Abstract
Purpose:
There is a lack of level I evidence to guide radiotherapy recommendations for patients receiving neoadjuvant chemotherapy for breast cancer. We utilized four neoadjuvant chemotherapy trials to determine which patients benefit from regional nodal irradiation (RNI).
Materials and Methods:
We obtained data from NSABP B-18, B-27, B-40, and B-41 clinical trials. B-40 and B-41 allowed RNI at physician’s discretion. We evaluated local-regional recurrence (LRR), distant recurrence (DR), disease-free survival (DFS), and overall survival (OS). Kaplan-Meier, Peto-Peto, Chi-squared, Fisher exact, and Wilcoxon rank-sum tests were used for survival estimates and comparison.
Results:
Median follow-up for B-18, B-27, B-40, and B-41 was 13.7, 9.7, 4.5, and 5.1 years, respectively, including 742, 2254, 1154, and 504 patients for analysis. On multivariable analysis, factors significantly associated with RNI included tumor size, ypN status, and tumor subtype; Hispanic patients were less likely to receive RNI. Patients with ypN+HER2+ disease who received RNI had improved OS. B-40 patients with ypN+HR+ disease had improved LRR. On multivariable analysis for the B-40 and B-41 study population, RNI was not associated with significantly improved OS, DFS, DR, or LRR.
Conclusion:
RNI was associated with a clinical benefit for patients with ypN+HER2+ and ypN+HR+ disease. RNI was not significantly associated with a clinically beneficial outcome for the entire cohort. Prospective phase III clinical trials are needed to establish guidelines for patients who should receive RNI following neoadjuvant treatment, and action is necessary to eliminate the disparity in care delivery shown for Hispanic women.
Keywords: regional nodal irradiation, breast cancer, radiation oncology, resource allocation, health disparities
Introduction
Breast cancer is the most common non-skin malignancy in the United States. Its management has evolved in the past 50 years from widespread radical mastectomy to a personalized approach that tailors surgery(1), adjuvant chemotherapy(2-4), and adjuvant radiation(5, 6), with the recognition that individualized treatment is paramount. In achieving this optimal personalized treatment, stepwise trials have established the indications for breast-conserving surgery, sentinel lymph node biopsy, adjuvant systemic therapy, and optimal radiotherapy recommendations. In particular, the evolution of radiotherapy design has allowed for shared decision-making with patients(7-9)—from radiotherapy omission to partial-breast treatments to a comprehensive treatment of lymphatics—and is supported by level I evidence for patients who receive surgery first followed by adjuvant systemic therapy and adjuvant radiotherapy. Most recently, recommendations for regional nodal irradiation (RNI) have expanded to include patients with any number of positive lymph nodes and to those with node-negative but high-risk disease, based on significant clinical benefits from targeting not only the breast/chest wall but also the regional lymphatics, such as the internal mammary nodes, supraclavicular nodes, and axilla(10, 11).
The introduction of neoadjuvant systemic therapy has also established new treatment paradigms for patients(12). However, recommendations for RNI after neoadjuvant chemotherapy are not supported by level I evidence. The recently completed National Surgical Adjuvant Breast and Bowel Project (NSABP) B-51/Radiation Therapy Oncology Group (RTOG) 1304 phase III trial evaluated the effect of adding RNI in patients with cN1 disease (pathologically proven by fine-needle aspiration or core needle biopsy) and are found to have axillary pathologic complete response(13). Trial results are not yet available. Thus, while we wait for this level I evidence, investigators have pursued retrospective analyses of RNI’s benefit in previously completed trials (B-18, B-27, B-40 and B-41) of neoadjuvant therapy to better guide personalized radiotherapy recommendations, inform the decision-making process, and avoid over- and under-treatment.
Previously reported were the findings associated with local-regional recurrence for patients enrolled on NSABP trials B-18 and B-27, on which neither radiotherapy nor HER2-directed therapy were allowed(14). NSABP B-40 and B-41 are contemporary trials with stratification by HER2 positivity and with HER2-directed therapy(15, 16). Both latter studies allowed for radiotherapy at the physician’s discretion, thus allowing for evaluation of radiotherapy’s efficacy depending on pathologic response. At present, patterns-of-care analyses show that almost half of patients with ypN+ breast cancer do not receive RNI (17). Our goal is to elucidate which patients undergoing neoadjuvant systemic treatment will benefit from the addition of RNI.
Materials and Methods
Following institutional review board approval, we obtained data from four large NSABP neoadjuvant therapy trials: B-18, B-27, B-40, and B-41. All four trials were designed to evaluate the efficacy of neoadjuvant chemotherapy for patients with operable breast cancer. NSABP B-18 and B-27 did not allow RNI therapy. NSABP B-40 and B-41 allowed RNI at the physician’s discretion.
The design of NSABP B-18 and B-27 have been previously reported (14). In NSABP B-18, patients were assigned to receive 4 cycles of doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 (AC) on day 1 of every 21-day cycle either before or after surgery. Patients of age 50 years received tamoxifen (10 mg orally twice per day for 5 years) starting after chemotherapy, regardless of hormone receptor status. In NSABP B-27, patients were assigned to receive 4 cycles of neoadjuvant AC similar to B-18’s cohort but either alone (group 1) or followed by 4 cycles of neoadjuvant docetaxel at 100 mg/m2 on day 1 of every 21-day cycle (group 2) or followed by the same docetaxel regimen postoperatively (group 3). All B-27 patients received tamoxifen (20 mg per day for 5 years) from the first day of chemotherapy, regardless of hormone receptor status (14). Median follow-up for B-18 and B-27 were 13.7 years and 9.7 years, respectively. Notably, neither hormone receptor nor HER2 status was reported for these two trials. In B-18 and B-27, radiation was only permitted for those receiving lumpectomy and limited to the breast.
Treatment Regimens
The more contemporary neoadjuvant trials, NSABP B-40 and B-41, stratified eligibility by HER2 positivity and reported on both hormone receptor and HER2 status. B-40 enrolled women with HER2-non-amplified invasive adenocarcinoma of the breast, clinical stage T1c-3, and cN0, cN1, or cN2a. Between January 5, 2007, and June 30, 2010, a total of 1206 patients were randomly assigned to one of three neoadjuvant docetaxel-based chemotherapy regimens (docetaxel alone, docetaxel plus capecitabine, or docetaxel plus gemcitabine) followed by neoadjuvant doxorubicin and cyclophosphamide with or without neoadjuvant and adjuvant bevacizumab (15). Results from the trial’s primary endpoint of pathological complete response and secondary endpoints of disease-free survival have been previously reported with a median follow-up of 4.5 years. Radiation was given at physician discretion and not protocolled.
NSABP B-41 included patients with operable HER2-positive breast cancer, clinical stage cT2-T3 cN0-N2a. Between 7/16/2007 – 6/30/2011, a total of 529 patients were randomized to receive four cycles of standard AC followed by weekly paclitaxel with trastuzumab, lapatinib, or both. All patients received postoperative weekly trastuzumab for 52 weeks. With a median follow-up of 5.1 years, results of the primary endpoint of pathologic complete response have been previously reported. Radiation was given at physician discretion and not protocolled.
Primary Aims of Investigation
Report the natural history of recurrence for women with breast cancer receiving neoadjuvant systemic therapy in contemporary national trials NSABP B-40 and B-41, and evaluate changes since historical trials B-18 and B-27 based on pathologic response in the breast and axilla.
With physician-directed RNI for trials B-40 and B-41, evaluate the factors associated with RNI receipt.
Evaluate the effect of RNI on cancer outcomes using multivariable analysis methodology for B-40 and B-41.
Statistical Analysis
We selected four endpoints for breast cancer outcomes: local-regional recurrence (LRR), distant recurrence (DR), disease-free survival (DFS), and overall survival (OS).
To answer Aim 1, we evaluated differences in breast cancer outcome by levels of breast pCR and axillary pCR, stratifying by clinical nodal status at time of diagnosis.
To answer Aim 2, we performed univariable analyses of clinical and demographic factors associated with receipt of RNI. Variables noted to be significant in univariable testing were selected for multivariable analysis.
For Aim 3, as RNI was given based on physician discretion, both univariable and multivariable approaches were used to evaluate RNI benefit, while adjusting for clinical and pathologic confounding factors such as grade, clinical tumor size, clinical nodal status, hormone receptor positivity, lymphovascular invasion, margin status, breast pCR, and axillary pCR. Margin status was not available for B-40, and lymphovascular invasion was not available for B-40 or B-41.
Chi-squared or Fisher exact tests were used to compare categorical variables and a Wilcoxon rank-sum test was used for continuous variables. Multivariable logistic regression was fitted to model receiving RNI or not for patients in B-40 and B-41. Kaplan-Meier (KM) curves were generated for OS and DFS, and cumulative incidence plots were generated for LRR and DR, treating death as the only competing event. A log-rank or Peto-Peto (when proportional hazards assumption did not hold) test was used for comparing Kaplan-Meier curves. Gray’s test was used for cumulative incidence function (CIF) comparison. A stepdown Bonferroni adjustment was used for multiple comparison adjustment. Multivariable Cox regressions were fitted for OS and DFS. Multivariable cause-specific Cox regressions were fitted for LRR and DR(18). In the survival models, RNI, age, and tumor subtype were forced in the model regardless of their P values.
Results
Patient Population
From the study populations, 742 (of 1523; 49%), 2254, 1154, and 504 patients were available for analysis from NSABP trials B-18, B-27, B-40, and B-41, respectively. Similar to a prior analysis(14), only those patients who received neoadjuvant chemotherapy in B-18 were included in our analysis. Table 1 provides the demographic characteristics of the study populations of the four included trials.
Table 1.
Characteristics of Patients Enrolled on NSABP B-18, B-27, B-40, and B-41 (N=4654)
| Characteristic | B-18 (n=742) | B-27 (n=2254) |
B-40 (n=1154) | B-41 (n=504) |
|---|---|---|---|---|
| Age, median (range) years | 49 (25-75) | 48 (25-75) | 49 (24-72) | 49 (18-73) |
| Node-positive at entry, n (%) | 201 (27·1%) | 705 (30·1%) | 534 (46·3%) | 252 (50·0%) |
| Clinical tumor size, median (range) cm |
3 (0·9-10·5) | 4 (0·1-25·4) | 4.5 (2-17·5) | 4 (2-20) |
| HR-positive, n (%) | N/A | N/A | 688 (59·6%) | 317 (62·9%) |
| Mastectomy, n (%) | 236 (31·8%) | 864 (37·3%) | 610 (52·9%) | 245 (48·6%) |
| pCR breast, n (%) | 86 (12·9%) | 398 (17·5%) | 356 (30·8%) | 277 (55·0%) |
| pCR node, n (%) | 434 (59·0%) | 1210 (53·7%) | 603 (53·8%) | 386 (77·5%) |
Abbreviations: HR, hormone receptor; NSABP, National Surgical Adjuvant Breast and Bowel Project; pCR, pathological complete response; N/A, not available
Aim 1: Natural History of Recurrence
Outcomes by pCR for the Populations of B-18 and B-27
Patients in B-18 and B-27 did not receive RNI. When examining patients who were clinically node-positive at presentation, OS was significantly higher for women who had pathologic complete response (ypT0N0) than those who had residual breast disease (ypT+N0; p=0.022). Similarly, OS was significantly higher for women with pathologic complete response than those with residual axillary disease (ypT0N+; p=0.002). This finding of higher OS in patients with pathologic complete response (ypT0N0) compared to patients with residual breast (ypT+N0) and residual axillary (ypT0N+) disease was also seen in patients who were clinically node-negative at presentation (p<0.001 and p=0.006, respectively; Supplemental Figure 1).
Outcomes by pCR for the Populations of B-40 and B-41
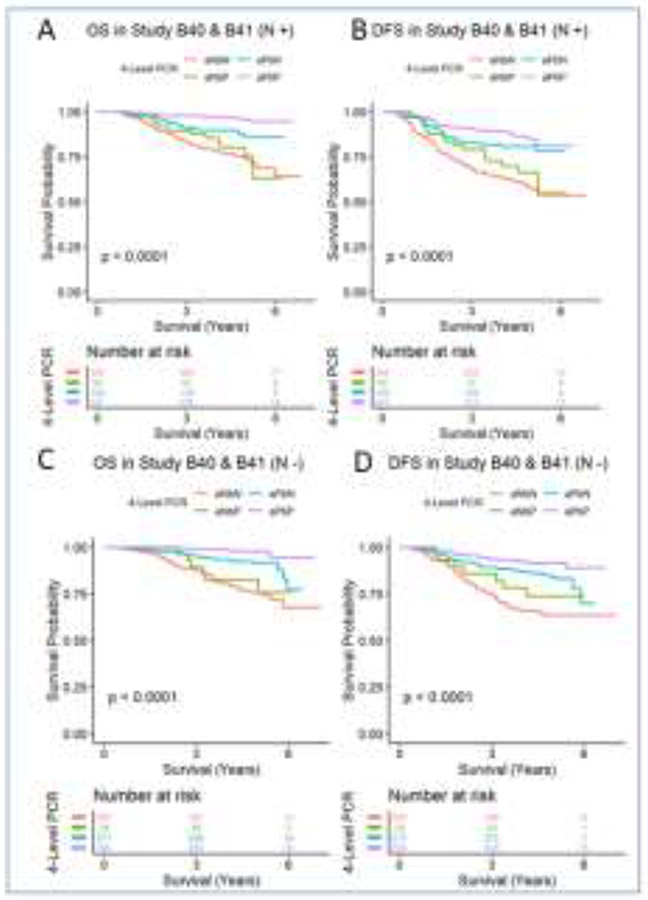
Patients enrolled in B-40 and B-41 received RN I at the physicians’ discretion. In B-40 and B-41, patients who were clinically node-positive at presentation but had a pathologic complete response (ypT0N0) experienced significantly higher OS than those with residual breast disease (ypT+N0; p=0.008). DFS and LRR were not significantly different between groups (p=0.312; p=0.353), respectively. However, those with residual breast (ypT+N0) disease experienced a higher cumulative incidence of distant recurrence compared to those who achieved a total complete response (ypT0N0; p=0.02). These differences were also seen in women with initially clinically node-negative breast cancer such that women with ypT0N0 response had significantly higher OS and DFS and a significantly lower cumulative incidence of DR compared to women with residual breast (ypT+N0) disease (p=0.003; p=0.022; p<0.001), respectively (Figure 1). When evaluating differences in outcome between women who were clinically node-positive with a pathologic complete response (ypT0N0) and those with residual axillary disease (ypT0N+), those with ypT0N0 disease experienced significantly higher OS (p<0.001), higher DFS (p=0.004), and a lower cumulative incidence of DR (p<0.001) without a significant difference in LRR (p>0.99), although with a statistically significant difference in competing events for LRR (p<0.001). These significant findings for OS, DFS, and DR for women with residual axillary disease were similarly observed for clinically node-negative women (p<0.001, p=0.014, and p<0.001, respectively). Likewise, LRR was not significantly different (p=0.227), although a significant difference in competing events for LRR was noted (p<0.001).
Figure 1.
Overall survival and disease-free survival by response to neoadjuvant therapy for patients enrolled on NSABP B-40 and B-41. (A) Overall survival for cN+ patients; (B) disease-free survival for cN+ patients; (C) overall survival for cN− patients; and (D) disease-free survival for cN− patients; aNbN = ypT+N+; aNbP = ypT0N+; aPbN= ypT+N0; aPbP = ypT0N0
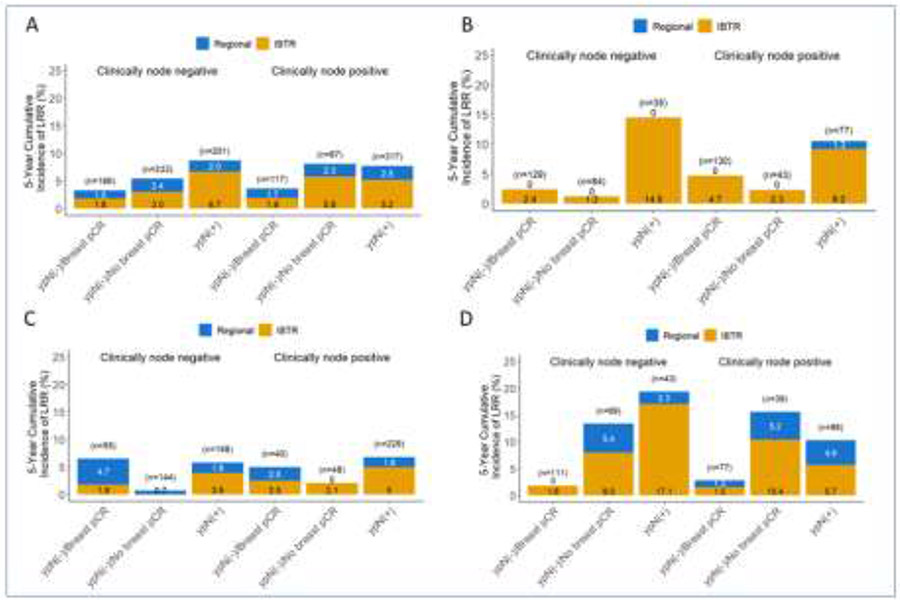
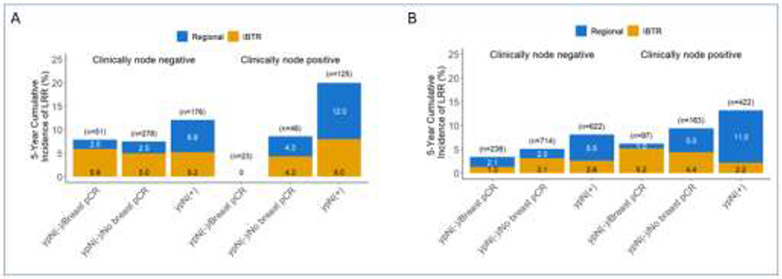
LRR was also evaluated by initial clinical nodal status, and breast and axillary pathologic response for the study populations of B-18, B-27, B-40 and B-41. The 5-year cumulative incidence of LRR exceeded 5% for patients with any residual disease, including breast (ypT+N0) or axillary (ypN+) disease in B-40 (Figure 2A), regardless of initial clinical nodal status. For B-41, LRR exceeded 5% for women with residual axillary disease (ypTanyN+), regardless of initial clinical nodal status (Figure 2B). Examining the cohort of patients in B-40 by HR+ expression (Figures 2C and 2D), LRR exceeded 5% for patients with HR+ disease with ypN+ and for those with triple-negative disease, LRR exceeded 10% for patients with residual breast or axillary disease. The 5-year LRR for historical trials B-18 and B-17 are shown in Figure 3 for comparison.
Figure 2.
Five-year local-regional recurrence for patients enrolled on NSABP B-40, and B-41 stratified by clinical node involvement and subsequent response to neoadjuvant chemotherapy. (A) B-40; (B) B-41; (C) HR+ patients from B40; and (D) TN patients from B40. 95% Confidence Intervals are provided in Supplementary Tables 10 and 11. Abbreviations: HR+ = Hormone-receptor positive; TN = triple negative
Figure 3.
Five-year local-regional recurrence for patients enrolled on NSABP B-18 (A) and B-27 (B) stratified by clinical node involvement and subsequent response to neoadjuvant chemotherapy
Aim 2: Evaluating Allocation of RNI
Characteristics of patients from B-40 and B-41 by receipt of RNI are displayed in Table 2. Univariable testing results indicated that patients with larger tumors, mastectomy, HR+ disease, clinically node-positive, residual breast disease, and residual axillary disease were significantly more likely to receive RNI. While there was no significant association detected between race and RNI receipt, Hispanic patients were significantly less likely to receive RNI. On multivariable analysis (Supplementary Table 1), factors significantly associated with receipt of RNI were clinical nodal status, axillary pCR, Hispanic ethnicity, and tumor subtype. Specifically, Hispanic patients were significantly less likely to receive RNI on multivariable analysis.
Table 2.
Characteristics of Patient Population Enrolled on NSABP B-40 and B-41 by Receipt of RNI (N=1542)
| Characteristic | Received RNI (n=783) | Did not receive RNI (n=759) |
P value |
|---|---|---|---|
| Age, median (range) years | 49 (25-73) | 49 (24-72) | 0·529 |
| Node-positive at entry, n (%) | 484 (61·8%) | 270 (35·6%) | <0·001 |
| Clinical tumor size, median (range) cm | 5 (2-17·5) | 4 (2-20) | <0·001 |
| Race, n (%) | 0·745 | ||
| White | 654 (84·3%) | 630 (84·9%) | |
| Black | 97 (12·5%) | 93 (12·5%) | |
| Other | 25 (3·2%) | 19 (2·6%) | |
| Ethnicity, n (%) | 0·045 | ||
| Hispanic | 73 (9·6%) | 96 (13.1%) | |
| Non-Hispanic | 684 (90·4%) | 639 (86·9%) | |
| HR-positive, n (%) | 514 (65·6%) | 427 (56·3%) | <0·001 |
| Mastectomy, n (%) | 419 (53·5%) | 338 (44·5%) | 0·001 |
| pCR breast, n (%) | 254 (32·4%) | 313 (41·2%) | <0·001 |
| pCR node, n (%) | 356 (45·9%) | 542 (73·7%) | <0·001 |
Abbreviations: HR, hormone receptor; NSABP, National Surgical Adjuvant Breast and Bowel Project; pCR, pathological complete response
Aim 3: Determining Efficacy of RNI on Cancer Outcomes
For the combined population of B-40 and B-41, receipt of RNI was not associated with statistically significant differences in DFS or OS. When restricting the analysis to the population of patients in B-40 and B-41 with ypN+ disease, there were no statistically significant differences in DFS and OS by RNI receipt (p=0.088 and p=0.094, respectively). RNI receipt was not associated with statistically significant differences in OS or DFS for those with triple-negative disease or OS for HR+ disease in B-40. The improvement in DFS for patients with HR+ disease was not statistically significant (p=0.086). In B-40, RNI was associated with a significant reduction in LRR for patients with HR+ypN+ disease (p=0.001). For the B-41 population with ypN+ disease, RNI was associated with statistically significant higher OS (p=0.025) without statistically significant improvement in DFS (p=0.21) or DR (p=0.274). There was a statistically significant reduction in competing events for LRR (p=0.014). Detailed estimates and sample sizes of LRR, DR, DFS, and OS stratified by cN status, tumor subtype, and receipt of RNI are presented in Supplementary Tables 2-5 and Supplementary Figure 2A-F.
In light of the above findings, we performed subgroup analyses to evaluate the association of RNI with LRR for HR+ypN+ women from B-40 and RNI with OS for ypN+ women from B-41 on multivariable analysis (Supplement Tables 6-9). RNI was significantly associated with lower LRR (p=0.005) for HR+ypN+ from B-40, and RNI was associated with improved OS for ypN+ women from B-41 (p=0.005).
We also sought to evaluate the effect of RNI in the specific population of women who were cN+ with ypN0/ypT+ disease. For the combined population of B40 and B41, 127 women were evaluable, of whom 75 received RNI. There was no statistically significant difference in OS, DFS, or LRR by RNI receipt. Multivariable analyses were run for the combined population of B-40 and B-41 for LRR, DR, DFS, and OS, and the results are displayed in Table 3. Tumor subtype, breast pathologic response, and axillary pathologic response were significant for all four outcomes, but RNI was not significant for any outcome.
Table 3.
Multivariable Analyses for the Combined Study Populations of NSABP B-40 and B-41 Evaluating the Association of Regional Nodal Irradiation with Local-regional Recurrence, Distant Recurrence, Disease-free Survival, and Overall Survival
| LRR | DR | DFS | OS | |||||
|---|---|---|---|---|---|---|---|---|
| Variables | HR | P value | HR | P value | HR | P value | HR | P value |
| RNI | 0·79 | 0·299 | 0·97 | 0·808 | 0·92 | 0·496 | 0·86 | 0·306 |
| Age | 0·98 | 0·069 | 0·99 | 0·045 | 0·99 | 0·047 | 0·99 | 0·274 |
| Tumor subtype | <0·001 | <0·001 | <0·001 | <0·001 | ||||
| HR+/HER2− | 1·94 | 0·023 | 1·12 | 0·523 | 1·29 | 0·101 | 0·91 | 0·691 |
| TN vs HR+/HER2− | 3·30 | <0·001 | 1·88 | <0·001 | 2·01 | <0·001 | 2·24 | <0·001 |
| Breast pCR | 0·48 | 0·008 | 0·46 | <0·001 | 0·56 | <0·001 | 0·45 | <0·001 |
| Axillary pCR | 0·39 | <0·001 | 0·30 | <0·001 | 0·32 | <0·001 | 0·23 | <0·001 |
| Clinical node positivity | NS | NS | 1·41 | 0·014 | NS | NS | NS | NS |
| Grade | NS | 0·011 | 0·004 | 0·001 | ||||
| Grade 2 vs 1 | NS | NS | 1·99 | 0·080 | 2·22 | 0·022 | 1·98 | 0·144 |
| Grade 3 vs 1 | NS | NS | 2·66 | 0·014 | 2·72 | 0·004 | 3·32 | 0·011 |
| Clinical tumor size | NS | NS | NS | NS | 1·11 | <0·001 | 1·11 | <0·001 |
Abbreviations: LRR, local-regional recurrence; DR, distant recurrence; DFS, disease-free survival; OS, overall survival; pCR, pathological complete response; RNI, regional nodal irradiation; TN, triple negative; NS, not significant
Discussion
We present the largest analysis examining the effect of RNI in a prospectively followed group of more than 1700 patients with breast cancer treated with neoadjuvant chemotherapy as participants in national cooperative group trials, NSABP B-40 and B-41. We demonstrated that in an era of modern HER2-targeted therapy, significant differences in OS persist, creating heterogeneity in outcomes for patients based on breast pathologic complete response, even when considering axillary complete response. Between the four possible strata created by binary responses of axillary and breast pathologic response, we demonstrate clear outcome differences not only in historical trials such as B-18 and B-27 but also in more contemporary trials such as B-40 and B-41. We show that persistence of breast disease following neoadjuvant therapy is associated with worse survival. In examining strata of patients by tumor subtype and pathologic response, we demonstrated an overall survival difference by RNI receipt for women with persistent axillary disease after HER2-directed neoadjuvant systemic treatment in univariable analysis. We also demonstrated a local-regional recurrence difference by RNI receipt for patients with persistent axillary disease with HR+ breast cancer in B-40. However, we did not detect any significant difference associated with receipt of RNI in OS, DFS, DR, or LRR on multivariable analysis for the overall cohort of B-40 and B-41.
Aim 1: Natural History of Recurrence
Foremost, we are able to provide more evidence describing the natural history of breast cancer for patients who receive neoadjuvant chemotherapy. We identify several populations at high risk of local-regional recurrence. Any patient with triple-negative subtype and residual disease (in the breast or axilla) was shown to have a local-regional recurrence rate greater than 10% at 5 years. We continue to see a high risk of local-regional recurrence approximating 10% at 5 years for patients with HER2+ breast cancer with residual axillary disease. Interestingly, the only subgroup for whom we saw a statistically significant difference in OS in the univariable analysis between patients who received RNI and those who did not, was the subgroup of patients with HER2+ disease and residual lymph node disease. However, we note low rates of LRR for patients with pathologic complete response in both the breast and nodes. Lack of pathologic complete response was associated with worse OS not only in the B-18 and B-27 trials but also in the recent B-40 and B-41 trials. This association existed despite the fact that RNI was administered in B-40 and B-41 to patients with more aggressive disease – that is, larger tumors and those without pathologic complete response of the axilla. Therefore, the rationale for RNI in patients with residual disease is two-fold: First, these patients have resistant disease that likely requires further targeting with treatment (e.g., breast/chest and RNI). Second, the evidence demonstrates poorer outcomes with or without RNI in these contemporary prospective studies if there is persistent disease in the breast regardless of nodal response.
Aim 2: Allocation of RNI
Our analyses to determine factors associated with RNI receipt confirmed expected clinical characteristics, such as clinical tumor size and axillary response, but also revealed a disparity in care delivery as Hispanic patients were less likely, even on multivariable analysis, to receive RNI. Hispanic women have been previously reported as less likely to receive adjuvant radiotherapy in a National Cancer Database (NCDB) analysis(19). The corroboration of this disparity in a large national prospective clinical trial more than merits further exploration—be it attributable to systematic barriers to access, biased referral patterns, or patient beliefs about radiotherapy. Recent debate within our field has discussed the value of real-world data versus clinical trial information. The treatment patterns evidenced in NSABP B-40 and B-41 corroborate the disparities in radiotherapy allocation associated with Hispanic women in real-world data.
Aim 3: Determining Efficacy of RNI on Cancer Outcomes
The addition of neoadjuvant systemic treatment to the management of breast cancer has greatly impacted the therapeutic landscape. In particular, with the publication of trials utilizing neoadjuvant response as a decision-maker for the use of adjuvant systemic therapy in patients with residual disease—such as TDM1 agents for HER2+ breast cancer and capecitabine for triple-negative breast cancer—neoadjuvant treatment has become a standard recommendation and further personalizes both surgery and adjuvant systemic therapy(19-21). Despite informed adjuvant systemic treatment decision-making gained from neoadjuvant treatment outcomes, radiotherapy recommendations have become more challenging. Radiotherapy trials for breast cancer have been stepwise in their planning: noting an OS benefit in patients with breast-conserving surgery(22), then for patients with 4+ lymph nodes, and more recently a DFS benefit (with improvements in distant-metastasis-free survival and breast-cancer specific-survival) for women with high-risk node-negative disease or 1-3 positive lymph nodes(10, 11). Those logical steps in radiotherapy decision-making have all proceeded with a treatment paradigm of surgery followed by adjuvant systemic therapy. Neoadjuvant systemic therapy for breast cancer management has rendered radiotherapy decision-making difficult, particularly in patients who present with clinical axillary lymph node involvement but are found to have pathologically negative axillary lymph nodes following neoadjuvant chemotherapy. Although results from a completed phase III trial on the subject (NSABP B-51/RTOG 1304) will be forthcoming, it is this gray area that we hoped to clarify through this analysis.
Other retrospective analyses have been conducted to similarly examine the benefit of radiotherapy in the setting of neoadjuvant systemic therapy. The largest have focused on patients with clinically node-positive disease. In the analysis of patients with cN+ disease treated with radiotherapy after neoadjuvant systemic treatment on the American College of Surgeons Oncology Group (ACOSOG) Z1071 trial, the authors similarly did not demonstrate a statistically significant benefit in OS or DFS with the addition of radiotherapy(23). Notably, the authors evaluated radiotherapy rather than RNI in particular. Out of 701 patients who underwent neoadjuvant chemotherapy, 28% had a pCR and, at 6 years of follow-up, receipt of radiotherapy at the treating physician’s discretion trended towards improved LRR but no significant survival or other benefits. However, this selection bias with RNI prevents answering whether patients who received radiotherapy would have fared as well had they not received it, or whether patients who did not receive RNI would have fared better had they received it. On the other hand, in a large separate single-institution analysis of >1200 patients with node-positive breast cancer treated with neoadjuvant systemic therapy(24), the authors noted in a multivariate analysis that RNI was significantly associated with significant 10-year reductions in LRR and DR. They also noted a particularly strong benefit of RNI with reducing DR in patients with HER2+ breast cancer who received trastuzumab, a finding that corroborates our own determination of a unique OS benefit for patients with HER2+ ypN+ breast cancer receiving targeted neoadjuvant systemic treatment. Finally, a combined analysis of 817 patients treated on three prospective German trials noted an association of postmastectomy radiotherapy (PMRT) with reduced LRR for women who receive neoadjuvant chemotherapy without a benefit in DFS. This reported experience was limited in the delivery of PMRT due to heterogeneity in treatment of the internal mammary nodes and inclusion of a trial which did not include HER2-directed therapy. Our series was distinct in including patients with both clinically node-positive and node-negative breast cancer, owing to the demonstration of RNI benefit in high-risk node-negative breast cancer. This heterogeneity in RNI effect(25) is noted in other analyses including a series of patients with stage III breast cancer that received neoadjuvant chemotherapy and breast-conserving surgery with a pathologic complete response and trended toward a significant benefit in LRR with RNI(26). In a separate analysis, patients with stage III breast cancer who underwent a mastectomy and had a pathologic complete response experienced a statistically significant improvement in LRR with RNI(27). These numerous reports highlight the continued void in knowledge and conflicting information regarding for whom RNI can be omitted after neoadjuvant chemotherapy, with some demonstrating very good long-term outcomes with the omission of RNI treatment and others showing significant benefits with RNI even in the presence of pathologic complete response. Nonetheless, a common theme emerges and further supported by these data, that patients with ypN+ disease experience the worst outcomes, warranting RNI consideration. In fact, patients with worse disease biology or Her2+ tumors that remain ypN+ have the most guarded outcomes where further treatment should be offered.
Our work has implications on the outcome and findings of NSABP B-51, which accrued patients with cN1 disease with subsequent axillary pCR. As we demonstrated that women with ypT+N0 disease have inferior overall survival compared to those with ypT0N0, it is possible that results may be biased without stratification by breast residual disease. This effect may be particularly marked for those with triple-negative disease, where the 5-year cumulative incidence of LRR for those with residual breast disease may be as high as 21% compared to 6% for those with breast and axillary pCR (Supplementary Table 2).
Our study has some limitations. Similar to the aforementioned analyses, this study aimed to clarify the efficacy of radiotherapy (specifically RNI) when it was not randomized or even dictated by protocol. Instead, RNI was left to the discretion of the treating physicians. As such, the results are prey to selection bias, which we aimed to mitigate through multivariable analysis. Nevertheless, it is possible that the results are still susceptible to type II error and were underpowered to detect a benefit of RNI, which may be possible by increasing the sample size. Furthermore, the systemic management of breast cancer has continued to evolve since the completion of trials B-40 and B-41 with the addition of pertuzumab in the neoadjuvant and adjuvant setting and the addition of trastuzumab emtansine and capecitabine for residual HER2+ and triple-negative breast cancer, respectively. Follow-up for B-40 and B-41 was approximately 5 years, and undoubtedly more events will occur with more time, particularly in patients with ER+ disease.
Prospective randomized clinical trials provide level I evidence by removing confounders to establish outcomes such as superiority or non-inferiority between different treatment approaches. We eagerly await the results of NSABP-51/RTOG 1304, a superiority trial evaluating RNI benefit in patients with biopsy-proven cN+ breast cancer who convert to ypN0 disease(13) after neoadjuvant chemotherapy. Our data demonstrate low LRR risk in patients with complete pathologic response in both the breast and axilla. Even so, B-51 does not answer the benefit of RNI in those patients with ypN+ disease. Although intuitively, such patients should receive RNI (since they would have been candidates to receive it if they were treated with surgery first), patterns-of-care analysis continue to show almost half of patients with ypN+ breast cancer do not receive adjuvant RNI(17). Finally, and importantly, our work demonstrates a disparity in RNI receipt for Hispanic women—echoing data seen in an NCDB analysis: investigation and action are needed to ensure equitable access for this minoritized patient population.
Data Sharing Statement:
The authors agree to share anonymized data upon reasonable request by researchers.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Paul G. Okunieff, MD is the scientific advisor and owns stock in DiaCarta Inc., Gain Pep Inc., and Entrinsic Health Inc. All remaining authors have declared no conflicts of interest.
Prior Submissions: Abstract submitted to 2021 Annual Meeting of the American Society of Therapeutic Radiation Oncology (ASTRO) in Chicago, IL on 10/24/21 – 10/27/21.
References
- 1.Halsted WS. I. The Results of Radical Operations for the Cure of Carcinoma of the Breast. Ann Surg 1907;46:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233–1241. [DOI] [PubMed] [Google Scholar]
- 3.Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017;389:1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N Engl J Med 2018;379:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997;337:949–955. [DOI] [PubMed] [Google Scholar]
- 6.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999;353:1641–1648. [DOI] [PubMed] [Google Scholar]
- 7.Vicini FA, Cecchini RS, White JR, et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet 2019;394:2155–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meattini I, Marrazzo L, Saieva C, et al. Accelerated Partial-Breast Irradiation Compared With Whole-Breast Irradiation for Early Breast Cancer: Long-Term Results of the Randomized Phase III APBI-IMRT-Florence Trial. J Clin Oncol 2020;38:4175–4183. [DOI] [PubMed] [Google Scholar]
- 9.Kunkler IH, Williams LJ, Jack WJ, et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol 2015;16:266–273. [DOI] [PubMed] [Google Scholar]
- 10.Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N Engl J Med 2015;373:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poortmans PM, Weltens C, Fortpied C, et al. Internal mammary and medial supraclavicular lymph node chain irradiation in stage I-III breast cancer (EORTC 22922/10925): 15-year results of a randomised, phase 3 trial. Lancet Oncol 2020;21:1602–1610. [DOI] [PubMed] [Google Scholar]
- 12.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008;26:778–785. [DOI] [PubMed] [Google Scholar]
- 13.NASBP Foundation. A Randomized Phase III Clinical Trial Evaluating Post-Mastectomy Chestwall and Regional Nodal XRT and Post-Lumpectomy Regional Nodal XRT in Patients With Positive Axillary Nodes Before Neoadjuvant Chemotherapy Who Convert to Pathologically Negative Axillary Nodes After Neoadjuvant Chemotherapy [NCT01872975]. August 26, 2019. Available at https://clinicaltrials.gov/ct2/show/NCT01872975. Accessed on March 1, 2021. Last accessed: [Google Scholar]
- 14.Mamounas EP, Anderson SJ, Dignam JJ, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol 2012;30:3960–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bear HD, Tang G, Rastogi P, et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med 2012;366:310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robidoux A, Tang G, Rastogi P, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. Lancet Oncol 2013;14:1183–1192. [DOI] [PubMed] [Google Scholar]
- 17.Fayanju OM, Ren Y, Suneja G, et al. Nodal Response to Neoadjuvant Chemotherapy Predicts Receipt of Radiation Therapy After Breast Cancer Diagnosis. Int J Radiat Oncol Biol Phys 2020;106:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation 2016;133:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parekh A, Fu W, Hu C, et al. Impact of race, ethnicity, and socioeconomic factors on receipt of radiation after breast conservation surgery: analysis of the national cancer database. Breast Cancer Res Treat 2018;172:201–208. [DOI] [PubMed] [Google Scholar]
- 20.von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med 2019;380:617–628. [DOI] [PubMed] [Google Scholar]
- 21.Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med 2017;376:2147–2159. [DOI] [PubMed] [Google Scholar]
- 22.Early Breast Cancer Trialists' Collaborative G, Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haffty BG, McCall LM, Ballman KV, et al. Impact of Radiation on Locoregional Control in Women with Node-Positive Breast Cancer Treated with Neoadjuvant Chemotherapy and Axillary Lymph Node Dissection: Results from ACOSOG Z1071 Clinical Trial. Int J Radiat Oncol Biol Phys 2019;105:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stecklein SR, Park M, Liu DD, et al. Long-Term Impact of Regional Nodal Irradiation in Patients With Node-Positive Breast Cancer Treated With Neoadjuvant Systemic Therapy. Int J Radiat Oncol Biol Phys 2018;102:568–577. [DOI] [PubMed] [Google Scholar]
- 25.Krug D, Lederer B, Seither F, et al. Post-Mastectomy Radiotherapy After Neoadjuvant Chemotherapy in Breast Cancer: A Pooled Retrospective Analysis of Three Prospective Randomized Trials. Ann Surg Oncol 2019;26:3892–3901. [DOI] [PubMed] [Google Scholar]
- 26.Caudle AS, Yu TK, Tucker SL, et al. Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res 2012;14:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGuire SE, Gonzalez-Angulo AM, Huang EH, et al. Postmastectomy radiation improves the outcome of patients with locally advanced breast cancer who achieve a pathologic complete response to neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys 2007;68:1004–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.