Abstract
The electrocardiogram (ECG) records the electrical activity in the heart in real-time, providing an important opportunity to detecting various cardiac pathologies. The 12-lead ECG currently serves as the “standard” ECG acquisition technique for diagnostic purposes for many cardiac pathologies other than arrhythmias. However, the technical aspects of acquiring a 12-lead ECG are not easy and its usage is currently restricted to trained medical personnel, limiting the scope of its usefulness. Remote and wearable ECG devices have attempted to bridge this gap by enabling patients to take their own ECG using a simplified method at the expense of a reduced number of leads, usually a single-lead ECG. In this review article, we summarize the studies which investigate the use of remote ECG devices and their clinical utility in diagnosing cardiac pathologies. Eligible studies discussed FDA-cleared, commercially available devices that were validated on an adult population. We summarize technical logistics of signal quality and device reliability, dimensional and functional features, and diagnostic value. In summary, our synthesis shows that reduced-set ECG wearables have huge potential for long-term monitoring, particularly if paired with real-time notification techniques. Such capabilities make them primarily useful for abnormal rhythm detection and there is sufficient evidence that a remote ECG device can be more superior to traditional 12-lead ECG in diagnosing specific arrhythmias such as atrial fibrillation. However, this review identifies important challenges faced by this technology, highlighting the limited availability of clinical research examining their usefulness.
Keywords: electrocardiogram, diagnosis, remote, wearable, device, atrial fibrillation
1. Introduction
The electrocardiogram (ECG) is the most widely used diagnostic tool in clinical cardiology and is also one of the most widely collected body signals in wearable devices intended for diagnostic use. The ECG is abnormal in a significant proportion of cardiac pathologies other than arrhythmias (coronary artery disease, heart failure, valvular heart disease, etc.), making it suitable for screening purposes prior to subsequent evaluation by more specific diagnostic tests (echocardiography, coronary angiography, etc.). The ECG is commonly acquired from 12 body surface leads to enable the spatial assessment of the heart in a three-dimensional model. However, the 12-lead ECG is traditionally acquired by clinicians and trained personnel via a highly regulated procedure, limiting the scope of its clinical utility beyond the clinic. If one could trigger ECG recordings at the onset of worrisome cardiac symptoms, anytime and anywhere, clinicians would be provided with evidence of cardiac diseases that might no longer be apparent on the 12-lead ECG taken later at a medical appointment1. The latter objective has led to a widespread use of consumer-oriented remote and wearable ECG devices in recent years.
Information provided by the 12-lead ECG are interpreted following recommendations and expert-consensus statements. Fortunately, identifying basic arrythmias only requires one ECG lead2, which drastically simplifies the task to the point of making possible its assignment to untrained individuals. Some solutions lie in the scope of wearables, which allow for a long-term ECG recording with an ergonomic design. There are also portable options, which do not provide continuous monitoring but allow a patient to quickly record one or multiple ECG leads in a range of non-clinical settings. These two types of devices–wearables and portables–make up the broader class of remote ECG devices.
In this review article, we summarize the recent contributions, examine the reliability, and discuss the limitations of commercially available remote ECG devices in adult population. In doing so, we restrict our investigation to wireless products that claim a diagnostic value with a reduced set of electrodes. This paper also elaborates on how remote ECG devices overcome the disadvantages of the standard 12-lead ECG, as well as their clinical utility in diagnosing cardiac disease.
1.1. Basic 12-lead ECG function
The 12-lead ECG can be used as a non-invasive assessment of a plethora of abnormalities, including arrhythmias and ectopic rhythm abnormalities, conduction defects and heart blocks, chamber hypertrophies and cardiomyopathies, inherited syndromes and channelopathies, myocardial ischemia and infarction, electrolyte abnormalities, medication toxicity, secondary cardiopulmonary manifestations, and other non-cardiac etiologies4. Thus, practice guidelines by the American Heart Association / American College of Cardiology grouped the diagnostic statements for automated ECG interpretation in a list to promote uniformity of ECG diagnosis, yielding 117 potential diagnostic statements 5. Figure 1 shows the ECG acquisition method and an example of the tracing and diagnostic statements available to clinicians.
Fig. 1: ECG acquisition method and tracing.
(a) Frontal ECG leads; (b) Precordial ECG leads acquisition methods.; (c) An example of a standard 10-second 12-lead ECG from a 60-year-old male evaluated for acute chest pain. The header of the ECG page shows global measurements of the ECG waveforms (left), diagnostic statements made by the machine and an overall automated interpretation (center), and the corresponding decision criteria (right). WCT: Wilson’s central terminal.
The ECG signal needs to be filtered before analyzing it for diagnostic purposes. This is done by keeping a frequency band that preserves important prognostic physiological signatures needed for proper diagnostic statements. Guidelines specify the lower and upper filtering frequency bounds to guarantee an interpretable signal, respectively equal to 0.05 Hz and 150 Hz for adults6. Measuring abrupt events such as peak amplitude is more accurate when higher frequencies are kept in the signal after filtering7.
1.2. Literature Search Strategy
This review was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines3. A database search was conducted using PubMed to screen research articles with the following search term: “(((wearable or portable) and (ECG or electrocardiogram)) and adult) and diagnosis) not PPG”. The most recent search was performed on 3/1/2021 and was limited to the past 5 years. The search yielded 243 articles, which were subsequently filtered, first based on their title and abstract, then based on whether they were suitable for the study of the review’s topic. The targeted studies were the ones discussing commercially available, FDA cleared devices validated on an adult population. Moreover, a manual search was carried out to identify commercially available remote ECG devices and link them to relevant research articles, forming a complete summary of the state-of-the-art products. Data extraction regarding study characteristics, device description and diagnostic utility metrics reported in that study was done by a single reviewer (ZB).
2. Summary of remote ECG devices
2.1. Commercially Available ECG devices
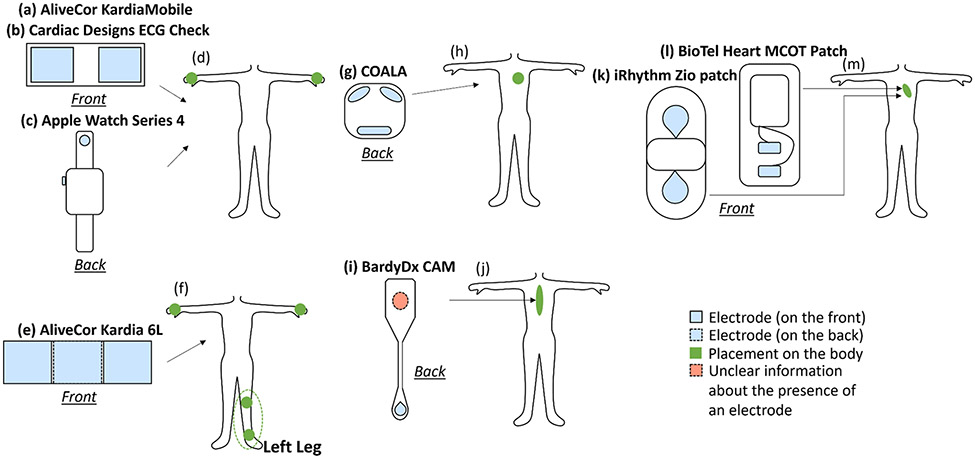
Table 1 summarizes the available remote ECG devices from published validation studies. Figure 2 illustrates the configuration and placement of these 8 remote ECG systems, and Figure 3 compares the functionality of these systems against the standard 12-lead ECG.
Table 1:
Examples of Remote ECG Devices
| Remote ECG Device |
Number Of ECG Leads |
Continuous Monitoring Duration |
Real-time | Require prescription? |
Data storage and manipulation | Diagnostic claims |
|---|---|---|---|---|---|---|
| Handheld | ||||||
| AliveCor KardiaMobile | 1 lead | NA | Yes | No | Stores data on the phone or emails it to doctor | Detects Atrial Fibrillation, Bradycardia, Tachycardia, Sinus Rhythm with SVE, Sinus Rhythm with Wide QRS, and Sinus Rhythm with PVCs |
| AliveCor Kardia 6L | 1 lead/6 leads | NA | Yes | No | Provides secured cloud storage and cardiologist reviews | |
| Cardiac Designs ECG Check | 1 lead | NA | Yes | No | Stores and transmits the ECG data to a medical professional via a secure cloud server or email for users with a prescription | Displays normal, Irregular HR, Unable to read |
| COALA | 2 leads | NA | Yes, with on demand ECG reports | Yes | Recordings are easy to access with automatic interpretation and real-time r ports in the cloud based COALA Care portal | Diagnoses symptomatic arrhythmias (9 of the most common arrhythmias) or murmurs, P-wave based AF detection |
| Patch | ||||||
| BardyDx CAM | 1 lead | 14 days | No | - | Upload data to the Cloud through a secure web-based portal | P-wave centric sternal ECG monitoring |
| BioTel Heart MCOT Patch | 2 leads | 5 days | Yes | Yes | Data transferred to trained technicians on 24/7 basis. Clinical reports are available to your clinician during and at the end of your service | Assists in diagnosing certain heart arrhythmias |
| iRhythm Zio patch | 1 lead | up to 14 days | No | Yes | Data are stored in the device and downloaded after completion of monitoring for offline analysis. | Generates main findings in a report |
| Smartwatch | ||||||
| Apple Watch Series 4 | 1 lead | NA | Yes | No | Saves the results (ECG and analysis) in the Health app of the iPhone and can be shared as a PDF with the doctor | Classifies the recording as sinus rhythm, bradycardia, tachycardia, or atrial fibrillation or as inconclusive |
Fig. 2: Commercialized remote ECG devices and their placement.
(d) the placement of the two sensors of (a), (b) and (c); (f) the placement of the three sensors of (e); (h) the placement of the three sensors of (g); (j) the placement of the sensors of (i); and (m) the placement of the sensors of (l) and (k) to record the ECG signal.
Fig. 3: Comparison of the features of standard vs. remote ECG monitoring systems.
2.2. Real-time capabilities
Many remote ECG devices have real-time monitoring capabilities. For example, intermittent portable ECGs recorded by AliveCor devices can be used as near real-time systems thanks to wireless communication through a web-based portal. However, many remote ECG systems that use patches are non-real-time (e.g., Zio Patch) with a median time from acquisition to physician notification of 19 days. This excessive delay is due to the time needed to return the device, analyze the ECG signals, create the report, and then notify the clinician8. Yet, most significant symptomatic arrhythmias were spotted on the patch within 7 days of the beginning of the monitoring, with all serious ones picked up within 4 days8. The patch has a great ability to capture significant arrhythmias in a timely way but there is a big gap between the time of detection and the time of diagnosis by the clinician. Real-time monitoring would address this problem.
A good representative of a real-time ECG patch system is the one implemented by the BioTel Heart MCOT Patch. Representative arrhythmia diagnostic strips are sent wirelessly to an independent diagnostic testing facility upon activation of threshold triggers based on the analysis of rate, rhythm irregularity, QRS morphology, and P-wave9. Notification criteria are set for a patient to alert the appointed physician and the patient9. In addition, clinical reports are made accessible to the health care provider during the monitoring period and when it is finished. The MCOT Patch had a significantly higher diagnostic yield than the auto-trigger looping event recorder for AF, bradycardia, ventricular pause, supraventricular tachycardia and ventricular tachycardia, as well as a significantly shorter mean time to diagnosis9. The MCOT patch thus pairs good diagnostic value with an efficient real-time use protocol and avoids the logistical problems associated with non-real-time monitoring devices.
3. Remote devices signal quality
3.1. Factors that jeopardize signal quality
An ECG signal can be seriously compromised by noise, which might be a result of baseline wander and abrupt drift, power line interference, or muscle artifact10. ECG signals corrupted with noise are unreliable and must be filtered using noise-specific signal processing techniques before any manipulation or discarded in case recovery of a good-quality signal is impossible10. Ambulatory data recording from wearable devices is more likely to result in signals with artifacts than data obtained from bed-bound patients11 because of the new challenges that daily-life movements introduce on the adherence of the electrodes and their placement. Also, external factors such as the contact with water while showering or swimming, or because of perspiration, may disrupt the recording system.
Remote ECG devices use alternative electrode positions to record a specific number of leads (Figure 2). However, the electrode placement impacts the quality of an ECG tracing12, and studies have demonstrated that a displacement as small as 20 mm might result in substantial modifications in ECG signal morphology13. Moreover, changes in the standard electrodes’ positions affect the ECG tracings, where alterations along the left arm were the most visible compared to the right arm because of their relative distance to the myocardium, and a lateral site along the lower limb was more vulnerable to modifications in electrode placement relative to an anterior site14.
3.2. Signal quality assessment
A few studies evaluated the signal quality of ambulatory ECGs. The Apple Watch Series 4 was developed to record lead I and has been shown to have good quality despite the absence of any skin preparation16. Physical instability on the recording location (wrist or abdomen) could, however, result in temporary artifacts16. When lead II and lead III were self-recorded, the signals obtained were accurate and consistent with the standard ECG leads15. There was no clinical difference between the values of intervals, amplitudes and polarity computed for ECG segments in both standard and watch-based ECG leads I, II and III16. Setting the positive electrode situated at the back of the watch against the mid-abdomen showed good agreement between watch-based ECGs and corresponding standard ECGs. This method suggested the potential of the device to generate a 6-lead ECG by deriving the augmented limb leads aVL, aVF and aVR17. A brief research report kept the reference point on the leg to record leads II and III but suggested recording bipolar chest leads as a substitute to the standard precordial leads (V1 to V6). Given the unavailability of WCT to connect the 3 limb electrodes, authors used the right arm as an alternative central terminal18. Such approach to calculate pseudo 12-lead ECG using Apple Watch yielded good diagnostic signal quality and morphology as compared to standard 12-lead ECG18, 19.
The fidelity of smartwatch ECG to calculate reliable and accurate measurement of the QT interval was also explored20. Similarly, a study using the AliveCor KardiaMobile 6L ECG device showed that interval duration measurements (QTcF, heart rate, PR, and QRS) based on lead II are comparable to standard ECG and, hence, useful in detecting clinically meaningful abnormalities21. Nevertheless, measurements from a single ECG lead must be scrutinized because waveform segmentation is ideally done using global measurements on standard 12 leads. Temporal superposition of the 12 leads allows the detection of the earliest onset and latest offset of waveforms to compute more accurate intervals than those resulting from segmenting individual leads6. Single-lead ECG systems are prone to miscalculations, and any attempt to segment a lead is not equivalent to that operated on a standard ECG.
3.3. Implemented solutions to improve signal quality
Remote ECG devices may compensate for lack of multi-lead recordings by capturing the ECG from alternative, non-standard positions. The BardyDx CAM, for instance, is placed on the sternum given that myocardial currents flow through the mediastinum to the skin overlying the sternum22-25. This has been shown to optimize P-wave quality, morphology and its relationship with QRS, which are key factors to elucidate the mechanism of any arrhythmia22. The signal quality of the CAM patch was shown to be comparable to that of a Holter monitor based on reported high correlation coefficients between the two systems22. In another study, the signal clarity of the CAM patch was significantly improved compared to the Zio-XT patch as indicated by the physicians’ degree of certainty for deciding on a diagnosis26. Thus, such non-standard positioning might compensate for the absence of multiple ECG leads.
4. Diagnostic value of remote ECG devices
4.1. Diagnostic capabilities of remote ECG devices
Most clinical guidelines are based on 12-lead ECGs, which limits the diagnostic capabilities of reduced-set ECGs. Thus, most reduced-set ECG devices have primarily focused on abnormal rhythm detection, namely the detection of AF. This was recently highlighted by a collaborative statement on mHealth in arrhythmia management by leading societies in the field27. Figure 4 summarizes the diagnostic significance of remote ECG devices for AF detection. Yet, some other studies focused on the role of remote ECG devices for QTc interval monitoring or myocardial ischemia detection. A critical appraisal of this literature is provided herein.
Fig. 4: Summary of the diagnostic accuracy of remote ECG devices for AF detection.
This figure summarizes the reported sensitivity and specificity metrics for detecting AF using a wearable device. Error bars indicate the 95% confidence interval as reported by parent study. Metrics without error bars indicate parent study did not report such values or the independent reviewer computed these metrics from data extracted from the parent study.
Despite the conceptual differences between single-lead and 12-lead ECG, single-lead ECG devices have been shown as useful tools for AF detection given that special consideration for interpretation and signal quality assessment are considered.28 A meta-analysis of 6 studies showed a pooled sensitivity and specificity of hand-held single-lead ECG devices of 89%-92% and 95%-99% when compared against 12-lead ECG29. Another review showed that an accumulative intermittent recordings of 19 minutes by handheld devices matches the AF diagnostic accuracy of a 24-hour Holter ECG recording30. AliveCor KardiaMobile has been specifically shown to have high sensitivity (87%) and specificity (98%) for automated AF detection when compared to a gold reference interpretation by cardiologists31. Similar benefits were observed for capturing 30-day AF recurrence in patients post ablation or cardioversion32. A multicenter randomized control trial has also shown that there is a significantly better identification of AF using a 30-day AliveCor KardiaMobile monitoring recorded 3 times a day compared to a repeated 24-hour Holter monitoring33. However, the it was noted that just a quarter of these detections are true AF33. This finding highlights a low positive predictive value and, thus, a need to formally confirm the diagnosis before starting any treatment based solely on AF detection algorithms.
To further improve sensitivity, a 3-lead recording would reveal signatures of arrhythmias featuring a shift in electrical axis possibly invisible on single-lead signals. It would also better identify aberrant/broader QRS complexes when the leading edge of the QRS complex is relatively isoelectric to the only recorded lead, which is especially more applicable in the case of broad complex tachycardia34. Even after the release of the 6-lead AliveCor Kardia 6L35, Frisch et al. slightly modified the single-lead AliveCor KardiaMobile to extend its function to multiple-lead recordings36. The authors used an alligator clip which enabled the device to record leads II and V1, in addition to lead I, for which the device is conceived36. It was demonstrated that the supplemental alternate leads significantly increased the accuracy of the ECG interpretation by cardiologists alongside their confidence in their decision36.
On the other hand, reduced-set leads are not conceptually tailored toward acute myocardial infarction detection. In particular, STEMI cannot be practically detected using a single-lead ECG since the diagnosis of STEMI requires the assessment of the ST-segment at the J-point in two contiguous leads as per the Fourth Universal Definition of Myocardial Infarction guidelines38. Lead I might show some changes with anterolateral infarcts or reciprocal changes with inferior infarcts, but it remains a poor screening tool, let alone, a diagnostic tool for STEMI. For instance, Avila examined the potential role of a 3-lead ECG taken by the smartwatch in unveiling myocardial ischemia, where further specificity and sensitivity analysis are needed17. By adopting the multichannel method to record not only leads I, II, and III, but also leads V1-V6, the Apple Watch Series 4 could spot signatures of acute coronary syndrome visible on a 12-lead ECG, and specifically localize ST-segment alterations39. However, such Wilson-like leads are conceptually not equivalent to precordial leads of a standard 12-lead ECG, 36, 39 which warrants caution.
Finally, remote ECG devices have been shown to play a role in QTc interval monitoring, especially during the current surge of COVID-19 pandemic 21, 40, 41. This was seen in a study using the AliveCor KardiaMobile 6L conducted in an inpatient setting during the COVID-19 era, in which stricter protocols for the monitoring of QTc intervals are required 42. In this early preliminary work, it was found that remote ECG devices with 6 leads can be a reliable mean for accurate QTc measurements. Yet, these findings lacked larger scale assessment, as well as a comparative study against the 12-lead ECG as a gold standard 42. Thus, in a more recent study comparing remote 6-lead ECG to a standard 12-lead ECG in a larger cohort (n=182), authors found that QTc measurements did not differ between the two approaches, but the overall reliability of remote ECG was moderate (ICC = 0.56) 43. Nevertheless, the authors noted the significantly lower time needed to obtain the remote-ECG compared to standard 12-lead ECG, suggesting a potential clinical utility for real-time QTc monitoring in ambulatory COVID-19 patients 43.
4.2. Advantages of longer monitoring periods and self-recorded ECG
Long-term monitoring provides an increase in the diagnostic yield for detecting incidental AF34, 44. A comprehensive study on the duration of rhythm monitoring and the detection rate of incidental AF, where the gold standard reference was an implanted loop recorder, showed that the 10-second ECG resulted in a sensitivity of 1.5% for AF detection45. This sensitivity increased to 11%, 13%, 15%, 21%, and 34% for a single 24-hour, 48-hour, 72-hour, 7-day, or 30-day continuous monitoring, respectively45. However, Holter monitors are cumbersome, and restrict the activities that can be performed while wearing them. Therefore, they are commonly used for 24 or 48 hours, or less frequently, a week. The sensitivity for a 14-day monitoring period during which a 30-second ECG was recorded twice a day was only 8.3%.
Smith et al. showed that BardyDx CAM patch outperforms standard Holter monitor over 24 hours22 in identifying clinically pertinent events, including arrhythmias misdiagnosed by the Holter. In the case of the Zio Patch, a study reported the inability of the patch to outperform the Holter over the 24-hour period, whereas the former identified significantly more events than the Holter monitor over a median wear time of 11.1 days34. Nevertheless, intermittent 30-second recordings using portable devices for a total of 19 minutes were as good as the 24-hour Holter ECG recording in detecting AF30.
4.3. Lack of studies on clinical utility
We found a limited number of research articles examining the clinical utility of remote ECG devices. The median sample size for the reviewed studies is 105 subjects (IQR = 50-214), suggesting that majority of studies are likely underpowered to detect significant clinical differences in rate of AF events. Only one study was looking at a large-scale sample (n=78,490 subjects)9. Conducting power analyses before data collection would provide a solid ground to draw statistically significant conclusions. Another problem is that some studies acquired remote and standard ECG (or Holter monitor) data sequentially rather than simultaneously.43 This lack of rigor might jeopardize the findings of these studies due to temporal bias in outcome assessment. A third limitations is the lack of consensus on rhythm interpretations in remote ECG devices. For instance, Apple Watch reads ECG rhythm strips as sinus rhythm, AF or inconclusive where nearly 31% rhythms are interpreted as inconclusive, yielding a low sensitivity of 41% 46.
5. Challenges and limitations of remote ECG devices
5.1. Signal filtering bandwidth
Recording higher frequency signal consumes more battery and memory, which is problematic for remote ECG systems. Guidelines relax the upper frequency to 60 Hz in ambulatory devices. Such frequency can suppress the excessive noise and interferences introduced by daily activities and excessive movement. The downside is that a frequency as low as 40 Hz affects the QRS complex and nullify amplitude measurements. This impacts the performance of R peak detection used in almost all ECG signal processing algorithms as the basis for AF detection, consequently leading to imprecise diagnostic decisions47. There are no studies investigating the impact of signal filtering on the adequacy of remote ECG for diagnostic purposes. For example, the ECG Check from Cardiac Designs has a very narrow bandwidth from 0.5 Hz to 25 Hz, which explains the limited statements that it can make (“Normal”, “Irregular HR”, “Unable to read ECG”). On the other hand, AliveCor KardiaMobile and BioTel Heart MCOT patch have sampling rates equal to 300 and 250 samples per second (Hz), respectively, which would allow an upper bandwidth filter of ~150 Hz as per current recommendations. Precise data about such technical aspect are very important in assessing the utility of the recorded signal, yet they are hard to find in published studies.
5.2. Real-time need
As mentioned in section 4.3, the high potential of remote ECG devices is hindered by both the absence of real-time interaction with the healthcare professionals and the lack of a prompt notification of the user in case of detection of possible abnormalities. The first week of monitoring using the Zio patch revealed most significant symptomatic arrhythmias8, but the report was only available after 14 days. This issue is extended to many remote ECG devices, where the lack of a timely alarm constitutes a missed opportunity to provide timely diagnosis and treatment.
5.3. Device acceptability
5.3.1. User acceptability and compliance
The main factors to investigate in the assessment of the user acceptability of the device depend on the nature of the device. Handheld devices and smartwatches are simple to manipulate as opposed to patch devices, for which it is challenging to identify the correct location; a solution for better compliance would be including a patch placement template in the kit as done for BioTel Heart MCOT Patch. However, it is worth noting that the use of portable ECG devices might be difficult for older adults. In the case of the AliveCor KardiaMobile 6L, for instance, the application of the device to the left knee or ankle might be challenging for elderly patients and the data acquisition might be hindered due to their clinical status which could result in a lack of collaboration from the subjects43.
Additionally, skin irritation is a big challenge to user acceptability and compliance in the case of wearable ECG patches, which are intended for an extended monitoring period. The BardyDx CAM, for instance, was found to be gentler on the skin with no severe skin irritation reported over 24-hour use compared to 3 cases of severe reactions in Holter patients, representing 6% of the studied sample22. Since the CAM patch interfered significantly less with daily activities and sleep, and has better adherence, the surveyed patients preferred it to the Holter monitor, and the technicians noted that it is easier to attach to patients22.
5.3.2. Acceptability among healthcare professionals
A survey of current perspectives on consumer-available remote ECG devices for detecting AF revealed that most clinicians do recommend a digital device for AF detection48. While 42.7% of clinicians indicated that 30-second single-lead ECG was enough to recommend oral anticoagulation for patients at high risk for stroke, a majority questioned the performance of such devices relative to standard ECG or Holter. More than half demanded professional societies to issue guidelines to regulate the clinical use of remote devices48. Pitman et al. found that relying solely on the automated interpretation algorithms for AF detection in remote ECG devices does not reach the desired performance, mandating an overread by expert cardiologists,49 which has been shown to yield 100% ascertainment rate31. Similar recommendations were suggested by another study reporting low positive predictive value of general practitioners for the identification of AF or flutter as compared to consensus by a panel of cardiologists. These findings again suggest the pressing need for cardiologist’s over-reading of remote ECG tracings50.
A main challenge of the increasing use of remote ECG devices is their burden on healthcare professionals as a potential consequence of the enormous increase in false positive referrals. For example, it has been shown that a diagnosis of AF could be confirmed by cardiologists in only 34% of cases identified by Apple Watch and up to 65% of cases identified by Kardia Mobile51. Thus, automated diagnosis of AF using mobile devices remains an approximation of the current reference standard. Nevertheless, the demonstrated benefits of mobile devices for early detection of AF in those with unknown history and in need of oral anti-coagulation is undeniable. Chan et al. report that the number needed to screen for a new AF diagnosis in adults older than 50 years of age is 145 at device detection rate of 2.6% and positive predictive value of 65%52. To interpret these numbers, if a cardiologist prescribes a device for 145 patients, a mobile device will identify roughly 3 patients with possible AF. Among those three: one would be a false positive referral; one would be a known history of AF; and one would be a newly diagnosed AF case. Yet, continuous refinement of current automated ECG interpretation algorithms, including the adoption of artificial intelligence (AI)-enabled classification algorithms, can play a significant role in improving the specificity of device-provided AF diagnosis and, hence, in reducing the absolute number of false positive referrals in the upcoming decade53.
Finally, little has been done about the integration of remote ECG devices with existing electronic health record (EHR) systems, which brings additional challenges, including the burden of a large amount of information on physicians54. For example, physicians might have to deal with too many false positive referrals, forcing them to look over great quantities of ultimately unimportant information.
5.4. Privacy, security and safety
Wearable biomedical devices inherently deal with confidential health information unavailable for public access by healthcare entities, which employ strict methods to enforce related regulations. This raises multiple issues especially with the poor integration of this data with EHR. Izmailova et al.55 provides methodological and logistical considerations for the implementation of wearable technologies in clinical trials, presenting data security as one of the challenges to the deployment of these devices. General issues also include guaranteeing secure data collection, storage, transmission and receipt, secure account management, and data encryption and blinding55. The practicality and diagnosis automation of the novel portable ECG devices come at the expense of an increased manipulation of health information, raising questions about the reliability of the storage medium and the access protocol.
One approach to address this problem may be by taking decisions at the hardware level. One can design a “physically secure communication” by opting for human body communication which constrains the signals to be in the body56. The large-scale Apple Heart Study examined measures taken to prevent any violation of data security and privacy regulations, such as Title 21 Part 11 of the Code of Federal Regulations, and made private health information inaccessible to Apple, the sponsor of the study and the manufacturer of the phone, the watch and the algorithm57.
6. Conclusion
Remote ECG devices provide incremental benefits over the standard 12-lead ECG or ambulatory Holter monitors in multiple aspects. They can facilitate the accurate and timely diagnosis of heart rhythm abnormalities, namely AF. The potential integration of these tools in clinical settings or at home conditions give an unprecedented flexibility for patients to self-monitor their heart health without interrupting daily activities or scheduling doctor visits. They also improve diagnostic value by allowing for long-term monitoring, particularly if paired with real-time notification techniques. Multiple aspects and applications of the devices, such as compliance issues for consistent use, are still to be investigated but their current use is promising 58.
Footnotes
Disclosures: None of the authors have any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ringwald M, Crich A, Beysard N. Smart watch recording of ventricular tachycardia: Case study. The American journal of emergency medicine 2020;38:849. e843–849. e845. [DOI] [PubMed] [Google Scholar]
- 2.Becker DE. Fundamentals of electrocardiography interpretation. Anesthesia progress 2006;53:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Annals of internal medicine 2018;169:467–473. [DOI] [PubMed] [Google Scholar]
- 4.Al-Zaiti SS, Faramand Z, Rjoob K, Finlay D, Bond R. The role of automated 12-lead ECG interpretation in the diagnosis and risk stratification of cardiovascular disease. Cardiovascular and Coronary Artery Imaging: Elsevier; 2022:45–87. [Google Scholar]
- 5.Mason JW, Hancock EW, Gettes LS. Recommendations for the standardization and interpretation of the electrocardiogram: part II: Electrocardiography diagnostic statement list. Circulation 2007;115:1325–1332. [DOI] [PubMed] [Google Scholar]
- 6.Kligfield P, Gettes LS, Bailey JJ, et al. Recommendations for the standardization and interpretation of the electrocardiogram: part I: the electrocardiogram and its technology. Journal of the American College of Cardiology 2007;49:1109–1127. [DOI] [PubMed] [Google Scholar]
- 7.Berson AS, Pipberger HV. Electrocardiographic distortions caused by inadequate high-frequency response of direct-writing electrocardiographs. American heart journal 1967;74:208–218. [DOI] [PubMed] [Google Scholar]
- 8.Reed MJ, Grubb NR, Lang CC, et al. Diagnostic yield of an ambulatory patch monitor in patients with unexplained syncope after initial evaluation in the emergency department: the PATCH-ED study. Emergency Medicine Journal 2018;35:477–485. [DOI] [PubMed] [Google Scholar]
- 9.Derkac WM, Finkelmeier JR, Horgan DJ, Hutchinson MD. Diagnostic yield of asymptomatic arrhythmias detected by mobile cardiac outpatient telemetry and autotrigger looping event cardiac monitors. Journal of cardiovascular electrophysiology 2017;28:1475–1478. [DOI] [PubMed] [Google Scholar]
- 10.Satija U, Ramkumar B, Manikandan MS. A review of signal processing techniques for electrocardiogram signal quality assessment. IEEE reviews in biomedical engineering 2018;11:36–52. [DOI] [PubMed] [Google Scholar]
- 11.Clifford GD, Clifton D. Wireless technology in disease management and medicine. Annual review of medicine 2012;63:479–492. [DOI] [PubMed] [Google Scholar]
- 12.Khunti K. Accurate interpretation of the 12-lead ECG electrode placement: A systematic review. Health Education Journal 2014;73:610–623. [Google Scholar]
- 13.Herman MV, Ingram DA, Levy JA, Cook JR, Athans RJ. Variability of electrocardiographic precordial lead placement: a method to improve accuracy and reliability. Clinical cardiology 1991;14:469–476. [PubMed] [Google Scholar]
- 14.Pahlm O, Haisty WK Jr, Edenbrandt L, et al. Evaluation of changes in standard electrocardiographic QRS waveforms recorded from activity-compatible proximal limb lead positions. The American journal of cardiology 1992;69:253–257. [DOI] [PubMed] [Google Scholar]
- 15.Samol A, Bischof K, Luani B, Pascut D, Wiemer M, Kaese S. Recording of bipolar multichannel ECGs by a smartwatch: modern ECG diagnostic 100 years after Einthoven. Sensors 2019;19:2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behzadi A, Sepehri Shamloo A, Mouratis K, Hindricks G, Arya A, Bollmann A. Feasibility and reliability of smartwatch to obtain 3-lead electrocardiogram recordings. Sensors 2020;20:5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avila CO. Novel use of Apple Watch 4 to obtain 3-lead electrocardiogram and detect cardiac ischemia. The Permanente Journal 2019;23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cobos Gil MA. Standard and precordial leads obtained with an Apple Watch. Annals of internal medicine 2020;173:249–250. [DOI] [PubMed] [Google Scholar]
- 19.Samol A, Bischof K, Luani B, Pascut D, Wiemer M, Kaese S. Single-lead ECG recordings including Einthoven and Wilson leads by a smartwatch: a new era of patient directed early ECG differential diagnosis of cardiac diseases? Sensors 2019;19:4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spaccarotella CAM, Migliarino S, Mongiardo A, et al. Measurement of the QT interval using the Apple Watch. Scientific reports 2021;11:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleiman R, Darpo B, Brown R, et al. Comparison of electrocardiograms (ECG) waveforms and centralized ECG measurements between a simple 6-lead mobile ECG device and a standard 12-lead ECG. Annals of Noninvasive Electrocardiology 2021;26:e12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith WM, Riddell F, Madon M, Gleva MJ. Comparison of diagnostic value using a small, single channel, P-wave centric sternal ECG monitoring patch with a standard 3-lead Holter system over 24 hours. American heart journal 2017;185:67–73. [DOI] [PubMed] [Google Scholar]
- 23.Jorgenson DB, Haynor DR, Bardy GH, Kim Y. Computational studies of transthoracic and transvenous defibrillation in a detailed 3-D human thorax model. IEEE transactions on biomedical engineering 1995;42:172–184. [DOI] [PubMed] [Google Scholar]
- 24.Jorgenson DB, Schimpf PH, Shen I, et al. Predicting cardiothoracic voltages during high energy shocks: methodology and comparison of experimental to finite element model data. IEEE transactions on biomedical engineering 1995;42:559–571. [DOI] [PubMed] [Google Scholar]
- 25.Shrinidhi N, Haynor DR, Wang Y, Jorgenson DB, Bardy GH, Kim Y. An efficient tissue classifier for building patient-specific finite element models from X-ray CT images. IEEE transactions on biomedical engineering 1996;43:333–337. [DOI] [PubMed] [Google Scholar]
- 26.Rho R, Vossler M, Blancher S, Poole JE. Comparison of 2 ambulatory patch ECG monitors: The benefit of the P-wave and signal clarity. American heart journal 2018;203:109–117. [DOI] [PubMed] [Google Scholar]
- 27.Varma N, Cygankiewicz I, Turakhia M, et al. 2021 ISHNE/ HRS/ EHRA/ APHRS collaborative statement on mHealth in Arrhythmia Management: Digital Medical Tools for Heart Rhythm Professionals. Annals of Noninvasive Electrocardiology 2021;26:e12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witvliet MP, Karregat EP, Himmelreich JC, de Jong JS, Lucassen WA, Harskamp RE. Usefulness, pitfalls and interpretation of handheld single-lead electrocardiograms. Journal of Electrocardiology 2021;66:33–37. [DOI] [PubMed] [Google Scholar]
- 29.Wong KC, Klimis H, Lowres N, von Huben A, Marschner S, Chow CK. Diagnostic accuracy of handheld electrocardiogram devices in detecting atrial fibrillation in adults in community versus hospital settings: a systematic review and meta-analysis. Heart 2020;106:1211–1217. [DOI] [PubMed] [Google Scholar]
- 30.Ramkumar S, Nerlekar N, D’Souza D, Pol DJ, Kalman JM, Marwick TH. Atrial fibrillation detection using single lead portable electrocardiographic monitoring: a systematic review and meta-analysis. BMJ open 2018;8:e024178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Himmelreich JC, Karregat EP, Lucassen WA, et al. Diagnostic accuracy of a smartphone-operated, single-lead electrocardiography device for detection of rhythm and conduction abnormalities in primary care. The Annals of Family Medicine 2019;17:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldenthal IL, Sciacca RR, Riga T, et al. Recurrent atrial fibrillation/flutter detection after ablation or cardioversion using the AliveCor KardiaMobile device: iHEART results. Journal of cardiovascular electrophysiology 2019;30:2220–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koh KT, Law WC, Zaw WM, et al. Smartphone electrocardiogram for detecting atrial fibrillation after a cerebral ischaemic event: a multicentre randomized controlled trial. EP Europace 2021. [DOI] [PubMed] [Google Scholar]
- 34.Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. The American journal of medicine 2014;127:95. e11–95. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stavrakis S, Garabelli PJ, Smith L, Albert D, Po SS. Clinical Validation of a Smartphone Based, 6-lead ECG Device. Circulation 2017;136:A15576–A15576. [Google Scholar]
- 36.Frisch DR, Weiss M, Dikdan SJ, Keith SW, Sarkar K. Improved accuracy and confidence with multiple-lead recordings from a single-lead mobile electrocardiographic device. Pacing and Clinical Electrophysiology 2019;42:1191–1196. [DOI] [PubMed] [Google Scholar]
- 37.Aljuaid M, Marashly Q, AlDanaf J, et al. Smartphone ECG monitoring system helps lower emergency room and clinic visits in post–atrial fibrillation ablation patients. Clinical Medicine Insights: Cardiology 2020; 14:1179546820901508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Journal of the American College of Cardiology 2018;72:2231–2264. [DOI] [PubMed] [Google Scholar]
- 39.Spaccarotella CAM, Polimeni A, Migliarino S, et al. Multichannel electrocardiograms obtained by a smartwatch for the diagnosis of ST-segment changes. JAMA cardiology 2020;5:1176–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strik M, Caillol T, Ramirez FD, et al. Validating QT-interval measurement using the apple watch ECG to enable remote monitoring during the COVID-19 pandemic. Circulation 2020;142:416–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Titus-Lay EN, Jaynes HA, Tomaselli Muensterman E, et al. Accuracy of a single-lead mobile smartphone electrocardiogram for QT interval measurement in patients undergoing maintenance methadone therapy. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 2021. [DOI] [PubMed] [Google Scholar]
- 42.Frisch DR, Frankel ES, Farzad DJ, Woo SH, Kubey AA. Initial Experience in Monitoring QT Intervals Using a Six-lead Contactless Mobile Electrocardiogram in an Inpatient Setting. The Journal of Innovations in Cardiac Rhythm Management 2021;12:4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minguito-Carazo C, Echarte-Morales J, Benito-Gonzalez T, et al. QT Interval Monitoring with Handheld Heart Rhythm ECG Device in COVID-19 Patients. Global Heart 2021;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reiffel JA, Schwarzberg R, Murry M. Comparison of autotriggered memory loop recorders versus standard loop recorders versus 24-hour Holter monitors for arrhythmia detection. The American journal of cardiology 2005;95:1055–1059. [DOI] [PubMed] [Google Scholar]
- 45.Diederichsen SZ, Haugan KJ, Kronborg C, et al. Comprehensive evaluation of rhythm monitoring strategies in screening for atrial fibrillation: insights from patients at risk monitored long term with an implantable loop recorder. Circulation 2020;141:1510–1522. [DOI] [PubMed] [Google Scholar]
- 46.Seshadri DR, Bittel B, Browsky D, et al. Accuracy of Apple Watch for detection of atrial fibrillation. Circulation 2020;141:702–703. [DOI] [PubMed] [Google Scholar]
- 47.Garson A Jr. Clinically significant differences between the “old” analog and the “new” digital electrocardiograms. American heart journal 1987;114:194–197. [DOI] [PubMed] [Google Scholar]
- 48.Ding EY, Svennberg E, Wurster C, et al. Survey of current perspectives on consumer-available digital health devices for detecting atrial fibrillation. Cardiovascular digital health journal 2020;1:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pitman BM, Chew S-H, Wong CX, et al. Performance of a Mobile Single-Lead Electrocardiogram Technology for Atrial Fibrillation Screening in a Semirural African Population: Insights From “The Heart of Ethiopia: Focus on Atrial Fibrillation”(TEFF-AF) Study. JMIR mHealth and uHealth 2021;9:e24470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karregat EP, Himmelreich JC, Lucassen WA, Busschers WB, van Weert HC, Harskamp RE. Evaluation of general practitioners’ single-lead electrocardiogram interpretation skills: a case-vignette study. Family practice 2021;38:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frisch DR. Diagnosing atrial fibrillation by mobile technology: physician decision or device provision? Vol 106: BMJ Publishing Group Ltd and British Cardiovascular Society; 2020:629–630. [DOI] [PubMed] [Google Scholar]
- 52.Chan N-Y, Choy C-C, Chan C-K, Siu C-W. Effectiveness of a nongovernmental organization–led large-scale community atrial fibrillation screening program using the smartphone electrocardiogram: an observational cohort study. Heart Rhythm 2018;15:1306–1311. [DOI] [PubMed] [Google Scholar]
- 53.Siontis KC, Yao X, Pirruccello JP, Philippakis AA, Noseworthy PA. How will machine learning inform the clinical care of atrial fibrillation? Circulation research 2020;127:155–169. [DOI] [PubMed] [Google Scholar]
- 54.Brisk R, Bond R, Finlay D, McEneaney D. Personal ECG Devices: How Will Healthcare Systems Cope? A Single Centre Case Study. Paper presented at: 2019 Computing in Cardiology (CinC) 2019. [Google Scholar]
- 55.Izmailova ES, Wagner JA, Perakslis ED. Wearable devices in clinical trials: hype and hypothesis. Clinical Pharmacology & Therapeutics 2018;104:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiang J, Dong Y, Xue X, Xiong H. Electronics of a wearable ECG with level crossing sampling and human body communication. IEEE transactions on biomedical circuits and systems 2018;13:68–79. [DOI] [PubMed] [Google Scholar]
- 57.Turakhia MP, Desai M, Hedlin H, et al. Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: The Apple Heart Study. American heart journal 2019;207:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Masterson Creber RM, Reading Turchioe M, Biviano A, et al. Cardiac symptom burden and arrhythmia recurrence drives digital health use: results from the iHEART randomized controlled trial. European Journal of Cardiovascular Nursing 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]