Abstract
Synchrotron radiation phase-contrast microtomography is sensitive to low attenuating tissues, giving an alternative visualisation of the sample and being useful for investigating microstructure inside biological specimens without staining them with a contrast medium. The phase-contrast technique has been widely used in the scientific community, as it is a technique associated with radiography and microscopy and able to enhance contrast in soft tissues, specifically at the edges, showing details that could not be seen by the absorption technique. This work aims to show the ability of synchrotron-based phase-contrast microtomography for the visualisation of soft tissues and hard internal structures of millimetre-sized biological organisms. Case studies of the anatomy of Rhodnius prolixus head and Thoropa miliaris tadpole are presented to illustrate the imaging technique.
Introduction
Characterisation and quantification of sub-tissue structures within a whole organism demand for non-destructive imaging methods, fast image acquisition and high-resolution 3D reconstruction. Histological slicing has been the gold standard for analysing biological tissues. Although histology can yield excellent discriminative power on both the tissue and cellular level, sectioning-induced artefacts, sample preparation, time-consuming during the histo-technical procedures used for manual cutting, embedding and staining tissue with consequences for the three-dimensional integrity of tissues and organs remains a challenging (Appel et al. 2013; Zehbe et al. 2010).
The ability of absorption-based X-ray imaging to provide important insight into the field of life sciences for the evaluation, spatial assessment, quantification of tissue structures, visualisation and segmentation of 3D volumes has been shown to overcome these drawbacks in biological samples (Albers et al. 2021; Keklikoglou et al. 2021; Weissleder and Nahrendorf 2015). One method that fulfils all of these requirements is X-ray microtomography (microCT) and a number of excellent reviews are available on this topic (Holdsworth and Thornton 2002; Ritman 2011; Shearer et al. 2016). However, absorption contrast is limited by the difficulties of visualising weakly absorbing objects (e.g. soft tissues) without the reliance of exogenous contrast agents (Descamps et al. 2020; Silva et al. 2015).
X-ray imaging techniques based on phase contrast (PC) are alternative approaches that have shown promise for biomedical application. X-ray PC imaging (PCI) methods might be more suited for structures with a low difference in absorption contrast due to their ability to involve a combination of phase and absorption contrast in terms of the complex X-ray refractive index in a single exposure. Appropriate algorithms can solve the reconstruction problem for the extraction of the relationship between the phase distribution and the amplitude information of the sample wave (Beltran et al. 2011; Paganin et al. 2002). Experimentally, propagation-based imaging (PBI) is the simplest phase contrast technique, relying on Fresnel diffraction. There have been a number of valuable reviews of phase-contrast synchrotron radiation microtomography (PhC-SR-µCT), and some especially in the context of using synchrotron radiation (SR) sources (Betz et al. 2007; Bravin et al. 2013; Groso et al. 2006; Liu et al. 2013; Momose 2005; Nesterets et al. 2006; Wilkins et al. 2014).
Advances in PhC-SR-µCT technology, in terms of detectors, sub-micron spatial resolution, faster scan acquisition, computational analysis, and in the development of imaging protocols have been crucial for evaluation non-destructively 3D anatomical and morphological data in life sciences. We and other groups have shown that the application of PhC-SR-µCT to millimetre-sized biological samples, even in the absence of X-ray contrast agents, is growing (Albers et al. 2021; Koch et al. 2017; Lak et al. 2008; Socha et al. 2007; Takeda et al. 2000). In this paper, we will explore how PhC-SR-µCT has been applied for revealing the detailed quantification and segmentation of various tissue of the head of a hematophagous bug—Rhodnius prolixus (Garcia et al. 2007) and of the internal anatomy of a whole tadpole—Thoropa miliaris (Barros-Battesti et al. 2015). We also discuss the future aspects of the utilisation of machine learning techniques for future assessments using deep neural networks (DNNs) applied to PhC-SR-µCT volumes, which is scarce in the literature, to our knowledge.
Discussion
In this letter we will describe the advantages provided by phase-contrast synchrotron microtomography (PhC-SR-µCT) as an imaging source in the study of biological samples. Case studies of the anatomy of Rhodnius prolixus head and Thoropa miliaris, all performed at synchrotron facilities, are presented to demonstrate the potential of PhC-SR-µCT for physiology and anatomical investigations.
Rhodnius prolixus
Rhodnius prolixus is a blood-feeding insect used as a model to study basic concepts of insect physiology and is the main insect vector of Trypanosoma cruzi, which causes Chagas disease, killing around 10,000 people every year (Nunes-da-Fonseca et al. 2017).
The studies involving the use of PhC-SR-µCT in Rhodnius prolixus started with Almeida et al. (2012) illustrating the physiology of this insect’s head. In this first paper, Almeida et al. (2012) evidenced the advantages of the use of phase-contrast in the study of insects’ anatomy. In the following years, Almeida et al. (2013, 2014) developed two anatomical studies of Rhodnius prolixus head using PhC-SR-µCT and the phase retrieval approach to improve the visualisation of the insect’s structures. These studies were carried out at the SYRMEP beamline of ELETTRA, Trieste, Italy, using a monochromatic beam with a resolution of 9 µm, and turned out to be the pillars to the use of µCT for the study of this insect.
In order to develop new methodologies and improve quality for a better understanding of the R. prolixus, Sena et al. (2014, 2015) pursued new investigations involving Rhodnius prolixus head using PhC-SR-µCT. The following studies were performed at the SYRMEP beamline of ELETTRA, however that time using a polychromatic beam, which allowed for a better resolution as well as a decrease in the acquisition time. The results highlighted the structures rich in chitin—a large, structural polysaccharide that is fundamental during the insect’s metamorphosis—however, there were important structures inside the insect’s head that were not visualised with the polychromatic beam images. To continue the research using a polychromatic beam (Sena et al. 2016a; 2019), they developed a staining protocol to try to visualise the soft structures in Rhodnius prolixus head structures. Different combinations of fixatives and staining were used and compared qualitatively and quantitatively, in order to find the best protocol that could yield enough contrast for the different soft tissues encapsulated into the hard chitinous shell. The scans were performed at the Brazilian Synchrotron Light Laboratory (LNLS), Campinas, Brazil, and the results have shown that Bouin’s fluid fixative in combination with iodine solution staining was the best protocol to study Rhodnius prolixus head using PhC-SR-µCT and a polychromatic beam. To validate the chosen protocol, some measurements were performed at SYRMEP beamline, at ELETTRA, Trieste, Italy, with a polychromatic beam and a resolution of 2 µm. The results can be found at Sena et al. (2018).
In addition to the results described above, Sena et al. (2021, 2016b) have published two papers using PhC-SR-µCT where they studied possible effects of pesticides on Rhodnius prolixus head. These results are very important for the studies of Chagas disease control. The first paper (Sena et al. 2016b) compared the control group of Rhodnius prolixus with a group treated with triflumurom, a pesticide that inhibits chitin synthesis, interrupting the metamorphosis. The images obtained with PhC-SR-µCT demonstrated in detail the difference between these two groups. In the second paper (Sena et al. 2021), it was compared the control group with the group treated with azadirachtin that acts as an antifeedant and growth disruptor. Here they used synchrotron X-ray microfluorescence at TwinMic beamline at ELETTRA. PhC-SR-µCT was used to help to find the best region to perform the X-ray microfluorescence measurements.
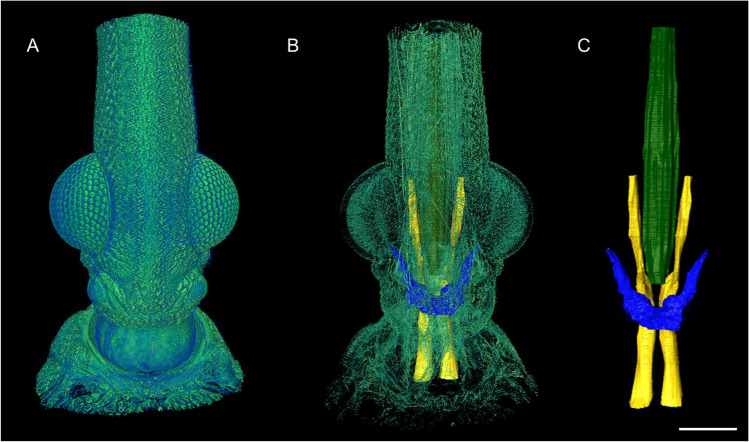
Figure 1 shows some of the images obtained after scans at SYRMEP beamline, at ELETTRA. The sample was prepared using the staining protocol described at Sena et al. (2019) and the microtomography was acquired with 2 µm resolution. Figure 1A shows an external view of Rhodnius prolixus head cuticle, highlighting mainly the compound eyes. The results allowed the segmentation of some structures such as the pharynx, trachea and protocerebrum, which can be seen in Fig. 1B and C.
Fig. 1.
Rhodnius prolixus: (A) the external view of Rhodnius prolixus head cuticle. (B) The protocerebrum (blue), the pharynx (yellow) and the trachea (green) inside Rhodnius prolixus head (faded green). (C) The three segmented structures isolated from the head. Scale bar: 200 μm. X-ray phase-contrast microCT scans here presented were performed at the microtomography beamline SYRMEP of the ELETTRA Sincrotrone Trieste, Italy
It is important to note that PhC-SR-µCT has been used in the study of many other insects (Betz et al. 2007; Soriano et al. 2010; Yao et al. 2017), proving the effectiveness of the technique for this type of sample.
Thoropa miliaris
The use of amphibians as models in ecological research has identified attributes that made them suitable for experimental manipulations (Feder and Burggren 1992; Inger et al. 1986; Wassersug 2000) and some studies have revealed that amphibians were useful models for understanding natural processes such as predation and competition, and how they could influence the structure of the community (Morin 1981; Wilbur 1972, 1977; Wilbur and Collins 1973; Wilbur et al. 1983).
In this context, a study was carried out on the species Thoropa miliaris, which is a leptodactylid endemic to the Brazilian Atlantic Forest, characterised mainly by the fact that adults and tadpoles occupy, reproduce and develop in rocks with low humidity (Feio et al. 2006).
The first study using PhC-SR-µCT on T. miliaris specimens was performed by Fidalgo et al. (2018). For that work, specimens in their larval stage were used, since 75% of the known amphibian species have the tadpole stage with development characteristics very close to each other and catalogued by developmental stages 1 to 46 (egg fertilisation until complete metamorphosis, respectively), when the animal becomes an adult (Gosner 1960).
The measurements were performed at the IMX beamline (LNLS), and in this first study, they aimed at setting up a protocol for scanning biological samples in a liquid medium. The initial attempts to scan this specimen presented some challenges. One of them was the fact that the biological tissues had densities very close to that of water, avoiding the generation of enough contrast within the internal structures; on the other hand, they also presented endolymphatic calcium deposits in their internal structures, which led to streaks artefacts when low energy had to be used.
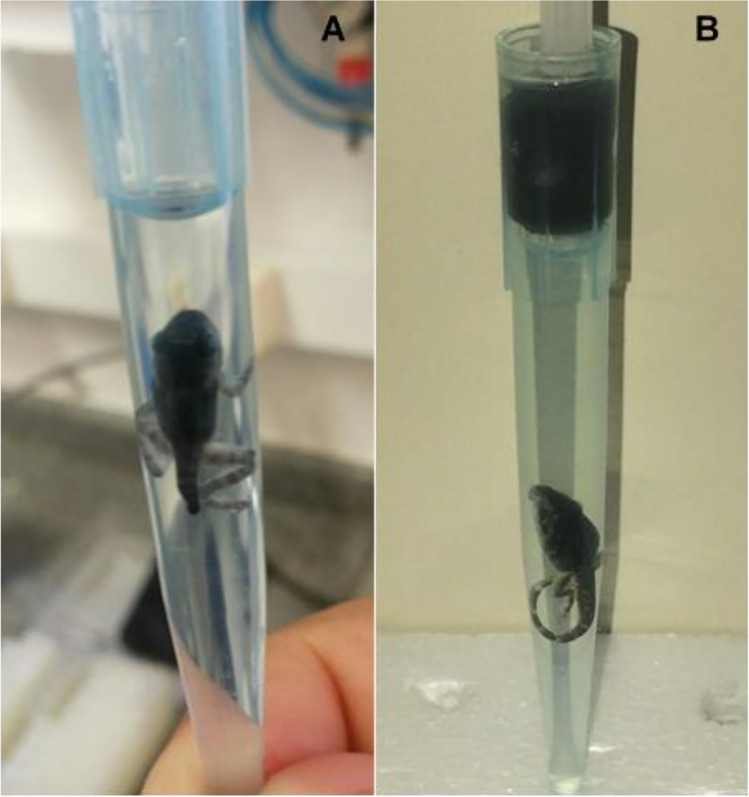
Another difficulty was the rapid dehydration (and consequent loss of morphological information) of the soft tissues when the sample was removed from the liquid medium. In the attempt to avoid dehydration of the sample during the scan, the specimens were transferred to 100% ethanol by series starting from 50% up to 100% in steps of 10% for 30 min each. At this point, due to the high intensity of the beam, the rise in temperature induced the formation of bubbles inside the specimen, causing movement artefacts in the CT reconstructions. As a solution for this, the samples were put in polypropylene tips containing absolute ethanol and a light pressure of a plunger was applied. Figure 2 shows the sample holder used.
Fig. 2.
T. miliaris specimen packed in polypropylene tips containing absolute ethanol (A) without pressure and (B) under light pressure of a plunger
In the IMX beamline, samples were measured at developmental stages 28, 37 and 42, with 4.11 µm pixel size, 0.18° rotation step, 600 ms exposure time, with the sample at 10 cm from the detector (SDD), for allowing phase-contrast effects. The dose rate was found to be 47.6 Gy/s at the sample.
This first work showed the possibility of segmenting soft tissues in biological samples in a liquid medium without the use of any staining methods. The structures that undergo most of the morphological changes during the development of the animal, such as the central nervous system, notochord (cartilage responsible for locomotion of the animal in the larval stage), eye lens and skeletal tissue, could be observed and segmented for further analysis.
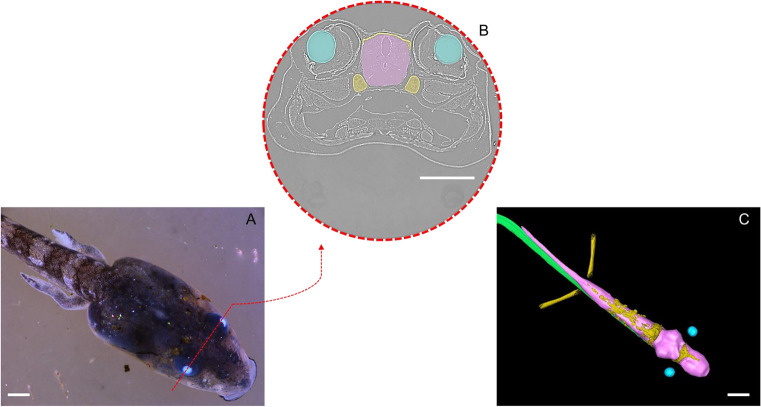
The quantification and comparison of these structures were further published in Fidalgo et al. (2020), where new samples of T. miliaris tadpoles were scanned by PhC-SR-µCT in the SYRMEP beamline (ELETTRA). Samples at developmental stages 28, 30, 32, 34, 37, 38, 40, 43 and 44 were used. Figure 3 shows the image of a specimen at stage 40 under an optical microscope, together with an example of the segmentation of the areas of interest.
Fig. 3.
T. miliaris stage 40: (A) optical microscopic image. (B) Tomographic cross-section at the red dashed line region, showing a virtual section at the eye level, with the different tissues segmented in colour. (C) Segmentation of the tissues of interest, where the skeletal tissue has been coloured in yellow, the eye lenses in blue, the notochord in green and the central nervous system in pink. Scale bar: 1 mm. X-ray phase-contrast microCT scans here presented were performed at the microtomography beamline SYRMEP of the ELETTRA Sincrotrone Trieste, Italy
Again, the image acquisition took place in a liquid medium under light external pressure and without staining. The acquisition setup used a 2.20 µm pixel size, with a rotation step of 0.20°, 100 ms exposure time, 10 cm SDD, 2.56 Gy/s dose rate and 0.025 mm Mo filter.
These acquisitions allowed for further morphometric quantification of the tissues of interest. The results provided a better understanding of how the animal develops in relation to morphological and volumetric changes in the tissues of interest, in addition to demonstrating the effectiveness of the PhC-SR-µCT technique in biological samples where tissues present very similar densities, without the need for staining, avoiding any possible change to the sample due to chemical staining.
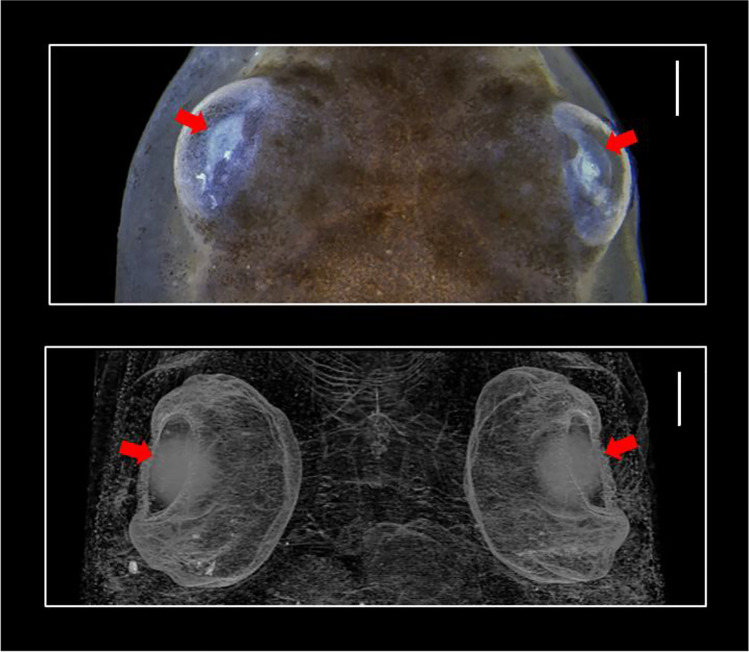
In order to optimise the image segmentation process, Paiva et al. (2022) worked on different approaches to segment the eye lenses of T. miliaris (Fig. 4) using two different semiautomatic segmentation methods, namely, interpolation and watershed, respectively, and an automatic segmentation method using U-Net architecture. The interpolation and watershed methods were performed with the commercial Thermo Scientific™ Amira™ software version 2020.1 (Stalling et al. 2005) whereas the method using U-Net architecture was based on deep neural networks (DNNs), with in-house software (Ronneberger et al. 2015). Their performances were evaluated by means of Dice similarity coefficient (DSC) (Dice 1945) and volume quantification.
Fig. 4.
Visualisation of the top of the T. miliaris’s head where the eye lenses are located (red arrows). These regions of interest (ROI) are composed of soft tissue surrounded by similar density structures. Images obtained by stereomicroscope (top) and by microCT (bottom). Scale bar: 300 µm
T. miliaris data scans were acquired at IMX beamline (LNLS) in the phase-contrast regime using propagation-based imaging technique according to the protocol shown in Fig. 2. With a 4.11 µm effective pixel size and 200 mm SDD, the sample was rotated in 0.18° steps to obtain 1000 projections with 1070 ms exposure time per projection.
For the segmentation, the interpolation method was divided into two parts: in the first part, a 3D rendered volume of the ROI was created from the manual segmentations of 40 slices (out of the 156 slices that made up each lens) followed by interpolating the remaining slices in between. This volume was used as a reference for further comparison. In the second part, a 3D rendered volume of the lenses was created from the 20 slices previously segmented manually. For the watershed method, seed markers were manually selected inside and outside of the ROI (Amira software), allowing further segmentation of the lenses and then the volume of the lenses was calculated. In the U-Net method, the network was trained and tested with different data sets of manually segmented slices, each data set containing 20 slices. After training, the whole volume was segmented then 3D rendered.
In the slices segmented by each method, it was observed that the detection of the edges of the region of interest was one of the main challenges that influenced the performance of each method, as shown in Fig. 5.
Fig. 5.
Visualisation of the slices in a sagittal view, for each segmentation method. In green: interpolation method—reference rendered volume, by 40 slices; in blue: interpolation method by 20 slices; in red: watershed method and in yellow: U-Net method. Scale bar: 100 µm
Compared to the reference rendered volume of each ROI (Fig. 6), the interpolation method (20 slices) showed a difference of around 0.8% below the reference volume; watershed method showed a volume 1.5% above while in the volume segmented using U-Net method, the volume was approximately 4.0% below the reference one. Regarding the statistical analysis, the interpolation method (20 slices) showed a DSC = 97.0%, watershed method DSC = 95.4% and the U-Net method, a DSC = 89.3%. Although the U-Net method showed the lowest DSC value compared to the other two methods, this value is consistent and above expected because the U-Net was trained with a low amount of ground truths (13%) compared to the literature. Furthermore, the segmentation of the lenses using the semiautomatic methods took about 1.5 h, while the U-Net method took about 17 s, showing an advantage over the semiautomatic methods.
Fig. 6.
Volume rendering of the reference volume (green) compared to the interpolation method (blue), watershed method (red) and U-Net method (yellow). Scale bar: 100 µm
The processes of automation and pattern recognition by deep learning (DL) have gained prominence in the scientific field, where publications have been growing exponentially, according to the publishers ScienceDirect and Springer databases. Articles have been published in several areas of science such as medicine and public health, life sciences, education, among others (Mayhew et al. 2011; Shafiei et al. 2021; Zhang et al. 2020; Zheng and Wang 2019). More specifically, when associated with microCT, DL has been used as a tool for automating the segmentation process, being a more reliable method for autonomous segmentation (Lorenzoni et al. 2020), creation/improvement of reconstruction algorithms (Schoppe et al. 2020) and in image filtering applications (Wang et al. 2020; Yang et al. 2018).
Conclusion
In this letter, we showed the importance of PhC-SR-µCT in the study of biological samples, highlighting the ability to obtain detailed structural information, with high spatial resolution and depth, without the necessity of dissection processes. The high brilliance and degree of coherence offered by synchrotron light sources enable the use of phase contrast technique, providing images with loads of details between the tissues, which allow the segmentation of different organs of Rhodnius prolixus and Thoropa miliaris, as demonstrated in this work. We described the importance of using deep learning in the segmentation process of images acquired with PhC-SR-µCT. This paper confirms the need for a continuous development and investigation of PhC-SR-µCT studies applied to biological imaging.
Acknowledgements
The authors acknowledge the Brazilian Government organisations CAPES Foundation, CNPq (The Brazilian National Council for Scientific and Technological Development) and FAPERJ (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro). The authors also acknowledge the ICTP (The Abdus Salam International Centre for Theoretical Physics). The authors would like to thank the IMX and SYRMEP beamlines staff for their assistance during the experiments. G. Sena acknowledges the ICTP for STEP program support. Lastly, we would like to pay our gratitude and our respects to our co-author and colleague, Dr. Delson Braz who passed away in January of 2021.
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Gabriela Sena, Gabriel Fidalgo, Katrine Paiva, Renan Barcelos, Liebert Parreiras Nogueira and Marcos Vinícius Colaço. The first draft of the manuscript was written by Regina Cély Barroso and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declarations
Ethics approval
This is an observational study. The Federal University Fluminense and Federal Rural University of Rio de Janeiro have confirmed that no ethical approval is required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Albers J, Svetlove A, Alves J, et al. Elastic transformation of histological slices allows precise co-registration with microCT data sets for a refined virtual histology approach. Sci Rep. 2021;11:10846. doi: 10.1038/s41598-021-89841-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida AP, Braz D, Nogueira LP, et al. Application of the SR-PhC-μCT technique with phase retrieval for the characterization of internal and external structures of Rhodnius prolixus. J Instrum. 2013;8:C07004–C07004. doi: 10.1088/1748-0221/8/07/c07004. [DOI] [Google Scholar]
- Almeida AP, Braz D, Nogueira LP, et al. Phase contrast X-ray microtomography of the Rhodnius prolixus head: comparison of direct reconstruction and phase retrieval approach. Radiat Phys Chem. 2014;95:243–246. doi: 10.1016/j.radphyschem.2013.02.015. [DOI] [Google Scholar]
- Almeida AP, Soares J, Meneses AA, et al. Phase contrast X-ray synchrotron imaging for assessing external and internal morphology of Rhodnius prolixus. Appl Radiat Isot. 2012;70:1340–1343. doi: 10.1016/j.apradiso.2011.11.028. [DOI] [PubMed] [Google Scholar]
- Appel AA, Anastasio MA, Larson JC, et al. Imaging challenges in biomaterials and tissue engineering. Biomaterials. 2013;34:6615–6630. doi: 10.1016/j.biomaterials.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros-Battesti DM, Landulfo GA, Luz HR, et al. Ornithodoros faccinii n. sp. (Acari: Ixodida: Argasidae) parasitizing the frog Thoropa miliaris (Amphibia: Anura: Cycloramphidae) in Brazil. Parasit Vectors. 2015;8:268. doi: 10.1186/s13071-015-0877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran MA, Paganin DM, Siu KK, et al. Interface-specific X-ray phase retrieval tomography of complex biological organs. Phys Med Biol. 2011;56:7353–7369. doi: 10.1088/0031-9155/56/23/002. [DOI] [PubMed] [Google Scholar]
- Betz O, Wegst U, Weide D, et al. Imaging applications of synchrotron X-ray phase-contrast microtomography in biological morphology and biomaterials science. I. General aspects of the technique and its advantages in the analysis of millimetre-sized arthropod structure. J Microsc. 2007;227:51–71. doi: 10.1111/j.1365-2818.2007.01785.x. [DOI] [PubMed] [Google Scholar]
- Bravin A, Coan P, Suortti P. X-ray phase-contrast imaging: from pre-clinical applications towards clinics. Phys Med Biol. 2013;58:R1–35. doi: 10.1088/0031-9155/58/1/R1. [DOI] [PubMed] [Google Scholar]
- Descamps E, Sochacka A, De Kegel B, et al (2020) Soft tissue discrimination with contrast agents using micro-CT scanning. Belg J Zool 144. 10.26496/bjz.2014.63.
- Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945;26:297–302. doi: 10.2307/1932409. [DOI] [Google Scholar]
- Feder ME, Burggren WW. Environmental physiology of the amphibians. Chicago: University of chicago Press; 1992. [Google Scholar]
- Feio R, Napoli M, Caramaschi U. Considerações taxonômicas sobre Thoropa miliaris (Spix, 1824), com revalidação e redescrição de Thoropa taophora (Miranda-Ribeiro, 1923) (Amphibia, Anura, Leptodactylidae) Arquivos Do Museu Nacional. 2006;64:41–60. [Google Scholar]
- Fidalgo G, Colaço MV, Nogueira LP, et al. Virtual dissection of Thoropa miliaris tadpole using phase-contrast synchrotron microtomography. J Instrum. 2018;13:C05012–C05012. doi: 10.1088/1748-0221/13/05/c05012. [DOI] [Google Scholar]
- Fidalgo G, Paiva K, Mendes G, et al. Synchrotron microtomography applied to the volumetric analysis of internal structures of Thoropa miliaris tadpoles. Sci Rep. 2020;10:18934. doi: 10.1038/s41598-020-75993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia ES, Ratcliffe NA, Whitten MM, et al. Exploring the role of insect host factors in the dynamics of Trypanosoma cruzi-Rhodnius prolixus interactions. J Insect Physiol. 2007;53:11–21. doi: 10.1016/j.jinsphys.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Gosner KL. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 1960;16:183–190. [Google Scholar]
- Groso A, Stampanoni M, Abela R, et al (2006) Phase contrast tomography: an alternative approach. Appl Phys Lett 8810.1063/1.2207221
- Holdsworth DW, Thornton MM. Micro-CT in small animal and specimen imaging. Trends Biotechnol. 2002;20:S34–S39. doi: 10.1016/s0167-7799(02)02004-8. [DOI] [Google Scholar]
- Inger RF, Szarski H, Kollros JJ, et al. Biology of amphibians. Copeia. 1986;1986:549–553. doi: 10.2307/1445022. [DOI] [Google Scholar]
- Keklikoglou K, Arvanitidis C, Chatzigeorgiou G, et al (2021) Micro-CT for biological and biomedical studies: a comparison of imaging techniques. J Imaging 710.3390/jimaging7090172 [DOI] [PMC free article] [PubMed]
- Koch RW, Elfarnawany M, Zhu N, et al. Evaluation of cochlear duct length computations using synchrotron radiation phase-contrast imaging. Otol Neurotol. 2017;38:e92–e99. doi: 10.1097/MAO.0000000000001410. [DOI] [PubMed] [Google Scholar]
- Lak M, Neraudeau D, Nel A, et al. Phase contrast X-ray synchrotron imaging: opening access to fossil inclusions in opaque amber. Microsc Microanal. 2008;14:251–259. doi: 10.1017/S1431927608080264. [DOI] [PubMed] [Google Scholar]
- Liu Y, Nelson J, Holzner C, et al (2013) Recent advances in synchrotron-based hard X-ray phase contrast imaging. J Phys D: Appl Phys 4610.1088/0022-3727/46/49/494001
- Lorenzoni R, Curosu I, Paciornik S, et al (2020) Semantic segmentation of the micro-structure of strain-hardening cement-based composites (SHCC) by applying deep learning on micro-computed tomography scans. Cement Concr Compos 10810.1016/j.cemconcomp.2020.103551
- Mayhew MJ, Seifert TA, Pascarella ET, et al. Going deep into mechanisms for moral reasoning growth: how deep learning approaches affect moral reasoning development for first-year students. Res High Educ. 2011;53:26–46. doi: 10.1007/s11162-011-9226-3. [DOI] [Google Scholar]
- Momose A. Recent advances in X-ray phase imaging. Jpn J Appl Phys. 2005;44:6355–6367. doi: 10.1143/jjap.44.6355. [DOI] [Google Scholar]
- Morin PJ. Predatory salamanders reverse the outcome of competition among three species of anuran tadpoles. Science. 1981;212:1284–1286. doi: 10.1126/science.212.4500.1284. [DOI] [PubMed] [Google Scholar]
- Nesterets YI, Coan P, Gureyev TE, et al. On qualitative and quantitative analysis in analyser-based imaging. Acta Crystallogr A. 2006;62:296–308. doi: 10.1107/S0108767306017843. [DOI] [PubMed] [Google Scholar]
- Nunes-da-Fonseca R, Berni M, Tobias-Santos V, et al (2017) Rhodnius prolixus: from classical physiology to modern developmental biology. Genesis 5510.1002/dvg.22995 [DOI] [PubMed]
- Paganin D, Mayo SC, Gureyev TE, et al. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J Microsc. 2002;206:33–40. doi: 10.1046/j.1365-2818.2002.01010.x. [DOI] [PubMed] [Google Scholar]
- Paiva K, Meneses AAM, Barcellos R, et al. Performance evaluation of segmentation methods for assessing the lens of the frog Thoropa miliaris from synchrotron-based phase-contrast micro-CT images. Phys Med. 2022;94:43–52. doi: 10.1016/j.ejmp.2021.12.013. [DOI] [PubMed] [Google Scholar]
- Ritman EL. Current status of developments and applications of micro-CT. Annu Rev Biomed Eng. 2011;13:531–552. doi: 10.1146/annurev-bioeng-071910-124717. [DOI] [PubMed] [Google Scholar]
- Ronneberger O, Fischer P, Brox T (2015) U-Net: convolutional networks for biomedical image segmentation. In Medical image computing and computer-assisted intervention – MICCAI 2015. pp 234–241
- Schoppe O, Pan C, Coronel J, et al. Deep learning-enabled multi-organ segmentation in whole-body mouse scans. Nat Commun. 2020;11:5626. doi: 10.1038/s41467-020-19449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena G, Almeida AP, Braz D, et al. Phase contrast X-ray synchrotron microtomography for virtual dissection of the head of Rhodnius prolixus. J Phys: Conf Ser. 2014;499:012018. doi: 10.1088/1742-6596/499/1/012018. [DOI] [Google Scholar]
- Sena G, Almeida AP, Braz D, et al. On the possibilities of polychromatic synchrotron radiation microtomography for visualization of internal structures of Rhodnius prolixus. Radiat Phys Chem. 2015;115:179–182. doi: 10.1016/j.radphyschem.2015.07.006. [DOI] [Google Scholar]
- Sena G, Barroso RC, Braz D, et al (2021) Evaluation of the effects of Azadirachtin on internal structures of Rhodnius prolixus head using low-energy X-ray microfluorescence. Spectrochim Acta B 177. ARTN 106064. 10.1016/j.sab.2020.106064
- Sena G, Nogueira LP, Almeida AP, et al. Effects of different fixation methods on the study of Rhodnius prolixus head using 3D microCT imaging. AIP Conf Proc. 2016;1764:030007. doi: 10.1063/1.4961141. [DOI] [Google Scholar]
- Sena G, Nogueira LP, Braz D, et al. Ecdysis period of Rhodnius prolixus head investigated using phase contrast synchrotron microtomography. Phys Med. 2016;32:812–817. doi: 10.1016/j.ejmp.2016.05.051. [DOI] [PubMed] [Google Scholar]
- Sena G, Nogueira LP, Braz D, et al. Improving image quality of Rhodnius prolixus head using different types of staining methods and synchrotron radiation phase contrast microtomography. Radiat Phys Chem. 2019;155:26–30. doi: 10.1016/j.radphyschem.2018.06.039. [DOI] [Google Scholar]
- Sena G, Nogueira LP, Braz D, et al. Application of synchrotron radiation phase-contrast microtomography with iodine staining to Rhodnius prolixus head during ecdysis period. J Instrum. 2018;13:C05007–C05007. doi: 10.1088/1748-0221/13/05/c05007. [DOI] [Google Scholar]
- Shafiei SB, Iqbal U, Hussein AA, et al. Utilizing deep neural networks and electroencephalogram for objective evaluation of surgeon's distraction during robot-assisted surgery. Brain Res. 2021;1769:147607. doi: 10.1016/j.brainres.2021.147607. [DOI] [PubMed] [Google Scholar]
- Shearer T, Bradley RS, Hidalgo-Bastida LA, et al. Three-dimensional visualisation of soft biological structures by X-ray computed micro-tomography. J Cell Sci. 2016;129:2483–2492. doi: 10.1242/jcs.179077. [DOI] [PubMed] [Google Scholar]
- Silva J, Zanette I, Noël PB, et al. Three-dimensional non-destructive soft-tissue visualization with X-ray staining micro-tomography. Sci Rep-Uk. 2015;5:14088. doi: 10.1038/srep14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socha JJ, Westneat MW, Harrison JF, et al. Real-time phase-contrast X-ray imaging: a new technique for the study of animal form and function. BMC Biol. 2007;5:6. doi: 10.1186/1741-7007-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano C, Archer M, Azar D, et al. Synchrotron X-ray imaging of inclusions in amber. Cr Palevol. 2010;9:361–368. doi: 10.1016/j.crpv.2010.07.014. [DOI] [Google Scholar]
- Stalling D, Westerhoff M, Hege H-C. amira: A Highly interactive system for visual data analysis. In: Hansen CD, Johnson CR, editors. Visualization handbook. Burlington: Butterworth-Heinemann; 2005. pp. 749–767. [Google Scholar]
- Takeda T, Momose A, Hirano K, et al. Human carcinoma: early experience with phase-contrast X-ray CT with synchrotron radiation—comparative specimen study with optical microscopy. Radiology. 2000;214:298–301. doi: 10.1148/radiology.214.1.r00ja08298. [DOI] [PubMed] [Google Scholar]
- Wang YD, Armstrong RT, Mostaghimi P (2020) Boosting resolution and recovering texture of 2D and 3D micro‐CT images with deep learning. Water Resour Res 5610.1029/2019wr026052
- Wassersug R. Tadpoles: the biology of anuran larvae. Copeia. 2000;2000:1125–1134. doi: 10.1643/0045-8511(2000)000[1125:Br]2.0.Co;2. [DOI] [Google Scholar]
- Weissleder R, Nahrendorf M. Advancing biomedical imaging. Proc Natl Acad Sci U S A. 2015;112:14424–14428. doi: 10.1073/pnas.1508524112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur HM. Competition, predation, and the structure of the Ambystoma-rana sylvatica community. Ecology. 1972;53:3–21. doi: 10.2307/1935707. [DOI] [Google Scholar]
- Wilbur HM. Density-dependent aspects of growth and metamorphosis in Bufo americanus. Ecology. 1977;58:196–200. doi: 10.2307/1935122. [DOI] [Google Scholar]
- Wilbur HM, Collins JP. Ecological aspects of amphibian metamorphosis: nonnormal distributions of competitive ability reflect selection for facultative metamorphosis. Science. 1973;182:1305–1314. doi: 10.1126/science.182.4119.1305. [DOI] [PubMed] [Google Scholar]
- Wilbur HM, Morin PJ, Harris RN. Salamander predation and the structure of experimental communities - anuran responses. Ecology. 1983;64:1423–1429. doi: 10.2307/1937496. [DOI] [Google Scholar]
- Wilkins SW, Nesterets YI, Gureyev TE, et al. On the evolution and relative merits of hard X-ray phase-contrast imaging methods. Philos Trans A Math Phys Eng Sci. 2014;372:20130021. doi: 10.1098/rsta.2013.0021. [DOI] [PubMed] [Google Scholar]
- Yang X, De Andrade V, Scullin W, et al. Low-dose X-ray tomography through a deep convolutional neural network. Sci Rep. 2018;8:2575. doi: 10.1038/s41598-018-19426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Zong Y, Fan J, et al. Synchrotron X-ray microtomography with improved image quality by ring artifacts correction for structural analysis of insects. Microsc Microanal. 2017;23:938–944. doi: 10.1017/S1431927617012387. [DOI] [PubMed] [Google Scholar]
- Zehbe R, Haibel A, Riesemeier H, et al. Going beyond histology. Synchrotron micro-computed tomography as a methodology for biological tissue characterization: from tissue morphology to individual cells. J R Soc Interface. 2010;7:49–59. doi: 10.1098/rsif.2008.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Jiang L, Yang D, et al. Correction to: urine sediment recognition method based on multi-view deep residual learning in microscopic image. J Med Syst. 2020;44:84. doi: 10.1007/s10916-020-01558-x. [DOI] [PubMed] [Google Scholar]
- Zheng J, Wang K. Emerging deep learning methods for single-cell RNA-seq data analysis. Quantitative Biology. 2019;7:247–254. doi: 10.1007/s40484-019-0189-2. [DOI] [Google Scholar]