Abstract
Buckwheat (Fagopyrum spp.) has immense nutritional and nutraceutical potential. All the plant parts of buckwheat possess various metabolites, such as rutin, quercetin, vitexin etc. The high content of rutin in this pseudo cereal crop strongly adapts it to grow under adverse environments. In the present study 50 germplasm lines of Fagopyrum tataricum were used for estimation of seed endosperm rutin content through HPLC. Furthermore, molecular analysis of PAL gene (Phenylalanine Ammonia Lyase), an upstream gene in rutin biosynthesis pathway was targeted for detection of SNPs to understand the variations in the concentrations of seed endosperm rutin content, among tartary buckwheat genotypes with highest and lowest seed endosperm rutin content. Three primer pairs were employed for amplification of PAL gene for F. tartaricum (covering whole gene) followed by sequencing. Rutin concentration in seed endosperm of F. tartaricum ranged from 194.86 to 1403.22 ppm with an average of 617.06 ppm. Highest rutin concentration was found in genotype BWZ90 and lowest in BWZ16. Significant variations were observed in the seed endosperm rutin content among the genotypes of tartary buckwheat. Furthermore, alignment of PAL gene sequences of genotypes with high seed endosperm rutin content and low seed endosperm rutin content revealed variations at 21 polymorphic sites. The amino acid sequences obtained from the nucleotide sequences were also aligned and the variations were detected at 19 positions. The putative protein structure showed conformational changes among predicted proteins from two contrasting genotypes for endosperm rutin content. We here established an inventory of seed endosperm rutin content of tartary buckwheat. This study also provided insights about role of these SNPs in rutin biosynthesis. Furthermore, this information can be used for breeding buckwheat for high metabolite contents.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-022-03218-y.
Keywords: Buckwheat, Rutin, PAL, Nutraceutical, Protein structure
Introduction
Buckwheat is originated from Anglo-Saxonboc (beech) and whoet (wheat) (Edwardson 1995). Buckwheat is a pseudo cereal as it is similar to the conventional cereals in its usage and chemical composition (Campbell 1997). However, presence of high content of metabolites such as rutin helps it to withstand very well in adverse environments (Li and Howard Zhang 2001; Zhang et al. 2017; Mukhtar et al. 2021). Despite being neglected during twentieth century, it is well acknowledged as a functional food in many countries such as China, Taiwan and Japan due to its high nutritional and nutraceutical potential (Cawoy et al. 2008). Out of many species of buckwheat grown across the world, only nine are used for agricultural and nutritional purposes (Krkošková and Mrázová 2005). For food purposes only two species are used around the world, common buckwheat (Fagopyrum esculentum) and tartary buckwheat (F. tataricum). The common and tartary buckwheat are referred as sweet and bitter buckwheat, respectively (Ohnishi 1993). Buckwheat is known for presence of bioactive compounds in its various plant tissues (Bashir et al. 2021; Sabreena et al. 2021; Mir et al. 2022). Among numerous bioactive compounds, rutin has been of great interest due its nutraceutical abilities.
Rutin (3, 3′, 4′, 5, 7-pentahydroxyflavone-3-rhamnoglucoside), a well-known flavonol di-glucoside is used in the manufacture of drugs (Kitabayashi et al. 1995). It has a very high antioxidant, vaso-protective, cardio-protective, neuroprotective and cyto-protective activities (Javed et al. 2012). Rutin can prevent formation of clots in both arteries and veins and is proving to be very effective in treating all the issues related to blood clot formation. Rutin and quercetin play a very important role in the cure of diabetes (Lee et al. 2012). Buckwheat is the only field crop that contains rutin (Kreft et al. 1999). Buckwheat plants that originated in locations exposed to high UV-B radiations have the ability to synthesize more rutin as an evolutionary response to extreme environmental conditions. The UV-B absorbing compounds such as rutin, quercetin, have the ability to protect vital molecules such as nucleic acids from the damage of UV-B radiations (Germ et al. 2004).
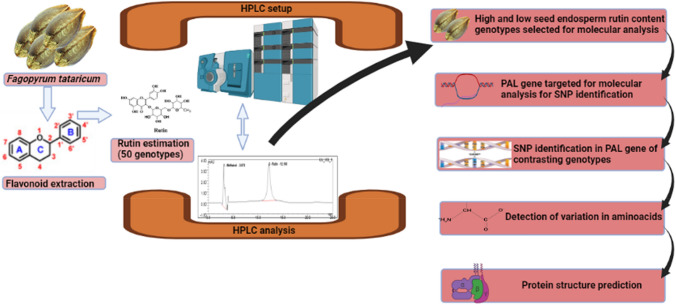
The pathway of rutin and quercetin involve a number of genes, PAL (Phenylalanine Ammonia Lyase) gene being the first in the pathway is an important upstream gene which catalyses the conversion of phenylalanine (amino acid precursor) to trans-cinnamic acid (Li et al. 2010; Thiyagarajan et al. 2016). Many other enzymes further catalyze each substrate and finally flavonol synthetase converts dihydroquercetin to quercetin and rutin (Li et al. 2010). The PAL gene is an essential candidate involved in the synthesis of rutin/quercetin. Allelic characterization of the PAL gene is must for genetic improvement of Fagopyrum spp. in terms of enhancing the rutin and quercetin contents (Thiyagarajan et al. 2016). The primary flavonoid biosynthesis genes in buckwheat, that include the PAL gene, was also found to be most heavily expressed in the stems and roots, according to a study (Li et al. 2010). PAL gene expression was also studied in three tissues (cotyledon, hypocotyl, and radicle) of buckwheat sprouts during germination, and a correlation was found between polyphenol compounds and PAL gene expression. Moreover, it was observed that during germination polyphenol components, and PAL gene expression significantly increased in all the three separate tissues of buckwheat sprouts; however, the higher expression of the PAL gene was observed in radicals compared to other two tissues, the rutin content in cotyledons was raised to 88.6 g kg−1, which was 7.7-times and 39.4-times more compared to those in buckwheat seeds and radicles. Therefore, this study reveals that there is a correlation between bioactive components and related gene expressions (Ling et al. 2018). In the present study, we focused on PAL gene of two genotypes (one with highest seed endosperm rutin content and another with lowest seed endosperm rutin content) to have a holistic information about possible SNPs that may have impact on rutin biosynthesis. Furthermore, the protein structure was predicted to visualize protein conformational changes among high and low rutin genotypes based on SNPs in their respective PAL gene sequences. Figure 1 gives an overview of the research undertaken.
Fig. 1.
Stepwise details of research work for allele mining of PAL gene in tartary buckwheat. Tartary buckwheat seed material was collected and the rutin content was estimated using HPLC. High and low seed endosperm rutin content germplasm lines were selected for PAL gene allele mining
Materials and methods
Materials
Seed material of 50 germplasm lines of tartary buckwheat used as experimental material, have been collected from hot spots of north western Himalayas of J&K and Ladakh (Kargil, Leh, Gurez and Kishtwar) and some germplasm lines have been procured from NBPGR, New Delhi (Table 1).
Table 1.
List of Tartary buckwheat germplasm lines used as experimental material, their places of collection and seed endosperm Rutin content
| Sr. no. | Germplasm | IC. No. | Collection site | Average Seed endosperm rutin content (ppm) ± S.E |
|---|---|---|---|---|
| 1 | BWZ3 | Not allotted | Gurez, J&K, India | 366.16 ± 0.14 |
| 2 | BWZ4 | Not allotted | Kupwara, J&K, India | 615.12 ± 0.04 |
| 3 | BWZ11 | IC- 0,637,166 | Kishtwar, J&K, India | 543.81 ± 0.04 |
| 4 | BWZ14 | IC-0637167 | Kargil, Ladakh, India | 757.46 ± 0.02 |
| 5 | BWZ16 | IC-0637168 | Gurez, J&K, India | 194.91 ± 0.05 |
| 6 | BWZ17 | IC-0642406 | Gurez, J&K, India | 401.81 ± 0.05 |
| 7 | BWZ18 | IC-0642407 | Gurez, J&K, India | 570.90 ± 0.03 |
| 8 | BWZ19 | IC-0642408 | Gurez, J&K, India | 609.01 ± 0.03 |
| 9 | BWZ20 | IC-0642409 | Gurez, J&K, India | 563.72 ± 0.06 |
| 10 | BWZ21 | IC-0637169 | Gurez, J&K, India | 754.77 ± 0.04 |
| 11 | BWZ22 | IC-0637170 | Gurez, J&K, India | 236.58 ± 0.08 |
| 12 | BWZ23 | IC-0637171 | Gurez, J&K, India | 935.47 ± 0.04 |
| 13 | BWZ25 | IC-0637173 | Gurez, J&K, India | 693.20 ± 0.21 |
| 14 | BWZ26 | IC-0637174 | Gurez, J&K, India | 712.21 ± 0.06 |
| 15 | BWZ27 | IC-0637175 | Gurez, J&K, India | 475.03 ± 0.05 |
| 16 | BWZ30 | Not allotted | Kargil, Ladhak, India | 565.80 ± 0.06 |
| 17 | BWZ32 | IC-0637176 | Leh, Ladhak, India | 654.84 ± 0.06 |
| 18 | BWZ33 | Not allotted | Kargil, Ladhak, India | 480.48 ± 0.01 |
| 19 | BWZ35 | IC-0637177 | Leh, Ladhak, India | 368.15 ± 0.04 |
| 20 | BWZ36 | Not allotted | Leh, Ladhak, India | 482.03 ± 0.04 |
| 21 | BWZ37 | IC-0642410 | Leh, Ladhak, India | 836.04 ± 0.02 |
| 22 | BWZ39 | Not allotted | Kargil, Ladhak, India | 489.64 ± 0.13 |
| 23 | BWZ41 | Not allotted | Leh, Ladhak, India | 438.75 ± 0.11 |
| 24 | BWZ42 | IC-0642411 | Leh, Ladhak, India | 406.91 ± 0.10 |
| 25 | BWZ43 | IC-0642412 | Leh, Ladhak, India | 539.60 ± 0.30 |
| 26 | BWZ51 | IC-13140 | NBPGR, New Delhi, India | 917.08 ± 0.01 |
| 27 | BWZ52 | IC-13143 | NBPGR, New Delhi, India | 281.64 ± 0.02 |
| 28 | BWZ54 | IC- 14,494 | NBPGR, New Delhi, India | 1072.2 ± 0.01 |
| 29 | BWZ56 | IC-17370 | NBPGR, New Delhi, India | 923.35 ± 0.10 |
| 30 | BWZ61 | IC-18751 | NBPGR, New Delhi, India | 503.53 ± 0.09 |
| 31 | BWZ62 | IC-18757 | NBPGR, New Delhi, India | 775.37 ± 0.24 |
| 32 | BWZ65 | IC-18889 | NBPGR, New Delhi, India | 681.15 ± 0.10 |
| 33 | BWZ66 | IC-22426 | NBPGR, New Delhi, India | 782.64 ± 0.10 |
| 34 | BWZ67 | IC-24296 | NBPGR, New Delhi, India | 767.78 ± 0.06 |
| 35 | BWZ68 | IC-24298 | NBPGR, New Delhi, India | 756.59 ± 0.08 |
| 36 | BWZ69 | IC-24299 | NBPGR, New Delhi | 546.65 ± 0.06 |
| 37 | BWZ70 | IC-24302 | NBPGR, New Delhi, India | 908.15 ± 0.07 |
| 38 | BWZ72 | IC-25999 | NBPGR, New Delhi, India | 762.24 ± 0.01 |
| 39 | BWZ75 | IC-26591 | NBPGR, New Delhi, India | 536.90 ± 0.05 |
| 40 | BWZ76 | IC-37277 | NBPGR, New Delhi, India | 487.37 ± 0.13 |
| 41 | BWZ77 | IC-37278 | NBPGR, New Delhi, India | 537.78 ± 0.07 |
| 42 | BWZ81 | EC-12537 | NBPGR, New Delhi, India | 654.30 ± 0.20 |
| 43 | BWZ83 | EC-18182 | NBPGR, New Delhi, India | 465.48 ± 0.11 |
| 44 | BWZ85 | EC-18629 | NBPGR, New Delhi, India | 465.87 ± 0.05 |
| 45 | BWZ86 | EC-18740 | NBPGR, New Delhi | 499.88 ± 0.06 |
| 46 | BWZ90 | EC-99945 | NBPGR, New Delhi, India | 1403.2 ± 0.05 |
| 47 | BWZ92 | EC-99948 | NBPGR, New Delhi, India | 770.54 ± 0.06 |
| 48 | BWZ94 | EC-104037 | NBPGR, New Delhi, India | 298.34 ± 0.02 |
| 49 | BWZ100 | EC-161415–16 | NBPGR, New Delhi, India | 680.01 ± 0.01 |
| 50 | BWZ102 | EC-21662 | NBPGR, New Delhi, India | 682.56 ± 0.08 |
| Average | 617.06 ± 0.074 |
Extraction and estimation of rutin
100 mg of finely ground seeds of buckwheat was prepared using mortar and pestle. 10 ml of methanol (HPLC grade) mixed with 10 ml of Dichloromethane (DCM) (HPLC grade) was added to it in a conical flask and was kept at room temperature for 24 h. Then the solvent was removed and was stored in a separate conical flask. A fresh mixture of 10 ml methanol (HPLC grade) and 10 ml DCM (HPLC grade) was added to the powder and was again kept for 24 h. The process was repeated thrice and a total of 60 ml solvent was obtained after 3 days. The solvent was filtered using filter paper (whatmann No. 1). It was then put in water bath at 60 °C and was allowed to evaporate. The extract left behind was dissolved in 2 ml methanol and was stored in 1.5 ml centrifuge tube and further used for HPLC analysis.
20 μl of the sample was injected in the HPLC. Acetonitrile: water (HPLC grade): orthophosphoric acid in 15:84.9:0.1 ratio, respectively, was used as mobile phase. The retention time of rutin was around 12.5 min at a wavelength of 257 nm which was confirmed by injecting the standards (Sigma Aldrich 250,249–75-3) (1 ppm, 5 ppm, 10 ppm, 15 ppm, 20 ppm, 25 ppm, 30 ppm). The flow rate was set at 1 ml/min. The chromatograms were obtained and the results were formulated accordingly. Figure 2, represents the chromatograms of standards. As such, rutin content of all the samples was estimated.
Fig. 2.
HPLC chromatograms of standards used for quantification of rutin from 50 diverse genotypes of tartary buckwheat. Six different standard were used for calibration and to determine the actual concentration of rutin content in seed endosperm of subjected samples
Genomic DNA extraction and quantification
The seeds of buckwheat genotypes having high and low seed endosperm rutin contents were grown in cups and the shoots were harvested after 3 weeks for extraction of genomic DNA by following CTAB (cetyl-trimethyl ammonium bromide) method (Doyle 1991). DNA was then quantified and further diluted to a concentration of 30 ng/μl and was then used for further analysis.
Polymerase chain reaction
Three primer pairs retrieved from early available literature Thiyagarajan et al. (2016) were used for PAL gene amplification. DNA amplification was carried out in a Thermocycler (Applied Biosystems). The total reaction volume of 100 µl was prepared that consists of 20 µl of 5X reaction buffer, 2 µl of 10 mM dNTPs, 5 µl of 10 pmol forward primer, 5 µl of 10 pmol reverse primer, 6 µl MgCl2, 3 µl DMSO, 7 µl genomic DNA, 1U Phusion high fidelity polymerase (M0530S) and the volume was made up by adding nuclease free water. PCR program was carried out in a thermocycler of 30 cycles loop with annealing temperature ranging from 55 to 57°C). The PCR products were then stored at 4 °C. Furthermore, PCR products were separated on 1.5% agarose gel. 20 µl of loading dye (Bromophenol blue) was added in each PCR tube having amplified products and after mixing gently was finally loaded in the wells. 100 bp DNA ladder (ALS-DM03) was also loaded as a molecular marker for determining the product size of amplified products. Electrophoresis was carried out at 90 V for 1.5 h and then visualized under gel documentation system (Syngene, UK).
Gel elution and purification
The gel elution and purification was carried out using wizard SV Gel and PCR clean-up Kit of Promega (A9281) by following the manufacturer’s guidelines. The eluted DNA was quantified at 260 nm wavelength using Nanodrop and was sent for sequencing.
Phylogenetic analysis
Purified PCR Products were sequenced at Genosys informatics (New Delhi, India). Sequences obtained were further assembled using CLUSTAL W (Bio-edit) and BLAST-X. MEGA-7 (Kumar et al. 2016) was used for construction of dendrogram in a bootstrap test with 1000 replicates.
Identification of SNPs and prediction of protein structure
The sequences obtained were aligned with the reference genome to ensure accuracy of the sequences. Furthermore, common sequences of each product were considered for alignment using CLUSTAL omega. Seaview software 3.2 was used for alignment of the final sequences and SNP detection (Galtier et al. 1996). Furthermore, complete PAL gene sequences (having combined sequences of 3 parts, sequenced separately) from both the genotypes with high and low seed endosperm rutin contents were then used for determination of their amino acid sequences followed by protein structure prediction using i-tasser software (Yang et al. 2014). The amino acid sequences were retrieved using expasy 2.0 software (Gasteiger et al. 2003). The amino acid sequences were determined and aligned using sea view software 3.2 (Galtier et al. 1996).
Results
Rutin profiling of Tartary buckwheat
The concentrations of rutin content in the seed endosperm of 50 genotypes of tartary buckwheat varied from 194.863 to 1403.22 ppm. The highest content of rutin was found in genotype BWZ 90 (1403.22 ppm) and lowest with BWZ 16 (194.22 ppm) with an average of 617.06 ppm. Table 1 represents the mean values of rutin content observed in seed endosperm of tartary buckwheat species. This helped us in establishing the inventory of seed endosperm rutin content of tartary buckwheat of north western Himalayas.
Identification of potential candidate germplasm lines
Seven tartary buckwheat genotypes with highest seed endosperm rutin content and seven with lowest rutin content were identified as detailed in Table 2.
Table 2.
Potential candidate germplasm lines of tartary buckwheat with higher and lower seed endosperm rutin content
| Sr. no. | Germplasm lines with higher seed endosperm rutin content | Rutin content (ppm) | Sr. no. | Germplasm lines with lower seed endosperm rutin content | Rutin content (ppm) |
|---|---|---|---|---|---|
| 1 | BWZ90 | 1403.23 | 1 | BWZ16 | 194.91 |
| 2 | BWZ54 | 1072.29 | 2 | BWZ22 | 236.58 |
| 3 | BWZ23 | 935.47 | 3 | BWZ52 | 281.64 |
| 4 | BWZ56 | 923.35 | 4 | BWZ94 | 298.34 |
| 5 | BWZ51 | 917.08 | 5 | BWZ3 | 366.16 |
| 6 | BWZ70 | 908.15 | 6 | BWZ35 | 368.15 |
| 7 | BWZ37 | 836.04 | 7 | BWZ17 | 401.81 |
PAL gene amplification
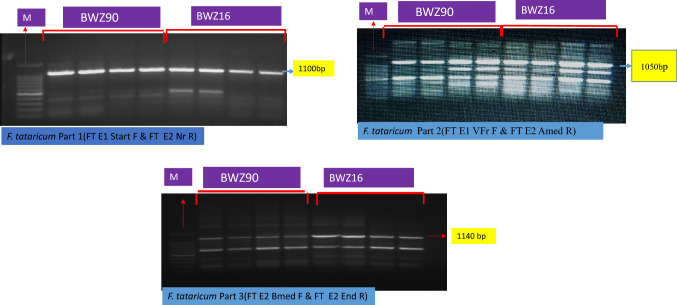
Three primer pairs were used in the present study for amplification of PAL gene (Table 3). The sequences of amplified products obtained using these three primer pairs when overlapped covered entire length of the gene. The amplified product obtained were 1100 bp for the primer FT E1 Start F & FT E2 Nr R; 1050 bp for primer FT E1 Start F & FT E2 Nr R and 1140 bp for primer FT E2 Bmed F & FT E2 End R. Figure 3 represents the results of PCR amplification using these three primers.
Table 3.
List of primers with Tm used for amplification of different parts of PAL gene (Thiyagarajan et al. 2016)
| S. no | Primer | Target buckwheat species | Sequence | Tm |
|---|---|---|---|---|
| 1 |
FT E1 Start F FT E2 Nr R |
F. tataricum |
[F]ATGGGGGTCTCAAACGGA [R]TCGCCAGAAGCAGTGATG |
56 °C |
| 2 |
FT E1 VFr F FT E2AMed R |
F. tataricum |
[F] TAAGGAAGGCGGTGCTCTT [R]GCTTCTGAGAGGATCGAGTTC |
55 °C |
| 3 |
FT E2 BMed F FT E2 End R |
F. tataricum |
[F]GAGCTTCACGAACTCGATCC [R]CTAGCAGATAGGCAGAGGAGCA |
56 °C |
Fig. 3.
Amplification of different parts of PAL gene from BWZ90 and BWZ16 genotype; M-marker; Part 1 product size is 1100 bp; Part 2 product size is 1050 bp and Part 3 product size is 1140 bp
SNPs and variation in amino acid sequence of PAL gene of F. tartaricum genotypes contrasting in seed endosperm rutin content
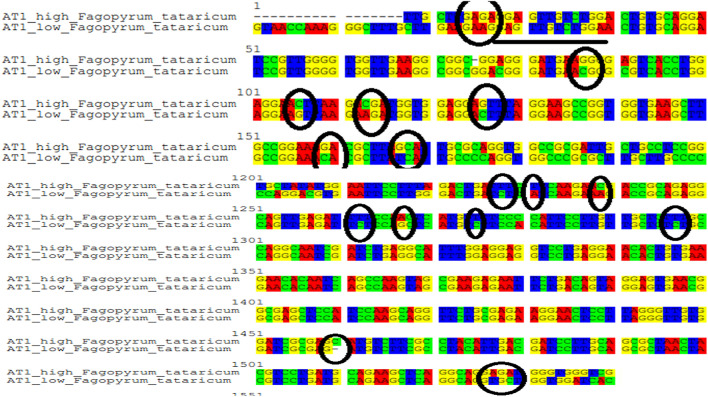
The sequences obtained were aligned and viewed using Seaview software 3.2. The aligned data showed variations among nucleotides at various places. Many point mutations (SNPs) were found along with a single frame shift mutation. A total number of 21 nucleotide variations among high and low rutin genotypes of F. tataricum were found (Fig. 4). Furthermore, these SNPs lead to nineteen amino acid changes among high and low seed endosperm rutin genotypes. It was observed that among 19 amino acid changes, nine were conservative and the remaining ten mutations were non-conservative (Fig. 5).
Fig. 4.
SNPs along with one frame shift mutation identified in PAL gene sequences' of tartary buckwheat genotype (BWZ90 with high endosperm rutin content and BWZ16 with low endosperm rutin content). A total number of 21 nucleotide variations among high and low rutin genotypes of F. tataricum were found
Fig. 5.
Variations in amino acid sequences of PAL gene of tartary buckwheat genotype (BWZ90 with high endosperm rutin content and BWZ16 with low endosperm rutin content). Among 19 amino acid changes, nine were conservative and the remaining ten mutations were non-conservative
Protein structure prediction
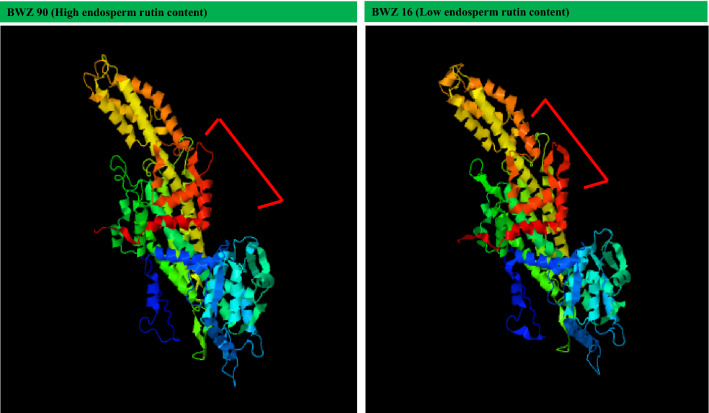
The 3-D protein structure was predicted based on PAL gene sequences obtained from high and low seed endosperm rutin yielding genotypes. I-tasser software was used for protein structure prediction that confirmed changes in predicted protein structure among contrasting genotypes. The putative protein of low rutin yielding genotype showed higher degree of coiling at two places, whereas putative protein of high rutin yielding genotype showed lower coiling in the same region (Fig. 6).
Fig. 6.
Conformational changes in predicted protein structure of BWZ90 and BWZ19 using i-tasser software. The 3-D protein structure was predicted based on PAL gene sequences obtained from high and low seed endosperm rutin yielding genotypes
Submission of sequences and phylogenetic analysis
Sequences retrieved were submitted to Genbank and the accession numbers obtained are (OK094676 and OK094675). Furthermore, BLASTX was performed against NCBI Non-Redundant Protein Sequence database (Fagopyrum–taxid: 3616) to ensure that the sequences obtained for (BWZ90 and BWZ16) were significantly similar (> 95%) to the already published sequences (Supplementary file 1). Furthermore, we employed MEGA-7 software to ensure the clustering of similar sequences and observed that the constructed dendrogram grouped the sequences of PAL gene of F. tataricum in one separate clade (Fig. 7).
Fig. 7.
Optimal dendrogram constructed using MEGA 7 that grouped the sequences of PAL gene of F. tataricum in one clade in a bootstrap test with1000 replicates
Discussion
In the present study, we have used different solvent combinations for rutin extraction and as per earlier reports the extractant used have performed better. Among two cultivated species (F. esculentum and F. tataricum), we have observed significant higher concentration of rutin in seed endosperm of tartary buckwheat compared to common buckwheat; therefore, we focused on tartary buckwheat PAL gene allele mining. Different scientific group around globe have revealed that there is variation in rutin content among different species and also among different plant parts of single species; however, its molecular regulation is not clear (Kreft et al. 2006). In the present study rutin content in the endosperm of the seeds of 50 diverse genotypes of tartary buckwheat was estimated to observe the variation among them (Bashir et al. 2021; Sabreena et al. 2021). Significant variation in rutin content was found in tartary buckwheat genotypes that range from 194.863 to 1403.22 ppm. The highest content of rutin was found in genotype BWZ 90 (1403.22 ppm) and BWZ 16 (194.863 ppm) possessed lowest content of rutin with an average of 617.06 ppm. Our results also confirmed that significantly higher quantity of rutin is present in F. tataricum as compared to F. esculentum. The genotypes with higher content of rutin do not have only nutraceutical potential but can combat harsh environments (Zhang et al. 2017). Moreover, we have selected two genotypes of tartary buckwheat with highest and lowest seed endosperm rutin content (BWZ 90 and BWZ 16) for PAL gene sequencing. F. tataricum whole PAL gene was targeted by amplifying it in three overlapping portions followed by their sequencing. 21 SNPs were detected between the PAL gene sequences of BWZ 90 and BWZ 16 that have led to changes in 19 amino acids. The changes in protein structure due to presence of SNPs in PAL gene sequences of two contrasting genotypes (for seed endosperm rutin content) were observed. In an earlier study, SNPs due to indel variations in PAL gene was also observed (Thiyagarajan et al. 2016). However, in the present study, we have evaluated different germplasm and we could identify more SNPs compared to earlier studies.
The amino acid sequences obtained from the nucleotide sequences were aligned. The alignment of amino acids in case of F. tataricum revealed variations at 19 places including 9 conservative variations (Phenylalanine to Leucine, Threonine to Lysine, Phenylalanine to Leucine, Histidine to Aspartic acid, Methionine to Leucine, Glycine to Aspartic acid, Lysine to Glutamic acid, Serine to Asparagine and Serine to Threonine) and the rest were non-conservative variations (Arginine to Glycine, Phenylalanine to serine, Proline to Threonine, Glutamic acid to Valine, Glycine to valine, Serine to Histidine, Glutamine to Alanine, Alanine to Asparagine, Lysine to Glycine, Alanine to Arginine). A similar study was carried out by (Thiyagarajan et al. 2016), in which it was observed that the identified SNPs in the PAL gene of contrasting genotypes of F. tataricum for rutin content, could not show any amino acid change, while in other two species (F. dibotrys and F. esculentum) both conservative and non-conservative variations were observed. The putative proteins showed variations in certain regions. The putative protein of low rutin yielding genotype showed higher coiling in two regions as compared to high rutin yielding genotype. The higher coiling was also observed at one place in high rutin yielding genotype and no such coiling was observed in low rutin yielding genotype. The effect on the putative protein is due to variations in amino acid sequences. The high and the low coiled regions could result in a change in binding sites. The variation in the putative protein structure could be the reason for high content of rutin in one genotype as compared to the other, owing to the fact that protein structure and its conformation determine its interaction with the substrate. However, in-depth bioinformatics research needs to be undertaken to have better understanding of interactions. Furthermore, these germplasm lines have been conserved and can be potential donors/ parental lines used in mapping QTLs and in varietal development programs. Furthermore, in our study, some of the candidate germplasm lines have already been deposited in the national gene bank (NBPGR, New Delhi) and their IC numbers are allotted as (BWZ 16 as IC-0637168, whereas BWZ 90 already procured from NBPGR, New Delhi with EC 99945).
Conclusions
In the present study, we have established rutin profile of 50 tartary buckwheat genotypes collected from various regions of north western Himalayas of Jammu and Kashmir and Ladakh, India and some procured from NBPGR, New Delhi. Further nucleotide polymorphisms in PAL gene of two contrasting genotypes for seed endosperm rutin content provided insights about role of these SNPs in rutin biosynthesis. 21 SNPs identified in the PAL gene sequence of these genotypes lead to variations in 19 amino acids having role in changing the structural conformation of proteins. This might have implications on rutin biosynthesis.
Furthermore, we could identify potential candidate lines as a genetic resource that can immediately be used in buckwheat breeding programs for QTL/gene mapping as well as selection/development of buckwheat varieties with high yield along with high metabolite content.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1: BLASTX against NCBI non-redundant protein sequence database (Fagopyrum–taxid:3616) (DOCX 13 kb)
Acknowledgements
SMZ acknowledges support of National Mission on Himalayan Studies [NMHS] implemented by Ministry of Environment, Forest & Climate Change [MoEF&CC] vide project sanction No.: GBPNI/NMHS-2017-18/SG24/622. SMZ acknowledges the support of Prof. R K Salgotra (SKUAST-Jammu) for helping in HPLC analysis.
Abbreviations
- HPLC
High-performance liquid chromatography
- PAL
Phenylalanine Ammonia Lyase
- SNP
Single nucleotide polymorphism
- UV-B
Type-B ultraviolet
- NBPGR
National Bureau of Plant Genetic Resources
- DCM
Dichloromethane
- CTAB
Cetyl-trimethyl ammonium bromide
- dNTP
Deoxynucleotide triphosphate
- MgCl2
Magnesium chloride
- DMSO
Dimethylsulfoxide
- QTL
Quantitative trait locus
Author contribution
AMT did biochemical and molecular research; AH and SAB helped in biochemical analysis; BB, MDS and MKK helped in bioinformatics analysis, SMZ conceived research idea, guided all laboratory and bioinformatics work, finalized the manuscript. AMT and AH wrote the first draft of manuscript which was further edited by KZM, MAB, MKK and SMZ. AH, MKK, BB and SMZ did revision of the manuscript.
Funding
The work was partly funded by National Mission on Himalayan Studies [NMHS] implemented by Ministry of Environment, Forest & Climate Change [MoEF&CC] vide project sanction No.: GBPNI/NMHS-2017–18/SG24/622.
Availability of data and materials
The data that support this study are available in the article.
Declarations
Conflict of interest
The Authors declare that they have no conflict of interests.
Ethics approval
Not Applicable.
Consent to participate
Not Applicable.
Consent for publication
We hereby give consent for publication if it is accepted.
References
- Bashir E, Mahajan R, Mir RA et al (2021) Unravelling the genetic variability and population structure of buckwheat (Fagopyrum spp.): a collection of north western Himalayas. Nucleus. 10.1007/s13237-020-00319-y
- Campbell CG (1997) Buckwheat. Fagopyrum esculentum Moench. In: Promoting the conservation and use of underutilized and neglected crops
- Cawoy V, Kinet JM, Jacquemart AL. Morphology of nectaries and biology of nectar production in the distylous species Fagopyrum esculentum. Ann Bot. 2008 doi: 10.1093/aob/mcn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle J (1991) DNA protocols for plants CTAB total DNA isolation. Mol Tech Taxon H57
- Edwardson SE (1995) 70 using growing degree days to estimate optimum windrowing time in buckwheat. Curr Adv Buckwheat Res 509–514
- Galtier N, Gouy M, Gautier C. Seaview and phylo_ win: two graphic tools for sequence alignment and molecular phylogeny. Bioinformatics. 1996 doi: 10.1093/bioinformatics/12.6.543. [DOI] [PubMed] [Google Scholar]
- Gasteiger E, Gattiker A, Hoogland C, et al. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003 doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germ M, Simčič T, Gaberščik A, et al. UV-B treated algae exhibiting different responses as a food source for Daphnia magna. J Plankton Res. 2004 doi: 10.1093/plankt/fbh111. [DOI] [Google Scholar]
- Javed H, Khan MM, Ahmad A, et al. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience. 2012;210:340–352. doi: 10.1016/J.NEUROSCIENCE.2012.02.046. [DOI] [PubMed] [Google Scholar]
- Kitabayashi H, Ujihara A, Hirose T, Minami M. Varietal differences and heritability for rutin content in common buckwheat Fagopyrum esculentum Moench. Breed Sci. 1995 doi: 10.1270/jsbbs1951.45.75. [DOI] [Google Scholar]
- Kreft S, Knapp M, Kreft I. Extraction of rutin from buckwheat (Fagopyrum esculentum moench) seeds and determination by capillary electrophoresis. J Agric Food Chem. 1999 doi: 10.1021/jf990186p. [DOI] [PubMed] [Google Scholar]
- Kreft I, Fabjan N, Yasumoto K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem. 2006 doi: 10.1016/j.foodchem.2005.05.081. [DOI] [Google Scholar]
- Krkošková B, Mrázová Z. Prophylactic components of buckwheat. Food Res Int. 2005 doi: 10.1016/j.foodres.2004.11.009. [DOI] [Google Scholar]
- Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed]
- Lee CC, Hsu WH, Shen SR, et al. Fagopyrum tataricum (Buckwheat) improved high-glucose-induced insulin resistance in mouse hepatocytes and diabetes in fructose-rich diet-induced mice. Exp Diabetes Res. 2012 doi: 10.1155/2012/375673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SQ, Howard ZQ (2001) Advances in the development of functional foods from buckwheat. Crit Rev Food Sci Nutr 41 [DOI] [PubMed]
- Li X, Il PN, Xu H, et al. Differential expression of flavonoid biosynthesis genes and accumulation of phenolic compounds in common buckwheat (Fagopyrum esculentum) J Agric Food Chem. 2010 doi: 10.1021/jf103310g. [DOI] [PubMed] [Google Scholar]
- Ling A, Li X, Hu X, et al. Dynamic changes in polyphenol compounds, antioxidant activity, and PAL gene expression in different tissues of buckwheat during germination. J Sci Food Agric. 2018 doi: 10.1002/jsfa.9119. [DOI] [PubMed] [Google Scholar]
- Mir RA, Nazir M, Sabreena S, et al. Utilizing the underutilized plant resources for development of life style foods: putting nutrigenomics to use. Plant Physiol Biochem. 2022;171:128–138. doi: 10.1016/J.PLAPHY.2021.12.038. [DOI] [PubMed] [Google Scholar]
- Mukhtar S, Bashir Z, Mir RA, Zargar SM (2021) Genomic approaches for the improvement and conservation of buckwheat. In: Neglected and underutilized crops—towards nutritional security and sustainability
- Ohnishi O (1993) Population genetics of cultivated common buckwheat, fagopyrum esculentum moench. VIII. Local differentiation of land races in Europe and the silk road. Jpn J Genet. 10.1266/jjg.68.303
- Sabreena NM, Mahajan R, et al. Deciphering allelic variability and population structure in buckwheat: an analogy between the efficiency of ISSR and SSR markers. Saudi J Biol Sci. 2021 doi: 10.1016/j.sjbs.2021.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiyagarajan K, Vitali F, Tolaini V, et al. Genomic characterization of phenylalanine ammonia lyase gene in buckwheat. PLoS ONE. 2016 doi: 10.1371/journal.pone.0151187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yan R, Roy A, et al (2014) The I-TASSER suite: protein structure and function prediction. Nat Methods 12 [DOI] [PMC free article] [PubMed]
- Zhang L, Li X, Ma B, et al. The tartary buckwheat genome provides insights into rutin biosynthesis and abiotic stress tolerance. Mol Plant. 2017 doi: 10.1016/j.molp.2017.08.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1: BLASTX against NCBI non-redundant protein sequence database (Fagopyrum–taxid:3616) (DOCX 13 kb)
Data Availability Statement
The data that support this study are available in the article.