunol 165, 4305–4311 (2000). [DOI] [PubMed] [Google Scholar]
- 263.Tough DF, Sun S. & Sprent J. T cell stimulation in vivo by lipopolysaccharide (LPS). J Exp Med 185, 2089–2094 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Tough DF, Borrow P. & Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 272, 1947–1950 (1996). [DOI] [PubMed] [Google Scholar]
- 265.Lee H-G, Cho M-Z & Choi J-M Bystander CD4+ T cells: crossroads between innate and adaptive immunity. Exp Mol Med 52, 1255–1263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Su LF, Kidd BA, Han A, Kotzin JJ & Davis MM Virus-Specific CD4+ Memory-Phenotype T Cells Are Abundant in Unexposed Adults. Immunity 38, 373–383 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Quintana FJ & Cohen IR The natural autoantibody repertoire and autoimmune disease. Biomedicine & Pharmacotherapy 58, 276–281 (2004). [DOI] [PubMed] [Google Scholar]
- 268.Noble A, Zhao Z-S & Cantor H. Suppression of Immune Responses by CD8 Cells. II. Qa-1 on Activated B Cells Stimulates CD8 Cell Suppression of T Helper 2 Responses. The Journal of Immunology 160, 566–571 (1998). [PubMed] [Google Scholar]
- 269.Kim H-J, Verbinnen B, Tang X, Lu L. & Cantor H. Inhibition of follicular T-helper cells by CD8 + regulatory T cells is essential for self tolerance. Nature 467, 328–332 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Gate D. et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 577, 399–404 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kattah NH et al. Tetramers reveal IL-17-secreting CD4+ T cells that are specific for U1–70 in lupus and mixed connective tissue disease. Proc. Natl. Acad. Sci. U.S.A 112, 3044–3049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Mohan JF, Calderon B, Anderson MS & Unanue ER Pathogenic CD4+ T cells recognizing an unstable peptide of insulin are directly recruited into islets bypassing local lymph nodes. J. Exp. Med 210, 2403–2414 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hoffman RW et al. Human T cell clones reactive against U-small nuclear ribonucleoprotein autoantigens from connective tissue disease patients and healthy individuals. J. Immunol 151, 6460–6469 (1993). [PubMed] [Google Scholar]
- 274.Bockenstedt LK, Gee RJ & Mamula MJ Self-peptides in the initiation of lupus autoimmunity. J. Immunol 154, 3516–3524 (1995). [PubMed] [Google Scholar]
- 275.Mohan C, Adams S, Stanik V. & Datta SK Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J. Exp. Med 177, 1367–1381 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ito Y. et al. Detection of T cell responses to a ubiquitous cellular protein in autoimmune disease. Science 346, 363–368 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Odegard JM et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med 205, 2873–2886 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lee SK et al. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J. Exp. Med 208, 1377–1388 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Facciotti F. et al. Evidence for a pathogenic role of extrafollicular, IL-10-producing CCR6+B helper T cells in systemic lupus erythematosus. Proc. Natl. Acad. Sci. U.S.A 117, 7305–7316 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Klein L, Hinterberger M, Wirnsberger G. & Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol 9, 833–844 (2009). [DOI] [PubMed] [Google Scholar]
- 281.Merkenschlager J. et al. Dynamic regulation of TFH selection during the germinal centre reaction. Nature 591, 458–463 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kotov DI et al. TCR Affinity Biases Th Cell Differentiation by Regulating CD25, Eef1e1, and Gbp2. The Journal of Immunology 202, 2535–2545 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bettini M. et al. TCR Affinity and Tolerance Mechanisms Converge To Shape T Cell Diabetogenic Potential. The Journal of Immunology 193, 571–579 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Liu GY et al. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity 3, 407–415 (1995). [DOI] [PubMed] [Google Scholar]
- 285.Harkiolaki M. et al. T Cell-Mediated Autoimmune Disease Due to Low-Affinity Crossreactivity to Common Microbial Peptides. Immunity 30, 348–357 (2009). [DOI] [PubMed] [Google Scholar]
- 286.Malkiel S, Barlev AN, Atisha-Fregoso Y, Suurmond J. & Diamond B. Plasma Cell Differentiation Pathways in Systemic Lupus Erythematosus. Frontiers in Immunology 9, 427 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Cerutti A, Cols M. & Puga I. Marginal zone B cells: virtues of innatelike antibody-producing lymphocytes. Nat Rev Immunol 13, 118–132 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Lopes-Carvalho T, Foote J. & Kearney JF Marginal zone B cells in lymphocyte activation and regulation. Current Opinion in Immunology 17, 244–250 (2005). [DOI] [PubMed] [Google Scholar]
- 289.Rao DA et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542, 110–114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Eisenbarth SC et al. CD4+ T cells that help B cells – a proposal for uniform nomenclature. Trends in Immunology 42, 658–669 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Yeh C-H, Finney J, Okada T, Kurosaki T. & Kelsoe G. Primary germinal center-resident T follicular helper cells are a physiologically distinct subset of CXCR5hiPD-1hi T follicular helper cells. Immunity 55, 272–289.e7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Shulman Z. et al. T Follicular Helper Cell Dynamics in Germinal Centers. Science 341, 673–677 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Morita R. et al. Human Blood CXCR5+CD4+ T Cells Are Counterparts of T Follicular Cells and Contain Specific Subsets that Differentially Support Antibody Secretion. Immunity 34, 108–121 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Choi J-Y et al. Circulating Follicular Helper–Like T Cells in Systemic Lupus Erythematosus: Association With Disease Activity. Arthritis & Rheumatology 67, 988–999 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Xu B. et al. The ratio of circulating follicular T helper cell to follicular T regulatory cell is correlated with disease activity in systemic lupus erythematosus. Clinical Immunology 183, 46–53 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Zhang X. et al. Circulating CXCR5+CD4+helper T cells in systemic lupus erythematosus patients share phenotypic properties with germinal center follicular helper T cells and promote antibody production. Lupus 24, 909–917 (2015). [DOI] [PubMed] [Google Scholar]
- 297.Liu C. et al. Increased circulating CD4+CXCR5+FoxP3+ follicular regulatory T cells correlated with severity of systemic lupus erythematosus patients. International Immunopharmacology 56, 261–268 (2018). [DOI] [PubMed] [Google Scholar]
- 298.Sage PT, Alvarez D, Godec J, von Andrian UH & Sharpe AH Circulating T follicular regulatory and helper cells have memory-like properties. J Clin Invest 124, 5191–5204 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Locci M. et al. Human Circulating PD-1+CXCR3−CXCR5+ Memory Tfh Cells Are Highly Functional and Correlate with Broadly Neutralizing HIV Antibody Responses. Immunity 39, 758–769 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Stephenson W. et al. Single-cell RNA-seq of rheumatoid arthritis synovial tissue using low-cost microfluidic instrumentation. Nat Commun 9, 791 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Caielli S. et al. A CD4+ T cell population expanded in lupus blood provides B cell help through interleukin-10 and succinate. Nat Med 25, 75–81 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Vu Van D. et al. Local T/B cooperation in inflamed tissues is supported by T follicular helper-like cells. Nat Commun 7, 10875 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Gu-Trantien C. et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Bocharnikov AV et al. PD-1hiCXCR5– T peripheral helper cells promote B cell responses in lupus via MAF and IL-21. JCI Insight 4, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Asashima H. et al. PD-1highCXCR5–CD4+ Peripheral Helper T (Tph) cells Promote Tissue-Homing Plasmablasts in COVID-19. 2021.03.13.21253527 (2021) doi: 10.1101/2021.03.13.21253527. [DOI]
- 306.Fischer J. et al. Effect of Clonally Expanded PD-1highCXCR5–CD4+ Peripheral T Helper Cells on B Cell Differentiation in the Joints of Patients With Antinuclear Antibody–Positive Juvenile Idiopathic Arthritis. Arthritis & Rheumatology 74, 150–162 (2022). [DOI] [PubMed] [Google Scholar]
- 307.Renand A. et al. Integrative molecular profiling of autoreactive CD4 T cells in autoimmune hepatitis. Journal of Hepatology 73, 1379–1390 (2020). [DOI] [PubMed] [Google Scholar]
- 308.Christophersen A. et al. Distinct phenotype of CD4+ T cells driving celiac disease identified in multiple autoimmune conditions. Nat Med 25, 734–737 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Son YM et al. Tissue-resident CD4+ T helper cells assist the development of protective respiratory B and CD8+ T cell memory responses. Science Immunology 6, eabb6852 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Swarnalekha N. et al. T resident helper cells promote humoral responses in the lung. Science Immunology 6, eabb6808 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Shi Y. et al. IL-21 Induces an Imbalance of Th17/Treg Cells in Moderate-to-Severe Plaque Psoriasis Patients. Frontiers in Immunology 10, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Chen JS et al. High-affinity, neutralizing antibodies to SARS-CoV-2 can be made without T follicular helper cells. Sci Immunol 7, eabl5652 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Miyauchi K. et al. Protective neutralizing influenza antibody response in the absence of T follicular helper cells. Nat Immunol 17, 1447–1458 (2016). [DOI] [PubMed] [Google Scholar]
- 314.Comte D. et al. SLAMF7 engagement restores defective effector CD8+ T cells activity in response to foreign antigens in systemic lupus erythematosus. Arthritis Rheumatol 69, 1035–1044 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Katsuyama E. et al. The CD38/NAD/SIRTUIN1/EZH2 Axis Mitigates Cytotoxic CD8 T Cell Function and Identifies Patients with SLE Prone to Infections. Cell Rep 30, 112–123.e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Stohl W. Impaired polyclonal T cell cytolytic activity. A possible risk factor for systemic lupus erythematosus. Arthritis Rheum 38, 506–516 (1995). [DOI] [PubMed] [Google Scholar]
- 317.Couzi L. et al. Predominance of CD8+ T lymphocytes among periglomerular infiltrating cells and link to the prognosis of class III and class IV lupus nephritis. Arthritis Rheum 56, 2362–2370 (2007). [DOI] [PubMed] [Google Scholar]
- 318.Tilstra JS et al. Kidney-infiltrating T cells in murine lupus nephritis are metabolically and functionally exhausted. J Clin Invest 128, 4884–4897 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Filaci G. et al. Impairment of CD8+ T suppressor cell function in patients with active systemic lupus erythematosus. J Immunol 166, 6452–6457 (2001). [DOI] [PubMed] [Google Scholar]
- 320.Kim H-J et al. CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. PNAS 108, 2010–2015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Mishra S. et al. TGF-β and Eomes control the homeostasis of CD8+ regulatory T cells. Journal of Experimental Medicine 218, e20200030 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Tulunay A, Yavuz S, Direskeneli H. & Eksioglu-Demiralp E. CD8+CD28-, suppressive T cells in systemic lupus erythematosus. Lupus 17, 630–637 (2008). [DOI] [PubMed] [Google Scholar]
- 323.Dolff S. et al. Urinary T cells in active lupus nephritis show an effector memory phenotype. Ann Rheum Dis 69, 2034–2041 (2010). [DOI] [PubMed] [Google Scholar]
- 324.Li J. et al. KIR+CD8+ T cells suppress pathogenic T cells and are active in autoimmune diseases and COVID-19. Science 376, eabi9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Valentine KM et al. CD8 Follicular T Cells Promote B Cell Antibody Class Switch in Autoimmune Disease. The Journal of Immunology 201, 31–40 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Chen Y. et al. CXCR5+PD-1+ follicular helper CD8 T cells control B cell tolerance. Nat Commun 10, 4415 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Yang R. et al. IL-6 promotes the differentiation of a subset of naive CD8+ T cells into IL-21–producing B helper CD8+ T cells. Journal of Experimental Medicine 213, 2281–2291 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Wagner UG et al. The Role of CD8+ CD40L+ T Cells in the Formation of Germinal Centers in Rheumatoid Synovitis. The Journal of Immunology 161, 6390–6397 (1998). [PubMed] [Google Scholar]
- 329.Kang YM et al. CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J Exp Med 195, 1325–1336 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Cui C. et al. Neoantigen-driven B cell and CD4 T follicular helper cell collaboration promotes anti-tumor CD8 T cell responses. Cell 184, 6101–6118.e13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Zander R. et al. Tfh-cell-derived interleukin 21 sustains effector CD8+ T cell responses during chronic viral infection. Immunity 55, 475–493.e5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Jenks SA, Cashman KS, Woodruff MC, Lee FE-H & Sanz I. Extrafollicular responses in humans and SLE. Immunol Rev 288, 136–148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Mandik-Nayak L. et al. MRL-lpr/lpr Mice Exhibit a Defect in Maintaining Developmental Arrest and Follicular Exclusion of Anti–double-stranded DNA B Cells. Journal of Experimental Medicine 189, 1799–1814 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Eaton-Bassiri AS et al. Alterations in splenic architecture and the localization of anti-double-stranded DNA B cells in aged mice. International Immunology 12, 915–926 (2000). [DOI] [PubMed] [Google Scholar]
- 335.Sweet RA et al. Facultative role for T cells in extrafollicular Toll-like receptor-dependent autoreactive B-cell responses in vivo. PNAS 108, 7932–7937 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Cunningham AF, Serre K, Mohr E, Khan M. & Toellner K-M Loss of CD154 impairs the Th2 extrafollicular plasma cell response but not early T cell proliferation and interleukin-4 induction. Immunology 113, 187–193 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.McAdam AJ et al. ICOS is critical for CD40-mediated antibody class switching. Nature 409, 102–105 (2001). [DOI] [PubMed] [Google Scholar]
- 338.Mohr E. et al. Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J Immunol 182, 2113–2123 (2009). [DOI] [PubMed] [Google Scholar]
- 339.Paus D. et al. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med 203, 1081–1091 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Bentebibel S-E, Schmitt N, Banchereau J. & Ueno H. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proceedings of the National Academy of Sciences 108, E488–E497 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Kraaij T. et al. The NET-effect of combining rituximab with belimumab in severe systemic lupus erythematosus. Journal of Autoimmunity 91, 45–54 (2018). [DOI] [PubMed] [Google Scholar]
- 342.Hale M, Rawlings DJ & Jackson SW The long and the short of it: insights into the cellular source of autoantibodies as revealed by B cell depletion therapy. Current Opinion in Immunology 55, 81–88 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Tew G. et al. Baseline autoantibody profiles predict normalization of complement and anti-dsDNA autoantibody levels following Rituximab treatment in systemic lupus erythematosus. Lupus 19, 146–157 (2010). [DOI] [PubMed] [Google Scholar]
- 344.Yusof MYM et al. Predicting and managing primary and secondary non-response to rituximab using B-cell biomarkers in systemic lupus erythematosus. Annals of the Rheumatic Diseases 76, 1829–1836 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Merrill JT et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: The randomized, double-blind, phase ii/iii systemic lupus erythematosus evaluation of rituximab trial. Arthritis & Rheumatism 62, 222–233 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Jacobi AM et al. Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis & Rheumatism 48, 1332–1342 (2003). [DOI] [PubMed] [Google Scholar]
- 347.Tipton CM et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol 16, 755–765 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Groom JR et al. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. Journal of Experimental Medicine 204, 1959–1971 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Castigli E. et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med 201, 35–39 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Tsao PY, Jiao J, Ji MQ, Cohen PL & Eisenberg RA T Cell-Independent Spontaneous Loss of Tolerance by Anti-Double-Stranded DNA B Cells in C57BL/6 Mice. The Journal of Immunology 181, 7770–7777 (2008). [DOI] [PubMed] [Google Scholar]
- 351.Jenks SA et al. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity 49, 725–739.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Rubtsova K. et al. B cells expressing the transcription factor T-bet drive lupuslike autoimmunity. J Clin Invest 127, 1392–1404 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Song W. et al. Development of Tbet- and CD11c-expressing B cells in a viral infection requires T follicular helper cells outside of germinal centers. Immunity 55, 290–307.e5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Palm A-KE & Kleinau S. Marginal zone B cells: From housekeeping function to autoimmunity? Journal of Autoimmunity 119, 102627 (2021). [DOI] [PubMed] [Google Scholar]
- 355.Sang A, Zheng Y-Y & Morel L. Contributions of B cells to lupus pathogenesis. Mol Immunol 62, 329–338 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Zhou Z, Niu H, Zheng Y-Y & Morel L. Autoreactive marginal zone B cells enter the follicles and interact with CD4+ T cells in lupus-prone mice. BMC Immunology 12, 7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Ramanujam M. et al. Interferon-α treatment of female (NZW × BXSB)F1 mice mimics some but not all features associated with the Yaa mutation. Arthritis & Rheumatism 60, 1096–1101 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Herlands RA, Christensen SR, Sweet RA, Hershberg U. & Shlomchik MJ T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity 29, 249–260 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Christensen SR et al. Toll-like Receptor 7 and TLR9 Dictate Autoantibody Specificity and Have Opposing Inflammatory and Regulatory Roles in a Murine Model of Lupus. Immunity 25, 417–428 (2006). [DOI] [PubMed] [Google Scholar]
- 360.Soni C. et al. Plasmacytoid Dendritic Cells and Type I Interferon Promote Extrafollicular B Cell Responses to Extracellular Self-DNA. Immunity 52, 1022–1038.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Dieu-Nosjean M-C, Goc J, Giraldo NA, Sautès-Fridman C. & Fridman WH Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 35, 571–580 (2014). [DOI] [PubMed] [Google Scholar]
- 362.Voswinkel J. et al. Single cell analysis of B lymphocytes from Wegener’s granulomatosis: B cell receptors display affinity maturation within the granulomatous lesions. Clin Exp Immunol 154, 339–345 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Voswinkel J. et al. B lymphocyte maturation in Wegener’s granulomatosis: a comparative analysis of VH genes from endonasal lesions. Ann Rheum Dis 65, 859–864 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Armengol MP et al. Thyroid autoimmune disease: demonstration of thyroid antigen-specific B cells and recombination-activating gene expression in chemokine-containing active intrathyroidal germinal centers. Am J Pathol 159, 861–873 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Armengol M-P et al. Chemokines determine local lymphoneogenesis and a reduction of circulating CXCR4+ T and CCR7 B and T lymphocytes in thyroid autoimmune diseases. J Immunol 170, 6320–6328 (2003). [DOI] [PubMed] [Google Scholar]
- 366.Howell OW et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 134, 2755–2771 (2011). [DOI] [PubMed] [Google Scholar]
- 367.Lovato L. et al. Related B cell clones populate the meninges and parenchyma of patients with multiple sclerosis. Brain 134, 534–541 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Magliozzi R. et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130, 1089–1104 (2007). [DOI] [PubMed] [Google Scholar]
- 369.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E. & Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 14, 164–174 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Magliozzi R, Columba-Cabezas S, Serafini B. & Aloisi F. Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. Journal of Neuroimmunology 148, 11–23 (2004). [DOI] [PubMed] [Google Scholar]
- 371.Sims GP, Shiono H, Willcox N. & Stott DI Somatic hypermutation and selection of B cells in thymic germinal centers responding to acetylcholine receptor in myasthenia gravis. J Immunol 167, 1935–1944 (2001). [DOI] [PubMed] [Google Scholar]
- 372.Humby F. et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med 6, e1 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Takemura S. et al. Lymphoid neogenesis in rheumatoid synovitis. J Immunol 167, 1072–1080 (2001). [DOI] [PubMed] [Google Scholar]
- 374.Salomonsson S. et al. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren’s syndrome. Arthritis Rheum 48, 3187–3201 (2003). [DOI] [PubMed] [Google Scholar]
- 375.Stott DI, Hiepe F, Hummel M, Steinhauser G. & Berek C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjögren’s syndrome. J Clin Invest 102, 938–946 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Chang A. et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol 186, 1849–1860 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Kang S. et al. BAFF Induces Tertiary Lymphoid Structures and Positions T Cells within the Glomeruli during Lupus Nephritis. J Immunol 198, 2602–2611 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Le Pottier L. et al. Ectopic germinal centers are rare in Sjogren’s syndrome salivary glands and do not exclude autoreactive B cells. J Immunol 182, 3540–3547 (2009). [DOI] [PubMed] [Google Scholar]
- 379.Vossenkämper A. et al. A role for gut-associated lymphoid tissue in shaping the human B cell repertoire. J Exp Med 210, 1665–1674 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Serafini B. et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J Exp Med 204, 2899–2912 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Cavalcante P. et al. Epstein-Barr virus persistence and reactivation in myasthenia gravis thymus. Ann Neurol 67, 726–738 (2010). [DOI] [PubMed] [Google Scholar]
- 382.Croia C. et al. Epstein-Barr virus persistence and infection of autoreactive plasma cells in synovial lymphoid structures in rheumatoid arthritis. Ann Rheum Dis 72, 1559–1568 (2013). [DOI] [PubMed] [Google Scholar]
- 383.Croia C. et al. Implication of Epstein-Barr virus infection in disease-specific autoreactive B cell activation in ectopic lymphoid structures of Sjögren’s syndrome. Arthritis Rheumatol 66, 2545–2557 (2014). [DOI] [PubMed] [Google Scholar]
- 384.Pikor NB et al. Integration of Th17- and Lymphotoxin-Derived Signals Initiates Meningeal-Resident Stromal Cell Remodeling to Propagate Neuroinflammation. Immunity 43, 1160–1173 (2015). [DOI] [PubMed] [Google Scholar]
- 385.Peters A. et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity 35, 986–996 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Chiang EY et al. Targeted depletion of lymphotoxin-alpha-expressing TH1 and TH17 cells inhibits autoimmune disease. Nat Med 15, 766–773 (2009). [DOI] [PubMed] [Google Scholar]
- 387.Bombardieri M. et al. Inducible tertiary lymphoid structures, autoimmunity, and exocrine dysfunction in a novel model of salivary gland inflammation in C57BL/6 mice. J Immunol 189, 3767–3776 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Albert LJ & Inman RD Molecular Mimicry and Autoimmunity. New England Journal of Medicine 341, 2068–2074 (1999). [DOI] [PubMed] [Google Scholar]
- 389.Rojas M. et al. Molecular mimicry and autoimmunity. Journal of Autoimmunity 95, 100–123 (2018). [DOI] [PubMed] [Google Scholar]
- 390.Kaplan MelvinH. & Meyeserian M. AN IMMUNOLOGICAL CROSS-REACTION BETWEEN GROUP-A STREPTOCOCCAL CELLS AND HUMAN HEART TISSUE. The Lancet 279, 706–710 (1962). [DOI] [PubMed] [Google Scholar]
- 391.Rees JH, Soudain SE, Gregson NA & Hughes RAC Campylobacter jejuni Infection and Guillain–Barré Syndrome. New England Journal of Medicine 333, 1374–1379 (1995). [DOI] [PubMed] [Google Scholar]
- 392.Bjornevik K. et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 375, 296–301 (2022). [DOI] [PubMed] [Google Scholar]
- 393.Nishimura K. et al. Meta-analysis: Diagnostic Accuracy of Anti–Cyclic Citrullinated Peptide Antibody and Rheumatoid Factor for Rheumatoid Arthritis. Ann Intern Med 146, 797–808 (2007). [DOI] [PubMed] [Google Scholar]
- 394.Terzoglou AG, Routsias JG, Avrameas S, Moutsopoulos HM & Tzioufas AG Preferential recognition of the phosphorylated major linear B-cell epitope of La/SSB 349–368 aa by anti-La/SSB autoantibodies from patients with systemic autoimmune diseases. Clin Exp Immunol 144, 432–439 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Seitz-Polski B. et al. High-Dose Rituximab and Early Remission in PLA2R1-Related Membranous Nephropathy. Clin J Am Soc Nephrol 14, 1173–1182 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Sokolove J. et al. Autoantibody Epitope Spreading in the Pre-Clinical Phase Predicts Progression to Rheumatoid Arthritis. PLOS ONE 7, e35296 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Robert L. et al. CTLA4 Blockade Broadens the Peripheral T-Cell Receptor Repertoire. Clinical Cancer Research 20, 2424–2432 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Memarnejadian A. et al. PD-1 Blockade Promotes Epitope Spreading in Anticancer CD8+ T Cell Responses by Preventing Fratricidal Death of Subdominant Clones To Relieve Immunodomination. The Journal of Immunology 199, 3348–3359 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Alspach E. et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 574, 696–701 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Disis ML et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol 27, 4685–4692 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Butterfield LH et al. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin Cancer Res 9, 998–1008 (2003). [PubMed] [Google Scholar]
- 402.Wierecky J. et al. Immunologic and clinical responses after vaccinations with peptide-pulsed dendritic cells in metastatic renal cancer patients. Cancer Res 66, 5910–5918 (2006). [DOI] [PubMed] [Google Scholar]
- 403.Ribas A, Timmerman JM, Butterfield LH & Economou JS Determinant spreading and tumor responses after peptide-based cancer immunotherapy. Trends in Immunology 24, 58–61 (2003). [DOI] [PubMed] [Google Scholar]
- 404.Menares E. et al. Tissue-resident memory CD8+ T cells amplify anti-tumor immunity by triggering antigen spreading through dendritic cells. Nat Commun 10, 4401 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Xin G. et al. Pathogen-Boosted Adoptive Cell Transfer Therapy Induces Endogenous Antitumor Immunity through Antigen Spreading. Cancer Immunology Research 8, 7–18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Läubli H. et al. The T cell repertoire in tumors overlaps with pulmonary inflammatory lesions in patients treated with checkpoint inhibitors. Oncoimmunology 7, e1386362 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Johnson DB et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med 375, 1749–1755 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.June CH, Warshauer JT & Bluestone JA Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med 23, 540–547 (2017). [DOI] [PubMed] [Google Scholar]
- 409.Kwek SS et al. Diversity of Antigen-Specific Responses Induced In Vivo with CTLA-4 Blockade in Prostate Cancer Patients. The Journal of Immunology 189, 3759–3766 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Berner F. et al. Association of Checkpoint Inhibitor–Induced Toxic Effects With Shared Cancer and Tissue Antigens in Non–Small Cell Lung Cancer. JAMA Oncology 5, 1043–1047 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Topalian SL et al. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol 5, 1411–1420 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Eggermont AMM et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival Among Patients With Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol 6, 519–527 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Maher VE et al. Analysis of the Association Between Adverse Events and Outcome in Patients Receiving a Programmed Death Protein 1 or Programmed Death Ligand 1 Antibody. J Clin Oncol 37, 2730–2737 (2019). [DOI] [PubMed] [Google Scholar]
- 414.Corbière V. et al. Antigen spreading contributes to MAGE vaccination-induced regression of melanoma metastases. Cancer Res 71, 1253–1262 (2011). [DOI] [PubMed] [Google Scholar]
- 415.Lo JA et al. Epitope spreading toward wild type melanocyte-lineage antigens rescues suboptimal immune checkpoint blockade responses. Sci Transl Med 13, eabd8636 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Mazor RD et al. Tumor-reactive antibodies evolve from non-binding and autoreactive precursors. Cell 0, (2022). [DOI] [PubMed]
- 417.Chaurio RA et al. TGF-β-mediated silencing of genomic organizer SATB1 promotes Tfh cell differentiation and formation of intra-tumoral tertiary lymphoid structures. Immunity 55, 115–128.e9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Hollern DP et al. B Cells and T Follicular Helper Cells Mediate Response to Checkpoint Inhibitors in High Mutation Burden Mouse Models of Breast Cancer. Cell 179, 1191–1206.e21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Bindea G. et al. Spatiotemporal Dynamics of Intratumoral Immune Cells Reveal the Immune Landscape in Human Cancer. Immunity 39, 782–795 (2013). [DOI] [PubMed] [Google Scholar]
- 420.Cabrita R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020). [DOI] [PubMed] [Google Scholar]
- 421.Cillo AR et al. Immune Landscape of Viral- and Carcinogen-Driven Head and Neck Cancer. Immunity 52, 183–199.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Garaud S. et al. Tumor infiltrating B-cells signal functional humoral immune responses in breast cancer. JCI Insight 5, 129641 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Germain C. et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am. J. Respir. Crit. Care Med 189, 832–844 (2014). [DOI] [PubMed] [Google Scholar]
- 424.Griss J. et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat Commun 10, 4186 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Gu-Trantien C. et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 123, 2873–2892 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Helmink BA et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Hennequin A. et al. Tumor infiltration by Tbet+ effector T cells and CD20+ B cells is associated with survival in gastric cancer patients. OncoImmunology 5, e1054598 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Kroeger DR, Milne K. & Nelson BH Tumor-Infiltrating Plasma Cells Are Associated with Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer. Clinical Cancer Research 22, 3005–3015 (2016). [DOI] [PubMed] [Google Scholar]
- 429.Lu Y. et al. Complement Signals Determine Opposite Effects of B Cells in Chemotherapy-Induced Immunity. Cell 180, 1081–1097.e24 (2020). [DOI] [PubMed] [Google Scholar]
- 430.Petitprez F. et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020). [DOI] [PubMed] [Google Scholar]
- 431.Wieland A. et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature 597, 274–278 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Rakaee M. et al. Tertiary lymphoid structure score: a promising approach to refine the TNM staging in resected non-small cell lung cancer. Br J Cancer 124, 1680–1689 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Ruffin AT et al. B cell signatures and tertiary lymphoid structures contribute to outcome in head and neck squamous cell carcinoma. Nat Commun 12, 3349 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Zander R. et al. CD4+ T Cell Help Is Required for the Formation of a Cytolytic CD8+ T Cell Subset that Protects against Chronic Infection and Cancer. Immunity 51, 1028–1042.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Kaneko N. et al. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell 183, 143–157.e13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Woodruff MC et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol 21, 1506–1516 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Painter MM et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity 54, 2133–2142.e3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Cavazzoni CB et al. Follicular T cells optimize the germinal center response to SARS-CoV-2 protein vaccination in mice. Cell Rep 38, 110399 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Lederer K. et al. Germinal center responses to SARS-CoV-2 mRNA vaccines in healthy and immunocompromised individuals. Cell 185, 1008–1024.e15 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Bastard P. et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370, eabd4585 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Zhang Q. et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 370, eabd4570 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Wang EY et al. Diverse functional autoantibodies in patients with COVID-19. Nature 595, 283–288 (2021). [DOI] [PubMed] [Google Scholar]
- 443.Chang SE et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat Commun 12, 5417 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Gruber CN et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell 183, 982–995.e14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Fujii H. et al. High levels of anti-SSA/Ro antibodies in COVID-19 patients with severe respiratory failure: a case-based review. Clin Rheumatol 39, 3171–3175 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Zuo Y. et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Science Translational Medicine 12, eabd3876 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Liang KP et al. Autoantibodies and the Risk of Cardiovascular Events. The Journal of Rheumatology 36, 2462–2469 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Tsimikas S. et al. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. Journal of Lipid Research 48, 425–433 (2007). [DOI] [PubMed] [Google Scholar]
- 449.Iseme RA et al. A role for autoantibodies in atherogenesis. Cardiovascular Research 113, 1102–1112 (2017). [DOI] [PubMed] [Google Scholar]
- 450.Roman MJ et al. Prevalence and Correlates of Accelerated Atherosclerosis in Systemic Lupus Erythematosus. New England Journal of Medicine 349, 2399–2406 (2003). [DOI] [PubMed] [Google Scholar]
- 451.Su Y. et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 185, 881–895.e20 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]