Abstract
Diabetic peripheral neuropathy (DPN) is the most common complication of diabetes, with several underlying pathophysiological mechanisms, some of which are still uncertain. The cornea is an avascular tissue and sensitive to hyperglycemia, resulting in several diabetic corneal complications including delayed epithelial wound healing, recurrent erosions, neuropathy, loss of sensitivity, and tear film changes. The manifestation of DPN in the cornea is referred to as diabetic neurotrophic keratopathy (DNK). Recent studies have revealed that disturbed epithelial-neural-immune cell interactions are a major cause of DNK. The epithelium is supplied by a dense network of sensory nerve endings and dendritic cell processes, and it secretes growth/neurotrophic factors and cytokines to nourish these neighboring cells. In turn, sensory nerve endings release neuropeptides to suppress inflammation and promote epithelial wound healing, while resident immune cells provide neurotrophic and growth factors to support neuronal and epithelial cells, respectively. Diabetes greatly perturbs these interdependencies, resulting in suppressed epithelial proliferation, sensory neuropathy, and a decreased density of dendritic cells. Clinically, this results in a markedly delayed wound healing and impaired sensory nerve regeneration in response to insult and injury. Current treatments for DPN and DNK largely focus on managing the severe complications of the disease. Cell-based therapies hold promise for providing more effective treatment for diabetic keratopathy and corneal ulcers.
Keywords: Corneal wound healing, diabetic keratopathy, Diabetic peripheral nerve degeneration
1. INTRODUCTION AND BACKGROUND
1.1. Corneal Structure and function.
The cornea is a transparent, avascular, and glandless tissue that provides the eye with most of its refractive power and serves as the main structural barrier to the remaining ocular tissues. The human cornea is made up of 3 cellular layers: epithelium, stromal keratocytes, and endothelium. These are separated by two membranes, the Bowman’s layer and Descemet’s membrane (Wilson et al., 2001).
The corneal epithelium is a stratified, non-keratinized squamous layer that is bathed in a tear film. The epithelium and tear film are anatomically and physiologically related. The tear film not only forms a smooth surface for light refraction but also acts as a shield from infection or irritants (McDermott, 2013). Within the stroma, keratocytes are distributed throughout, with the highest cell density in the anterior stroma (Berlau et al., 2002). These cells maintain the unique extracellular matrix environment required for transparency (Espana and Birk, 2020). On the other end of the Descemet’s membrane, adjacent to the anterior chamber is the corneal endothelial layer, which contributes to corneal transparency by maintaining the cornea in a deturgescent state (Jeang et al., 2021; Okumura and Koizumi, 2020).
Unlike the corneal endothelium, the epithelium is renewed throughout life from a stem cell population (Cotsarelis et al., 1989; Lehrer et al., 1998; Schermer et al., 1986). The corneal stem cells are believed to reside at the corneal limbus and may be maintained by a variety of intrinsic and extrinsic factors. The corneal epithelium provides physical protection to the eye through several mechanisms. These include the production of membrane-bound mucins (Argueso et al., 2006), apical tight junctions (Sugrue and Zieske, 1997), barrier effect of the basal lamina (Gao et al., 2015b; Torricelli et al., 2013), and continuous epithelial sloughing and replacement (Fleiszig et al., 2020). Moreover, the corneal epithelium is also a sentinel in detecting and responding to external perturbations, such as prolonged exposure to hyperglycemia through recognition of the so-called damage-associated molecular patterns (DAMPs) or alarmins such as advanced glycation end-products (AGEs) (Kaji et al., 2000; Zhang et al., 2003; Zhao et al., 2019).
Although recognized as an immune-privileged site, the cornea contains two types of resident immune cells: dendritic cells (DCs), which are distributed in the epithelium and anterior part of the stroma, and macrophages, which are primarily located at the posterior part of the stroma (Liu and Li, 2021). DCs and macrophages are antigen-presenting cells and are characterized as innate immune cells (Iwasaki, 2007; Soloff and Barratt-Boyes, 2010). DCs are classified as plasmacytoid DCs (pDC), conventional (resident) DCs (cDCs), and monocyte-derived DCs (mDCs). cDCs line the mucosal tissues such as the skin and the epithelia of the lung (Tournier and Mohamadzadeh, 2010), gut (Tezuka and Ohteki, 2010), and cornea (Hamrah and Dana, 2007; Hamrah et al., 2003; Hamrah et al., 2002; Hattori et al., 2011; Segawa, 1964). In the corneal epithelium, DCs residing under the basal epithelial layer are more numerous in the peripheral than in the central cornea (Lee et al., 2010a). Two studies revealed that DCs respond to epithelial injury or proinflammatory cytokines by changing cell orientation or migrating towards the site of stimulation (Lee et al., 2010a; Ward et al., 2007). pDCs were recently shown to populate the homeostatic cornea and their numbers are increased during sterile injury or acute herpes simplex virus 1 (HSV-1) keratitis (Jamali et al., 2020). mDCs, acting alongside cDCs, play a key role in inflammation and infection (Marzaioli et al., 2020). In the cornea, mDCs accumulate in the subbasal nerve plexus and may contribute to nerve fiber damage and low-grade ocular surface inflammation (Lagali et al., 2018; Leppin et al., 2014). Alongside DCs, macrophages are another type of corneal resident immune cell and are located more posteriorly in the cornea (Brissette-Storkus et al., 2002). Macrophages are heterogenous phagocytic cells with an important role in innate immunity (Abdelaziz et al., 2020).
The cornea is the most densely innervated mammalian tissue, primarily innervated by small-diameter C-fiber sensory neurons from the ophthalmic division of the trigeminal nerve, via the anterior ciliary nerves and to a lesser degree from the maxillary nerve (Al-Aqaba et al., 2019; Rozsa and Beuerman, 1982). Moreover, the limbus and the peripheral cornea also receive autonomic sympathetic innervation from the superior cervical ganglion (Srinivasan and Lyall, 2013). Axons from the trigeminal ganglia (TG) terminate in delicate endings among the epithelial cells of the cornea (Abdelkader et al., 2011). The sensory nerves are responsible for the sensation of dryness, temperature, touch, and pain, and also play an important role in the blink reflex, wound healing, and tear production (Dartt, 2004; Garcia-Hirschfeld et al., 1994; Heigle and Pflugfelder, 1996; Inoue et al., 2005; Millodot, 1984; Nishida et al., 2012; Wang et al., 2012).
Corneal innervation has also been shown to play a critical role in maintaining stem cells and/or the stem cell niche (Ueno et al., 2012). Nerves in the cornea are known to secrete neuropeptides. Substance P (SP) and calcitonin gene-related peptide (CGRP) are produced from sensory nerves, and vasoactive intestinal polypeptide (VIP) is mostly produced from autonomic nerves (He and Bazan, 2016; Zhang et al., 2020a). In addition to nourishing the surrounding epithelial cells, these neuropeptides have profound effects on resident immune cells which actively interact in a paracrine or contact-dependent manner (Chavan et al., 2018). These communications precede the recruitment of non-resident immune cells to the site of injured corneas (Gasteiger and Rudensky, 2014; Iwasaki and Medzhitov, 2015).
1.2. Diabetes and corneal abnormalities in human and experimental animals.
The global increase in the prevalence of diabetes mellitus (DM) is an important public health burden due to its significant morbidity and mortality (Khan et al., 2020; Lin et al., 2020). DM results in damage to the ocular tissues, which occurs early in the disease process. The ocular complications resulting from DM are the leading causes of blindness in the developed world and have become major public health problems (Nentwich and Ulbig, 2015). While diabetic retinopathy is the most common and best-known ophthalmic complication, DM also has profound clinically relevant effects on the cornea with sight-threatening consequences (Bikbova et al., 2012; Frank, 2004; Lockwood et al., 2006; Sanchez-Thorin, 1998; Shah et al., 2021).
These changes are generally termed diabetic keratopathy or diabetic neurotrophic keratopathy (DNK, for consistency DNK will be used throughout) and the consequences on the quality of life for patients are often underestimated (Barsegian et al., 2018). It has been estimated to be present in 47–64% of diabetic patients throughout the course of this chronic disease (Barsegian et al., 2018; Schultz et al., 1981). DNK is a component of diabetic polyneuropathy (DPN) and is recognized as the major cause of corneal morbidity in diabetic patients (Bikbova et al., 2012). Clinical features of DNK include superficial punctate keratopathy, delayed epithelial wound healing, persistent epithelial defects/recurrent erosions, neuropathy/loss of sensitivity or sensation, tear film changes, and corneal ulceration, all of which can be found frequently in clinic and are often resistant to routine clinical management (Abdelkader et al., 2011; Priyadarsini et al., 2020). To date, there have been only a limited number of studies that focused on the importance of corneal diseases in DM; our laboratories are ones that have focused on understanding the mechanisms underlying these changes. Our results suggest that the healthy cornea relies on proper functioning and communication between epithelial cells, sensory neurons, and resident immune cells (Gao et al., 2016c), and that DM perturbs the interaction and interdependencies of these three types of cells.
The cornea is an excellent model for studying the underlying mechanisms of and testing efficacy of treatments for DPN. This is because it has a simple structure, lacks appendages (e.g., glands) and blood vessels, possesses immune privilege (Griffith et al., 1995; Streilein, 2003), is easily accessible, and there is a battery of tests used clinically that can be adapted to animal models of DM (Wang et al., 2012; Xu and Yu, 2011; Yin et al., 2011). During the last several years, based on advances in ocular imaging, in vivo confocal microscopy (IVCM) and OCT, as well as in basic research methodologies such as microarray, RNA-seq, gene therapy, great progress regarding DNK has been made. Many of these findings also have great implications for understanding the pathogenesis of DPN in other tissues such as the skin. Importantly, the degree of corneal nerve damage is directly related to the severity of somatic neuropathy in diabetic patients (Boulton et al., 2004). Assessing this damage noninvasively in the cornea has been proposed as an effective method for the early detection, diagnosis, staging severity, and monitoring the progression of DPN (Efron, 2011; Pritchard et al., 2012b; Tavakoli et al., 2013a; Tavakoli et al., 2012).
Because epithelial cells, immune cells (particularly DCs), and sensory neurons are anatomically in close proximity and structurally intertwined, we propose a concept of the Epithelium-Nerve-DC (Epineuroimmune) function unit, analogous to the function neurovascular unit consisting of neurons, glia, and vasculature in the retina (Simo et al., 2018) and to the neuro-immune axis found in the skin. Similarly, to how the retinal function unit is perturbed in DM resulting in diabetic retinopathy, we will focus on how hyperglycemia disturbs the cell-cell interactions in the corneal function unit, causing DNK. Figure 1 shows confocal images of a DC with several processes intimately interconnected with sensory nerve endings; these intertwined DC and nerve processes are within the space of the epithelium and its basement membrane (Gao et al., 2016a). This sub-basal nerve-DC interaction within the epithelium is the structural basis of the Epineuroimmune function unit, which is sensitive to perturbations both external (e.g. microbial infection) and internal (e.g. reactive oxygen species and hyperglycemia), which will be discussed in the following sections.
Figure 1. Intimate contacts between an intraepithelial DC and sensory nerve endings at sub-basal space between basal side of epithelium and its basement membrane.
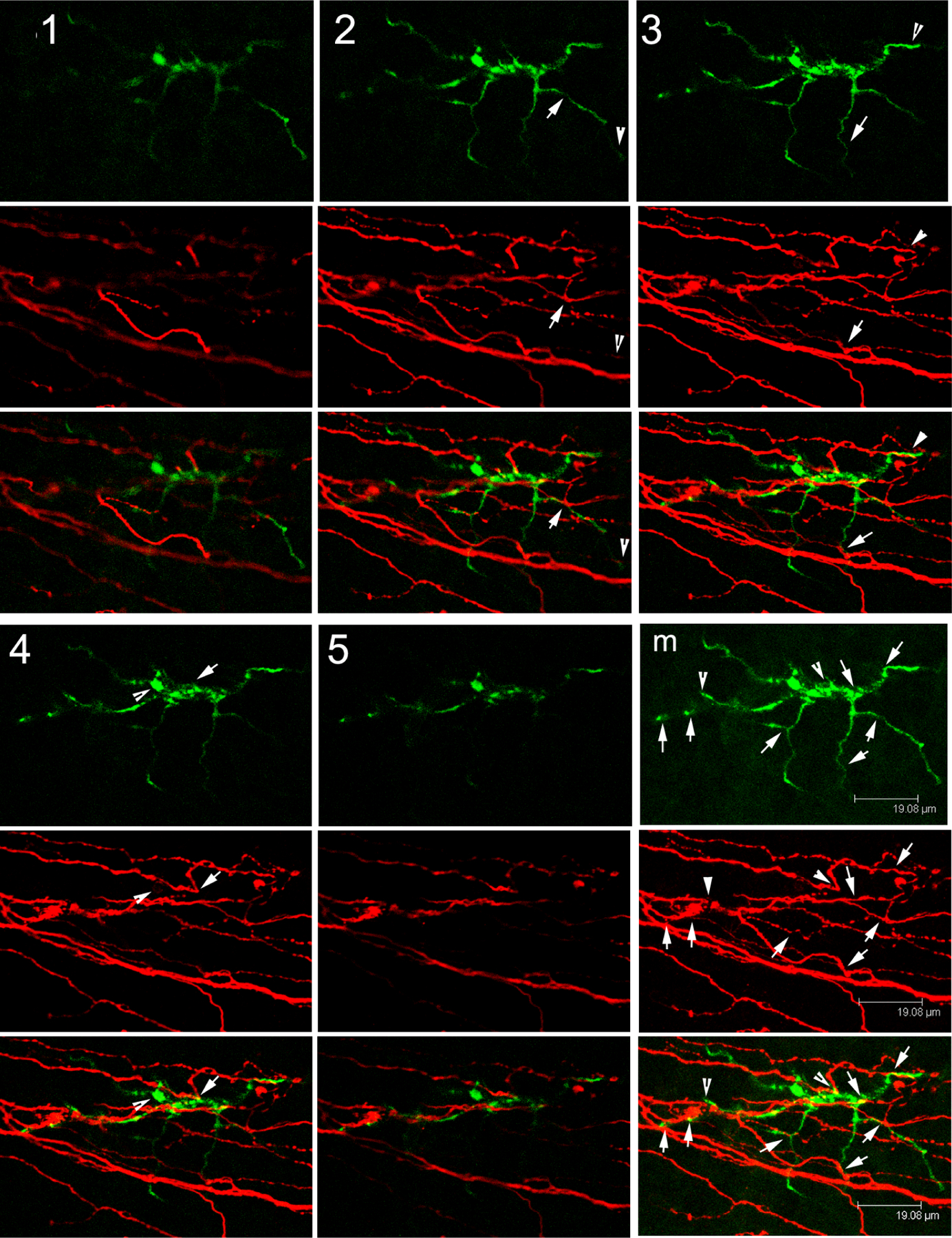
Confocal images of a mouse cornea stained with CD11c (green for DC) and β-tubulin 3 (red for neuron). Intimate contacts of DC body and processes with sensory nerve endings which are likely derived from the same epithelium insertion site of a sensory nerve fiber. This Figure wis originally published in (Gao et al., 2016a).
The Epineuroimmune function unit includes three types of cells: epithelia, sensory nerves, and dendritic cells. DM causes defects in all three cell types, making the investigation of the dysfunctional wound healing response, altered inflammation, and neuropathy on the ocular surface in human epidemiological studies challenging. Early studies focused directly on the epithelial component, and, using transformed human corneal epithelial cells, revealed that elevated extracellular glucose modulates migration, adhesion, and proliferation of human corneal epithelial cells in a glucose concentration-, but not osmolality-dependent manner (McDermott et al., 1998). To more closely mimic the effects of hyperglycemia, a model using cultured porcine corneas was developed, allowing the assessment of the effects of high glucose on epithelial wound healing and signal transduction (Xu et al., 2009). Although rodent corneas structurally differ from human corneas, similar effects of hyperglycemia on the distribution of phosphorylated Akt was observed in both T1DM and T2DM of the human corneas (Figure 2) (Xu et al., 2009), as well as in healing corneas of diabetic rats (Xu and Yu, 2011). Not surprisingly, corneas from diabetic patients exhibited a similar impaired response to epithelial wounding, which can be normalized by adenoviral delivery of gene therapy (Kramerov et al., 2016b; Saghizadeh et al., 2013; Saghizadeh et al., 2010). The Saghizadeh and Ljubimov group also investigated the role of corneal stem cells, leading to the discovery that microRNAs, such as miR-146a, were differntially expressed in corneal stem cells and targeting this microRNA restored delayed wound healing in diabetic human corneas (Poe et al., 2020; Winkler et al., 2014). The role of miR-146a in impaired epithelial wound healing was confirmed in diabetic mouse skin wound healing (Bi et al., 2021), indicating the fidelity of the organ culture model.
Figure 2. Altered pAkt staining pattern in diabetic human corneal epithelium.
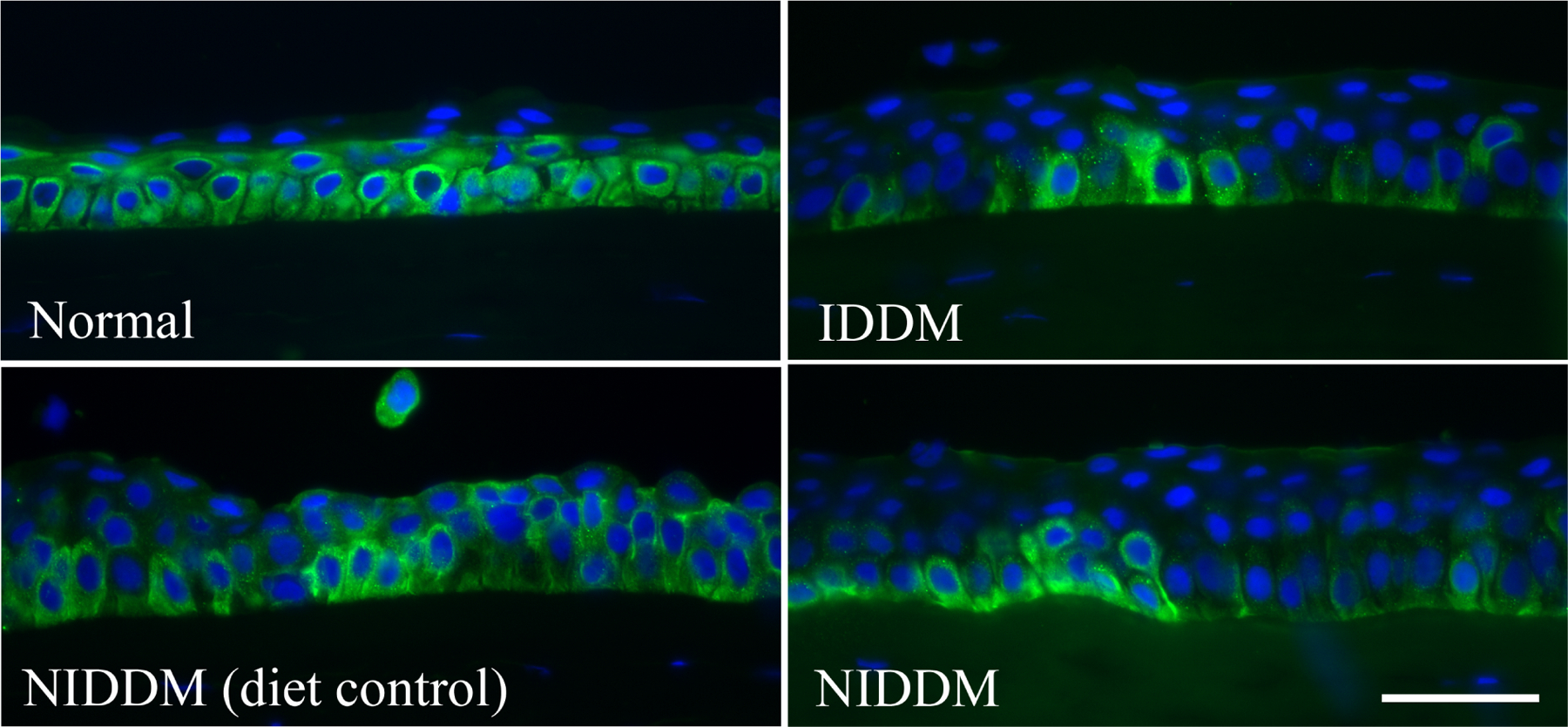
Human corneal frozen sections from patients with type 1 diabetes (IDDM) and non–insulin-dependent type 2 diabetes (NIDDM), with normal subjects and diet-controlled type 2 diabetic patients as the control subjects, were stained by immunofluorescence with antibody against pAkt. Photos show merged images of immunoreactivity of pAkt and nuclear staining of DAPI. Scale bar 50 μm. This Figure wis originally published in (Gao et al., 2016a).
While human corneal organ cultures have greatly increased our understanding of epithelial wound healing as well as stem cell biology, there are no neuronal or immune elements in the system. Thus, the importance of in vivo rodent models, including rats (Xu and Yu, 2011; Yin et al., 2011; Yin and Yu, 2010) and mice (Bettahi et al., 2014a; Gao et al., 2016c; Gao et al., 2011) are recognized. As reviewed by King, diabetes has been induced using several methods, including the beta cell toxin streptozotocin (STZ), leptin- (ob/ob) and leptin receptor-deficient (db/db) obese mouse models, or the lean T2DM Goto-Kakizaki (GK) model (King, 2012). While these models allow for a better understanding of the alterations induced by diabetes on all three components of the Epineuroimmune function unit, they each have caveats and considerations regarding their use. For instance, STZ injections produce a model of diabetes that more closely resembles T1DM or late-stage T2DM, when insulin production has declined. The toxin may have effects in other organs, including liver, kidney, lung, intestine, and brain (Lee et al., 2010b; Zhang et al., 2017). The obese leptin- and receptor-deficient mice more closely resemble patients with T2DM and “metabolic syndrome”, with insulin resistance developing in these rodents. However, some differences from T2DM in humans still exist. Leptin and leptin receptor deficiencies are not the predominant driver of T2DM in humans. Leptin-deficient mice also continue to produce insulin, in contrast to the ultimate failure of beta cells in humans with chronic uncontrolled T2DM (King, 2012; Wang et al., 2014). Similarly, while GK rodents are considered to be models for T2DM, their hyperglycemia appears to be due to abnormal beta cell functioning rather than insulin resistance, which play an important role for T2DM in patients (Ostenson and Efendic, 2007; Portha et al., 2001).
In addition to its effect on the cornea, DM is also expected to affect the surrounding tissue, including limbal stem cells (Kramerov et al., 2015), limbal vasculature (Didenko et al., 1999), tear film (Yoon et al., 2004), and aqueous humor (Hayashi et al., 1989). The effects of DM on limbal stem cells and tear film will be discussed in section 2.2 and 3.7, respectively. (Whelchel et al., 2021)The stroma is known to play a role in the corneal scarring and edema, which can become severe and threaten vision acuity (Priyadarsini et al., 2016b).
Finally using murine model of stem cell tracing and labeling, cell surface protein ABCB5+ was identified as a marker for human limbal stem cells (LSCs) (Ksander et al., 2014) which can be isolated and expanded from human cadaveric limbal tissue. An international multicenter phase I/IIa clinical trial (NCT03549299) to evaluate the safety and therapeutic efficacy of ABCB5+ positive LSC as advanced-therapy medicinal product to treat patients with LSCD is on the way. As alterations of stem cells have been identified in DM corneas (Kulkarni et al., 2017), therapy using allogeneic corneal stem cells has great potential in treating DNK in both T1DM and T2DM. This, however, may also require the use of immunosuppressants such as cyclosporine, which can exacerbate the hyperglycemia. Though diabetes is not a contraindication to its use, this side effect must be taken into consideration for any diabetic patient.
1.3. Type 1 and Type 2 DM and Diabetic keratopathy.
Generally, diabetes can be classified into two types: Type 1 (T1DM) and Type 2 (T2DM). T1DM is a chronic autoimmune disease against pancreatic β cells, resulting in insulin deficiency and hyperglycemia. T2DM is a complex metabolic disease with varying degrees of insulin resistance and β-cell dysfunction. Obesity is a major contributor to the development of T2DM. While pathogenesis of T1DM differs from T2DM, hyperglycemia is common feature, with low grade inflammation as an underlying mechanism for the development of diabetic complications. However, there are differences found in diabetic corneas between T1DM and T2DM. For example, Black et al reported impaired collagen deposition in healing human skin wounds in T1DM, but not T2DM, possibly due to decreased fibroblast proliferation (Black et al., 2003). Interestingly, in an in vitro study, Whelchel et al showed that nerves influence the metabolism of the corneal stroma, and that this is altered in both T1 and T2DM, but to different extents. For instance, glucose-6-phosphate and oxaloacetate were higher in T2DM compared to T1DM, suggesting decreased glucose metabolism capacity in T2DM corneas (Whelchel et al., 2021). Recently, Jende et al demonstrated that in patients with distal symmetric diabetic neuropathy, T1DM nerve lesions are associated with poor glycemic control and loss of nerve conduction, whereas T2DM nerve lesions are associated with changes in lipid metabolism (Jende et al., 2018). Importantly, diabetic foot ulcers, similar to corneal ulcers, are more commonly found in patients with T2DM compared with T1DM (Schreml and Berneburg, 2017). One of the biochemical abnormalities found in diabetic tissues is impaired nicotinamide adenine dinucleotide (NAD+) metabolism; a decline in NAD+ levels in T1DM is the result of the activation of poly adenine nucleotide diphosphate-ribose polymerase, while in T2DM, is due to the inhibition of adenine nucleotide monophosphate-activated protein kinase. In the cornea, Hence, although nicotinamide and related compounds have been and are currently being tested for treating both T1 and T2DM in humans, insulin sensitizers have been suggested as an adjunctive therapy for T2DM (Fan et al., 2020).
Although the mechanisms driving T1DM and T2DM differ, their corneal complications, which this review deals with, develop similarly. We reported that in human NL corneas, all basal epithelial cells are phospho-AKT positive, indictive of active PI3K/ATK pathway, while only a few cells in cluster are Phospho-AKT positive in both T1 and T2DM human corneas. In the tight diet control of T2DM patient, more phospho-AKT positive cells were observed than that of uncontrolled T2DM patient (Figure 2). There is only very limited evidence for differences in presentation and progression of corneal complications between DM types. One example is that in cornea buttons of cadavers, tissue levels of γ-glutamyl transpeptidase, an enzyme that protects against oxidative stress via glutathione recapture, were lower in T1DM than in T2DM and controls (Burnham et al., 2013), suggesting a loss of antioxidant protection in T1DM patients. Future comparative investigations regarding ocular surface diseases in T1DM and T2DM are necessary.
2. DIABETIC KERATOPATHY
2.1. Clinical Presentation of Diabetic Keratopathy
Hyndiuk et al first described DNK in three patients with T1DM in 1977 (Hyndiuk et al., 1977). The next report of this occurred in 2006, when Lockwood et al reported three additional cases of DNK (Lockwood et al., 2006). In the first case, a 26-year-old woman with 10-year history of T1DM presented with epithelial irregularities, recurrent erosions, and decreased corneal sensation. Case 2 was a 33-year-old woman with a 24-year history of T1DM who had a neuropathic foot ulcer and corneal epithelial defects; after one-year of extensive treatment, a stromal scar remained. The final case was a 44-year-old man lacking significant ocular or medical history who was found to have bilateral corneal epithelial ulcers that significantly impaired vision. Consequently, the patient was diagnosed with T2DM, with the ulcers being the sole presentation of the disease.
Clinically, the features of DNK include edema, irregularity, fragility, superficial punctate keratopathy, delayed and incomplete wound repair, and persistent corneal epithelial erosions. DNK is classified into 3 stages (Semeraro et al., 2014b). The first stage of diabetic keratopathy consists of superficial punctate keratitis, stromal scarring, and neovascularization. In the second stage, persistent epithelial defects are observed. These are surrounded by epithelium that is poorly attached to the underlying stroma, which also exhibits swelling. The third and final stage of DNK is characterized by increased involvement of the deeper stromal layer, with deep ulcerations and stromal infiltrates that thin the cornea. Representative photographs of these clinical stages can be seen in Figure 3.
Figure 3. Representative images of diabetic keratopathy progression.
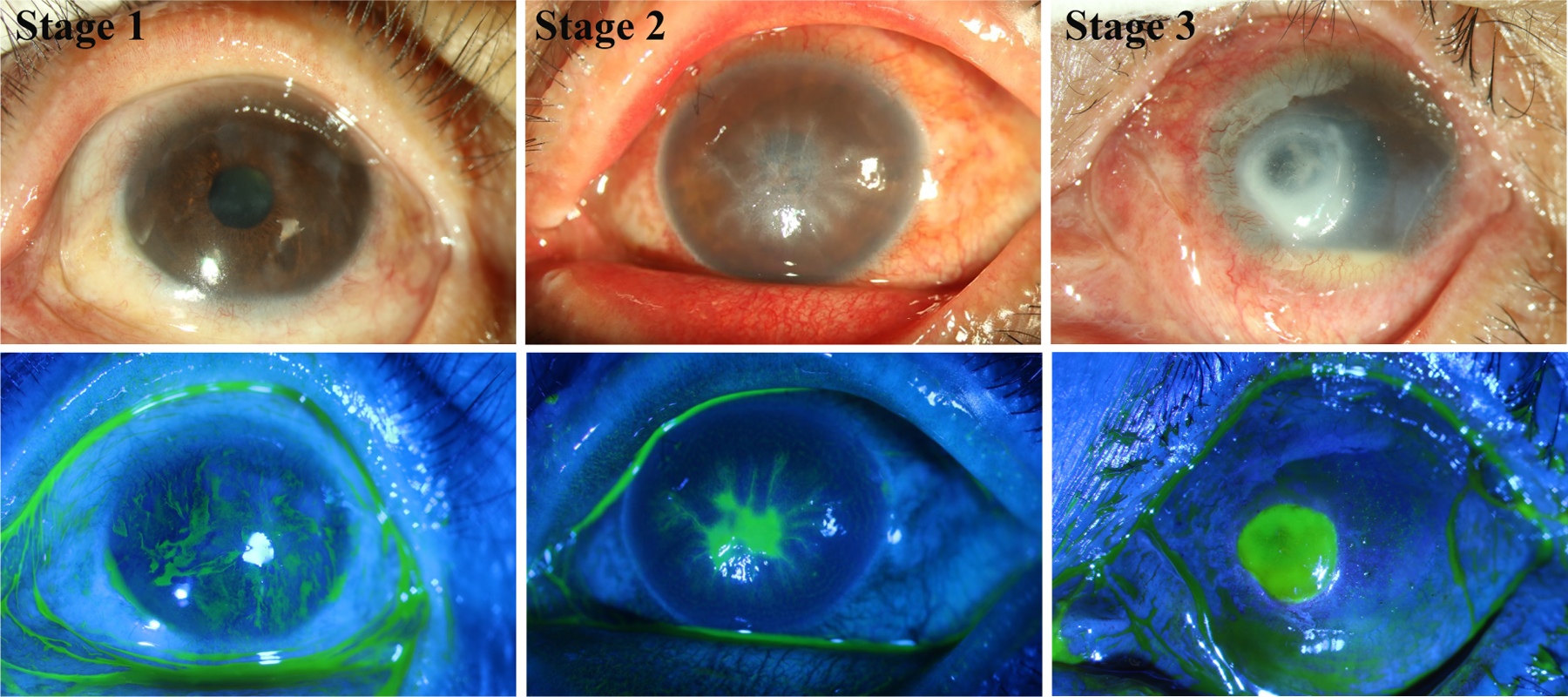
Slit lamp images with or without sodium fluorescein staining showing different severity of DNK. A cornea at first stage with scattered superficial stromal scarring and neovascularization in sclerotic scatter illumination photograph (top) and adjacent irregular hyperplastic epithelium and superficial punctate staining in cobalt blue scatter illumination photograph (bottom). A cornea at second stage with diffuse stromal edema and Descemet folds in sclerotic scatter illumination photograph (top) and lamellar staining indicating epithelial lamellar defect in cobalt blue scatter illumination photograph (bottom). A cornea at third stage with stromal infiltrate, neovascularization and hypopyon in sclerotic scatter illumination photograph (top) and stromal deep ulcerations in cobalt blue scatter illumination photograph (bottom), indicating excessive inflammation. The images were taken at the Qingdao Eye Hospital of Shandong First Medical University clinic.
Early studies suggested that the underlying cause of diabetic keratopathy was due to basement membrane abnormalities (Ljubimov et al., 1998; Taylor and Kimsey, 1981). Degradative processes due to proteinases such as MMP-10 and cathepsin F are thought to be responsible for these alterations. Changes in the epithelial cell adhesion complex are primarily responsible for wound healing abnormalities and recurrent erosions following abrasion in diabetic corneas, though this theory remains controversial (Ljubimov et al., 1998; Zagon et al., 2007). Later studies revealed more complicated alterations to gene expression (Bettahi et al., 2014a), to the breakdown of epithelial barrier (Yin et al., 2011), and to the increased susceptibility to microbial infection (Keay et al., 2009; Stapleton and Carnt, 2012; Ting et al., 2021). More recently, corneal epithelial stem cell deficiency has been closely linked to the abnormalities manifested by decreased epithelial wound healing and decreased expression of a number of putative stem cell markers (Saghizadeh et al., 2013; Saghizadeh et al., 2011a; Ueno et al., 2014). Targeting the stem cells with adenoviral gene therapy in organ-cultured corneas accelerated wound healing in human diabetic corneas (Kramerov et al., 2016b).
2.2. Defects of Limbal Stem Cells (LSCs)
Maintaining full corneal epithelial cell coverage depends on a balance between cell proliferation, migration, differentiation, and cell death. Renewal of the corneal epithelium depends on the LSC population, located in the limbus (Bonnet et al., 2021). LSCs regulate homeostatic cell turnover and wound healing (Ksander et al., 2014; Yoon et al., 2014). Upon injury to the epithelium, LSCs divide to quickly repopulate the defect. DM patients demonstrate changes in basal epithelial cell maturation and/or proliferation, resulting in a decrease in basal epithelial density (Chang et al., 2006; Quadrado et al., 2006; Tsubota et al., 1991).
Chronic diabetes results in stem cell dysfunction that contributes to the clinical presentation of DNK (Vemuganti et al., 2009). Using human cadaveric corneas and organ culture, Saghizadeh and Ljubimov demonstrated a decrease in hepatocyte growth factor receptor (c-Met) and an increase in proteinases such as MMP-10 and cathepsin F in human diabetic corneas, compared to normoglycemic corneas (Saghizadeh et al., 2005). In the following studies, they showed that epithelial stem cell markers ABCG2, N-cadherin, ΔNp63α, K15, K17, K19, and β1 integrin were significantly decreased (either by immunostaining intensity or number of cells) in diabetic limbal basal epithelia compared to non-diabetic human corneas (Saghizadeh et al., 2011a). Adenovirus vector-transduced expression of c-Met or silencing MMP-10 or cathepsin-F normalized diabetic marker expression and epithelial wound closure, compared to vector-transduced diabetic corneas (Saghizadeh et al., 2013; Saghizadeh et al., 2011a).
Using microarray analysis, the same group identified several miRNAs with increased expression in human diabetic central corneas (Funari et al., 2013). Overexpression of two such miRNAs, h-miRNA-146a or h-miRNA-424, inhibited cultured corneal epithelial cell wound healing, suggesting these miRNAs are important mediators of the abnormal wound healing seen in diabetic corneas (Funari et al., 2013; Winkler et al., 2014). The group went on to use deep sequencing analysis to identify differentially-expressed miRNAs in the limbus versus central cornea in normal (NL) (34 miRNA) and diabetic (DM) corneas (36 miRNAs) including in both T1 and T2DM (20, 13 upregulated and 7 downregulated in DM versus NL limbus) (Kulkarni et al., 2017). Seven miRNAs were found to be upregulated whereas 12 miRNAs were downregulated in the T1DM versus T2DM limbus. Among these differentially expressed miRNAs, miRNA-10b is of particular interest as it was upregulated in limbus versus central cornea and in DM versus NL limbus. Overexpression of miRNA-10b increased Ki-67 staining in human organ-cultured corneas and proliferation rates in cultured corneal epithelial cells, suggesting that miRNA-10b could be involved in LSC maintenance and/or early differentiation and that miRNA-10b upregulation may be an important mechanism of corneal diabetic alterations, particularly in T1DM patients (Kulkarni et al., 2017). These results suggest a therapeutic potential in targeting miRNA-10b, with its antagomir for restoring the LSC population and corneal regenerative function. Interestingly, miR-10b is a well-known oncogenic miRNA and can promote growth and metastasis of cancer cells (Sheedy and Medarova, 2018). The development of therapeutic reagents targeting miRNA-10B for treating DNK may also have applications in cancer therapy.
2.3. Aberrant Response of Diabetic Corneal Epithelium
Using a streptozotocin (STZ)-induced rat model of T1DM, Yin et al reported that STZ rats showed stronger Rose Bengal staining for defects in tear film, decreased tear secretion, slightly attenuated sensitivity, less innervation, delayed epithelial wound healing, and delayed formation of adherent and tight junctions after epithelial wound closure (Yin et al., 2011). Corneal epithelium-debridement wounding is an ideal model to study re-epithelialization and delayed wound healing in diabetic cornea (Ljubimov and Saghizadeh, 2015; Xu and Yu, 2011).
Using this model, we performed a genome-wide cDNA array analysis and found 1,888 differentially expressed genes in the healing epithelia of NL versus DM rat corneas (Bettahi et al., 2014a). Analysis of cDNA array data revealed marked wounding-induced expression of interleukin-1 (IL-1β) and the secreted form of the IL-1 receptor antagonist (sIL-1Ra). Diabetes suppressed this wounding-induced expression of sIL1Ra, but augmented the expression of IL-1β in healing epithelia (Bettahi et al., 2014a). Taken together, this suggests a disturbance of the balanced expression of IL-1β and its natural inhibitor in DM epithelium in favoring excessive inflammation during wound healing. In normoglycemic mice, IL-1β or sIL-1Ra blockade delayed wound healing and influenced one another’s expression, suggesting the importance of controlled and balanced expression and signaling of IL-1β, one of the major proinflammatory cytokines (Yan et al., 2016). In diabetic mice, in addition to delayed re-epithelialization, diabetes weakened PI3K-AKT signaling, caused cell apoptosis, diminished cell proliferation, suppressed neutrophil and natural killer (NK) cell infiltration, and impaired sensory nerve re-innervation in healing mouse corneas. Local administration of recombinant IL-1Ra partially, but significantly reversed these pathological changes in the diabetic corneas (Yan et al., 2016). Using the Proteome Profiler Mouse XL Cytokine Array (R&D Systems), analysis revealed that CXCL10, CXCL5 and CCL5 exhibited a wound-induced and diabetes-suppressed expression in the healing corneas; IL-1Ra partially restored the diabetes-suppressed expressions of these three proteins. CXCL5 is a chemokine that recruits and activates neutrophils while CCL5 is a chemoattractant for blood monocytes and eosinophils. CXCL10 acts as a downstream modulator of IL-1β-IL-1Ra-mediated signaling (Yan et al., 2016). CXCL10 has been shown to recruit NK cells to the normal mouse cornea underneath the basement membrane (Liu et al., 2014). Functionally, topical application of CXCL10 promotes corneal epithelial wound healing in diabetic mouse corneas. Finally, experiments using mice with a reporter gene controlled by the IL-1β promotor revealed that, in addition to a higher density of IL-1β-expressing cells in the cornea during epithelial wound closure, these cells disappeared rapidly in NL but lasted several days in DM corneas, indicating that resolution of wound-induced inflammation is severely impaired the DM corneas (Figure 4) (Yan et al., 2016). These results indicate the importance of proper levels of inflammation mediated primarily by IL-1β and balanced by IL-1Ra. Anakinra, a human recombinant IL-1Ra, was originally designed for treating rheumatoid arthritis and has therapeutic value in an array of autoinflammatory disorders, including gout, pericarditis, heart failure, diabetes, and myocarditis (Cavalli and Dinarello, 2018). Compared to other biologics, Anakinra has an unparalleled record of safety. In a Phase II randomized clinical trial, the effects of Anakinra on moderate to severe dry eye disease revealed that topical Anakinra was well tolerated and was able to significantly reduce dry eye disease symptoms (Amparo et al., 2013). To date, phase III results have been not released (Baiula and Spampinato, 2021). Whether Anakinra can be used, alone or in combination with other type of medications such as NGF, for treating DNK- and/or DM-associated eye dry remains to be determined.
Figure 4. Inflammatory responses of the cornea in response to epithelial injury.
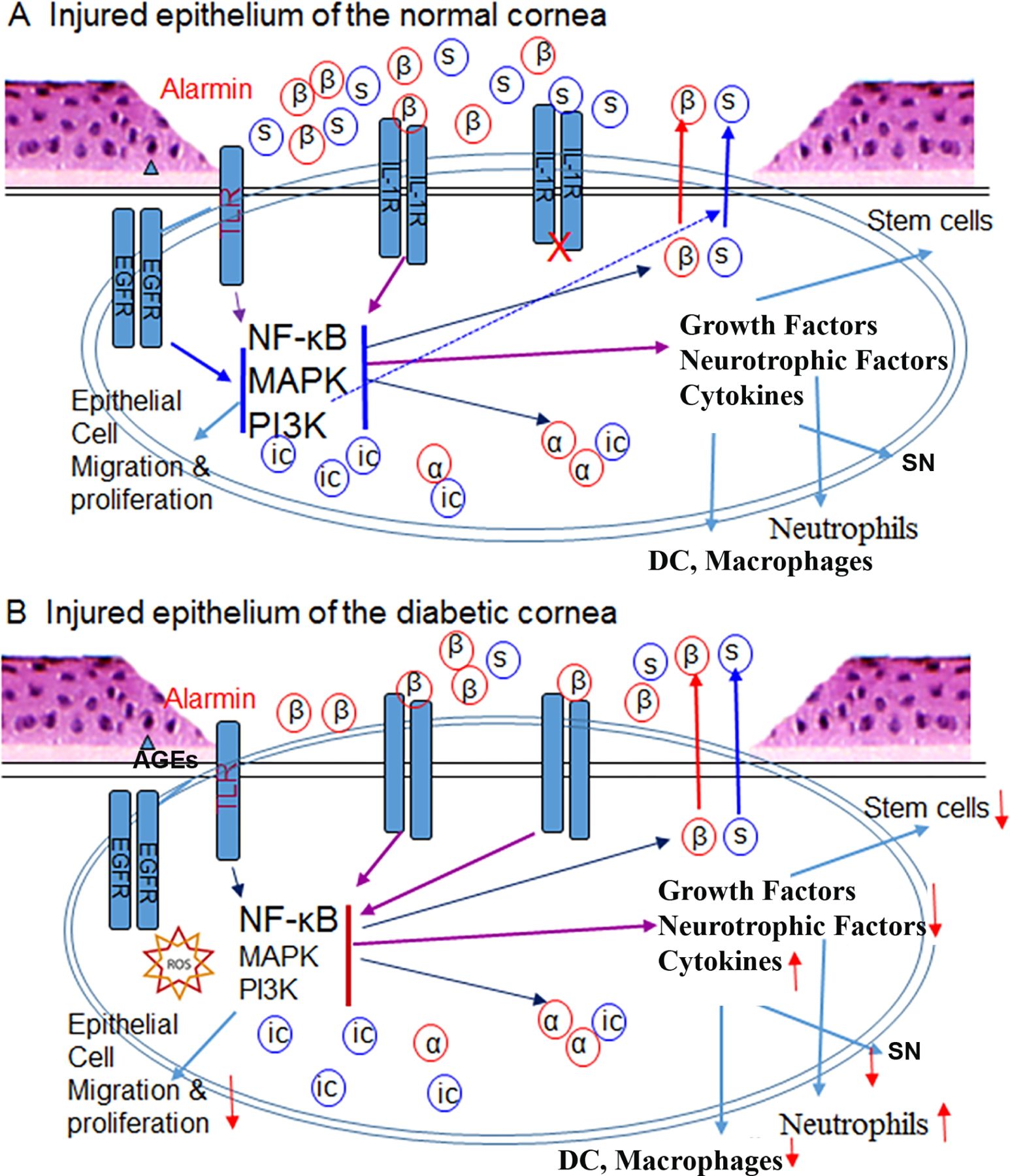
(A) In a normal cornea, injury causes the release of alarmins such as IL-1α (a) and ATP, resulting in activation of intracellular signaling pathways which trigger the expression and secretion of growth factors, neurotrophic factors, and cytokines, including IL-1β and soluble IL-1Ra (sIL-1Ra). The balanced expression of both IL-1β and its antagonist sIL-1Ra ensures the controlled inflammation and infiltration of neutrophils, dendritic cells (DCs), and macrophages. Growth factors stimulate limbal stem cell proliferation to replenish lost epithelial cells. (B) In diabetic corneas, hyperglycemia causes accumulation of Advanced Glycation End-Products (AGEs) extracellularly and generation of reactive oxygen species (ROS) intracellularly. AGEs and ROS inhibits epithelial proliferation and migration, resulting in delayed wound healing. Hyperglycemia promotes IL-1β secretion and neutrophil infiltration but suppresses sIL-1Ra expression and DC and macrophage infiltration, resulting in an imbalance favoring excessive inflammation and increased cell death, further delaying epithelial wound closure and sensory nerve regeneration (not shown in this diagram). This figure is adapted from supplemental Figure 1 of (Yan et al., 2016).
The TGFβ family of growth factors has a profound influence on physiological and pathological processes in the body. TGFβ1 has been implicated in the pathogenesis of an array of human diseases including diabetes and wound healing (Hathaway et al., 2015; Kajdaniuk et al., 2013). In the cornea, TGFβ1 induces fibrosis in laser-ablated mouse corneas (Robinson et al., 2013). The functions of TGFβ isoforms, particularly TGFβ1 and β3 which share receptors, are thought to be mostly indistinguishable. However, some data suggest that TGFβ1 and TGFβ3 are profibrotic and antifibrotic agents, respectively (Ferguson and O’Kane, 2004; Fujio et al., 2016; Shah et al., 1995). The experimental manipulation of TGFβ levels in the cornea has established a strong correlation between the rates of epithelial wound closure and the levels of TGFβ1 or 3. TGFβ1 and 3 also differentially affect the expression of their target genes/effectors in the epithelium, such as Serpine1 (plasminogen activator inhibitor 1, PAI-1)/Plat (tissue PA or tPA)/Plau (urokinase PA or uPA). Exogenous TGFβ3 restores hyperglycemia-suppressed expression of the plasminogen activators tPA and uPA in DM corneas. tPA and uPA enhance proteolytic degradation of collagen and other extracellular matrix proteins, preventing fibrosis (Ghosh and Vaughan, 2012) In contrast, TGFβ1 restores the expression of PAI-1, an inhibitor of fibrin degradation that contributes to fibrosis (Oda et al., 2001). Both plasminogen activation and inhibition were shown to play an important role in cell migration and wound healing (Eddy and Fogo, 2006; Ma and Fogo, 2009; Sun et al., 2015) (Yu and Gao, unpublished results). Hence, TGFβ1 and β3 act collaboratively and are both required for plasminogen activation during wound healing. This collaboration is disrupted by hyperglycemia via TGFβ3 repression, resulting in the pathogenesis of keratopathy. Figure 5 shows balanced expression of paired genes, TGFβ1/3, IL-1β/Ra, PAI/tPA,uPA, and Sema3A/3C, with distinctive or opposing functions, and disturbance of these balanced expressions by hyperglycemia contributing to the pathogenesis of diabetic keratopathy.
Figure 5. Epithelium response to wounding in normal and diabetic corneas.
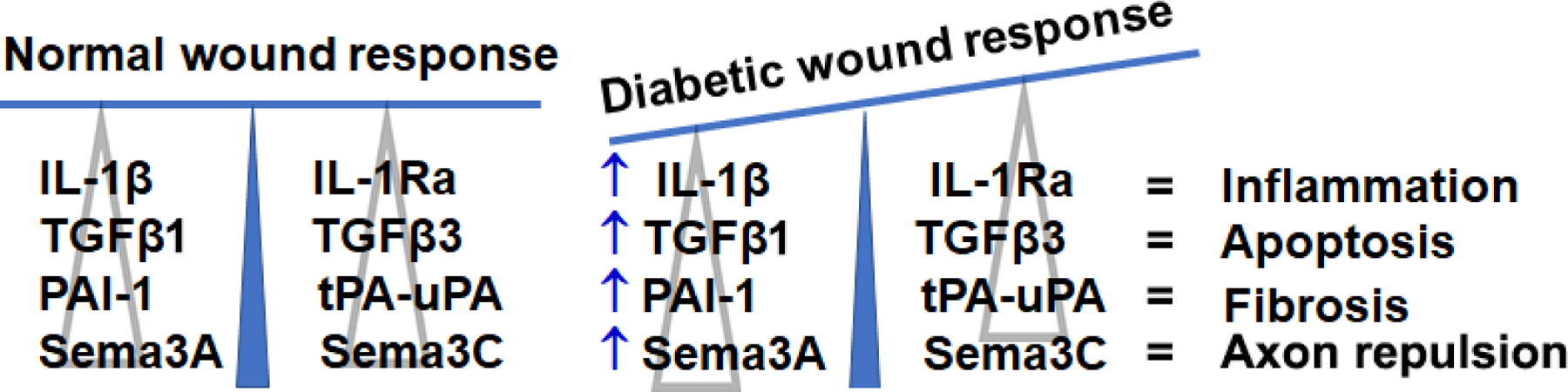
In normal corneas, wounding induces the balanced expressions of IL-1β/1Ra, transforming growth factor (TGF) β1/β3, PAI/tPA-uPA, and Semaphorin (Sema) 3A/3C. In diabetic corneas, wounding sufficiently induces the expressions of IL-1β, TGFβ1, PAI, and Sema3A, but not IL-1Ra, TGFβ3, uPA-tPA, and Sema3C, resulting in increased inflammation, apoptosis, fibrosis, and repulsion of regenerating sensory nerves, hence delaying corneal epithelial wound closure and sensory nerve regeneration seen in diabetic patients. This diagram has not been published and was made by FS Yu.
IGF-I is a multifunctional regulatory peptide that shares structural homology with proinsulin (Pollak, 2008). IGF-I regulates cell proliferation, differentiation, and survival. IGF-I and its receptors are expressed by both epithelial cells and fibroblasts in human corneas (Li and Tseng, 1995) Though IGF-I alone did not affect corneal wound healing ex vivo or in vivo (Nakamura et al., 1997; Nishida et al., 1996), SP and IGF-I synergistically enhance corneal wound closure in organ cultures and in a rat model of neurotrophic keratopathy (Nagano et al., 2003; Nakamura et al., 2003). Hence, the epithelial-produced IGF-1 may have synergistic effects with nerve-derived SP on corneal epithelial wound healing and sensory nerve regeneration in DM corneas. Hepatocyte growth factor (HGF) is known to be involved in tissue morphogenesis and regeneration. In a diabetic mouse model, HGF was found to accelerate skin (Yoshida et al., 2004) and corneal wound healing (Miyagi et al., 2018). Saghizadeh and Ljubimov identified specific epithelial proteins with altered expression in human diabetic central corneas, and the expression of HGF receptor (c-met) was decreased in diabetic corneas. Importantly, gene therapy to express c-Met was shown to accelerate epithelial wound closure in cultured human diabetic corneas (Saghizadeh et al., 2010; Saghizadeh et al., 2011b). Interestingly, the IGF-1 signal transduction cascade upregulates microRNA-1 expression in cardiac and skeletal muscle in physiological and pathological conditions through a feedback loop (Elia et al., 2009).
DM also has a significant impact on the corneal stroma, which accounts for 90% of the thickness of the cornea and therefore its tensile strength and biomechanical properties (Hager et al., 2009). Using the Ocular Response Analyser, several studies reported a higher corneal hysteresis (suggesting higher rigidity), and a greater corneal thickness in both T1DM and T2DM, compared with age-matched controls (for more references see (Shih et al., 2017)). The reason for this is not completely known; epitheliopathy and the accumulation of AGEs in the corneal stroma are speculated to be potential factors (Goldin et al., 2006; Shih et al., 2017). At the molecular levels, significantly increased expression of collagen I and III, and altered lipidomics and metabolomics were reported and suggested to contribute to corneal haze and scarring associated with diabetes (Lam et al., 2021; Priyadarsini et al., 2016a; Whelchel et al., 2021). Whelchel et al used 3-dimensional cell cultures of T1DM and T2DM corneal stroma and neuroblastoma cells; metabolic analysis revealed that the glucose-6-phosphate and oxaloacetate was higher in T2DMs compared to T1DMs (Whelchel et al., 2021). Additionally, a reduction in stromal keratocyte density appears to be correlated to the degree of diabetic neuropathy in T1 and T2DM patients, though the factors responsible for this change have not been identified (Kalteniece et al., 2018). Unlike diabetic epitheliopathy, only a few studies can be found in the literature that focused on the impact of DM on corneal stroma, with no direct data on stromal wound healing in diabetic corneas. Further study on the subject is warranted.
2.4. Changes of MicroRNAs in the Diabetic Cornea
MicroRNAs (miRNAs) are endogenous short chain noncoding RNAs that inhibit protein translation through binding to target mRNAs, resulting in gene regulation (Carrington and Ambros, 2003). Recent studies demonstrated that miRNAs play a regulatory role in corneal wound healing as well as in the pathogenesis of diabetic keratopathy (Ljubimov and Saghizadeh, 2015). MicroRNAs thought to play a role in corneal wound healing and diabetic keratopathy include miRNA-34c (Hu et al., 2019), −205 (Yu et al., 2010), −133b (Robinson et al., 2013), −146a (Bi et al., 2021; Funari et al., 2013), −204–5p (Gao et al., 2015a), −206 (Li et al., 2015), −182 (Wang et al., 2016), −184 (Cao et al., 2020; Yu et al., 2008), −129–5p (Yang et al., 2019), and 181a (Funari et al., 2013; Hu et al., 2020; Ryan et al., 2006).
Longevity-associated Sirtuin-1 (Sirt1) is a key gene involved in neuroprotection and tissue repair and plays an important role in promoting epithelial wound closure in diabetic corneas (Wang et al., 2016). Gao et al. used bioinformatics methods to predict and verify the possible miRNAs that regulate Sirt1, a class III NAD+ dependent protein deacetylase that may play a role in a variety of models of neurodegenerative disorders. Overexpression of Sirt1 promoted epithelial wound healing in diabetic corneas (Wang et al., 2013). It was found that miRNA-204-5p directly regulates Sirt1 expression in corneal epithelial cells (Gao et al., 2015a). Inhibition of miRNA-204-5p promotes cell cycle progression by enhancing Sirt1 expression, thereby increasing corneal epithelial wound healing. Work from the same group further confirmed that miRNA-182 is a downstream effector of Sirt1 through microRNA microarray analysis. Sirt1 overexpression in TG neurons upregulates the expression of miRNA-182, which results in enhanced nerve regeneration and functioning in diabetes, likely through a reduction of expression of its target gene, NOX4 (Wang et al., 2016) (Figure 6). These findings indicate that miRNA-204-5p and miRNA-182 function upstream and downstream of the key modulator Sirt1, respectively, in regulating the regeneration of diabetic corneal epithelium and sensory nerves. Since activation of SIRT1 is a potential new target of therapeutics for osteoporosis and other bone related disorders (Zainabadi, 2019), targeting miRNA-204-5p and/or miRNA-182 may also provide an alternative for pharmacological activation of SIRT1 in treating DNK. Interestingly, animal and human experimental data suggest that upregulation/activation of SIRT1 can prevent sensory neuronal degeneration pathways leading to distal axonopathy (Chandrasekaran et al., 2019). Therefore, its application to treating DNK is a promising avenue for further research.
Figure 6. The downregulation of Sirt1/miR-182 in the trigeminal ganglion of diabetic type 2 db/db mice.
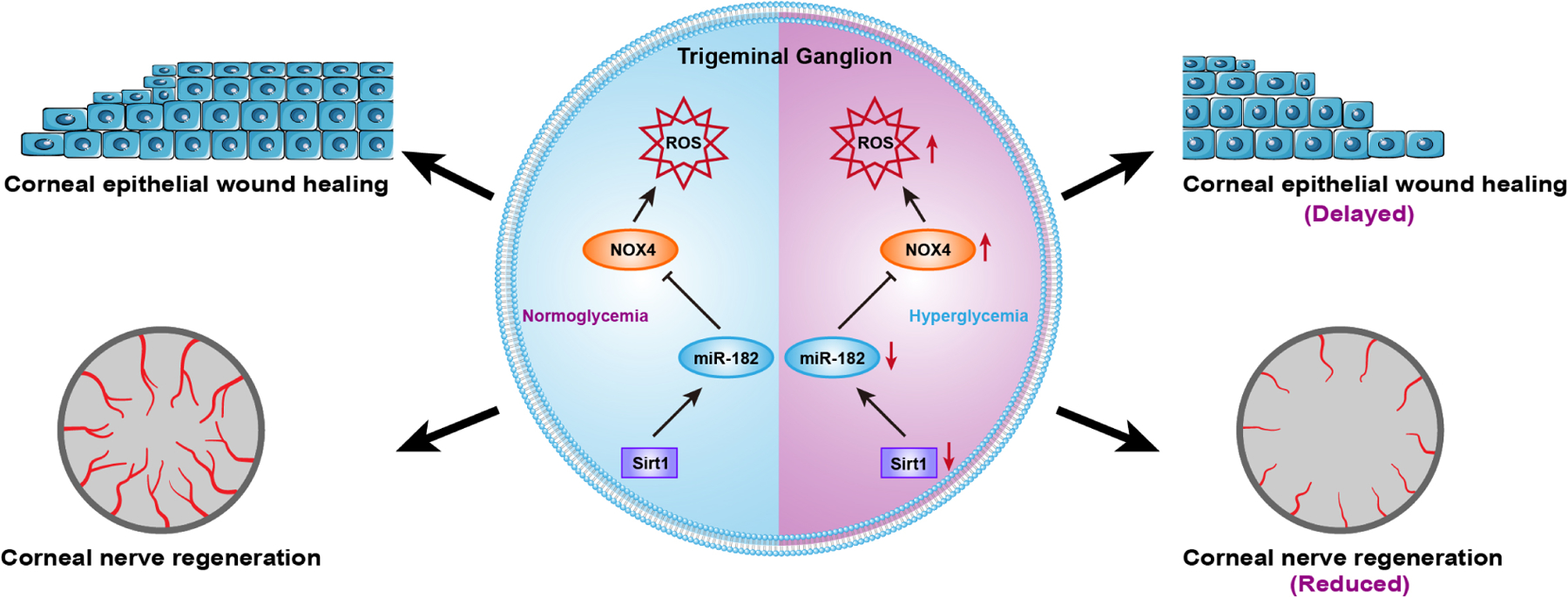
In diabetic trigeminal ganglion, the normal expression of sirt1 was impaired, resulting in inhibiting expression of its downstream miRNA miR-182, causing excess expression of the target gene NOX4 of miR-182, thereby induing an overexpression of ROS. These changes eventually lead to the delay of corneal epithelial wound healing and corneal nerve regeneration. This diagram has not been published and was made by B Zhang.
Recently, a study from Dr. Xie using RNA-seq demonstrated that the expression of several micro-RNAs including miR-350-5p and miR-592-5 was altered in the trigeminal nerve during diabetic corneal neuropathy (Zhang et al., 2020b).
3. DIABETIC NEUROPATHY IN THE CORNEA
3.1. Sensitivity and Neuropathy
Corneal nerves, which are branches of the ophthalmic division of the TG nerve, enter the peripheral cornea at the level of the mid-stroma and run in a radial fashion. As they run superficially, they then penetrate Bowman’s layer to form the corneal sub-basal nerve plexus before terminating as free nerve endings within the corneal epithelium (Muller et al., 2003). In humans, these nerves are either the larger diameter and myelinated Aδ fibers (high threshold to gentle stimuli), which transmit mechanical stimuli, or the smaller nonmyelinated C fibers (low threshold to gentle stimuli) that transmit thermal and chemical stimuli (Muller et al., 1997).
Corneal sensitivity declines with age (Millodot and Owens, 1984), as well as with other pathologies and factors such as herpes simplex keratitis, myasthenia gravis, chemical or drug exposure, contact lens usage (Murphy et al., 2001), and refractive surgery (Kohlhaas, 1998; Martin and Safran, 1988). It has been well-understood that hyperglycemia is a major cause of TG nerve damage and decreased sensitivity in patients (Nielsen, 1978; Schwartz, 1974), and impairment in corneal sensitivity has been identified in nearly 20% of diabetic patients (Mansoor et al., 2020; Neira-Zalentein et al., 2011). Rapid in-office evaluation of corneal sensitivity is critical for both diagnosing and assessing the severity of diabetic corneal neuropathy in these patients. Corneal esthesiometers have been used widely in clinics for the quantitative assessment of sensitivity (Mansoor et al., 2020). The Cochet-Bonnet esthesiometer is a commonly used tactile measuring device, which is composed of a retractable nylon monofilament with a fixed diameter. The length of the monofilament can be extended outwards from the pen-shaped device to a distance from 0 to 6 cm, and in doing so, the pressure exerted by the monofilament on the corneal surface is changed. The length of the monofilament that triggers the blink response is an indicator of the corneal sensitivity (Sacchetti and Lambiase, 2014; Sitompul, 2017). Though relatively inexpensive and portable, this device is variable, may cause apprehension for patients, and has the potential to cause trauma to the cornea. To address this, noncontact esthesiometers that use pulses of air have been devised, though these are more expensive and less portable (Efron, 2012). Corneal sensitivity has been proposed as a potential marker of DPN, with increasing loss of corneal sensitivity or sensation correlated to severity of DPN (Pritchard et al., 2012a; Tavakoli et al., 2013b).
To understand the overall role of sensory nerves in the cornea, local destruction of corneal sensory nerves was achieved by either surgical TG denervation (Gao et al., 2016b) or topical resiniferatoxin (Zhang et al., 2020a). Resiniferatoxin is a naturally derived, ultrapotent capsaicin analog that activates the vanilloid receptor (TRPV1) in a subpopulation of primary afferent sensory neurons involved in nociception (Szallasi, 1995). TG denervation via topical resiniferatoxin resulted in a significant decrease in corneal sensitivity and a reduced density of nerve endings at the center of the cornea. Moreover, local depletion resulted in epithelial defects with or without total tarsorrhaphy, decreased tear secretion, and loss of dendriform DCs at the ocular surface (Gao et al., 2016b). Temporary local corneal denervation by topical resiniferatoxin resulted in decreases in the density and length of sensory nerve endings and in epithelial healing rate (Zhang et al., 2020a). Functional deficiencies of resiniferatoxin-denervated corneas may be partially restored by topical application of neuropeptides SP, CGRP, as well as VIP (Zhang et al., 2020a), suggesting that sensory nerves participate in corneal homeostasis and wound healing by releasing neuropeptides, which target nearby epithelial cells and intraepithelial DCs.
Nicotinamide adenine dinucleotide (NAD) is an essential biological molecule in biological processes and diseases, including aging, cardiomyopathy, metabolic disorders, and neurodegeneration (Katsyuba and Auwerx, 2017). Corneal innervation contributes to the epithelial homeostasis by regulating NAD+ biosynthesis. The epithelial NAD+ content is decreased in neurotrophic keratopathy mice, while replenishment of NAD or nicotinamide mononucleotide (NMN) partially reverses the corneal nerve fiber degeneration and epithelial defect (Li et al., 2019b). In diabetic rats and mice, the NAD+ levels were significantly decreased in multiple organs and cells, such as the pancreatic islets, bone marrow, and bone marrow-derived endothelial progenitor cells. It was found that NMN could restore NAD+ levels by regulating hepatic insulin sensitivity, glucose tolerance and lipid profiles (Yoshino et al., 2011). In the cornea, the epithelial NAD+ content is decreased in T1DM mice and T2DM patients, which impairs epithelial wound healing. However, NAD+, its precursors NMN and nicotinamide riboside ameliorate the delayed corneal wound healing and nerve regeneration by restoring the expression of SIRT1, the nicotinamide-adenine dinucleotide (NAD)-dependent-deacetylase enzyme (Li et al., 2021).
Although corneal denervation either through tarsorrhaphy (Gao et al., 2016b) or by topical resiniferatoxin (Zhang et al., 2020a) results in severely impaired corneal wound healing, the causal relationship between diabetic neuropathy and DNK remains inconclusive. As non-neural mechanisms, such as depletion of DCs (Gao et al., 2011) and microphages (Koh and DiPietro, 2011; Li et al., 2013), can lead to poor epithelial migration and adhesion, it is unclear if diabetic neuropathy is the aggravating mechanism or the instigating driver. However, a recent human studies revealed that topical insulin (Tong et al., 2020) or 50% autologous serum eye drops (Semeraro et al., 2014a) may benefit patients with neurotrophic corneal ulcers of heterogeneous etiologies, other than diabetic neuropathy, suggesting more complicated pathologies in DNK than other types of neurotrophic keratopathy. Moreover, Two clinical cohort studies have concluded that once DM (type 1 and type 2) was established, good glycemic control was able to improve but not completely reverse corneal neuropathy (Shih et al., 2017; Yorek et al., 2015; Yorek et al., 2014), suggesting some aspects of diabetic neuropathy may not be reversible. Early detection and treatment of diabetic corneal neuropathy are critical in preventing diabetic corneal ulceration and blindness at later stages of DM progression.
3.2. In vivo Detection of Neuropathy
The cornea is unique, as nerve fibers can be examined and quantitated in the transparent tissue by in vivo corneal IVCM (Roszkowska et al., 2021; Tervo et al., 2002). IVCM is a noninvasive scanning modality providing detailed information on the corneal nerve plexus, and has become a valuable tool and gold standard for ophthalmologists to evaluate the status of corneal nerve fibers (Mansoor et al., 2020; Roszkowska et al., 2021). In recent years, IVCM has gained increasing importance in studying morphological changes of corneal nerves, due to its ability to resolve fine details in the tissue (Jalbert et al., 2003; Markoulli et al., 2018). Images can be analyzed to obtain various measures of the corneal nerve plexus, including nerve fiber density, nerve branch density, nerve fiber length, and tortuosity (Liu et al., 2021). While limitations of IVCM exist, including cost, availability, and a limited resolution and size of the images generated (though improved technologies) (Bondugulapati, 2020), IVCM continues to show promise in improving patient care. Figure 7 shows diabetic neuropathy in the corneas of T1D and T2DM patients.
Figure 7. In vivo corneal confocal microscopy of sensory fibers in human normal and T1DM and T2DM corneas.
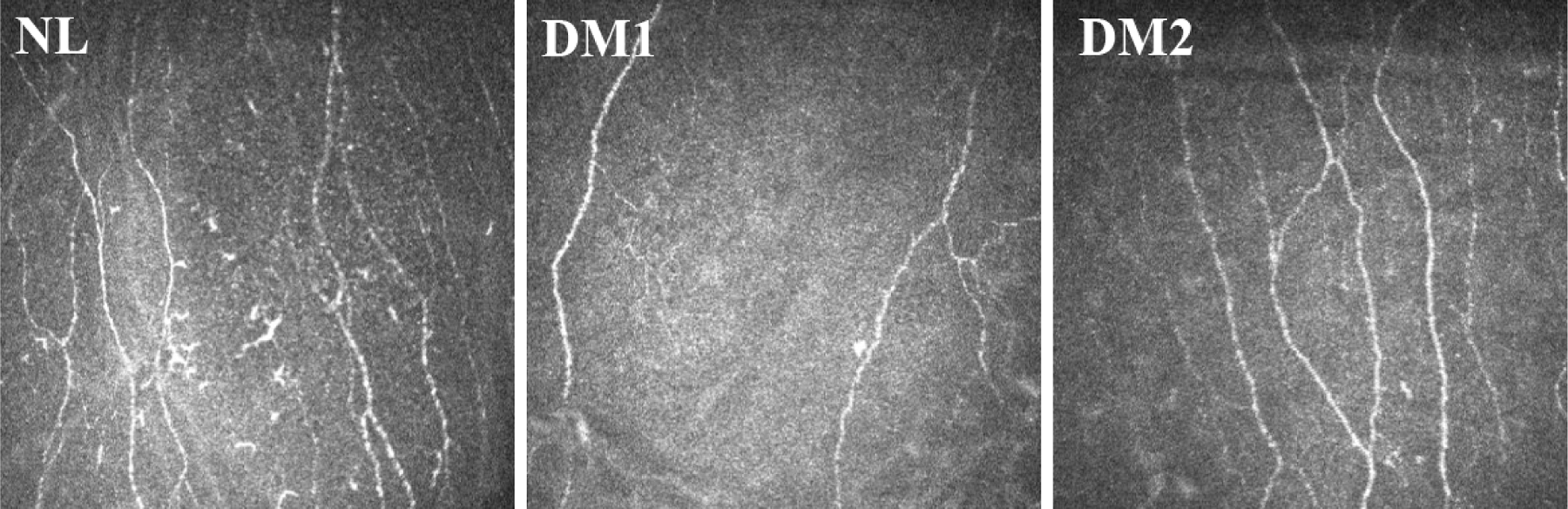
In vivo confocal microscopy images of the cornea in a healthy control subject (NL), an age-matched patient with type 1 (T1DM), and an age-matched patient with type 2 (T2DM) diabetes. The images were taken at the Shandong Eye Institute clinic. The images were taken at the Qingdao Eye Hospital of Shandong First Medical University clinic.
Petropoulos et al. (Petropoulos et al., 2013) reported that corneal nerve fiber density, branch density and nerve fiber length were significantly different in both T1DM and T2DM patients relative to controls, and these corneal nerve indices deteriorated with increasing severity of DPN. In addition, corneal nerve branch density and fiber length determined by IVCM were decreased in diabetic patients with mild DPN compared with diabetic patients without DPN. Hence, IVCM measurements including nerve fiber density, branch density, nerve fiber length can be used as noninvasive indicators for DPN evaluation (Cruzat et al., 2017). Using IVCM, Ferdousi et al identified more severe corneal nerve loss in patients with T1DM compared with T2DM and demonstrated its diagnostic accuracy for DPN (Ferdousi et al., 2021). Moreover, Lewis et al showed that in 17% of diabetic patients, an abnormally rapid annual loss in corneal nerve fiber length of at least 6% occurs. These patients also have the highest risk for the development and progression of diabetic distal symmetric polyneuropathy (Lewis et al., 2020). Hence, the noninvasive measurement by IVCM of corneal nerve fibers may also spare the patient from undergoing invasive measures to assess DPN elsewhere in the body. Figure 7 shows images of sensory nerves of human nondiabetic and diabetic corneas detected by IVCM, illustrating diabetic neuropathy in both T1DM and T2DM patients. The detectable decrease in sensory nerves suggests a corresponding decrease in neuropeptides in DM corneas. Recently, IVCM has been used to quantify the density of DCs in relation to corneal nerve morphology and the presence of diabetic neuropathy in T1DM and T2DM. An increase in DC density was reported to be correlated with corneal nerve loss in patients with T1DM patients (D’Onofrio et al., 2021).
In addition to IVCM, anterior segment optical coherence tomography (AS-OCT) has been evolved over the years, and hence a detailed evaluation of anterior segment (AS) structures such as corneas has been possible in a noncontact and safe procedure.(Sridhar and Martin, 2018). Diabetic patients have significantly thicker central corneal thickness regardless of retinopathy status whereas anterior chamber width was significantly narrower in DM with non-proliferative diabetic retinopathy group compared to DM with no diabetic retinopathy (Suraida et al., 2018). A 2020 ARVO Annual Meeting Abstract suggests that IVCM, but not OCT, allows the detection of progressive worsening of neuropathy in T1DM (Akil et al., 2020).
3.3. Changes in Neuropeptide Secretion in Diabetic Corneas.
Corneal nerves maintain the integrity and homeostasis of the cornea by interacting with resident cells including the epithelium, keratocytes, endothelium, DCs and macrophages (Gao et al., 2016a; Maruyama et al., 2007). Communication is mediated through the release of neuropeptides including Substance P (SP) (Suvas, 2017; Twardy et al., 2011; Yang et al., 2014) Calcitonin Gene-Related Peptide (CGRP) (Mikulec and Tanelian, 1996; Tran et al., 2000), Vasoactive Intestinal Peptide (VIP) (Jiang et al., 2012), and Neuropeptide Y (Ekstrand et al., 2003). SP and CGRP are the most well-studied neuropeptides in corneal sensory nerve fibers (He and Bazan, 2016) (Figure 8). The neurotransmitter and neuromodulator SP, derived from sensory C-fibers, mediates a pro-inflammatory response (Wu et al., 2007). In contrast, CGRP is considered an anti-inflammatory neuropeptide (Holzmann, 2013a). Both TG and dorsal root ganglia in DM rat models express decreased levels of SP and CGRP (Li et al., 2017; Troger et al., 1999).
Figure 8. Co-staining of CGRP and SP with β-tubulin-3.
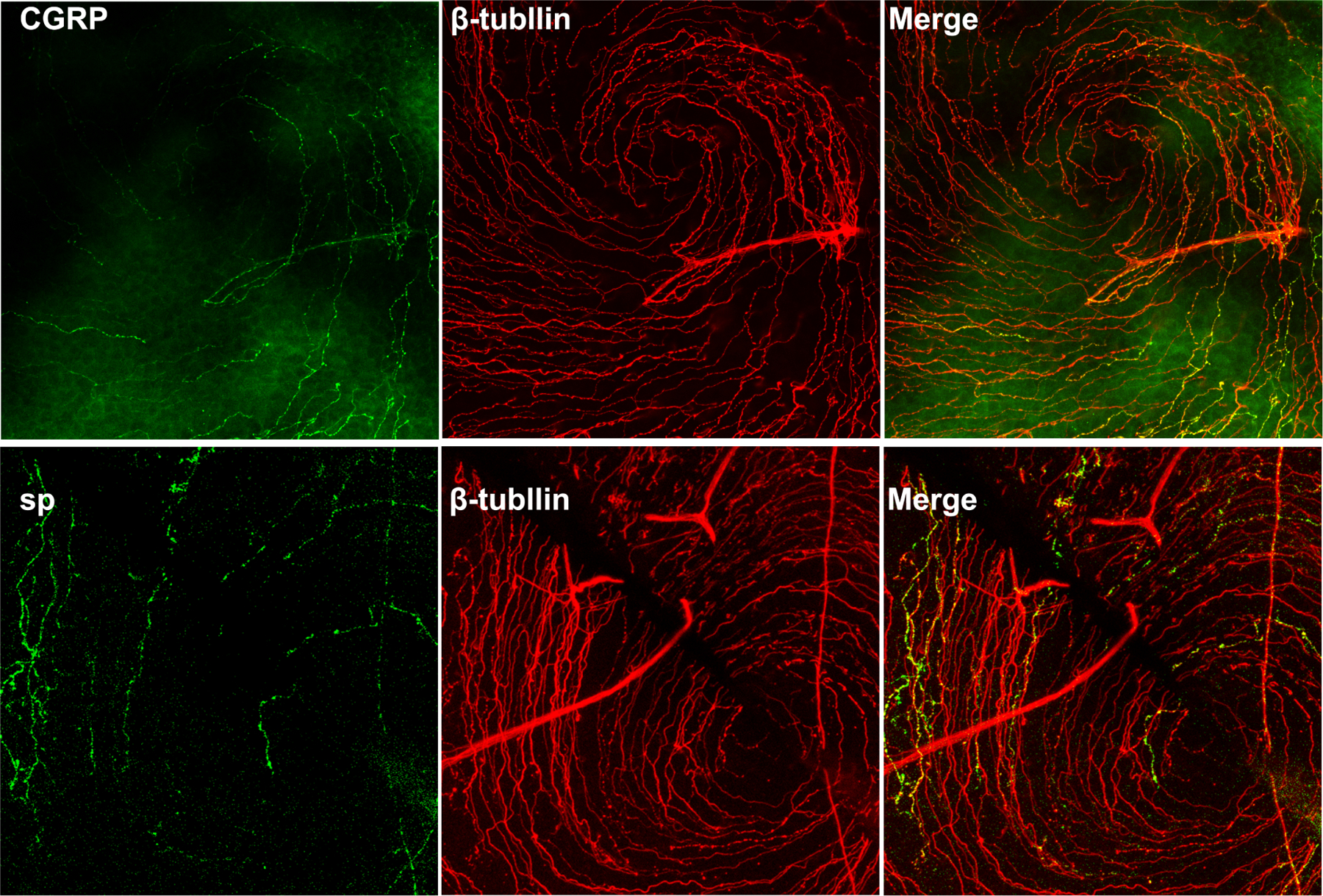
B6 mouse corneas were staining with CGRP/Tubulin3 or SP/Tubulin-3 and the center of the corneas were photographed and the images of sensory nerve ending and nerve expressing CGRP or SP were merged. Note only small portion of sensory nerve endings express CGRP or SP. Unpublished results (Gao and Yu).
SP has been found to be expressed in corneal nerves, epithelium and keratocytes and appears to mobilize bone marrow-derived stem cells to participate in corneal wound healing (Li and Zhao, 2014; Yang et al., 2014). SP has proinflammatory effects in immune and epithelial cells and participates in inflammatory diseases of the respiratory, gastrointestinal, musculoskeletal systems, and cornea (Scott et al., 2008; Suvas, 2017; Wu et al., 2007).Diabetic rats showed significant increases of infarct size and myocyte apoptosis after acute myocardial ischemia/reperfusion, compared to non-diabetic controls (Li et al., 2017). The diabetic rats had a significant elevation of noxious thermal thresholds, with obvious reduction of the contents of SP and CGRP in the dorsal root ganglion and myocardium. Furthermore, exogenous CGRP and SP attenuated glucose and hypoxic/reoxygenation induced myocyte injury (Li et al., 2017). CGRP and SP are also known to play a critical role in corneal homeostasis and its response to injury.(Cortina et al., 2012; Jones and Marfurt, 1991; Yang et al., 2014).
Early studies revealed that SP, in combination with insulin-like growth factor −1 (IGF-1), promotes the migration and attachment of corneal epithelial cells to the extracellular matrix. Binding of NK-1R stimulates the expression of integrins α5 and β1, and the phosphorylation of focal adhesion kinase, paxillin, and P38 MAP kinase (Nakamura et al., 1997; Nishida et al., 1996). Our study revealed the hyperglycemia-suppressed expression of SP in both nonwounded and wounded corneas of diabetic mice (Yang et al., 2014). This suggests that the lack of SP might be an underlying mechanism for the delayed wound healing in diabetic corneas. To investigate the protective mechanism of SP against hyperglycemia-induced corneal defects, STZ diabetic mice were used. Hyperglycemia was found to delay corneal epithelial wound healing, accompanied by attenuated corneal sensation, mitochondrial dysfunction, and impairments of Akt, epidermal growth factor receptor (EGFR), and Sirt1 signal transduction. Exogenous SP treatment promoted corneal epithelial wound healing and restored corneal sensitivity, as well as increased ROS scavenging capacity. The effects of SP on diabetic corneal epithelial healing were completely abolished by a neurokinin-1 (NK-1) receptor antagonist. Moreover, the subconjunctival injection of NK-1 receptor antagonist also caused pathological changes that resembled those of DNK in normal mice. These results suggest that SP ameliorates the delayed wound healing of diabetic corneas by rescuing activation of Akt, EGFR, and Sirt1, improving mitochondrial function, and increasing ROS scavenging capacity, and has therapeutic potential for treating DNK (Yang et al., 2014).
CGRP is also released by sensory nerves. It directly acts on innate immune cells such as macrophages and DCs and inhibits the capacity of these cells to produce inflammatory cytokines (Holzmann, 2013a, b). CGRP decreases inflammation through several distinct pathways, including increasing IL-10 production, induction of the inducible cAMP early repressor, and inhibiting NF-kB activity (Holzmann, 2013a). The number of CGRP-positive neurons in dorsal root ganglia was significantly reduced in T1DM rats (Adeghate et al., 2006). Similarly, the number of CGRP-positive nerves was correlated with more units of insulin given to the animals. Similar findings were reported by others as well (Baum et al., 2021). However, the involvement of CGRP in diabetic keratopathy, and whether a similar change is seen in the cornea, remains to be investigated.
Interestingly, in contrast to SP and CGRP, which are found in sensory nerves, VIP appears to be expressed predominantly in autonomic nerves, which modulate inflammation and epithelial renewal through the activation of distinct local macrophages (Xue et al., 2018). While autonomic innervation is present in mammalian corneas, it is to a lesser extent than that of sensory fibers. The degree of coverage is variable between species; 10–15% of the innervation to rabbit corneas consists of sympathetic fibers, while a much smaller proportion innervates the human cornea (Marfurt et al., 1989).
In our resiniferatoxin-denervated mouse corneal model, VIP was found to be the most effective (compared to SP or CGRP) in restoring corneal nerve function and in inducing expression of the anti-inflammatory cytokine IL-10 (Zhang et al., 2020a). Wounding induced VIP and VIP receptor upregulation, and these effects were suppressed in DM corneas. In the NL corneas, the blockade of VIP Receptor (VIPR)-1 resulted in a decrease in the healing rate, compared with untreated eyes. Administration of bioactive recombinant VIP accelerated wound healing and increased sensory regeneration in the DM corneas compared to the untreated DM corneas. Presence of VIP1R antagonist downregulated the expression of the neurotrophic factors NGF and CNTF in NL healing corneas whereas exogenous VIP partially reversed the suppressing effects of diabetes in B6 mice (Figure 9). The upregulation of the pro-inflammatory cytokines IL-1β and CXCL2 was augmented by VIP1R antagonist in NL corneas, but was significantly attenuated by exogenous VIP in diabetic corneas. Anti-inflammatory cytokines, IL-1Ra, IL-10, as well as CXCL5, on the other hand, exhibited an opposing pattern of expression compared to that of pro-inflammatory cytokines: VIP1R antagonist suppressed in NL corneas, while VIP enhanced their expressions in diabetic corneas. These results suggest the anti-inflammatory nature of VIP and a positive feedback loop of neuropeptides and neurotrophic factors in maintenance of normal corneas, which is disturbed by hyperglycemia (Zhang et al., 2020a).
Figure 9. VIP accelerates diabetic wound healing and nerve regeneration in healing corneas through VIPR1.
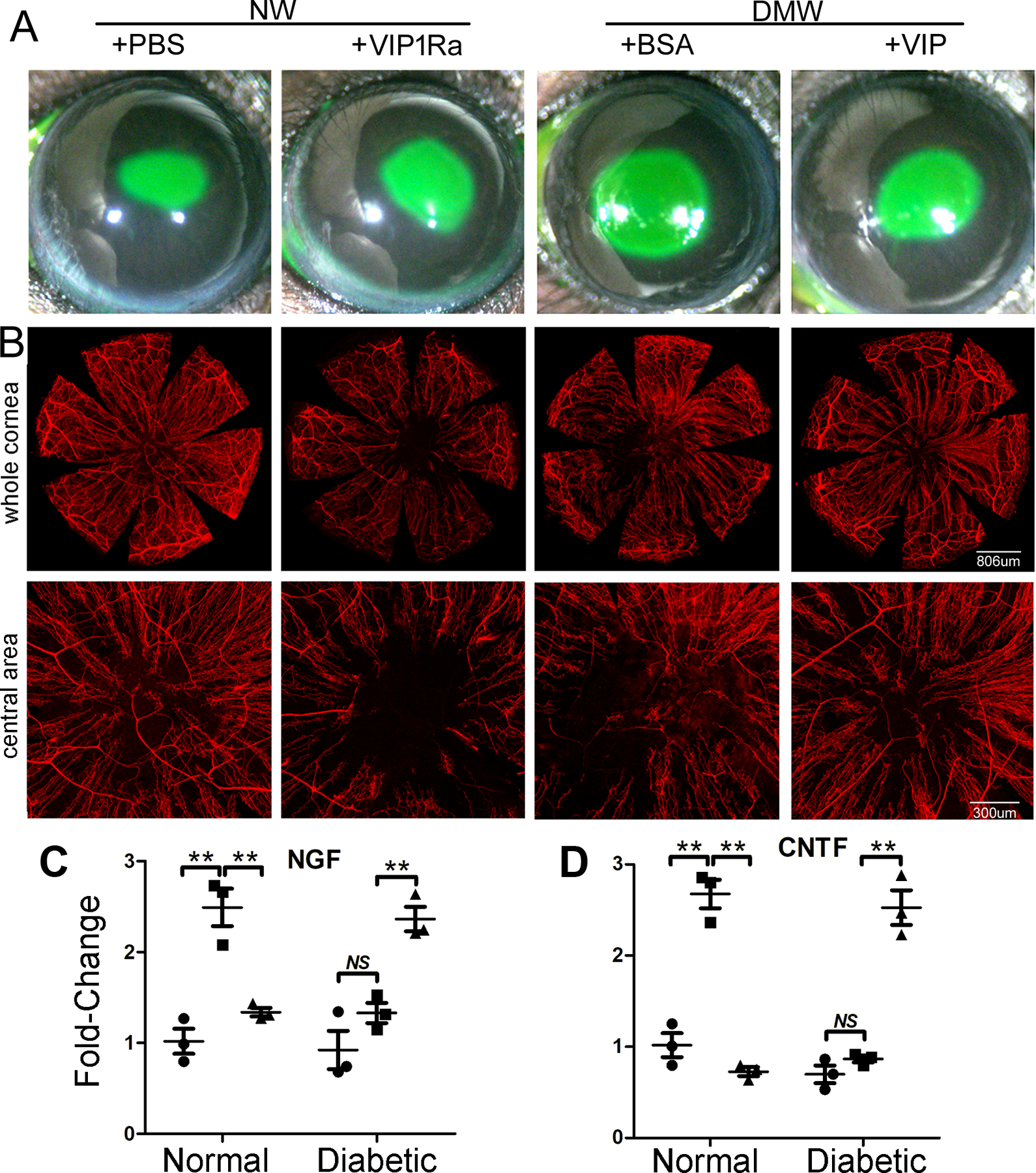
(A) NL corneas were pretreated with VIPR1 antagonist or PBS and diabetic corneas with recombinant VIP or BSA as the control 4h prior to epithelial debridement. At 0h, the corneas were wounded by epithelium-debridement (2 mm diameter). At 22 hpw, the remaining wounds were stained with fluorescein and photographed. The wound sizes were calculated and presented as percent of healed area over the size of original wounds. (B) Another set of mice were allowed to heal for 3 days and the corneas were processed for WMCM with beta-tubulin III staining for nerve fibers and endings. The images of whole corneas (upper panels) and high-magnification images of central area (bottom panels) were shown. The Figure shows that VIP regulates epithelial wound healing and nerve regeneration in the corneas, suggesting a therapeutic potential for these molecules in treating diabetic keratopathy. (C) The expression of NGF in wounded, with unwounded (●) as the control, NL or DM corneas treated with (▲) or without VIP antagonist (■). (D) The expression of CNTF in wounded, with unwounded (●) as the control, NL or DM corneas treated with (▲) or without VIP antagonist (■). VIP antagonist suppresses and exogenous VIP promotes wound-induced NGF and CNTF expression in NL and DM corneas, respectively. This figure was originally published in (Zhang et al., 2020a).
Neuropeptides also play a vital role in lacrimal gland functioning. In addition to maintaining hydration of the ocular surface, the lacrimal gland produces several antimicrobial proteins (McDermott, 2013) and growth factors (Klenkler et al., 2007) crucial in establishing a healthy cornea. Stimulation of afferent corneal sensory fibers results in the activation of efferent autonomic fibers that innervate the gland, forming a reflex arc. These efferent fibers produce several neuropeptides, including VIP, SP, and CGRP, which influence the secretions produced by the gland (Dartt, 1989; Walcott, 1998). Given the systemic effects of DM on sensory and autonomic fibers throughout the body, we expect that hyperglycemia results in neural dysfunction of the lacrimal gland. Indeed, the tear film is decreased and unstable in diabetic patients (Eissa et al., 2016; Goebbels, 2000). While the DM-induced loss of afferent fibers is a significant contributor, little is known about the direct effects of DM on the efferent fibers, and how the resulting neuropeptide abnormalities contributes to DNK. Further investigation into this subject will likely yield novel insights.
3.4. Changes in Neurotrophic Factors in Diabetic Corneas
Neuropeptides produced by corneal sensory nerves establish a homeostatic environment through acting on neighboring cells (Markoulli et al., 2020). In turn, corneal epithelial cells secrete neurotrophins, neurotrophic and growth factors that accelerate the outgrowth and survival of corneal nerves (Di et al., 2017a; Sacchetti and Lambiase, 2017). Neurotrophic factors found in the cornea and their role in the modulation of the wound healing response are listed in Table 1. Epithelial debridement results in the loss of sensory nerve endings and the retraction of nerve fibers in the stroma (Yu et al., 2015). Healing epithelia secrete neuroprotective factors such as nerve growth factor (NGF) and ciliary neurotrophic factor (CNTF), and Mesencephalic astrocyte-derived neurotrophic factor (Wang et al., 2020), as well as axon guidance molecules such as the Sema3 family of proteins (Lee et al., 2019). . Our labs have shown that NGF is upregulated in response to epithelial wounding in NL but not DM corneas at the mRNA levels (Di et al., 2017a; Gao et al., 2016c).
Table 1.
Summary of neurotrophic factors in the diabetic cornea
| Name | Secretion | Functions | References |
|---|---|---|---|
| SP | Nerves | (1) Accelerate epithelial wound healing. (2) Recovery of corneal sensation. (3) Improve the mitochondrial function. (4) Activate of Akt, EGFR, and Sirt1 through NK-1R. |
(Yang et al., 2014) |
| VIP | Nerves | (1) Stimulate corneal wound healing. (2) Promote nerve regeneration. (3) Regulate the wounding inflammatory response. (4) Activate the Sonic Hedgehog signaling pathway. |
(Zhang et al., 2020a) |
| MANF | CEC | (1) Stimulate corneal wound healing. (2) Promote nerve regeneration. (3) Inhibit ER stress and apoptosis. (4) Activate AKT signaling pathway. |
(Wang et al., 2020) |
| IGF-1 | CEC | (1) Promote the expression of stem cell markers. (2) Increase corneal subbasal nerve density. |
(Ueno et al., 2014) |
| NGF | Nerves, CEC | (1) Promote neurite outgrowth and nerve regeneration. (2) Stimulate corneal epithelial wound healing. |
(Di et al., 2017a) |
| GDNF | Nerves and CEC | (1) Promote neurite outgrowth and nerve regeneration. (2) Induce corneal epithelial regeneration. |
(Di et al., 2017a) |
| PEDF | Nerves and CEC | (1) Increase nerve regeneration. (2) Elevate corneal sensitivity and tear production. (3) Accelerate corneal wound healing, selectively recruit type 2 macrophages, and prevent neutrophil infiltration. |
(He et al., 2017) |
| VEGF-B | CEC | (1) Promote nerve regeneration. (2) Activate PI-3K/Akt-Gsk3ß-mTOR signaling pathway. (3) Elevate the corneal content of PEDF. |
(Di et al., 2017b) |
| CNTF | Nerves and Immune cells | (1) Accelerate corneal wound healing. (2) Promote nerve regeneration. (3) Stimulate the mitosis of epithelial stem/progenitor cells. |
(Zhou et al., 2015) |
| EGF | CEC | (1) Stimulate corneal wound closure. (2) Activate Akt signaling pathway. |
(Xu et al., 2009) |
| Netrin-1 | Nerves | (1) Promote corneal wound healing. (2) Reactivate the phosphorylation of ERK and EGFR signaling. (3) Decrease inflammation (4) Promote M2 macrophage transition. |
(Zhang et al., 2018) |
| HGF | CEC | (1) HGF receptor c-met accelerates corneal wound healing. (2) Reverse alterations the epithelial stem cell marker patterns |
(Saghizadeh et al., 2005) |
| Il-22 | Immune cells | (1) Promote epithelial cell regeneration (2) Control tissue Inflammation through IL-10 expression |
(Gao and Xiang, 2019) |
SP: substance P; VIP: Vasoactive intestinal peptide; MANF: Mesencephalic Astrocyte Derived Neurotrophic Factor; IGF-1: Insulin-like growth factor-1; NGF: Nerve growth factor; GDNF: Glial Cell Derived Neurotrophic Factor; PEDF: Pigment epithelium-derived factor; VEGF-BVascular endothelial growth factor B; CNTF: Ciliary Neurotrophic Factor; EGF: Epidermal Growth Factor; HGF: hepatocyte growth factor; IL-22: interleukin-22.
NGF is the prototypical neurotrophic/growth factor and is thought to mediate the deficits in diabetic neuropathy. NGF is required for the maintenance, survival, and regeneration of peripheral sensory nerves (Pittenger and Vinik, 2003). A study in human patients found that NGF production by keratinocytes is decreased with DM; these changes are correlated with sensory dysfunction, suggesting that decrease in epithelial NGF is partly responsible for the deficits in DPN (Anand et al., 1996). In the cornea, NGF production is upregulated in response to insults such as wounding, surgery (Chaudhary et al., 2012; Pan et al., 2018), dry eye disease (Lee et al., 2006), or inflammation (Lambiase et al., 1995). Di et al showed that NGF, alongside Glial Cell-Derived Neurotrophic Factor (GDNF), in the conditioned media of cultured corneal epithelial cells was responsible for promoting TG neurite growth. They also identified that the wound-induced upregulation of NGF is suppressed in vivo in diabetic mice, suggesting that this abnormal expression is a contributing factor for DNK. Indeed, Cenegermin (Oxervate™), an ophthalmic solution containing recombinant human NGF was recently FDA-approved for treating neurotrophic keratopathy, although not specifically DNK (Pflugfelder et al., 2020).
Another neurotrophic factor that appears to play a role in DNK is ciliary neurotrophic factor (CNTF). Our study using STZ mice showed that DCs are the major sources of CNTF in the cornea and a decrease in the DC population in DM corneas during wound healing resulted in the reduction of tissue levels of CNTF, a potential underlying cause of diabetic corneal neuropathy (Figure 10) (Gao et al., 2016c). While CNTF neutralization retards reinnervation in normal corneas, exogenous CNTF accelerated nerve regeneration in wounded corneas of diabetic mice. As CNTF is produced by DCs in the cornea, it plays a potential role in mediating neuroimmune crosstalk. Hence, decreases in numbers of resident and infiltrating DCs in nonwounded and healing corneas of diabetic mice may be intertwined with defects in sensory nerve regeneration. Moreover, soluble CNTFRα can restore sensory nerve integrity in unwounded diabetic corneas and reverse the impaired sensory nerve regeneration in wounded DM corneas (Gao et al., 2016c). Human and mouse CNTF signaling requires the formation of CNTF-CNTFRα dimers before the formation of the CNTFRα/p130/LIFRβ tripartite signaling complex (Pasquin et al., 2015). In addition to CNTFRa, CNTF may also use IL-6 receptor-α as a substitute for its cognate alpha-receptor (Schuster et al., 2003). The CNTF-induced formation of IL-6Ra/gp130/LIFRβ signaling complex may be inflammatory and contribute to the side effects observed in patients upon systemic administration of CNTF (Pasquin et al., 2015; Schuster et al., 2003). Hence, therapy using exogenous soluble CNTFRα may be more preferable than that using CNTF as soluble can increase CNTF signaling efficiency in repairing impaired sensory nerve regeneration in diabetic corneas without undesired side effects of CNTF-IL-6Rα/gp130/LIFRβ signaling complex. Soluble CNTFRα has been used in treating neuron degenerative diseases such as DPN (Saleh et al., 2013), diabetic retinopathy (Skundric and Lisak, 2003), and age-related macular degeneration (Leung and Landa, 2013; Rhee et al., 2013; Zhang et al., 2011). While neurotrophic factors are important for nourishing sensory nerve axons in homeostasis and support neuron regeneration in injured tissues, re-innovation requires the collaborative action of a large group of genes collectively termed axon guidance molecules that can be subdivided into attractive and repulsive cues (Stoeckli, 2018).
Figure 10. CNTF expression and co-localization with CD11c-positive cells in the normal and diabetic corneas with or without epithelium debridement.
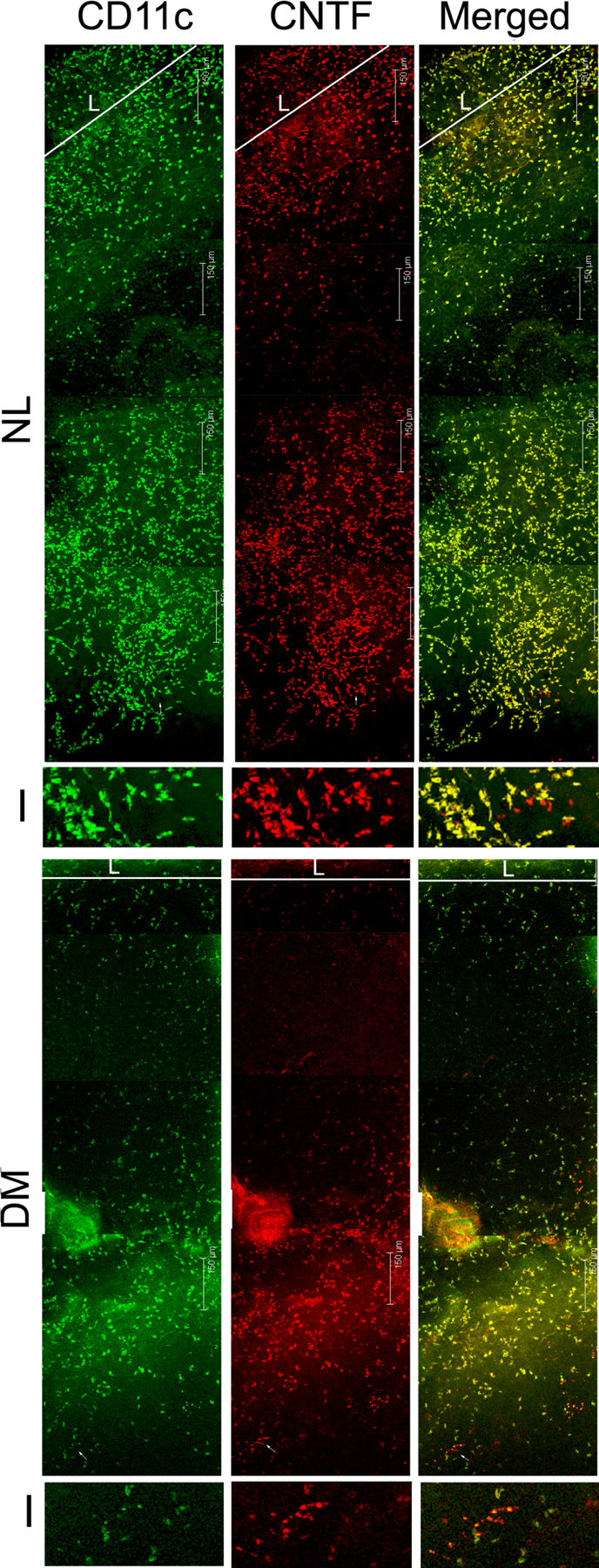
Whole mount confocal microscopy showing DC and CNTF co-localization in healing corneas of normal and diabetic mice. I: 3.8x magnification of the images showing CD11c-negative, CNTF-positive cells; L: limbal region. Note the shorter distance between limbal region of leading edge and significantly less numbers of CD11c and CNTF positive cells in DM versus NL corneas. This figure was originally published in (Gao et al., 2016c).
3.5. Axon Guidance Molecules and Semaphorin-3 Signaling
In the embryo, axonal pathfinding is regulated by several families of neuronal guidance molecules consisting of Netrins, Slits, Ephrins, and Semaphorins (Giger et al., 2010). Although a great deal is known about extracellular molecules and signaling pathways that regulate axonal pathfinding in the embryo, relatively little is known regarding the mechanisms underlying the regenerating nerves in the adult. Netrin-1 was found to promote corneal epithelial wound healing and regeneration of nerve fibers in diabetic mice (Zhang et al., 2018). Eph receptors and multiple ephrins were found within the human cornea and limbus and may play multiple potential roles in the maintenance of normal corneal architecture (Hogerheyde et al., 2013).
In our genome-wide cDNA array study (Bettahi et al., 2014b), we identified that Semaphorin 3C (SEMA3C) was upregulated by epithelial cells in response to wounding, and that this upregulation was suppressed in diabetic corneas. The semaphorins are a large family of guidance cues that were originally found to allow axons to target specific locations of the developing embryo by providing attractant or repulsive signals (Goshima et al., 2016; Mecollari et al., 2014; O’Malley et al., 2014). In contrast, SEMA3A expression is also induced by wounding, but is unaffected by diabetes. Suppression of Sema3C using siRNA subconjunctival injections resulted in delayed wound healing and nerve regeneration in nondiabetic corneas, mimicking the diabetic phenotype, while application of exogenous SEMA3C in diabetic corneas enhanced wound healing and nerve regeneration (Lee et al., 2019). This suggests that the wounded corneal epithelium can regulate wound healing and regeneration of injured axons through its expression of SEMA3C, and that diabetes-induced suppression of SEMA3C is partly responsible for the deficits in diabetic corneas.
While members of the Plexin receptor family bind the semaphorin ligands, the class 3 semaphorins also require the presence of either the neuropilin (NRP) −1 or −2 coreceptor for proper signaling. SEMA3A binds only NRP-1, while SEMA3C is thought to bind either NRP-1 or −2. In our studies, we demonstrated that corneal nerves express both isoforms. Intriguingly, NRP-2 expression in the epithelium mirrored that of SEMA3C and was induced with wounding but suppressed in diabetes. Functional studies utilizing neutralizing antibodies showed that blockade of NRP-2, like Sema3C-specific siRNA, resulted in delayed epithelial wound healing and nerve regeneration, suggesting that SEMA3C-NRP2 signaling is critical in mediating the wound healing process and is perturbed in diabetes. Unexpectedly, we observed that NRP1 expression in the epithelia was predominantly upregulated in the unwounded diabetic epithelium, relative to the unwounded nondiabetic and wounded nondiabetic/diabetic conditions. In contrast to NRP2, blockade of NRP1 signaling greatly accelerated both wound healing and nerve regeneration in diabetic corneas, suggesting a detrimental role in the wound healing process. Taken together, wounding in the normal cornea appears to result in an upregulation of NRP2 that may mediate additional SEMA3C signaling to direct the wound healing and nerve regeneration process. In the diabetic wounded cornea, however, a failure of NRP2 upregulation results in a relative abundance of NRP1 complexes. Ligands such as SEMA3C that are capable of binding to either of the NRP isoforms may therefore be shifted towards binding NRP-1 instead of NRP-2, resulting in the abnormal healing process observed in the diabetic cornea. Figure 11 illustrated the involvement and defects of these signaling molecules in NL versus DM corneas during epithelial wound healing. In addition to functioning as the receptors of SEMA3A and C, NRP-1 and 2 are known to be coreceptors for vascular endothelial growth factor (VEGF). As targeting VEGFR/VEGF has resulted in the development of anti-VEGF drugs for treating wet age-related macular degeneration and diabetic retinopathy, targeting NRP-1 and NRP-2 or their interactions with VEGFR is now a novel focus for the development of anti-angiogenesis drugs (Djordjevic and Driscoll, 2013; Peng et al., 2019).
Figure 11. Proposed Mechanism of Sema3-Neuropilin Signaling in the Diabetic and Nondiabetic Wounded Cornea.
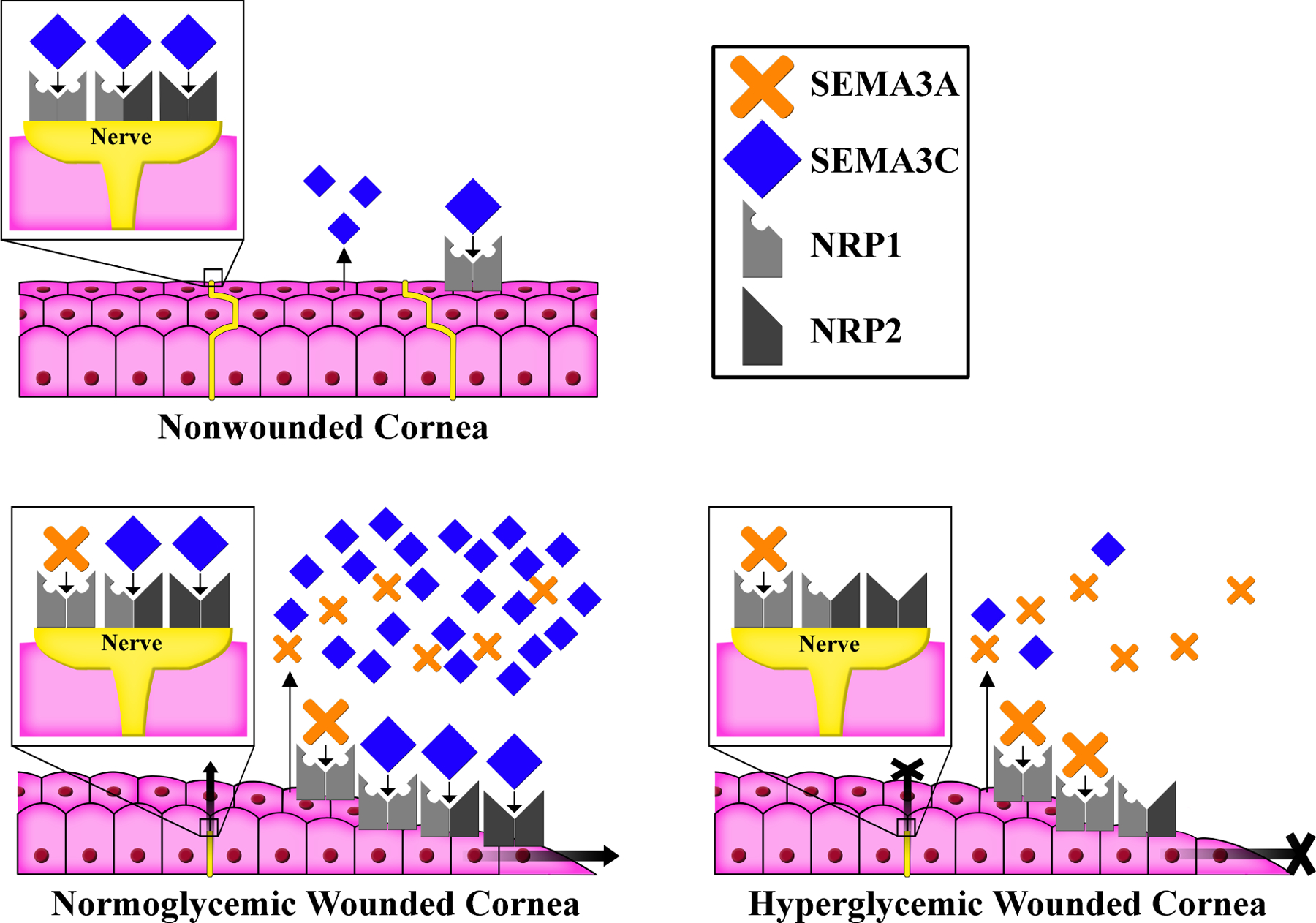
In the nonwounded cornea, low amounts of Semaphorin (Sema) 3C signal through neuropilin (NRP) 1 complexes of the epithelium and NRP1 and/or NRP2 complexes on sensory nerves. Corneal wounding induces expression of both SEMA3A and its preferred receptor, NRP1, as well as additional SEMA3C and its preferred receptor, NRP2. SEMA3C-NRP2 signaling is crucial for both epithelial wound healing and sensory nerve regeneration. In the diabetic cornea, reduced expression of both SEMA3C and NRP2 results in a relative excess of SEMA3A-NRP1 signaling (resulting in axon repulsion), compared to SEMA3C-NRP2 signaling. This imbalance leads to delayed epithelial wound healing and nerve regeneration. This original figure was made by P SY Lee.
3.6. Defects of Tear Secretion and Sensory Neuropathy
The tear film is the interface between the ocular surface epithelium and the environment, and consists of water, electrolytes, mucins, and an array of proteins and lipids (Pflugfelder and Stern, 2020). The tear film has three layers: an innermost mucin layer (from goblet cells) to lower the hydrophobicity of the epithelial cells; an intermediate aqueous layer (from lacrimal glands) to provide lubrication, nutrients, antimicrobial proteins and appropriate osmolarity; and an outermost lipid layer (from meibomian glands) to prevent evaporation of the aqueous layer (Holly and Lemp, 1977; Holly et al., 1977). The basic physiological functions of the tear film are: to protect the ocular surface from the influence of bacteria or other harmful substances; (2) to maintain the lubrication of the ocular surface and maintain the physiological functions of the cornea and conjunctiva. Thus, a stable preocular tear film is a hallmark of ocular health.
The lacrimal function unit (LFU) consists of the conjunctiva, cornea, and main and accessory lacrimal glands (Bron et al., 2017). Dysfunction of the LFU leads to tear instability, increased evaporation, inflammation, and blurred vision. The lacrimal gland, a major contributor to the tear film, is critical for maintaining the homeostasis of the ocular surface microenvironment through secretion of aqueous tears (Rocha et al., 2008). In a diabetic mouse model, lacrimal gland weight and tear film volume were lower compared to those of non-diabetic controls. Oxygen consumption rate and basal extracellular acidification rate detection results suggested that the early onset of diabetic dry eye may be due to the susceptibility of mitochondrial bioenergetic deficit in diabetic lacrimal gland, while application of mitochondria targeted antioxidant SKQ1 may ameliorate diabetic dry eye and keratopathy (Qu et al., 2021). Likewise, Misra et. al found that higher severity of neuropathy in T1DM patients was correlated with significantly poorer tear film quality - namely, reduced stability, thickness, and quantity (Misra et al., 2014). Tear secretions in diabetic patients were usually significantly lower than in the normal population (Cousen et al., 2007), and the osmolality of the tear film was significantly increased (Beckman, 2014). The lacrimal gland has also been found to be susceptible to AGE-mediated damage and disordered autonomic control secondary to DM (Nakata et al., 2014; Zhang et al., 2016).
The meibomian gland synthesizes and produces lipids and proteins which form the outermost layer of the tear film. Meibomian gland dysfunction (MGD) is a chronic disease with significant worsening of the symptoms in T2DM (GS et al., 2019; Watters et al., 2017), suggesting a high correlation between T2DM and MGD. Ding et al. demonstrated that insulin stimulates the proliferation of meibomian gland epithelial cells whereas high glucose was toxic to these cells (Ding et al., 2015). The recent studies showed the disruption of lipid homeostasis in T2DM patients (Yang et al., 2021) and in T1DM mice (Wang et al., 2021) with dry eye syndromes.
Secretion of tears is under tight neural control, including the afferent sensory nerves, and efferent parasympathetic and sympathetic nerves of the cornea and conjunctiva. Neuropeptides such as Substance P (SP), Calcitonin Gene-Related Peptide (CGRP), Vasoactive Intestinal Peptide (VIP), Neuropeptide Y (NPY), Acetylcholine (Ach), Norepinephrine (NE) are found in tears (Dartt, 2009). Mouse lacrimal and meibomian glands are innervated by parasympathetic (VIP), sympathetic (tyrosine hydroxylase (TH)), and sensory (CGRP) nerves (Cox and Nichols, 2014; Dartt, 2009).
Sensory nerves densely innervate the cornea and undergo early damage in diabetic patients with peripheral neuropathy (Coppey et al., 2012; Liu et al., 2011). The corneal sensory neurons express the thermal TRP channels, polymodal TRPV1 and TRPA1, osmotic and mechanosensitive TRPV4, and cold thermosensitive TRPM8 (Guerrero-Moreno et al., 2020). TRPV1 is a well-characterized channel expressed by a subset of peripheral sensory neurons, and canonically mediates inflammatory and neuropathic pain (Huang et al., 2020). Corneal TRPV1 is involved in the maintenance of the corneal structure, re-epithelialization, and inflammation upon corneal injury (Okada et al., 2015). TRPV1 was shown to play a significant role in mediating enhanced nociceptive behavior in dry eye, while TRPM8 may play a lesser role (Bereiter et al., 2018). Recently, TRPV1 activity and SP release were shown to be required for corneal cold nociception (Li et al., 2019a). A novel siRNA for TRPV1, tivanisiran, was shown to reduce ocular pain and to improve ocular hyperemia and tear quality in dry eye in human and animal models (Moreno-Montanes et al., 2018). Activation of the TRPV1 receptor was suggested to contribute to preferential stress in sensory neurons relatively early in diabetic sensory neuropathy (Hong et al., 2008).
TRPM8 and TRPA1 respond to cold, although cold-dependent activation of TRPA1 remains controversial (Schecterson et al., 2020). TRPM8 is widely expressed in corneal afferent fibers, and its deletion abolished cold responsiveness and reduced basal tearing and blinking frequency, indicating that TRPM8 cold receptor contributes to regulating tear secretion and osmolarity (Alcalde et al., 2018; Hirata and Oshinsky, 2012). DPN was associated with increased TRPV1 and decreased TRPM8 expression (Pabbidi and Premkumar, 2017). In a model of dry eye disease, the expression of cold-sensing fibers was enhanced, resulting in sensitization, cold allodynia, and increased release of SP (Li et al., 2019a). The investigation of the role of SP and CGRP in DNK revealed that the concentration of SP in tears was reduced in T1DM and this was associated with corneal neuropathy, but these abnormalities were not mirrored in T2DM patients or in the analysis of CGRP in tears (Tummanapalli et al., 2019).
4. Diabetic Immune Deficiency in the Corneas
4.1. Epithelial Effects on Immune Cell Infiltration and Activation
Epithelium debridement produces necrotic cells, leading to the release of cell contents including ATP, HMGB1, IL-1α, and alarmins (Yin et al., 2007). Epithelial-, but not bone marrow derived-HMGB1 is required for sterile inflammation following injury. Epithelial HMGB1, through its receptor RAGE (Ekanayaka et al., 2018) and Toll-like receptor-4 (Heim et al., 2018), triggers the expression/secretion of proinflammatory cytokines including IL-1β, IL-6, and IL-8, the recruitment of neutrophils, and death via necrosis (Huebener et al., 2015) Our in vitro study showed that one of the earliest signaling molecules release by injured epithelium is ATP (Yin et al., 2007) This ATP binds to its receptor P2X7 (Kurashima et al., 2012) and mediates tissue inflammation (Kurashima et al., 2012). In a diet-induced obesity (DIO) mouse model of pre-Type 2 diabetes, the onset of diet induces the expression of P2X7 (Kneer et al., 2018), consistent with the high levels of P2X7 mRNA in diabetic human corneas (Mankus et al., 2011). Epithelial necrosis requires immediate removal of the cellular debris, which would delay healing and regeneration, and promote further collateral inflammatory damage (Galluzzi et al., 2012; Westman et al., 2019). Our recent unpublished study has also demonstrated the defects of phagocytosis and efferocytosis in DM corneas in response to bacterial challenge.
Our genome-wide cDNA microarray analysis revealed TGFβ-signaling as a major altered pathway in healing B6 mouse corneas in DM versus NL corneas. As mentioned previously, at the molecular levels TGFβ1 and β3 were upregulated in response to wounding in NL corneas whereas the latter was greatly suppressed by hyperglycemia in rat T1DM (STZ) and T2DM models (GK-Wister) (Figure 12A & B). Figure 12C shows that TGF-β3 was abundantly expressed throughout the entire healing corneal epithelial sheet, and only a few TGF-β3 positive cells were found at the leading edge of the migratory epithelial sheet in T1DM rat corneas. Exogenous TGFβ3 accelerated epithelial wound closure in T2DM rat and T1DM mouse corneas via Smad and PI3K-AKT signaling pathways. The wound-induced epithelial TGFβ may control the innate immune system by inhibiting natural killer (NK) cells and regulating the complex behavior of macrophages and neutrophils (Batlle and Massague, 2019). We observed that the population of neutrophils is increased greatly in DM, when compared to NL corneas, during corneal epithelial wound healing, while the density of DCs decreased in DM healing corneas (Gao et al., 2016c). Recently we observed that, like DCs, the population of macrophages in the healing corneas was decreased drastically, coinciding with a decrease in TGFβ3 expression. The addition of exogenous TGFβ1 increased the epithelial healing rate and restored the macrophage population moderately, whereas the ability of TGFβ3 to accelerate re-epithelialization and macrophage population was significantly higher than that of TGFβ1 (Gao and Yu, unpublished results). TGF-β3 was shown to be positively associated with macrophage M2 polarization, an anti-inflammatory phenotype (Chen et al., 2018). Moreover, an increase in corneal M2 macrophages has been shown to accelerate healing of ocular herpetic disease following treatment with FGF-1 (Dhanushkodi et al., 2021). TGFβ3 is also known to distinctively regulate adaptive immunity such as TH17 polarization and B cell function (Komai et al., 2018).
Figure 12. mRNA Expression of TGFβ isoforms detected by RT-PCR and verification of TGFβ3 expression by real-time PCR.
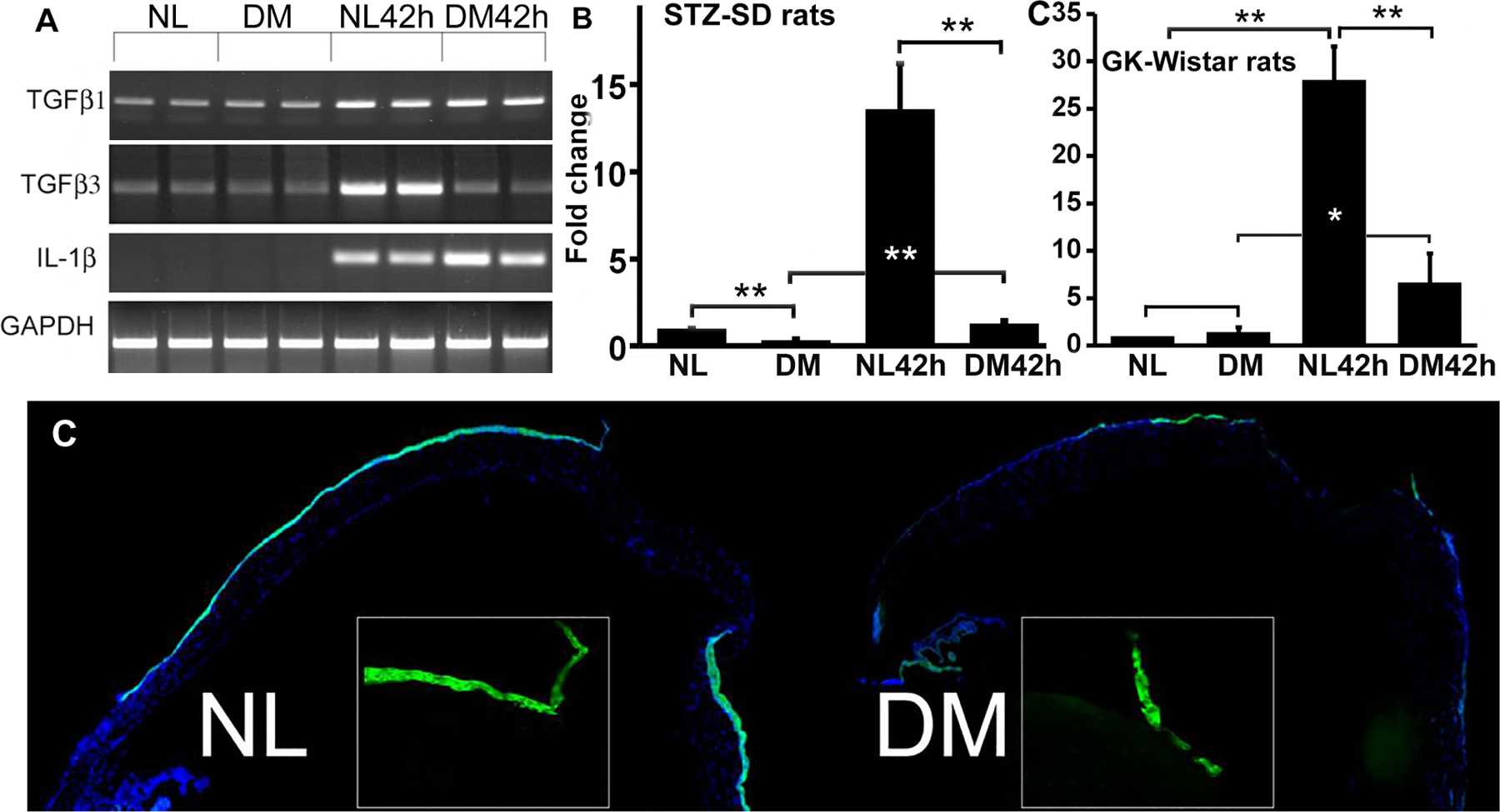
The RNA samples were obtained as described in Fig. 1. The scraped CECs from NL and STZ-DM rat and mouse corneas for creating a wound were marked as UW and from the wound bed at 42 hpw (rats) or 24 hpw (mice) and were subjected to RT-PCR with GAPDH as the internal control and IL-1β as a positive control. The data were presented as fold changes over non-DM, homeostatic CECs (1). For each condition three samples were collected from three rats/mice. Four independent experiments were performed: two with SD and STZ-SD, one with Wistar and GK rats, and one with B6 and STZ-B6 mice.*P < 0.05; **P < 0.01. Immunohistochemistry of TGF-β3 distribution in healing and UW corneas. The corneas of SD and STZ-SD were wounded and O.C.T. snap frozen at 42 hpw, followed by sectioning and immunostaining with antibodies against TGF-β3; DAPI was used to stain nuclei. Low magnification (×5) images of the entire cornea were taken and images stitched to present the whole from limbus to wound center. Inserts are high magnification (×20) images of the leading edge. This figure was originally published in (Bettahi et al., 2014a).
In addition to these common proinflammatory cytokines, other epithelial-derived cytokines, namely thymic stromal lymphopoietin (TSLP), IL-25, and IL-33, have come to the forefront of recent investigations (Patel et al., 2019). TSLP-conditioned DCs were shown to induce regulatory T-cell differentiation and protect non-obese diabetic (NOD) mice against diabetes (Besin et al., 2008). Our research demonstrated that while TSLP was mostly expressed by corneal epithelial cells, CD11c-positive cells expressed TSLP receptors, suggesting an avenue for communication between epithelium and immune system. TSLP plays a protective role in the corneas against P. aeruginosa keratitis through targeting of DCs and in an IL-23/IL-17 signaling pathway-related manner (Cui et al., 2018). IL-25, the newest member of the IL-17 cytokine family, acts as an alarmin and initiates, promotes, and augments Th2 cell-mediated immune responses (Yan et al., 2020).
Importantly, the immune cells responding to epithelial-derived cytokines also play a role in maintaining epithelial homeostasis. Reducing the inflammatory response to corneal wounding slows the migration of the epithelial sheet over the wound bed. Through IL-22, γδ T cells communicate with corneal epithelial cells, which highly express IL-22 receptors. IL-22 promotes corneal wound healing, while also inducing CXCL1 production by the epithelium, which attracts neutrophils to the cornea and further promotes inflammation (Li et al., 2011b). Wound-induced accumulation of γδ T cells also appears to be important in nerve regeneration, as TCRδ−/− mice demonstrate significantly diminished nerve regeneration, as well as decreased epithelial wound healing (Li et al., 2011a).
4.2. Defects in Immune Cell infiltration and/or activation in Diabetic
4.2.1. Neutrophils
Neutrophils are part of the innate immune system, and these cells carry out a variety of functions during the normal wound repair process. Neutrophils are not frequently observed in normal corneas, but are recruited in high numbers after tissue injury and/or infection. They are the first circulating immune cell to move to the site of injury, consistent with their primary role in defending against infection. At the injury site, neutrophils phagocytose cell debris and produce antimicrobial substances and proteases to eradicate pathogens. As an integral part of the inflammatory response, neutrophils can secrete signals which amplify inflammation including the expression of genes encoding proteins that recruit and activate more neutrophils and other inflammatory cells such as macrophages, γδT-cells, and NK cells at the early stages of healing. Taken together, neutrophils can kill invading microorganisms and stimulate other immune cells to effectively eliminate threats of infection while supporting epithelial wound closure.
In a normally healing wound, neutrophils undergo apoptosis after performing their function at the wound site. Apoptotic neutrophils are eventually engulfed by macrophages by a process termed efferocytosis, and the uptake of apoptotic cells by macrophages provides a strong signal for the resolution of inflammation (Widgerow, 2012; Wong et al., 2015). However, diabetes is known to impair the function and metabolism of neutrophils (Alba-Loureiro et al., 2006), resulting in accumulation of neutrophils at the injury site. An overactive or prolonged neutrophil response is detrimental to wound healing (Wilgus et al., 2013). In addition to apoptosis, neutrophils can also die through the release of chromatins loaded with antimicrobial molecules as neutrophil extracellular trap (NETs) that kill various pathogens (Brinkmann et al., 2004). However, NETs have been shown to elicit harmful effects on the host in noninfectious settings, and promote tumor progression and spread (Nakazawa et al., 2017; Thalin et al., 2018). Diabetes is known to prime neutrophils to undergo this process, which severely impairs wound healing (Wong et al., 2015). Consistent with these studies, we showed a great increase in the numbers of neutrophils, and a decrease in numbers of macrophages and dendritic cells in diabetic wounded corneas (Gao et al., 2016c). Interestingly, a high density of neutrophils is located outside of the healing epithelial sheet and on the central, denuded wound bed of diabetic healing corneas; treatment with exogenous VIP decreases the presence of neutrophils on the denuded wound bed (Figure 13). These neutrophils on the surface of wounded are likely most inflammatory. This decrease in the number of DCs may reduce the capacity of macrophages to effectively remove dying neutrophils.
Figure 13. VIP dampens neutrophil infiltration in NL and diabetic healing corneas.
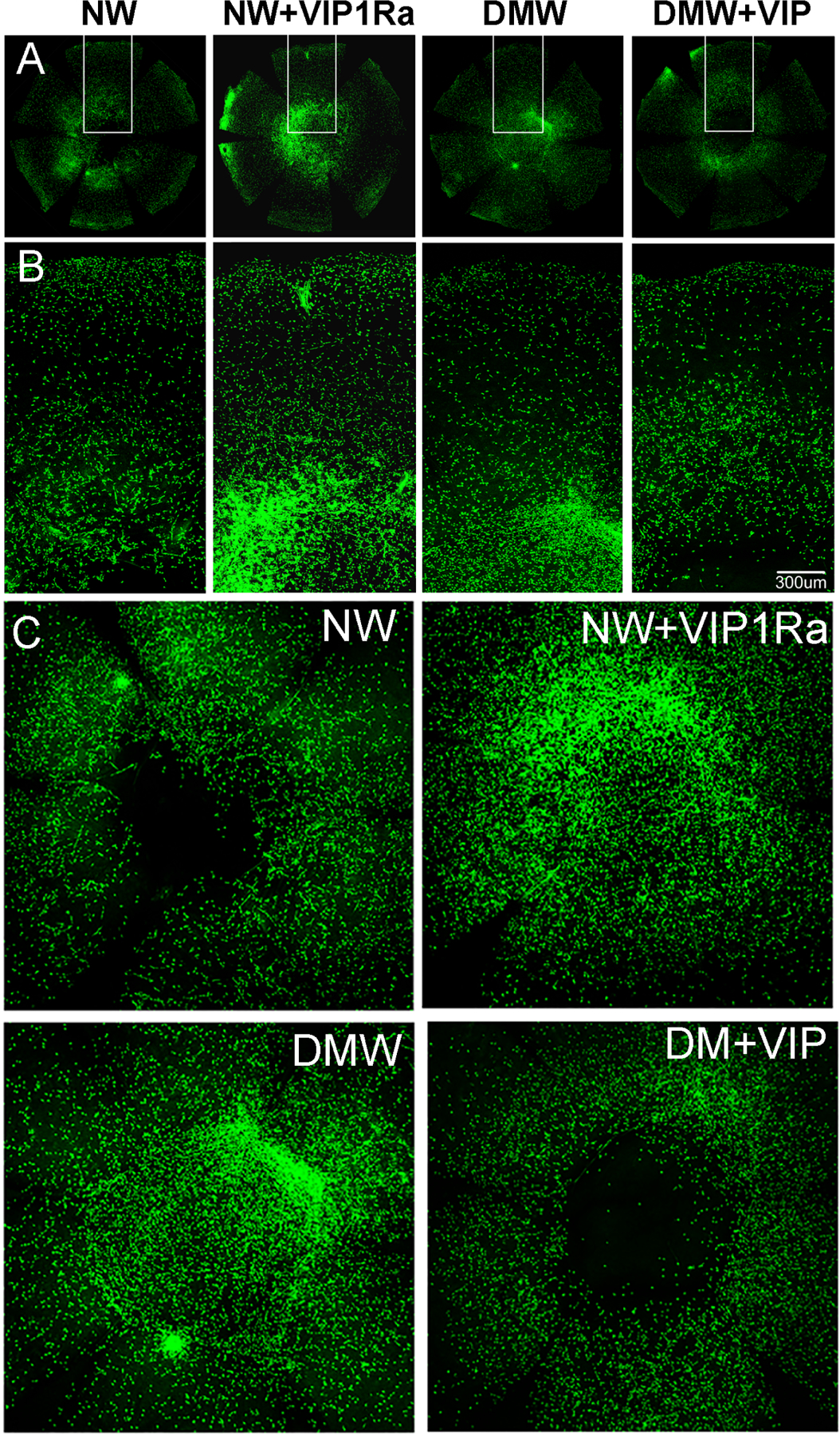
NL (NW), pretreated with VIP1Ra (NW1VIP1Ra), and diabetic (DMW), pretreated with recombinant VIP (DMW1VIP), mouse corneas were wounded by epithelium debridement (2-mm diameter). Healing corneas collected at 22 hpw from NL and diabetic mice were subjected to WMCM using Ly6G-FITC antibody for mouse neutrophil staining. The images of the whole cornea were captured (A), the marked areas of whole corneas were amplified (B), and central areas were shown (C). (D): Cell numbers in the whole corneas (A) were calculated with ImageJ and presented (mean 6 SD; n 5 3). *P>0.05 (one-way ANOVA). This figure was originally published in (Zhang et al., 2020a).
4.2.2. Macrophages
Most tissues (including corneas) contain macrophages (Watanabe et al., 2019). Macrophages represent a heterogeneous population of nonmigratory cells that respond to injury or infection by sensing DAMPs. Macrophages are continuously replenished by blood monocytes (Bain et al., 2014). Recently, Langerhans cells, previously considered as DCs, are now considered as a specialized subset of macrophages (Brazil et al., 2019; Doebel et al., 2017) because of a shared ontogeny (Ginhoux et al., 2006; Hoeffel et al., 2012). Langerhans cells are self-replicating and can migrate to lymph nodes to present antigens to T cells (Doebel et al., 2017). Importantly, Langerhans cells were reported to repopulate the epidermis during re-epithelialization of acute skin wounds (Stojadinovic et al., 2013). The reported DCs associated with sensory nerve fibers and detected by IVCM (Figure 7) might be Langerhans cells, a local resident macrophages (Bitirgen et al., 2018a; D’Onofrio et al., 2021).
Recently, Liu et al identified two populations of macrophages in the cornea: CCR2+ and CCR2- cells (Liu et al., 2017). The CCR2- corneal macrophages, which were thought to originate from myeloid progenitors in the embryonic yolk sac, exhibit local proliferation capacity at a steady state and are repopulated by monocytes following corneal epithelial abrasion. These cells likely represent the Langerhans cells, and it is of great interest to establish whether this is the case. Conversely, the CCR2+ cells are continuously replenished by blood monocytes, starting at later stages of development. While the loss of either cell type results in a delay in corneal wound healing, depletion of CCR2- macrophages increases inflammation of the injured cornea, suggesting an anti-inflammatory role of this subtype of macrophages. In contrast, it appears that the CCR2+ counterparts may serve in an opposing pro-inflammatory role.
Macrophages contribute to diabetic neuropathy through neuroimmune cell-cell interactions and the release of pro-inflammatory cytokines, chemokines to induce inflammatory cell recruitment and cell apoptosis (Meshkani and Vakili, 2016). Functionally, experiments using STZ-induced diabetic mice demonstrated a significant decrease in the phagocytic activity of macrophages 12 weeks after induction of diabetes compared with age-matched control mice. This reduced phagocytic activity is correlated inversely with tissue levels of AGEs (Liu et al., 1999). While this suggests that macrophage activity is significantly altered in diabetes, the role of resident macrophages in diabetic complications of the cornea is elusive.
CD11c-positive immune cells have been categorized and identified as conventional DCs (Gao et al., 2016b; Gao et al., 2011), Langerhans cells (Jager, 1992), and plasmacytoid DCs (Jamali et al., 2021). The role of plasmacytoid DCs in corneal immunity was recently reviewed (Jamali et al., 2021). While Langerhans cells were historically considered DCs, they are now believed to represent a specialized subset of tissue-resident macrophages (TRM) (Brazil et al., 2019; Doebel et al., 2017). We propose that the corneal Langerhans cells are also a special type of TRM. These cells can be viewed with IVCM and there is an increase in corneal Langerhans cells in diabetic patients, particularly in the earlier phases of corneal nerve damage, suggesting a role of altered immune mechanisms in human diabetic neuropathy (Tavakoli et al., 2011). As for wounding, there is a rapid initial influx of neutrophils, followed by successive waves of infiltrating monocytes that differentiate within the wound into MΦs and dendritic cells. Depletion of both macrophages and DCs greatly attenuates epithelial wound closure in NL and DM corneas (Gao et al., 2016a; Gao et al., 2011).
Finally, the contribution of myofibroblasts to DNK have yet to be elucidated. Diabetes was found toinduce the transformation of cardiac fibroblasts into a myofibroblast phenotype, leading to the increased deposition of collagen and other matrix proteins (Fowlkes et al., 2013). In the retina, infiltrated macrophages in the subretinal space can also convert to collagen-producing myofibroblasts, contributing to neovascular age-related macular degeneration (Little et al., 2020). A similar process has also been identified in human and experimental kidney disease (Meng et al., 2016). Although this has not yet been demonstrated in diabetic corneas during wound healing, the conversion of macrophages/monocytes to myofibroblasts and their contribution to corneal ulceration are expected. Hence, the impact of DM on the corneal stroma may also result from altered phenotypes of macrophages derived from infiltrated monocytes at the injury site (Sinha et al., 2018). The contribution of infiltrated immune cells to corneal stromal degradation in DNK should be investigated in diabetic animal models and human corneas.
4.2.3. Dendritic Cells
Conventional DCs are found in the superficial layers of the cornea. DCs residing under the basal epithelial layer are more numerous in the peripheral versus central cornea (Lee et al., 2010a). Some of the DCs in the cornea insert processes between epithelial cells, similar to that of the vertically-oriented sensory nerve endings (Lee et al., 2010a; Ward et al., 2007). Using CD11c as a marker, our group identified two morphologically different DCs, dendriform and round-shaped, within the corneal epithelium (Gao et al., 2016b). The dendriform DCs were located at the sub-basal space where the nerve plexus resides, with DC dendrites crossing several nerve endings. The round-shaped DCs were closely associated with nerve fiber branching points, penetrating the basement membrane and reaching into the stroma. Trigeminal denervation resulted in epithelial defects, decreased tear secretion, and the loss of these dendriform DCs at the ocular surface. Local DC depletion resulted in a significant decrease in corneal sensitivity, an increase in epithelial defects, and a reduced density of nerve endings at the center of the cornea. Importantly, the numbers of both dendriform and round-shaped DCs were greatly reduced, associated with marked decrease in the density of sub-basal nerve plexus in DM corneas (Gao et al., 2016c). These studies demonstrated a potential unidentified role for DCs in accompanying sensory nerve fibers to penetrate the basement membrane and innervate the epithelia. Moreover, prolonged hyperglycemia may be detected by DCs, resulting in apoptosis and a general decrease in density of both DCs and their associated sensory nerve fibers and endings. Whether DCs or nerves initially sense hyperglycemia or AGEs is of great interest, as they may allow for earlier interventions for DNK or DPN.
Our studies also have revealed that DCs migrate along with the epithelial sheet to cover a wound and that local depletion of DCs resulted in a significant delay in epithelial wound closure (Gao et al., 2011). In response to wounding, migratory epithelia produce CXCL10, thymic stromal lymphopoietin, IL-1β and sIL-1Ra; depletion of corneal DCs resulted in a reversal of these elevated expressions to different extents, suggesting a DC-mediated positive feedback loop in epithelial gene expression. Moreover, DC depletion resulted in an increase in cell apoptosis and a decrease in neutrophil infiltration in healing normoglycemic corneas. This decrease in neutrophil infiltration may be related to the decreased expression of cytokines such as IL-1β and/or CXCL10. In healing diabetic corneas, the number of infiltrating DCs was greatly reduced (Gao et al., 2016c). Direct involvement of DCs in accelerating epithelial wound healing and sensory nerve degeneration suggests the potential for the use of bone marrow generated DCs and/or their exosomes as therapy for DNK (see Section 5.5.).
4.2.4. Defects in Epineuroimmune Interactions in Response to Wounding
Figure 14 (Gao et al., 2016a) summarize the Epineuroimmune function unit and its defects in diabetic corneas (STZ-induced T1DM) with or without epithelial wounding. It shows that intraepithelial DCs and sensory nerves are structurally associated and functionally interdependent within the platform in the cornea. In steady state conditions, there were numerous dendriform (arrows) and round-shaped (arrowheads) DCs located near the limbus, with many nerve fiber branching points in NL corneas. A few round-shaped and dendriform DCs from which endings were branching were detected in the DM cornea, indicating neuropathy and decreased resident DCs. In healing corneas, the re-epithelization is much delayed in DM compared to the NL corneas (white lines: distances traveled by NL and DM corneas). There are infiltrated DCs that migrate behind the epithelial leading edge in which with high density of DCs was found. Corneal innervation was robust and the regenerating nerve fibers were frequently branching to form nerve endings with numerous DCs evenly distributed among regenerating nerve endings. There is one DC in front of each growing nerve fiber/ending. Hence, DCs in NL corneas appear to stabilize newly formed sensory nerve fibers/endings and guide sensory nerve regeneration during healing. In healing DM corneas, fewer nerve insertion sites were observed near the limbus and the density of regenerating nerve fibers was less than in the NL corneas. Strikingly, these nerve fibers were tortuous, discontinuous or fragmented, similar to that observed in diabetic human corneas (Kallinikos et al., 2004). There were few DCs in the region behind the leading edge (DM1). The regenerating nerve fibers were fewer in number and free-ended without nearby DCs. These results demonstrate the functional interdependence of three types of cells in maintaining homeostasis and mediating the corneal response to epithelial wounding. Prolonged exposure to hyperglycemia disturbs these interactions, including cell-cell contacts, autocrine and paracrine signaling through growth factors, cytokines, neurotrophic factors, and neuropeptides (Table 1). Because small-fiber neuropathy can be detected in a patient diagnosed with prediabetes (Divisova et al., 2012) and peripheral neuropathy is the most prevalent chronic complication of diabetes (Feldman et al., 2019), we propose that among three type of cells, sensory neuron exons are the most sensitive sentinels for detecting elevated glucose levels. This would trigger cascade reactions including increased secretion of neuropeptides and altered cell-cell contacts within the sub-basal plexus of the corneas and initiate DNK pathogenesis. Hence, preserving small fiber neurons and restoring their function might be the most effective in treating DNK. This will be discussed in the next section.
Figure 14. DCs and sensory nerve fibers/endings in healing normal and diabetic mouse corneas.
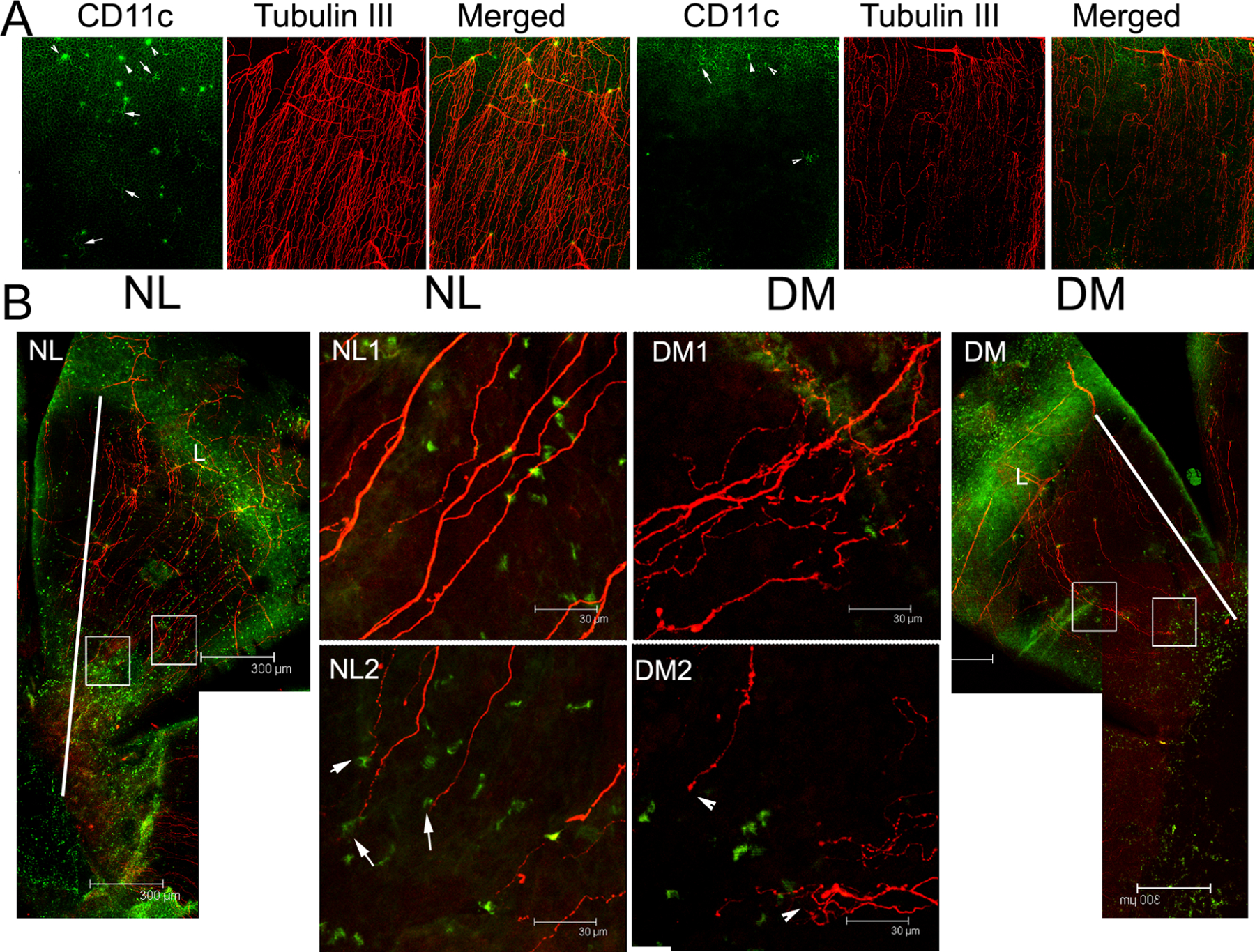
(A) Unwounded corneas were double-stained for β-tubulin III (nerve marker) and CD11c (dendritic cell marker) and the limbal region was photographed. Normal (NL, left) corneas showed dendritic cells (DCs) located where sensory nerves of the basal plexus enter the superficial epithelium. In the diabetic (DM, right) cornea, the number of DCs and nerves are diminished, and this anatomical relationship appears to be lost. Arrows, dendriform DCs; arrowheads, round-shaped DCs. (B) The NL and DM corneas were wounded using epithelial debridement. The whole cornea from the limbus (L) to the leading edge was photographed (10x, lateral panels). Higher magnification images (40x) at the middle (NL1, DM1) and the leading edge (NL2, DM2) of healing migratory sheets are also shown. DCs appear at each end of the healing nerve fibers in the NL cornea, but this relationship is lost in the DM cornea. Arrow, co-localization of nerve endings with a DC in NL cornea; Arrowhead, free nerve ending in the DM cornea. The results are representative of two independent experiments (N=3). Diabetes delays epithelial wound closure, and disturbs the interactions of DC and regenerative sensory nerves. This figure was originally published in (Gao et al., 2016c).
5. EXPERIMENTAL THERAPIES FOR DIABETIC KERATOPATHY
An effective therapy for management of DNK is to address the systemic cause of the problem, hyperglycemia, by controlling blood glucose levels with agents such as metformin or insulin injections and lifestyle changes such as weight loss that reduce insulin resistance. Current management of DNK focuses on ensuring patient comfort and protecting the cornea while allowing the epithelial defects to heal. Well-established therapies include the use of topical lubricants, antibiotic ointments, bandage soft contact lenses, and tarsorrhaphy (Priyadarsini et al., 2020). While amniotic membrane grafts may also be utilized in severe cases, corneal transplants are reserved for the most severe, ulcerated and scarred corneas (Siu et al., 2015). However, these options are all palliative and seek to reduce patient symptoms rather than halt or reverse the underlying disease. There are several experimental therapies that may hold promise for effective treatment DNK.
5.1. Growth Factors/Cytokines as Therapeutic Reagents
Many growth factors have been suggested to possess therapeutic effects in enhancing corneal wound healing, including EGF (Xu and Yu, 2011), TGFβ (Bettahi et al., 2014a), PDGF (Vij et al., 2008), CNTF (Gao et al., 2016c; Zhou et al., 2015), HGF (Kramerov et al., 2016a; Saghizadeh et al., 2011a), and NGF (Aloe et al., 2008; Lambiase et al., 2003). Recently, cenegermin (OXERVATE™), a topically-applied recombinant human NGF, was approved by FDA as the first drug for treating neurotrophic keratopathy (Pflugfelder et al., 2020; Sheha et al., 2019). Although its usefulness in treating DNK was not specified in the clinical trial, cenegermin (OXERVATE™) topical eye drops were used successfully to cure diabetic and neurotrophic ulcers after other treatments options were exhausted (Ahuja et al., 2020). A broad use of cenegermin to treat DNK is expected. Our study showed that diabetes disturbs the balance of IL-1β/IL-1Ra by suppressing IL-1Ra expression. Hence, local application of anakinra, monotherapy or added-on therapy, such as with cenegermin, may be used for more effective treatment of chronic corneal and potential skin wounds of diabetic patients.
5.2. microRNA based therapy
In recent years, plenty of miRNAs have been identified to be involved in the development of epithelial and neuro-degenerative disorders, thus making them an attractive option for therapy. miRNA-based therapeutic approaches can be divided into two fundamental groups: miRNA inhibition to diminish the expression of disease-induced miRNAs and miRNA replacement to reestablish the expression of disease repressed miRNAs (Paul et al., 2020). In principle, all the microRNAs listed in section 2.5. may be targeted for treating diabetic keratopathy, including our reported studies (Gao et al., 2015a; Wang et al., 2016; Zhang et al., 2020b). To date, while two small interferon RNA drugs, Patisiran (Hoy, 2018) and Givosiran (Scott, 2020), have been approved by US FDA in 2018 and 2019, respectively, 10 obtainable miRNA drugs have been in clinical trials, none of which have entered phase III. This may be related to too many off-target effects (Zhang et al., 2021).
5.3. Limbal Stem/Progenitor Cell Modification
In T1DM and T2DM mouse models, as well as organ and cell cultures, diabetes has been shown to cause alterations of LSCs (Ueno et al., 2014; Zhou et al., 2015). Thus, the loss or dysfunction of the resident LSCs may be responsible for clinically observed delay of corneal epithelial wound closure in diabetic corneas. Therefore, improving the function of diabetic LSCs through gene therapy or exogenous modulators is expected to be an effective means to promote diabetic corneal wound healing in vivo.
Gene therapy has been utilized to restore the normal function of diabetic LSCs which in turn accelerate diabetic corneal epithelial wound healing. Adenoviral c-Met gene transduction in organ-cultured human diabetic corneas normalized wound healing, epithelial and stem cell marker expression patterns in organ-cultured human diabetic corneas (Saghizadeh et al., 2011a). Moreover, over expression of c-Met and miR-146a in LSC by gene therapy enhanced or delayed diabetic corneal epithelial wound healing of cultured human corneas, respectively (Saghizadeh et al., 2014; Winkler et al., 2014). Moreover, silencing of MMP-10 and Cathepsin F, proteinases up-regulated in DM corneas, in diabetic organ-cultured human corneas enhanced expression of corneal epithelial stem cell markers and reduced corneal epithelial wound healing time (Saghizadeh et al., 2013). Since adenoviral gene therapy showed toxicity to cultured limbal epithelial cells (LEC), the group designed a novel non-toxic nanobiopolymer that that carried antisense oligonucleotides suppressing MMP-10, Cathepsin F, and miRNA-409-3p, which inhibits c-Met, and demonstrated that these treatments normalized levels of LSC markers, and accelerated wound healing in diabetic human LEC and organ-cultured corneas (Shah et al., 2021). It should be noted that the nanobiopolymer needs an enhancer to effectively penetrate the epithelial layer and affect the basal LSCs. Although many enhancers for ophthalmic drugs are being studied, currently, there are no FDA-approved lipophilic drug delivery enhancers (Moiseev et al., 2019).
5.4. Limbal stem cell therapy for treating limbal stem cell deficiency (LSCD).
While gene therapy to modify limbal stem/progenitor cells is primarily in the preclinical phase, ABCB5+ LSCs, isolated and expanded ex vivo from human corneal rims, were approved for an international multicenter phase I/IIa clinical trial by the German Paul Ehrlich Institute and the U.S. FDA (Norrick et al., 2021). Although LSCs have been identified over 30 years ago (Cotsarelis et al., 1989; Lehrer et al., 1998; Schermer et al., 1986), their application to treat LSCD has been limited by the lack of a marker able to unequivocally identify LSCs and the treatment of total, bilateral LSCD which requires other sources of stem cells for ocular surface reconstruction (Sacchetti et al., 2018). ABCB5, a member of the ATP-binding cassette transporter superfamily of integral membrane proteins, was identified as a marker of human limbal stem cells and was shown to be required for corneal development and repair of corneal epithelium (Ksander et al., 2014). In the following studies, ABCB5+ LSCs were isolated from human cadaveric corneal rims and expanded in culture. These in vitro expanded cells contained comparably high percentages of cells expressing transcription factors critical for maintenance of LSC stemness (p63) and corneal epithelial differentiation (PAX6). Preclinical studies confirmed the local engraftment potential of these cells and showed no signals of toxicity and tumorgenicity. ABCB5+ LSCs have great potential for treating DNK, particularly at the later stages of the disease, when persistent epithelial defects occur. The major advantage of clinical trials is that the human LSCs are derived from donor rims and bilateral LSCD associated with DM can be treated (Norrick et al., 2021).
5.5. Exosomes as Novel Therapeutic Agents for Treating DNK
Exosomes are nano-sized lipid bilayer vesicles (30–150 nm) that are formed from intraluminal vesicles produced in the lumen from late endosomes. Once released into the extracellular environment, they serve as mediators for intercellular communication with recipient cells through the delivery of bioactive cargo, including proteins, lipids, and nucleic acids (Jing et al., 2018; Valadi et al., 2007; van Niel et al., 2018; Yanez-Mo et al., 2015). Exosomes derived from MSCs and DCs have been under intense investigation for their potential use as an alternative to cell therapy (Baghaei et al., 2018). Exosomes have many advantages as a form of therapy. They are relatively easily isolated by centrifugation and filtration, compared to the standards required for processing cell therapies. Additionally, they do not have the risks of immunological rejection and malignant transformation seen with stem cell therapies (Vizoso et al., 2017). The presence of the lipid bilayer also serves to protect its contents, allowing easier production methods and a product that is more stable in the clinical environment (Wahlgren et al., 2016).
Endogenous exosomes of various cellular origins play a role in both corneal homeostasis and pathology, suggesting a potential avenue for the use of exogenous exosomes as therapy (Figure 15). Exosomes derived from healing mouse corneal epithelium induce transformation of keratocytes into fibroblasts, suggesting their importance in the wound healing process (Han et al., 2017). Samaeekia et al found that exosomes from cadaveric corneal MSCs were taken up by both mouse epithelium in vivo as well as human macrophages in vitro. In both scratch wounds on cultured epithelial cells and mouse corneas, topical application of the exosomes accelerated wound healing (Samaeekia et al., 2018). These results highlight the potential use of exosomes for delayed wound healing, though their effects in a DNK model remain to be seen. Our unpublished results showed that exosomes derived from bone marrow-derived DCs are sufficient to restore severely impaired wound healing in DC-depleted corneas (Yu, unpublished results). Leszczynska et al explored the regulatory roles of stromal limbal stem cell (LSC)-derived exosomes and found that wound healing and proliferation rates in primary normal limbal epithelial cells were significantly enhanced upon treatment with exosomes derived from normal, but not diabetic LSCs. Next generation sequencing of exosome contents revealed that microRNA-146a and −184 were enriched in normal but not diabetic LSC-derived exosomes (Leszczynska et al., 2018). While exosomes isolated from healing normal glucose-cultured mouse corneal epithelial cells accelerate corneal wound healing in DM mice, exosomes isolated from high glucose cultured cells induce corneal sensory nerve denervation in normoglycemic mice (Yu, unpublished results). Thus, exosomes may serve as novel therapeutic tools for treatment of the diabetic corneal defects (Liu et al., 2020).
Figure 15. Effect of N- or DM-Exos on LESC marker expression in normal organ-cultured corneas and primary LEC.
(A) Normal Exo treatment in normal organ-cultured corneas led to increased expression of putative LESC markers, K15 and FZ7, and no significant change in K17 protein level compared to fellow corneas treated with DM-Exos (immunofluorescent staining of limbal corneal sections). The same exposure time was used for each set of compared stained sections, and the assessment was done by more than one observer. (B) Western analysis shows that N-Exos treatment increased, whereas DM-Exo treatment decreased K17 protein expression level in primary LEC compared to control treated cells, which did not reach significance. Antibody to β-actin was used as equal loading control and for semi-quantitation. This figure was originally published in (Leszczynska et al., 2018)
There is an increasing number of clinical trials (19 listed in clinicaltrials.gov up to 2021) investigating exosomes as therapeutic agents in a wide range of diseases including cancer, immunomodulation, neurodegeneration and infectious diseases. Mesenchymal stem cells and DC are the main sources for therapeutic exosomes in either their naïve (i.e. unmodified) or engineered forms (Perocheau et al., 2021). As previously discussed, DCs play a critical role in corneal homeostasis and innervation, and their defects in diabetic corneas contribute to the pathogenesis of DNK. Thus, exosomes derived from bone marrow-derived or blood monocyte-differentiated DCs might be developed as topical therapies for treating DNK. Because of their potential high therapeutic value, excellent stability, and ability to be stored long-term in a refrigerator, the DC-derived therapy might also be used to treat corneal/eye injuries caused in many settings, from the emergency room to the battlefield (Araj et al., 2020; Yeung et al., 2020).
6. Conclusion and Future Directions
Significant advances have been made in the last decade in understanding the pathophysiology of and potential treatments for DNK. We have summarized the progress of research in this field by describing the adverse effects of hyperglycemia on three types of cells vital to maintaining corneal homeostasis: epithelium, immune cells, and sensory nerves. How these cells are altered in diabetes both at steady state and during wound healing is critical for understanding the pathophysiology of DNK.
DCs and sensory nerves are functionally interdependent within the corneal epithelium, forming an “epineuroimmune” function unit to respond to wounding in a coordinated fashion. In addition to creating an anatomical protective barrier and layer for these interactions to occur, the epithelium secretes factors to communicate with sensory nerves and immune cells. Neurotrophic factors such as NGF maintain the innervation of the cornea, while axon guidance molecules such as the semaphorins promote proper regrowth of axons after injury. Various cytokines such as IL-1β/IL-1Ra are produced by the epithelium to control and signal to immune cells during states of insult and injury. Immune cells, meanwhile, not only protect the cornea from infection, but also themselves produce neurotrophic factors such as CNTF to communicate with sensory nerves and epithelium. Lastly, the dense network of sensory nerve fibers serves to guard against potential insult (such as the tearing reflex to foreign bodies), while secreting neuropeptides such as substance P to communicate with immune cells and the epithelium. These anatomical and functional relationships are therefore critical in maintaining homeostasis of the cornea (summarized in Figure 16).
Figure 16. The Functional “Epineuroimmune Unit”.
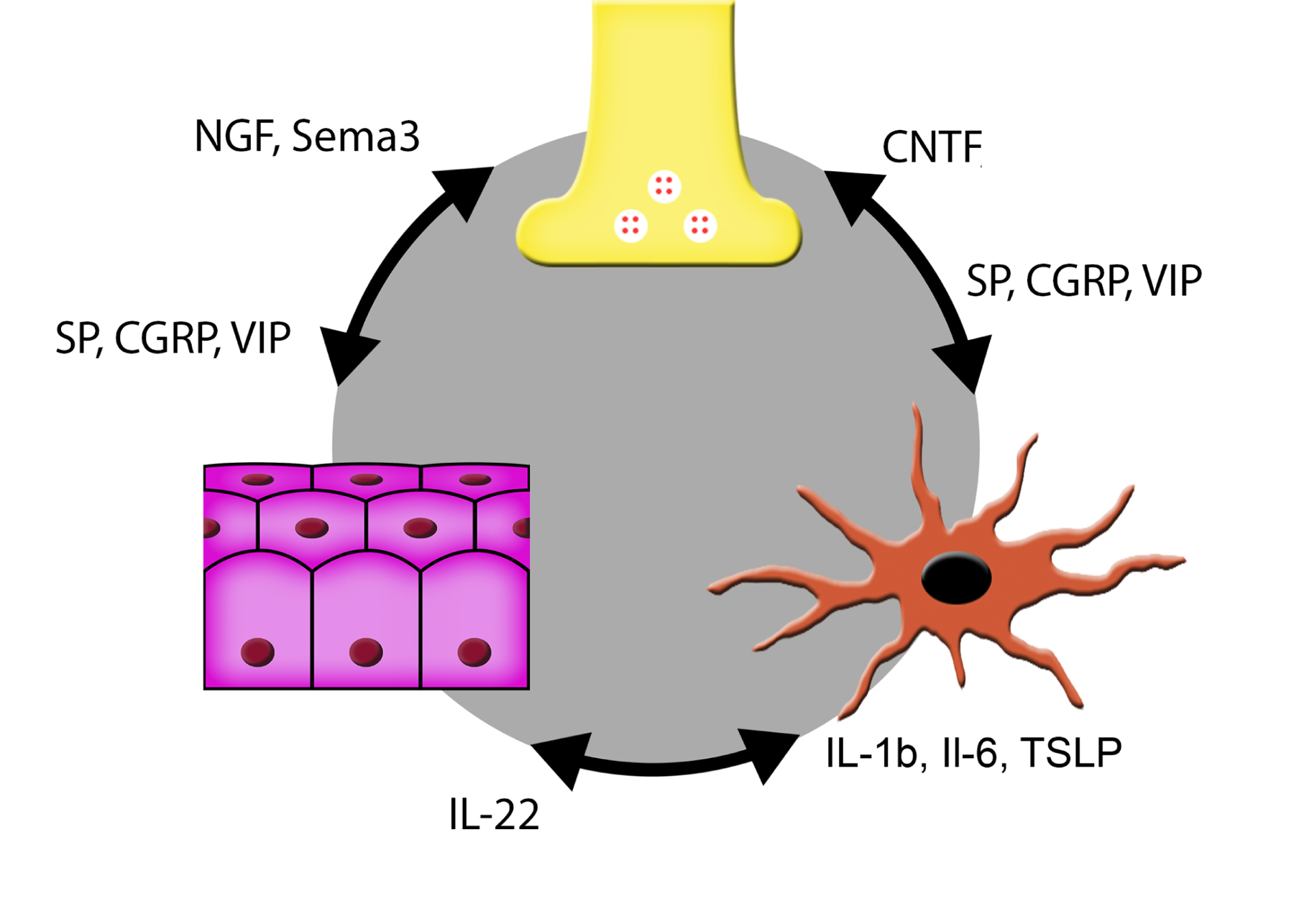
Corneal epithelium, sensory nerves, and immune cells are anatomically and functionally interdependent in maintaining the corneal barrier. Epithelial-derived cytokines and neurotrophic factors/axonal guidance factors allow regulation of immune cells and nerves, respectively. CNTF produced by dendritic cells promotes epithelial healing and nerve regeneration. Neuropeptides derived from sensory nerves allow regulation of both epithelium and immune cells. Details regarding interactions are described in the text. CGRP, Calcitonin Gene-Related Peptide; CNTF, Ciliary Neurotrophic Factor; IL, Interleukin; MANF, Mesencephalic Astrocyte-Derived Neurotrophic Factor; NGF, Nerve Growth Factor; Sema3, class 3 semaphorins; SP, Substance P; TGF, Transforming Growth Factor α; TSLP, Thymic Stromal Lymphopoietin; VIP, Vasoactive Intestinal Peptide. This original figure was made by P SY Lee.
Diabetes disturbs the interaction of these cells, resulting in delayed wound healing and impaired sensory nerve regeneration in the cornea. Disturbance of this unit by hyperglycemia, and its correction by mechanism-based therapies will be of paramount importance in improving clinical outcomes of DNK treatment. The future directions of the field should include, but not be limited to the following.
In the clinical setting, DNK can be observed both under the slit lamp as well as more precisely through use of newly developed diagnostic tools. As previously described, several groups have shown that corneal IVCM is able to identify and track diabetes-induced corneal neuropathy in vivo, and that these changes correlate with progression of disease elsewhere in the body (Ahmed et al., 2012; Mehra et al., 2007; Rosenberg et al., 2000; Tavakoli et al., 2010). The observation of corneal resident immune cells in other disorders has also been described, and would likely be feasible for DNK as well (Bitirgen et al., 2017; Bitirgen et al., 2018b). These allow better detection and monitoring of inflammatory and neurodegenerative changes in diabetic eyes, which, in turn, have led to laboratory investigations of new underlying mechanisms for the pathogenesis of DNK.
First, a few questions regarding the pathogenesis of DNK should and can be addressed using currently available technologies and research tools: 1) While all three components of the corneal function unit may be considered as “sentinels” that respond to hyperglycemia, which component is the initial detector of hyperglycemia? What is the initial instigating “trigger” (e.g. intracellular reactive oxygen species, extracellular advanced glycation end products) for the response? How do these pathogenic factors converge into inflammatory response(s), which are exacerbated by prolonged hyperglycemia, and lead to the development of DNK?
Second, there is a critical need to elucidate the transcriptomes of individual cells (i.e., single cell RNA-seq), including limbal stem cells, basal and wing epithelial cells, resident DCs and macrophages, and sensory nerves in normal and diabetic corneas, both before and during wound healing. This will include mRNA, microRNA, small interfering RNA, Piwi-interacting RNAs, and long ncRNAs; these non-coding RNAs are involved in the regulation of gene expression of somatic cells through epigenetic programming, DNA rearrangements, mRNA turnover, and translational control. Limbal stem cells and basal epithelial cells can be isolated using cell lineage tracing; immune cells by cell surface markers, and corneal sensory nerves by retrograde transport labeling using stromal injection. This will lead to a more complete understanding of mechanisms underlying the pathogenesis of diabetic corneal complications and sensory nerve degeneration/regeneration.
Third, diabetic eyes are at increased risk of pathologies such as dry eye, superficial punctate keratitis, recurrent corneal erosion syndrome and persistent epithelial defects, all of which can lead to microbial infection. As we potentially move into a post-antibiotic era, studies that aim at understanding the mechanisms underlying the increased susceptibility and rapid progression of microbial keratitis in DM corneas are of paramount importance.
Lastly, mRNA delivered by lipid nanoparticles (most recently utilized in some COVID-19 vaccines) and CRISPR–Cas9 gene editing tools, which have been used both in research and the clinic, are likely to change the landscape of biomedical research and medicine. The use of these technologies will also provide more detailed insights into the pathogenesis of DNK and lead to new and more efficient treatments for this and other diabetic complications.
Table 2.
Summary of miRNAs in the diabetic cornea and TG
| Name | Change (DM vs. NL) | Functions | References |
|---|---|---|---|
| miR-146a | ↑ in cornea | Delay epithelial wound healing. Inhibit epithelial cells migration. Limbal epithelial cells (LEC) maintenance. Reduce p-p38 and p-EGFR expression. |
(Funari et al., 2013; Winkler et al., 2014) |
| miR-424 | ↑ in cornea | Delay epithelial wound healing. Reduce p-p38 and p-EGFR expression |
(Funari et al., 2013) |
| miR-10b | ↑ in corneal limbus | Increase corneal epithelial cell proliferation. LESC maintenance and/or their early differentiation. |
(Kulkarni et al., 2017) |
| miR-182 | ↓ in TG | Promote nerve regeneration. Recovery of corneal sensation. Decrease its target gene NOX4. |
(Wang et al., 2016) |
| miR-204–5p | ↑ in corneal epithelia | Upregulate Sirt1. Delay epithelial cell cycle. Inhibit corneal epithelial wound healing. |
(Gao et al., 2015a) |
| miR-409–3p | / | Upregulate c-Met. Accelerate epithelial wound healing |
(Kramerov et al., 2021) |
| miR-181a | ↑ in TG | Decrease nerve regeneration. Alleviate corneal epithelium healing. Decrease ATG5-mediated autophagic activation. Reduce BCL-2-mediated inhibition of apoptosis. |
(Hu et al., 2020) |
| miR-214 | ↓ in corneal endothelium | Attenuate high glucose induced pyroptosis. | (Zhang et al., 2020c) |
| miR-34c | ↑ in TG | Affect the growth of trigeminal sensory neurons. Affect the repair of diabetic corneal nerve endings. Inhibit autophagy by acting directly on Atg4B. |
(Hu et al., 2019) |
Highlights.
Within the cornea, the epithelium, sensory nerves, and resident immune cells form a function “epineuroimmune” unit to maintain tissue homeostasis and coordinate tissue responses to environmental challenges, such as diabetes mellitus (DM).
DM causes corneal epithelial abnormalities, including delayed wound healing, epithelial fragility, punctate keratopathy, and persistent epithelial defects/recurrent erosions.
Diabetic sensory neuropathy may be both an underlying cause and a consequence of diabetic keratopathy, with both nerve and epithelial damage exacerbating the other. A decrease in nerve density reduces the release of neuropeptides, potentially contributing to heightened inflammation and delayed wound healing in diabetic corneas.
Interactions within the “epineuroimmune” unit occur through cell-cell contacts and paracrine signaling among the three cellular components.
DM-induced immune dysfunction contributes to diabetic neuropathy and keratopathy. Diabetes decreases the numbers of resident immune cells but increases immune cell infiltration - particularly of neutrophils. This results in an exacerbation of underlying inflammation and neurotrophic keratopathy.
Cell-based therapies including stem cells, in vitro-induced dendritic cells, and their exosomes hold promise for novel avenues in treating DNK.
Grant support:
NIH/NEI R01-EY010869, R01-EY017960 (to FSY), F30-EY025923 (to PSYL); R01-EY013431, R01-EY031377 (to AVL); P30-EY04068 (to Linda Hazlett), Research to Prevent Blindness (to Mark Juzych); the Academic Promotion Program and Innovation Project of Shandong First Medical University (to LX).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Statement
The authors declare that there is no duality of interest associated with this manuscript.
References
- Abdelaziz MH, Abdelwahab SF, Wan J, Cai W, Huixuan W, Jianjun C, Kumar KD, Vasudevan A, Sadek A, Su Z, Wang S, Xu H, 2020. Alternatively activated macrophages; a double-edged sword in allergic asthma. J Transl Med 18, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelkader H, Patel DV, McGhee C, Alany RG, 2011. New therapeutic approaches in the treatment of diabetic keratopathy: a review. Clin Experiment Ophthalmol 39, 259–270. [DOI] [PubMed] [Google Scholar]
- Adeghate E, Rashed H, Rajbandari S, Singh J, 2006. Pattern of distribution of calcitonin gene-related Peptide in the dorsal root ganglion of animal models of diabetes mellitus. Ann N Y Acad Sci 1084, 296–303. [DOI] [PubMed] [Google Scholar]
- Ahmed A, Bril V, Orszag A, Paulson J, Yeung E, Ngo M, Orlov S, Perkins BA, 2012. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes Care 35, 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja AS, Bowden FW 3rd, Robben JL, 2020. A Novel Treatment for Neurotrophic Corneal Ulcer Using Topical Cenegermin (OXERVATE) Containing Recombinant Human Nerve Growth Factor. Cureus 12, e11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil H, Lim J, Gao D, Burgess J, Stylianides A, Ooi C, Finana M, Burgess P, Zheng Y, Alam U, 2020. Corneal confocal microscopy and not optical coherence tomography detects progressive worsening of neuropathy in Type 1 Diabetes. IOVS 61, https://iovs.arvojournals.org/article.aspx?articleid=2769946. [Google Scholar]
- Al-Aqaba MA, Dhillon VK, Mohammed I, Said DG, Dua HS, 2019. Corneal nerves in health and disease. Prog Retin Eye Res [DOI] [PubMed] [Google Scholar]
- Alba-Loureiro TC, Hirabara SM, Mendonca JR, Curi R, Pithon-Curi TC, 2006. Diabetes causes marked changes in function and metabolism of rat neutrophils. J Endocrinol 188, 295–303. [DOI] [PubMed] [Google Scholar]
- Alcalde I, Inigo-Portugues A, Gonzalez-Gonzalez O, Almaraz L, Artime E, Morenilla-Palao C, Gallar J, Viana F, Merayo-Lloves J, Belmonte C, 2018. Morphological and functional changes in TRPM8-expressing corneal cold thermoreceptor neurons during aging and their impact on tearing in mice. J Comp Neurol 526, 1859–1874. [DOI] [PubMed] [Google Scholar]
- Aloe L, Tirassa P, Lambiase A, 2008. The topical application of nerve growth factor as a pharmacological tool for human corneal and skin ulcers. Pharmacological research : the official journal of the Italian Pharmacological Society 57, 253–258. [DOI] [PubMed] [Google Scholar]
- Amparo F, Dastjerdi MH, Okanobo A, Ferrari G, Smaga L, Hamrah P, Jurkunas U, Schaumberg DA, Dana R, 2013. Topical interleukin 1 receptor antagonist for treatment of dry eye disease: a randomized clinical trial. JAMA Ophthalmol 131, 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P, Terenghi G, Warner G, Kopelman P, Williams-Chestnut RE, Sinicropi DV, 1996. The role of endogenous nerve growth factor in human diabetic neuropathy. Nat Med 2, 703–707. [DOI] [PubMed] [Google Scholar]
- Araj H, Tumminia SJ, Yeung DT, 2020. Ocular Surface - Merging Challenges and Opportunities. Translational vision science & technology 9, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso P, Tisdale A, Spurr-Michaud S, Sumiyoshi M, Gipson IK, 2006. Mucin characteristics of human corneal-limbal epithelial cells that exclude the rose bengal anionic dye. Investigative ophthalmology & visual science 47, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghaei K, Tokhanbigli S, Asadzadeh H, Nmaki S, Reza Zali M, Hashemi SM, 2018. Exosomes as a novel cell-free therapeutic approach in gastrointestinal diseases. J Cell Physiol [DOI] [PubMed] [Google Scholar]
- Bain CC, Bravo-Blas A, Scott CL, Perdiguero EG, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM, 2014. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 15, 929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiula M, Spampinato S, 2021. Experimental Pharmacotherapy for Dry Eye Disease: A Review. J Exp Pharmacol 13, 345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsegian A, Lee J, Salifu MO, McFarlane SI, 2018. Corneal Neuropathy: An Underrated Manifestation of Diabetes Mellitus. J Clin Endocrinol Diabetes 2. [Google Scholar]
- Batlle E, Massague J, 2019. Transforming Growth Factor-beta Signaling in Immunity and Cancer. Immunity 50, 924–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum P, Koj S, Kloting N, Bluher M, Classen J, Paeschke S, Gericke M, Toyka KV, Nowicki M, Kosacka J, 2021. Treatment-Induced Neuropathy in Diabetes (TIND)-Developing a Disease Model in Type 1 Diabetic Rats. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman KA, 2014. Characterization of dry eye disease in diabetic patients versus nondiabetic patients. Cornea 33, 851–854. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Rahman M, Thompson R, Stephenson P, Saito H, 2018. TRPV1 and TRPM8 Channels and Nocifensive Behavior in a Rat Model for Dry Eye. Invest Ophthalmol Vis Sci 59, 3739–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlau J, Becker HH, Stave J, Oriwol C, Guthoff RF, 2002. Depth and age-dependent distribution of keratocytes in healthy human corneas: a study using scanning-slit confocal microscopy in vivo. J Cataract Refract Surg 28, 611–616. [DOI] [PubMed] [Google Scholar]
- Besin G, Gaudreau S, Menard M, Guindi C, Dupuis G, Amrani A, 2008. Thymic stromal lymphopoietin and thymic stromal lymphopoietin-conditioned dendritic cells induce regulatory T-cell differentiation and protection of NOD mice against diabetes. Diabetes 57, 2107–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettahi I, Sun H, Gao N, Wang F, Mi X, Chen W, Liu Z, Yu FS, 2014a. Genome-wide transcriptional analysis of differentially expressed genes in diabetic, healing corneal epithelial cells: hyperglycemia-suppressed TGFbeta3 expression contributes to the delay of epithelial wound healing in diabetic corneas. Diabetes 63, 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettahi I, Sun H, Gao N, Wang F, Mi X, Chen W, Liu Z, Yu FS, 2014b. Genome-wide transcriptional analysis of differentially expressed genes in diabetic, healing corneal epithelial cells: hyperglycemia-suppressed TGFbeta3 expression contributes to the delay of epithelial wound healing in diabetic corneas. Diabetes 63, 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Zhou L, Liu Y, Gu J, Mi Q, 2021. MicroRNA-146a deficiency delays wound healing in normal and diabetic mice. Advances in wound care [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikbova G, Oshitari T, Tawada A, Yamamoto S, 2012. Corneal changes in diabetes mellitus. Current diabetes reviews 8, 294–302. [DOI] [PubMed] [Google Scholar]
- Bitirgen G, Akpinar Z, Malik RA, Ozkagnici A, 2017. Use of Corneal Confocal Microscopy to Detect Corneal Nerve Loss and Increased Dendritic Cells in Patients With Multiple Sclerosis. JAMA Ophthalmol 135, 777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitirgen G, Tinkir Kayitmazbatir E, Satirtav G, Malik RA, Ozkagnici A, 2018a. In Vivo Confocal Microscopic Evaluation of Corneal Nerve Fibers and Dendritic Cells in Patients With Behcet’s Disease. Front Neurol 9, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitirgen G, Turkmen K, Malik RA, Ozkagnici A, Zengin N, 2018b. Corneal confocal microscopy detects corneal nerve damage and increased dendritic cells in Fabry disease. Sci Rep 8, 12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black E, Vibe-Petersen J, Jorgensen LN, Madsen SM, Agren MS, Holstein PE, Perrild H, Gottrup F, 2003. Decrease of collagen deposition in wound repair in type 1 diabetes independent of glycemic control. Arch Surg 138, 34–40. [DOI] [PubMed] [Google Scholar]
- Bondugulapati LRN, Nitesh, 2020. Corneal confocal microscopy: potential usage in the context of diabetes mellitus. Practical Diabetes 38, 20–22. [Google Scholar]
- Bonnet C, Gonzalez S, Roberts JS, Robertson SYT, Ruiz M, Zheng J, Deng SX, 2021. Human limbal epithelial stem cell regulation, bioengineering and function. Prog Retin Eye Res, 100956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton AJ, Malik RA, Arezzo JC, Sosenko JM, 2004. Diabetic somatic neuropathies. Diabetes Care 27, 1458–1486. [DOI] [PubMed] [Google Scholar]
- Brazil JC, Quiros M, Nusrat A, Parkos CA, 2019. Innate immune cell-epithelial crosstalk during wound repair. J Clin Invest 129, 2983–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A, 2004. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535. [DOI] [PubMed] [Google Scholar]
- Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL, 2002. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci 43, 2264–2271. [PMC free article] [PubMed] [Google Scholar]
- Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, Knop E, Markoulli M, Ogawa Y, Perez V, Uchino Y, Yokoi N, Zoukhri D, Sullivan DA, 2017. TFOS DEWS II pathophysiology report. Ocul Surf 15, 438–510. [DOI] [PubMed] [Google Scholar]
- Burnham JM, Sakhalkar M, Langford MP, Liang C, Redens TB, Jain SK, 2013. Diabetic and non-diabetic human cornea and tear gamma-glutamyl transpeptidase activity. Clin Ophthalmol 7, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Xu W, Chen W, Peng D, Liu Q, Dong J, Reinach PS, Yan D, 2020. MicroRNA-184 negatively regulates corneal epithelial wound healing via targeting CDC25A, CARM1, and LASP1. Eye and vision 7, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington JC, Ambros V, 2003. Role of microRNAs in plant and animal development. Science 301, 336–338. [DOI] [PubMed] [Google Scholar]
- Cavalli G, Dinarello CA, 2018. Anakinra Therapy for Non-cancer Inflammatory Diseases. Front Pharmacol 9, 1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran K, Salimian M, Konduru SR, Choi J, Kumar P, Long A, Klimova N, Ho CY, Kristian T, Russell JW, 2019. Overexpression of Sirtuin 1 protein in neurons prevents and reverses experimental diabetic neuropathy. Brain 142, 3737–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PY, Carrel H, Huang JS, Wang IJ, Hou YC, Chen WL, Wang JY, Hu FR, 2006. Decreased density of corneal basal epithelium and subbasal corneal nerve bundle changes in patients with diabetic retinopathy. Am J Ophthalmol 142, 488–490. [DOI] [PubMed] [Google Scholar]
- Chaudhary S, Namavari A, Yco L, Chang JH, Sonawane S, Khanolkar V, Sarkar J, Jain S, 2012. Neurotrophins and nerve regeneration-associated genes are expressed in the cornea after lamellar flap surgery. Cornea 31, 1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan SS, Ma P, Chiu IM, 2018. Neuro-immune interactions in inflammation and host defense: Implications for transplantation. Am J Transplant 18, 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yang B, Tian J, Hong H, Du Y, Li K, Li X, Wang N, Yu X, Wei X, 2018. Dental Follicle Stem Cells Ameliorate Lipopolysaccharide-Induced Inflammation by Secreting TGF-beta3 and TSP-1 to Elicit Macrophage M2 Polarization. Cell Physiol Biochem 51, 2290–2308. [DOI] [PubMed] [Google Scholar]
- Coppey LJ, Holmes A, Davidson EP, Yorek MA, 2012. Partial replacement with menhaden oil improves peripheral neuropathy in high-fat-fed low-dose streptozotocin type 2 diabetic rat. J Nutr Metab 2012, 950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortina MS, He J, Li N, Bazan NG, Bazan HE, 2012. Recovery of corneal sensitivity, calcitonin gene-related peptide-positive nerves, and increased wound healing induced by pigment epithelial-derived factor plus docosahexaenoic acid after experimental surgery. Arch Ophthalmol 130, 76–83. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM, 1989. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell 57, 201–209. [DOI] [PubMed] [Google Scholar]
- Cousen P, Cackett P, Bennett H, Swa K, Dhillon B, 2007. Tear production and corneal sensitivity in diabetes. J Diabetes Complications 21, 371–373. [DOI] [PubMed] [Google Scholar]
- Cox SM, Nichols JJ, 2014. The neurobiology of the meibomian glands. Ocul Surf 12, 167–177. [DOI] [PubMed] [Google Scholar]
- Cruzat A, Qazi Y, Hamrah P, 2017. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul Surf 15, 15–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Gao N, Me R, Xu J, Yu FX, 2018. TSLP Protects Corneas From Pseudomonas aeruginosa Infection by Regulating Dendritic Cells and IL-23-IL-17 Pathway. Invest Ophthalmol Vis Sci 59, 4228–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio L, Kalteniece A, Ferdousi M, Azmi S, Petropoulos IN, Ponirakis G, Alam U, Asghar O, Marshall A, Boulton AJM, Efron N, Buzzetti R, Soran H, Malik RA, 2021. Small Nerve Fiber Damage and Langerhans Cells in Type 1 and Type 2 Diabetes and LADA Measured by Corneal Confocal Microscopy. Invest Ophthalmol Vis Sci 62, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt DA, 1989. Signal transduction and control of lacrimal gland protein secretion: a review. Curr Eye Res 8, 619–636. [DOI] [PubMed] [Google Scholar]
- Dartt DA, 2004. Dysfunctional neural regulation of lacrimal gland secretion and its role in the pathogenesis of dry eye syndromes. The ocular surface 2, 76–91. [DOI] [PubMed] [Google Scholar]
- Dartt DA, 2009. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res 28, 155–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanushkodi NR, Srivastava R, Coulon PA, Prakash S, Roy S, Bagnol D, David ED, BenMohamed L, 2021. Healing of Ocular Herpetic Disease Following Treatment With an Engineered FGF-1 Is Associated With Increased Corneal Anti-Inflammatory M2 Macrophages. Front Immunol 12, 673763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di G, Qi X, Zhao X, Zhang S, Danielson P, Zhou Q, 2017a. Corneal Epithelium-Derived Neurotrophic Factors Promote Nerve Regeneration. Invest Ophthalmol Vis Sci 58, 4695–4702. [DOI] [PubMed] [Google Scholar]
- Di G, Zhao X, Qi X, Zhang S, Feng L, Shi W, Zhou Q, 2017b. VEGF-B promotes recovery of corneal innervations and trophic functions in diabetic mice. Sci Rep 7, 40582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didenko TN, Smoliakova GP, Sorokin EL, Egorov VV, 1999. [Clinical and pathogenetic features of neurotrophic corneal disorders in diabetes]. Vestn Oftalmol 115, 7–11. [PubMed] [Google Scholar]
- Divisova S, Vlckova E, Hnojcikova M, Skorna M, Nemec M, Dubovy P, Dusek L, Jarkovsky J, Belobradkova J, Bednarik J, 2012. Prediabetes/early diabetes-associated neuropathy predominantly involves sensory small fibres. J Peripher Nerv Syst 17, 341–350. [DOI] [PubMed] [Google Scholar]
- Djordjevic S, Driscoll PC, 2013. Targeting VEGF signalling via the neuropilin co-receptor. Drug Discov Today 18, 447–455. [DOI] [PubMed] [Google Scholar]
- Doebel T, Voisin B, Nagao K, 2017. Langerhans Cells - The Macrophage in Dendritic Cell Clothing. Trends Immunol 38, 817–828. [DOI] [PubMed] [Google Scholar]
- Eddy AA, Fogo AB, 2006. Plasminogen activator inhibitor-1 in chronic kidney disease: evidence and mechanisms of action. Journal of the American Society of Nephrology : JASN 17, 2999–3012. [DOI] [PubMed] [Google Scholar]
- Efron N, 2011. The Glenn A. Fry award lecture 2010: Ophthalmic markers of diabetic neuropathy. Optometry and vision science : official publication of the American Academy of Optometry 88, 661–683. [DOI] [PubMed] [Google Scholar]
- Efron N, 2012. Anterior Eye Examination, Contact Lens Complications (Third Edition) [Google Scholar]
- Eissa IM, Khalil NM, El-Gendy HA, 2016. A Controlled Study on the Correlation between Tear Film Volume and Tear Film Stability in Diabetic Patients. J Ophthalmol 2016, 5465272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekanayaka SA, McClellan SA, Peng X, Barrett RP, Francis R, Hazlett LD, 2018. HMGB1 Antagonist, Box A, Reduces TLR4, RAGE, and Inflammatory Cytokines in the Cornea of P. aeruginosa-Infected Mice. J Ocul Pharmacol Ther [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand AJ, Cao R, Bjorndahl M, Nystrom S, Jonsson-Rylander AC, Hassani H, Hallberg B, Nordlander M, Cao Y, 2003. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc Natl Acad Sci U S A 100, 6033–6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia L, Contu R, Quintavalle M, Varrone F, Chimenti C, Russo MA, Cimino V, De Marinis L, Frustaci A, Catalucci D, Condorelli G, 2009. Reciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions. Circulation 120, 2377–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana EM, Birk DE, 2020. Composition, structure and function of the corneal stroma. Exp Eye Res 198, 108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Cacicedo JM, Ido Y, 2020. Impaired nicotinamide adenine dinucleotide (NAD(+) ) metabolism in diabetes and diabetic tissues: Implications for nicotinamide-related compound treatment. Journal of diabetes investigation 11, 1403–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V, 2019. Diabetic neuropathy. Nat Rev Dis Primers 5, 41. [DOI] [PubMed] [Google Scholar]
- Ferdousi M, Kalteniece A, Azmi S, Petropoulos IN, Ponirakis G, Alam U, Asghar O, Marshall A, Fullwood C, Jeziorska M, Abbott C, Lauria G, Faber CG, Soran H, Efron N, Boulton AJM, Malik RA, 2021. Diagnosis of Neuropathy and Risk Factors for Corneal Nerve Loss in Type 1 and Type 2 Diabetes: A Corneal Confocal Microscopy Study. Diabetes Care 44, 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson MW, O’Kane S, 2004. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci 359, 839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SMJ, Kroken AR, Nieto V, Grosser MR, Wan SJ, Metruccio MME, Evans DJ, 2020. Contact lens-related corneal infection: Intrinsic resistance and its compromise. Prog Retin Eye Res 76, 100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes V, Clark J, Fix C, Law BA, Morales MO, Qiao X, Ako-Asare K, Goldsmith JG, Carver W, Murray DB, Goldsmith EC, 2013. Type II diabetes promotes a myofibroblast phenotype in cardiac fibroblasts. Life Sci 92, 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RN, 2004. Diabetic retinopathy. The New England journal of medicine 350, 48–58. [DOI] [PubMed] [Google Scholar]
- Fujio K, Komai T, Inoue M, Morita K, Okamura T, Yamamoto K, 2016. Revisiting the regulatory roles of the TGF-beta family of cytokines. Autoimmun Rev 15, 917–922. [DOI] [PubMed] [Google Scholar]
- Funari VA, Winkler M, Brown J, Dimitrijevich SD, Ljubimov AV, Saghizadeh M, 2013. Differentially expressed wound healing-related microRNAs in the human diabetic cornea. PLoS One 8, e84425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nunez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G, 2012. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19, 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Xiang X, 2019. Interleukin-22 from bench to bedside: a promising drug for epithelial repair. Cell Mol Immunol 16, 666–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wang Y, Zhao X, Chen P, Xie L, 2015a. MicroRNA-204–5p-Mediated Regulation of SIRT1 Contributes to the Delay of Epithelial Cell Cycle Traversal in Diabetic Corneas. Invest Ophthalmol Vis Sci 56, 1493–1504. [DOI] [PubMed] [Google Scholar]
- Gao N, Kumar A, Yu FS, 2015b. Matrix Metalloproteinase-13 as a Target for Suppressing Corneal Ulceration Caused by Pseudomonas aeruginosa Infection. J Infect Dis 212, 116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N, Lee P, Yu F-S, 2016a. Intraepithelial dendritic cells and sensory nerves are structurally associated and functional interdependent in the cornea. Sci Rep 6, 36414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N, Lee P, Yu FS, 2016b. Intraepithelial dendritic cells and sensory nerves are structurally associated and functional interdependent in the cornea. Sci Rep 6, 36414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N, Yan C, Lee P, Sun H, Yu FS, 2016c. Dendritic cell dysfunction and diabetic sensory neuropathy in the cornea. J Clin Invest 126, 1998–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N, Yin J, Yoon GS, Mi QS, Yu FS, 2011. Dendritic cell-epithelium interplay is a determinant factor for corneal epithelial wound repair. The American journal of pathology 179, 2243–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Hirschfeld J, Lopez-Briones LG, Belmonte C, 1994. Neurotrophic influences on corneal epithelial cells. Experimental eye research 59, 597–605. [DOI] [PubMed] [Google Scholar]
- Gasteiger G, Rudensky AY, 2014. Interactions between innate and adaptive lymphocytes. Nat Rev Immunol 14, 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, Vaughan DE, 2012. PAI-1 in tissue fibrosis. J Cell Physiol 227, 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger RJ, Hollis ER 2nd, Tuszynski MH, 2010. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol 2, a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, Randolph GJ, Merad M, 2006. Langerhans cells arise from monocytes in vivo. Nat Immunol 7, 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels M, 2000. Tear secretion and tear film function in insulin dependent diabetics. Br J Ophthalmol 84, 19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin A, Beckman JA, Schmidt AM, Creager MA, 2006. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 114, 597–605. [DOI] [PubMed] [Google Scholar]
- Goshima Y, Yamashita N, Nakamura F, Sasaki Y, 2016. Regulation of dendritic development by semaphorin 3A through novel intracellular remote signaling. Cell Adh Migr 10, 627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA, 1995. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 270, 1189–1192. [DOI] [PubMed] [Google Scholar]
- GS J, Antonio L, Andrés G, 2019. Correlation between type 2 diabetes, dry eye and Meibomian glands dysfunction. J Optom 12, 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Moreno A, Baudouin C, Melik Parsadaniantz S, Reaux-Le Goazigo A, 2020. Morphological and Functional Changes of Corneal Nerves and Their Contribution to Peripheral and Central Sensory Abnormalities. Frontiers in cellular neuroscience 14, 610342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A, Wegscheider K, Wiegand W, 2009. Changes of extracellular matrix of the cornea in diabetes mellitus. Graefes Arch Clin Exp Ophthalmol 247, 1369–1374. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Dana MR, 2007. Corneal antigen-presenting cells. Chem Immunol Allergy 92, 58–70. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Liu Y, Zhang Q, Dana MR, 2003. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci 44, 581–589. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Zhang Q, Liu Y, Dana MR, 2002. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Invest Ophthalmol Vis Sci 43, 639–646. [PubMed] [Google Scholar]
- Han KY, Tran JA, Chang JH, Azar DT, Zieske JD, 2017. Potential role of corneal epithelial cell-derived exosomes in corneal wound healing and neovascularization. Sci Rep 7, 40548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway CK, Gasim AM, Grant R, Chang AS, Kim HS, Madden VJ, Bagnell CR Jr., Jennette JC, Smithies O, Kakoki M, 2015. Low TGFbeta1 expression prevents and high expression exacerbates diabetic nephropathy in mice. Proc Natl Acad Sci U S A 112, 5815–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Chauhan SK, Lee H, Ueno H, Dana R, Kaplan DH, Saban DR, 2011. Characterization of Langerin-expressing dendritic cell subsets in the normal cornea. Investigative ophthalmology & visual science 52, 4598–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Yablonski ME, Boxrud C, Fong N, Berger C, Jovanovic LJ, 1989. Decreased formation of aqueous humour in insulin-dependent diabetic patients. Br J Ophthalmol 73, 621–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Bazan HE, 2016. Neuroanatomy and Neurochemistry of Mouse Cornea. Invest Ophthalmol Vis Sci 57, 664–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Pham TL, Kakazu A, Bazan HEP, 2017. Recovery of Corneal Sensitivity and Increase in Nerve Density and Wound Healing in Diabetic Mice After PEDF Plus DHA Treatment. Diabetes 66, 2511–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heigle TJ, Pflugfelder SC, 1996. Aqueous tear production in patients with neurotrophic keratitis. Cornea 15, 135–138. [DOI] [PubMed] [Google Scholar]
- Heim KR, Mulla MJ, Potter JA, Han CS, Guller S, Abrahams VM, 2018. Excess glucose induce trophoblast inflammation and limit cell migration through HMGB1 activation of Toll-Like receptor 4. Am J Reprod Immunol 80, e13044. [DOI] [PubMed] [Google Scholar]
- Hirata H, Oshinsky ML, 2012. Ocular dryness excites two classes of corneal afferent neurons implicated in basal tearing in rats: involvement of transient receptor potential channels. Journal of neurophysiology 107, 1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, Choy SH, Grisotto M, Renia L, Conway SJ, Stanley ER, Chan JK, Ng LG, Samokhvalov IM, Merad M, Ginhoux F, 2012. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med 209, 1167–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogerheyde TA, Stephenson SA, Harkin DG, Bray LJ, Madden PW, Woolf MI, Richardson NA, 2013. Evaluation of Eph receptor and ephrin expression within the human cornea and limbus. Exp Eye Res 107, 110–120. [DOI] [PubMed] [Google Scholar]
- Holly FJ, Lemp MA, 1977. Tear physiology and dry eyes. Surv Ophthalmol 22, 69–87. [DOI] [PubMed] [Google Scholar]
- Holly FJ, Patten JT, Dohlman CH, 1977. Surface activity determination of aqueous tear components in dry eye patients and normals. Exp Eye Res 24, 479–491. [DOI] [PubMed] [Google Scholar]
- Holzmann B, 2013a. Antiinflammatory activities of CGRP modulating innate immune responses in health and disease. Curr Protein Pept Sci 14, 268–274. [DOI] [PubMed] [Google Scholar]
- Holzmann B, 2013b. Modulation of immune responses by the neuropeptide CGRP. Amino Acids 45, 1–7. [DOI] [PubMed] [Google Scholar]
- Hong S, Agresta L, Guo C, Wiley JW, 2008. The TRPV1 receptor is associated with preferential stress in large dorsal root ganglion neurons in early diabetic sensory neuropathy. Journal of neurochemistry 105, 1212–1222. [DOI] [PubMed] [Google Scholar]
- Hoy SM, 2018. Patisiran: First Global Approval. Drugs 78, 1625–1631. [DOI] [PubMed] [Google Scholar]
- Hu J, Hu X, Kan T, 2019. MiR-34c Participates in Diabetic Corneal Neuropathy Via Regulation of Autophagy. Invest Ophthalmol Vis Sci 60, 16–25. [DOI] [PubMed] [Google Scholar]
- Hu J, Huang Y, Lin Y, Lin J, 2020. Protective effect inhibiting the expression of miR-181a on the diabetic corneal nerve in a mouse model. Exp Eye Res 192, 107925. [DOI] [PubMed] [Google Scholar]
- Huang YK, Lu YG, Zhao X, Zhang JB, Zhang FM, Chen Y, Bi LB, Gu JH, Jiang ZJ, Wu XM, Li QY, Liu Y, Shen JX, Liu XJ, 2020. Cytokine activin C ameliorates chronic neuropathic pain in peripheral nerve injury rodents by modulating the TRPV1 channel. Br J Pharmacol 177, 5642–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebener P, Pradere JP, Hernandez C, Gwak GY, Caviglia JM, Mu X, Loike JD, Schwabe RF, 2015. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J Clin Invest 125, 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyndiuk RA, Kazarian EL, Schultz RO, Seideman S, 1977. Neurotrophic corneal ulcers in diabetes mellitus. Arch Ophthalmol 95, 2193–2196. [DOI] [PubMed] [Google Scholar]
- Inoue K, Okugawa K, Amano S, Oshika T, Takamura E, Egami F, Umizu G, Aikawa K, Kato S, 2005. Blinking and superficial punctate keratopathy in patients with diabetes mellitus. Eye 19, 418–421. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, 2007. Mucosal dendritic cells. Annu Rev Immunol 25, 381–418. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R, 2015. Control of adaptive immunity by the innate immune system. Nat Immunol 16, 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager MJ, 1992. Corneal Langerhans cells and ocular immunology. Reg Immunol 4, 186–195. [PubMed] [Google Scholar]
- Jalbert I, Stapleton F, Papas E, Sweeney DF, Coroneo M, 2003. In vivo confocal microscopy of the human cornea. The British journal of ophthalmology 87, 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamali A, Hu K, Sendra VG, Blanco T, Lopez MJ, Ortiz G, Qazi Y, Zheng L, Turhan A, Harris DL, Hamrah P, 2020. Characterization of Resident Corneal Plasmacytoid Dendritic Cells and Their Pivotal Role in Herpes Simplex Keratitis. Cell Rep 32, 108099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamali A, Kenyon B, Ortiz G, Abou-Slaybi A, Sendra VG, Harris DL, Hamrah P, 2021. Plasmacytoid dendritic cells in the eye. Prog Retin Eye Res 80, 100877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang LJ, Margo CE, Espana EM, 2021. Diseases of the corneal endothelium. Exp Eye Res 205, 108495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jende JME, Groener JB, Oikonomou D, Heiland S, Kopf S, Pham M, Nawroth P, Bendszus M, Kurz FT, 2018. Diabetic neuropathy differs between type 1 and type 2 diabetes: Insights from magnetic resonance neurography. Ann Neurol 83, 588–598. [DOI] [PubMed] [Google Scholar]
- Jiang X, McClellan SA, Barrett RP, Zhang Y, Foldenauer ME, Hazlett LD, 2012. The role of VIP in cornea. Invest Ophthalmol Vis Sci 53, 7560–7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H, He X, Zheng J, 2018. Exosomes and regenerative medicine: state of the art and perspectives. Transl Res 196, 1–16. [DOI] [PubMed] [Google Scholar]
- Jones MA, Marfurt CF, 1991. Calcitonin gene-related peptide and corneal innervation: a developmental study in the rat. J Comp Neurol 313, 132–150. [DOI] [PubMed] [Google Scholar]
- Kajdaniuk D, Marek B, Borgiel-Marek H, Kos-Kudla B, 2013. Transforming growth factor beta1 (TGFbeta1) in physiology and pathology. Endokrynol Pol 64, 384–396. [DOI] [PubMed] [Google Scholar]
- Kaji Y, Usui T, Oshika T, Matsubara M, Yamashita H, Araie M, Murata T, Ishibashi T, Nagai R, Horiuchi S, Amano S, 2000. Advanced glycation end products in diabetic corneas. Invest Ophthalmol Vis Sci 41, 362–368. [PubMed] [Google Scholar]
- Kallinikos P, Berhanu M, O’Donnell C, Boulton AJ, Efron N, Malik RA, 2004. Corneal nerve tortuosity in diabetic patients with neuropathy. Investigative ophthalmology & visual science 45, 418–422. [DOI] [PubMed] [Google Scholar]
- Kalteniece A, Ferdousi M, Azmi S, Marshall A, Soran H, Malik RA, 2018. Keratocyte Density Is Reduced and Related to Corneal Nerve Damage in Diabetic Neuropathy. Invest Ophthalmol Vis Sci 59, 3584–3590. [DOI] [PubMed] [Google Scholar]
- Katsyuba E, Auwerx J, 2017. Modulating NAD(+) metabolism, from bench to bedside. EMBO J 36, 2670–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay L, Edwards K, Stapleton F, 2009. Signs, symptoms, and comorbidities in contact lens-related microbial keratitis. Optom Vis Sci 86, 803–809. [DOI] [PubMed] [Google Scholar]
- Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J, 2020. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health 10, 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, 2012. The use of animal models in diabetes research. Br J Pharmacol 166, 877–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenkler B, Sheardown H, Jones L, 2007. Growth factors in the tear film: role in tissue maintenance, wound healing, and ocular pathology. Ocul Surf 5, 228–239. [DOI] [PubMed] [Google Scholar]
- Kneer K, Green MB, Meyer J, Rich CB, Minns MS, Trinkaus-Randall V, 2018. High fat diet induces pre-type 2 diabetes with regional changes in corneal sensory nerves and altered P2X7 expression and localization. Exp Eye Res 175, 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh TJ, DiPietro LA, 2011. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med 13, e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhaas M, 1998. Corneal sensation after cataract and refractive surgery. J Cataract Refract Surg 24, 1399–1409. [DOI] [PubMed] [Google Scholar]
- Komai T, Okamura T, Inoue M, Yamamoto K, Fujio K, 2018. Reevaluation of Pluripotent Cytokine TGF-beta3 in Immunity. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramerov AA, Saghizadeh M, Ljubimov AV, 2016a. Adenoviral Gene Therapy for Diabetic Keratopathy: Effects on Wound Healing and Stem Cell Marker Expression in Human Organ-cultured Corneas and Limbal Epithelial Cells. J Vis Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramerov AA, Saghizadeh M, Ljubimov AV, 2016b. Adenoviral Gene Therapy for Diabetic Keratopathy: Effects on Wound Healing and Stem Cell Marker Expression in Human Organ-cultured Corneas and Limbal Epithelial Cells. J Vis Exp, e54058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramerov AA, Saghizadeh M, Maguen E, Rabinowitz YS, Ljubimov AV, 2015. Persistence of reduced expression of putative stem cell markers and slow wound healing in cultured diabetic limbal epithelial cells. Mol Vis 21, 1357–1367. [PMC free article] [PubMed] [Google Scholar]
- Kramerov AA, Shah R, Ding H, Holler E, Turjman S, Rabinowitz YS, Ghiam S, Maguen E, Svendsen CN, Saghizadeh M, Ljubimova JY, Ljubimov AV, 2021. Novel nanopolymer RNA therapeutics normalize human diabetic corneal wound healing and epithelial stem cells. Nanomedicine 32, 102332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksander BR, Kolovou PE, Wilson BJ, Saab KR, Guo Q, Ma J, McGuire SP, Gregory MS, Vincent WJ, Perez VL, Cruz-Guilloty F, Kao WW, Call MK, Tucker BA, Zhan Q, Murphy GF, Lathrop KL, Alt C, Mortensen LJ, Lin CP, Zieske JD, Frank MH, Frank NY, 2014. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature 511, 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni M, Leszczynska A, Wei G, Winkler MA, Tang J, Funari VA, Deng N, Liu Z, Punj V, Deng SX, Ljubimov AV, Saghizadeh M, 2017. Genome-wide analysis suggests a differential microRNA signature associated with normal and diabetic human corneal limbus. Sci Rep 7, 3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurashima Y, Amiya T, Nochi T, Fujisawa K, Haraguchi T, Iba H, Tsutsui H, Sato S, Nakajima S, Iijima H, Kubo M, Kunisawa J, Kiyono H, 2012. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat Commun 3, 1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagali NS, Badian RA, Liu X, Feldreich TR, Arnlov J, Utheim TP, Dahlin LB, Rolandsson O, 2018. Dendritic cell maturation in the corneal epithelium with onset of type 2 diabetes is associated with tumor necrosis factor receptor superfamily member 9. Sci Rep 8, 14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TN, Nicholas SE, Choi A, Ma JX, Karamichos D, 2021. Cellular Contractility Profiles of Human Diabetic Corneal Stromal Cells. Anal Cell Pathol (Amst) 2021, 9913210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Bonini S, Bonini S, Micera A, Magrini L, Bracci-Laudiero L, Aloe L, 1995. Increased plasma levels of nerve growth factor in vernal keratoconjunctivitis and relationship to conjunctival mast cells. Invest Ophthalmol Vis Sci 36, 2127–2132. [PubMed] [Google Scholar]
- Lambiase A, Manni L, Rama P, Bonini S, 2003. Clinical application of nerve growth factor on human corneal ulcer. Arch Ital Biol 141, 141–148. [PubMed] [Google Scholar]
- Lee EJ, Rosenbaum JT, Planck SR, 2010a. Epifluorescence intravital microscopy of murine corneal dendritic cells. Invest Ophthalmol Vis Sci 51, 2101–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Ryu IH, Seo KY, Hong S, Kim HC, Kim EK, 2006. Topical 0.1% prednisolone lowers nerve growth factor expression in keratoconjunctivitis sicca patients. Ophthalmology 113, 198–205. [DOI] [PubMed] [Google Scholar]
- Lee JH, Yang SH, Oh JM, Lee MG, 2010b. Pharmacokinetics of drugs in rats with diabetes mellitus induced by alloxan or streptozocin: comparison with those in patients with type I diabetes mellitus. J Pharm Pharmacol 62, 1–23. [DOI] [PubMed] [Google Scholar]
- Lee PS, Gao N, Dike M, Shkilnyy O, Me R, Zhang Y, Yu FX, 2019. Opposing Effects of Neuropilin-1 and −2 on Sensory Nerve Regeneration in Wounded Corneas: Role of Sema3C in Ameliorating Diabetic Neurotrophic Keratopathy. Diabetes 68, 807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer MS, Sun TT, Lavker RM, 1998. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J Cell Sci 111 ( Pt 19), 2867–2875. [DOI] [PubMed] [Google Scholar]
- Leppin K, Behrendt AK, Reichard M, Stachs O, Guthoff RF, Baltrusch S, Eule JC, Vollmar B, 2014. Diabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the cornea. Invest Ophthalmol Vis Sci 55, 3603–3615. [DOI] [PubMed] [Google Scholar]
- Leszczynska A, Kulkarni M, Ljubimov AV, Saghizadeh M, 2018. Exosomes from normal and diabetic human corneolimbal keratocytes differentially regulate migration, proliferation and marker expression of limbal epithelial cells. Sci Rep 8, 15173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung E, Landa G, 2013. Update on current and future novel therapies for dry age-related macular degeneration. Expert review of clinical pharmacology 6, 565–579. [DOI] [PubMed] [Google Scholar]
- Lewis EJH, Lovblom LE, Ferdousi M, Halpern EM, Jeziorska M, Pacaud D, Pritchard N, Dehghani C, Edwards K, Srinivasan S, Mintz Shtein R, Efron N, Tavakoli M, Bril V, Malik RA, Perkins BA, 2020. Rapid Corneal Nerve Fiber Loss: A Marker of Diabetic Neuropathy Onset and Progression. Diabetes Care 43, 1829–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DQ, Tseng SC, 1995. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. Journal of cellular physiology 163, 61–79. [DOI] [PubMed] [Google Scholar]
- Li F, Yang W, Jiang H, Guo C, Huang AJW, Hu H, Liu Q, 2019a. TRPV1 activity and substance P release are required for corneal cold nociception. Nat Commun 10, 5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Zhao SZ, 2014. Mesenchymal stem cells: Potential role in corneal wound repair and transplantation. World J Stem Cells 6, 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Li B, Jiang H, Wang Y, Qu M, Duan H, Zhou Q, Shi W, 2013. Macrophage depletion impairs corneal wound healing after autologous transplantation in mice. PLoS One 8, e61799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TP, Guo Z, Liu CJ, Sun T, Chen L, Zhao X, 2017. Association of down-regulation of calcitonin gene-related peptide and substance P with increase of myocardial vulnerability in diabetic neuropathic rats. Peptides 96, 1–7. [DOI] [PubMed] [Google Scholar]
- Li X, Zhou H, Tang W, Guo Q, Zhang Y, 2015. Transient downregulation of microRNA-206 protects alkali burn injury in mouse cornea by regulating connexin 43. Int J Clin Exp Pathol 8, 2719–2727. [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li J, Zhao C, Yang L, Qi X, Wang X, Zhou Q, Shi W, 2021. Hyperglycemia-reduced NAD(+) biosynthesis impairs corneal epithelial wound healing in diabetic mice. Metabolism 114, 154402. [DOI] [PubMed] [Google Scholar]
- Li Y, Ma X, Li J, Yang L, Zhao X, Qi X, Zhang X, Zhou Q, Shi W, 2019b. Corneal Denervation Causes Epithelial Apoptosis Through Inhibiting NAD+ Biosynthesis. Invest Ophthalmol Vis Sci 60, 3538–3546. [DOI] [PubMed] [Google Scholar]
- Li Z, Burns AR, Han L, Rumbaut RE, Smith CW, 2011a. IL-17 and VEGF are necessary for efficient corneal nerve regeneration. Am J Pathol 178, 1106–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Burns AR, Miller SB, Smith CW, 2011b. CCL20, gammadelta T cells, and IL-22 in corneal epithelial healing. FASEB J 25, 2659–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, Song X, Ren Y, Shan PF, 2020. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep 10, 14790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little K, Llorian-Salvador M, Tang M, Du X, Marry S, Chen M, Xu H, 2020. Macrophage to myofibroblast transition contributes to subretinal fibrosis secondary to neovascular age-related macular degeneration. J Neuroinflammation 17, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BF, Miyata S, Kojima H, Uriuhara A, Kusunoki H, Suzuki K, Kasuga M, 1999. Low phagocytic activity of resident peritoneal macrophages in diabetic mice: relevance to the formation of advanced glycation end products. Diabetes 48, 2074–2082. [DOI] [PubMed] [Google Scholar]
- Liu J, Jiang F, Jiang Y, Wang Y, Li Z, Shi X, Zhu Y, Wang H, Zhang Z, 2020. Roles of Exosomes in Ocular Diseases. Int J Nanomedicine 15, 10519–10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Li Z, 2021. Resident Innate Immune Cells in the Cornea. Front Immunol 12, 620284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Xue Y, Dong D, Xiao C, Lin C, Wang H, Song F, Fu T, Wang Z, Chen J, Pan H, Li Y, Cai D, Li Z, 2017. CCR2(−) and CCR2(+) corneal macrophages exhibit distinct characteristics and balance inflammatory responses after epithelial abrasion. Mucosal Immunol 10, 1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QS, Pang ZR, Liu R, He GR, Cui J, Yin XY, 2011. Effective compounds group of Mongolian prescriptions BAIMAI-SAN protect against peripheral neuropathy in lower limbs of rats through neuro protective effect. J Ethnopharmacol 135, 786–791. [DOI] [PubMed] [Google Scholar]
- Liu X, Gao N, Dong C, Zhou L, Mi QS, Standiford TJ, Yu FS, 2014. Flagellin-induced expression of CXCL10 mediates direct fungal killing and recruitment of NK cells to the cornea in response to Candida albicans infection. Eur J Immunol 44, 2667–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YC, Lin MT, Mehta JS, 2021. Analysis of corneal nerve plexus in corneal confocal microscopy images. Neural regeneration research 16, 690–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubimov AV, Huang ZS, Huang GH, Burgeson RE, Gullberg D, Miner JH, Ninomiya Y, Sado Y, Kenney MC, 1998. Human corneal epithelial basement membrane and integrin alterations in diabetes and diabetic retinopathy. J Histochem Cytochem 46, 1033–1041. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Saghizadeh M, 2015. Progress in corneal wound healing. Prog Retin Eye Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood A, Hope-Ross M, Chell P, 2006. Neurotrophic keratopathy and diabetes mellitus. Eye (Lond) 20, 837–839. [DOI] [PubMed] [Google Scholar]
- Ma LJ, Fogo AB, 2009. PAI-1 and kidney fibrosis. Front Biosci (Landmark Ed) 14, 2028–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankus C, Rich C, Minns M, Trinkaus-Randall V, 2011. Corneal epithelium expresses a variant of P2X(7) receptor in health and disease. PLoS One 6, e28541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoor H, Tan HC, Lin MT, Mehta JS, Liu YC, 2020. Diabetic Corneal Neuropathy. Journal of clinical medicine 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfurt CF, Kingsley RE, Echtenkamp SE, 1989. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Invest Ophthalmol Vis Sci 30, 461–472. [PubMed] [Google Scholar]
- Markoulli M, Colorado LH, Edwards K, 2020. The Relationship between Corneal Nerve Morphology and Inflammatory Mediators and Neuropeptides in Healthy Individuals. Optom Vis Sci 97, 145–153. [DOI] [PubMed] [Google Scholar]
- Markoulli M, Flanagan J, Tummanapalli SS, Wu J, Willcox M, 2018. The impact of diabetes on corneal nerve morphology and ocular surface integrity. The ocular surface 16, 45–57. [DOI] [PubMed] [Google Scholar]
- Martin XY, Safran AB, 1988. Corneal hypoesthesia. Surv Ophthalmol 33, 28–40. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D’Amore PA, 2007. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol 170, 1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzaioli V, Canavan M, Floudas A, Wade SC, Low C, Veale DJ, Fearon U, 2020. Monocyte-Derived Dendritic Cell Differentiation in Inflammatory Arthritis Is Regulated by the JAK/STAT Axis via NADPH Oxidase Regulation. Front Immunol 11, 1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott AM, 2013. Antimicrobial compounds in tears. Exp Eye Res 117, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott AM, Kern TS, Murphy CJ, 1998. The effect of elevated extracellular glucose on migration, adhesion and proliferation of SV40 transformed human corneal epithelial cells. Current eye research 17, 924–932. [DOI] [PubMed] [Google Scholar]
- Mecollari V, Nieuwenhuis B, Verhaagen J, 2014. A perspective on the role of class III semaphorin signaling in central nervous system trauma. Frontiers in cellular neuroscience 8, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra S, Tavakoli M, Kallinikos PA, Efron N, Boulton AJ, Augustine T, Malik RA, 2007. Corneal confocal microscopy detects early nerve regeneration after pancreas transplantation in patients with type 1 diabetes. Diabetes Care 30, 2608–2612. [DOI] [PubMed] [Google Scholar]
- Meng XM, Wang S, Huang XR, Yang C, Xiao J, Zhang Y, To KF, Nikolic-Paterson DJ, Lan HY, 2016. Inflammatory macrophages can transdifferentiate into myofibroblasts during renal fibrosis. Cell Death Dis 7, e2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshkani R, Vakili S, 2016. Tissue resident macrophages: Key players in the pathogenesis of type 2 diabetes and its complications. Clin Chim Acta 462, 77–89. [DOI] [PubMed] [Google Scholar]
- Mikulec AA, Tanelian DL, 1996. CGRP increases the rate of corneal re-epithelialization in an in vitro whole mount preparation. J Ocul Pharmacol Ther 12, 417–423. [DOI] [PubMed] [Google Scholar]
- Millodot M, 1984. A review of research on the sensitivity of the cornea. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians 4, 305–318. [PubMed] [Google Scholar]
- Millodot M, Owens H, 1984. The influence of age on the fragility of the cornea. Acta Ophthalmol (Copenh) 62, 819–824. [DOI] [PubMed] [Google Scholar]
- Misra SL, Patel DV, McGhee CN, Pradhan M, Kilfoyle D, Braatvedt GD, Craig JP, 2014. Peripheral neuropathy and tear film dysfunction in type 1 diabetes mellitus. J Diabetes Res 2014, 848659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi H, Thomasy SM, Russell P, Murphy CJ, 2018. The role of hepatocyte growth factor in corneal wound healing. Exp Eye Res 166, 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseev RV, Morrison PWJ, Steele F, Khutoryanskiy VV, 2019. Penetration Enhancers in Ocular Drug Delivery. Pharmaceutics 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Montanes J, Bleau AM, Jimenez AI, 2018. Tivanisiran, a novel siRNA for the treatment of dry eye disease. Expert Opin Investig Drugs 27, 421–426. [DOI] [PubMed] [Google Scholar]
- Muller LJ, Marfurt CF, Kruse F, Tervo TM, 2003. Corneal nerves: structure, contents and function. Exp Eye Res 76, 521–542. [DOI] [PubMed] [Google Scholar]
- Muller LJ, Vrensen GF, Pels L, Cardozo BN, Willekens B, 1997. Architecture of human corneal nerves. Invest Ophthalmol Vis Sci 38, 985–994. [PubMed] [Google Scholar]
- Murphy PJ, Patel S, Marshall J, 2001. The effect of long-term, daily contact lens wear on corneal sensitivity. Cornea 20, 264–269. [DOI] [PubMed] [Google Scholar]
- Nagano T, Nakamura M, Nakata K, Yamaguchi T, Takase K, Okahara A, Ikuse T, Nishida T, 2003. Effects of substance P and IGF-1 in corneal epithelial barrier function and wound healing in a rat model of neurotrophic keratopathy. Invest Ophthalmol Vis Sci 44, 3810–3815. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Kawahara M, Nakata K, Nishida T, 2003. Restoration of corneal epithelial barrier function and wound healing by substance P and IGF-1 in rats with capsaicin-induced neurotrophic keratopathy. Investigative ophthalmology & visual science 44, 2937–2940. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Ofuji K, Chikama T, Nishida T, 1997. Combined effects of substance P and insulin-like growth factor-1 on corneal epithelial wound closure of rabbit in vivo. Curr Eye Res 16, 275–278. [DOI] [PubMed] [Google Scholar]
- Nakata M, Okada Y, Kobata H, Shigematsu T, Reinach PS, Tomoyose K, Saika S, 2014. Diabetes mellitus suppresses hemodialysis-induced increases in tear fluid secretion. BMC Res Notes 7, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa D, Kumar S, Desai J, Anders HJ, 2017. Neutrophil extracellular traps in tissue pathology. Histol Histopathol 32, 203–213. [DOI] [PubMed] [Google Scholar]
- Neira-Zalentein W, Holopainen JM, Tervo TM, Borras F, Acosta MC, Belmonte C, Gallar J, 2011. Corneal sensitivity in diabetic patients subjected to retinal laser photocoagulation. Investigative ophthalmology & visual science 52, 6043–6049. [DOI] [PubMed] [Google Scholar]
- Nentwich MM, Ulbig MW, 2015. Diabetic retinopathy - ocular complications of diabetes mellitus. World journal of diabetes 6, 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen NV, 1978. Corneal sensitivity and vibratory perception in diabetes mellitus. Acta Ophthalmol (Copenh) 56, 406–411. [DOI] [PubMed] [Google Scholar]
- Nishida T, Chikama T, Sawa M, Miyata K, Matsui T, Shigeta K, 2012. Differential contributions of impaired corneal sensitivity and reduced tear secretion to corneal epithelial disorders. Japanese journal of ophthalmology 56, 20–25. [DOI] [PubMed] [Google Scholar]
- Nishida T, Nakamura M, Ofuji K, Reid TW, Mannis MJ, Murphy CJ, 1996. Synergistic effects of substance P with insulin-like growth factor-1 on epithelial migration of the cornea. Journal of cellular physiology 169, 159–166. [DOI] [PubMed] [Google Scholar]
- Norrick A, Esterlechner J, Niebergall-Roth E, Dehio U, Sadeghi S, Schroder HM, Ballikaya S, Stemler N, Ganss C, Dieter K, Dachtler AK, Merz P, Sel S, Chodosh J, Cursiefen C, Frank NY, Auffarth GU, Ksander B, Frank MH, Kluth MA, 2021. Process development and safety evaluation of ABCB5(+) limbal stem cells as advanced-therapy medicinal product to treat limbal stem cell deficiency. Stem Cell Res Ther 12, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley AM, Shanley DK, Kelly AT, Barry DS, 2014. Towards an understanding of semaphorin signalling in the spinal cord. Gene 553, 69–74. [DOI] [PubMed] [Google Scholar]
- Oda T, Jung YO, Kim HS, Cai X, Lopez-Guisa JM, Ikeda Y, Eddy AA, 2001. PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int 60, 587–596. [DOI] [PubMed] [Google Scholar]
- Okada Y, Reinach PS, Shirai K, Kitano-Izutani A, Miyajima M, Yamanaka O, Sumioka T, Saika S, 2015. Transient Receptor Potential Channels and Corneal Stromal Inflammation. Cornea 34 Suppl 11, S136–141. [DOI] [PubMed] [Google Scholar]
- Okumura N, Koizumi N, 2020. Regeneration of the Corneal Endothelium. Curr Eye Res 45, 303–312. [DOI] [PubMed] [Google Scholar]
- Ostenson CG, Efendic S, 2007. Islet gene expression and function in type 2 diabetes; studies in the Goto-Kakizaki rat and humans. Diabetes Obes Metab 9 Suppl 2, 180–186. [DOI] [PubMed] [Google Scholar]
- Pabbidi MR, Premkumar LS, 2017. Role of Transient Receptor Potential Channels Trpv1 and Trpm8 in Diabetic Peripheral Neuropathy. J Diabetes Treat 2017 [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Liu F, Qi X, Hu Y, Xu F, Jia H, 2018. Nerve Growth Factor Changes and Corneal Nerve Repair after Keratoplasty. Optom Vis Sci 95, 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquin S, Sharma M, Gauchat JF, 2015. Ciliary neurotrophic factor (CNTF): New facets of an old molecule for treating neurodegenerative and metabolic syndrome pathologies. Cytokine Growth Factor Rev. [DOI] [PubMed] [Google Scholar]
- Patel NN, Kohanski MA, Maina IW, Workman AD, Herbert DR, Cohen NA, 2019. Sentinels at the wall: epithelial-derived cytokines serve as triggers of upper airway type 2 inflammation. Int Forum Allergy Rhinol 9, 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Bravo Vazquez LA, Perez Uribe S, Roxana Reyes-Perez P, Sharma A, 2020. Current Status of microRNA-Based Therapeutic Approaches in Neurodegenerative Disorders. Cells 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K, Bai Y, Zhu Q, Hu B, Xu Y, 2019. Targeting VEGF-neuropilin interactions: a promising antitumor strategy. Drug Discov Today 24, 656–664. [DOI] [PubMed] [Google Scholar]
- Perocheau D, Touramanidou L, Gurung S, Gissen P, Baruteau J, 2021. Clinical applications for exosomes: Are we there yet? Br J Pharmacol 178, 2375–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos IN, Alam U, Fadavi H, Asghar O, Green P, Ponirakis G, Marshall A, Boulton AJ, Tavakoli M, Malik RA, 2013. Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes care 36, 3646–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC, Massaro-Giordano M, Perez VL, Hamrah P, Deng SX, Espandar L, Foster CS, Affeldt J, Seedor JA, Afshari NA, Chao W, Allegretti M, Mantelli F, Dana R, 2020. Topical Recombinant Human Nerve Growth Factor (Cenegermin) for Neurotrophic Keratopathy: A Multicenter Randomized Vehicle-Controlled Pivotal Trial. Ophthalmology 127, 14–26. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC, Stern ME, 2020. Biological functions of tear film. Exp Eye Res 197, 108115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger G, Vinik A, 2003. Nerve growth factor and diabetic neuropathy. Exp Diabesity Res 4, 271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe AJ, Kulkarni M, Leszczynska A, Tang J, Shah R, Jami-Alahmadi Y, Wang J, Kramerov AA, Wohlschlegel J, Punj V, Ljubimov AV, Saghizadeh M, 2020. Integrated Transcriptome and Proteome Analyses Reveal the Regulatory Role of miR-146a in Human Limbal Epithelium via Notch Signaling. Cells 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M, 2008. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 8, 915–928. [DOI] [PubMed] [Google Scholar]
- Portha B, Giroix MH, Serradas P, Gangnerau MN, Movassat J, Rajas F, Bailbe D, Plachot C, Mithieux G, Marie JC, 2001. beta-cell function and viability in the spontaneously diabetic GK rat: information from the GK/Par colony. Diabetes 50 Suppl 1, S89–93. [DOI] [PubMed] [Google Scholar]
- Pritchard N, Edwards K, Vagenas D, Russell AW, Malik RA, Efron N, 2012a. Corneal sensitivity is related to established measures of diabetic peripheral neuropathy. Clinical & experimental optometry 95, 355–361. [DOI] [PubMed] [Google Scholar]
- Pritchard N, Edwards K, Vagenas D, Russell AW, Malik RA, Efron N, 2012b. Corneal sensitivity is related to established measures of diabetic peripheral neuropathy. Clinical & experimental optometry : journal of the Australian Optometrical Association 95, 355–361. [DOI] [PubMed] [Google Scholar]
- Priyadarsini S, McKay TB, Sarker-Nag A, Allegood J, Chalfant C, Ma JX, Karamichos D, 2016a. Complete metabolome and lipidome analysis reveals novel biomarkers in the human diabetic corneal stroma. Exp Eye Res 153, 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarsini S, Sarker-Nag A, Rowsey TG, Ma JX, Karamichos D, 2016b. Establishment of a 3D In Vitro Model to Accelerate the Development of Human Therapies against Corneal Diabetes. PLoS One 11, e0168845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarsini S, Whelchel A, Nicholas S, Sharif R, Riaz K, Karamichos D, 2020. Diabetic keratopathy: Insights and challenges. Surv Ophthalmol 65, 513–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu M, Wan L, Dong M, Wang Y, Xie L, Zhou Q, 2021. Hyperglycemia-induced severe mitochondrial bioenergetic deficit of lacrimal gland contributes to the early onset of dry eye in diabetic mice. Free Radic Biol Med 166, 313–323. [DOI] [PubMed] [Google Scholar]
- Quadrado MJ, Popper M, Morgado AM, Murta JN, Van Best JA, 2006. Diabetes and corneal cell densities in humans by in vivo confocal microscopy. Cornea 25, 761–768. [DOI] [PubMed] [Google Scholar]
- Rhee KD, Nusinowitz S, Chao K, Yu F, Bok D, Yang XJ, 2013. CNTF-mediated protection of photoreceptors requires initial activation of the cytokine receptor gp130 in Muller glial cells. Proc Natl Acad Sci U S A 110, E4520–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PM, Chuang TD, Sriram S, Pi L, Luo XP, Petersen BE, Schultz GS, 2013. MicroRNA signature in wound healing following excimer laser ablation: role of miR-133b on TGFbeta1, CTGF, SMA, and COL1A1 expression levels in rabbit corneal fibroblasts. Invest Ophthalmol Vis Sci 54, 6944–6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha EM, Alves M, Rios JD, Dartt DA, 2008. The aging lacrimal gland: changes in structure and function. Ocul Surf 6, 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg ME, Tervo TM, Immonen IJ, Muller LJ, Gronhagen-Riska C, Vesaluoma MH, 2000. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci 41, 2915–2921. [PubMed] [Google Scholar]
- Roszkowska AM, Licitra C, Tumminello G, Postorino EI, Colonna MR, Aragona P, 2021. Corneal nerves in diabetes-The role of the in vivo corneal confocal microscopy of the subbasal nerve plexus in the assessment of peripheral small fiber neuropathy. Surv Ophthalmol 66, 493–513. [DOI] [PubMed] [Google Scholar]
- Rozsa AJ, Beuerman RW, 1982. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain 14, 105–120. [DOI] [PubMed] [Google Scholar]
- Ryan DG, Oliveira-Fernandes M, Lavker RM, 2006. MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity. Mol Vis 12, 1175–1184. [PubMed] [Google Scholar]
- Sacchetti M, Lambiase A, 2014. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol 8, 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti M, Lambiase A, 2017. Neurotrophic factors and corneal nerve regeneration. Neural regeneration research 12, 1220–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti M, Rama P, Bruscolini A, Lambiase A, 2018. Limbal Stem Cell Transplantation: Clinical Results, Limits, and Perspectives. Stem Cells Int 2018, 8086269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Dib CM, Brunken WJ, Ljubimov AV, 2014. Normalization of wound healing and stem cell marker patterns in organ-cultured human diabetic corneas by gene therapy of limbal cells. Exp Eye Res 129, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Epifantseva I, Hemmati DM, Ghiam CA, Brunken WJ, Ljubimov AV, 2013. Enhanced wound healing, kinase and stem cell marker expression in diabetic organ-cultured human corneas upon MMP-10 and cathepsin F gene silencing. Invest Ophthalmol Vis Sci 54, 8172–8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Kramerov AA, Tajbakhsh J, Aoki AM, Wang C, Chai NN, Ljubimova JY, Sasaki T, Sosne G, Carlson MR, Nelson SF, Ljubimov AV, 2005. Proteinase and growth factor alterations revealed by gene microarray analysis of human diabetic corneas. Invest Ophthalmol Vis Sci 46, 3604–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Kramerov AA, Yu FS, Castro MG, Ljubimov AV, 2010. Normalization of wound healing and diabetic markers in organ cultured human diabetic corneas by adenoviral delivery of c-Met gene. Invest Ophthalmol Vis Sci 51, 1970–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Soleymani S, Harounian A, Bhakta B, Troyanovsky SM, Brunken WJ, Pellegrini G, Ljubimov AV, 2011a. Alterations of epithelial stem cell marker patterns in human diabetic corneas and effects of c-met gene therapy. Mol Vis 17, 2177–2190. [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Soleymani S, Harounian A, Bhakta B, Troyanovsky SM, Brunken WJ, Pellegrini G, Ljubimov AV, 2011b. Alterations of epithelial stem cell marker patterns in human diabetic corneas and effects of c-met gene therapy. Molecular vision 17, 2177–2190. [PMC free article] [PubMed] [Google Scholar]
- Saleh A, Roy Chowdhury SK, Smith DR, Balakrishnan S, Tessler L, Martens C, Morrow D, Schartner E, Frizzi KE, Calcutt NA, Fernyhough P, 2013. Ciliary neurotrophic factor activates NF-kappaB to enhance mitochondrial bioenergetics and prevent neuropathy in sensory neurons of streptozotocin-induced diabetic rodents. Neuropharmacology 65, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaeekia R, Rabiee B, Putra I, Shen X, Park YJ, Hematti P, Eslani M, Djalilian AR, 2018. Effect of Human Corneal Mesenchymal Stromal Cell-derived Exosomes on Corneal Epithelial Wound Healing. Invest Ophthalmol Vis Sci 59, 5194–5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Thorin JC, 1998. The cornea in diabetes mellitus. International ophthalmology clinics 38, 19–36. [PubMed] [Google Scholar]
- Schecterson LC, Pazevic AA, Yang R, Matulef K, Gordon SE, 2020. TRPV1, TRPA1, and TRPM8 are expressed in axon terminals in the cornea: TRPV1 axons contain CGRP and secretogranin II; TRPA1 axons contain secretogranin 3. Mol Vis 26, 576–587. [PMC free article] [PubMed] [Google Scholar]
- Schermer A, Galvin S, Sun TT, 1986. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol 103, 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreml S, Berneburg M, 2017. The global burden of diabetic wounds. Br J Dermatol 176, 845–846. [DOI] [PubMed] [Google Scholar]
- Schultz RO, Van Horn DL, Peters MA, Klewin KM, Schutten WH, 1981. Diabetic keratopathy. Trans Am Ophthalmol Soc 79, 180–199. [PMC free article] [PubMed] [Google Scholar]
- Schuster B, Kovaleva M, Sun Y, Regenhard P, Matthews V, Grotzinger J, Rose-John S, Kallen KJ, 2003. Signaling of human ciliary neurotrophic factor (CNTF) revisited. The interleukin-6 receptor can serve as an alpha-receptor for CTNF. J Biol Chem 278, 9528–9535. [DOI] [PubMed] [Google Scholar]
- Schwartz DE, 1974. Corneal sensitivity in diabetics. Arch Ophthalmol 91, 174–178. [DOI] [PubMed] [Google Scholar]
- Scott JR, Tamura RN, Muangman P, Isik FF, Xie C, Gibran NS, 2008. Topical substance P increases inflammatory cell density in genetically diabetic murine wounds. Wound Repair Regen 16, 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LJ, 2020. Givosiran: First Approval. Drugs 80, 335–339. [DOI] [PubMed] [Google Scholar]
- Segawa K, 1964. Electron Microscopic Studies on the Human Corneal Epithelium: Dendritic Cells. Arch Ophthalmol 72, 650–659. [DOI] [PubMed] [Google Scholar]
- Semeraro F, Forbice E, Braga O, Bova A, Di Salvatore A, Azzolini C, 2014a. Evaluation of the efficacy of 50% autologous serum eye drops in different ocular surface pathologies. Biomed Res Int 2014, 826970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semeraro F, Forbice E, Romano V, Angi M, Romano MR, Filippelli ME, Di Iorio R, Costagliola C, 2014b. Neurotrophic keratitis. Ophthalmologica 231, 191–197. [DOI] [PubMed] [Google Scholar]
- Shah M, Foreman DM, Ferguson MW, 1995. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci 108 ( Pt 3), 985–1002. [DOI] [PubMed] [Google Scholar]
- Shah R, Amador C, Tormanen K, Ghiam S, Saghizadeh M, Arumugaswami V, Kumar A, Kramerov AA, Ljubimov AV, 2021. Systemic diseases and the cornea. Exp Eye Res 204, 108455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy P, Medarova Z, 2018. The fundamental role of miR-10b in metastatic cancer. Am J Cancer Res 8, 1674–1688. [PMC free article] [PubMed] [Google Scholar]
- Sheha H, Tighe S, Hashem O, Hayashida Y, 2019. Update On Cenegermin Eye Drops In The Treatment Of Neurotrophic Keratitis. Clin Ophthalmol 13, 1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih KC, Lam KS, Tong L, 2017. A systematic review on the impact of diabetes mellitus on the ocular surface. Nutr Diabetes 7, e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo R, Stitt AW, Gardner TW, 2018. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia 61, 1902–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M, Sen CK, Singh K, Das A, Ghatak S, Rhea B, Blackstone B, Powell HM, Khanna S, Roy S, 2018. Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue. Nat Commun 9, 936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitompul R, 2017. Corneal Sensitivity as a Potential Marker of Diabetic Neuropathy. Acta medica Indonesiana 49, 166–172. [PubMed] [Google Scholar]
- Siu GD, Young AL, Cheng LL, 2015. Long-term symptomatic relief of bullous keratopathy with amniotic membrane transplant. Int Ophthalmol 35, 777–783. [DOI] [PubMed] [Google Scholar]
- Skundric DS, Lisak RP, 2003. Role of neuropoietic cytokines in development and progression of diabetic polyneuropathy: from glucose metabolism to neurodegeneration. Exp Diabesity Res 4, 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff AC, Barratt-Boyes SM, 2010. Enemy at the gates: dendritic cells and immunity to mucosal pathogens. Cell Res 20, 872–885. [DOI] [PubMed] [Google Scholar]
- Sridhar MS, Martin R, 2018. Anterior segment optical coherence tomography for evaluation of cornea and ocular surface. Indian J Ophthalmol 66, 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Lyall D, 2013. Neurotrophic Keratopathy. Ocular Surface Disease: Cornea, Conjunctiva and Tear Film 10.1016/B978-1-4557-2876-3.00027-4. [DOI] [Google Scholar]
- Stapleton F, Carnt N, 2012. Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye (Lond) 26, 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckli ET, 2018. Understanding axon guidance: are we nearly there yet? Development 145. [DOI] [PubMed] [Google Scholar]
- Stojadinovic O, Yin N, Lehmann J, Pastar I, Kirsner RS, Tomic-Canic M, 2013. Increased number of Langerhans cells in the epidermis of diabetic foot ulcers correlates with healing outcome. Immunol Res 57, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein JW, 2003. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol 3, 879–889. [DOI] [PubMed] [Google Scholar]
- Sugrue SP, Zieske JD, 1997. ZO1 in corneal epithelium: association to the zonula occludens and adherens junctions. Experimental eye research 64, 11–20. [DOI] [PubMed] [Google Scholar]
- Sun H, Mi X, Gao N, Yan C, Yu FS, 2015. Hyperglycemia-suppressed expression of serpine1 contributes to delayed epithelial wound healing in diabetic mouse corneas. Invest Ophthalmol Vis Sci 56, 3383–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suraida AR, Ibrahim M, Zunaina E, 2018. Correlation of the anterior ocular segment biometry with HbA1c level in type 2 diabetes mellitus patients. PLoS One 13, e0191134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvas S, 2017. Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J Immunol 199, 1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A, 1995. Autoradiographic visualization and pharmacological characterization of vanilloid (capsaicin) receptors in several species, including man. Acta Physiol Scand Suppl 629, 1–68. [PubMed] [Google Scholar]
- Tavakoli M, Boulton AJ, Efron N, Malik RA, 2011. Increased Langerhan cell density and corneal nerve damage in diabetic patients: role of immune mechanisms in human diabetic neuropathy. Contact lens & anterior eye : the journal of the British Contact Lens Association 34, 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli M, Mitu-Pretorian M, Petropoulos IN, Fadavi H, Asghar O, Alam U, Ponirakis G, Jeziorska M, Marshall A, Efron N, Boulton AJ, Augustine T, Malik RA, 2013a. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes 62, 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli M, Petropoulos IN, Malik RA, 2012. Assessing corneal nerve structure and function in diabetic neuropathy. Clinical & experimental optometry : journal of the Australian Optometrical Association 95, 338–347. [DOI] [PubMed] [Google Scholar]
- Tavakoli M, Petropoulos IN, Malik RA, 2013b. Corneal confocal microscopy to assess diabetic neuropathy: an eye on the foot. J Diabetes Sci Technol 7, 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli M, Quattrini C, Abbott C, Kallinikos P, Marshall A, Finnigan J, Morgan P, Efron N, Boulton AJ, Malik RA, 2010. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care 33, 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HR, Kimsey RA, 1981. Corneal epithelial basement membrane changes in diabetes. Invest Ophthalmol Vis Sci 20, 548–553. [PubMed] [Google Scholar]
- Tervo TM, Moilanen JA, Rosenberg ME, Tuominen IS, Valle T, Vesaluoma MH, 2002. In vivo confocal microscopy for studying corneal diseases and conditions associated with corneal nerve damage. Adv Exp Med Biol 506, 657–665. [DOI] [PubMed] [Google Scholar]
- Tezuka H, Ohteki T, 2010. Regulation of intestinal homeostasis by dendritic cells. Immunol Rev 234, 247–258. [DOI] [PubMed] [Google Scholar]
- Thalin C, Lundstrom S, Seignez C, Daleskog M, Lundstrom A, Henriksson P, Helleday T, Phillipson M, Wallen H, Demers M, 2018. Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer. PLoS One 13, e0191231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting DSJ, Ho CS, Deshmukh R, Said DG, Dua HS, 2021. Infectious keratitis: an update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye (Lond) 35, 1084–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong CM, Iovieno A, Yeung SN, 2020. Topical insulin for neurotrophic corneal ulcers. Can J Ophthalmol 55, e170–e172. [DOI] [PubMed] [Google Scholar]
- Torricelli AA, Singh V, Santhiago MR, Wilson SE, 2013. The corneal epithelial basement membrane: structure, function, and disease. Invest Ophthalmol Vis Sci 54, 6390–6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JN, Mohamadzadeh M, 2010. Key roles of dendritic cells in lung infection and improving anthrax vaccines. Trends Mol Med 16, 303–312. [DOI] [PubMed] [Google Scholar]
- Tran MT, Ritchie MH, Lausch RN, Oakes JE, 2000. Calcitonin gene-related peptide induces IL-8 synthesis in human corneal epithelial cells. J Immunol 164, 4307–4312. [DOI] [PubMed] [Google Scholar]
- Troger J, Humpel C, Kremser B, Kralinger M, Teuchner B, Kunze C, Philipp W, Kieselbach G, 1999. The effect of streptozotocin-induced diabetes mellitus on substance P and calcitonin gene-related peptide expression in the rat trigeminal ganglion. Brain Res 842, 84–91. [DOI] [PubMed] [Google Scholar]
- Tsubota K, Chiba K, Shimazaki J, 1991. Corneal epithelium in diabetic patients. Cornea 10, 156–160. [DOI] [PubMed] [Google Scholar]
- Tummanapalli SS, Willcox MDP, Issar T, Yan A, Pisarcikova J, Kwai N, Poynten AM, Krishnan AV, Markoulli M, 2019. Tear film substance P: A potential biomarker for diabetic peripheral neuropathy. Ocul Surf 17, 690–698. [DOI] [PubMed] [Google Scholar]
- Twardy BS, Channappanavar R, Suvas S, 2011. Substance P in the corneal stroma regulates the severity of herpetic stromal keratitis lesions. Invest Ophthalmol Vis Sci 52, 8604–8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H, Ferrari G, Hattori T, Saban DR, Katikireddy KR, Chauhan SK, Dana R, 2012. Dependence of corneal stem/progenitor cells on ocular surface innervation. Invest Ophthalmol Vis Sci 53, 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H, Hattori T, Kumagai Y, Suzuki N, Ueno S, Takagi H, 2014. Alterations in the corneal nerve and stem/progenitor cells in diabetes: preventive effects of insulin-like growth factor-1 treatment. Int J Endocrinol 2014, 312401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO, 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9, 654–659. [DOI] [PubMed] [Google Scholar]
- van Niel G, D’Angelo G, Raposo G, 2018. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19, 213–228. [DOI] [PubMed] [Google Scholar]
- Vemuganti GK, Fatima A, Madhira SL, Basti S, Sangwan VS, 2009. Limbal stem cells: application in ocular biomedicine. International review of cell and molecular biology 275, 133–181. [DOI] [PubMed] [Google Scholar]
- Vij N, Sharma A, Thakkar M, Sinha S, Mohan RR, 2008. PDGF-driven proliferation, migration, and IL8 chemokine secretion in human corneal fibroblasts involve JAK2-STAT3 signaling pathway. Mol Vis 14, 1020–1027. [PMC free article] [PubMed] [Google Scholar]
- Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R, 2017. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlgren J, Statello L, Skogberg G, Telemo E, Valadi H, 2016. Delivery of Small Interfering RNAs to Cells via Exosomes. Methods Mol Biol 1364, 105–125. [DOI] [PubMed] [Google Scholar]
- Walcott B, 1998. The Lacrimal Gland and Its Veil of Tears. News Physiol Sci 13, 97–103. [DOI] [PubMed] [Google Scholar]
- Wang B, Chandrasekera PC, Pippin JJ, 2014. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev 10, 131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Gao N, Yin J, Yu FS, 2012. Reduced innervation and delayed re-innervation after epithelial wounding in type 2 diabetic Goto-Kakizaki rats. Am J Pathol 181, 2058–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhou Q, Wan L, Guo M, Chen C, Xue J, Yang L, Xie L, 2021. Lipidomic analysis of meibomian glands from type-1 diabetes mouse model and preliminary studies of potential mechanism. Exp Eye Res 210, 108710. [DOI] [PubMed] [Google Scholar]
- Wang X, Li W, Zhou Q, Li J, Wang X, Zhang J, Li D, Qi X, Liu T, Zhao X, Li S, Yang L, Xie L, 2020. MANF Promotes Diabetic Corneal Epithelial Wound Healing and Nerve Regeneration by Attenuating Hyperglycemia-Induced Endoplasmic Reticulum Stress. Diabetes 69, 1264–1278. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao X, Shi D, Chen P, Yu Y, Yang L, Xie L, 2013. Overexpression of SIRT1 promotes high glucose-attenuated corneal epithelial wound healing via p53 regulation of the IGFBP3/IGF-1R/AKT pathway. Invest Ophthalmol Vis Sci 54, 3806–3814. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao X, Wu X, Dai Y, Chen P, Xie L, 2016. microRNA-182 Mediates Sirt1-Induced Diabetic Corneal Nerve Regeneration. Diabetes 65, 2020–2031. [DOI] [PubMed] [Google Scholar]
- Ward BR, Jester JV, Nishibu A, Vishwanath M, Shalhevet D, Kumamoto T, Petroll WM, Cavanagh HD, Takashima A, 2007. Local thermal injury elicits immediate dynamic behavioural responses by corneal Langerhans cells. Immunology 120, 556–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Alexander M, Misharin AV, Budinger GRS, 2019. The role of macrophages in the resolution of inflammation. J Clin Invest 129, 2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters GA, Turnbull PR, Swift S, Petty A, Craig JP, 2017. Ocular surface microbiome in meibomian gland dysfunction. Clin Exp Ophthalmol 45, 105–111. [DOI] [PubMed] [Google Scholar]
- Westman J, Grinstein S, Marques PE, 2019. Phagocytosis of Necrotic Debris at Sites of Injury and Inflammation. Front Immunol 10, 3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelchel AE, Nicholas SE, Ma JX, Karamichos D, 2021. Nerve influence on the metabolism of type I and type II diabetic corneal stroma: an in vitro study. Sci Rep 11, 13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widgerow AD, 2012. Cellular resolution of inflammation--catabasis. Wound Repair Regen 20, 2–7. [DOI] [PubMed] [Google Scholar]
- Wilgus TA, Roy S, McDaniel JC, 2013. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Advances in wound care 2, 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Mohan RR, Ambrosio R Jr., Hong J, Lee J, 2001. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res 20, 625–637. [DOI] [PubMed] [Google Scholar]
- Winkler MA, Dib C, Ljubimov AV, Saghizadeh M, 2014. Targeting miR-146a to treat delayed wound healing in human diabetic organ-cultured corneas. PLoS One 9, e114692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, Kahn CR, Wagner DD, 2015. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med 21, 815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Guan C, Qin X, Xiang Y, Qi M, Luo Z, Zhang C, 2007. Upregulation of substance P receptor expression by calcitonin gene-related peptide, a possible cooperative action of two neuropeptides involved in airway inflammation. Pulm Pharmacol Ther 20, 513–524. [DOI] [PubMed] [Google Scholar]
- Xu K, Yu FS, 2011. Impaired Epithelial Wound Healing and EGFR Signaling Pathways in the Corneas of Diabetic Rats. Invest Ophthalmol Vis Sci 52, 3301–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu KP, Li Y, Ljubimov AV, Yu FS, 2009. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes 58, 1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, He J, Xiao C, Guo Y, Fu T, Liu J, Lin C, Wu M, Yang Y, Dong D, Pan H, Xia C, Ren L, Li Z, 2018. The mouse autonomic nervous system modulates inflammation and epithelial renewal after corneal abrasion through the activation of distinct local macrophages. Mucosal Immunol 11, 1496–1511. [DOI] [PubMed] [Google Scholar]
- Yan B, Gao J, Guo J, Yang D, Li D, 2020. Interleukin-28B dampens protease-induced lung inflammation via IL-25 and TSLP inhibition in epithelial cells. Sci Rep 10, 20973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Gao N, Sun H, Yin J, Lee P, Zhou L, Fan X, Yu FS, 2016. Targeting Imbalance between IL-1beta and IL-1 Receptor Antagonist Ameliorates Delayed Epithelium Wound Healing in Diabetic Mouse Corneas. Am J Pathol 186, 1466–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kramer-Albers EM, Laitinen S, Lasser C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lotvall J, Mancek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pallinger E, Del Portillo HA, Reventos J, Rigau M, Rohde E, Sammar M, Sanchez-Madrid F, Santarem N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O, 2015. Biological properties of extracellular vesicles and their physiological functions. Journal of extracellular vesicles 4, 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Di G, Qi X, Qu M, Wang Y, Duan H, Danielson P, Xie L, Zhou Q, 2014. Substance P promotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor. Diabetes 63, 4262–4274. [DOI] [PubMed] [Google Scholar]
- Yang Q, Li B, Sheng M, 2021. Meibum lipid composition in type 2 diabetics with dry eye. Exp Eye Res 206, 108522. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gong B, Wu ZZ, Shuai P, Li DF, Liu LL, Yu M, 2019. Inhibition of microRNA-129–5p expression ameliorates ultraviolet ray-induced corneal epithelial cell injury via upregulation of EGFR. J Cell Physiol 234, 11692–11707. [DOI] [PubMed] [Google Scholar]
- Yeung DT, Araj H, Harper JR, Platoff GE Jr., 2020. Considerations in developing medical countermeasures against chemical ocular toxicity. Toxicol Lett 334, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Huang J, Chen C, Gao N, Wang F, Yu FS, 2011. Corneal complications in streptozocin-induced type I diabetic rats. Invest Ophthalmol Vis Sci 52, 6589–6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Xu K, Zhang J, Kumar A, Yu FS, 2007. Wound-induced ATP release and EGF receptor activation in epithelial cells. J Cell Sci 120, 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Yu FS, 2010. LL-37 via EGFR transactivation to promote high glucose-attenuated epithelial wound healing in organ-cultured corneas. Invest Ophthalmol Vis Sci 51, 1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JJ, Ismail S, Sherwin T, 2014. Limbal stem cells: Central concepts of corneal epithelial homeostasis. World J Stem Cells 6, 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KC, Im SK, Seo MS, 2004. Changes of tear film and ocular surface in diabetes mellitus. Korean journal of ophthalmology : KJO 18, 168–174. [DOI] [PubMed] [Google Scholar]
- Yorek MS, Obrosov A, Shevalye H, Holmes A, Harper MM, Kardon RH, Yorek MA, 2015. Effect of diet-induced obesity or type 1 or type 2 diabetes on corneal nerves and peripheral neuropathy in C57Bl/6J mice. J Peripher Nerv Syst 20, 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorek MS, Obrosov A, Shevalye H, Lupachyk S, Harper MM, Kardon RH, Yorek MA, 2014. Effect of glycemic control on corneal nerves and peripheral neuropathy in streptozotocin-induced diabetic C57Bl/6J mice. J Peripher Nerv Syst 19, 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Matsumoto K, Tomioka D, Bessho K, Itami S, Yoshikawa K, Nakamura T, 2004. Recombinant hepatocyte growth factor accelerates cutaneous wound healing in a diabetic mouse model. Growth Factors 22, 111–119. [DOI] [PubMed] [Google Scholar]
- Yoshino J, Mills KF, Yoon MJ, Imai S, 2011. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab 14, 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Yin J, Lee P, Hwang F, McDermott M, 2015. Sensory nerve regeneration after epithelium wounding in normal and diabetic corneas. Expert Rev Ophthalmol 10, 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Peng H, Ruan Q, Fatima A, Getsios S, Lavker RM, 2010. MicroRNA-205 promotes keratinocyte migration via the lipid phosphatase SHIP2. FASEB J 24, 3950–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Ryan DG, Getsios S, Oliveira-Fernandes M, Fatima A, Lavker RM, 2008. MicroRNA-184 antagonizes microRNA-205 to maintain SHIP2 levels in epithelia. Proc Natl Acad Sci U S A 105, 19300–19305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagon IS, Sassani JW, Myers RL, McLaughlin PJ, 2007. Naltrexone accelerates healing without compromise of adhesion complexes in normal and diabetic corneal epithelium. Brain Res Bull 72, 18–24. [DOI] [PubMed] [Google Scholar]
- Zainabadi K, 2019. Drugs targeting SIRT1, a new generation of therapeutics for osteoporosis and other bone related disorders? Pharmacol Res 143, 97–105. [DOI] [PubMed] [Google Scholar]
- Zhang C, Li J, Hu C, Wang J, Zhang J, Ren Z, Song X, Jia L, 2017. Antihyperglycaemic and organic protective effects on pancreas, liver and kidney by polysaccharides from Hericium erinaceus SG-02 in streptozotocin-induced diabetic mice. Sci Rep 7, 10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xu K, Ambati B, Yu FS, 2003. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Invest Ophthalmol Vis Sci 44, 4247–4254. [DOI] [PubMed] [Google Scholar]
- Zhang K, Hopkins JJ, Heier JS, Birch DG, Halperin LS, Albini TA, Brown DM, Jaffe GJ, Tao W, Williams GA, 2011. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci U S A 108, 6241–6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Cheng Z, Wang Y, Han T, 2021. The Risks of miRNA Therapeutics: In a Drug Target Perspective. Drug design, development and therapy 15, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhao L, Deng S, Sun X, Wang N, 2016. Dry Eye Syndrome in Patients with Diabetes Mellitus: Prevalence, Etiology, and Clinical Characteristics. J Ophthalmol 2016, 8201053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen P, Di G, Qi X, Zhou Q, Gao H, 2018. Netrin-1 promotes diabetic corneal wound healing through molecular mechanisms mediated via the adenosine 2B receptor. Sci Rep 8, 5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gao N, Wu L, Lee PSY, Me R, Dai C, Xie L, Yu FX, 2020a. Role of VIP and Sonic Hedgehog Signaling Pathways in Mediating Epithelial Wound Healing, Sensory Nerve Regeneration, and Their Defects in Diabetic Corneas. Diabetes 69, 1549–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Jiang H, Dou S, Zhang B, Qi X, Li J, Zhou Q, Li W, Chen C, Wang Q, Xie L, 2020b. Comprehensive analysis of differentially expressed microRNAs and mRNAs involved in diabetic corneal neuropathy. Life Sci 261, 118456. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Song Z, Li X, Xu S, Zhou S, Jin X, Zhang H, 2020c. Long noncoding RNA KCNQ1OT1 induces pyroptosis in diabetic corneal endothelial keratopathy. American journal of physiology. Cell physiology 318, C346–C359. [DOI] [PubMed] [Google Scholar]
- Zhao H, He Y, Ren YR, Chen BH, 2019. Corneal alteration and pathogenesis in diabetes mellitus. Int J Ophthalmol 12, 1939–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Chen P, Di G, Zhang Y, Wang Y, Qi X, Duan H, Xie L, 2015. Ciliary neurotrophic factor promotes the activation of corneal epithelial stem/progenitor cells and accelerates corneal epithelial wound healing. Stem Cells 33, 1566–1576. [DOI] [PubMed] [Google Scholar]


