Abstract
BACKGROUND:
Penicillin allergy labels are often inaccurate in children and removing unnecessary labels results in improved outcomes and lower healthcare costs. While the hospital setting is a frequent point of contact for children, strategies to evaluate penicillin allergies in the hospital are lacking.
METHODS:
We performed a prospective pilot study to determine the feasibility of centralized, pharmacy-led approach to penicillin allergy evaluation. Children with a reported history of penicillin allergy admitted our Children’s Hospital were risk stratified and those stratified as low risk underwent a single dose oral challenge by a central pharmacist, regardless of need for antibiotics. Upon completion of each patient’s delabeling process, surveys were distributed to healthcare personnel involved in the patient’s care to collect perceptions on the acceptability, appropriateness, and feasibility of this intervention. Measures were scored using a 5-point Likert scale.
RESULTS:
Of the 23 patients who screened as low risk, 20 underwent a penicillin allergy evaluation and an oral challenge. Of these, the penicillin allergy label was removed in 19 (95%) patients (Figure 1). Median age was 7 years (range 11 months – 18 years). Participants overall rated the risk stratification and delabeling favorably, with high ratings on all three implementation measures: Acceptability (mean 4.55, ± STD 0.65), Appropriateness (mean 4.58, STD ± 0.6), and Feasibility (mean 4.51, STD ± 0.73). Measures of acceptability, appropriateness, and feasibility remained high when stratified by healthcare worker type and provider type.
CONCLUSIONS:
Our findings provide support for systemic implementation of penicillin allergy delabeling strategies in hospitalized children.
INTRODUCTION
Approximately 10% of the US population is labeled as allergic to a penicillin antibiotic.1 However, penicillin allergy labels are often inaccurate, especially in children. Over 90% of children with a penicillin allergy label do not have an IgE- mediated allergy and can safely be prescribed one of these first-line, narrow spectrum antibiotics.1 Penicillin allergy labels result in delayed antibiotic administration and higher rates of broad spectrum antibiotic use, adverse drug events, and treatment failure1, while appropriately delabeling penicillin allergies improves health outcomes and lowers health care costs.2,3 However, efforts to implement penicillin allergy assessments in children are lacking, especially in hospitalized patients4 and recent studies demonstrate that de-centralized, provider-led approaches result in only modest increases in penicillin allergy delabeling in children’s hospitals.1 We performed a prospective study to determine the feasibility, appropriateness, and acceptability of a centralized, pharmacy-led approach to penicillin allergy risk-stratification and delabeling of hospitalized children at low risk for true allergy.
METHODS
Study Population and Patient Identification
A convenience sample of patients with a reported history of penicillin allergy admitted to our academic children’s hospital were identified by a central pharmacist. Patients with a penicillin allergy label were identified during the intake process by nurses, who then documented a penicillin allergy into the patient’s electronic medical record (EMR). We developed an internal EMR penicillin allergy dashboard visible to the pharmacy team that displayed all patients within the hospital with a penicillin allergy label. All patients on a non-psychiatric or behavioral health team (including pediatric hospital medicine and surgery specialties) were evaluated for enrollment and eligible patients were randomly selected for risk stratification. Patients excluded from risk stratification included those who were pregnant, had a previous positive penicillin skin test, those admitted with concern for allergic reaction or non-accidental trauma, unstable wheezing or rash on admission and those with antihistamine use in the previous 24 hours.
Penicillin Allergy Risk Stratification and Oral Challenge
Once a patient was enrolled and selected for intervention, the pharmacist contacted the primary team and obtained permission to perform risk stratification and oral challenge if patient stratified as low- risk. Decision to perform risk stratification and oral challenge was at the sole discretion of the primary team upon consent by parent or legal guardian. Patients were risk stratified using a previously developed and validated penicillin allergy-specific questionnaire (regardless of need for antibiotics)(Supplemental Figure 1).5,6 Those screened as low-risk underwent an amoxicillin oral challenge following informed consent from a parent or guardian. The amoxicillin oral challenge was performed using an institutional standard-of-care protocol consisting of a 250 mg oral amoxicillin dose followed by 60 minutes of monitoring for reactions while on a cardiorespiratory monitor and pulse oximetry. Vital signs were obtained at 0, 30 and 60 minutes following the amoxicillin administration. An adult (≥ 18 years of age) other than the patient was required to be present in the room during the monitoring period. This could be a family member or hospital staff.
Implementation Measures
Upon completion of each patient’s risk stratification and delabeling process, the Acceptability of Intervention Measure, Intervention Appropriateness Measure, and Feasibility of Intervention Measure surveys7 were distributed to healthcare personnel (pharmacists, nurses, nurse practitioners, housestaff and attendings) involved in the patient’s care that day. Measures were scored using a 5-point Likert scale ranging from strongly disagree (1) to strongly agree (5).7 The study was approved by the our Institutional Review Board (#201337, ClinicalTrials.gov Identifier: NCT04441021).
RESULTS
Of the 23 patients who screened as low risk, 20 underwent a penicillin allergy evaluation and an oral challenge. Of these, the penicillin allergy label was removed in 19 (95%) patients (Figure 1). Median age was 7 years (range 11 months – 18 years). One 13-month-old patient had pruritus reported by her mother one hour into the oral challenge observation period. There were no vital sign changes associated with the parent-reported reaction, and medication intervention with antihistamines or epinephrine was not required. The patient’s original penicillin allergy label was due to a family history of reported penicillin allergy but the patient had never received a penicillin prior to the amoxicillin oral challenge and suspicion for true amoxicillin IgE-mediated reaction was low. She was referred to allergy clinic for skin testing after discharge but was lost to follow-up.
Figure 1.
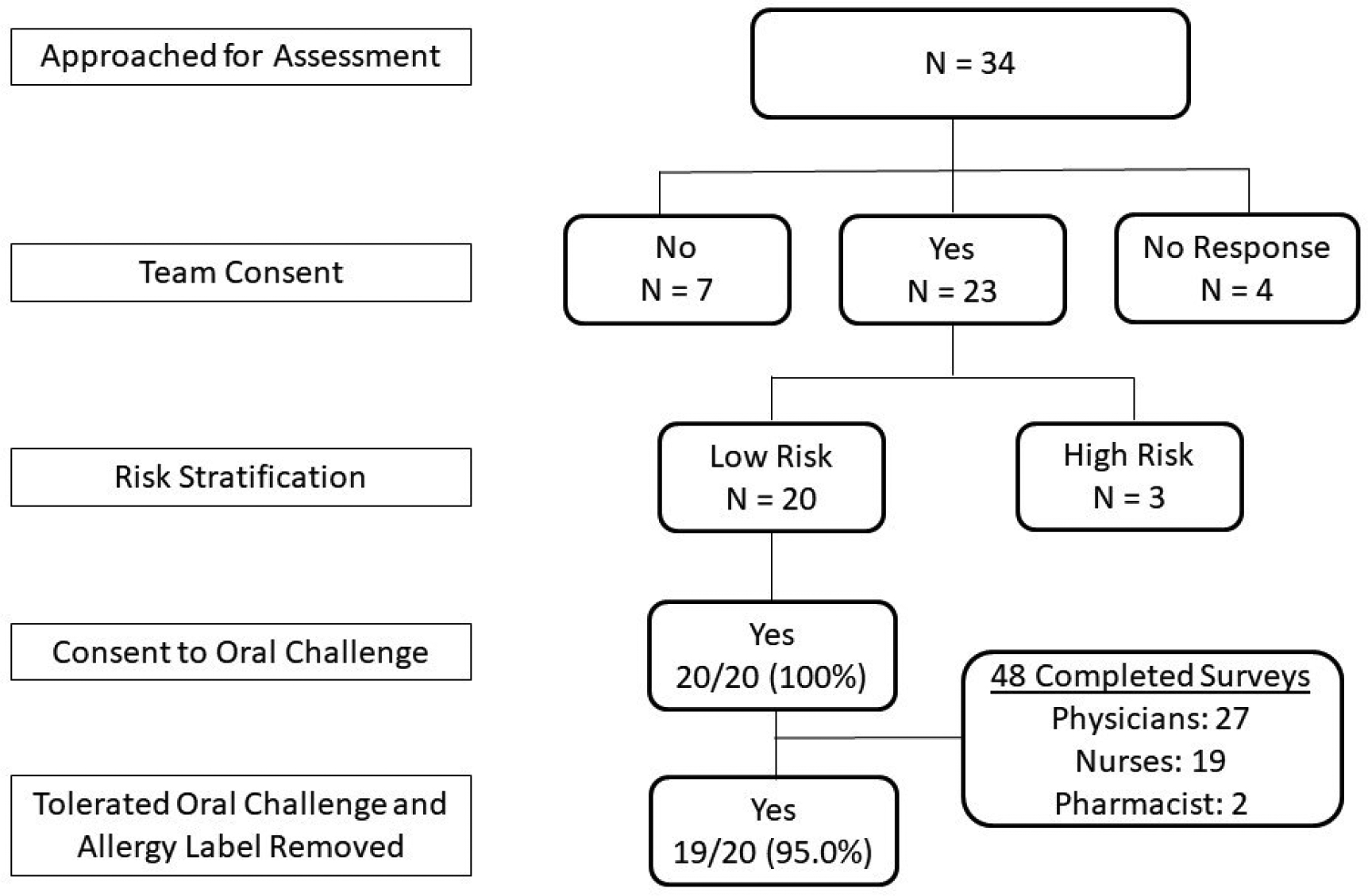
Enrollment flow diagram. Note: Reason for primary team declined consent was “imminent patient discharge” in all 7 patients.
A total of 48 out of 52 (92.3%) healthcare personnel approached completed the survey. Participants overall rated the risk stratification and delabeling favorably, with high ratings on all three implementation measures: Acceptability (mean 4.55, ± STD 0.65), Appropriateness (mean 4.58, STD ± 0.6), and Feasibility (mean 4.51, STD ± 0.73). Measures of acceptability, appropriateness, and feasibility remained high when stratified by healthcare worker type (Figure 2A) and provider type (Figure 2B). Comments from the survey are represented in Supplemental Table 1 and revolved around timing, communication, clinical need, and required effort.
Figure 2.
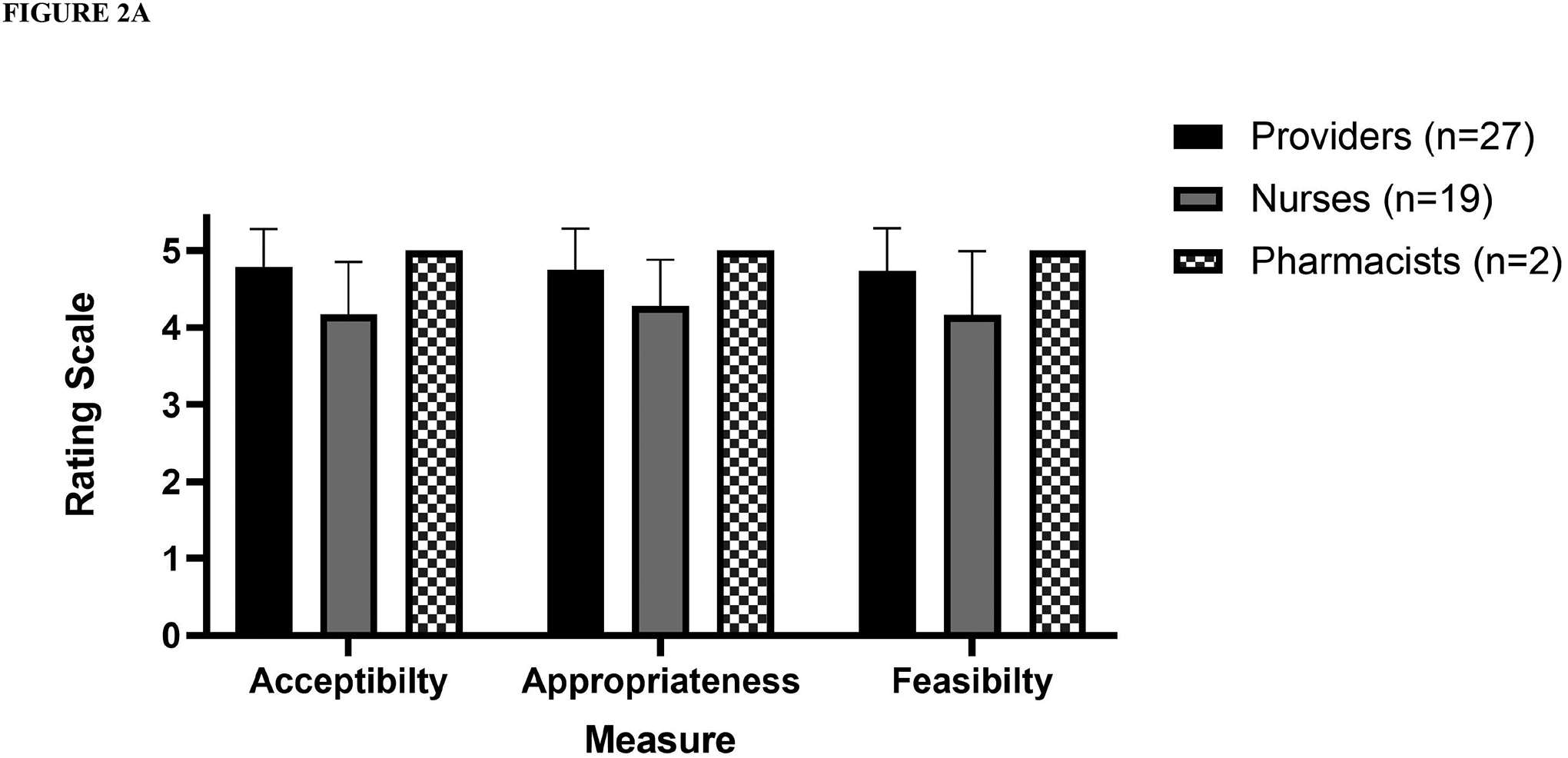
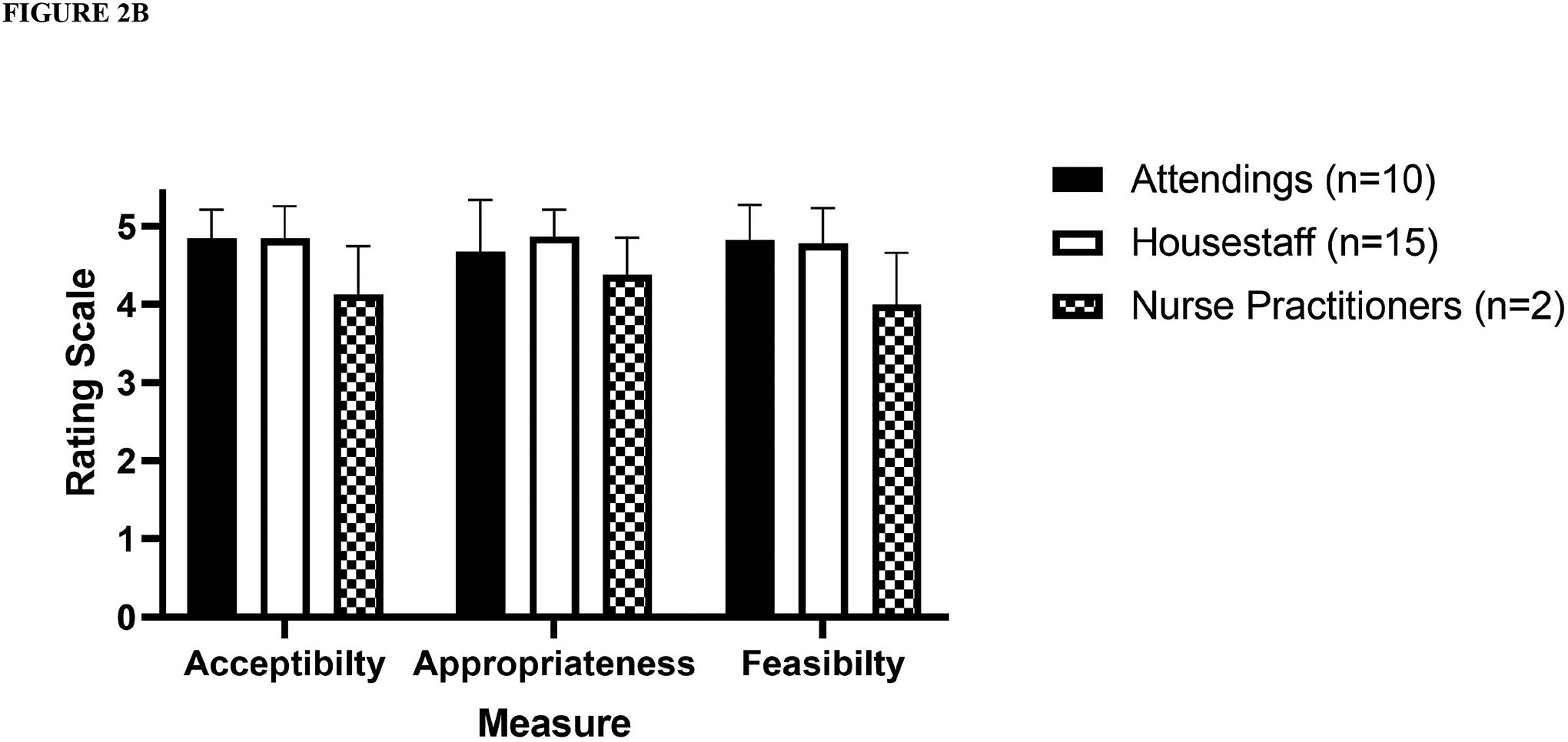
Acceptability, appropriateness, and feasibility of penicillin allergy risk- stratification and the labeling of low- risk patients by (A) healthcare personnel (B) provider type. Note: pharmacists rated all measures with the highest possible rating.
DISCUSSION
Given the high prevalence of inaccurate penicillin allergies and the significant benefits of removing such labels, improved approaches are needed to identify and safely delabel inappropriate allergy labels in children. In this study, we piloted a centralized, pharmacy-led penicillin allergy risk- stratification and delabeling program that was demonstrated to be safe and effective, and perceived as acceptable, appropriate, and feasible by associated healthcare workers. Hospitalized patients provide an ideal point- of- care for allergy evaluation as hospitals are a location of frequent patient interaction with the healthcare system, provide close patient supervision, and often include sufficient downtime in which to perform allergy evaluations, regardless of need for antibiotics.8,9
There are several aspects of this pilot intervention that were key to the success of this new delabeling process. Importantly, the pharmacy, hospital medicine, nursing and allergy/immunology teams were intimately involved in the development of the study protocol. This multidisciplinary, unified approach across subspecialties was crucial in obtaining early buy-in for the project. Second, this intervention was pharmacist-led. A pharmacist identified patients with a penicillin allergy using an EMR dashboard, consented families, ordered medications and provided documentation. All of these were performed in coordination with the primary team and often through routine interactions with the primary provider. Next, the use of a single dose, rather than a graded oral challenge requires less monitoring and is less of a burden to nurses and pharmacy staff. There was also face-to-face communication with nurses and families that included education on the benefits of delabeling and the likelihood of reaction related to oral challenge. Specifically, discussion regarding the availability of medications and protocols for managing allergic reactions as well as the likelihood of allergic reaction with or without need for epinephrine or respiratory support and duration of monitoring were judged as very important. As a result, as needed orders for epinephrine, antihistamines, steroids, and beta-agonists were added to the protocol and included for every patient prior to amoxicillin administration. Enhanced team communication also allowed real-time nursing staff feedback to optimally schedule oral challenges within daily workflows. Finally, prior to implementation, we engaged hospital leadership, including nursing, pharmacy, unit medical directors, clinical informatics, and hospital medicine to develop internal support for the process to improve patient care of children with drug allergies.
Findings from this project suggest that a centralized, pharmacy-led delabeling program can be successfully implemented within the workflow of a children’s hospital. Additional study is needed to evaluate the scalability of this intervention at a hospital-wide level. Our intervention could be improved by leveraging the EMR to improve and automate patient identification, notification of allergy labels, delabeling orders and documentation support through the use of clinical decision support tools to improve the efficiency of the delabeling process within hospital workflow. Limitations of this pilot demonstration include the number of patients evaluated as well as the academic hospital setting, which may limit generalizability of our findings.
Appropriate delabeling of penicillin allergy in children will lower health care costs and improve health outcomes by lowering rates of treatment failure, multi-drug-resistant infections, infection-related mortality, antibiotic related drug-drug interactions, and adverse drug events.1,10 These promising results provide support for future prospective studies of implementation strategies to most effectively and safely delabel patients at low risk for true allergy.
Supplementary Material
Table 1.
Representative thematic comments From Health Care Worker Survery
| Oral Challenge | “Timing of challenge best in the morning” (Nurse) |
| “It would be great to have a student or medical professional at the bedside to complete the tasks needed for the allergy assessment. It was difficult to complete the task as a nurse without a care partner because of short staffing. It added a lot to my workload within that hour with the fear of the patient possibly having an allergic reaction” (Nurse) | |
| “It was easy to perform and I felt confident in monitoring my patient for allergic reactions however I had a slower assignment today. I do think this could be difficult for nurses to have time to do when their assignment is high acuity and very busy” (Nurse) | |
| “Easy order set for nursing staff to follow with emergency meds, etc” (Nurse) | |
| Provider Effort | “The best thing about the allergy delisting is that it required minimal involvement on my part; the use of pharmacist-driven cosigned orders that I didn’t have to enter made the experience quite frictionless, and key to why I think it’s very feasible.” (Housestaff provider) |
| Clinical Need | “I was glad to be part of risk assessment process it is a problem we deal with and this will help put those antibiotics back into treatment of infections for those who have thought they were allergic” (Nurse) |
| Multidisciplinary Communication | “Great information from team to make administrative coordinator and nursing staff more comfortable with procedure” (Nurse) |
Note: Heathcare worker type noted in bold for each comment
Funding Disclosure:
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K12 HL137943 (Dr. Antoon), National Institute for Allergy and Infectious Diseases K24 AI148459 (Dr. Grijalva) and R01 AI125642 (Dr. Williams) and the Agency for Healthcare Research and Quality 1K12HS026395 (Dr. Stone).
Funding Role:
The funder/sponsor did not participate in the work
Abbreviations:
- EMR
electronic medical record
- STD
standard deviation
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest relevant to this article to disclose.
REFERENCES
- 1.Vyles D, Antoon JW, Norton A, et al. Children with reported penicillin allergy: Public health impact and safety of delabeling. Ann Allergy Asthma Immunol. 2020;124(6):558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. J Allergy Clin Immunol. 2014;133(3):790–796. [DOI] [PubMed] [Google Scholar]
- 3.Vyles D, Chiu A, Routes J, et al. Antibiotic Use After Removal of Penicillin Allergy Label. Pediatrics. 2018;141(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffin SE, Abanyie F, Bryant K, et al. Pediatric research priorities in healthcare-associated infections and antimicrobial stewardship. Infect Control Hosp Epidemiol. 2021;42(5):519–522. [DOI] [PubMed] [Google Scholar]
- 5.Vyles D, Adams J, Chiu A, Simpson P, Nimmer M, Brousseau DC. Allergy Testing in Children With Low-Risk Penicillin Allergy Symptoms. Pediatrics. 2017;140(2). [DOI] [PubMed] [Google Scholar]
- 6.Vyles D, Chiu A, Routes J, et al. Oral amoxicillin challenges in low-risk children during a pediatric emergency department visit. J Allergy Clin Immunol Pract. 2020;8(3):1126–1128 e1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiner BJ, Lewis CC, Stanick C, et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci. 2017;12(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone CA Jr., Stollings JL, Lindsell CJ, et al. Risk-stratified Management to Remove Low-Risk Penicillin Allergy Labels in the ICU. Am J Respir Crit Care Med. 2020;201(12):1572–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoon JW, Grisso AG, Stone CA. Breaking the Mold: Safely Delabeling Penicillin Allergies in Hospitalized Children. Hosp Pediatr. 2021;11(5):e70–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antoon JW, Hall M, Herndon A, et al. Prevalence of Clinically Significant Drug-Drug Interactions Across US Children’s Hospitals. Pediatrics. 2020;146(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


